Abstract
Cardiac fibrosis is implicated in numerous physiologic and pathologic conditions, including scar formation, heart failure and cardiac arrhythmias. However the specific cells and signaling pathways mediating this process are poorly understood. Lysine acetylation of nucleosomal histone tails is an important mechanism for the regulation of gene expression. Additionally, proteomic studies have revealed that thousands of proteins in all cellular compartments are subject to reversible lysine acetylation, and thus it is becoming clear that this post-translational modification will rival phosphorylation in terms of biological import. Acetyl groups are conjugated to lysine by histone acetyltransferases (HATs) and removed from lysine by histone deacetylases (HDACs). Recent studies have shown that pharmacologic agents that alter lysine acetylation by targeting HDACs have the remarkable ability to block pathological fibrosis. Here, we review the current understanding of cardiac fibroblasts and the fibrogenic process with respect to the roles of lysine acetylation in the control of disease-related cardiac fibrosis. Potential for small molecule HDAC inhibitors as antifibrotic therapeutics that target cardiac fibroblasts is highlighted.
1. Introduction
Heart failure constitutes a major medical and financial burden in the U.S. and other developing countries, where it remains the leading hospital discharge diagnosis, and accounts for 400,000–700,000 deaths and $20–$40 billion in yearly healthcare costs in the US alone (www.cdc.gov/dhdsp). This situation exists despite impressive advances in our understanding of cardiac biology, disease pathophysiology, and medical therapy. These circumstances therefore justify and motivate continuing basic research in this field and highlight the need for ongoing exploration of novel therapeutic approaches.
Whether originating from genetic abnormality, viral infection, toxic insult, atherosclerosis, long-standing hypertension, or diabetes, heart failure was historically viewed as a disease of the cardiac myocyte, where failure reflects a final common pathway of myocyte hypertrophy, pathological gene expression, and apoptosis [1]. This focus on the myocyte was understandable given the bottom-line inability of the failing heart to meet the metabolic needs of peripheral tissues. Moreover, experiments in genetically manipulated mouse models and also human heart failure have demonstrated that single gene defects in myocyte contractile proteins are sufficient to trigger cardiac hypertrophy and failure [2]. More recently, though, it has become clear that there are other significant “players” in addition to the cardiac myocyte that are involved in the myocardial response to injury, and to the progression and severity of heart failure [3]. In this context, the cardiac fibroblast represents a compelling and understudied contributor to cardiac remodeling in myocardial injury and failure.
While significant advancements have been made in our understanding of the pathologic structure and function of the cardiac myocyte in disease, it has only been recently that various groups have started to focus their investigations on what could arguably be viewed as “the elephant in the room” – cardiac fibrosis. Unlike myocyte or endothelial cell function, fibrosis is a biologic process implicated in virtually all forms of cardiovascular disease, ranging from hypertension and atherosclerosis, to hereditary and even toxin-related cardiomyopathies. Because of this broad scope, research into the molecular mechanisms underlying the development of cardiac fibrosis has the potential to drastically change our siloed view of cardiovascular disease processes and identify therapeutic targets for a wide variety of disease states.
Fibrosis and its relationship to cardiovascular disease is not a new discovery. As early as the 1850s when pathologist Rudolf Virchow first described how the extracellular space around what we now refer to as fibroblasts becomes “fibrillated”, we have at least in a basic sense understood that there is a significant relationship between fibroblasts, fibrosis and disease [4]. This observation has been made in numerous organs and tissue types, including the lung parenchyma, bone marrow, kidneys and liver. Unfortunately, despite over a century of research, our understanding of the fibrogenic process remains very limited and there are still no FDA-approved medications for the prevention or treatment of fibrosis in any organ.
Why has there been such slow progress in the field of fibrotic diseases? Possibly the largest barrier has been our lack of understanding about what exactly a fibroblast is and the identification of reliable, distinct and defining characteristics capable of distinguishing fibroblasts from other cell types. In addition, since the increase in extracellular matrix (ECM) that characterizes fibrosis is involved in such a wide variety of both pathologic and physiologic processes, it has been difficult to clearly identify the mechanisms underlying its development in these distinctly different settings. In part this stems from the redundancy seen between pathways that lead to physiologic fibrosis (“repair”) and those that lead to pathologic fibrosis.
Research into the molecular basis of cardiac fibrosis is now rapidly evolving, and several potential therapeutic targets have been identified. Such targets include regulators of matrix components themselves (collagen, fibronectin, and elastin), enzymes involved in matrix degradation (matrix metalloproteinases and their inhibitors the TIMPs), and also cell surface receptors that promote cardiac fibroblast activation and differentiation. Here, we review the molecular mechanisms controlling cardiac fibrosis, with an emphasis on characteristics and origins of the cardiac fibroblasts. Furthermore, we highlight emerging data suggesting that enzymes that control reversible lysine acetylation are ideal drug targets for the treatment of pathological fibrosis of the heart.
2. Cardiac Fibroblasts
As Virchow alluded to in the 1800s, fibroblasts are a phenotypically distinct subset of cells generically defined as being from a mesenchymal origin and producing a variety of ECM components, such as collagen and fibronectin [4]. Over a century later, our definition of a fibroblast is only marginally more refined. Although several markers, including vimentin, fibronectin, periostin, and β1-integrin, have been found to be highly expressed in fibroblasts [5–7], none are truly fibroblast-specific [8–10]. Herein lays one of the largest barriers to the field of fibrosis research – the lack of a fibroblast-specific marker. As an approach to this problem, several groups have sought to find fibroblast markers that are not necessarily globally-specific for fibroblasts, but are at least organ-specific. In the heart, this work has resulted in the proposal of discoidin domain receptor (DDR) 2 [11, 12], cadherin-11 [5, 13, 14] and fibroblast-specific protein-1 [5, 15] as potential cardiac fibroblast-specific markers. Unfortunately, each of these markers has fallen short, as none are truly expressed exclusively in cardiac fibroblasts.
Fibroblasts, therefore, both generally and in the field of cardiac fibroblast research, are routinely classified solely based on morphology, culture characteristics and lack of specific markers for cells from other lineages (e.g., striated muscle). Collagen expression is also typically used as a fibroblast marker and, as will be described further below, α-smooth muscle actin (αSMA) defines a subset of fibroblasts known as myofibroblasts. Some groups are looking into other ways of identifying and tracking fibroblasts in vivo and in vitro by using reporter genes (e.g., green or red fluorescent protein) under the regulation of promoters for fibroblast markers such as collagen or αSMA [16, 17]. While this strategy represents a potentially exciting system to assess mechanisms of cardiac or other organ fibrosis, its use thus far has been limited to studies of liver and skin fibrosis [18–20].
Further complicating the issue is a large body of evidence that supports the existence of fibroblast heterogeneity. For example, Chang and co-workers found that distinct differences in fibroblast gene expression exist depending on the tissue from which the cells originated as well as the local microenviroment [21]. Additional evidence for tissue-specific fibroblast characteristics was provided by studies showing that, when fibroblasts from different tissues are cultured in vitro, they orient themselves in a tissue-specific manner reflective of the organ from which they were derived [22]. As a correlate to this, in vitro studies have revealed distinct profiles of cytokine signaling in fibroblasts derived from different tissues [23]. Finally, in a very recently published article, Driskell and co-workers were able to show with transplantation assays and lineage tracing in mice that within skin fibroblasts there are different fibroblast subsets that in fact arise from distinct lineages, as opposed to merely differing in their differentiation patterns [24].
For the scope of this paper, however, we will focus on the cardiac fibroblast because in the heart, fibroblasts represent a particularly unique population of cells. Unlike cardiomyocytes, which have only minimal if any proliferative capacity beyond the neonatal stage, fibroblasts retain the ability to proliferate and differentiate throughout a human’s lifespan. In this way, cardiac fibroblasts are a unique cellular component of the heart that functions in a dynamic way to adapt as the heart grows developmentally and as the adult heart responds to stress [11, 25]. The plasticity of fibroblasts is also particularly important in allowing the heart to respond to acute insults and chronic stressors such as myocardial infarctions, longstanding hypertension and heart failure [3, 26–29].
It is commonly accepted that cardiac fibroblasts are both phenotypically and functionally different than other fibroblasts. At a developmental level, cardiac fibroblasts differentiate from multi-potent progenitor cells or mesenchymal stem cells that are from a specific spatiotemporal locus; the cells ultimately undergo an epithelial-to-mesenchymal transition (EMT) to become mature cardiac fibroblasts [3, 27, 30]. Mature cardiac fibroblasts are more elongated and have higher cellular activity than immature cardiac fibroblasts, as evidenced by a highly elaborated endoplasmic reticulum [27, 31]. In adults, there are also differences between ventricular and atrial fibroblasts, which are constituents of the conduction system [11, 32, 33]. Additionally, there is evidence for unique subpopulations of fibroblasts that serve specialized functions in the control of electromechanical coupling [34, 35], autocrine and paracrine signaling, remodeling and angiogenesis [11, 36–41]. Finally, adult cardiac fibroblasts have been found to possess a functional intracellular renin-angiotensin aldosterone system (RAAS) that is of particular interest as a target for the treatment of cardiac fibrosis, as discussed below [42]. Collectively, research to date strongly supports the notion that cardiac fibroblasts are both different than other fibroblasts, and that within the heart, there exist subpopulations of fibroblasts with unique characteristics and functions.
3. Myofibroblasts
Of the different cardiac fibroblast subpopulations, myofibroblasts have been the focus of most studies because of their purported role in pathological fibrosis. Myofibroblasts have a phenotype more closely resembling smooth muscle cells (SMCs), with expression of αSMA and other SMC markers not typically found in cardiac fibroblasts; this SMC-like phenotype enables myofibroblasts to promote wound contracture [5, 43]. Myofibroblasts are commonly referred to as “activated” fibroblasts because their differentiation from resident fibroblasts (and other precursor cell types discussed below) is triggered by various cellular stresses, including exposure to TGF-β1 [44], IL-18 [45], and platelet-derived growth factor (PDGF)-D [46]. In addition, mechanical stress can lead to differentiation of fibroblasts into myofibroblasts, both in vivo, as seen in pressure overload models of heart failure, and in vitro, with stretching or extended culture duration [47–50]. It should be noted as well that in vitro culture conditions alone rapidly stimulate the differentiation of fibroblasts to myofibroblasts, as evidenced by increased αSMA expression with each cell passage. As a result, virtually all in vitro studies done with “fibroblasts” are more accurately a mix of fibroblasts and myofibroblasts, depending on cell passage number [51–53]. Although the vast majority of the pathways shown to mediate fibroblast to myofibroblast differentiation involve changes in TGF-β, there is growing evidence to support alternative pathways, such as those functioning through transient receptor potential (TRP) ion channels [54, 55].
Although myofibroblasts express αSMA, similar to SMCs, and contain an extensive rough endoplasmic reticulum, similar to fibroblasts, they are distinctly different from both cell types in terms of gene expression and phenotype. For example, work by Gan and co-workers showed that myofibroblasts and SMCs utilize distinctly different transcriptional control mechanisms to regulate αSMA expression [56]. Likewise, myofibroblasts also have been found to express the embryonic form of smooth muscle myosin heavy chain (SMemb) and focal adhesion components, which are not expressed in cardiac fibroblasts [43, 50, 57]. Phenotypically, in contrast to cardiac fibroblasts, myofibroblasts have a high level of exocytotic vesicles and can routinely be distinguished from fibroblasts using electron microscopy [31, 44]. Perhaps most importantly, however, there are distinct functional differences that set myofibroblasts apart from cardiac fibroblasts. In vitro, myofibroblasts have the ability to contract collagen gels and exhibit decreased motility [5, 50, 58], which in vivo translates to their ability to stabilize scar tissue at an infarct site as well as mediate normal valve function [59–62]. It is also important to note that myofibroblasts are never found in normal heart tissue, apart from in valve leaflets. Furthermore, myofibroblasts in the diseased heart persist for a longer period and are found at sites that are remote from the initial injury compared to myofibroblasts from other organs [5, 31, 63, 64].
4. Extra-Cardiac Sources of Cardiac Fibroblasts and Myofibroblasts
During development, cardiac fibroblasts originate from mesenchymal cells primarily derived from the embryologic epicardium. It was previously thought that this source of mesenchymal stem cells does not persist into adulthood [65, 66]. However, using an infarct mouse model, mesenchymal stem cells have been found to significantly contribute to wound healing post-MI by differentiating into cardiac fibroblasts and myofibroblasts [67, 68]. In addition, as depicted in Figure 1, there is evidence for other sources of cardiac fibroblasts, both in the developing and adult heart [65, 69, 70]. There is evidence to support bone marrow-derived fibrocytes [71–77], endothelial cells [7, 78, 79], epithelial cells [80], vascular smooth muscle cells [81], epicardial derived cells [82–86] and pericytes of the microvasculature [87] all as sources of activated fibroblasts and myofibroblasts that contribute to cardiac fibrosis. Nonetheless, although it is now more widely accepted that there are other sources of myofibroblasts beyond resident cardiac fibroblasts, there is still debate about the extent to which these cells contribute to cardiac fibrosis [88, 89].
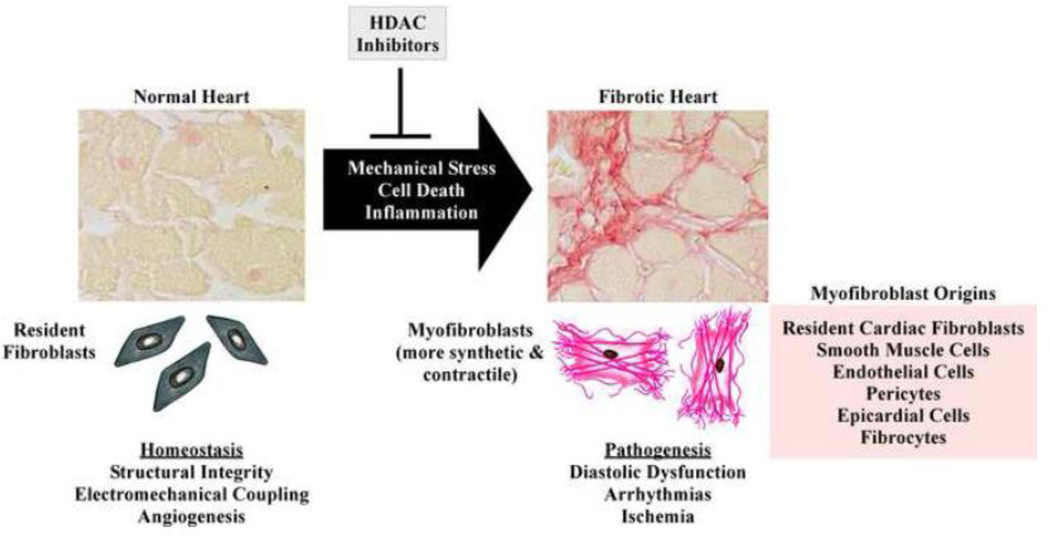
Figure 1. Processes mediating the development of cardiac fibrosis.
Cardiac fibrosis is triggered by diverse cues, including mechanical stress, myocyte death and inflammation. Shown are representative images of left ventricles stained with picrosirius red dye to assess interstitial fibrosis from an untreated mouse (left image) and a mouse treated with angiotensin II (Ang II) for two weeks. Pharmacologic inhibition of class I HDACs blocks development of Ang II-mediated cardiac fibrosis. Under normal conditions, resident cardiac fibroblasts contribute to various homeostatic mechanisms in the heart, including maintenance of structural integrity, electromechanical coupling and angiogenesis. Development of pathological cardiac fibrosis is dependent on differentiation of resident cardiac fibroblasts, as well as other cell types listed, into phenotypically and functionally distinct myofibroblasts, which contribute to arrhythmias, ischemia and diastolic dysfunction.
5. Myofibroblasts as Therapeutic Targets
The observation that myofibroblasts persist in the heart for prolonged periods after acute injury, and in locations distant from the insult, has led to the hypothesis that this cell type is crucially involved in the pathogenesis of heart failure and arrhythmia. As such, there is intense interest in pharmacologically targeting cardiac myofibroblasts. Since myofibroblasts express renin and ACE (the molecules necessary for the formation of Ang II), as well as AT1 receptors, which bind Ang II [90–92], effects of ACE-inhibition and AT1-receptor blockade on cardiac fibrosis have been studied extensively. Although most findings are promising, the delicate interactions between ACE and angiotensin subtypes, as well as the dynamic differences in their expression patterns, make this a challenging pharmacological approach [60, 93, 94]. For example, a large clinical trials revealed that the AT-1 receptor blocker, losartan, failed to reduce fibrosis in many patients [95], highlighting the need for alternative anti-fibrotic strategies for the heart.
6. Histone Deacetylases (HDACs)
With the multitude of mechanisms discovered that mediate the development of cardiac fibrosis, it has become clear that many of the signaling pathways, such as those mediated by TGF-β and Ang II, are highly redundant. This functional redundancy has prompted many groups to search for common downstream mediators of cardiac fibrosis, so-called ‘nodal’ regulators. Recent studies suggest that histone deacetylases (HDACs), which remove acetyl groups from lysine residues in a vast array of proteins [96, 97], may represent such targets. Indeed, small molecule HDAC inhibitors have been shown to block cardiac fibrosis in response to diverse upstream signaling cascades.
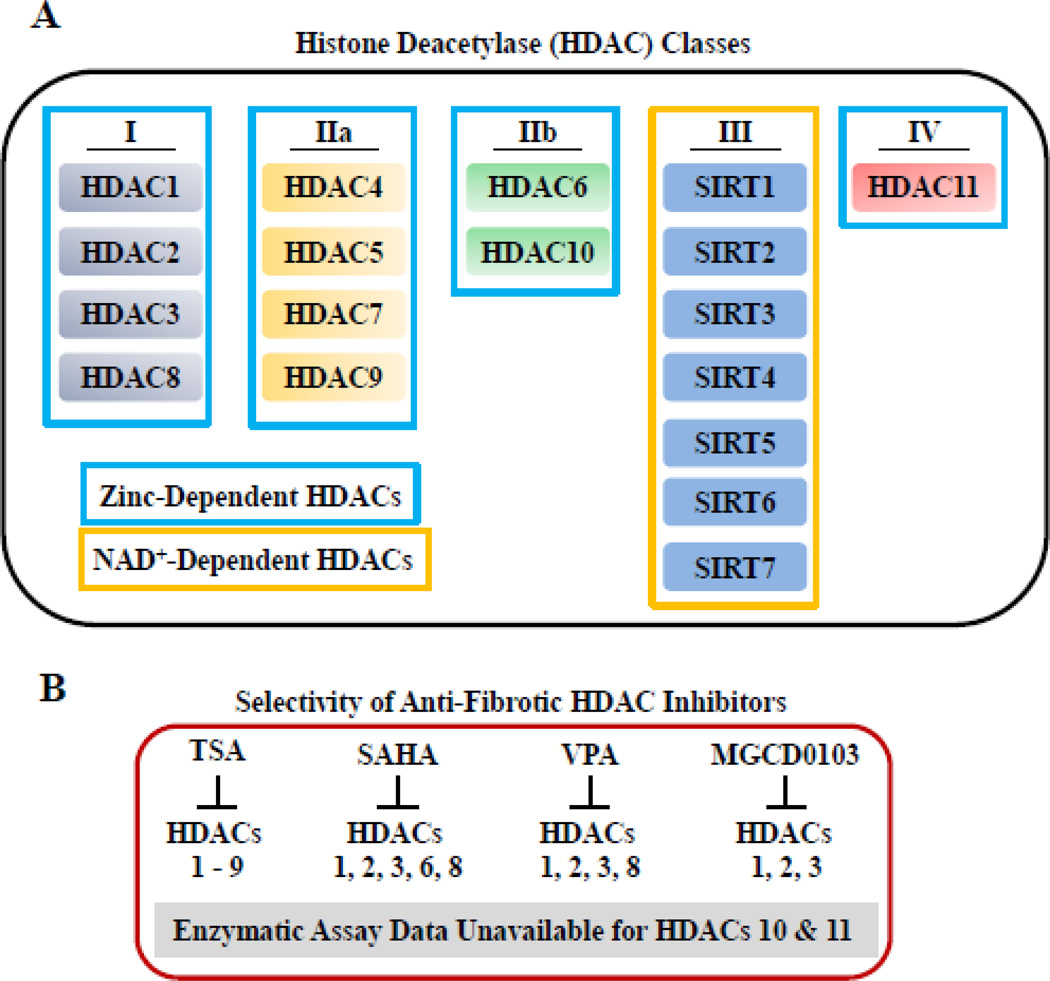
As shown in Figure 2A, there are four classes of mammalian HDACs encompassing 18 different isoforms, each encoded by distinct genes. Class I (1, 2, 3 and 8), class II (4, 5, 6, 7, 9 and 10) and class IV (HDAC11) HDACs are all zinc-dependent enzymes. These HDACs are distinct from the class III HDACs (SirT 1–7), referred to as sirtuins, that require nicotinamide adenine dinucleotide (NAD+) for catalytic activity [98]. Although sirtuins clearly regulate cardiac fibrosis [99], the remainder of this review will be focused on zinc-dependent HDACs, since these are the targets of the small molecule inhibitors that have been shown to block fibrosis in the heart (Fig. 2B).
Figure 2. HDAC classification and selectivity of inhibitors.
(A) Class I HDACs include HDAC1, -2, -3 and -8. Class II HDACs are divided into IIa, HDAC4, -5, -7, -9, and IIb, consisting of HDAC6 and -10. Class III HDACs are also referred to as sirtuins (SIRT) and include SIRT1-7. HDAC11 represents the only class IV HDAC. Class III HDACs require NAD+ for catalytic activity while the all other HDACs are zinc-dependent (outlined in blue). (B) Routinely used and available HDAC inhibitors target class I, IIb and IV HDACs by chelating zinc in the active sites of the enzymes. Shown are the selectivity profiles for the anti-fibrotic HDAC inhibitors highlighted in this review: trichostatin A (TSA), SAHA, valproic acid (VPA) and MGCD0103.
7. Suppression of Cardiac Fibrosis by HDAC Inhibitors
HDAC inhibitors have been shown to block fibrosis in diverse organ systems in response to a variety of stress stimuli. In the heart, HDAC inhibitors have been shown to reduce pressure overload-driven interstitial cardiac fibrosis and to reverse pre-established atrial fibrosis and arrhythmic inducibility in Hop transgenic mice [100–103]; these early studies were performed primarily with trichostatin A (TSA), a ‘pan’ inhibitor of all zinc-dependent HDACs (Fig.2B) Despite these promising findings, however, the molecular basis for the anti-fibrotic actions of HDAC inhibitors remains incompletely understood. It seems likely that HDAC inhibitors block cardiac fibrosis by multiple mechanisms, including inhibition of cardiac fibroblast proliferation and/or migration, induction of genes that suppress extracellular matrix production from fibroblasts, suppression of pro-inflammatory cues for fibrosis, and blockade of endothelial-to-mesenchymal transition (EndoMT). In addition, our recent work has demonstrated that HDAC inhibitors have remarkable ability to block differentiation of monocytic precursors into mature, collagen-producing fibrocytes (see below).
Endo-MT defines the process of pathological de-differentiation of vascular endothelial cells into matrix-producing mesenchymal cells. This process has emerged as another mechanism for production of excessive numbers of cardiac fibroblasts in adult hearts in response to stress [7, 104]. Cardiac Endo-MT is stimulated by transforming growth factor-beta (TGF-β) and suppressed by Bone Morphogenic Protein-7 (BMP-7), which is known to block fibrosis [105]. Endothelin-1, a potent vasoconstrictor with pro-mitogenic properties, was also shown to stimulate cardiac fibrosis by promoting EndoMT [79]. The anti-oncogenic action of HDAC inhibitors has been attributed, in part, to blockade of a related process, epithelial-to-mesenchymal transition (EMT) [106]. As such, future studies should address whether HDAC inhibition alters EndoMT in the heart.
HDAC inhibitors also likely have direct effects on cardiac fibroblasts. TSA blocks TGF-β-mediated induction of collagen synthesis in cultured rat ventricular fibroblasts [102]. HDAC inhibitors do not, however, affect TGF-β-mediated phosphorylation or nuclear translocation of SMAD transcription factors, which control collagen gene expression, but do appear to suppress other signaling mediators (e.g., ERK, AKT and PI3K) that impact collagen synthesis [107]. Studies in models of renal fibrosis have suggested that HDAC inhibitors also suppress TGF-β protein expression [108].
Recently, we showed that selective class I HDAC inhibition potently blocks Ang II-mediated cardiac fibrosis [109], in part by suppressing cardiac fibroblast proliferation. MGCD0103, a selective small molecule inhibitor of class I HDACs [110], blocked cultured neonatal and adult rat cardiac fibroblasts in the G0/G1 phase of the cell cycle via inhibition of Rb phosphorylation, which is mediated by cyclin-dependent kinases (CDKs) and is required to stimulate downstream expression of E2F target genes that drive the G1-to-S transition. A major mechanism for inhibition of cancer cell proliferation by HDAC inhibitors involves induction of expression of the p21 CDK inhibitor [111–115]. Surprisingly, class I HDAC inhibition failed to stimulate expression of p21 in cardiac fibroblasts. Instead, a survey of expression of the six other endogenous CDK inhibitors revealed that class I HDAC inhibition selectively upregulates p15 and p57, suggesting a previously unrecognized role for these genes in cardiac fibrosis. The results suggest that one mechanism by which class I HDACs stimulate fibrosis in the heart is by repressing expression of anti-proliferative genes in cardiac fibroblasts, resulting in expansion of the pool of ECM-producing cells in the myocardium in response to stress.
Pro-inflammatory cytokines activate cardiac fibroblasts to produce extracellular matrix [27]. At least part of the anti-fibrotic action of HDAC inhibitors may be due to anti-inflammatory actions of the compounds. In spontaneously hypertensive rats (SHR), treatment with valproic acid, a weak inhibitor of class I HDACs [116], for 20 weeks led to reduced LV expression of the pro-inflammatory cytokines IL-1β and TNFα, which correlated with inhibition of cardiac fibrosis and improved cardiac function [117]. In a related study, HDAC inhibitors were shown to reduce plasma cytokine levels in a rat deoxycorticosterone acetate (DOCA)-salt model of hypertensive cardiomyopathy [118]. Four weeks of treatment, SAHA, which potently inhibits class I and IIb HDACs [119], significantly reduced circulating levels of multiple pro-inflammatory cytokines, including IL-1β, IL-6 and TNFα, and these decreases correlated with reduced cardiac hypertrophy and suppression of interstitial fibrosis in the LV. Together, these data support a significant role for HDACs in cytokine-mediated development of cardiac fibrosis.
8. Targeting Bone Marrow-Derived Fibrocytes with HDAC Inhibitors
Cardiac fibroblasts have long been viewed as the major producers of ECM during the fibrotic process. However, recent studies have revealed an important role for a population of bone marrow-derived cells, termed fibrocytes, in the control of cardiac fibrosis. Fibrocytes have features of both monocytes and fibroblasts, and are able to adopt a mesenchymal phenotype and contribute to tissue remodeling in response to pathological stress [120–122]. Studies by the Entman lab have demonstrated that age-related cardiac fibrosis and diastolic dysfunction coincide with accumulation of fibrocytes in ventricular interstitial space [123]. Additional studies in mice have demonstrated roles for fibrocytes in Ang II-meditated cardiac fibrosis [73, 124, 125], and in fibrosis due to intermittent ischemia [72]. More recently, patients with hypertensive heart disease were found to have elevated levels of circulating, activated fibrocytes, and fibrocyte numbers correlated with disease severity [126].
We recently described results of flow cytometry studies focused on elucidating effects of HDAC inhibitors on cellular infiltration into the heart in response to chronic Ang II signaling in mice [109]. Interestingly, despite having known anti-inflammatory properties, HDAC inhibitors did not reduce Ang II-mediated leukocyte infiltration in the heart. Remarkably, however, class I HDAC-selective inhibition completely blocked Ang II-mediated increases in fibrocyte numbers in the heart, and also decreased circulating levels of fibrocytes; fibrocytes were defined by co-expression of CD34 (stem cell marker), CD45 (hematopoietic cell marker), a monocyte markers (e.g., CD11), and either collagen or α-smooth muscle actin (mesenchymal markers). HDAC inhibitor-mediated suppression of fibrocytes did not appear to be due to blockade of recruitment to the heart, since monocytic fibrocyte precursors were equally abundant in hearts of mice treated with Ang II in the absence or presence of HDAC inhibitor. Consistent with this, cardiac expression of monocyte chemoattractant protein-1 (MCP-1), which is critical for fibrocyte recruitment to the heart [73], was induced by Ang II despite class I HDAC inhibition.
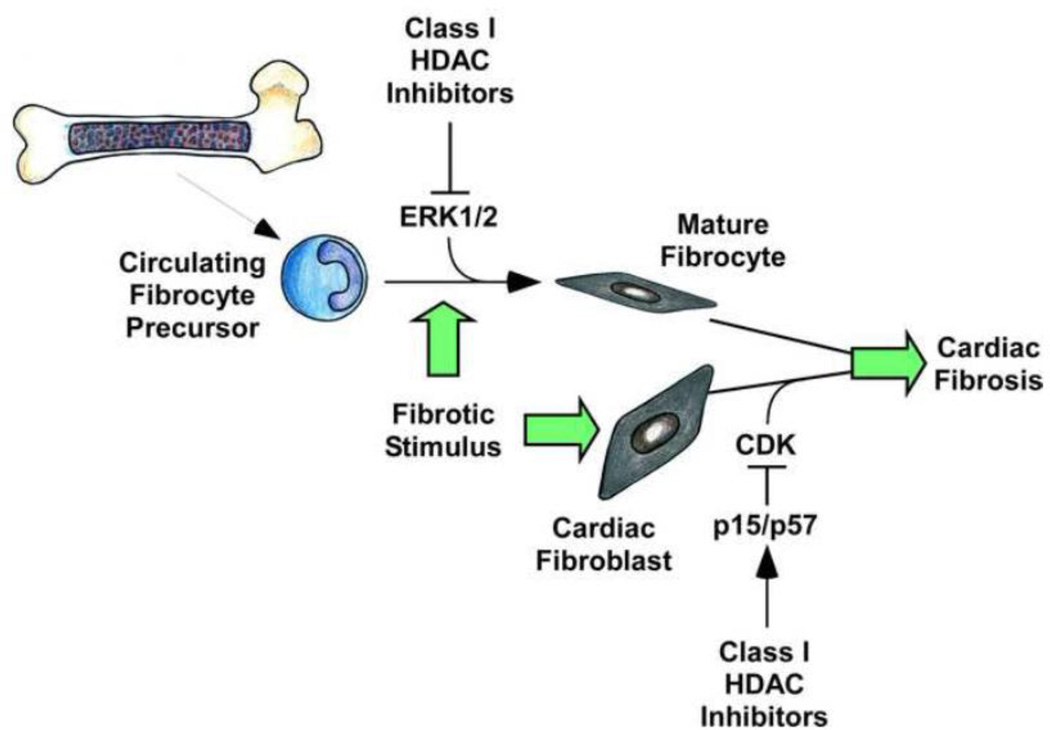
Using an in vitro assay, we found that class I HDAC inhibition blocks fibrocyte differentiation as efficiently as serum amyloid P, an Fcγ receptor antagonist that is currently in clinical development for idiopathic pulmonary fibrosis [127]. Compared to cardiac fibroblasts, little is known about the molecular mechanisms that control fibrocyte differentiation and growth. Several receptor agonists have been shown to stimulate fibrocyte differentiation, including TGF-β, ET-1, IL-4 and IL-13 [120–122]; TNFα signaling also appears to be involved [128]. Recently, ERK1/2 was also found to be a critical downstream effector of fibrocyte differentiation [129]. Rho kinase [130], p38 kinase [131] and STAT transcription factors [132] have also been implicated in the control of fibrocyte differentiation. A survey of pathways known to control fibrocyte differentiation demonstrated that class I HDAC inhibition selectively blocked activation of ERK1/2 [109]. The mechanism by which class I HDAC inhibition blocks ERK activation in fibrocytes remains unknown, but could be related to our recent finding that class I HDAC inhibitors suppress ERK signaling in cardiac myocytes by derepressing expression of an ERK-specific phosphatase, termed DUSP5 [133]. Together, our findings establish class I HDACs as key regulators of cardiac fibrosis that serve dual fibrogenic functions by promoting cardiac fibroblast activation and controlling differentiation of bone marrow-derived fibrocytes (Fig. 3).
Figure 3. Mechanisms by which class I HDAC inhibition blocks cardiac fibrosis.
Class I HDAC inhibition prevents the differentiation of bone-marrow derived fibrocytes into active fibrocytes and fibroblasts through inhibition of ERK1/2 activation. Class I HDAC inhibition arrests cardiac fibroblasts in G0/G1 of the cell cycle via upregulation of the cyclin-dependent kinase (CDK) inhibitors p15 and p57. Both of these processes result in decreased numbers of activated ECM-producing fibroblasts and myofibroblasts in the myocardium, leading to decreased fibrosis.
9. Summary
As a consequence of recent efforts to define regulatory mechanisms controlling fibroblast function and differentiation, there is an expanding list of potential targets for the treatment of cardiac fibrosis. Among the list of targets, HDACs are particularly promising because these enzymes serve as a nexus for multiple pro-fibrotic signaling networks. It is now recognized that thousands of proteins are subject to reversible lysine acetylation [96, 97], and thus there is no doubt that effects of HDAC inhibitors on cardiac fibroblasts will be mediated by genomic (histone targets) and non-genomic (non-histone targets) mechanisms. The development of isoform-selective HDAC inhibitors will facilitate efforts to more specifically define the mechanisms by which HDACs control fibrosis, and should establish HDAC inhibition as a realistic and viable therapeutic option for cardiac fibrosis. Given the absence of FDA-approved drugs to treat fibrosis in any organ, rapid pre-clinical and clinical assessment of isoform-selective HDAC inhibitors for the treatment of pathological fibrosis is paramount.
Highlights.
Cardiac fibroblasts are a unique cell population.
Fibroblasts and myofibroblasts are involved in pathologic fibrosis of the heart.
HDAC inhibition block cardiac fibrosis by targeting fibroblasts and myofibroblasts.
Acknowledgements
We thank M.K. McKinsey for assistance with graphics. K.B.D. received funding from a T32 training grant from the NIH (5T32HL007822-12). T.A.M. was supported, in part, by NIH grant RO1HL116848.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
No conflicts of interest exist for the authors.
References
- 1.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 2.Maass AH, Leinwand LA. Mechanisms of the pathogenesis of troponin T-based familial hypertrophic cardiomyopathy. Trends Cardiovasc Med. 2003;13:232–237. doi: 10.1016/s1050-1738(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 3.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 4.Virchow R. Die Cellularpathologie in ihrer Begrundung auf physiologische und pathologische Gewebelehre. Berlin. 1858 [PubMed] [Google Scholar]
- 5.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland AE, Calarco PG, Damsky CH. Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development. 1993;119:1175–1186. doi: 10.1242/dev.119.4.1175. [DOI] [PubMed] [Google Scholar]
- 7.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 8.Malara A, Currao M, Gruppi C, Celesti G, Viarengo G, Buracchi C, et al. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen and laminin. Stem Cells. 2013 doi: 10.1002/stem.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merle B, Bouet G, Rousseau JC, Bertholon C, Garnero P. Periostin and transforming growth factor beta-induced protein (TGFbetaIp) are both expressed by osteoblasts and osteoclasts. Cell Biol Int. 2013 doi: 10.1002/cbin.10219. [DOI] [PubMed] [Google Scholar]
- 10.Puschmann TB, Zanden C, Lebkuechner I, Philippot C, de PY, Liu J, et al. HB-EGF affects astrocyte morphology, proliferation, differentiation, and the expression of intermediate filament proteins. J Neurochem. 2013 doi: 10.1111/jnc.12519. [DOI] [PubMed] [Google Scholar]
- 11.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, et al. Organization of fibroblasts in the heart. Dev Dyn. 2004;230:787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 13.Simonneau L, Kitagawa M, Suzuki S, Thiery JP. Cadherin 11 expression marks the mesenchymal phenotype: towards new functions for cadherins? Cell Adhes Commun. 1995;3:115–130. doi: 10.3109/15419069509081281. [DOI] [PubMed] [Google Scholar]
- 14.Valencia X, Higgins JM, Kiener HP, Lee DM, Podrebarac TA, Dascher CC, et al. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J Exp Med. 2004;200:1673–1679. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzucchelli L. Protein S100A4: too long overlooked by pathologists? Am J Pathol. 2002;160:7–13. doi: 10.1016/S0002-9440(10)64342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding S, Walton KL, Blue RE, McNaughton K, Magness ST, Lund PK. Mucosal healing and fibrosis after acute or chronic inflammation in wild type FVB-N mice and C57BL6 procollagen alpha1(I)-promoter-GFP reporter mice. PLoS One. 2012;7:e42568. doi: 10.1371/journal.pone.0042568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151–1159. doi: 10.1002/hep.20427. [DOI] [PubMed] [Google Scholar]
- 18.Higashiyama R, Moro T, Nakao S, Mikami K, Fukumitsu H, Ueda Y, et al. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology. 2009;137:1459–1466. doi: 10.1053/j.gastro.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Higashiyama R, Nakao S, Shibusawa Y, Ishikawa O, Moro T, Mikami K, et al. Differential contribution of dermal resident and bone marrow-derived cells to collagen production during wound healing and fibrogenesis in mice. J Invest Dermatol. 2011;131:529–536. doi: 10.1038/jid.2010.314. [DOI] [PubMed] [Google Scholar]
- 20.Barisic-Dujmovic T, Boban I, Clark SH. Fibroblasts/myofibroblasts that participate in cutaneous wound healing are not derived from circulating progenitor cells. J Cell Physiol. 2010;222:703–712. doi: 10.1002/jcp.21997. [DOI] [PubMed] [Google Scholar]
- 21.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doane KJ, Birk DE. Fibroblasts retain their tissue phenotype when grown in three-dimensional collagen gels. Exp Cell Res. 1991;195:432–442. doi: 10.1016/0014-4827(91)90394-a. [DOI] [PubMed] [Google Scholar]
- 23.Brown RD. Proinflammatory cytokines and cardiac extracellular matrix: regulation of fibroblast phenotype. In: Villarreal FJ, editor. Interstitial Fibrosis in Heart Disease. New York: Springer; 2005. pp. 57–81. [Google Scholar]
- 24.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 26.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res. 2004;62:415–425. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 29.van Nieuwenhoven FA, Turner NA. The role of cardiac fibroblasts in the transition from inflammation to fibrosis following myocardial infarction. Vascul Pharmacol. 2013;58:182–188. doi: 10.1016/j.vph.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Wessels A, Perez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 31.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tveito A, Lines G, Artebrant R, Skavhaug O, Maleckar MM. Existence of excitation waves for a collection of cardiomyocytes electrically coupled to fibroblasts. Math Biosci. 2011;230:79–86. doi: 10.1016/j.mbs.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013;304:C216–C225. doi: 10.1152/ajpcell.00328.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camelliti P, McCulloch AD, Kohl P. Microstructured cocultures of cardiac myocytes and fibroblasts: a two-dimensional in vitro model of cardiac tissue. Microsc Microanal. 2005;11:249–259. doi: 10.1017/S1431927605050506. [DOI] [PubMed] [Google Scholar]
- 35.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 36.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 37.Darland DC, D'Amore PA. Cell-cell interactions in vascular development. Curr Top Dev Biol. 2001;52:107–149. doi: 10.1016/s0070-2153(01)52010-4. [DOI] [PubMed] [Google Scholar]
- 38.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 39.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 40.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 41.Sussman MA, McCulloch A, Borg TK. Dance band on the Titanic: biomechanical signaling in cardiac hypertrophy. Circ Res. 2002;91:888–898. doi: 10.1161/01.res.0000041680.43270.f8. [DOI] [PubMed] [Google Scholar]
- 42.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol. 2008;294:H1675–H1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 43.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 44.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–263. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 45.Valente AJ, Sakamuri SS, Siddesha JM, Yoshida T, Gardner JD, Prabhu R, et al. TRAF3IP2 mediates interleukin-18-induced cardiac fibroblast migration and differentiation. Cell Signal. 2013 doi: 10.1016/j.cellsig.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao T, Zhao W, Chen Y, Li VS, Meng W, Sun Y. Platelet-derived growth factor-D promotes fibrogenesis of cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2013;304:H1719–H1726. doi: 10.1152/ajpheart.00130.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adapala RK, Thoppil RJ, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, et al. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol. 2013;54:45–52. doi: 10.1016/j.yjmcc.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalla Costa AP, Clemente CF, Carvalho HF, Carvalheira JB, Nadruz W, Jr, Franchini KG. FAK mediates the activation of cardiac fibroblasts induced by mechanical stress through regulation of the mTOR complex. Cardiovasc Res. 2010;86:421–431. doi: 10.1093/cvr/cvp416. [DOI] [PubMed] [Google Scholar]
- 49.Herum KM, Lunde IG, Skrbic B, Florholmen G, Behmen D, Sjaastad I, et al. Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J Mol Cell Cardiol. 2013;54:73–81. doi: 10.1016/j.yjmcc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 51.Khouw IM, van Wachem PB, Plantinga JA, Vujaskovic Z, Wissink MJ, de Leij LF, et al. TGF-beta and bFGF affect the differentiation of proliferating porcine fibroblasts into myofibroblasts in vitro. Biomaterials. 1999;20:1815–1822. doi: 10.1016/s0142-9612(99)00077-0. [DOI] [PubMed] [Google Scholar]
- 52.Rohr S. Cardiac fibroblasts in cell culture systems: myofibroblasts all along? J Cardiovasc Pharmacol. 2011;57:389–399. doi: 10.1097/FJC.0b013e3182137e17. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285:H1871–H1881. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- 54.Turner NA, Porter KE. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair. 2013;6:5. doi: 10.1186/1755-1536-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue Z, Zhang Y, Xie J, Jiang J, Yue L. Transient receptor potential (TRP) channels and cardiac fibrosis. Curr Top Med Chem. 2013;13:270–282. doi: 10.2174/1568026611313030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gan Q, Yoshida T, Li J, Owens GK. Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle alpha-actin expression. Circ Res. 2007;101:883–892. doi: 10.1161/CIRCRESAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 57.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovasc Res. 2000;48:89–100. doi: 10.1016/s0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 58.Sun Y, Weber KT. RAS and connective tissue in the heart. Int J Biochem Cell Biol. 2003;35:919–931. doi: 10.1016/s1357-2725(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 59.Blankesteijn WM, Essers-Janssen YP, Verluyten MJ, Daemen MJ, Smits JF. A homologue of Drosophila tissue polarity gene frizzled is expressed in migrating myofibroblasts in the infarcted rat heart. Nat Med. 1997;3:541–544. doi: 10.1038/nm0597-541. [DOI] [PubMed] [Google Scholar]
- 60.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 61.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 62.Filip DA, Radu A, Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 1986;59:310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- 63.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 64.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 65.Vasquez C, Morley GE. The origin and arrhythmogenic potential of fibroblasts in cardiac disease. J Cardiovasc Transl Res. 2012;5:760–767. doi: 10.1007/s12265-012-9408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, et al. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol. 2005;14:241–246. doi: 10.1016/j.carpath.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 67.Carlson S, Trial J, Soeller C, Entman ML. Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction. Cardiovasc Res. 2011;91:99–107. doi: 10.1093/cvr/cvr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dubois C, Liu X, Claus P, Marsboom G, Pokreisz P, Vandenwijngaert S, et al. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol. 2010;55:2232–2243. doi: 10.1016/j.jacc.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 69.Potts JD, Runyan RB. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989;134:392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- 70.Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci. 2008;1123:30–40. doi: 10.1196/annals.1420.005. [DOI] [PubMed] [Google Scholar]
- 71.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 72.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mollmann H, Nef HM, Kostin S, von KC, Pilz I, Weber M, et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 75.Odorfer KI, Walter I, Kleiter M, Sandgren EP, Erben RG. Role of endogenous bone marrow cells in long-term repair mechanisms after myocardial infarction. J Cell Mol Med. 2008;12:2867–2874. doi: 10.1111/j.1582-4934.2008.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sato D, Otani H, Enoki C, Fujita M, Minato N, Iwasaka T. Phenotypic modulation and turnover of bone marrow-derived cells after myocardial infarction in rats. Cardiovasc Pathol. 2011;20:146–155. doi: 10.1016/j.carpath.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 77.van Amerongen MJ, Bou-Gharios G, Popa E, van AJ, Petersen AH, van Dam GM, et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214:377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 78.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–2418. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 80.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonzalez-Rosa JM, Peralta M, Mercader N. Pan-epicardial lineage tracing reveals that epicardium derived cells give rise to myofibroblasts and perivascular cells during zebrafish heart regeneration. Dev Biol. 2012;370:173–186. doi: 10.1016/j.ydbio.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 83.Di MF, Castaldo C, Nurzynska D, Romano V, Miraglia R, Bancone C, et al. Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. J Mol Cell Cardiol. 2010;49:719–727. doi: 10.1016/j.yjmcc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Russell JL, Goetsch SC, Gaiano NR, Hill JA, Olson EN, Schneider JW. A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res. 2011;108:51–59. doi: 10.1161/CIRCRESAHA.110.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 86.van TJ, Atsma DE, Winter EM, van der Velde-van Dijke, Pijnappels DA, Bax NA, et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells. 2007;25:271–278. doi: 10.1634/stemcells.2006-0366. [DOI] [PubMed] [Google Scholar]
- 87.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pichler M, Rainer PP, Schauer S, Hoefler G. Cardiac fibrosis in human transplanted hearts is mainly driven by cells of intracardiac origin. J Am Coll Cardiol. 2012;59:1008–1016. doi: 10.1016/j.jacc.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 89.Wu GD, Tuan TL, Bowdish ME, Jin YS, Starnes VA, Cramer DV, et al. Evidence for recipient derived fibroblast recruitment and activation during the development of chronic cardiac allograft rejection. Transplantation. 2003;76:609–614. doi: 10.1097/01.TP.0000066362.37931.6D. [DOI] [PubMed] [Google Scholar]
- 90.Sun Y, Zhang JQ, Zhang J, Ramires FJ. Angiotensin II, transforming growth factor-beta1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998;30:1559–1569. doi: 10.1006/jmcc.1998.0721. [DOI] [PubMed] [Google Scholar]
- 91.Katwa LC, Campbell SE, Tyagi SC, Lee SJ, Cicila GT, Weber KT. Cultured myofibroblasts generate angiotensin peptides de novo. J Mol Cell Cardiol. 1997;29:1375–1386. doi: 10.1006/jmcc.1997.0376. [DOI] [PubMed] [Google Scholar]
- 92.Weber KT, Sun Y, Campbell SE. Structural remodelling of the heart by fibrous tissue: role of circulating hormones and locally produced peptides. Eur Heart J. 1995;16(Suppl N):12–18. doi: 10.1093/eurheartj/16.suppl_n.12. [DOI] [PubMed] [Google Scholar]
- 93.Takeda Y, Zhu A, Yoneda T, Usukura M, Takata H, Yamagishi M. Effects of aldosterone and angiotensin II receptor blockade on cardiac angiotensinogen and angiotensin-converting enzyme 2 expression in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2007;20:1119–1124. doi: 10.1016/j.amjhyper.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 94.Zhao YX, Yin HQ, Yu QT, Qiao Y, Dai HY, Zhang MX, et al. ACE2 overexpression ameliorates left ventricular remodeling and dysfunction in a rat model of myocardial infarction. Hum Gene Ther. 2010;21:1545–1554. doi: 10.1089/hum.2009.160. [DOI] [PubMed] [Google Scholar]
- 95.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 96.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 97.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, et al. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2:419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 100.Gallo P, Latronico MV, Gallo P, Grimaldi S, Borgia F, Todaro M, et al. Inhibition of class I histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc Res. 2008;80:416–424. doi: 10.1093/cvr/cvn215. [DOI] [PubMed] [Google Scholar]
- 101.Kee HJ, Sohn IS, Nam KI, Park JE, Qian YR, Yin Z, et al. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation. 2006;113:51–59. doi: 10.1161/CIRCULATIONAHA.105.559724. [DOI] [PubMed] [Google Scholar]
- 102.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–2588. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu F, Levin MD, Petrenko NB, Lu MM, Wang T, Yuan LJ, et al. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol. 2008;45:715–723. doi: 10.1016/j.yjmcc.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goumans MJ, van Zonneveld AJ, ten DP. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 105.Weiskirchen R, Meurer SK, Gressner OA, Herrmann J, Borkham-Kamphorst E, Gressner AM. BMP-7 as antagonist of organ fibrosis. Front Biosci (Landmark Ed) 2009;14:4992–5012. doi: 10.2741/3583. [DOI] [PubMed] [Google Scholar]
- 106.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barter MJ, Pybus L, Litherland GJ, Rowan AD, Clark IM, Edwards DR, et al. HDAC-mediated control of ERK- and PI3K-dependent TGF-beta-induced extracellular matrix-regulating genes. Matrix Biol. 2010;29:602–612. doi: 10.1016/j.matbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 108.Liu N, He S, Ma L, Ponnusamy M, Tang J, Tolbert E, et al. Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling. PLoS One. 2013;8:e54001. doi: 10.1371/journal.pone.0054001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams SM, Golden-Mason L, Ferguson BS, Douglas KB, Cavasin MA, Demos-Davies K, et al. Class I HDACs regulate angiotensin II-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J Mol Cell Cardiol. 2013 doi: 10.1016/j.yjmcc.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boumber Y, Younes A, Garcia-Manero G. Mocetinostat (MGCD0103): a review of an isotype-specific histone deacetylase inhibitor. Expert Opin Investig Drugs. 2011;20:823–829. doi: 10.1517/13543784.2011.577737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci U S A. 2004;101:1241–1246. doi: 10.1073/pnas.0307708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li H, Wu X. Histone deacetylase inhibitor, Trichostatin A, activates p21WAF1/CIP1 expression through downregulation of c-myc and release of the repression of c-myc from the promoter in human cervical cancer cells. Biochem Biophys Res Commun. 2004;324:860–867. doi: 10.1016/j.bbrc.2004.09.130. [DOI] [PubMed] [Google Scholar]
- 113.Marks PA. The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin Investig Drugs. 2010;19:1049–1066. doi: 10.1517/13543784.2010.510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sambucetti LC, Fischer DD, Zabludoff S, Kwon PO, Chamberlin H, Trogani N, et al. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J Biol Chem. 1999;274:34940–34947. doi: 10.1074/jbc.274.49.34940. [DOI] [PubMed] [Google Scholar]
- 116.Fass DM, Shah R, Ghosh B, Hennig K, Norton S, Zhao WN, et al. Effect of Inhibiting Histone Deacetylase with Short-Chain Carboxylic Acids and Their Hydroxamic Acid Analogs on Vertebrate Development and Neuronal Chromatin. ACS Med Chem Lett. 2010;2:39–42. doi: 10.1021/ml1001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM, et al. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56:437–444. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Iyer A, Fenning A, Lim J, Le GT, Reid RC, Halili MA, et al. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Br J Pharmacol. 2010;159:1408–1417. doi: 10.1111/j.1476-5381.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol. 2010;38:548–556. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peng H, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Curr Opin Pharmacol. 2012;12:491–496. doi: 10.1016/j.coph.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol. 2011;50:248–256. doi: 10.1016/j.yjmcc.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sopel M, Falkenham A, Oxner A, Ma I, Lee TD, Legare JF. Fibroblast progenitor cells are recruited into the myocardium prior to the development of myocardial fibrosis. Int J Exp Pathol. 2012;93:115–124. doi: 10.1111/j.1365-2613.2011.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu J, Lin SC, Chen J, Miao Y, Taffet GE, Entman ML, et al. CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol. 2011;301:H538–H547. doi: 10.1152/ajpheart.01114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keeley EC, Mehrad B, Janardhanan R, Salerno M, Hunter JR, Burdick MM, et al. Elevated circulating fibrocyte levels in patients with hypertensive heart disease. J Hypertens. 2012;30:1856–1861. doi: 10.1097/HJH.0b013e32835639bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duffield JS, Lupher ML., Jr PRM-151 (recombinant human serum amyloid P/pentraxin 2) for the treatment of fibrosis. Drug News Perspect. 2010;23:305–315. doi: 10.1358/dnp.2010.23.5.1444206. [DOI] [PubMed] [Google Scholar]
- 128.Duerrschmid C, Crawford JR, Reineke E, Taffet GE, Trial J, Entman ML, et al. TNF receptor 1 signaling is critically involved in mediating angiotensin-II-induced cardiac fibrosis. J Mol Cell Cardiol. 2013 doi: 10.1016/j.yjmcc.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nikam VS, Wecker G, Schermuly R, Rapp U, Szelepusa K, Seeger W, et al. Treprostinil inhibits the adhesion and differentiation of fibrocytes via the cyclic adenosine monophosphate-dependent and Ras-proximate protein-dependent inactivation of extracellular regulated kinase. Am J Respir Cell Mol Biol. 2011;45:692–703. doi: 10.1165/rcmb.2010-0240OC. [DOI] [PubMed] [Google Scholar]
- 130.Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, Trial J, et al. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res. 2009;83:511–518. doi: 10.1093/cvr/cvp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kokubo S, Sakai N, Furuichi K, Toyama T, Kitajima S, Okumura T, et al. Activation of p38 mitogen-activated protein kinase promotes peritoneal fibrosis by regulating fibrocytes. Perit Dial Int. 2012;32:10–19. doi: 10.3747/pdi.2010.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferguson BS, Harrison BC, Jeong MY, Reid BG, Wempe MF, Wagner FF, et al. Signal-dependent repression of DUSP5 by class I HDACs controls nuclear ERK activity and cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2013;110:9806–9811. doi: 10.1073/pnas.1301509110. [DOI] [PMC free article] [PubMed] [Google Scholar]