Abstract
Objective
The risk for cardiovascular diseases is elevated in persons with bipolar disorder. However, it remains unknown how much of this excess risk is secondary to pharmacologic treatment. We tested the hypothesis that current and cumulative antipsychotic drug exposure is associated with increased cardiovascular risk as indicated by lower heart rate variability (HRV) and increased blood pressure variability (BPV).
Methods
55 individuals with bipolar disorder (33±7 years; 67% female) underwent non-invasive electrocardiogram assessment of time- and frequency-domain HRV, as well as BPV analysis. Medication histories were obtained through systematic review of pharmacy records for the past five years.
Results
Current antipsychotic exposure was associated with lower SDNN. Second generation antipsychotics were associated with lower SDNN and RMSSD. There was no significant relationship between five-year antipsychotic exposure and HRV in subjects with bipolar disorder. Exploratory analysis revealed a possible link between SSRI exposure and increased low frequency spectral HRV.
Conclusions
Current antipsychotic use (particularly second generation antipsychotics with high affinities for the D2S receptor) is associated with reduced autonomic-mediated variability of heart rate. The absence of an association with cumulative exposure suggests that the effects are acute in onset, and may therefore relate more to altered autonomic function than structural cardiovascular abnormalities. Future studies should prospectively examine effects of these antipsychotics on autonomic function.
Keywords: cardiovascular disease, SSRI, blood pressure variability, heart rate variability
INTRODUCTION
Cardiovascular pathologies are a prominent cause of death in patients with bipolar disorder (Murray et al., 2009; Weiner et al., 2011). Autonomic nervous system input (ANS) to the heart and the vasculature plays a key role in the regulation of normal cardiovascular function. Altered cardiac or vascular autonomic innervation or impaired cardiac or vascular responsiveness to autonomic inputs are common features of cardiovascular disease. Since autonomic innervation of the heart and the vasculature causes characteristic patterns of heart rate and blood pressure variability, the analysis of heart rate and blood pressure variability provides a non-invasive tool to indirectly assess cardiovascular health (Bar et al., 2008a; Mujica-Parodi et al., 2005; Stauss, 2007). In addition, changes in cardiovascular structure can alter heart rate variability (HRV), for example through increased vascular stiffness leading to reduced carotid baroreflex modulation of heart rate.
The focus of our study was to examine the effects of antipsychotic (AP) drug exposure on heart rate and blood pressure variability in individuals with bipolar disorder, in which there has been only limited study (Latalova et al., 2010). The bulk of the existing literature examining antipsychotic drug effects on HRV has focused on samples with schizophrenia, and the results have been generally inconclusive with exposure variably defined (Bar et al., 2008a; Chang et al., 2010; Cohen et al., 2001; Hempel et al., 2009; Latalova et al., 2010; Malaspina et al., 2002; Mujica-Parodi et al., 2005; Silke et al., 2002; Wang et al., 2008). Several studies found a negative correlation between AP exposure and parasympathetic activity (Bar et al., 2008a; Mujica-Parodi et al., 2005). Others found AP exposure to increase parasympathetic tone (Chang et al., 2010; Hempel et al., 2009), preventing any definitive conclusions. Small samples and variability in the classification of exposure may explain these conflicting conclusions. Some inconsistencies between previous studies could also be due to differences in the extent of psychiatric disease progression or differences in the pharmacodynamics of the studied agents (Bar et al., 2007; Bar et al., 2008b; Chang et al., 2012b; Chang et al., 2012a).
Although a prior small study in bipolar disorder did not show any relationship between HRV and current medication dose (Latalova et al., 2010),in an analysis of a well-characterized sample with mood disorders (Fiedorowicz et al., 2012), we found long-term antipsychotic exposure to be associated with increased arterial stiffness and speculated this resulted from sympathetic-mediated increases in blood pressure. Increased arterial stiffness would be hypothesized to lead to reduced HRV through changes in baroreflex sensitivity. In addition, it is possible that these drugs may have direct autonomic actions that would be reflected in altered HRV.
Presynaptic D2 receptors have a role in the inhibition of sympathetic nerve activity (Mannelli et al., 1997). These receptors are primarily composed of the short isoform (D2S) with high receptor density (Usiello et al., 2000; Tadori et al., 2011), and antagonism could increase sympathetic tone. In this follow-up study, we hypothesized that chronic antipsychotic exposure would be associated with lower HRV, which would be indicated by an increased LF/HF ratio and decreased standard deviation of NN intervals (SDNN), as well as decreased root-mean-square of successive differences (RMSSD) values. SDNN is the standard deviation of all intervals between consecutive heartbeats over a fixed amount of time. RMSSD is the square root of the mean of the squared differences between successive NN intervals. Sympathetic-mediated increases in blood pressure would result in increased LF blood pressure variability (BPV), which we also assessed during an exploratory analysis.
METHODS
Sample
Informed consent was obtained from each participant in this IRB-approved study. Participants were between the ages of 20 and 46 and had all received care from a faculty physician at the University of Iowa Hospitals and Clinics. We recruited participants with the diagnosis of bipolar disorder (type I or type II), by cross-referencing the relevant ICD-9 diagnoses codes and billing data. Only diagnoses of bipolar type I and bipolar type II were allowed for inclusion in this study. Chart diagnosis was confirmed in clinical interview. We retrospectively assessed the burden of mood symptoms using previously reported methods (Sodhi et al., 2012). No participants were included if they had history of cancer, untreated thyroid disease, pregnancy, or peripheral vascular disease. This sample represents a subset of participants from a prior analysis (Sodhi et al., 2012), who additionally completed the HRV and BPV measurements described below.
A member of the research team confirmed each participant’s diagnosis during an interview assessing prior mood syndromes. Age of onset, duration of illness, number of psychiatric hospitalizations, and chronicity of depression and mania over the shorter of course of illness or past decade were also assessed during this interview. Current medication use was recorded during the interview and detailed histories of medication exposures were acquired through systematic review of pharmacy records for the previous five years. We contacted all pharmacies at which participants reported filling prescriptions in the past five years. Medication exposure was quantified as the number of weeks taking the medication over the prior five years and divided into the following medication classes: first generation antipsychotics, second generation antipsychotics, valproic acid derivatives, lithium, and serotonin reuptake inhibitors. First generation antipsychotics were not commonly prescribed and the first and second generation categories were subsequently pooled for primary analysis. Valproic acid derivatives and other medication classes were also found to have more limited exposure frequencies and subsequently excluded from the analyses. Tobacco exposure (pack per day*years) was also assessed.
HRV and BPV Assessment
Heart rate and blood pressure were measured simultaneously, by electrocardiogram and the Finometer MIDI (Finapres Medical Systems, Amsterdam, The Netherlands), respectively. All participants were lying down throughout data acquisition and were breathing normally. A consistent room temperature was maintained across studies. Both measures sampled at 1000 HZ. HR and BP measures were stored as equidistant data in a separate text file for each patient, and then assessed using the HemoLab software suite (http://www.haraldstauss.com/HaraldStaussScientific/hemolab). The analyzer software in the HemoLab suite was utilized to derive beat-by-beat measurements for HR from the electrocardiogram reading. Systolic, mean, and diastolic blood pressure values were obtained using the same software.
Systolic BP and HR values exhibiting recording artifacts or cardiac arrhythmias were visually detected and replaced by interpolated values. The following analyses were performed using the Batch Processor software from the HemoLab suite. Time-domain HRV parameters were calculated from the beat-by-beat HR time series using an artifact free segment of 5-minute duration. For frequency-domain analysis, the beat-by-beat systolic BP and HR time series were interpolated (cubic spline) to obtain equidistant time series at 10 Hz sampling rate. Spectral powers were calculated in the VLF (0.02–0.05 Hz), LF (0.05 – 0.15 Hz), and HF (0.15–0.50 Hz) bands by applying the fast Fourier transform (FFT, ~410 s long segments, 50% overlap). Spectral powers were defined as the area under the curve of the power spectra in the respective frequency bands. Spectral analysis of all patients was carried out using the Batch Processor.
Statistical Analyses
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at The University of Iowa. REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources (Harris et al., 2009).
Data was exported from REDCap for statistical analyses using SAS 9.3. Descriptive statistics were compiled from the sample on sociodemographic and clinical variables. Multivariate linear regression models tested the primary, secondary, and exploratory hypotheses of the manuscript. These models carried the form:
Where Y represents the dependent outcome variable (variability), X1 is age (continuous variable, linear effect), X2 is gender, X3 is tobacco exposure in pack years, and X4 is medication exposure (total weeks exposed to class in past 5 years). Covariates were selected based on potential for confounding given their association with variability measures in other studies (Dinas et al., 2011; McNarry and Lewis, 2012; Sookan and McKune, 2012). Sensitivity analyses were performed with reduced and expanded models. Reduced models included only age as a covariate. Expanded models replaced pack*years, the variable least associated with LF/HF ratio in our primary model, with body mass index and duration of affective disorder as potential covariates. These covariates were not included in the initial models to mitigate the risk of over-fitting based on the number of observations included in this sample. Our primary hypothesis, based on prior study (Fiedorowicz et al., 2012), was that five-year antipsychotic exposure would be associated with a higher LF/HF ratio and our secondary hypothesis was that cumulative antipsychotic exposure would be associated with reduced HRV as measured by SDNN and RMSSD. Exploratory analyses assessed the effect of five-year exposure to other medication classes and current use of each medication class on measures of HRV (time and frequency domain) and blood pressure variability. Blood pressure variability data is missing on N=16 (29% of the sample) due to equipment failure prior to completion of the study.
Our primary analytic models examined the assumption that cumulative, long-term exposure to various medication classes impacted measures of autonomic activity. Alternatively, it is plausible that any impact might be related to the immediate, short-term pharmacodynamic effects of the agents. We subsequently conducted a secondary analysis looking at any exposure to medication classes in the two weeks prior to intake, as opposed to cumulative exposure over a five-year period. Positive findings for any medication class were pursued with refined analyses of medications based on pharmacodynamic affinity for the D2S receptor at high density (Tadori et al., 2011). Aripiprazole, risperidone, and paliperidone were classified as high potency; clozapine (no current use in sample) and quetiapine as low potency; and all others as intermediate potency. The latter groups were collapsed due to low cell counts.
RESULTS
Sociodemographic and clinical characteristics of our sample are outlined in Table 1, along with a breakdown of medication exposures for the drugs in our analysis and concomitant drug exposure. Our sample was relatively young with a mean (SD) age of 33 (7) and a mean (SD) duration of mood disorder of 15(9) years. A slight majority (56%) carried a diagnosis of bipolar I disorder and participants had a mean age of onset of 19 years of age, consistent with the median age of onset reported in nationally representative samples (Merikangas et al., 2007). Participants estimated spending a mean (median; SD) of 28.4 (22.0; 25.2) percent of their time with depressive symptoms, and 12.2 (7.0, 0.14) percent of the time with manic symptoms over their course of illness. All 55 individuals had HRV data for time domain analysis, and 39 individuals had usable data for HR spectral analysis or blood pressure variability spectral analysis.
Table 1.
Sociodemographic and Clinical Characteristics (N = 55)
| Mean (SD) | |
|---|---|
| Age | 33 (7) |
| Age of mood disorder onset | 19 (6) |
| Pack years (smoking) | 8 (12) |
| Body mass index (kg/m2) | 30.9 (7.1) |
| Heart Rate | 66 (12) |
| Systolic blood pressure (mmHg) | 117 (10) |
| Diastolic blood pressure (mmHg) | 69 (9) |
| Heart Rate Variability, Time Domain | |
| SDNN | 70.3 (48.2) |
| RMSSD | 58.0 (53.1) |
| Heart Rate Variability, Frequency Domain (N=39) | |
| High Frequency Relative Spectral Power | 17.7 (10.1) |
| Low Frequency Relative Spectral Power | 16.5 (6.2) |
| Low Frequency: High Frequency Ratio | 1.23 (0.80) |
| Very low frequency Relative spectral Power | 22.3 (7.8) |
| Blood Pressure Variability (N=39) | |
| High Frequency Absolute Spectral Power | 2.42 (2.47) |
| Low Frequency Absolute Spectral Power | 3.58 (3.50) |
| Very low frequency Absolute spectral power | 8.33 (8.65) |
| N (%) | |
| Female gender | 37 (67%) |
| White, not Hispanic | |
| Unemployed | 22 (40%) |
| Bipolar I diagnosis | 31 (56%) |
| Ever smoked tobacco | 30 (55%) |
| Medication History (Any Prior Use) | |
| Antipsychotics | 37 (67%) |
| First Generation | 10 (18%) |
| High Potency | 5 (9%) |
| Other | 7 (13%) |
| Second Generation | 33 (60%) |
| High Potency (D2S) | 28 (51%) |
| Intermediate Potency | 12 (22%) |
| Low Potency | 14 (25%) |
| Valproic acid derivatives | 8 (15%) |
| Lithium | 24 (44%) |
| Serotonin Reuptake Inhibitor | 20 (36%) |
| Concomitant Medications | |
| Anticholinergics | 25 (45%) |
| Benzodiazepines | 39 (71%) |
| Thyroid Product | 12 (22%) |
| Stimulants | 4 (7%) |
| Tricyclic Antidepressants | 11 (20%) |
| Hypnotics | 14 (25%) |
| Beta-blocker | 3 (5%) |
In the primary analysis, antipsychotic exposure did not appear to be associated with the LF/HF ratio in multivariate models controlling for age, gender, and pack*years (F1,34=0.02, p=0.90). This remained non-significant in the reduced and expanded sensitivity analysis models. In secondary analyses, exposure to antipsychotics was not associated with the time domain measure of heart rate variability RMSSD (F1,50=2.61, p=0.11) and SDNN (F1,50=1.23, p=0.27) in analogous multivariate models. Similar results were observed in the reduced and expanded models. In exploratory analyses, antipsychotics were not related to VLF, LF, or HF power upon spectral analysis of the blood pressure variability data.
Exploratory analyses similarly assessed the relationship between lithium and serotonin reuptake inhibitors on HRV and BPV. Lithium was not associated (F1,34=0.00, p=0.98) and serotonin reuptake inhibitors (F1,34=3.11, p=0.087) were marginally but not significantly associated with LF/HF ratio. This appeared to be related to an association between serotonin reuptake exposure and LF power of HRV (F1,34=5.07, p=0.03) such that greater exposure to SSRIs was associated with lower power in the LF spectrum (univariate r=−0.40, p=0.01). This finding remained significant in reduced and expanded models. Serotonin reuptake inhibitor exposure was not associated with SDNN (F1,50=1.21, p=0.28) or RMSSD (F1,50=1.63, p=0.21), nor was exposure to lithium.
Current antipsychotic use was reported for 21 (38%) of the sample with the following breakdown: first generation use in 5 (9%, 2 high potency, 3 not high potency) and second generation use in 16 (29%, 12 high potency, 4 not high potency as defined in methods). SDNN was substantially lower in current users of antipsychotics, with a mean (SD) of 55 (46) as compared to non-users [80 (48)]. Controlling for age, gender, and tobacco exposure (pack years), SDNN was significantly associated with current antipsychotic exposure (F1,50=4.23, p=0.05), while RMSSD was not (F1,50=2.56, p=0.11). SDNN (F1,50=5.96, p=0.02)and RMSSD (F1,50=4.37, p=0.04) were both associated with current exposure to second generation antipsychotics in analogous multivariate models. This finding persisted in the expanded model that replaced tobacco exposure with body mass index and duration of illness. Additional inclusion of an indicator for current use of an anticholinergic medication did not substantively alter findings.
SDNN was lower in current second generation antipsychotic users [mean (SD): 45 (31)] compared to non-users [81 (50)]. RMSSD was also lower in current second generation antipsychotic users [32 (32)] compared to non-users [69 (56)]. On univariate non-parametric analyses, current users of second generation antipsychotics had lower SDNN (Wilcoxon Rank Sum Z= −2.83, p=0.005) and RMSSD (Wilcoxon Rank Sum Z= −2.47, p=0.007). Groups did not significantly differ on age, gender, and tobacco exposure. Univariate results are portrayed in Figure 1.
Figure 1. Heart rate variability measures by second generation antipsychotic use.
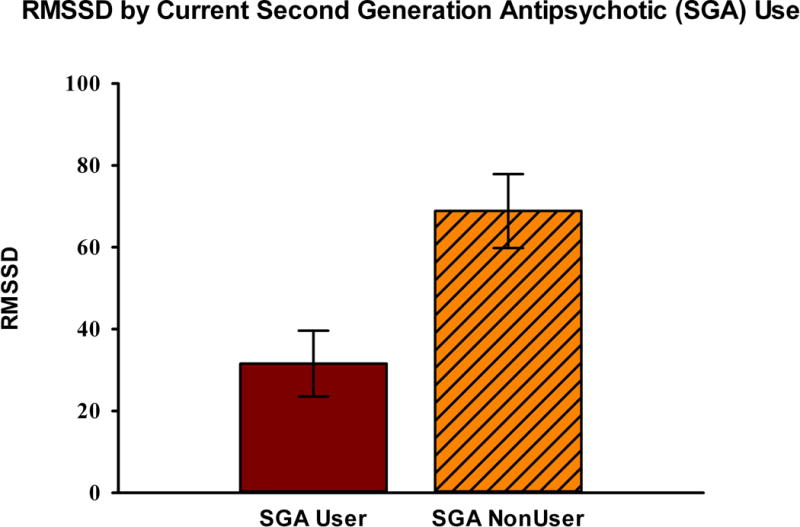
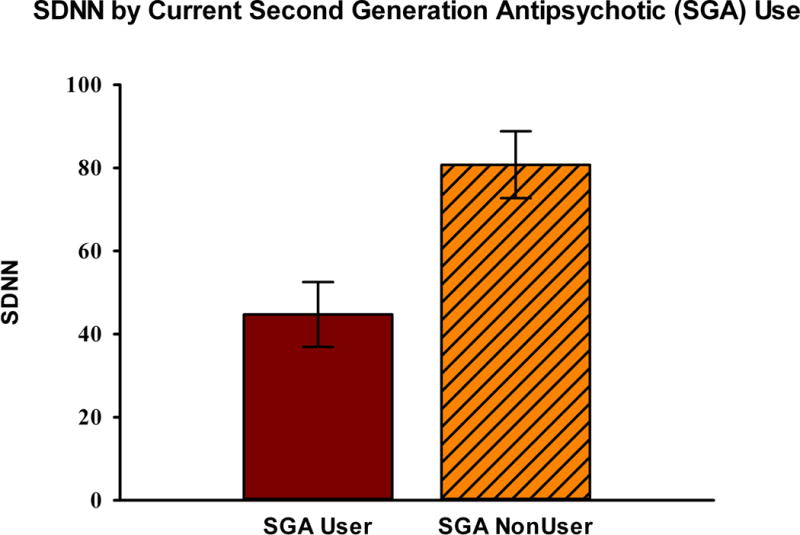
Current (last two weeks) users of second-generation antipsychotics (SGAs) had lower measures of time-domain heart rate variability for RMSSD, and SDNN as shown in Figure 1a and 1b below.
These associations with current second generation antipsychotic use were explored based on relative potency for antagonism of presynaptic D2S receptors and the finding was indeed confined to users of these high potency agents (N=12).
DISCUSSION
This cross-sectional study of individuals with bipolar disorder suggests the possibility that antipsychotics may have an acute effect on HRV during current use. Although limited by the cross-sectional nature of the design, the negative findings of our cumulative primary models suggest that these effects are confined to periods of use and that cumulative exposure does not cause any lasting effects. These findings were not confirmed by spectral analysis data, though spectral analysis was hampered by more limited power given the smaller sample size. The difference in effects on HRV between current treatment and longer-term cumulative exposure is intriguing and offers potential mechanistic insight. It would suggest, for example, that any effects of antipsychotics on HRV wash out even after prolonged or high dose exposure. This would be inconsistent with an effect of these drugs on HRV that is mediated by increased arterial stiffness due to structural changes. However, short-term changes in endothelial function could still influence arterial stiffness and thus HRV, as could acute effects on autonomic function (such as reduced parasympathetic tone).
Our primary model failed to find an association between long-term antipsychotic exposure over the last five years and autonomic dysfunction as assessed through measures of heart rate and blood pressure variability. This fails to support the hypothesis that our prior findings of a relationship between first generation antipsychotic exposure and arterial stiffness was mediated by ANS activity, although our current sample had limited exposure to first generation antipsychotics and these broad classes of antipsychotics were subsequently pooled. As previously noted, we did find a relationship between current use of second generation antipsychotics and measures of heart rate variability. This finding appeared to be explained by those agents which most strongly antagonize high density D2S receptors, consistent with our mechanistic hypothesis. The lack of a corresponding finding for first generation antipsychotics, which also antagonize these receptors, could represent Type II error given limited exposure or other confounding factors differentially related to exposure to this antipsychotic class (Barbui et al., 2006). The low and intermediate potency second generation antipsychotics were also infrequently prescribed and this interesting finding is subsequently best construed as hypothesis generating, given the limitations in study design for such a refined analysis.
In exploratory analyses, we also found reduced low, but not high frequency spectral power of HRV among those with greater exposure to SSRIs. Given that sympathetic activity contributes to low and high spectral power HRV while parasympathetic activity contributes only to high spectral power, one might speculate that SSRIs contribute to a dampening of sympathetic outflow to the heart. Existing literature on the relationship between SSRI exposure and HRV indicates that SSRIs may decrease parasympathetic tone (Licht et al., 2010) and contribute to decreases in both low and high frequency power spectral HRV (Henje Blom et al., 2010). We did not see a significant effect of SSRIs on HF variability, but did observe LF to be affected by SSRI exposure, which lends further credibility to claims that SSRI exposure reduces HRV. Studies in the future will want to find samples with a higher number of participants with SSRI exposure to further corroborate these findings.
Although our results coincide with a majority of the existing literature, consideration of several study limitations must be taken into account. While we obtained records from all reported pharmacies, the acquisition of medication histories for some patients might have been incomplete if a participant used multiple pharmacies and forgot to report one. We were unable to explore the effects of individual medications and therefore medication exposure data was pooled across classes, which display some heterogeneity. In this cross-sectional study, temporality cannot be established. Findings from our relatively young, mostly Caucasian clinical sample may not generalize to all populations. Treatment was not randomly assigned and potential for confounding exists. While we attempted to address potential for confounding in multivariate models, the potential for residual confounding exists. ANS activity is further indirectly inferred by HRV and blood pressure variability measures and was not directly assessed. Assessments were made at rest and not following any provocation or challenge and thus the impact of exposure on any such dynamic measures could not be discerned. Current antipsychotic exposure was assessed in secondary models and our findings related to SSRI exposure were exploratory in nature and these findings should subsequently be considered hypothesis generating and require subsequent hypothesis-testing study.
Despite these limitations, given the limited previous examinations of this topic, our study contributes incrementally to the evidence base. The sample size for this study is considerably larger than similar existing studies of the relationship between antipsychotic exposure and HRV. Our assessment of exposure was not limited to current medication use and pharmacy records tend to be very accurate and allow for some inference of adherence. We rigorously assessed exposure over the past five years with data obtained from multiple pharmacies for each individual participant. Our findings suggest that D2 receptor antagonism may be one mechanism, beyond metabolic adverse effects, by which antipsychotic drugs could worsen vascular risk. If replicated, these findings may also have implications related to use of these medications in patients with dysautonomias.
Future studies to investigate the acute effects of these agents on HRV are warranted, ideally using an experimental design to establish temporality and address potential for confounding, which we attempted to address in our multivariate models. This would additionally facilitate examination of specific agents instead of analysis of broader classes of medications. Including complexity measures of HRV, in addition to time- and frequency-domain analyses may yield additional insights, particularly using the aforementioned experimental design.
Contrary to our initial hypothesis, we found a significant relationship between current, but not long-term antipsychotic exposure and HRV. This may indicate acute effects of antipsychotics on the ANS, vascular tone or cardiac rhythm centers. Exploratory analyses indicated decreased low frequency spectral power HRV was correlated with higher SSRI exposure, which could also be a focus for future study.
Acknowledgments
This work was supported by the National Institutes of Health (1K23MH083695-01A210), the Nellie Ball Research Trust, and the Institute for Clinical and Translational Science at the University of Iowa (5UL1RR024979-05). We would like to thank Harald Stauss for intellectual contributions to this paper.
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- Bar KJ, Boettger MK, Koschke M, Schulz S, Chokka P, Yeragani VK, Voss A. Non-linear complexity measures of heart rate variability in acute schizophrenia. Clin Neurophysiol. 2007;118:2009–2015. doi: 10.1016/j.clinph.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Koschke M, Berger S, Schulz S, Tancer M, Voss A, Yeragani VK. Influence of olanzapine on QT variability and complexity measures of heart rate in patients with schizophrenia. J Clin Psychopharmacol. 2008a;28:694–698. doi: 10.1097/JCP.0b013e31818a6d25. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Wernich K, Boettger S, Cordes J, Boettger MK, Loffler S, Kornischka J, Agelink MW. Relationship between cardiovagal modulation and psychotic state in patients with paranoid schizophrenia. Psychiatry Res. 2008b;157:255–257. doi: 10.1016/j.psychres.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Barbui C, Nose M, Mazzi MA, Bindman J, Leese M, Schene A, Becker T, Angermeyer MC, Koeter M, Gray R, Tansella M. Determinants of first- and second-generation antipsychotic drug use in clinically unstable patients with schizophrenia treated in four European countries. Int Clin Psychopharmacol. 2006;21:73–79. doi: 10.1097/01.yic.0000185022.48279.db. [DOI] [PubMed] [Google Scholar]
- Chang JS, Ha K, Yoon IY, Yoo CS, Yi SH, Her JY, Ha TH, Park T. Patterns of cardiorespiratory coordination in young women with recurrent major depressive disorder treated with escitalopram or venlafaxine. Prog Neuropsychopharmacol Biol Psychiatry. 2012a;39:136–142. doi: 10.1016/j.pnpbp.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Chang JS, Yoo CS, Yi SH, Her JY, Choi HM, Ha TH, Park T, Ha K. An integrative assessment of the psychophysiologic alterations in young women with recurrent major depressive disorder. Psychosom Med. 2012b;74:495–500. doi: 10.1097/PSY.0b013e31824d0da0. [DOI] [PubMed] [Google Scholar]
- Chang JS, Yoo CS, Yi SH, Hong KH, Lee YS, Oh HS, Jung DC, Kim YS, Ahn YM. Changes in heart rate dynamics of patients with schizophrenia treated with risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:924–929. doi: 10.1016/j.pnpbp.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Cohen H, Loewenthal U, Matar M, Kotler M. Association of autonomic dysfunction and clozapine. Heart rate variability and risk for sudden death in patients with schizophrenia on long-term psychotropic medication. Br J Psychiatry. 2001;179:167–171. doi: 10.1192/bjp.179.2.167. [DOI] [PubMed] [Google Scholar]
- Dinas PC, Koutedakis Y, Flouris AD. Effects of active and passive tobacco cigarette smoking on heart rate variability. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.10.140. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz JG, Coryell WH, Rice JP, Warren LL, Haynes WG. Vasculopathy related to manic/hypomanic symptom burden and first-generation antipsychotics in a sub-sample from the collaborative depression study. Psychother Psychosom. 2012;81:235–243. doi: 10.1159/000334779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel RJ, Tulen JH, Van Beveren NJ, Roder CH, Hengeveld MW. Cardiovascular variability during treatment with haloperidol, olanzapine or risperidone in recent-onset schizophrenia. J Psychopharmacol. 2009;23:697–707. doi: 10.1177/0269881108091254. [DOI] [PubMed] [Google Scholar]
- Henje Blom E, Olsson EM, Serlachius E, Ericson M, Ingvar M. Heart rate variability (HRV) in adolescent females with anxiety disorders and major depressive disorder. Acta Paediatr. 2010;99:604–611. doi: 10.1111/j.1651-2227.2009.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latalova K, Prasko J, Diveky T, Grambal A, Kamaradova D, Velartova H, Salinger J, Opavsky J. Autonomic nervous system in euthymic patients with bipolar affective disorder. Neuro Endocrinol Lett. 2010;31:829–836. [PubMed] [Google Scholar]
- Licht CM, De Geus EJ, Van Dyck R, Penninx BW. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol Psychiatry. 2010;68:861–868. doi: 10.1016/j.biopsych.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Dalack G, Leitman D, Corcoran C, Amador XF, Yale S, Glassman A, Gorman JM. Low heart rate variability is not caused by typical neuroleptics in schizophrenia patients. CNS Spectr. 2002;7:53–57. doi: 10.1017/s1092852900022264. [DOI] [PubMed] [Google Scholar]
- Mannelli M, Lazzeri C, Ianni L, La Villa G, Pupilli C, Bellini F, Serio M, Franchi F. Dopamine and sympathoadrenal activity in man. Clin Exp Hypertens. 1997;19:163–179. doi: 10.3109/10641969709080813. [DOI] [PubMed] [Google Scholar]
- Mcnarry MA, Lewis MJ. Interaction between age and aerobic fitness in determining heart rate dynamics. Physiol Meas. 2012;33:901–914. doi: 10.1088/0967-3334/33/6/901. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujica-Parodi LR, Yeragani V, Malaspina D. Nonlinear complexity and spectral analyses of heart rate variability in medicated and unmedicated patients with schizophrenia. Neuropsychobiology. 2005;51:10–15. doi: 10.1159/000082850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DP, Weiner M, Prabhakar M, Fiedorowicz JG. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? Curr Psychiatry Rep. 2009;11:475–480. doi: 10.1007/s11920-009-0072-3. [DOI] [PubMed] [Google Scholar]
- Silke B, Campbell C, King DJ. The potential cardiotoxicity of antipsychotic drugs as assessed by heart rate variability. J Psychopharmacol. 2002;16:355–360. doi: 10.1177/026988110201600410. [DOI] [PubMed] [Google Scholar]
- Sodhi SK, Linder J, Chenard CA, Miller Del D, Haynes WG, Fiedorowicz JG. Evidence for accelerated vascular aging in bipolar disorder. J Psychosom Res. 2012;73:175–179. doi: 10.1016/j.jpsychores.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookan T, Mckune AJ. Heart rate variability in physically active individuals: reliability and gender characteristics. Cardiovasc J Afr. 2012;23:67–72. doi: 10.5830/CVJA-2011-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol. 2007;34:362–368. doi: 10.1111/j.1440-1681.2007.04588.x. [DOI] [PubMed] [Google Scholar]
- Tadori Y, Forbes RA, Mcquade RD, Kikuchi T. Functional potencies of dopamine agonists and antagonists at human dopamine D(2) and D(3) receptors. Eur J Pharmacol. 2011;666:43–52. doi: 10.1016/j.ejphar.2011.05.050. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, Lemeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Wang YC, Yang CC, Bai YM, Kuo TB. Heart rate variability in schizophrenic patients switched from typical antipsychotic agents to amisulpride and olanzapine. 3-month follow-up. Neuropsychobiology. 2008;57:200–205. doi: 10.1159/000149818. [DOI] [PubMed] [Google Scholar]
- Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23:40–47. [PMC free article] [PubMed] [Google Scholar]


