Abstract
The specialized cell cycles that characterize various aspects of the differentiation of germ cells provide a unique opportunity to understand heretofore elusive aspects of the in vivo function of cell cycle regulators. Key components of the cell cycle machinery are the regulatory sub-units, the cyclins, and their catalytic partners, the cyclin-dependent kinases. Some of the cyclins exhibit unique patterns of expression in germ cells that suggest possible concomitant distinct functions, predictions that are being explored by targeted mutagenesis in mouse models. A novel, meiosis-specific function has been shown for one of the A-type cyclins, cyclin A1. Embryonic lethality has obviated understanding of the germline functions of cyclin A2 and cyclin B1, while yet other cyclins, although expressed at specific stages of germ cell development, may have less essential function in the male germline.
Keywords: cell cycle machinery, spermatogenesis, germ cell differentiation, meiosis, male germ cell stem cells, mitosis, cyclin-dependent kinases, apoptosis
Introduction
Understanding the genetic program controlling the mitotic and meiotic divisions of the germ line presents a unique opportunity for providing insight into cell cycle control in vivo, during development and differentiation. Elucidating the key control points and proteins involved in this regulation may also enhance our understanding of the etiology of human infertility and ultimately, provide new directions for contraception. Meiosis is a biological process that is restricted to germ line cells and as such, likely requires mechanisms of cell cycle control that do not function or even exist in somatic cells. One striking difference, for example, is that there are two metaphase segregation events in the absence of an intervening round of DNA synthesis. Another aspect of meiosis that distinguishes it from mitosis is the behavior of sister chromatids during the first meiotic division.
Higher organisms are characterized by having sexually dimorphic gametes. While male and female germ cells have stages of cell cycle regulation in common, including a mitotic proliferative stage, entry into meiosis, completion of a reductive division, and entry into a quiescent state prior to fertilization, the timing of these events and the stage of development at which these events occur differ in the two sexes (reviewed in refs. 1-4). Germ cells of both sexes undergo mitotic divisions in the embryonic gonad but the female germ cells enter meiosis during fetal development, whereas this is a post-natal event in the male. Once the male germ cell has entered meiosis, the process continues without interruption until the haploid sperm is produced. In contrast, the oocyte is arrested in the diplotene stage of meiotic prophase I, where it can remain for months or years, depending on the species. Following a growth period, the oocyte resumes meiosis, but arrests a second time, at metaphase II. Fertilization then triggers the completion of meiosis and extrusion of the second polar body. It is likely that there will be genes uniquely involved in these regulatory check points that will further exhibit sexual dimorphism.1,4
Spermatogenesis in the mouse is particularly attractive as a model system in which to study genes important in the in vivo regulation of the mammalian mitotic and meiotic cell cycles. In the adult testis, one can examine cells in various stages of differentiation within a single lineage, from the self-renewing stem cells and mitotically dividing spermatogonia, to the meiotically dividing spermatocytes, as well as the post-meiotic germ cells, the spermatids. Further, the progression of differentiation of these events occurs in a temporally orchestrated manner, such that classical cellular associations have been defined and deviations in these associations upon loss of gene function can be readily ascertained. Finally, the power of genetic analysis can be brought into play, whether utilizing strains carrying existing mutations that result in characteristic phenotypes, such as loss of the germ cell lineage (the W alleles encoding the c-kit tyrosine kinase receptor), or generating new mutant strains using targeted mutagenesis in embryonic stem (ES) cells (reviewed in refs. 5-7).
Cyclins: Key Components of the Cell Cycle Machinery
Cell cycle progression is regulated in part by the sequential activity of various cyclins. The cyclins are regulatory subunits that bind, activate and provide substrate specificity for their catalytic partner serine-threonine kinases, collectively called cyclin-dependent kinases (Cdks) (reviewed in refs. 8 and 9). The activity of cyclin-Cdk complexes is tightly regulated by a complex network of other proteins that function as activators and inhibitors as well as influencing their transcription, sub-cellular localization and degradation.
Several classes of cyclins have been described in mammalian cells, designated A to I, and also T. During the mitotic cell cycle, cyclins from the D-type family (D1, D2 and D3) regulate progression of cells through the G1 phase.10 D-type cyclins bind and activate Cdk4 and Cdk6. These cyclin D-Cdk4/6 complexes phosphorylate and functionally inactivate the retinoblastoma protein, pRB and pRB-related proteins p107 and p130, thereby contributing to cell cycle progression. Cyclins of the E-type family (E1 and E2) are expressed during late G1 and during S-phase progression. E-type cyclins activate primarily Cdk2, but they can also associate with Cdk1 and Cdk3. Later during the S-phase cyclin A2 becomes activated. Cyclin A2 associates with both Cdk1 and Cdk2, and phosphorylates substrates similar to those of cyclin E-Cdk. Lastly, M-phase progression is driven by the B-type cyclins which activate Cdk1. In addition to activating Cdks, cyclins also play kinase-independent functions. In particular, D-type cyclins have been reported to have Cdk-independent roles as co-activators or co-repressors of tissue-specific transcription factors (reviewed in ref. 11) and recent studies for the E-type cyclins also implicate kinase-independent functions.12
A-Type Cyclins
It was discovered now over ten years ago that there are two distinct A-type cyclins in the mouse (and human) genome, one of which, cyclin A1, is testis-specific and restricted to the germ line.13-15 The originally identified A-type cyclin, cyclin A2, is ubiquitously expressed in cultured cells and is upregulated in a variety of cancers.16,17 The two A-type cyclins exhibit strikingly different patterns of expression: cyclin A2 is ubiquitously expressed in mitotically dividing cells while expression of cyclin A1 is highly restricted, being most abundant in the testis in both mice and humans.14,15 Mouse cyclin A2 is expressed in a broad variety of tissues in the adult mouse and during embryogenesis.13,14
The two A-type cyclins exhibit distinct and sexually dimorphic patterns of expression in the mammalian germ line
In mice, cyclin A1 is expressed specifically in testis in pachytene and diplotene spermatocytes spermatocytes in stage IX to XII tubules at both the mRNA and protein levels.14,18 In contrast, cyclin A2 is expressed in spermatogonia and pre-leptotene spermatocytes and its expression is downregulated early in meiotic prophase, well before cyclin A1 is expressed.14,19 The A-type cyclins are differentially regulated in the ovary, with cyclin A1 being totally repressed and cyclin A2 being expressed in both granulosa cells and oocytes in a developmentally regulated manner.20 It is therefore likely that there are specific regulatory elements unique to each A-type cyclin that are critical for their distinct regulation of expression, and further, that the two A-type cyclins may play unique functions in cell cycle progression in the male and female germ lines.
Genetic approaches to understanding the function of the two A-type cyclins
Targeted mutagenesis of Ccna2, the mouse cyclin A2 gene, resulted in early embryonic lethality, around the peri-implantation stage.21 While this observation dramatically underscores the essential nature of cyclin A2 function in vivo, it obviates understanding its role in either the embryonic or adult germ line. In striking contrast, loss of cyclin A1 function did not affect viability but did result in male (but not female) sterility.18,22 Histological and cytogenetic analysis revealed normal meiotic progression until mid-diplotene, with normal formation and resolution of chiasmata.18,23 However in late diplotene, rather than undergoing diakinesis and proceeding to metaphase I, spermatocytes in the cyclin A1-deficient mice arrested and underwent apoptosis.18,24 This arrest was distinct from and occurred later than the meiotic arrest observed in mice deficient in the putative cyclin A1 kinase partner, Cdk2 (see below).
Molecular consequences and possible targets of loss of cyclin A1 function
Although the molecular basis of this arrest remains to be elucidated, several aspects of the phenotype provide insight into some of the cellular processes and components involved. For example, MPF kinase activity was shown to be greatly inhibited in cyclin A1-deficient testes, although both cyclin B and Cdk1 are present, suggesting that cyclin A-containing complexes are involved in activating MPF.18,25
A distinctive feature of pachytene spermatocytes involves the localization of the Ser139-phosphorylated form of H2AX, or γH2AX, in a characteristic staining pattern at each stage of meiosis and in particular, in the XY body.26 In cyclin A1-deficient spermatocytes, the localization of γH2AX was indistinguishable from that of the control spermatocytes.23 However, at the point of meiotic arrest in diplotene, γH2AX foci were observed first at the centromere and subsequently along the length of the chromosomal axes. The appearance of γH2AX foci was concurrent with an aggregation of centromeric heterochromatin.
The late diplotene stage of meiosis has also been reported to be coincident with robust phosphorylation of histone H3 at serine 10.27 Interestingly, phosphorylation of H3 serine 10 was dramatically reduced in Ccna1+/- spermatocytes and undetectable in spermatocytes totally lacking cyclin A1.23 Concomitantly, cyclin A1-deficient spermatocytes show reduced staining of aurora B kinase at the pericentromeric heterochromatin.23 While immunoblot analysis of whole testicular lysates did not indicate a significant difference in levels of aurora B protein between control and A1-deficinet testicular lysates, the amount of aurora B protein associated with meiotic chromosomes was clearly different. Interstingly, histone H3 serine 10 is a known target of phosphorylation by the aurora B component of the passenger protein complex28 and the point of meiotic arrest in cyclin A1 mice also overlaps with the assembly of the passenger protein complex.
B-Type Cyclins
Multiple B-type cyclin family members have been identified in organisms as evolutionarily divergent as yeast, frog, mouse and human and there may be as many as nine B1-related sequences in the mouse genome.29,30 The roles of the different B-type cyclins are not understood, but in general, the B-type cyclins appear during the G2/M phase transition of the cell cycle. We had previously reported distinct developmentally regulated patterns of expression of the mouse cyclin B1 (Ccnb1) and B2 (Ccnb2) genes in both the male and female germ cells.31,32 In the adult testis, Ccnb2 was present at highest levels in the meiotically dividing spermatocytes. This is in contrast to the distribution of Ccnb1 transcripts, which were most abundant in the post-meiotic spermatids. Lower levels of Ccnb2 mRNAs were also detected in the early round spermatids. It is curious that neither Ccnb1 nor Ccnb2 transcripts was detected in the mitotically dividing spermatogonia. This could be because the levels of transcripts were simply below the level of sensitivity of the in situ hybridization used in these studies. Alternatively, another B-type could be responsible for activating Cdk1 in these cells. The finding of high levels of Ccnb1 transcripts in the post-meiotic male germ cells was surprising.32 These results may implicate a function for cyclin B1 which does not involve cell cycle progression; rather, cyclin B1 could be involved in directing kinase activity to specific substrates involved in germ cell differentiation, or as mentioned before, in other kinase-independent functions.
A third B-type cyclin, cyclin B3, has been identified in several organisms. In Drosophila, cyclin B3 is expressed in both mitotic and meiotic cells.33 In mitotic cells, it is downregulated during the metaphase to anaphase transition, following degradation of cyclin B1.33 Overexpression of a non-degradable cyclin B3 is associated with chromatin decondensation defects at the end of mitosis.34 Drosophila cyclin B3 is essential for fertility; but unlike cyclin B1 deficiency, infertility of cyclin B3-deficient flies is restricted to the female germ line and does not appear to involve defects in ovary structure or in meiosis I entry.33 No endogenous cyclin B3 could be detected in a variety of mammalian cell lines; however, cyclin B3 mRNA and protein was observed specifically in leptotene and zygotene spermatocytes (and in nests of female oocytes during embryonic development).35 Forced expression of cyclin B3 in cultured cells showed that is nuclear, is degraded upon anaphase entry following cyclin B1 degradation, and it is a poor activator of Cdk2 kinase.35 Further, prolonged expression of cyclin B3 after the zygotene stage led to aberrant spermatogenesis and apoptosis in transgenic mice.36
D-Type Cyclins
The family of D-type cyclins was the first mammalian cyclin family shown to have multiple members, D1, D2 and D3.37 The D-type cyclins play pivotal roles in the entry of cells into G1/S of the mitotic cell cycle,38,39 and hence a role in proliferation, but they have been implicated in diverse cellular events, including differentiation,40-42 apoptosis,43 and as mentioned above, in non-cell cycle related functions in activating transcription factors.44 Given their potential diverse functions, there was great interest to define their cellular sites of expression in a variety of tissues and during tumori-genesis, including the testis,45-50 and further, each family member has been knocked out in mouse models.39 Although there appear to be stages of spermatogenesis when one family member is more prevalent, for example cyclin D3 in proliferating gonocytes at embryonic day 14,49 all three D-type cyclins are expressed in proliferating spermatogonia. Less predicted was the observation of cyclin D2 and D3 in spermatocytes45,48,49 and quite clear expression of cyclin D3 in round spermatids.48 With regard to the somatic compartment of the testis, cyclin D3 appears to predominate in Sertoli cells48 and D2 and D3 in the Leydig cells.49 Interestingly, the Sertoli cell expression of cyclin D3 persists even after the cells no longer divide in the adult testis and it is readily detected in the slowly dividing Leydig cells.
Regardless of the clear expression of the cyclin D family in various stages of spermatogenesis, deletion of specific D function in mouse models has not revealed an essential function in the germ line. Whether this is due to functional redundancy among the family members in those cells that express all three cyclin D’s or a non-essential role in cells where a single family member is expressed remains to be determined. As the ablation of all three D-type cyclins resulted in embryonic lethality, albeit at a relatively advanced stage,39 conditional knockouts will be required to address these questions (see below).
E-Type Cyclins
Like the A-type cyclins, there are also two members of the E-type cyclin family in the mammalian genome. Genetic studies to dissect the functions of the E-type cyclins in development and in neoplasia, revealed a surprising function for these genes in spermatogenesis.51,52 That is, the only obvious phenotype in the single knock-out models was in the male germ line: cyclin E2-deficient males had reduced fertility, with ~50% of the males being sterile. On average, cyclin E2-deficinet males displayed nearly a four-fold reduction in their sperm counts, as compared to wild-type controls. Preliminary histological examination of the affected testes revealed greatly reduced cellularity within the tubules and the presence of giant cells51 and further that spermatogenesis is not uniformly arrested nor at a unique stage (Chung SSW, Sutton SS, Geng Y, Sincinski P and Wolgemuth DJ, unpublished observations). Further, there is a gradation in the level of disruption of spermatogenesis and the loss of cells among the testes of the infertile males. In the more severely disrupted tubules, spermatocytes appear to advance as far as the zygotene stage but later stage spermatocytes and round spermatids were missing. In the less severely affected tubules, more advanced stages, including fully elongated spermatids were detected. This phenotype is quite different from that observed in the absence of cyclin A1, where spermatogenesis is halted precisely in late diplotene and is 100% penetrant even on a mixed background.
To further test the requirement for the E-type cyclins in development and in cell proliferation, mice lacking both cyclin E genes have been generated.12 Although embryos lacking both E-type cyclins died during mid-gestation due to placental abnormalities, this lethality could be rescued by providing the double-deficient embryos with wild-type placentas through tetraploid blastocyst complementation (although the progeny died shortly after birth). Mouse embryonic fibroblasts (MEFs) from cyclin E1, E2-deficient embryos proliferated normally under conditions of continuous cell cycling, but were unable to re-enter the cell cycle from quiescence. Molecular analyses revealed that during cell cycle re-entry, cyclin E is loaded onto DNA pre-replication complexes, where it facilitates loading of the MCM replicative helicase onto DNA and interestingly, that this function of cyclin E is Cdk-independent.
Cdk2
It is also of interest that Cdk2, a major kinase partner for both A- and E-type cyclins, has been shown to be essential for meiosis in both the male and female germ lines.53,54 Cdk2-deficient male mice also exhibit an arrest in meiotic prophase, similar (but not identical to) cyclin A1-deficient spermatocytes. In Cdk2-deficient mice, arrest was observed in mid-pachytene spermatocytes and was accompanied by thin threads of SCP3 staining, perhaps indicating aberrant pairing,53 while cyclin A1-deficient mice were normal with respect to staining of SCP3.23 Further, synaptonemal complexes were indistinguishable between normal and cyclin A1-deficient spermatocytes,18 and when pachytene cyclin A1-deficinet spermatocytes were artificially driven into a meiotic configuration by treatment with okadaic acid, metaphase I preparations from mutant and normal spermatocytes appeared similar, with no obvious defects in chaismata.25
A possible reason for these distinct phenotypes may lie in the temporal appearance and nuclear distribution of cyclin A1 versus Cdk2. Cdk2 protein is localized in the centromeric region, at telomeres, and at foci along chromosomes during pachytene to diplotene,55 and is not altered in cyclin A1-deficient mice.23 However, cyclin A1 did not completely co-localize with its putative Cdk2 partner at the centromeres.23 Further, although cyclin A1 is capable of activating Cdk2,25 it cannot represent the regulatory subunit for Cdk2 at these early stages of meiotic prophase as it is not expressed until later in meiotic prophase.14
Apoptosis is a Hallmark of Germ Cells Lacking Cyclin A1 as well as CDK2
Another striking aspect of the phenotype of the cyclin A1-deficient mice was that the spermatocytes underwent a highly synchronized wave of apoptosis just before the first meiotic division.18 This was further shown to be a primary response to the loss of cyclin A1 function, as it occurred in the first cells to reach the diplotene to metaphase I transition, and not due to overall degeneration of the testicular tubules.24 Along with activation of caspase-3, an increase in the levels and change of sub-cellular localization of Bax protein was observed, which coincided with the onset of apoptosis. As p53 is implicated in the activation of Bax-mediated cell death, mice lacking both cyclin A1 and p53 were generated.24 Although the absence of p53 did not rescue the meiotic arrest, there was a decrease in the number of apoptotic cells in the doubly-mutant testes. This suggested that p53 may be involved in the induction of apoptosis in the cell cycle arrested cells, but that other pathways function as well to insure removal of the arrested spermatocytes. Apoptosis was also induced quite robustly in Cdk2-deficient spermatocytes.56
A curious clustering of centromeres of cyclin A1-deficient late pachytene chromosomes was observed and noted to occur just as the cells were entering apoptosis.23 This clustering was concomitant with an aggregation of centromeric heterochromatin into large clumps as visualized by DAPI staining. It remains to be determined whether this aggregation occurred as part of the apoptotic response, but it is of interest that the γH2AX foci accumulated after this aggregation.
Some Unresolved Questions
As mentioned above, the embryonic lethality that resulted from loss of either cyclin A2 or cyclin B1 function has precluded understanding their function in the germ line, where they are both abundantly expressed. Clearly, the generation of floxed alleles of both genes is a critical next step, combined with the use of strains of mice expressing Cre recombinases in specific stages of germ cell differentiation. For example, the Ngn3-Cre mice developed by Yoshida and colleagues57 could be used to generated null alleles for floxed genes specifically in type A spermatogonia. Targeted mutagenesis later in meiosis may be achieved by use of mice expressing Scyp3-Cre.58
Although cyclin E2-null mice did exhibit reduced fertility and abnormalities in spermatogenesis51,52 and loss of an additional cyclin E allele (i.e., Ccne1+/-, Ccne2-/- mice) further compromises spermatogenesis (Sicinski P, personal communication), the combination of targeted germline ablation of cyclin E1 on a cyclin E2-deficient background would be the most direct approach to determining its function in the germ line. This approach, however, requires the clear identification of the cellular sites of expression of cyclin E1 and E2 in the testis, which remains to be determined.
Another intriguing question lies in the mode of regulation of cyclin function, whether at the level of transcription, translation, post-translational modifications, or altered sub-cellular distribution, in germ cells. The genes encoding cyclins A1 and A2 as well as cyclins B1, B2 and B3 have already been shown to exhibit highly regulated transcription.13,14,19,31,32,35 Finally, almost nothing is known with regard to either potential substrates of the various cyclin/Cdk complexes or to other potential interacting proteins during germ cell proliferation or differentiation.
Figure 1.
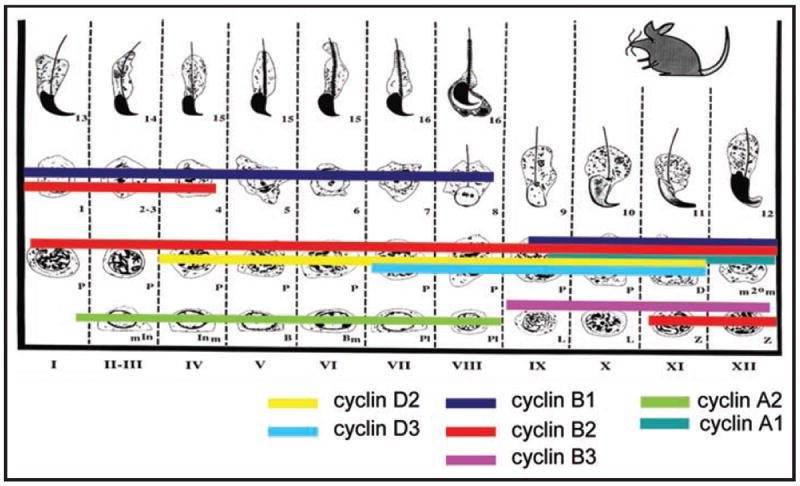
Cartoon summarizing the meiotic stages of spermatogenesis in which cyclin expression has been documented. The bars are color-coded to depict expression of specific cyclins, as indicated, that have been detected at the mRNA and/or protein level. No attempt is made to convey relative levels since the methods used for detection were not quantitative.
Acknowledgments
This work was supported in part by grants from the NIH to D.J.W, HD034915 and an Indo-US Program on Contraception and Reproductive Health.
References
- 1.Wolgemuth DJ, Laurion E, Lele KM. Regulation of the mitotic and meiotic cell cycles in the male germ line. Recent Prog Horm Res. 2002;57:75–101. doi: 10.1210/rp.57.1.75. [DOI] [PubMed] [Google Scholar]
- 2.Wolgemuth DJ, Rhee K, Wu S, Ravnik SE. Genetic control of mitosis, meiosis and cellular differentiation during mammalian spermatogenesis. Reproduction, Fertility and Development. 1995;7:669–83. doi: 10.1071/rd9950669. [DOI] [PubMed] [Google Scholar]
- 3.Hunt T. Maturation promoting factor, cyclin and the control of M-phase. Curr Opin Cell Biol. 1989;1:268–74. doi: 10.1016/0955-0674(89)90099-9. [DOI] [PubMed] [Google Scholar]
- 4.Handel MA, Eppig JJ. Sexual dimorphism in the regulation of mammalian meiosis. Current Topics in Developmental Biology. 1998;37:333–58. doi: 10.1016/s0070-2153(08)60179-9. [DOI] [PubMed] [Google Scholar]
- 5.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4:41–9. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 6.de Rooij DG, de Boer P. Specific arrests of spermatogenesis in genetically modified and mutant mice. Cytogenet Genome Res. 2003;103:267–76. doi: 10.1159/000076812. [DOI] [PubMed] [Google Scholar]
- 7.Venables JP, Cooke HJ. Lessons from knockout and transgenic mice for infertility in men. J Endocrinol Invest. 2000;23:584–91. doi: 10.1007/BF03343780. [DOI] [PubMed] [Google Scholar]
- 8.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–34. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 9.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell cycle control. Nat Rev Mol Cell Biol. 2007;8:149–60. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 10.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 11.Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–15. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 12.Geng Y, Lee YM, Welcker M, Swanger J, Zagozdzon A, Winer JD, Roberts JM, Kaldis P, Clurman BE, Sicinski P. Kinase-independent function of cyclin E. Mol Cell. 2007;25:127–39. doi: 10.1016/j.molcel.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Ravnik SE, Wolgemuth DJ. The developmentally restricted pattern of expression in the male germ line of a murine cyclin A, cyclin A2, suggests roles in both mitotic and meiotic cell cycles. Developmental Biology. 1996;173:69–78. doi: 10.1006/dbio.1996.0007. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney C, Murphy M, Kubelka M, Ravnik SE, Hawkins CF, Wolgemuth DJ, Carrington M. A distinct cyclin A is expressed in germ cells in the mouse. Development. 1996;122:53–64. doi: 10.1242/dev.122.1.53. [DOI] [PubMed] [Google Scholar]
- 15.Yang R, Morosetti R, Koeffler HP. Characterization of a second human cyclin A that is highly expressed in testis and in several leukemic cell lines. Cancer Research. 1997;57:913–20. [PubMed] [Google Scholar]
- 16.Wang J, Chenivesse X, Henglein B, Brechot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343:555–7. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- 17.Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990;346:760–3. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Matzuk MM, Sung KW, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nature Genetics. 1998;20:377–80. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- 19.Ravnik SE, Wolgemuth DJ. Regulation of meiosis during mammalian spermatogenesis: the A-type cyclins and their associated cyclin-dependent kinases are differentially expressed in the germ-cell lineage. Developmental Biology. 1999;207:408–18. doi: 10.1006/dbio.1998.9156. [DOI] [PubMed] [Google Scholar]
- 20.Persson J, Zhang Q, Wang XY, Ravnik SE, Muhlrad S, Wolgemuth DJ. Distinct roles for the mammalian A-type cyclins during oogenesis. Reproduction. 2005 doi: 10.1530/rep.1.00719. [DOI] [PubMed] [Google Scholar]
- 21.Murphy M, Stinnakre MG, Senamaud-Beaufort C, Winston NJ, Sweeney C, Kubelka M, Carrington M, Brechot C, Sobczak-Thepot J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nature Genetics. 1997;15:83–6. doi: 10.1038/ng0197-83. published erratum appears in Nat Genet 1999; 23:481. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer T, Chan WY, Palazon LS, Nieduszynski C, Murphy M, Sobczak-Thepot J, Carrington M, Colledge WH. Cyclin A1 protein shows haplo-insufficiency for normal fertility in male mice. Reproduction. 2004;127:503–11. doi: 10.1530/rep.1.00131. [DOI] [PubMed] [Google Scholar]
- 23.Nickerson HD, Joshi A, Wolgemuth DJ. Cyclin A1-deficient mice lack histone H3 serine 10 phosphorylation and exhibit altered aurora B dynamics in late prophase of male meiosis. Dev Biol. 2007;306:725–35. doi: 10.1016/j.ydbio.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salazar G, Joshi A, Liu D, Wei H, Persson JL, Wolgemuth DJ. Induction of apoptosis involving multiple pathways is a primary response to cyclin A1-deficiency in male meiosis. Dev Dyn. 2005;234:114–23. doi: 10.1002/dvdy.20533. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Liao C, Wolgemuth DJ. A Role for Cyclin A1 in the Activation of MPF and G2-M Transition during Meiosis of Male Germ Cells in Mice. Developmental Biology. 2000;224:388–400. doi: 10.1006/dbio.2000.9776. [DOI] [PubMed] [Google Scholar]
- 26.Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–6. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- 27.Handel MA, Cobb J, Eaker S. What are the spermatocyte’s requirements for successful meiotic division? Journal of Experimental Zoology. 1999;285:243–50. [PubMed] [Google Scholar]
- 28.Chen J, Jin S, Tahir SK, Zhang H, Liu X, Sarthy AV, McGonigal TP, Liu Z, Rosenberg SH, Ng SC. Survivin enhances Aurora-B kinase activity and localizes Aurora-B in human cells. J Biol Chem. 2003;278:486–90. doi: 10.1074/jbc.M211119200. [DOI] [PubMed] [Google Scholar]
- 29.Hanley-Hyde J, Mushinski JF, Sadofsky M, Huppi K, Krall M, Kozak CA, Mock B. Expression of murine cyclin B1 mRNAs and genetic mapping of related genomic sequences. Genomics. 1992;13:1018–30. doi: 10.1016/0888-7543(92)90015-k. [DOI] [PubMed] [Google Scholar]
- 30.Lock LF, Pines J, Hunter T, Gilbert DJ, Gopalan G, Jenkins NA, Copeland NG, Donovan PJ. A single cyclin A gene and multiple cyclin B1-related sequences are dispersed in the mouse genome. Genomics. 1992;13:415–24. doi: 10.1016/0888-7543(92)90262-q. [DOI] [PubMed] [Google Scholar]
- 31.Chapman DL, Wolgemuth DJ. Isolation of the murine cyclin B2 cDNA and characterization of the lineage and temporal specificity of expression of the B1 and B2 cyclins during oogenesis, spermatogenesis and early embryogenesis. Development. 1993;118:229–40. doi: 10.1242/dev.118.1.229. [DOI] [PubMed] [Google Scholar]
- 32.Chapman DL, Wolgemuth DJ. Identification of a mouse B-type cyclin which exhibits developmentally regulated expression in the germ line. Mol Reprod Dev. 1992;33:259–69. doi: 10.1002/mrd.1080330305. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs HW, Knoblich JA, Lehner CF. Drosophila Cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev. 1998;12:3741–51. doi: 10.1101/gad.12.23.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parry DH, O’Farrell PH. The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Curr Biol. 2001;11:671–83. doi: 10.1016/s0960-9822(01)00204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen TB, Manova K, Capodieci P, Lindon C, Bottega S, Wang XY, Refik-Rogers J, Pines J, Wolgemuth DJ, Koff A. Characterization and expression of mammalian cyclin b3, a prepachytene meiotic cyclin. J Biol Chem. 2002;277:41960–9. doi: 10.1074/jbc.M203951200. [DOI] [PubMed] [Google Scholar]
- 36.Refik-Rogers J, Manova K, Koff A. Misexpression of cyclin B3 leads to aberrant spermatogenesis. Cell Cycle. 2006;5:1966–73. doi: 10.4161/cc.5.17.3137. [DOI] [PubMed] [Google Scholar]
- 37.Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–13. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 38.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–5. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 39.Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, Akashi K, Sicinski P. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–91. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 40.Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–42. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 41.Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–30. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 42.Bartkova J, Lukas J, Strauss M, Bartek J. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene. 1998;17:1027–37. doi: 10.1038/sj.onc.1202016. [DOI] [PubMed] [Google Scholar]
- 43.Freeman RS, Estus S, Johnson EM., Jr Analysis of cell cycle-related gene expression in post-mitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron. 1994;12:343–55. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 44.Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–98. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravnik SE, Rhee K, Wolgemuth DJ. Distinct patterns of expression of the D-type cyclins during testicular development in the mouse. Dev Genet. 1995;16:171–8. doi: 10.1002/dvg.1020160209. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama H, Nishiyama H, Higuchi T, Kaneko Y, Fukumoto M, Fujita J. Change of cyclin D2 mRNA expression during murine testis development detected by fragmented cDNA subtraction method. Dev Growth Differ. 1996:141–51. doi: 10.1046/j.1440-169X.1996.t01-1-00003.x. [DOI] [PubMed] [Google Scholar]
- 47.Houldsworth J, Reuter V, Bosl GJ, Chaganti RS. Aberrant expression of cyclin D2 is an early event in human male germ cell tumorigenesis. Cell Growth Differ. 1997;8:293–9. [PubMed] [Google Scholar]
- 48.Zhang Q, Wang X, Wolgemuth DJ. Developmentally regulated expression of cyclin D3 and its potential in vivo interacting proteins during murine gametogenesis. Endocrinology. 1999;140:2790–800. doi: 10.1210/endo.140.6.6756. [DOI] [PubMed] [Google Scholar]
- 49.Beumer TL, Roepers-Gajadien HL, Gademan IS, Kal HB, de Rooij DG. Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol Reprod. 2000;63:1893–8. doi: 10.1095/biolreprod63.6.1893. [DOI] [PubMed] [Google Scholar]
- 50.Freemantle SJ, Vaseva AV, Ewings KE, Bee T, Krizan KA, Kelley MR, Hattab EM, Memoli VA, Black CC, Spinella MJ, Dmitrovsky E. Repression of cyclin D1 as a target for germ cell tumors. Int J Oncol. 2007;30:333–40. [PubMed] [Google Scholar]
- 51.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, Bronson RT, Gardner H, Sicinski P. Cyclin E ablation in the mouse. Cell. 2003;114:431–43. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 52.Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z, Amati B. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO Journal. 2003;22:4794–803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nature Genetics. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 54.Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Current Biology. 2003;13:1775–85. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 55.Ashley T, Walpita D, de Rooij DG. Localization of two mammalian cyclin dependent kinases during mammalian meiosis. J Cell Sci. 2001;114:685–93. doi: 10.1242/jcs.114.4.685. [DOI] [PubMed] [Google Scholar]
- 56.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima Y. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269:447–58. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 58.Chung SS, Cuzin F, Rassoulzadegan M, Wolgemuth DJ. Primary spermatocyte-specific Cre recombinase activity in transgenic mice. Transgenic Res. 2004;13:289–94. doi: 10.1023/b:trag.0000034716.73957.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]


