Abstract
Rationale
To protect against HIV infection, passively transferred and/or vaccine elicited neutralizing antibodies (NAbs) need to effectively target diverse subtypes that are transmitted globally. These variants are a limited subset of those present during chronic infection and display some unique features. In the case of mother-to-child transmission (MTCT), transmitted variants tend to be resistant to neutralization by maternal autologous NAbs.
Method
To investigate whether variants transmitted during MTCT are generally resistant to HIV-1 specific NAbs, 107 maternal or infant variants representing the dominant HIV-1 subtypes were tested against six recently identified HIV-1-specific broadly neutralizing monoclonal antibodies (bNAbs), NIH45-46W, VRC01, PGT128, PGT121, PG9, and PGT145.
Results
Infant and maternal variants did not differ in their neutralization sensitivity to individual bNAbs, nor did viruses from transmitting versus non-transmitting mothers, although there was a trend for viruses from transmitting mothers to be less sensitive overall. No single bNAb neutralized all viruses, but a combination of bNAbs that target distinct epitopes covered 100% of the variants tested. Compared to heterosexually transmitted variants, vertically transmitted variants, were significantly more sensitive to neutralization by PGT128 and PGT121 (p=0.03 in both cases) but there were no differences for the other bNAbs. Overall, subtype A variants were significantly more sensitive to NIH45-46 (p=0.04), VRC01 (p=0.002) and PGT145 (p=0.03) compared to the non-subtype A and less sensitive to PGT121 than subtype Cs (p=0.0001).
Conclusion
A combination of bNAbs against distinct epitopes may be needed to provide maximum coverage against viruses in different modes of transmission and diverse subtypes.
Keywords: HIV, MTCT, monoclonal, neutralizing, antibodies
Introduction
Neutralizing antibodies (NAbs) are a common feature of successful viral vaccines [1-3] and thus have been the subject of intense study for HIV-1. Passive immunization studies in the macaque model have provided proof-of-concept that NAbs can protect against HIV-1 infection [4]. However, because these studies were performed using select subtype B HIV-1 variants, and monoclonal antibodies (mabs) that were known to effectively neutralize the challenge virus, the potential of this approach to limit the spread of diverse circulating HIV-1 variants remains unclear. In order for passively transferred or vaccine elicited NAbs to halt the spread of HIV-1, they will have to effectively target transmitted variants from the major global subtypes, particularly those common in sub-Saharan Africa such as A, C and D [5-7]
Recently, HIV-1-specific NAbs with improved breadth (bNAbs) and potency across major subtypes were isolated from HIV-1 chronically infected individuals. Some of the new bNAbs interact with the CD4 binding site (CD4bs)[8-10] similar to the less potent HIV-1 mab, b12, which demonstrated protection in passive studies in macaques [11, 12]. Examples of CD4bs antibodies include VRC01 and NIH45-46, which were isolated from the same individual. NIH45-46W is an engineered form of NIH45-46 and displays enhanced breadth and potency through improved interaction with the hydrophobic CD4 binding pocket in gp120 [13]. Another class of bNAbs target the variable regions, the prototype of which are PG9 and PGT145. These antibodies show specificity for a quaternary epitope formed in the context of the envelope (Env) trimer in V1/V2, and are dependent on conserved N-linked glycans at position 156 and 160 [14-16]. Two other bNAbs, PGT121 and PGT128, target an epitope in the V3 loop, which includes conserved N- linked glycans at positions 301 and 332 [17, 18]. Breadth and potency of these bNAbs has been defined by screening against viruses from different stages of infection representing the major circulating subtypes worldwide [13, 14, 17, 19-21] and in a more recent study, variants from acute heterosexual transmission cases [22].
For NAbs to block HIV-1 infection, they must neutralize transmitted strains of HIV-1, which are a subset of relatively unique variants because only a subset of the variants in the index person is transmitted to the exposed recipient [23]. Several studies have suggested that the bottleneck imposed during transmission has an effect on sensitivity of transmitted variants to plasma NAbs [24-28]. Indeed, a recent study identified some bNAbs that displayed a breadth profile for viruses from acute heterosexual transmission that was distinct from what was observed in earlier studies with chronic stage viruses [22]. For example, glycan dependent antibodies PGT121, PGT128 and PGT145 had 2-3 fold lower breadth against variants from acute heterosexual infection, while no differences were noted for CD4bs antibodies [22].
MTCT occurs in the presence of passively acquired antibodies and transmitted variants have been shown to be less sensitive to maternal plasma autologous neutralizing antibodies (aNAbs) than maternal variants in some studies, suggesting a role for maternal antibodies in selecting for transmitted variants[26-28]. These variants also have fewer potential N-linked glycosylation sites (PNLGs), which can impact bNAb recognition, particularly those dependent on glycans [26, 29]. Thus far, the breadth of bNAbs against early stage MTCT variants remains unknown, yet this is relevant for considering the likely efficacy of passive antibody approaches for prevention of MTCT.
There is interest in whether HIV-1-specific bNAbs can contribute to prevention of MTCT [30]. The utility of such bNAbs however, depends on their ability to provide maximum coverage to match or exceed the current successful standard of care, which includes anti-retroviral prophylaxis for mothers and infants [31]. Thus, a better understanding of the relative breadth and potency of these new bNAbs against vertically transmitted viruses, and whether transmitted and chronic variants of diverse subtypes have distinct neutralization profiles will be important in considering how best to harness their potential. Here, we determined differences in neutralization sensitivity of envelope variants of diverse subtypes obtained from infants and from transmitting and non-transmitting mothers infected with subtypes A, C and D. The viruses transmitted to infants were generally similar in sensitivity to variants from their chronically infected mothers as well as variants from non-transmitting mothers. However, the breadth and potency of the bNAbs varied by subtype.
Materials and methods
Study subjects and viruses
Envelope variants (envs) were obtained from mothers and infants who participated in the Nairobi randomized breastfeeding clinical trial [32]. Envs were isolated directly from maternal PBMCs (n= 75), breast milk cells (n=10) or infant PBMCs (n=22) and were obtained using limiting dilution single copy PCR and cloning methods as described in [26, 33] (and Majiwa, unpublished). PBMC-derived virus may provide the best representative sequences of the transmitted variants in the case of infants and our prior studies have shown that the dominant PBMC variant sequences are representative of the predominant replication-competent virus population [34]. Maternal envs were obtained in late pregnancy, delivery, or by week 14 post-partum. Infant envs were obtained from 10 breastfeeding infants at the time they first tested HIV positive [26]; the infants were HIV-1 negative at birth and tested positive at 6 weeks (n=9) and 6 months (n=1) postpartum. The ethical review committees of the University of Nairobi, the University of Washington and the Fred Hutchinson Cancer Research Center approved this study (IRB of record, University of Washington; 11540).
Neutralization assays
Neutralization sensitivity was tested using a single cycle assay in TZM-bl target cells as described [26, 33]. In this HeLa cell-based assay, 6 × 2-fold serial dilutions of antibody were tested with each virus in duplicate, which we have found gives comparable results to triplicate testing in prior studies. bNAbs tested included: NIH 45-46W, VRC01, PGT121, PGT128, PG9, PGT145 and b12. Because the bNAbs are highly potent and were only available in limited supply, the highest concentration of antibody tested was 1μg/ml for all bNAbs except for b12 (10μg/ml). Viruses that were neutralized at greater than 50% at the lowest dilution tested (0.033μg/ml) were retested starting at 0.5μg/ml. Fifty percent inhibitory concentration (IC50) was defined as the concentration of NAb that resulted in 50% inhibition, as previously described [26]. At least two independent experiments were performed and the mean of the two IC50s was used for the analysis. In cases where IC50s from independent runs showed a difference of greater than 2.5 fold, a third run was performed and the most divergent value was excluded. In cases where 50% neutralization wasn't achieved at the highest bNAb concentration tested, an IC50 value corresponding to the highest concentration tested was used in the analysis.
Statistical analysis
Neutralization IC50 values were dichotomized, using the highest bNAb concentration tested of 1μg/ml as the cut-off. Generalized estimating equation (GEE) with a logit link and exchangeable correlation structure was used to analyze the majority of the data. For some specific analyses where the GEE model did not converge due to small sample size, Fisher's exact test was used. To compare differences in sensitivities between maternal and infant variants, only data from the 10 mother-infant pairs with matched variants were used (n=75). Only maternal variants were used to compare differences in sensitivities of variants from transmitters versus non-transmitters. All maternal and infant viruses (n=107) were used to compare differences in sensitivities between subtypes. IC50 values from a heterosexual transmission cohort of adult women were also included in some subtype comparisons [22]. All analyses were done using R version 2.10.1.
Results
Neutralization sensitivity of mother-infant variants
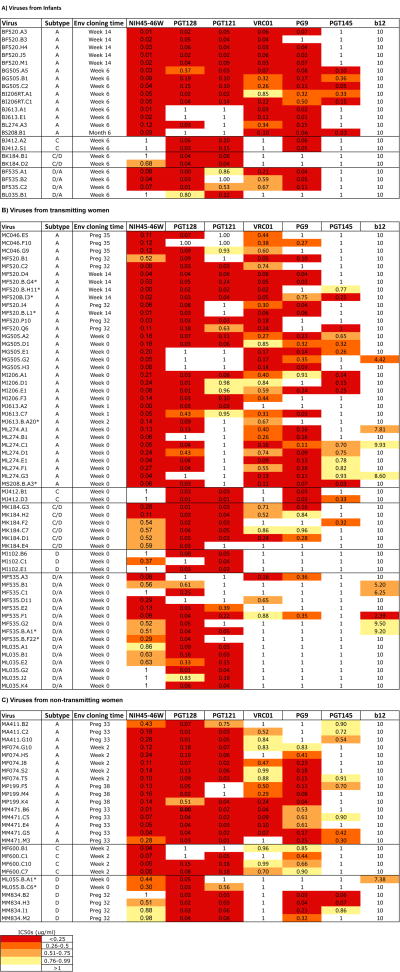
To examine the neutralization profiles of infant (transmitted acute) and maternal (circulating chronic) variants, env clones from mothers and infants enrolled in the Nairobi randomized breastfeeding clinical trial [32] were screened. A total of 107 variants consisting of 59 envs from 12 transmitting mothers, 26 envs from 7 non-transmitting mothers, and 22 envs from 10 infants were included in this study. These included 75 variants from 10 mother-infant pairs. We examined the neutralization profile of several variants from each individual in most cases, which provides a measure of neutralization sensitivity of representative viruses in the individual although it may not cover the full spectrum of diversity in the virus population. The virus panel comprised of envs from 3 major subtypes (A, C and D) and some inter-subtype recombinants (C/D and D/A) (Fig. 1).
Figure 1. Summary of neutralization sensitivity profiles of all mother –infant viruses against bNAbs.
Viruses have been grouped into three panels representing (A) viruses obtained from infants, (B) viruses obtained from transmitting mothers and (C) viruses obtained from non-transmitting mothers. Each row represents (from left to right) the virus name, virus subtype based on V1-V5 envelope sequence, the time of sample from which envelope was isolated and the IC50 values for all bNAbs tested. Viruses were obtained as described previously [26, 33]. Maternal PBMC variants were obtained from blood samples collected at various weeks of pregnancy (preg) and after delivery (week) and the time ranged from preg 32 to week 14 (Week 0 refers to the first week post-partum). Breast milk variants were obtained from week 0, 2 and 14 and are indicated with an asterisk. All infants were HIV-DNA negative at birth. Infant samples were obtained from the first time-point at which infants tested HIV-DNA positive (week 6 or 14 and month 6). IC50 values range from 0.001-1μg/ml except for b12 (2.39-10μg/ml) and are grouped by quartiles as shown in the key to the upper right. Darker shading indicates increasing bNAb potency defined in the key at the bottom left. White color indicates that 50% neutralization was not achieved at the highest concentration of bNAb tested 1μg/ml or 10μg/ml for the case of b12. IC50s are an average of at least two independent experiments performed in duplicates.
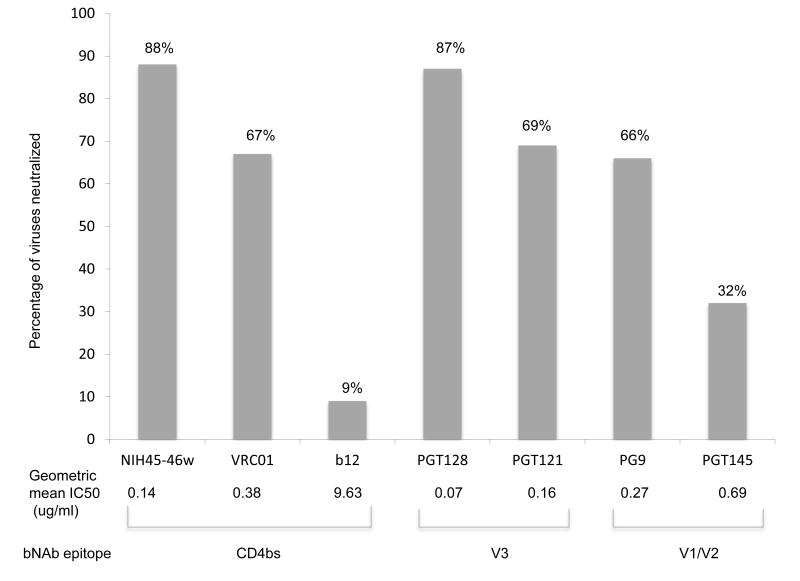
All variants were sensitive to at least two of the bNAbs tested and several (n=13) were neutralized by all 6 bNAbs; however no variants were sensitive to all 7 MAbs including b12, which exhibited little breadth and potency (Fig 2). NIH45-46W neutralized the highest percentage of viruses (88%) with a geometric mean IC50 of 0.14μg/ml. The glycan-dependent PGT128, neutralized 87% of the variants, and was the most potent (geometric mean IC50 of 0.07μg/ml). PGT121, VRC01 and PG9 showed similar coverage, neutralizing 69%, 67% and 66% of the variants with geometric mean IC50s of 0.16, 0.38 and 0.27μg/ml, respectively. PGT145, which shares a target site with PG9 [35], neutralized only 32% of the variants and was also the least potent of the new bNAbs (geometric mean IC50 of 0.69μg/ml). Only 9% of the variants were neutralized by b12 (Fig. 2). As observed in prior studies, bNAbs targeting distinct epitopes displayed considerable neutralization complementarity while neutralization profiles from those targeting similar epitopes were mainly overlapping (Supplementary Fig 1 and 2) [20, 22]
Figure 2. Neutralization sensitivity of mother-infant variants to bNAbs.
The Y-axis represents percentage of variants that achieved 50% neutralization to the bNAb indicated at the bottom of each bar on the X- axis. All bNAbs were tested at a starting dilution of 1μg/ml while b12 was tested at a starting dilution of 10μg/ml. Results are from the average of two duplicate experiments of each virus/bNAb combination. The geometric mean IC50 for each bNAb is shown at the bottom of each corresponding bar. Bars from bNAbs that target similar epitopes have been grouped together as shown at the bottom of the graph.
Neutralization sensitivity of variants from mother-infant pairs and transmitting and non-transmitting mothers
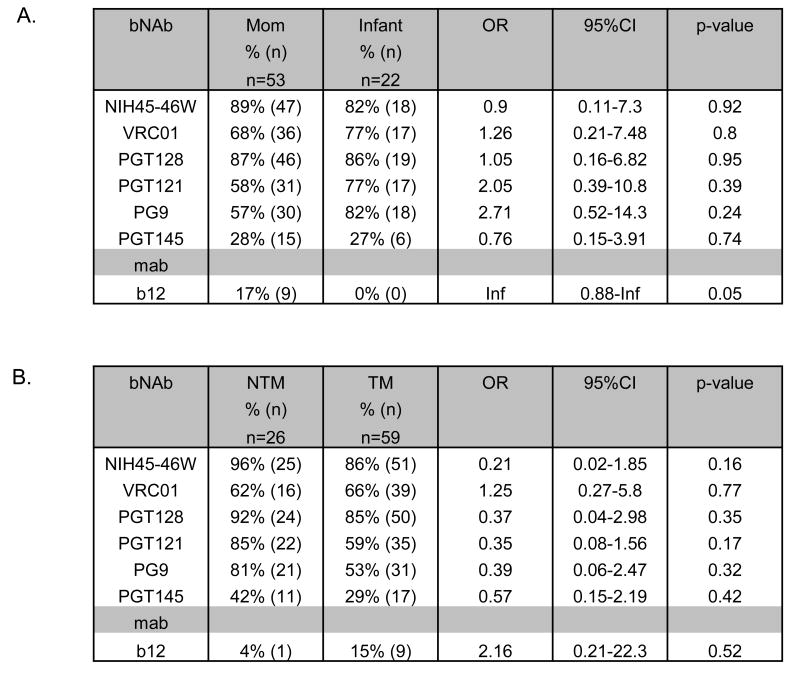
To examine differences in neutralization sensitivity between maternal and infant env variants, we compared IC50s for 75 variants from matched mother-infant pairs, including 53 from mothers and 22 from infants. Overall, we did not find any statistically significant differences between neutralization sensitivity of maternal and infant variants against the new bNAbs (Fig. 3A). However, only maternal but not infant variants were neutralized by b12 (p = 0.05). Similarly, we did not observe statistically significant differences between vertically transmitted variants from infants (n=22) and all the variants obtained from chronic infection from transmitting and non-transmitting mothers (n= 85) (Supplementary Fig. 3). There was no consistent pattern of infant variants being more resistant to the bNAbs than maternal variants in either analysis; there were several cases where the percentage of infant viruses neutralized was higher and some where it was lower than that of maternal viruses.
Figure 3. Summary of neutralization sensitivity of infant and maternal variants to bNAbs.
Comparison of neutralization IC50s for variants obtained from mother- infant pairs (A) and from non-transmitting mothers (NTM) and transmitting mothers (TM) (B). “n” indicates number of individual variants. All analyses were performed by generalized estimating equations (GEE) using a logit link and exchangeable correlation structure, except where marked with *, which indicates that Fisher's exact test was performed. “+” indicates the reference group used for the analyses. The p values for each bNAb are shown. IC50s are an average of at least 2 independent experiments
We also determined whether there were differences in neutralization sensitivity between variants from non-transmitting (n=26) and transmitting mothers (n=59). Interestingly, in all cases except VRC01 and b12, the percentage of viruses neutralized was higher in the non-transmitters compared to the transmitters, although these differences did not achieve statistical significance when bNAbs were considered individually (Fig 3B). We therefore performed an aggregate analysis considering neutralization sensitivity to all the bNAbs and observed that transmitting mothers showed a trend for having viruses that were more resistant to bNAbs compared to viruses from non-transmitting mothers (p = 0.06, data not shown).
Influence of mode of transmission on neutralization sensitivity of infant variants to bNAbs
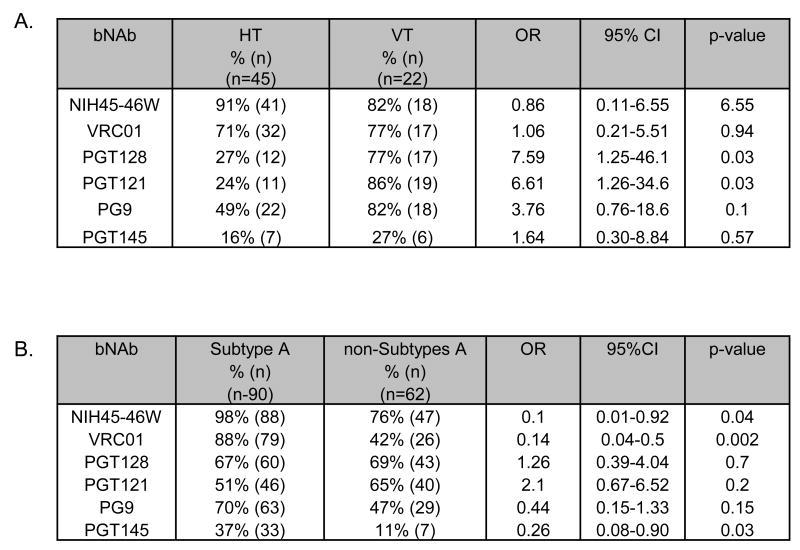
To examine whether there are differences in sensitivity between vertically and heterosexually transmitted variants, we performed an analysis comparing the sensitivity of 45 variants from acute heterosexual transmission isolated from individuals in Kenya to the same bNAbs [22] and the 22 variants from vertical transmission. Interestingly, vertically transmitted variants were significantly more sensitive to PGT128 and PGT121 (p = 0.03 in both cases); there were no differences for the other bNAbs (Fig 4A).
Figure 4. Summary of neutralization sensitivity of variants to bNAbs by transmission mode and subtype.
Comparison of neutralization IC50s for variants obtained from heterosexual transmission (HT) [22] and vertical transmission (VT) (A). Comparison of a results from viruses examined in this study as well as 45 variants from recently infected women in the prior study (the HT viruses in panel A) grouped as either subtype A or non-subtype A (includes C,D and inter-subtype recombinants C/D)(B) . “n” indicates number of individual variants. All analyses were performed by generalized estimating equations (GEE) using a logit link and exchangeable correlation structure. IC50s used are an average of at least 2 independent experiments. “+” indicates the reference group used for the analyses. The p values for each bNAb are shown.
Influence of subtype on neutralization sensitivity to bNAbs
Differences in neutralization sensitivity were explored in relation to subtype using the data from the 107 envelopes examined here, as well as 45 heterosexually transmitted variants isolated from individuals in Kenya analyzed in a prior study [22]. Subtype A variants were significantly more sensitive to the CD4bs antibodies NIH45-46W (p < 0.04) and VRC01 (p <0.002) compared to the non-subtype A variants (Fig. 4B). In addition, subtype A variants were more sensitive to PGT145 and PG9, which target a quaternary epitope formed by V1/V2 loops, compared to non-subtype A variants, although the latter did not achieve statistical significance (p = 0.03 and 0.15, respectively). Similar results were observed for NIH45-46W and VRC01 when we excluded subtype A recombinants from the non-A group (supplementary Fig 4A). Similar differences in neutralization by subtype were observed when we considered only the 107 mother-infant variants (data not shown).
Subtype A variants were significantly more sensitive to VRC01 (p = 0.03) and PGT145 (p = 0.007) but not to NIH45-46W (p = 0.11) when compared to subtype C variants (C and C/D), and also significantly more sensitive to NIH45-46W (p = 0.03), VRC01 (p = 0.002) but not PGT145 (p = 0.42) when compared to subtype D variants (D and C/D; Supplementary Fig 4B and C respectively). Thus, differences in neutralization of subtype A versus non-A appears to be due to reduced sensitivity of both subtype C and D viruses depending on the bNAb. Subtype A variants were significantly less sensitive to PGT121 compared to the combination of subtype C (C and C/D, p = 0.0001; Supplementary Fig 4B), but there were no differences between subtype A and variants encoding subtype D sequences (D and C/D) (p = 0.4; Supplementary Fig 4C), suggesting that the enhanced sensitivity of non-A variants may be largely due to the sensitivity of subtype C to PGT121. Among the 16 non-subtype A variants resistant to NIH45-46W, the broadest bNAb tested, 14 were sensitive to PGT121 (Supplementary Fig. 5A), whereas of the 2 subtype A variants that were resistant to NIH45-46W, none were neutralized by PGT121 (Supplementary Fig. 5B).
Discussion
Circulating HIV-1 variants found in HIV-1 endemic regions such as subtype A, C and D viruses, are critical targets for vaccines. Recently, new HIV-1 bNAbs have been isolated that show breadth against all HIV-1 subtypes but their neutralization profile against vertically transmitted circulating variants representing diverse subtypes from endemic regions has not been defined. We determined the neutralization sensitivities of 107 HIV-1 variants from a MTCT cohort from Nairobi, Kenya against bNAbs that target the CD4bs and glycan-dependent and/or quaternary epitopes in V1/V2 and V3. These variants displayed high sensitivity profiles similar to those observed in studies of other virus panels primarily composed of chronic strains of HIV-1 variants. We did not observe any significant differences in neutralization sensitivity between the transmitted variants from the infants and variants obtained from the transmitting mothers, but there was a trend for variants from transmitting mothers to be more resistant to neutralization by the bNAbs than those from non-transmitting mothers. We observed significant differences in neutralization sensitivity between subtypes, with subtype A being significantly more sensitive than non-subtype A to the CD4bs antibodies, NIH45-46W and VRC01, and glycan dependent PGT145, but less sensitive to PGT121.
NIH45-46W, PGT128 and PG9 showed the greatest breadth and potency in their respective antibody class among available bNAbs. Mother-infant viruses were most sensitive to antibodies NIH45-46W and PGT128, which target CD4bs and V3, respectively [13, 17]. Interestingly, we observed that neutralization sensitivity of variants to these 2 bNAbs was complementary, resulting in 100% coverage of all variants tested, as was observed for recently transmitted variants from adult women [22]. We also observed that the neutralization profiles of PGT128 and PG9 in combination would result in neutralization of 98% of viruses. Overall, these observations support previous data showing that broad neutralization coverage of diverse variants from both chronic and acute infection can be achieved when combining bNAbs that target independent epitopes [20, 22].
The subtypes commonly found in sub-Saharan Africa display limited sensitivity to first generation antibodies directed at the CD4bs and glycans such as b12 and 2G12, respectively [14, 19, 36, 37]. Consistent with this, b12 could only neutralize 9% of variants tested in this screen. Compared to non-subtype A variants, we found that subtype A variants were significantly more sensitive to neutralization by CD4bs antibodies, NIH45-46W and VRC01, and V1/V2 directed antibody PGT145. Further analysis suggested that the differences in neutralization were driven primarily by differences between subtypes A and D for NIH 45-46W, and differences in both C and D versus A for VRC01. It remains unclear why subtype As are particularly sensitive to these antibodies, which were obtained from a subtype B infected individual, but the data may suggest there are differences in the CD4bs among the various subtypes.
Glycan-dependent antibodies displayed varied neutralization profiles depending on subtype. Subtype A variants were more sensitive to PGT145 and PG9 compared to non-subtype As, although the difference did not reach statistical significance for the latter. The difference observed with PGT145 primarily reflected poor neutralization of subtype Cs. This result is somewhat surprising given a prior study showing that PGT145 neutralized a higher percentage of subtype C viruses compared to subtype As at 1μg/ml (59% vs 48% respectively), although the statistical significance of this difference was not evaluated [17].
In contrast to the CD4bs bNAbs, NIH45-46W-and VRC01, and V1/V2 directed PGT145, V3-directed, PGT121 displayed broader coverage and PGT128 showed a trend towards better coverage of non-subtype As, particularly subtype C. This difference in subtype preference could explain why PGT121 and PGT128 could effectively complement the CD4bs bNAbs. Thus, a combination of CD4bs and V3-directed bNAbs could overcome the constraints resulting from subtype differences.
We did not observe any significant differences in sensitivity profiles of transmitted variants from infants and variants from transmitting mothers with the bNAbs, although we did find that maternal variants were more sensitive to the mab b12 compared to infant variants. These findings indicate that although infant variants are transmitted in the face of aNAbs and tend to be escape variants [26, 27, 38, 39], they are not inherently resistant to neutralization by bNAbs. Likewise, there were no significant differences in neutralization sensitivity to any particular bNAb between variants from transmitting and non-transmitting mothers. However, in an aggregate analysis, viruses from non-transmitting mothers were generally more sensitive to neutralization, suggesting that there could be subtle differences in the neutralization properties of variants from transmitting mothers. Larger studies will be needed to explore this possibility.
In general, the sensitivity profile of our panel of chronic and acute viruses was comparable to the panels tested previously, which included mainly viruses from chronic infection [13, 14, 17, 19-21]. Similarly, the breadth of CD4bs antibodies NIH45-46W and VRC01 against variants from acute heterosexual infection (71-91%) and variants tested here was similar (overall, 67-88% and infant variants only, 77-82%) [22]. However, the glycan dependent antibodies PGT121, PGT128, PGT145, and PG9 had greater breadth against our panel of variants than against those from acute heterosexual transmission (32-87% versus 16-49%). The presence or absence of the reported critical glycans was not in itself sufficient to explain the activity of these antibodies, an observation consistent with previous reports, although the presence of N332 significantly increased sensitivity to PGT128 (p= 0.002) but not PGT121 (p=0.09) (data not shown) [22, 40]. PGT128 displayed the largest difference in breadth for mother-infant (87%) versus acute heterosexual variants (27%). Two recent studies suggested that heterosexually transmitted viruses from the early stage of HIV-1 infection are often resistant to PGT128 [22, 41]; in the case of subtype C, early heterosexually transmitted viruses were shown to be less sensitive to PGT128 than chronic stage viruses [41]. In this study, the percentage of variants neutralized was similar for infants (vertical transmission) versus mothers (chronic infection). Thus, our data suggest that, in contrast to heterosexual transmission, there is not a selective bottleneck against transmission of PGT128-sensitive variants in MTCT. Consistent with this hypothesis, infant variants were significantly more sensitive to PGT128 than viruses transmitted heterosexually. This was also true for PGT121, which targets a similar epitope. Importantly, PGT128 was found to effectively complement the activity of CD4bs bNAbs for maternal-infant variants, and this was also true for heterosexually acquired viruses, despite the lower sensitivity of these variants to PGT128[22].
In conclusion, we have shown that representative HIV-1 transmitted variants from infants and variants obtained from transmitting and non-transmitting mothers are susceptible to newly isolated bNAbs. A combination of bNAbs that target different epitopes leads to broad neutralization coverage of both maternal and infant variants, as was observed with other virus panels [22, 40]. Our data suggest that the broad coverage attained by combining antibodies may be partly due to the fact that the bNAbs show distinct subtype biases. Thus, an immunogen that elicits multiple antibody specificities similar to those represented here may be needed to provide the required neutralization breadth to block diverse HIV-1 subtypes.
Supplementary Material
Supplementary Figure 1. A heatmap showing sensitivity profiles of the most broad and potent bNAbs in each class. Each row shows the IC50s of maternal and infant variants tested against the bNAb that target distinct epitopes indicated at the bottom. Darker shading indicates increasing bNAb potency and white denotes variants resistant to neutralization by corresponding bNAb at highest concentration tested (1μg/ml). Color key values are in the range from 0.001-1μg/ml and are grouped by quartiles as shown in the key to the upper left. Viruses resistant to either NIH45-46W or PGT128 have been clustered together to emphasize the complete complementary activity of these bNAbs. IC50s are an average of at least two independent experiments.
Supplementary Figure 2: A heatmap comparing the neutralization profiles bNAbs that target similar epitopes. Each row shows the IC50s from maternal and infant variants for pairs of bNAbs that target similar epitopes indicated at the bottom. Panels represent (A) pairs of bNAbs that target the CD4bs, (B) a glycan dependent epitope in the V3 loop and (C) a glycan dependent epitope in the V1/V2 loop. Darker shading indicates increasing bNAb potency and white denotes variants resistant to neutralization by corresponding bNAb at highest concentration tested (1μg/ml). Color key values are in the range from 0.001-1μg/ml and are grouped by quartiles as shown in the key to the upper left. IC50s are an average of at least two independent experiments.
Supplementary Figure 3. Comparison of neutralization sensitivity of infant variants versus all maternal variants to bNAbs. Analyses were performed by GEE using a logit link and exchangeable correlation structure, except where marked by *, which indicates Fisher's exact test. “+” indicates the reference group for analyses. The p values for each bNAb are shown. IC50s are an average of at least 2 independent experiments.
Supplementary Figure 4. Detailed analysis of the neutralization sensitivity of different HIV-1 subtypes to bNAbs. Comparison of subtype A variants against non-subtype A variants (excluding subtype A recombinants) (A). Comparison of subtype A variants against C and C/D recombinants (excluding subtype A recombinants and pure subtype D) (B). Comparison of subtype A against D and C/D recombinants (excluding subtype A recombinants and pure subtype C) (C). Analysis was performed by GEE using a logit link and exchangeable correlation structure. “+” indicates the reference group for analyses. The p values for each bNAb are shown. IC50s are an average of at least 2 independent experiments.
Supplementary Figure 5. Subtype complementary activity of CD4bs bNAb, NIH45-46W with V3-specific bNAb, PGT121. Each row shows the IC50s of maternal -infant and heterosexually transmitted variants against bNAb NIH45-46W or PGT121. Neutralization profiles of NIH45-46W and PGT 121 against non-subtype A variants (A), or those of NIH45-46W and PGT 121 against subtype A variants (B) are shown. Darker shading indicates increasing bNAb potency and white denotes variants resistant to neutralization by corresponding bNAb at highest concentration tested (1μg/ml). Color key values are in the range from 0.001-1μg/ml and are grouped by quartiles as shown in the key in the upper right. IC50s are an average of at least two independent experiments.
Acknowledgments
We thank Xueling Wu, Stephanie Rainwater, and Dylan Peterson for generating some of the envelope plasmids used in our panel; Xueling Wu and John Mascola for providing bNAb VRC01; the IAVI Neutralizing Antibody Consortium for providing bNAbs PG9, PGT121, PGT128, PGT145; and Ron Diskin, Paola Marcovecchio and Pamela Bjorkman for providing NIH45-46W. We would like to thank Barbra Richardson and Katie-Odem Davis for their thoughtful input on the analyses. We also thank all the women who participated in the Nairobi breastfeeding randomized trial and the numerous investigators who carried out this trial.
This study was supported by NIH grant AI076105 to JO. JM and MOM were supported in part by a training grant from the Fogarty International center, NIH (grant D43- TW000007), and LG was supported in part by the Fred Hutchinson Cancer Research Center Interdisciplinary Research Fellowship.
References
- 1.Amanna IJ, Messaoudi I, Slifka MK. Protective immunity following vaccination: how is it defined? Hum Vaccin. 2008;4:316–319. doi: 10.4161/hv.4.4.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J. 2001;20:63–75. doi: 10.1097/00006454-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 4.Kramer VG, Siddappa NB, Ruprecht RM. Passive immunization as tool to identify protective HIV-1 Env epitopes. Current HIV Research. 2007;5:642–655. doi: 10.2174/157016207782418506. [DOI] [PubMed] [Google Scholar]
- 5.Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS. 2006;20:W13–23. doi: 10.1097/01.aids.0000247564.73009.bc. [DOI] [PubMed] [Google Scholar]
- 6.Hemelaar J, Gouws E, Ghys PD, Osmanov S, Characterisation WU. Global trends in molecular epidemiology of HIV-1 during 2000-2007. AIDS. 2011;25:679–689. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS. Report on Global HIV/AIDS Epidemic. 2010 [Google Scholar]
- 8.Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, et al. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Olivera TYK, et al. Sequence and Structural Convergence of Broad and Potent HIV Antibodies That Mimic CD4 Binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 12.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diskin R, Scheid JF, Marcovecchio PM, West AP, Klein F, Gao H, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker L. Broad and potent neutralizing antibodies from an African donor supp. Science. 2009:1–27. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1 neutralizing antibodies PG9 and PG16. J Virol. 2010 doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011 doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doria-Rose NA, Louder MK, Yang Z, O'Dell S, Nason M, Schmidt SD, et al. HIV-1 neutralization coverage is improved by combining monoclonal antibodies that target independent epitopes. J Virol. 2012;86:3393–3397. doi: 10.1128/JVI.06745-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Euler Z, Bunnik EM, Burger JA, Boeser-Nunnink BDM, Grijsen ML, Prins JM, et al. Activity of Broadly Neutralizing Antibodies, Including PG9, PG16, and VRC01, against Recently Transmitted Subtype B HIV-1 Variants from Early and Late in the Epidemic. J Virol. 2011;85:7236–7245. doi: 10.1128/JVI.00196-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goo L, Jalalian-Lechak Z, Richardson BA, Overbaugh J. A combination of broadly neutralizing HIV-1 monoclonal antibodies targeting distinct epitopes effectively neutralizes variants found in early infection. J Virol. 2012 doi: 10.1128/JVI.01414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagar M. HIV-1 transmission biology: selection and characteristics of infecting viruses. J Infect Dis. 2010;202(Suppl 2):S289–296. doi: 10.1086/655656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 25.Gray ES, Moore PL, Choge IA, Decker JM, Bibollet-Ruche F, Li H, et al. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J Virol. 2007;81:6187–6196. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Parast AB, Richardson BA, Nduati R, John-Stewart G, Mbori-Ngacha D, et al. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol. 2006;80:835–844. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickover R, Garratty E, Yusim K, Miller C, Korber B, Bryson Y. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J Virol. 2006;80:6525–6533. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Tully DC, Hoffmann FG, He J, Kankasa C, Wood C. Restricted genetic diversity of HIV-1 subtype C envelope glycoprotein from perinatally infected Zambian infants. PLoS ONE. 2010;5:e9294. doi: 10.1371/journal.pone.0009294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baan E, De Ronde A, Luchters S, Vyankandondera J, Lange JM, Pollakis G, et al. HIV Type 1 Mother-to-Child Transmission Facilitated by Distinctive Glycosylation Sites in the gp120 Envelope Glycoprotein. AIDS Res Hum Retroviruses. 2011:111024075717000. doi: 10.1089/AID.2011.0023. [DOI] [PubMed] [Google Scholar]
- 30.Bansal P. A summary of the workshop on passive immunization using monoclonal antibodies for HIV/AIDS, held at the National Institute of Allergy and Infectious Diseases, Bethesda, 10 March 2006. Biologicals. 2007;4:367–371. doi: 10.1016/j.biologicals.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sturt AS, Dokubo EK, Sint TT. Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database Syst Rev. 2010:CD008440. doi: 10.1002/14651858.CD008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 33.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-Specific Antibodies Capable of ADCC are Common in Breastmilk and are Associated with Reduced Risk of Transmission in Women with High Viral Loads. PLoS Pathog. 2012 doi: 10.1371/journal.ppat.1002739. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voronin Y, Chohan B, Emerman M, Overbaugh J. Primary isolates of human immunodeficiency virus type 1 are usually dominated by the major variants found in blood. J Virol. 2007;81:10232–10241. doi: 10.1128/JVI.01035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, et al. Broad and Potent Neutralizing Antibodies from an African Donor Reveal a New HIV-1 Vaccine Target. Science. 2009 doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blish C, Nedellec R, Mandaliya K, Mosier D, Overbaugh J. HIV-1 Subtype A Envelope Variants from Early in Infection Have Variable Sensitivity to Neutralization and to inhibitors of Viral Entry. AIDS. 2007;21:693–702. doi: 10.1097/QAD.0b013e32805e8727. [DOI] [PubMed] [Google Scholar]
- 37.Blish CA, Jalalian-Lechak Z, Rainwater S, Nguyen MA, Dogan OC, Overbaugh J. Cross-subtype neutralization sensitivity despite monoclonal antibody resistance among early subtype A, C, and D envelope variants of human immunodeficiency virus type 1. J Virol. 2009;83:7783–7788. doi: 10.1128/JVI.00673-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kliks SC, Wara DW, Landers DV, Levy JA. Features of HIV-1 that could influence maternal-child transmission. JAMA. 1994;272:467–474. [PubMed] [Google Scholar]
- 39.Zhang H, Rola M, West JT, Tully DC, Kubis P, He J, et al. Functional properties of the HIV-1 subtype C envelope glycoprotein associated with mother-to-child transmission. Virology. 2010;400:164–174. doi: 10.1016/j.virol.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doria-Rose NA, Georgiev I, O'Dell S, Chuang GY, Staupe RP, McLellan JS, et al. A Short Segment of the HIV-1 gp120 V1/V2 Region Is a Major Determinant of Resistance to V1/V2 Neutralizing Antibodies. J Virol. 2012 doi: 10.1128/JVI.00696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, et al. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med. 2012 doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. A heatmap showing sensitivity profiles of the most broad and potent bNAbs in each class. Each row shows the IC50s of maternal and infant variants tested against the bNAb that target distinct epitopes indicated at the bottom. Darker shading indicates increasing bNAb potency and white denotes variants resistant to neutralization by corresponding bNAb at highest concentration tested (1μg/ml). Color key values are in the range from 0.001-1μg/ml and are grouped by quartiles as shown in the key to the upper left. Viruses resistant to either NIH45-46W or PGT128 have been clustered together to emphasize the complete complementary activity of these bNAbs. IC50s are an average of at least two independent experiments.
Supplementary Figure 2: A heatmap comparing the neutralization profiles bNAbs that target similar epitopes. Each row shows the IC50s from maternal and infant variants for pairs of bNAbs that target similar epitopes indicated at the bottom. Panels represent (A) pairs of bNAbs that target the CD4bs, (B) a glycan dependent epitope in the V3 loop and (C) a glycan dependent epitope in the V1/V2 loop. Darker shading indicates increasing bNAb potency and white denotes variants resistant to neutralization by corresponding bNAb at highest concentration tested (1μg/ml). Color key values are in the range from 0.001-1μg/ml and are grouped by quartiles as shown in the key to the upper left. IC50s are an average of at least two independent experiments.
Supplementary Figure 3. Comparison of neutralization sensitivity of infant variants versus all maternal variants to bNAbs. Analyses were performed by GEE using a logit link and exchangeable correlation structure, except where marked by *, which indicates Fisher's exact test. “+” indicates the reference group for analyses. The p values for each bNAb are shown. IC50s are an average of at least 2 independent experiments.
Supplementary Figure 4. Detailed analysis of the neutralization sensitivity of different HIV-1 subtypes to bNAbs. Comparison of subtype A variants against non-subtype A variants (excluding subtype A recombinants) (A). Comparison of subtype A variants against C and C/D recombinants (excluding subtype A recombinants and pure subtype D) (B). Comparison of subtype A against D and C/D recombinants (excluding subtype A recombinants and pure subtype C) (C). Analysis was performed by GEE using a logit link and exchangeable correlation structure. “+” indicates the reference group for analyses. The p values for each bNAb are shown. IC50s are an average of at least 2 independent experiments.
Supplementary Figure 5. Subtype complementary activity of CD4bs bNAb, NIH45-46W with V3-specific bNAb, PGT121. Each row shows the IC50s of maternal -infant and heterosexually transmitted variants against bNAb NIH45-46W or PGT121. Neutralization profiles of NIH45-46W and PGT 121 against non-subtype A variants (A), or those of NIH45-46W and PGT 121 against subtype A variants (B) are shown. Darker shading indicates increasing bNAb potency and white denotes variants resistant to neutralization by corresponding bNAb at highest concentration tested (1μg/ml). Color key values are in the range from 0.001-1μg/ml and are grouped by quartiles as shown in the key in the upper right. IC50s are an average of at least two independent experiments.