Abstract
Objective
To demonstrate criterion (concurrent and predictive) and construct validity of the Walking Index for Spinal Cord Injury (WISCI) scale and other walking measures in the Spinal Cord Injury Locomotor Trial (SCILT).
Design
Prospective multicenter clinical trial of a walking intervention for patients with acute traumatic spinal cord injury (SCI).
Participants/Methods
Body weight–supported treadmill training was compared to overground mobility training in 146 patients with incomplete SCI (C4 to L3) enrolled within 8 weeks of onset and treated for 12 weeks. Primary outcome measures were the Functional Independence Measure (FIM), 50-foot walking speed (50FW-S), and 6-minute walking distance (6MW-D), tested 3, 6, and 12 months after entry. Secondary measures were the Lower Extremity Motor Score (LEMS), Berg Balance Scale (BBS), WISCI, and FIM locomotor score (LFIM), assessed at 6 centers by blinded observers. Data for the 2 arms were combined since no significant differences in outcomes had been found.
Results
Correlations with WISCI at 6 months were significant with BBS (r = .90), LEMS (r = .85), LFIM (r = .89), FIM (r = .77), 50FW-S (r = .85), and 6MW-D (r = .79); similar correlations occurred at 3 and 12 months. Correlations of change scores from baseline WISCI were significant for change scores from baseline of LEMS/BBS/LFIM. Correlation of baseline LEMS and WISCI at 12 months were most significant (r = .73). The R2 of baseline LEMS explained 57% of variability of WISCI levels at 3 months.
Conclusion
Concurrent validity of the WISCI scale was supported by significant correlations with all measures at 3, 6, and 12 months. Correlation of change scores supports predictive validity. The LEMS at baseline was the best predictor of the WISCI score at 12 months and explained most of the variance, which supported both predictive and construct validity. The combination of the LEMS, BBS, WISCI, 50FW-S, and LFIM appears to encompass adequate descriptors for outcomes of walking trials for incomplete SCI.
Keywords: Spinal cord injuries, Ambulation, Outcome assessment, Validity
The recovery of walking function after spinal cord injury (SCI) for individuals with incomplete injuries is one of their primary goals1 and has become a major focus in the design of recent clinical trials2–4 and outcome studies.5,6 Outcome studies to determine the effects of rehabilitation and regeneration interventions must examine improvement in impairment (neurological examination), functional capacity (activity in a standardized environment), and function at home and in the community (participation/disability).
The use of global disability scales in large databases7 characterizes mobility outcomes as they are performed in the person’s current environment. The testing environment is probably fairly standardized during inpatient assessments but more likely to mimic real-life situations such as different terrains and walking conditions during outpatient evaluations. While a disability scale is necessary to plan an appropriate rehabilitation program, results may vary for the same impairment or functional capacity because the environment and the testing conditions are not standardized. Walking functional capacity scales, walking speed,8 and walking efficiency, however, test ambulation under standardized environmental conditions and, for SCI, correlate with recovery of lower extremity strength.8,9
The Walking Index for Spinal Cord Injury (WISCI), which was developed as a 21-level functional capacity scale, integrates the level of physical assistance with the need for braces and walking aids.5,6 The distinction between a walking functional capacity scale and a disability scale that tests individuals in the environment is important to clinical trials that attempt to demonstrate improvement in neurological recovery, because a functional limitation/capacity measure may be more sensitive to changes in improvements of strength. A recent prospective randomized clinical trial to evaluate the effect of locomotor training following SCI10 utilized multiple endpoints (outcome measures) to assess the improvement in walking function. The SCILT is the first prospective study to correlate gains in the domains11 of impairment, functional capacity, and disability for walking. These endpoints reflect changes in physical impairment (increased strength), functional capacity in terms of balance (Berg Balance Scale [BBS]), functional capacity for walking (WISCI, walking speed, walking distance), and disability related to walking (Functional Independent Measure [FIM], FIM Locomotor Scale [LFIM], and FIM Motor Scale).
The purpose of this article is to demonstrate concurrent and predictive validity of the WISCI scale in relation to improvements in physical impairment and other measures of walking function, taking advantage of the lack of assessment bias provided by a prospective multisite clinical trial with blinded observers. We hypothesized that the concurrent, predictive, and construct validity of the WISCI scale would be supported by this study.
METHODS
Design
The Spinal Cord Injury Locomotor Trial (SCILT) was a single-blinded, parallel-group, multicenter randomized clinical trial that compared 2 interventions for walking. Participants from 6 regional SCI inpatient rehabilitation centers, who had sustained an incomplete (American Spinal Injury Association [ASIA] B, C, or D) upper motor neuron (UMN) lesion (C4-T11) or lower motor neuron (LMN) lesion (T11 to L3 with no UMN neurological signs), were assigned to either treadmill training with partial body weight support plus a defined program of overground walking therapy (BWSTT) or only overground standing and walking therapy (CONT) at a similar intensity of practice time.2 Inclusion and exclusion criteria have been reported.2 Institutional review board approval was obtained from each site, and written informed consent was obtained from each patient prior to inclusion.
Outcome Measures
All outcomes were assessed by trained observers who were blinded to treatment arm. The main outcome measures (primary) in the SCILT were the LFIM and the overground walking speed for patients who regained the ability to perform this task. The LFIM, a subset of the motor FIM,2,13,14 describes the assistance needed for walking 50 to 150 feet on a 7-point scale. Walking speed (50-foot walking speed [50FW-S]) was calculated for the faster of two 50-foot (15.2-meter) walks in patients who were able to walk that distance with moderate or less assistance. Patients were asked to walk as fast as safely possible. The speed of the 50-foot distance was converted to meters per second.
The secondary measures included the Lower Extremity Motor Score (LEMS), BBS, WISCI, and 6-minute walking distance (6MW-D). LEMS motor scores (range 0 to 50; maximum of 5 for each of 5 key muscles of each leg) were obtained by a trained physician or physical therapist following the guidelines of the ASIA scale.15 The blinded observer administered the BBS by scoring the patient’s level of balance during 14 balance activities. The tasks are performed in increasing level of difficulty by asking patients to change positions and challenging the limits of their base of support. Scores are based on the ability to maintain positions, speed in performing movements, and the need for supervision or assistance. The test assesses functional coordination, strength, flexibility, and balance. Patients were evaluated on the WISCI scale during the 15.2-m walk using the 21-level scale (Appendix A). Walking distance in 6 minutes (6MW-D) was assessed in patients who could walk with moderate or less assistance with turns on a 30-m walkway. The LFIM, 50FW-S, LEMS, BBS, WISCI, and 6MW-D were obtained at entry, if feasible for the patient; 12 weeks (end of treatment); and 6 and 12 months after entry.
Participant Characteristics
Table 1 lists the characteristics of the entire sample studied for this report. The data for the BWSTT and CONT patients have been combined, since no differences were found between groups at entry or in any of the outcomes obtained at 3, 6, and 12 months.10,12 These patients were entered within 8 weeks of onset of SCI and within 1 week of admission for rehabilitation. The majority of excluded patients had a complete SCI, onset beyond 8 weeks prior to admission for rehabilitation, or LFIM > 3. Eight UMN patients (BWSTT n = 6, CONT n = 2) and 5 LMN patients (BWSTT n = 4, CONT n = 1) dropped out prior to completing 6 weeks of intervention, mostly within the first week, so no 3 months or longer data were available. All participants received their center’s standard inpatient and outpatient rehabilitation therapies for mobility and self-care skills in addition to the hour of mobility treatment defined by the study protocol for a mean of 45 completed sessions (range, 35–60). One entry criterion was that the patients had to have a score on the LFIM for walking of <4 on entry. In 16 randomized participants, this was violated. Fourteen of the 16 patients were not able to walk well enough to obtain a measurable walking speed or distance at entry, however. These patients are included in this study but were not included in the report of the primary outcomes analysis for the trial.12 No baseline, preintervention WISCI was obtained because almost none of the patients were able to walk without maximal assistance.
Table 1.
Baseline Data for All Randomized Upper and Lower Motor Neuron Participants
| Gender | % | |||
| % Male | 78 | |||
| % Female | 22 | |||
| Level | % | |||
| % Cervical | 58 | |||
| % Thoracic | 18 | |||
| % Lumbar | 24 | |||
| ASIA | N | |||
| B | 36 | |||
| C | 90 | |||
| D | 20 | |||
| N | Mean | Median | Range | |
| Age | 146 | 32 | 16–69 | |
| LFIM (1–7) | 141 | 1.48 | 1.00 | 1–5 |
| LEMS (0 to 50) | 143 | 15.81 | 14.00 | 0–50 |
| Walking speed, m/s | 20 | 0.39 | 0.39 | |
| 6-min walking distance | N/A | N/A | N/A | N/A |
| Berg Balance (0–56) | 142 | 4.85 | 4.00 | 0–42 |
| WISCI (0–20) | 142 | 1.49 | 0.00 | 0–17 |
N = number of participants; ASIA B,C,D refers to the American Spinal Injury Association Impairment Scale; and NA refers to participants unable to walk at baseline. LFIM = Functional Independence Measure locomotor score; LEMS = Lower Extremity Motor Score; WISCI = Walking Index for Spinal Cord Injury.
STATISTICAL METHODS
In choosing statistical methods, we sought to treat all our outcome measures as ordinal to the extent possible. For correlations of WISCI to the other measures at 3, 6, and 12 months, we thus used Spearman correlations. We deviated from complete ordinality in 2 ways. First, we did compute differences from baseline for WISCI, FIM, LFIM, BBS, and LEMS. Correlations of changes from baseline were computed using Spearman correlations. Spearman’s correlation coefficient was interpreted according to the following guidelines: poor (r < .3), moderate (.3 < r < .6), good (.6 < r < .8), and excellent (r > .8) correlation.16 Second, to obtain some sense of the extent to which WISCI values at later testing intervals could be predicted by WISCI, LEMS, and BBS at a prior time, we estimated the regressions with WISCI scores as the dependent variable and WISCI, LEMS, and BBS at prior times as independent variables. As a check on the reasonableness of the normality assumption, residuals were checked by histogram and normal probability plots. All plots suggested reasonable symmetry of the residuals, even though the distribution of the measures is skewed. Since these regression analyses treat these 3 measures as ratio rather than ordinal, they should not be overinterpreted. They are intended to give a general sense of the extent to which variability in WISCI values are predictable from patients’ status at earlier times. R2s shown are sample-size adjusted (unbiased). All analyses were done with SAS 9.1 (SAS Inc, Cary, NC) on a Windows-based PC.
Some of the x-y plots show a trend line that is a non-parametric smoother (lowess). The nonparametric smoother is a means of suggesting the trend, if any, of the relationship without a priori specification of a model. As will be seen, a more traditional linear trend line would not have been appropriate. Many data points in these data will overlap and show as a single point on standard x-y plots. To avoid this and show the actual number of points, the plots were done with added “jitter.” That is, points are offset a small random amount in order to not overlap, with the consequence that they appear to be plotted at points that are not possible values of the measures. Figures 1 to 3 were done in Systat 11 (Systat Software Inc, Richmond, CA).
Figure 1.
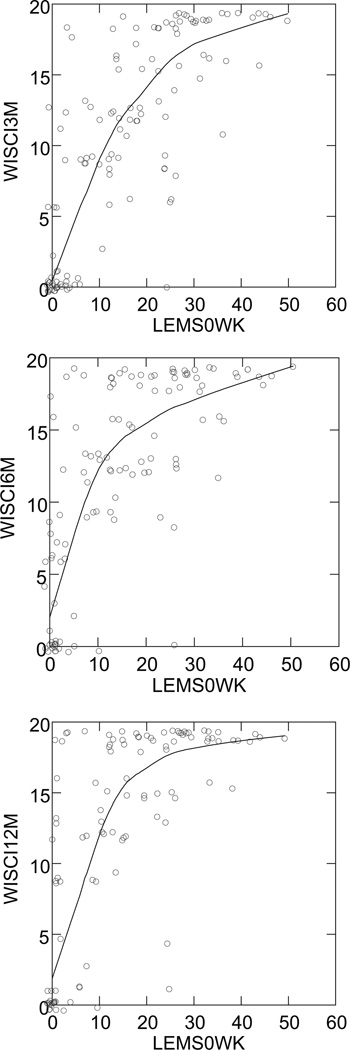
Comparison of baseline lower extremity motor scores (LEMS0WK) and Walking Index for Spinal Cord Injury (WISCI) scores at 3 (r = .80), 6 (r = .74), and 12 (r = .73) months. Many data points in these data will overlap and show as a single point. To avoid this and show the actual number of points, the plots were done with added “jitter.” That is, points are offset a small amount from their actual values in order to not overlap. The trend line is a nonparametric smoother to show trend without an a priori assumption as to the form of the trend.
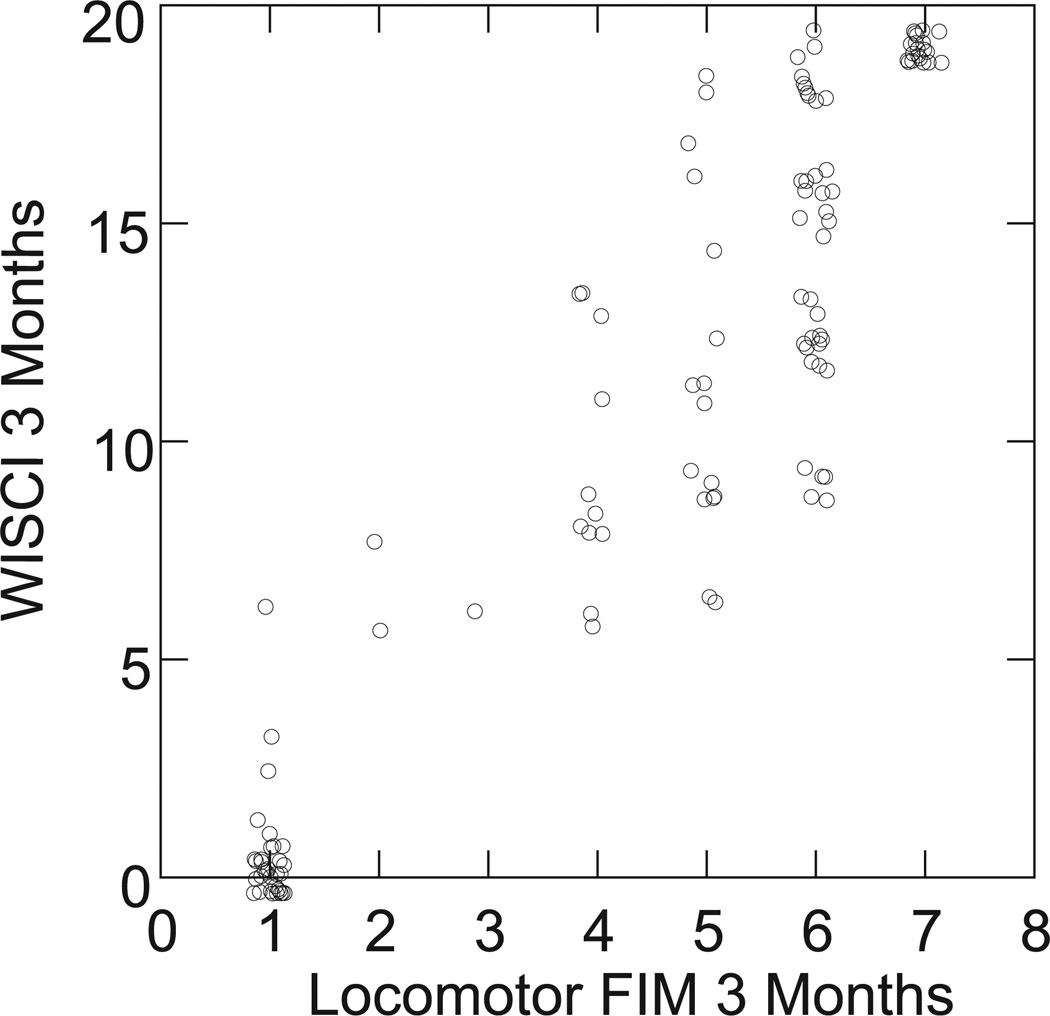
Figure 3.
Comparison of Walking Index for Spinal Cord Injury (WISCI) levels to Functional Independence Measure (FIM) locomotor scores at 3 months (n = 121).
RESULTS
Results are presented for correlations between WISCI and impairment measures (LEMS), between WISCI and functional capacity measures (BBS, 50FW-S, 6MW-D), and between WISCI and disability measures (LFIM, FIM). Subsequently, variance as examined by regression analysis, and finally correlations among the measures other than the WISCI are reported.
Relationship Between WISCI (Functional Capacity Measure) and LEMS (Impairment Measure)
Table 2 shows an excellent correlation between WISCI and LEMS at 3 months, 6 months, and 12 months. The relationships between changes from baseline of LEMS and change from baseline of WISCI at 3 months, 6 months, and 12 months show generally moderate correlations at each time period. The LEMS at baseline shows good correlations to WISCI level (Figure 1) at 3 months, at 6 months, and at 12 months (12-month data shown, Table 3). The trends in Figure 1 are monotone but not linear, with increasing nonlinearity as the number of weeks for the WISCI measurement increases. Change in baseline to subsequent WISCI could not be correlated with changes in 50FW-S or 6MW-D because few patients could walk at baseline.
Table 2.
Spearman Correlations of WISCI With Other Endpoints at 3, 6, and 12 Months and on Change From Baseline
| N | Outcome Measure |
|||||
|---|---|---|---|---|---|---|
| LEMS (n = 107–121) |
Speed (n = 80–88) |
6MW-D (n = 76–78) |
LFIM (n = 104–121) |
Total FIM (n = 106–117) |
BBS (n = 107–120) |
|
| At 3 months | 0.85 | 0.78 | 0.76 | 0.92 | 0.73 | 0.91 |
| At 6 months | 0.85 | 0.85 | 0.68 | 0.89 | 0.77 | 0.89 |
| At 12 months | 0.88 | 0.77 | 0.69 | 0.88 | 0.74 | 0.92 |
| Change baseline: | ||||||
| to 3 months | 0.55 | * | * | 0.87 | 0.54 | 0.85 |
| to 6 months | 0.60 | * | * | 0.88 | 0.48 | 0.87 |
| to 12 months | 0.64 | * | * | 0.87 | 0.51 | 0.85 |
N is sample size. All correlations are statistically significantly different from 0 (P < .001). WISCI = Walking Index for Spinal Cord Injury; LEMS = Lower Extremity Motor Score; 6MW-D = 6-minute walk distance; FIM = Functional Independence Measure; LFIM = FIM locomotor score; BBS = Berg Balance Scale.
Insufficient data; only 14 participants had any ability to walk at baseline; none could complete 6MW.
Table 3.
Best Predictors of the Walking Index for Spinal Cord Injury at 12 Months (Spearman rho)
| Baseline | 3 mos | 6 mos | |
|---|---|---|---|
| Lower Extremity Motor Score | 0.73 | 0.81 | 0.86 |
| Berg Balance Scale | 0.47 | 0.84 | 0.89 |
| FIM Locomotor | 0.30 | 0.79 | 0.85 |
| FIM Total | 0.12 | 0.63 | 0.69 |
| Speed | 0.71 | 0.81 | |
| Distance | 0.77 | 0.80 |
FIM = Functional Independence Measure.
Relationships Between WISCI and Other Functional Capacity Measures
The relationship between the BBS and the WISCI at 3, 6, and 12 months was also excellent (Table 2). In addition, the correlations between the change from baseline of the BBS and change from baseline of the WISCI at 3 months, at 6 months, and at 12 months were excellent, and higher than those of change in LEMS and change in WISCI. However, in comparison to the LEMS, the BBS at baseline was a poor predictor of the WISCI at 12 months (Table 3). This may be due to the fact that the baseline BBS falls within a narrow low range of scores since most patients had cervical lesions and were quite impaired initially. The predictive relationship of the BBS with the 12-month WISCI did improve at 3 and 6 months along with the other measures (Table 3).
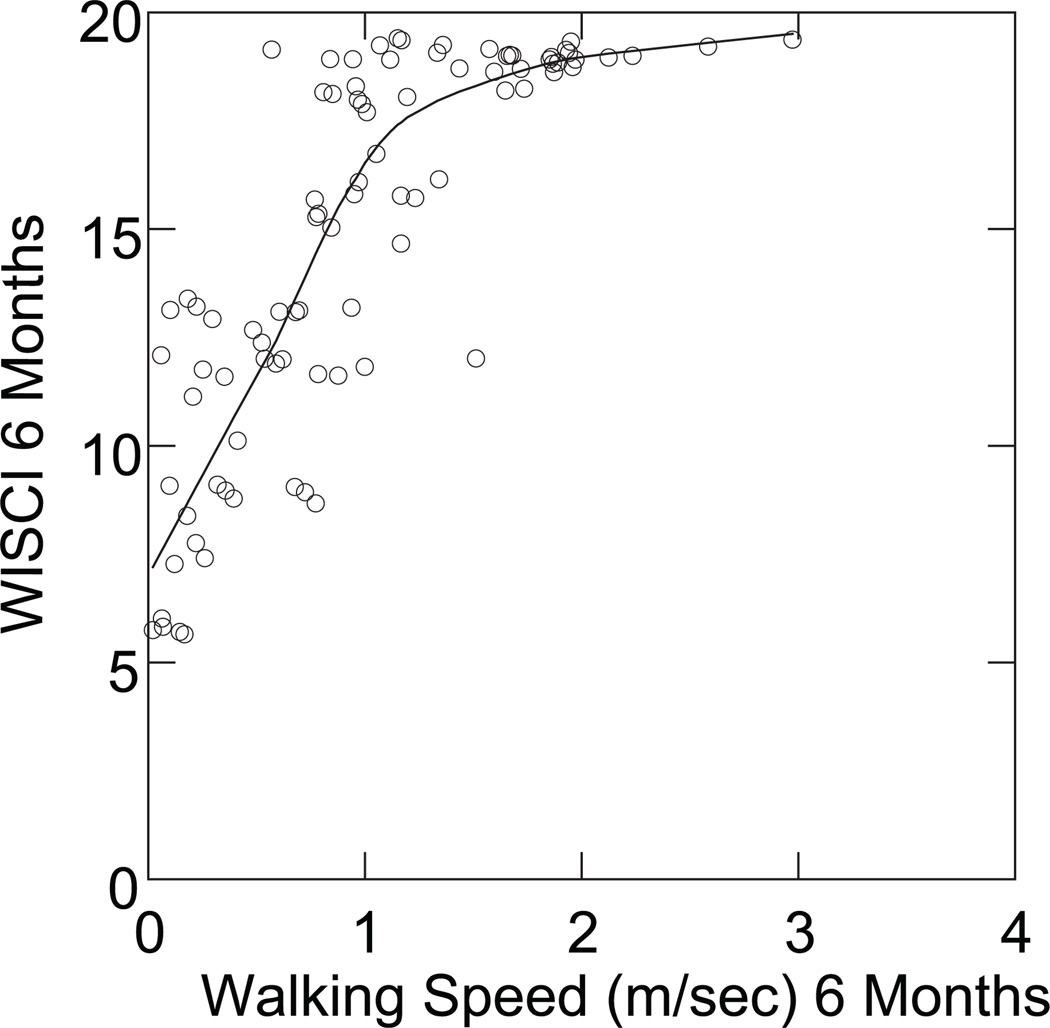
Correlations between the WISCI and the 50FW-S (a speed measure) were in the good to excellent range (Table 2) at 3 months, at 6 months, and at 12 months. The plot of walking speed in meters/second against the WISCI at 6 months shows a linear trend to the point of 1 to 1.5 meters/second, and subsequently, a ceiling effect on the WISCI (Figure 2), with walking speed continuing to improve after WISCI, was at or near its maximum possible value. The 50FW-S at 3 and 6 months was a good predictor of 12-month WISCI, but not as good as LEMS or BBS (Table 3).
Figure 2.
Comparison of Walking Index for Spinal Cord Injury (WISCI) levels to the speed of the 50-foot walk at 6 months (r = .73), demonstrating a ceiling effect of the WISCI scale. Many data points in these data will overlap and show as a single point. To avoid this and show the actual number of points, the plots were done with added “jitter.” That is, points are offset a small amount from their actual values in order to not overlap. The trend line is a nonparametric smoother to show trend without an a priori assumption as to the form of the trend.
Correlations between the WISCI and the 6MW-D (a distance measure) were good at 3, 6, and 12 months, but somewhat lower than that of the other measures (Table 2). As a predictor of the WISCI at 12-months, the 6MW-D is poorer than the other measures tested, although the correlations were still in the good range (Table 3).
Relationships Between WISCI and Disability Measures (Locomotor FIM, Total FIM)
Frequency data from baseline values of the WISCI illustrate that 97/140 patients were in a wheelchair (WISCI = 0) and only 3/140 patients were above a WISCI = 10. At 12 months, however, 18/106 patients had still not progressed above WISCI = 0, but 67/106 were above WISCI = 10. As expected, 112/140 patients were at LFIM = 1 at baseline, but 75/100 achieved an LFIM = 6/7 at 12 months. Thus, there is a strong relationship between the WISCI and the LFIM at 3 months, at 6 months, and at 12 months. However, the relationship between the WISCI and the total FIM was not as strong. As a predictor of 12-month WISCI levels, the LFIM does better than the total FIM at both 3 months and 6 months (Table 3).
Although the correlations are strong between the WISCI levels and LFIM levels, Figure 3 reveals that at 3 months, there are multiple WISCI levels (range 8–19) for an LFIM of 6 and a range of 6 to 18 for an LFIM of 5. The clustering of scores at 0 and 20, however, should be noted.
A general sense of variability is shown by calculation of R2 for the set LEMS, BBS, and prior WISCI. The combination of WISCI, Berg, and LEMS at baseline explains 57% of variability of the WISCI levels at 3 months, with LEMS being the only statistically significant predictor (P < .001). The 3-month values of these 3 explain 78% of the WISCI at 6 months, with LEMS (P = .043) and WISCI (P < .001) being significant. Similarly, the 3 predictors at 6 months explain 85% of the 12-month WISCI, with again LEMS (P = .020) and WISCI (P < .001) being significant. The baseline WISCI, when analyzed together with the BBS and LEMS, is not a significant predictor of the 12-month WISCI.
Interrelationships of BBS, Speed and Distance, LEMS, LFIM, and FIM
The BBS correlated with the 50FW-S at 3 months (r = .81, P < .001), at 6 months (r = .86), and at 12 months (r = .78). The BBS also had excellent correlations with the disability measures: the LFIM at 3 months (r = .89), at 6 months (r = .86), and at 12 months (r= .86). Good correlations were found for the total FIM at these times (r= .76 at 3 months, r= .72 at 6 months, and r= .77 at 12 months).
At 3 months, speed correlated with the LFIM (r= .80), BBS (r= .81), the 6MW-D (r= .95), LEMS (r= .64), and total FIM(r= .57). It continued to have strong correlations with LFIM, BBS, and 6MW-D at 6 months (r> .80), but at 12 months, the strong correlation only existed with the 6MW-D (r = .92), whereas a moderate correlation existed with the LFIM (r= .66) and the BBS (r= .78).
At 3 months, the distance measure (6MW) correlated with speed (r = .95), the LFIM (r = .78), and the BBS (r = .79). The 6MW maintained similar correlations at 6 and 12 months with the LFIM (r = .69, 6 months; r = .62, 12 months). The correlations with LEMS at each of the time periods was rather consistent but moderate (r= .56-.63).
DISCUSSION
The results of this prospective multicenter clinical trial (MCT) support the construct validity of the WISCI scale based on several analyses. First, the very high correlations of the WISCI to impairment (LEMS); the other functional capacity measures of balance (BBS), speed (50FW-S), and distance (6MW-D); the disability scale for walking (LFIM); and global disability (FIM) at 3, 6, and 12 months support concurrent validation (Table 2). In addition, this study represents the first prospective MCT in acute SCI that has demonstrated improved impairments (ASIA LEMS score) that correlated with functional capacity and disability assessments of walking. The correlation over time of predictors of the 12-month WISCI levels shows that the LEMS (impairment) is the best predictor at baseline. The finding that LEMS is the most important determinant for outcome of WISCI supports the predictive validity of LEMS and supports that muscle strength is highly related with walking ability. In addition, the high correlations of the other walking measures with the WISCI are further evidence of construct validity.
One reason for the superiority of the baseline LEMS over the other measures in predicting WISCI outcome may be due to the inclusion criteria of the study, which limited enrollment to poor walkers. As a result, baseline values of BBS, LFIM, total FIM, and WISCI tended to cluster at the lower range (Table 1), and walking speed and distance could not be measured on most participants. In a sample with a wider range of baseline values, these other measures may be better predictors of walking outcome. However, given the generally good outcomes of participants in the SCILT trial, trials of interventions to improve walking function would be likely to enroll patients with even poorer baseline function than seen here. In such studies, the LEMS may be the only measure with enough baseline variability to be useful for predicting walking outcome and may provide a better cutoff value for enrollment than LFIM or BBS.
In the NASCIS 3 Trial, Bracken17 showed a significant improvement in the experimental arm for both the impairment of the upper extremities (motor scores) and disability (FIM self-care), but the study did not report an improvement in the disability for walking (LFIM). The lower extremity motor score, which was derived from the International Spinal Cord Society (ISCoS)/ASIA Standards for Neurological Classification of SCI,15 represents the one measure that has become the standard neurological outcome measure for use in clinical trials for SCI.4,18 The BBS, the 6MW-D, and the 50FW-S are measures that had not been previously used in SCI clinical trials but had served studies of the elderly and trials in stroke and musculoskeletal disorders. During and following this trial, a number of studies were reported regarding the validity of several of these measures, including the WISCI,19 walking speed, and the 6-minute walk8,9,20 for SCI, which will be discussed in more detail below.
The National Center for Medical Rehabilitation Research of the National Institute of Child Health and Human Development (NICHD of the National Institutes of Health) has established an important “terminology in disability classification” that permits clinicians to study human disease within 5 domains of analysis: Pathophysiology, Impairment, Functional Limitation, Disability, and Societal Limitations.11 Therefore the outcome measures will be discussed utilizing the NICHD classification.
Impairment Measures: Lower Extremity Motor Scores
The ISCoS/ASIA International Neurological Standards have been utilized in part17,23 or in whole2,24–26 for most randomized clinical trials to date for SCI. Marino and Graves27 demonstrated the superiority of LEMS over the total motor score and that a separation of the upper from the LEMS more accurately reflects the impairment. The Lower Extremity Motor Scores have been shown to correlate with ambulatory capacity in several studies8,28 and is further validated in this trial. Not only was the LEMS highly correlated with the other outcome measures for each time period in this study (Table 2) and was the best predictor of the WISCI level at 12 months (Table 3), but it offered the best “sense of variance.” Although calculations of variance (R2) are usually applied to parametric data, the LEMS at baseline explained 57% of the variance of the WISCI at 12 months, and calculations of previous WISCI scores improved this to 85%. This analysis must be interpreted with caution, but it gives a sense of variance and has some value for explaining the relationship of the measure of impairment with WISCI levels and adds further support to the construct validity of the WISCI scale.
Functional Limitation/Capacity Measures: The BBS, the 50FW-S, the 6MW-D, and the WISCI
The BBS showed excellent correlations with all other measures for each time period and, as a change from baseline, correlated more highly than the LEMS with the 12-month WISCI (Table 2). It did not, however, at baseline predict the WISCI at 12 months very well as compared to the LEMS. The BBS is a functional capacity scale, which measures balance and strength indirectly. The BBS was developed to test balance in a geriatric population and has been validated for this purpose29,30 and vestibular dysfunction.31,32 It subsequently was applied to stroke patients in a clinical setting.33 A change score of 5–7/56 in this measure of 14 actions was considered clinically significant. It has been shown to correlate with improvements in ambulation speed and walking with less assistance in clinical populations and trials.34 Stroke patients, however, may manifest a number of impairments that may not be operable in the geriatric population that Berg tested, such as impaired position sense and/or numbness plus neurological weakness and spasticity. Perry35 reported that active extension of the knee while standing by stroke patients with or without balance assistance was highly correlated with ambulatory outcomes, which suggests that strength in certain muscles may be important by itself. While it is not the purpose of this article to report on the correlations of the BBS with walking tests in stroke patients, of which there is a substantial literature, it appears prudent to examine the role of muscle weakness, which may contribute to balance but does not represent a test of balance when performed in a static position by SCI patients. It is interesting to note that of the 14 categories tested for balance in the BBS, the strength of at least 1 extremity appears to be required in 50% of the tasks. No instructions appear on whether braces or brace substitutes (ace bandages at the ankle or knee stabilizers) should be included in the assessment as are indicated in the WISCI assessment.5
Walking speed and walking endurance have been utilized in clinical trials for stroke,36–38 cardiac,39,40 and respiratory training41 but have had limited use in SCI trials.42 Previous studies in individuals with chronic SCI show that muscle strength is an important component of walking function.28,43–46 A recent study8 examined the 6MW-D and lower extremity muscle strength in chronic SCI patients and found a high correlation for both the more and less affected side hip flexors, hip extensors, and hip abductors.
The 50FW-S has been most often used in muscu-loskeletal disorders47–49 and is included in a current stroke trial,50 but it had not been used in an SCI trial prior to this study. Speed and velocity for other distances than a 50-foot walk have been reported in SCI populations, including velocity over courses of 10, 50, and 100 meters51 in chronic SCI patients. The 10-meter walk usually includes a 2-meter warm-up and cool-down span on either end, but the speed is calculated for the middle 10 meters. It therefore requires a similar distance of 14 meters or 46 feet to the 50-foot walk. In the SCILT, patients started their walk 2 strides behind the start line and the speed was calculated from the time the lead foot crossed the start line until the lead foot crossed the finish line. While there are no studies that compare the 2 measurement techniques, it appears that they are quite similar. van Hedel examined the validity and reliability of the timed-up-and-go with walking speed measured in a 10-meter walk test and the 6-minute walk test in hospitalized patients. Both acute and chronic patients showed a high correlation between the various measures, and a moderate correlation with the WISCI II.9 Wirz used the same measures to study automated locomotor training in chronic SCI patients.20 Both studies used the WISCI as the standard for assessing walking function. The walking speed used over 10–15 m has been shown to be very similar to the velocity used by patients with stroke who perform a 6-minute walk.10 Although the speeds may differ for patients with SCI, the differences do not appear to be clinically significant.42 Recently, van Hedel52 reported on the responsiveness of walking speed and found it to be superior to the WISCI II used alone. This is consistent with the ceiling effect of the WISCI as compared to speed (Figure 2) in this study. The emerging data support the need for measures of speed, distance, and efficiency to be added to the WISCI scale to more fully characterize improved walking function.5,19,51
The 6MW-D was used as a secondary endpoint in the randomized MCT on locomotor training in SCI10 and correlated well with improvement in Lower Extremity Motor Scores, balance, speed, the WISCI, and the LFIM in ASIA C/D patients (N = 75), which supports criterion validity of the measure and would rank as Class I evidence. The 6MW-D was originally developed and has been very well studied as a measure for patients with chronic obstructive pulmonary disease (COPD).41 It has also been utilized in studies of heart disease53 and stroke in addition to SCI. The 6MW-D was first reported in acute/subacute patients9,52 and chronic patients with SCI8,20 following the report of the methodology of this trial in 2003,2 but this is the first RCT to report its use. Although the American Thoracic Society has recommended several distances of 20 to 30 meters with turns or use of an oval track, only 2 studies8 of SCI defined an 18.5-meter8 and 30.0-meter42 distance with turns. It may be necessary to standardize the distance/turns/use of oval tracks in the future to permit comparison between studies and for improved reliability.
It is also important to recognize that while the 6-minute walking distance is purported to be a measure of “endurance,” it evaluates the integrated response of pulmonary, cardiovascular, and circulatory systems, plus level of motor control, functional neuromuscular units, and muscle metabolism involved during exercise, and it does not provide specific information of the function of each of the organs/systems.41 Although it has no sub-scales, it is often used with several other timed tests such as walking speed.8,9,20 The sensitivity to change has only been reported for COPD in the American Thoracic Society Guidelines41—a 70-meter increase is associated with clinical improvement for COPD, and a 43-meter increase for heart failure patients. There have been no studies reporting the amount of change in the 6-minute walk, which indicates clinical improvement in SCI.
In a recent study,52 the responsiveness of the 6MW-D to change from 3 to 12 months in 22 patients with incomplete SCI showed continued improvement after the WISCI had reached maximum improvement in most patients at 12 months. These findings suggest the need to combine measures of distance and speed with the WISCI in patients who have rapid recovery or high LEMS at initial assessment. The patients chosen for our study, however, differed from this trial in regard to baseline walking capacity. In the current study, the correlation of change from baseline at 3 months to the WISCI was strongest in the BBS and LFIM, but most of the patients were unable to perform the 6MW-D, and only 14/124 were able to do the 50-foot walk due to severity of paralysis. Since all 22 patients in the van Hedel study were required to walk at 3 months, as an inclusion criterion, a comparison is not possible.
Although the 10MW-S and the 6MW-D may appear redundant in that nearly the same velocity is used by patients for both,42 the WISCI may require additional components4,51 of speed, distance, and efficiency, since no one measure appeared superior to the others in this trial. The WISCI scale, as a functional capacity measure, enables assessment at baseline, because it integrates devices with assistance and a distance less than 10 meters, which is not possible with most other capacity measures. In addition, it emerges in this trial as a well-validated functional capacity measure and highly correlated with other measures and domains. The SCILT could not determine whether one measure of efficacy would be more useful than another because the 2 arms of the trial had rather dichotomous outcomes—patients either could not walk or walked well at each assessment.
Disability Measures: Functional Independence Measure, FIM Locomotor Score
The FIM is a well-validated disability measure that determines burden of care but has several limitations when applied to SCI.5,54 The motor FIM55 utilized in this trial includes the domains of self-care, mobility, bladder and bowel function but does not include cognitive elements, which were included in the original global FIM. The global FIM was validated as a measure of burden of care for the rehabilitation field, but the cognitive items were not responsive to change in SCI and were therefore excluded. As would be expected, it showed moderately good correlation (r = .78) but was the lowest of all other functional capacity/disability measures to the WISCI in this trial.
The LFIM is a subset of the motor FIM and has been examined in stroke14,15 and SCI.2,52 It measures walking on a 7-point scale ranging from complete dependence (1) to maximal assistance (2) to complete independence without devices (7). Although used in clinical practice to test the assistance required for walking in any type of environment, as a disability measure, it was employed in a standardized environment, as were all other measures in this trial. The correlations (Table 2) of the LFIM were very strong with the WISCI at all time periods and comparable to BBS and LEMS. The results may be partially a reflection of the standardized measurement technique. The comparison of the tests of the WISCI and the FIM performed at the same time, however, showed multiple WISCI levels for 1 FIM level (Figure 3). An LFIM = 6 is defined as independent for walking with devices, and Figure 3 shows a range of WISCI = 9 (walks independently with a walker and braces) to WISCI = 19 (walks independently with a cane and no braces) in patients tested at an LFIM of 6. The very large difference between the use of a walker and braces compared to only 1 cane to walk is intuitively obvious to any patient or clinician but reveals the limitation in specificity of the LFIM. It has also been suggested that use of different devices by patients with incomplete SCI may result in different forces, cadence, velocity, and energy requirement.56–58
A new disability scale developed specifically for spinal cord lesions (traumatic and nontraumatic causes), the Spinal Cord Independence Measure (SCIM), has been reported as more precise than the FIM and has been validated in a multicenter study of more than 400 patients.59 Morganti19 reported a high correlation (r = .97) with the WISCI from retrospective analysis of more than 200 patients but suggests the SCIM may lack the same level of precision for walking function as the WISCI, because it lacks one of the levels commonly seen at discharge. The purpose of the SCIM and its development and validation, however, differ from the WISCI.19 The WISCI was developed for SCI clinical trials in an effort to reflect most of the factors such as walking aids, braces, and physical assistance utilized for restoration of upright mobility with a reciprocal gait. The SCIM54 also reflects the use of devices in the walking subscales, but it is a global disability scale that has not been validated in the same way as the WISCI. The WISCI scale integrates the use of walking aids, braces, and physical assistance. For several levels of the WISCI scale (levels 14 and 17; Appendix A), walking with the physical assistance of one person is ranked higher with fewer devices than lower levels with no physical assistance, because the scale is driven by the severity of the impairment rather than level of physical assistance. Independence from physical assistance, however, is always a clinical priority in a disability scale and ranks highest in the LFIM and SCIM. A similar difference is seen in the assessment of braces, where the SCIM always ranks no braces as higher than the use of a device, which is not the same in the WISCI scale. The differences in these measures do not indicate that one is better than the other but suggest that they measure different domains and possibly will serve different applications.
The high correlations of the functional capacity and disability measures with motor recovery of the lower extremities revealed by unbiased assessors in the SCILT, a randomized clinical trial that included multiple centers, provides strong support for their concurrent validation as outcome measures. They all showed a significant response to change at 3, 6, and 12 months. The specificity of their response in a clinical trial must await interventions that produce differences in efficacy. It appears prudent to use multiple measures to test strength, balance, speed, distance, and disability, as well as the need for assistive devices, because they apparently reflect different domains of mobility.
ACKNOWLEDGMENTS
This study was funded by the National Institutes of Health at the National Institute for Child Health and Human Development grants R01 H37439 and R24 HD39629. Supported in part by funds from the National Institute on Disability and Rehabilitation Research, Office of Special Education and Rehabilitative Services, US Department of Education (grant #H133 N000023) to the Regional Spinal Cord Injury Center of the Delaware Valley, Thomas Jefferson University.
APPENDIX A
Walking Scale for Spinal Cord Injury Levels
| Level | Devices | Braces | Assistance | Distance |
|---|---|---|---|---|
| 0 | Unable | |||
| 1 | Parallel bars | Braces | 2 persons | Less than 10 meters |
| 2 | Parallel bars | Braces | 2 persons | 10 meters |
| 3 | Parallel bars | Braces | 1 person | 10 meters |
| 4 | Parallel bars | No braces | 1 person | 10 meters |
| 5 | Parallel bars | Braces | No assistance | 10 meters |
| 6 | Walker | Braces | 1 person | 10 meters |
| 7 | Two crutches | Braces | 1 person | 10 meters |
| 8 | Walker | No braces | 1 person | 10 meters |
| 9 | Walker | Braces | No assistance | 10 meters |
| 10 | One cane/crutch | Braces | 1 person | 10 meters |
| 11 | Two crutches | No braces | 1 person | 10 meters |
| 12 | Two crutches | Braces | No assistance | 10 meters |
| 13 | Walker | No braces | No assistance | 10 meters |
| 14 | One cane/crutch | No braces | 1 person | 10 meters |
| 15 | One cane/crutch | Braces | No assistance | 10 meters |
| 16 | Two crutches | No braces | No assistance | 10 meters |
| 17 | No devices | No braces | 1 person | 10 meters |
| 18 | No devices | Braces | No assistance | 10 meters |
| 19 | One cane/crutch | No braces | No assistance | 10 meters |
| 20 | No devices | No braces | No assistance | 10 meters |
| Level assigned___________________ | ||||
Ditunno PL, Ditunno JF. Spinal Cord 2001. Scale revision.6
APPENDIX B
THE SCILT GROUP
Principal Investigator: Bruce H. Dobkin, MD (UCLA Department of Neurology)
Statistical Coordinating Unit: Robert Elashoff, PhD (co-I), Joanie Chung, MPH, and Xiaohong Yan (UCLA Department of Biomathematics)
Trainers Group: Susan Harkema, PhD (co-I, UCLA), Andrea Behrman, PT, PhD (co-I, U Florida), Lisa Fugate, MD, and Michele Basso, PT, EdD (co-Is, OSU)
Safety and Data Monitoring Committee: Carolee Winstein, PT, PhD, and Ann Xiang, PhD (University of Southern California), Patricia Nance, MD (VAMC Long Beach), Beth Ansel, PhD (NIH/NICHD)
Clinical Unit Sites: Magee Rehabilitation Center/ Jefferson University: Michael Saulino, MD (co-I), John Ditunno, MD (co-I), Amy Bratta, MPT, Mary Schmidt-Read, PT, MS; McGill University/Institut de Read-aptation de Montreal: Hugues Barbeau, PhD (co-I), Brigitte Bazinet, MD, Christiane Garneau, PT, Michael Danakas, PT
The Ohio State University: Lisa Fugate, MD, Michele Basso, PT, PhD, Rachel Botkin, PT
University of Ottawa Rehabilitation Hospital: Dan Deforge, MD (co-I), Jennifer Nymark, PT, Michelle Badour, PT
Rancho Los Amigos Rehabilitation Center: Michael Scott, MD (co-I), Jeanine Yip-Menck, PT, Claire Beekman, PT
Shepherd Rehabilitation Center: David Apple, MD (co-I), Gary Dudley, PhD (co-I, U Georgia), Leslie van Hiel, PT, Scott Bickel, PT, PhD
REFERENCES
- 1.Patrick M, Ditunno PL, Ditunno JF., Jr A comparison of spinal cord injury (SCI) consumers/staff preference for walking: a pilot study. J Spinal Cord Med. 2003;26(Suppl 1):S41. [Google Scholar]
- 2.Dobkin BH, Apple D, Barbeau H, et al. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehabil Neural Repair. 2003;17(3):153–167. doi: 10.1177/0888439003255508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curt A, Schwab ME, Dietz V. Providing the clinical basis for new interventional therapies: refined diagnosis and assessment of recovery after spinal cord injury. Spinal Cord. 2004;42(1):1–6. doi: 10.1038/sj.sc.3101558. [DOI] [PubMed] [Google Scholar]
- 4.Steeves J. Outcome measures to be used during SCI clinical trials. [Accessed September 18, 2006];International Campaign for Care and Cure of Paralysis (ICCP) 2006 Available at: http://www.icord.org/ICCP/ICCP_SCI_Guide-lines2.doc.
- 5.Ditunno JF, Jr, Ditunno PL, Graziani V, et al. Walking index for spinal cord injury (WISCI): an international multicenter validity and reliability study. Spinal Cord. 2000;38(4):234–243. doi: 10.1038/sj.sc.3100993. [DOI] [PubMed] [Google Scholar]
- 6.Ditunno PL, Ditunno JF., Jr Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39(12):654–656. doi: 10.1038/sj.sc.3101223. [DOI] [PubMed] [Google Scholar]
- 7.Spinal Cord Injury: Clinical Outcomes From the Model Systems. Gaithersburg, MD: Aspen; 1995. [Google Scholar]
- 8.Kim CM, Eng JJ, Whittaker MW. Level walking and ambulatory capacity in persons with incomplete spinal cord injury: relationship with muscle strength. Spinal Cord. 2004;42(3):156–162. doi: 10.1038/sj.sc.3101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil. 2005;86(2):190–196. doi: 10.1016/j.apmr.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Dobkin B, Barbeau H, Deforge D, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the SCILT multi-center randomized trial. Neurorehabil Neural Repair. 2007;21(1):25–35. doi: 10.1177/1545968306295556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Research Plan for the National Center for Medical Rehabilitation Research. National Center for Medical Rehabilitation Research. Washington, DC: National Institutes of Health; 1993. [Google Scholar]
- 12.Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66(4):484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh R, Hunter J, Philip A, et al. Predicting those who will walk after rehabilitation in a specialist stroke unit. Clin Rehabil. 2006;20(2):149–152. doi: 10.1191/0269215506cr887oa. [DOI] [PubMed] [Google Scholar]
- 14.Shaughnessy M, Michael KM, Sorkin JD, et al. Steps after stroke: capturing ambulatory recovery. Stroke. 2005;36(6):1305–1307. doi: 10.1161/01.STR.0000166202.00669.d2. [DOI] [PubMed] [Google Scholar]
- 15.Marino RJ, Barros T, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S50–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 16.Bellamy N, Wells G, Campbell J. Relationship between severity and clinical importance of symptoms in osteoarthritis. Clin Rheumatol. 1991;10(2):138–143. doi: 10.1007/BF02207652. [DOI] [PubMed] [Google Scholar]
- 17.Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up—results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89(5):699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- 18.Steeves J, Fawcett J, Tuszynski M. Report of international clinical trials workshop on spinal cord injury February 20–21, 2004, Vancouver, Canada. Spinal Cord. 2004;42(10):591–597. doi: 10.1038/sj.sc.3101669. [DOI] [PubMed] [Google Scholar]
- 19.Morganti B, Scivoletto G, Ditunno P, et al. Walking index for spinal cord injury (WISCI): criterion validation. Spinal Cord. 2005;43(1):27–33. doi: 10.1038/sj.sc.3101658. [DOI] [PubMed] [Google Scholar]
- 20.Wirz M, Zemon DH, Rupp R, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil. 2005;86(4):672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Ditunno JF, Jr, Burns AS, Marino RJ. Neurological and functional capacity outcome measures: essential to spinal cord injury clinical trials. J Rehabil Res Dev. 2005;42(3 Suppl 1):35–41. doi: 10.1682/jrrd.2004.08.0098. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Functional limitations research in rehabilitation science and engineering. In: Brandt EN, Pope AM, editors. Enabling America: Assessing the Role of Rehabilitation Science and Engineering. Washington, DC: National Academy Press; 1997. pp. 100–146. [PubMed] [Google Scholar]
- 23.Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 24.Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury—a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med. 1991;324(26):1829–1838. doi: 10.1056/NEJM199106273242601. [DOI] [PubMed] [Google Scholar]
- 25.Geisler FH, Coleman WP, Grieco G, et al. Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine. 2001;26(24 Suppl):S68–S86. doi: 10.1097/00007632-200112151-00014. [DOI] [PubMed] [Google Scholar]
- 26.Geisler FH, Coleman WP, Grieco G, et al. The Sygen multicenter acute spinal cord injury study. Spine. 2001;26(24 Suppl):S87–S98. doi: 10.1097/00007632-200112151-00015. [DOI] [PubMed] [Google Scholar]
- 27.Marino RJ, Graves DE. Metric properties of the ASIA motor score: subscales improve correlation with functional activities. Arch Phys Med Rehabil. 2004;85(11):1804–1810. doi: 10.1016/j.apmr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Waters RL, Adkins R, Yakura J, et al. Prediction of ambulatory performance based on motor scores derived from standards of the American Spinal Injury Association. Arch Phys Med Rehabil. 1994;75(7):756–760. [PubMed] [Google Scholar]
- 29.Berg KO, Maki BE, Williams JI, et al. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. 1992;73(11):1073–1080. [PubMed] [Google Scholar]
- 30.Berg KO, Wood-Dauphinee SL, Williams JI, et al. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(Suppl 2):S7–S11. [PubMed] [Google Scholar]
- 31.Whitney S, Wrisley D, Furman J. Concurrent validity of the Berg Balance Scale and the Dynamic Gait Index in people with vestibular dysfunction. Physiother Res Int. 2003;8(4):178–186. doi: 10.1002/pri.288. [DOI] [PubMed] [Google Scholar]
- 32.Badke MB, Shea TA, Miedaner JA, et al. Outcomes after rehabilitation for adults with balance dysfunction. Arch Phys Med Rehabil. 2004;85(2):227–233. doi: 10.1016/j.apmr.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Stevenson TJ. Detecting change in patients with stroke using the Berg Balance Scale. Aust J Physiother. 2001;47(1):29–38. doi: 10.1016/s0004-9514(14)60296-8. [DOI] [PubMed] [Google Scholar]
- 34.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34(9):2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 35.Mulroy S, Gronley J, Weiss W, et al. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture. 2003;18(1):114–125. doi: 10.1016/s0966-6362(02)00165-0. [DOI] [PubMed] [Google Scholar]
- 36.Visintin M, Barbeau H, Korner-Bitensky N, et al. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29(6):1122–1128. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- 37.Pohl PS, Perera S, Duncan PW, et al. Gains in distance walking in a 3-month follow-up poststroke: what changes? Neurorehabil Neural Repair. 2004;18(1):30–36. doi: 10.1177/0888439003260494. [DOI] [PubMed] [Google Scholar]
- 38.Dobkin BH. Short-distance walking speed and timed walking distance: redundant measures for clinical trials? Neurology. 2006;66(4):584–586. doi: 10.1212/01.wnl.0000198502.88147.dd. [DOI] [PubMed] [Google Scholar]
- 39.Kelly JO, Kilbreath SL, Davis GM, et al. Cardiorespiratory fitness and walking ability in subacute stroke patients. Arch Phys Med Rehabil. 2003;84(12):1780–1785. doi: 10.1016/s0003-9993(03)00376-9. [DOI] [PubMed] [Google Scholar]
- 40.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 41.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 42.Barbeau H, Dobkin B. SCILT Study Group. Comparison of velocities for a 15-meter and 6-minute walk over the first year in patients with incomplete spinal cord injury: the SCILT trial. 2007 doi: 10.1177/1545968306298937. Unpublished Work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teixeira-Salmela LF, Nadeau S, Mcbride I, et al. Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors. J Rehabil Med. 2001;33(2):53–60. doi: 10.1080/165019701750098867. [DOI] [PubMed] [Google Scholar]
- 44.Curt A, Dietz V. Ambulatory capacity in spinal cord injury: significance of somatosensory evoked potentials and ASIA protocol in predicting outcome. Arch Phys Med Rehabil. 1997;78(1):39–43. doi: 10.1016/s0003-9993(97)90007-1. [DOI] [PubMed] [Google Scholar]
- 45.Waters RL, Yakura JS, Adkins R, et al. Determinants of gait performance following spinal cord injury. Arch Phys Med Rehabil. 1989;70(12):811–818. [PubMed] [Google Scholar]
- 46.Hussey RW, Stauffer ES. Spinal cord injury: requirements for ambulation. Arch Phys Med Rehabil. 1973;54(12):544–547. [PubMed] [Google Scholar]
- 47.Ware JE, Jr, Keller SD, Hatoum HAT, et al. The SF-36 Arthritis-Specific Health Index (ASHI): I. Development and cross-validation of scoring algorithms. Med Care. 1999;37(5 Suppl):MS40–MS50. doi: 10.1097/00005650-199905001-00004. [DOI] [PubMed] [Google Scholar]
- 48.Lee CE, Simmonds MJ, Novy DM, et al. Self-reports and clinician-measured physical function among patients with low back pain: a comparison. Arch Phys Med Rehabil. 2001;82(2):227–231. doi: 10.1053/apmr.2001.18214. [DOI] [PubMed] [Google Scholar]
- 49.Bilek LD, Venema DM, Camp KL, et al. Evaluation of the human activity profile for use with persons with arthritis. Arthritis Rheum. 2005;53(5):756–763. doi: 10.1002/art.21455. [DOI] [PubMed] [Google Scholar]
- 50.Cramer SC. Trial of Ropinirole in motor recovery after stroke. [Accessed May 10, 2006];NIH Clinical Trials site. 2005 Available at: http://www.clinical-trials.gov/ct/search.
- 51.Kim MO, Marino RJ, Burns AS. Comparison of the self-selected to maximal levels of the Walking Index in Spinal Cord Injury (WISCI) [Abstract] J Spinal Cord Med. 2005;28(2):161–162. [Google Scholar]
- 52.van Hedel HJ, Wirz M, Curt A. Improving walking assessment in subjects with an incomplete spinal cord injury: responsiveness. Spinal Cord. 2006;44(6):352–356. doi: 10.1038/sj.sc.3101853. [DOI] [PubMed] [Google Scholar]
- 53.Demers C, McKelvie RS, Negassa A, et al. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 2001;142(4):698–703. doi: 10.1067/mhj.2001.118468. [DOI] [PubMed] [Google Scholar]
- 54.Catz A, Itzkovich M, Agranov E, et al. SCIM—spinal cord independence measure—a new disability scale for patients with spinal cord lesions. Spinal Cord. 1997;35(12):850–856. doi: 10.1038/sj.sc.3100504. [DOI] [PubMed] [Google Scholar]
- 55.Cardenas DD, Hoffman JM, Kirshblum S, et al. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a mul-ticenter analysis. Arch Phys Med Rehabil. 2004;85(11):1757–1763. doi: 10.1016/j.apmr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Haubert LL, Gutierrez DD, Newsam CJ, et al. A comparison of shoulder joint forces during ambulation with crutches versus a walker in persons with incomplete spinal cord injury. Arch Phys Med Rehabil. 2006;87(1):63–70. doi: 10.1016/j.apmr.2005.07.311. [DOI] [PubMed] [Google Scholar]
- 57.Melis EH, Torres-Moreno R, Barbeau H, et al. Analysis of assisted-gait characteristics in persons with incomplete spinal cord injury. Spinal Cord. 1999;37(6):430–439. doi: 10.1038/sj.sc.3100850. [DOI] [PubMed] [Google Scholar]
- 58.Ulkar B, Yavuzer G, Guner R, et al. Energy expenditure of the paraplegic gait: comparison between different walking aids and normal subjects. Int J Rehabil Res. 2003;26(3):213–217. doi: 10.1097/00004356-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Catz A, Itzkovich M, Tesio L, et al. A multi-center international study on the Spinal Cord Independence measure, Version III: Rasch Psychometric Validation. Spinal Cord. 2007;45(4):275–291. doi: 10.1038/sj.sc.3101960. [DOI] [PubMed] [Google Scholar]