Abstract
Vascular endothelial cells from 3- to 10-day-old chicken embryos were identified by the uptake of acetylated low density lipoprotein (Ac-LDL) and the presence of a von Willebrand-like factor. These were determined on cross sections of aortic arches as well as in cell cultures prepared from the arches. To visualize the uptake of Ac-LDL, the fluorescent probe l,r-dioctadecyl-l-3,3,3’,3’-tetramethyl-indo-carbocyanine perchlorate-Ac-LDL (DiI-Ac-LDL) was used. Following injection of the DiI-Ac-LDL probe into the embryonic heart, the endothelium of the aortic arches became specifically labeled. Also, following the administration of the probe to cell cultures, about 5–10% of the cells became DiI-positive. Indirect immunofluorescence with an antibody against von Willebrand (vW) factor also revealed specific staining of the endothelium of the aortic arches as well as of a subset of cells in cultures from aortic arches. These two histochemical markers were further used to identify the emergence of the endothelial cell lineage in the chicken blastodisc. Cultured cells from embryos incubated in ovo for 16 hr exhibited both uptake of DiI-Ac-LDL and expression of a vW-like factor. The proportion of these cells was about 30% of the total cultured cells and increased to over 50% in cultures of embryos incubated in ovo for 20 hr. However, cells positive for uptake of DiI-Ac-LDL and expression of vW-like factor were lacking in cultures of unincubated eggs or eggs incubated for 6–10 hr. We conclude that the very early endothelial cells in the chick blastodisc are already capable of expressing characteristic properties of vascular endothelium.
INTRODUCTION
The initial formation of the vasculature during early embryogenesis is marked by the segregation of the hemangioblastic cell lineage from the mesoderm shortly after its establishment. The hemangioblasts give rise to embryonic blood islands and found both the blood system and the vascular endothelium. This process was described for both avian and mammalian species (Romanoff, 1960; Wagner, 1980). Whereas this early vasculature formation is initiated by differentiation of cells from the mesoderm, the second stage of vascular development is initiated during organogenesis by the budding of preformed endothelial cells from the early vasculature (Wagner, 1980; Pardanaud et al., 1987; Coffin and Poole, 1988).
In the chick embryo, identification of early endothelial cells has most often relied on morphological and autoradiographic methods (Sabin, 1920; Romanoff, 1960; Gonzales-Crussi, 1971; Ausprunk et al., 1974; Murphy and Carlson, 1978; Hirakow and Hiruma, 1981; Shumko et al., 1988). However, studies on early events during the formation of the vascular endothelial cell lineage require means such as immunological or histochemical markers to facilitate the identification of the cells prior to their incorporation into microvessels. Monoclonal antibodies which recognize endothelial cells during vasculogenesis in the early quail embryo have been previously described (Peault et al., 1983; Peault, 1987; Pardanaud et al., 1987; Coffin and Poole, 1988). These antibodies, which also react with cells of the hematopoietic lineage, do not cross-react with chick endothelial cells (Peault et al., 1983; Pardanaud et al., 1987).
In mammalian species the uptake of chemically modified low density lipoprotein has been extensively used to identify vascular endothelial cells (Stein and Stein, 1980; Voyta et al., 1984; Pitas et al., 1985). This uptake, which is also expressed by macrophages (Pitas et al., 1981), operates via receptors that are different from those participating in the internalization of low density lipoprotein (Goldstein et al., 1979). Mammalian endothelial cells have also been identified by the presence of von Willebrand (vW) factor (or factor Vlll-related antigen) (Jaffe et al., 1973; Hormia et al., 1984; Voyta et al., 1984; Sporn et al., 1986). This factor is a large, adhesive glycoprotein and its presence is documented in megakaryocytes and platelets (Nachman et al., 1977; Piovella et al., 1978) in addition to endothelial cells. The vW factor circulates in plasma complexed with factor VIII and has a role following vessel wall damage (Ewenstein et al., 1987).
Brief descriptions of the in situ labeling of capillaries in the chorioallantoic membranes and in the brain of chicken embryos with a fluorescent probe of acetylated low density lipoprotein (Ac-LDL) have been published (Netland et al., 1985; Risau et al., 1986). Cultured capillary endothelial cells from the extraembryonic membranes of quails internalized Ac-LDL as well (Labastie et al., 1986). The use of this approach to study vascular endothelia in avian embryos prior to the formation of capillaries, and in vessels other than capillaries, has not been reported. Moreover, the identification of vascular endothelial cells using the expression of vW factor as a marker has not been reported for avian embryos.
During our studies on chicken cell lineages it became necessary to obtain a simple means for the identification of embryonic endothelial cells. The study presented here describes the identification, both in situ and in culture, of vascular endothelial cells from the aortic arches of embryonic chickens. This identification is based on the uptake of the fluorescent probe l,l’-dioc-tadecyl-l-3,3,3’,3’-tetramethyl-indo-carbocyanine per-chlorate-Ac-LDL (DiI-Ac-LDL) and the presence of a protein which specifically reacts with an antibody against human vW factor. Using these markers it is possible to identify endothelial cells during early developmental stages of the chick blastodisc.
MATERIALS AND METHODS
Animals
Fertile chicken eggs (White Leghorn) were used throughout the study and were obtained from Biological Supply (Bothell, WA).
Cell culture
To culture vascular endothelial cells from 7- and 10-day-old embryos the breast muscles of the embryos were removed to expose the heart and aortic arches. The aortic arches were then excised, finely minced, incubated with trypsin (final concentration 0.1%, GIBCO, Grand Island, NY) for 30–45 min at 37°C, and centrifuged at approximately 300g for 5 min. The trypsin solution was decanted and the pellet was resuspended in complete medium which consisted of 85 parts Eagle’s minimal essential medium (MEM, GIBCO), 10 parts horse serum (GIBCO), 5 parts chicken embryo extract (from 10- and 11-day-old embryos), and antibiotics (Yablonka-Reuveni et al., 1987). The digested tissue was then mechanically dissociated by five passages through a Pasteur pipet followed by five passages through an 18-gauge needle. The resulting cell suspension was filtered through a double layer of lens tissue and plated onto tissue culture dishes which had been coated with 2% gelatin. Cultures were initiated with 5 × 104 cells per 35-mm dish and maintained at 37.5° C in a water-saturated atmosphere containing 5% CO2 in air. Medium was changed 24 hr after plating and every other day thereafter. Vascular endothelial cells from 3-day-old embryos were prepared as described above except that the heart was sometimes included in the preparation. For cell preparation from early embryos (0- to 24-hr-incubation in ovo) the blastodiscs were isolated by means of a circular incision, carefully cleaned of yolk, and separated from the vitelline membrane. Each individual embryo was then triturated with a Pasteur pipet and further subjected to a trypsin digestion as described above for older embryos. Suspensions of individual embryos were then cultured in gelatincoated, 35-mm tissue culture dishes as described above. After 10–16 hr, the cultures were extensively rinsed with complete medium to eliminate leftover yolk and further processed for DiI-Ac-LDL uptake or expression of vW factor. Early embryos were processed and assayed individually due to the developmental variations between embryos which are very apparent at early stages (Patten and Carlson, 1974). Hence, by following such a protocol we ensured that the positive cells were not merely a contribution of just a small proportion of the embryos.
Labeling of cell cultures with DiI-Ac-LDL
DiI-Ac-LDL was from Biomedical Technologies (Cambridge, MA) and did not contain any DiI-LDL or LDL. Culture medium was removed and replaced with fresh medium containing 5 µg/ml DiI-Ac-LDL. Cultures were then incubated for 4 hr as described above. At the end of the labeling period cultures were rinsed (X3) with complete medium. The medium was then removed and the cells were covered with glass coverslips and microscopically examined.
Labeling of cell cultures with anti-vW factor
An antibody against vW factor from human plasma (rabbit IgG fraction, lot No. 015) was obtained from Dako Corp. (Santa Barbara, CA). Indirect immunofluorescence was performed as previously described (Yablonka-Reuveni et al., 1987; Yablonka-Reuveni and Nameroff, 1987) except that the fixation of cells was performed with −20°C methanol for 5 min. Cultures were exposed to the primary antibody (1:25 dilution) for 1 hr at room temperature followed by a reaction with fluorescein-labeled goat anti-rabbit IgG (1:50) (Cappel, West Chester, PA) at room temperature. Cultures reacting with normal rabbit serum instead of anti-vW factor or with fluorescein-labeled goat anti-rabbit IgG only were used as controls. The specificity of the antibody was also tested by preexposing the antibody to a highly purified human vW factor which was prepared according to Roth et al. (1986) and kindly provided by Dr. Gerald Roth (Veterans Administration Medical Center, Seattle). vW factor (0.25 ml at a concentration of 0.046 mg/ml) in Tris-buffered saline (0.05 MTris, 0.9% NaCl, pH 7.6, referred to below as TBS) was first allowed to adhere to a 35-mm tissue culture dish at 4°C for 16–20 hr. Bovine serum albumin (1 ml at a concentration of 1 mg/ml in TBS) was then added to the plate for 5–10 hr at 4°C to block nonspecific binding of the anti-vW factor to the plastic surface. The plate was then rinsed with TBS and reacted with the antibody (0.2 ml at a concentration of 0.44 mg/ml in TBS) for 16–20 hr at 4°C. The supernatant was then collected and reacted with cell cultures as described above, employing an indirect immunofluorescence assay. Cultures reacted with such a supernatant did not exhibit the presence of a vW factor. Reacting the vW factor antibody with bovine serum albumin only did not eliminate the stainability of the cultures with the treated antibody. This has suggested that the antibody against vW factor is indeed vW factor-specific and does not react with other cellular proteins.
Labeling of intact endothelium with DiI-Ac-LDL and preparation of Vibratome sections
The breast muscles of 10-day-old embryos were removed and the hearts and aortic arches were exposed. Employing a dissecting microscope about 0.01 ml DiI-Ac-LDL (20 µg/ml in complete medium containing carbon black) was injected into the embryonic heart with a Hamilton syringe. A minimal volume of carbon black was added to the LDL solution so that the injected material could be traced visually throughout the aortic arches. The injected embryos were immersed in complete medium and maintained in a tissue culture incubator for about 1–2 hr under conditions as described above for cells. The aortic arches were still dark (due to the presence of carbon black) following 1 hr of incubation. However, after 2 hr of incubation the carbon black marker was not detected. At the end of the incubation period complete medium was injected into the heart to wash away traces of unbound fluorescent probe. Treated aortic arches were then removed from the embryo, rinsed with MEM, and fixed for 1 min in 3% formaldehyde in TBS. Following fixation the tissue was rinsed with TBS, embedded in 15% gelatin, sectioned with a Vibratome (Ted Pella, Tustin, CA) into 30 µm sections, and mounted on egg white-treated slides.
Cryostat sections of aortic arches and labeling with anti-vW factor
Aortic arches were first frozen on a flat surface at −20°C to facilitate handling and transfer of the tissue into semifrozen O.C.T (Lab-Tek, Naperville, IL). The sample was maintained for an additional 30 min at −20° C and then sectioned at 30 µm using a cryostat. Sections were mounted on egg white-treated slides and kept for 30 min in Tris-buffered saline containing normal goat serum (TBS-NGS; 0.05 MTris, 0.9% NaCl, 1% normal goat serum, pH 7.6) to minimize nonspecific binding of the antibody. Sections were then reacted with the anti-vW factor antibody as described above for cultures. The specificity of the antibody against vW factor was also tested on frozen sections following the preexposing of the antibody to purified human vW factor as described above for cultures.
Fluorescence microscopy
Cultures and sections were analyzed with a Zeiss photomicroscope equipped for epifluorescence. Dil was visualized using the standard rhodamine excitation/emission filter combinations. Kodak Ektachrome film (EL 135, 400 ASA) was used for photography and exposure time was 5 sec for DiI-labeled samples and 10 sec for fluorescein-labeled samples.
RESULTS
Labeling of Intact Aortic Arches and Cultured Cells from 3- to 10-Day-Old Embryos with DiI-Ac-LDL
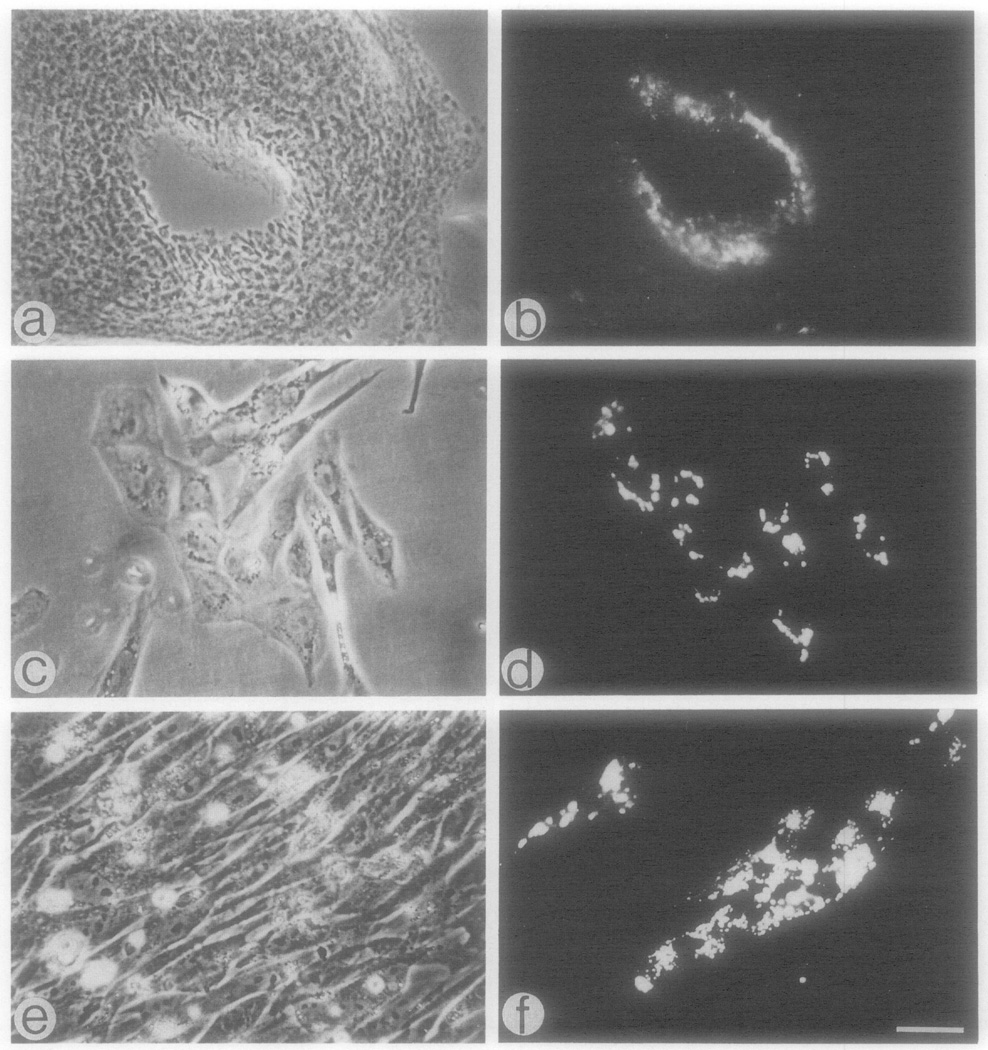
Microscopic analysis of the Vibratome sections of the aortic arches clearly demonstrated that only the cells in close proximity to the vessel lumen (presumably endothelial cells) exhibited the presence of Dil following the administration of the DiI-Ac-LDL to the heart (Figs, 1a and lb). Uptake of DiI-Ac-LDL was further examined to distinguish, in culture, endothelial cells from other cells isolated from the aortic arches. When cells were labeled 15–30 hr after the initial plating, the fluorescence could be detected in cells which appeared either individually or grouped as small colonies. This is shown in Figs, 1c and 1d for cultures from 10-day-old embryos. Similar results were observed in cultures from 7- and 3-day-old embryos. The DiI-labeled cells in the colonies were closely associated and all or part of them exhibited a cobblestone morphology which distinguished them from other cells in the culture. When cultures were maintained for 5–6 days and reached confluency, the cells which could internalize the DiI-Ac-LDL would sometimes become indistinguishable from neighboring cells (Figs, 1e and 1f) which were unlabeled and presumably were descendants of fibroblast-like cells or smooth muscle cells. In other cases, the cells in the 5- to 6-day-old cultures appeared as colonies identical to those shown in Fig. 2e and 2f. The colonies of endothelial cells tended to round up as one unit and detach from the plate if initial cells had been plated at higher densities so that confluency was reached in 1 day. It was estimated that about 5–10% of the cultured cells exhibited uptake of DiI-Ac-LDL during the first 2 days of incubation.
Fig. 1.
Uptake of DiI-Ac-LDL by endothelial cells from aortic arches of 10-day-old embryos, (a, b) Phase and fluorescence micrographs of a 30-µm-thick Vibratome section of an aortic arch which was perfused with DiI-Ac-LDL by administration of the DiI probe to the heart; (c, d) phase and fluorescence micrographs of 1-day-old culture exposed to the DiI probe for 4 hr; (e, f) as in c and d, respectively, but cultures were 6 days old. Bar = 30 µm.
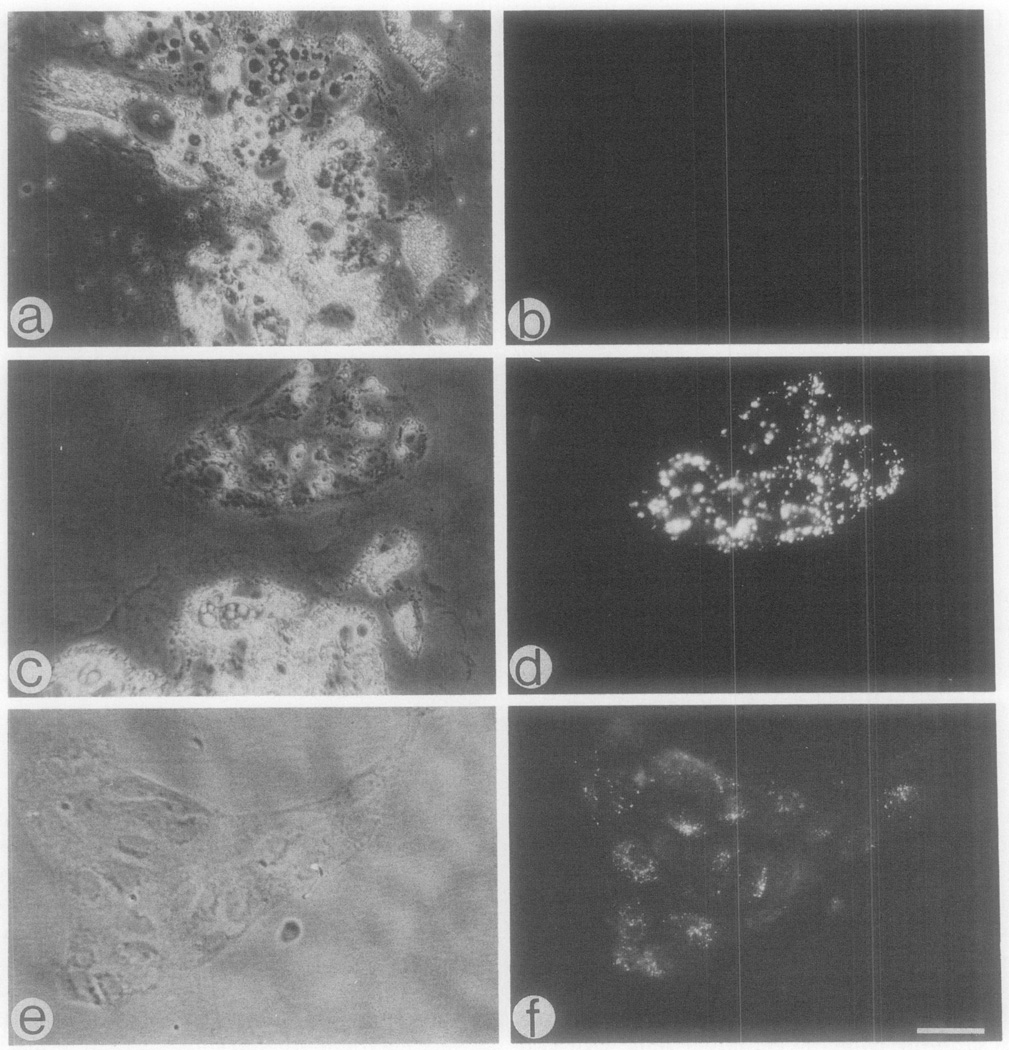
Fig. 2.
Immunofluorescence staining of aortic arch cells from 10-day-old embryos with an antibody against human von Willebrand factor. Specimens were reacted sequentially with anti-von Willebrand factor and fluorescein-conjugated goat anti-rabbit IgG. (a, b) Phase and fluorescence micrographs of a 30-µm-thick cryostat section; (c, d) phase and fluorescence micrographs of a 1-day-old culture prepared from the aortic arches; (e, f) as in c and d, respectively, but cultures were 3-day-old. Bar = 30 µm for b–f and 34 µm for a.
It is noteworthy that the presence of smooth muscle cells could be detected immunocytochemically in the aortic arches of 10-day-old chick embryos both on cross sections and in culture with anti-desmin antibody (data not shown; for description of antibody and methods see Yablonka-Reuveni and Nameroff, 1986, 1987, and submitted for publication). The latter, desmin-positive cells did not take up the DiI-Ac-LDL under the conditions described here. We have also shown in the past that skeletal myogenic cells and muscle fibroblasts from embryonic chicken do not exhibit uptake of DiI-Ac-LDL when the same labeling conditions are used (Yablonka-Reuveni and Nameroff, 1987).
Labeling of Sections and Cultures of Aortic Arches with Anti-vW Factor
Antibodies against vW factor have been frequently used to identify endothelial cells from mammalian sources immunohistochemically. However, the presence of vW factor in avian endothelial cells has not been established. When screening various commercial preparations of anti-vW factor using fixed tissues from chicken embryos, we could not identify vW factor regardless of the fixative used. In contrast, an antihuman vW factor from Dako which reacts with endothelial cells from several mammalian species (Clowes et al., 1986; Reidy et al., 1986; Ewenstein et al., 1987) could detect vW-like factor in chicken endothelial cells as shown below. (The antibody lot used in the former two mammalian cell studies was identical to the one used in the current study.) When the anti-vW factor antibody was added to frozen sections of aortic arches from 10-day-old embryos, cells in close proximity to the vessel lumen were positively stained (Fig. 2a and 2b). The appearance of cells positive for vW-like factor in regions further away from the lumen (presumably adventitia) is primarily due to the collapse of the vessel wall during freezing and sectioning and the partial detachment of the endothelium which folds over the vessel wall. The appearance of such cells could be almost completely eliminated by fixing the arches prior to the reaction with the antibody. However, the staining with anti-vW factor was significantly better when fixation of the arches was avoided. In addition, capillaries of the vasa vasorum may react with the antibody and contribute to the appearance of some vW factor-positive cells in the adventitia.
The reactivity of chicken vascular endothelial cells with the anti-vW factor was further tested in cultured cells that were isolated from aortic arches of 3-, 7-, and 10-day-old embryos. As with DiI-positive cells, cells which stained for vW factor appeared either individually or closely associated in colonies (Fig. 2c–2f). The frequency of cells demonstrating the vW-like factor was similar to that of cells which internalized the DiI-Ac-LDL. However, the methanol fixation had an effect on the morphology of the endothelial cells when compared to that of live cells treated with DiI-Ac-LDL (Fig. 2 versus Fig. 1). Fibroblast-like cells in the cultures did not stain with the anti-vW factor (Fig. 2e and 2f). Also, fibroblasts, myoblasts, and myotubes from embryonic chick skeletal muscle did not react with the antibody (data not shown).
It should be noted that Dil is soluble in organic solvents. Therefore, when fixation of DiI-positive cells is desired, formaldehyde but not methanol or acetone can be used. On the other hand, methanol fixation was significantly better for indirect immunofiuorescent staining of cultured cells with anti-vW factor. Therefore, when double labeling of cells with both Dil and anti-vW factor was desired, the cultures were first reacted with DiI-Ac-LDL and the positive cells were marked. Then the cultures were reacted with the anti-vW factor. In these experiments, the vast majority of DiI-positive cells also demonstrated the presence of vW-like factor (data not shown).
Labeling of Cultured Blastodisc Cells with DiI-Ac-LDL and Anti-vW Factor
Trypsin-dissociated blastodisc cells from 0- to 24-hr-old embryos were cultured and the uptake of DiI-Ac-LDL and the expression of vW factor were studied. Cultures from unincubated embryos (0-hr embryos) consisted of large, highly vesiculated cells which appeared as groups of closely opposed cells (Fig. 3). Cells positive for Dil or for vW factor were not detected in these cultures. This was true for cultures assayed within the first 24 hr of culturing or during the next 10–14 consecutive days. Cultures from embryos incubated in ovo for 6 and 10 hr (6- and 10-hr embryos) resembled those of 0-hr embryos and were lacking any of the endothelial cell characteristics.
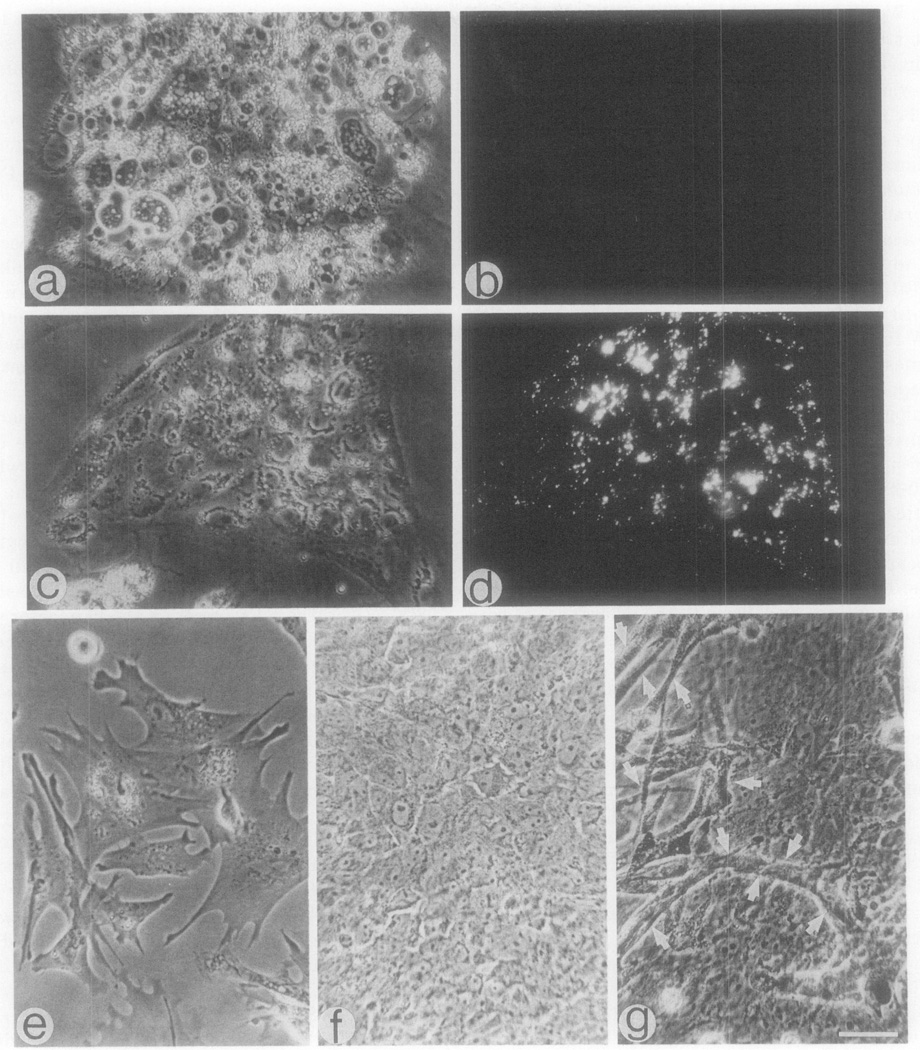
Fig. 3.
Identification of endothelial cell characteristics in cultures of early chick blastodiscs. Cultures were prepared from 0-hr embryos (a, b) and from 16-hr embryos (c–f). About 12 hr after the establishment of the cultures the cells were examined for the uptake of DiI-Ac-LDL and the expression of von Willebrand-like factor. (b, d) Representative fluorescence micrographs of cultures from 6- and 16-hr embryos, respectively, reacted with DiI-Ac-LDL; (f) a representative fluorescence micrograph of a culture from 16-hr embryo reacted with antibody against von Willebrand factor. Please note that in addition to the absence of DiI-positive cells in cultures from 6-hr embryos (b), cells expressing the von Willebrand-like factor were also absent in these cultures. Bar = 30 µm.
Cultures of 16-hr embryos exhibited cells with two different morphologies following the initial culturing. Over 50% of the cells were large and highly vesiculated. However, an additional predominant cell type was observed which amounted to about 30% of the cells. These cells appeared as islands or colonies of closely apposed cells which were separated from the large, vesiculated cells, exhibited uptake of Dil, and expressed the vW factor (Fig. 3c–3f). Examination of these cultures on consecutive days did not reveal the appearance of any new cell types or morphological changes. Similar to Holtzer et al., (1983b) we also observed in advanced cultures of this embryonic stage various branching tubes. The latter investigators suggested that these tubes might be composed of endothelial-like cells. However, in the current study such tubes never exhibited uptake of DiI-Ac-LDL or stained with the antibody against the vW factor.
Cultures from 20-hr-old embryos revealed on the first day of culture cells with morphologies similar to those detected in 1-day-old cultures from 16-hr-old embryos, but the proportion of DiI-positive cells was higher and accounted for about 50% of the cells (Fig. 4). However, in contrast to the cultures from the 16-hr-old embryos, cells with different morphologies appeared in the cultures of 20-hr-old embryos within the next few days following the initial culturing. The most obvious cells were spread out cells (Fig. 4e), epithelial-like cells (Fig. 4f), and melanoblast-like cells which were highly pigmented and which formed dendrite-like structures in older cultures (Fig. 4g). The appearance of additional cell lineages in progressively older cultures from chick blastodiscs fits well with the results reported by Holtzer et al. (1983a,b) using a similar cell culture approach. However, these investigators had no means for identifying the appearance of the endothelial cell lineage using this experimental system.
Fig. 4.
Representative cells detected in cultures of 22-hr-old chick embryos. (a–d) A 12-hr-old culture consisting of large cells which do not incorporate DiI-Ac-LDL (a, b) and islands of cells which are DiI-positive (c, d); (e–g) representative cells detected in a 7-day-old culture in addition to the cells in a and b. Bar – 30 µm.
Cultures from 24-hr-old embryos exhibit all the morphologies described for cultures from 20-hr-old embryos, including the DiI-positive/vW factor-positive cells. However, the various morphologies could be readily detected during the first 1–2 days following culturing.
DISCUSSION
This study demonstrates that vascular endothelial cells from aortic arches of embryonic chickens internalize Ac-LDL and express a vW-like factor. These properties are exhibited by the intact tissue as well as in culture. The identification of vW-like factor in chicken cells is novel and has not been previously reported. Moreover, the study indicates that the uptake of Ac-LDL and the expression of vW-like factor are properties of very early endothelial cells that can be used to follow the emergence of the endothelial cell lineage during early development of the chick blastodisc. The first endothelial cells can be detected in cultures of 16-hr embryos. These cells can be detected in culture within several hours after the initial plating, which may suggest that the endothelial cells are already present in the intact embryo at the time of culturing. It is still possible, however, that endothelial cells are absent in the embryo, but following culture founder cells divide quickly, giving rise to endothelial cells. In fact, Biehl et al. (1985) have reported that many of the very large cultured cells from the chick blastodisc have cell cycles of approximately 4 hr. However, since the endothelial cell colonies in the current study sometimes contain over 50 cells, it is more probable that the cells are already present in the embryo and sort each other out during cell isolation and culturing. It should be noted that the eggs used for the study are stored cold after laying (“cold-fertile”). Once placed in the incubator it takes about 3–4 hr for the eggs to become warmed to the point at which development begins (Patten and Carlson, 1974). Hence, the emergence of the endothelial cell lineage in fresh, fertile eggs probably occurs several hours earlier than the time reported here. Obviously, the study cannot rule out the possibility that in the early embryo the uptake of Ac-LDL and the presence of the vW factor may be expressed by other cell types in addition to endothelial cells.
Sabin (1920), studying vasculogenesis in living blastodiscs, could identify endothelial cells beginning with the four- to five-somite stage. Gonzales-Crussi (1971) followed blood island formation at the ultrastructural level, monitoring both hemopoiesis and endothelium formation, but only after the structures were formed. Similarly, Hirakow and Hiruma, (1981, 1983) in their ultrastructural studies of vascular formation were able to observe endothelial cells only after their incorporation into recognizable structures. In more recent studies by Pardanaud et al. (1987) and by Coffin and Poole (1988), where a monoclonal antibody that specifically identifies quail endothelial cells was employed, the first positive cells were detected during the head process stage. In the current chick study, the majority of the 16-hr embryos were in the intermediate or definitive streak stages (HH stages 3 and 4; Hamburger and Hamilton, 1951) as determined on parallel-fixed embryos. Hence, it is possible that the methods used here identify endothelial cells earlier than the monoclonal antibody approach. This may be due to the fact that the cultured endothelial cells appear as colonies rather than individual cells which enhances the detection level. Additionally, it is possible that the markers used to identify endothelial cells in the current study are characteristics of presumptive endothelial cells even before they acquire antigens such as the QH1 used in the two quail studies. We also acknowledge the possibility that in the current study some of the observed endothelial cells might have been originated in the yolk sac since traces of yolk were associated with the isolated blastodiscs. It should be noted, however, that whole mount studies on the expression of QHl-positive cells in the yolk sac using immunofluorescence are unreliable due to high background levels (D. Coffin, personal communication). At any rate, in contrast to the quail studies which rely on the availability of monoclonal antibodies, the current study offers more universal tools for the early identification of endothelial cells. These tools (uptake of DiI-Ac-LDL and immunohistochemical staining with anti-vW factor) are easily obtained and can be applied for studies of both mammalian and avian species.
The appearance of islands of cells with cobblestone morphology which exhibit an uptake of DiI-Ac-LDL and express a vW-like factor was detected in cultures from 16-hr embryos. Cultures from younger embryos (6- to 10-hr-old) did not express any of the latter three properties. This suggests that some event is taking place within the first 16 hr of incubation which allows the appearance of cells with characteristics of endothelial cells. The current studies suggest that the endothelial cell lineage emerges prior to the emergence of several other cell types which are documented in Fig. 4. Holtzer and collaborators (Holtzer et al., 1983a,b; Biehl et al. (1985) have previously reported on the appearance of several cell lineages during chick blastodisc development using the cell culture approach. In these studies the endothelial cell lineage was not studied directly. Erythropoiesis and the emergence of the erythrogenic lineage have been thoroughly described by various investigators for primitive streak and older stage embryos (Sabin, 1920; Romanoff, 1960; Hagopian et al., 1972; Groudine and Weintraub, 1981; Holtzer et al., 1983a,b; Biehl et al. (1985). Moreover,Zagris (1980), following culture of whole unincubated chick blastoderms (0-hr embryos) for several days, observed blood islands containing hemoglobin. Therefore, it is possible that the entire embryo is required rather than dissociated cells (which were used in the current study) in order to obtain the endothelial cell lineage in cultures of 0-hr embryos. For example, it is possible that cell-cell interactions or the availability of some growth factors are required for the emergence of the endothelial cell lineage. Alternatively, it is possible that the endothelial cell lineage is predetermined in the 0- to 10-hr embryos but that the culturing and progression of the founder cells which lead to the appearance of endothelial cells require additional nutrients and interactions that are not available in the current experimental system. Additional cell culture and whole embryo experiments will be required to clarify this issue.
The receptor-mediated uptake of modified low density lipoprotein such as Ac-LDL has been observed in mammalian species in both macrophages (Goldstein et al., 1979; Nagelkerke et al., 1983) and vascular endothelial cells (Stein and Stein, 1980; Voyta et al., 1984), whereas the expression of vW factor in mammalian species is documented for megakaryocytes, platelets, and vascular endothelial cells (Nachman et al., 1977; Sporn et al., 1985; Jaffe et al., 1973; Piovella et al., 1978; Voyta et al., 1984). Hence, in mammalian species only vascular endothelial cells exhibit both an uptake of Ac-LDL and an expression of vW factor. In avian species, an uptake of DiI-Ac-LDL was previously demonstrated both in vivo and in culture for capillary endothelial cells (Netland et al., 1985; Risau et al., 1986; Labastie et al., 1986). This is the first study demonstrating that cells capable of uptake of Ac-LDL are already present in the very early chicken embryo. Moreover, these cultured cells are capable of giving rise to colonies in which the cells are closely associated and resemble the cobblestone morphology of cultured endothelial cells from mammalian species (Folkman et al., 1979; Voyta et al., 1984; Gaffney et al., 1985; Sanan, 1985) as well as from the extraembryonic membranes of quail (Labastie et al., 1986). Also, these cells express a vW-like factor which is a marker of mammalian vascular endothelial cells.
The presence of vW-like factor has never been reported for avian endothelial cells. Risau et al. (1986) reported on the nonreactivity of “available” antibodies against vW factor with blood vessels from embryonic chicks. We also observed that some commercially available antibodies against vW factor did not react with endothelial cells from embryonic chicks, but we detected one antibody from Dako which reacted positively. This Dako antibody is a polyclonal antibody produced in rabbits against vW factor from human plasma. The Dako antibody, in contrast to similar preparations, is also unique in its ability to react with endothelial cells from a number of mammalian species in addition to the original source (rat, Clowes et al., 1986; baboon, Reidy et al., 1986; human, Ewenstein et al., 1987). Hence, it is possible that the Dako antibody reacts with a region in the vW factor which is common to various species. Some polyclonal antibodies developed against supposedly pure vW factor have been shown to react with fibronectin which is a common contaminant of the isolated factor. However, the ability to block the stainability of the antibody against vW factor by prior exposure of the antibody to highly purified human vW factor (as described under Methods and Materials) and the intracellular staining pattern which is totally different from that of fibronectin indicate that the antibody lacks such impurities. Moreover, the antibody does not react with fibronectin separated by polyacrylamide gels as has been demonstrated by immunoblot assays (M. Reidy, manuscript in preparation).
We conclude that vascular endothelial cells from embryonic chickens resemble those from mammalian species in both uptake of Ac-LDL and expression of vW-like factor. These characteristic properties of endothelial cells distinguish very early endothelial cells from other cells in the chicken blastodisc. Whether the early endothelial cells are different than vascular endothelial cells from mature animals and whether the endothelial cell lineage traverses through various lineage compartments as is documented for the erythropoietic lineage remain to be studied.
Acknowledgments
I am grateful to Dr. M. Nameroff for continuous support. I also thank Dr. R. L. Heimark for valuable discussions, Dr. A. G. Farr and Ms. S. K. Anderson for advice and help in preparing the Vibratome sections, Dr. G. J. Roth for providing the human von Willebrand factor, Dr. H. Sage and Ms. R. S. Hartley for thoughtful suggestions on the manuscript, and Ms. D. Ringer for editing the manuscript. This work was supported by grants from the American Heart Association Washington Affiliate and the University of Washington Research Fund to the author, and by a grant from the National Institutes of Health (AR28154) to Dr. M. Nameroff.
REFERENCES
- Ausprunk DH, Knighton DR, Folkman J. Differentiation of vascular endothelium in the chick chorioallantois: A structural and autoradiographic study. Dev Biol. 1974;38:237–248. doi: 10.1016/0012-1606(74)90004-9. [DOI] [PubMed] [Google Scholar]
- Biehl J, Holtzer S, Bennett G, Sun T, Holtzer H. Cultured chick blastodisc cells diverge into lineages with different IF isoforms. In: Wang E, Fischman D, Liem RKH, Sun TT, editors. In “Intermediate Filaments”. Vol. 455. New York: Ann. N.Y. Acad. Sci; 1985. pp. 158–166. [DOI] [PubMed] [Google Scholar]
- Clowes AW, Clowes MM, Reidy MA. Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab. Invest. 1986;54:295–303. [PubMed] [Google Scholar]
- Coffin DJ, Poole TJ. Embryonic vascular development: Immunohistochemical identification of the origin and subsequent morphogenesis of the major vessel primordia in quail embryos. Development. 1988;102:735–748. doi: 10.1242/dev.102.4.735. [DOI] [PubMed] [Google Scholar]
- Ewenstein BM, Warhol MJ, Handin RI, Pober JS. Composition of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells. Cell Biol. 1987;104:1423–1433. doi: 10.1083/jcb.104.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Haudenschild CC, Zetter BR. Long-term culture of capillary endothelial cells. Proc. Natl. Acad. Sci. USA. 1979;76:5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney J, West D, Arnold F, Sattar A, Kumar S. Differences in the uptake of modified low density lipoproteins by tissue cultured endothelial cells. J. Cell Sci. 1985;79:317–325. doi: 10.1242/jcs.79.1.317. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales-Crussi F. Vasculogenesis in the chick embryo: An ultrastructural study. Amer. J. Anat. 1971;130:441–460. doi: 10.1002/aja.1001300406. [DOI] [PubMed] [Google Scholar]
- Groudine M, Weintraub H. Activation of globin genes during chicken development. Cell. 1981;24:393–401. doi: 10.1016/0092-8674(81)90329-9. [DOI] [PubMed] [Google Scholar]
- Hagopian HK, Lippke JA, Ingram VM. Erythropoietic cell cultures from chick embryos. J. Cell Biol. 1972;54:98–106. doi: 10.1083/jcb.54.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hirakow R, Hiruma T. Scanning electron microscopic study on the development of the primitive blood vessels in chick embryos at the early somite stage. Anat. Embryol. 1981;163:299–306. doi: 10.1007/BF00315706. [DOI] [PubMed] [Google Scholar]
- Hirakow R, Hiruma T. TEM-studies on development and canalization of the dorsal aorta in the chick embryo. Anat. Embryol. 1983;166:299–306. doi: 10.1007/BF00305920. [DOI] [PubMed] [Google Scholar]
- Holtzer H, Biehl J, Antin P, Tokunaka S, Sasse J, Pacifici M, Holtzer S. Quantal and proliferative cell cycles: How lineages generate cell diversity and maintain fidelity. In: Stamatoyanno-poulos G, Neinhuis AW, editors. In “Progress in Clinical and Biological Research”. Vol. 134. New York: A. R. Liss; 1983a. pp. 213–227. [PubMed] [Google Scholar]
- Holtzer H, Biehl J, Payette R, Sasse J, Pacifici M, Holtzer S. Cell diversification: Differing roles of cell lineages and cell-cell interactions. In: Kelley RO, Goetinck PF, MacCabe JA, editors. In “Progress in Clinical and Biological Research”. Vol. 110. New York: A. R. Liss; 1983b. pp. 271–280. Part B. [PubMed] [Google Scholar]
- Hormia M, Lehto V-P, Virtanen I. Intracellular localization of factor Vlll-related antigen and fibronectin in cultured human endothelial cells: Evidence for divergent routes of intracellular translocation. Eur. J. Cell Biol. 1984;33:217–228. [PubMed] [Google Scholar]
- Jaffe EA, Hoyer LW, Nachman RL. Synthesis of anti-hemophilic factor antigen by cultured human endothelial cells. J. Clin. Invest. 1973;52:2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labastie M-C, Poole J, Peault BM, Le Douarin NM. MB1, a quail leukocyte-endothelium antigen: Partial characterization of the cell surface and secreted forms in cultured endothelial cells. Proc. Natl. Acad. Sci. USA. 1986;83:9016–9020. doi: 10.1073/pnas.83.23.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ME, Carlson EC. An ultrastructural study of developing extracellular matrix in vitelline blood vessels of the early chick embryo. Amer. J. Anat. 1978;151:345–376. doi: 10.1002/aja.1001510304. [DOI] [PubMed] [Google Scholar]
- Nachman R, Levine R, Jaffe EA. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J. Clin. Invest. 1977;60:914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke JF, Koen PB, van Berkel TJC. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J. Biol. Chem. 1983;258:12,221–12,227. [PubMed] [Google Scholar]
- Netland PA, Zetter BR, Via DP, Voyta JC. In situ labelling of vascular endothelium with fluorescent acetylated low density lipoprotein. Histochem J. 1985;17:1309–1320. doi: 10.1007/BF01002528. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Altmann C, Kitos P, Dieterlen-Lievre F, Buck CA. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development. 1987;100:339–349. doi: 10.1242/dev.100.2.339. [DOI] [PubMed] [Google Scholar]
- Patten BM, Carlson BM. In “ Embryology Foundation of ”. New York: McGraw-Hill; 1974. Reproductive organs and gametogenesis; pp. 67–71. [Google Scholar]
- Peault B. MB1, a quail leukocyte/vascular endothelium antigen: Characterization of the lymphocyte-surface form and identification of its secreted counterpart as α2-macroglobulin. Cell Differ. 1987;21:175–187. doi: 10.1016/0045-6039(87)90454-4. [DOI] [PubMed] [Google Scholar]
- Peault BM, Thiery J-P, Le Douarin NM. Surface marker for hemopoietic and endothelial cell lineages in quail that is defined by a monoclonal antibody. Proc. Natl. Acad. Sci USA. 1983;80:2976–2980. doi: 10.1073/pnas.80.10.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovella F, Nalli G, Malamani GD, Majolino I, Frassoni F, Sitar GM, Ruggeri A, Dell Orbo C, Ascari E. The ultrastructural localization of factor Vlll-antigen in human platelets, megakaryocytes and endothelial cells utilizing a ferritin-labelled antibody. Brit. J. Haematol. 1978;39:209–213. doi: 10.1111/j.1365-2141.1978.tb01090.x. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles J, Mahley RW, Bissell DM. Uptake of chemically modified low density lipoproteins in vivo is mediated by specific endothelial cells. J. Cell Biol. 1985;100:103–117. doi: 10.1083/jcb.100.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas RE, Innerarity TL, Weinstein JN, Mahley RW. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981;1:177–185. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- Reidy MA, Chao SS, Kirkman TR, Clowes AW. Endothelial regeneration. VI. Chronic nondenuding injury in baboon vascular grafts. Amer. J. Pathol. 1986;123:432–439. [PMC free article] [PubMed] [Google Scholar]
- Risau W, Kallmann R, Albrecht U. Differentiation-dependent expression of proteins in brain endothelium during development of the blood-brain barrier. Dev. Biol. 1986;117:537–545. doi: 10.1016/0012-1606(86)90321-0. [DOI] [PubMed] [Google Scholar]
- Romanoff AL. The Avian Embryo: Structural and Functional Development. New York: Macmillan; 1960. [Google Scholar]
- Roth GJ, Titani K, Hoyer LW, Hickey MJ. Localization of binding sites within human von Willebrand factor for monomeric type III collagen. Biochemistry. 1986;25:8357–8361. doi: 10.1021/bi00374a004. [DOI] [PubMed] [Google Scholar]
- Sabin FR. Studies on the origin of blood vessels and of red corpuscles as seen in the living blastoderm of the chick during the second day of incubation. Carnegie Contrib. Embryol. 1920;9:213–259. [Google Scholar]
- Sanan DA. Native and acetylated low density lipoprotein in metabolism in proliferating and quiescent bovine endothelial cells in culture. Eur. J. Cell Biol. 1985;36:81–90. [PubMed] [Google Scholar]
- Shumko JZ, Defouw D0, Feinberg RN. Vascular histodifferentiation in the chick chorioallantoic membrane: A morphometric study. Anal. Rec. 1988;220:179–189. doi: 10.1002/ar.1092200209. [DOI] [PubMed] [Google Scholar]
- Sporn LA, Chavin SI, Marder VJ, Wagner DD. Biosynthesis of von Willebrand protein by human megakaryocytes. J. Clin. Invest. 1985;76:1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46:185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Stein O, Stein Y. Bovine aortic endothelial cells display macrophage-like properties towards acetylated 125I-labelled low density lipoprotein. Biochim. Biophys. Acta. 1980;620:631–635. doi: 10.1016/0005-2760(80)90155-1. [DOI] [PubMed] [Google Scholar]
- Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated low-density lipoprotein. J. Cell Biol. 1984;99:2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RC. Endothelial cell embryology and growth. Adv. Microcirc. 1980;9:45–75. [Google Scholar]
- Yablonka-Reuveni Z, Nameroff M. Immunocyto-chemical studies on the expression of desmin by dividing cells from skeletal muscle. In: Emerson C, Fischman D, Nadal-Ginard B, Siddiqui MAQ, editors. “Molecular Biology of Muscle Development”. New York: A. R. Liss; 1986. pp. 47–60. [Google Scholar]
- Yablonka-Reuveni Z, Nameroff M. Skeletal muscle cell populations: Separation and partial characterization of fibro-blast-like cells from embryonic tissue using density centrifugation. Histochemistry. 1987;87:27–38. doi: 10.1007/BF00518721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Quinn LS, Nameroff M. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev. Biol. 1987;119:252–259. doi: 10.1016/0012-1606(87)90226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagris N. Erythroid cell differentiation in unincubated chick blastoderm in culture. J. Embryol Exp. Morphol. 1980;58:209–216. [PubMed] [Google Scholar]