Abstract
Purpose
This study examined whether locomotor training, which included body weight–supported treadmill therapy, improved walking and induced cortical representational adaptations using functional magnetic resonance imaging in the remaining sensorimotor network after cerebral hemispherectomy.
Methods
Hemispherectomy patients (n = 12) under-went 2 weeks of gait training for at least 30 hours each. They were tested pre- and posttraining with the Fugl-Meyer Motor Assessment, unassisted single-limb stance time, and usual and fastest walking speeds. Three patients performed voluntary ankle movements as the functional magnetic resonance imaging activation task pre- and posttraining. Control subjects included 5 healthy children tested 2 weeks apart, 2 of whom trained on the treadmill, and 2 hemispherectomy patients who received upper extremity rehabilitation and no gait therapy.
Results
Although patients reported improvements with gait training, behavioral outcomes did not significantly change. Training was associated with increased volume and intensity of cortical activation in the primary sensorimotor (S1M1), supplementary motor, motor cingulate, and secondary soma-tosensory cortex for the paretic foot, along with greater overlap in the representation for each moving foot in S1M1 and the supplementary motor area of the remaining hemisphere. Control subjects showed a decrease in activation in these cortical regions after training.
Conclusions
Locomotor training of hemispherectomy patients improved mobility subjectively in association with functional magnetic resonance imaging evidence of cortical remodeling with ankle dorsiflexion. These findings support the notion that hemispherectomy patients may respond to rehabilitation interventions through mechanisms of activity-dependent cortical plasticity. The authors hypothesize that developmentally persistent descending ipsilateral and contralateral corticospinal tracts may allow the remaining hemisphere to maintain bilateral lower extremity motor control after surgery.
Keywords: Gait, Epilepsy, Functional recovery, fMRI, Plasticity, Rehabilitation, Walking
Cerebral hemispherectomy is an increasingly used surgical procedure to treat therapy-resistant epilepsy.1 Hemispherectomy often controls seizures, but patients are left with a residual hemiparesis. Earlier age at the time of brain injury or surgery results in less distal motor weakness than when lesions occur at older ages.2 Although most children after hemispherectomy eventually learn to walk, they display hip circumduction and other gait deviations to advance and clear the affected foot. Whereas the motor deficits after cerebral hemispherectomy have been well documented,3,4 no studies have attempted to show whether task-oriented rehabilitation can improve motor functions such as walking many years following surgery.
In patients with hemiparetic stroke or spinal cord trauma, recent studies support the concept that locomotor interventions can improve walking even if therapy begins years after injury.5–9 The common factor that seems to lead to successful rehabilitation is repeated practice of task-related movements.10,11 One such task-oriented intervention for walking is body weight–supported treadmill training (BWSTT).12,13 This technique partially supports the weight of the patient to prevent the paretic leg from buckling at the knees and enables therapists to safely optimize the kinematic, kinetic, and temporal components of gait that are tied to the stance and swing phases of walking. Studies also suggest that motor gains with locomotor therapy are accompanied by cortical reorganization as observed by functional magnetic resonance imaging (fMRI).14 For walking, fMRI during ankle dorsiflexion appears to be a good marker of training-induced cerebral plasticity.15
The purpose of this study was to pursue these concepts in children long after hemispherectomy. We hypothesized that locomotor training that included BWSTT would be associated with better motor control for walking, accompanied by fMRI changes in cortical activations in the residual hemisphere during ankle dorseflexion.16,17
METHODS
Clinical Cohort
Twelve hemispherectomy subjects were recruited from the University of California, Los Angeles (UCLA) Pediatric Epilepsy Surgery Program.18 The study was approved by the institutional review board, and informed consent was obtained from patients and legal guardians. The clinical epilepsy protocols have been previously published.1,19 In brief, the presurgery evaluation included detailed history and neurologic examinations, interictal and ictal scalp electroencephalogram (EEG) recordings, high-resolution MRI (1.5 T), and 18fluoro-2-deoxyglucose (FDG) positron emission tomography (PET).
Inclusion criteria for locomotor training were the ability to walk without physical assistance at baseline, mental age 3 years or higher so patients were capable of following instructions, no behavioral problems that would jeopardize patient safety during therapy, commitments from the child and family for the 2 weeks of rehabilitation treatment and fMRI testing, and no metal objects in the body. Mental age was estimated using the Peabody Picture Vocabulary Tests (PPVT).20 Additional clinical data abstracted from the medical record included age at seizure onset (years), surgery and entry into study, gender, hemisphere resected, pathology of resected hemisphere, and whether the patient was seizure free at the time of therapy or needed antiepileptic drugs (AEDs). Seizure duration was calculated as age at surgery minus age at seizure onset, and surgery to therapy duration was calculated as age at therapy minus age at surgery.21
Control subjects were healthy right-handed volunteers (n = 5; 1 female; ages 10–12 years), of whom 2 had fMRI scans before and after treadmill training. The other 3 controls were assessed 2 weeks apart without any training. Another control groups consisted of 2 hemispherectomy children (1 female; ages 13 and 14 years) who received upper extremity rehabilitation and no gait therapy. They had pre-and post-fMRI studies of voluntary ankle dorsiflexion.
Locomotor Therapy
The typical gait training session consisted of stretching, 30 minutes of treadmill walking, over-ground practice walking for another 30 minutes, and encouragement for the parents to practice what the therapists had been doing indoors and outdoors on uneven surfaces for at least 1 hour daily. Physical therapists provided therapy in morning and afternoon sessions 5 days per week for 2 weeks.12 When necessary (n = 6), hemispherectomy patients wore a climbing harness (Robertson, Henderson, Nev) attached to a lift suspended over a treadmill (Robomedica, Irvine, Calif). The lift allows vertical displacement during stepping. Harness supports were adjusted from 10% to 30% of body weight to keep the knee from buckling while walking, aid foot clearance, and improve symmetry of stance and swing duration for a portion of each BWSTT session. Patients were systematically trained at increasingly faster walking speeds and reduced weight support. The other 6 hemispherectomy patients practiced walking without the aid of weight support. Therapists manually assisted the paretic leg during stepping or stood behind the individual to aid pelvic rotation and trunk control (Fig. 1). Treadmill speeds were adjusted to equal or exceed each patient’s off-treadmill comfortable over-ground walking velocity.9 During the treadmill sessions, heart rate was monitored with a goal of achieving 65% of maximum predicted values (defined as (220 – age) × 0.65) for at least 10 minutes each session. The healthy controls practiced fast walking and running to provide a challenge in parallel to the expected challenges for hemispherectomy patients.
Figure 1.
Body weight–supported treadmill training (BWSTT) in a right hemispherectomy patient more than 5 years post-surgery. Therapists manipulate the level of unloading of the legs and treadmill speed and provide physical and cognitive cues to improve temporal and kinematic aspects of the gait cycle.
Pre- and Posttraining Assessment of Motor Strength and Gait
Tests included the motor portion of the Fugl-Meyer scale for the lower extremity (maximum 22 points),22,23 stance time without assistance for support on the paretic leg (in seconds), and usual and fastest safe walking speeds over a tiled floor for 15.2 m.13 The Fugl-Meyer scale was modified to include only the motor/movement assess-ments, excluding the sensory and reflex scores, because this reflects lower extremity motor control with minimal burden on attention, as previously found for hemispherectomy patients.2
fMRI Activation for Ankle Dorsiflexion
Hemispherectomy and control patients who were able to cooperate were asked to voluntarily move their ankle during an fMRI scan. If the patient was incapable of voluntary dorsiflexion of the paretic leg, then the ankle was passively moved by an investigator. The fMRI studies were performed pre- and posttraining for the paretic and nonparetic leg. Patients were positioned supine, with the knee flexed 20 degrees and the heel slightly elevated off the gurney, and asked to voluntarily dorsiflex their foot at the ankle from the neutral relaxed position. Because of their severe hemiparesis, hemispherectomy patients were unable to perform this task with the foot in an articulated orthotic to control movement kinematics.15 Instead, to ensure reproducible task execution, cued ankle movements were practiced outside and inside the magnet core, and the outside of the foot was manually stabilized during the fMRI procedures so that movements were uniform. A reproducible angle and rate of ankle movement was achieved across trials for each patient. Patients and controls had at least 1 practice session in the fMRI machine to control for novelty. During each 3.5-minute fMRI scan, participants performed voluntary ankle dorsiflexion (mean 13 movements per 30-second period) over 3 trials inter-leaved with four 30-second rest periods, and the process was repeated for the other foot. Patients were trained to minimize mirror movements, and children were monitored in the scanner so that fMRI activations reflect movement of a single ankle.
Functional MRI scans were obtained using a 1.5 T Siemens Sonata scanner (Erlangen, Germany) at the UCLA Brain Mapping Center. Head motion was minimized by foam pads and head straps. A 3-dimensional T1-weighted data set was obtained for anatomical localization and registration (1 × 1 × 1-mm voxels, TR = 1970 ms, TE = 4.38, TI = 1100, flip angle = 15 degrees, matrix = 256 × 256). Functional imaging used a gradient echo planar imaging sequence (EPI; matrix = 64 × 64, 3 × 3 × 5-mm voxels, TR = 2500 ms, TE = 60 ms, flip angle = 80 degrees). For each subject, 25 axial slices were acquired with a slice thickness of 4 mm and a 1-mm gap oriented parallel to the anterior commissure (AC) to posterior commissure (PC) line.
The fMRI data were analyzed using FSL (FMRIB Software Library, Release 3.1, University of Oxford, www.fmrib.ox.ac.uk/fsl). Preprocessing of the functional imaging data included manual skull stripping (MultiTracer, LONI, UCLA) and correction for head motion (AIR 5, www.bishopw.loni.ucla.edu/AIR5). The analysis was based on the general linear model (GLM), and the basis was a boxcar convolved with a model of the homodynamic response function (HRF). Functional scans were coregistered onto the high-resolution scans (6-parameter rigid body transformation), and spatial smoothing was performed using a 5-mm Gaussian filter. The missing hemisphere was masked prior to analysis, and results were displayed as a cluster-based Z-map using FSL tools. For each patient, the analysis phase correlated the actual signal intensity within each voxel over time with the predicted increase in signal intensity during ankle dorsiflexion and the decrease during rest periods. A cutoff value of 0.30 Pearson’s r coefficient (corresponding to a P value of less than .01; uncorrected) was used to calculate the number of activated voxels above threshold in an a priori defined region of interest (ROI). Four cerebral ROI were selected based on known cortical networks that participate in ankle dorsiflexion.15 The ROI were 1) the primary sensorimotor foot area (S1M1), defined as the region between the postcentral and precentral sulci and from the cingulate sulcus medially to the hand area notch of the precentral sulcus laterally24; 2) the supplementary motor area (SMA), defined as the superior frontal gyrus, including the medial surface lying behind the vertical plane passing through the anterior commissure; 3) the cingulate motor area (Cing) on the medial surface of the cingulate gyrus; and 4) the secondary somatosensory area (SII) of the ventral inferior parietal lobule at the caudal end of the Sylvian fissure. Two investigators drew the ROIs and determined the spatial extent (number of voxels active over a threshold of 2.3, P < .01) and magnitude/intensity of activation (percent signal change), comparing the rest conditions with the voluntary ankle movement. Interrater reliability was R = 0.95. For displaying the overlap between the paretic and nonparetic ankle movements, Z-cluster maps for the nonparetic foot were used as a prethreshold mask when registering to structural space and analyzing statistical maps for the paretic foot.
Data Analysis
Data were entered into a database and analyzed using a statistical program (StatView 5; SAS Institute, Cary, NC). Differences between controls and hemispherectomy patients involving continuous dependent variables were statistically compared using t tests and repeated-measure analysis of variance (ANOVA). Comparisons using nominal variables were performed using chi-square tests. Results were considered different at a minimal level of significance of P < .05.
RESULTS
Hemispherectomy Cohort Characteristics
All 12 hemispherectomy patients completed locomotor training. The cohort included 4 females and 10 left-sided resections. Surgery occurred from 1991 to 2001, and training was carried out from 2003 to 2004. Histopathology of the resected hemispheres indicated that seizure etiologies were prenatal stroke (n = 4), cortical dysplasia (n = 3), and Rasmussen syndrome (n = 5). At the time of locomotor therapy, 10 (83%) were seizure free, and 7 (58%) were taking antiepilepsy medications. The mean age (years ± SD) at training was 13 ± 2.5 (range, 11–19 years), age at surgery was 5.0 ± 3.9 (range, 0.67–12.75 years), and age at seizure onset was 3.1 ± 2.9 years (range, birth to 10.3 years). The mean time from surgery to locomotor training was 7.2 ± 3.9 years (range, 3–12.5 years), and seizure duration prior to surgery was 2.8 ± 2.2 years (range, 0.6–7.4 years). At the time of gait therapy, mental age determined by PPVT was 8.5 ± 3.1 (range, 3–13 years). The average number of BWSTT sessions was 16 over 2 weeks. Per patient, the mean active treadmill session time was 25 minutes, and the mean total practice time (treadmill training plus over-ground practice) with a therapist over 2 weeks was 30 hours. In addition, patients practiced walking in the community with parents for a minimum of 40 hours of total locomotor training. Six patients required a harness for BWSTT with starting and ending weight support that averaged 38.3 lbs (range, 20–50 lbs) and 19.2 lbs (range, 0–50 lbs), respectively. For all patients, average treadmill speed at the start of BWSTT was 1.3 m/s (range, 0.8–2.2), then increased to 2.3 m/s at the end of training (range, 1.3–3.8).
Gait and Motor Functions With Locomotor Training
Following training, hemispherectomy patients and physical therapists described subjective improvements in walking abilities. Parents and family members indicated that their children appeared to walk straighter with less apparent limping. Physical therapists reported increased quadriceps and calf muscle strength, as well as improved gait kinematics with less circumduction of the paretic leg in 10 subjects (83%). All 8 patients using an ankle-foot orthosis prior to training were able to discontinue use after 2 weeks of therapy. One hemispherectomy child who was incapable of ankle movement before therapy was able to voluntarily dorsiflex the ankle posttherapy, and another patient who was able to voluntarily move the ankle before training was able to actively move the ankle and large toe about 10 degrees posttherapy.
Although subjective observations suggested improved walking abilities, the behavioral measures used in this study demonstrated only minimal changes after locomotor therapy (Table 1). Compared with control subjects, all hemispherectomy patients pre- and posttraining had lower Fugl-Meyer scores (−39%; P < .0001), reflecting severe motor impairment, briefer paretic limb stance time (−92%; P < 0.0001), and slower fast walking speeds (−27%; P < 0.0001). By contrast, usual walking speeds before therapy (−14%; P = .10) did not differ from controls. Compared to pretherapy, 3 of the 4 behavioral measures were numerically better after gait therapy in hemispherectomy patients, but the improvements were minimal and not statistically significant. Fugl-Meyer scores (+7%; P = .43), paretic limb stance time (+34%; P = .23), and usual walking speeds (+9%; P = .30) increased and fast walking decreased (−8%; P = .13) in hemispherectomy patients after therapy.
Table 1.
Fugl-Meyer Scores, Limb Stance Time, and Normal and Fast Walking Speeds Pre- and Post-BWSTT in Hemispherectomy Patients (Mean ± SD)
| Test | Controls | Hemi Pre-BWSTT |
Hemi Post- BWSTT |
Controls vs Hemi, P Value |
Pre- vs Post- BWSTT, P Value |
|---|---|---|---|---|---|
| Fugl-Meyer score | 22.0 ± 0.0 | 13.4 ± 1.9 | 14.3 ± 1.8 | <.0001 | .45 |
| Limb stance, s | 20.0 ± 0.0 | 1.6 ± 1.0 | 2.1 ± 1.1 | <.0001 | .23 |
| Normal walking, m/s | 1.2 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.2 | .10 | .32 |
| Fast walking, m/s | 2.4 ± 0.3 | 1.8 ± 0.2 | 1.6 ± 0.2 | <.0001 | .13 |
BWSTT, body weight–supported treadmill training.
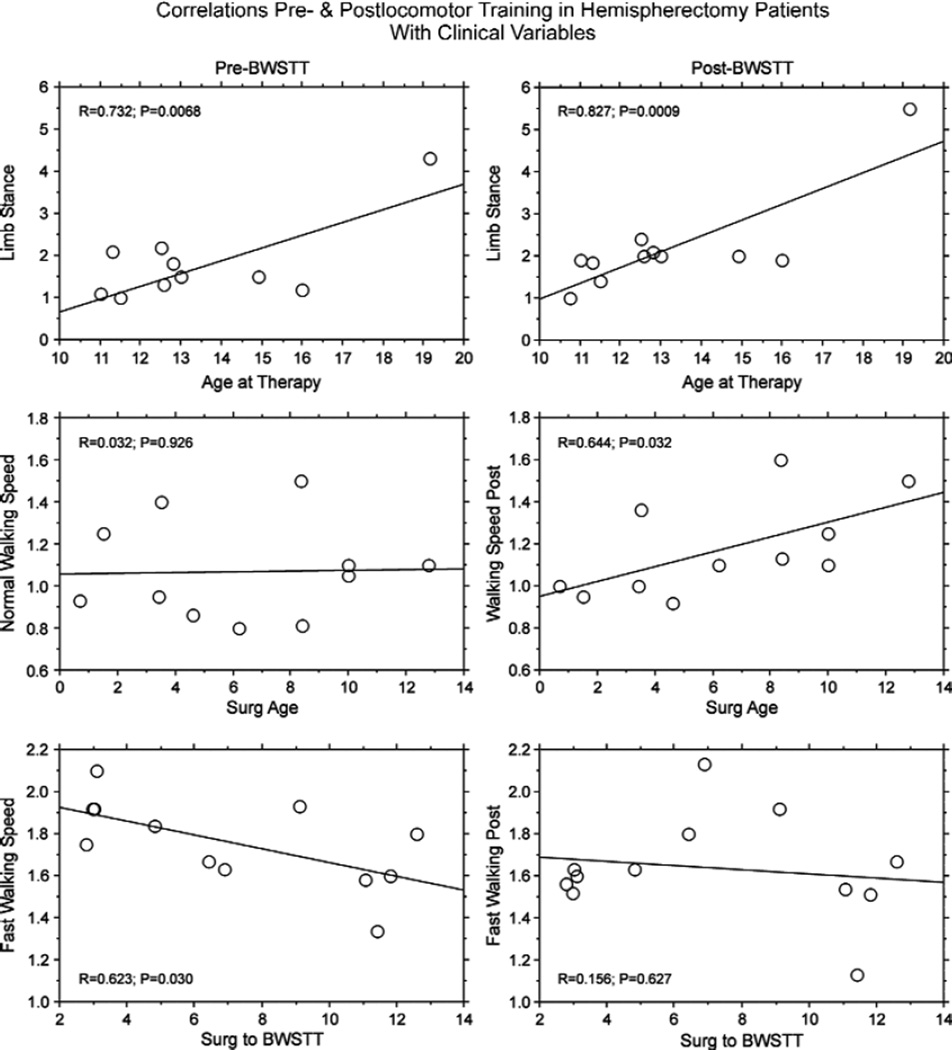
The lack of significant change associated with gait therapy for our behavioral measures could be explained, in part, by correlations of these tests with other clinical variables (Fig. 2). Pre- and posttraining limb stance time positively correlated with age at therapy in hemispherectomy patients (Fig. 2, first row; P< .0068). Usual walking speeds, which did not correlate with age at surgery before training, positively correlated after therapy (Fig. 2, second row; P= .032). Likewise, a negative correlation between fast walking speed and the interval from surgery to therapy before training (P = .030) was not statistically significant after training (Fig. 2, third row; P = .63). Other clinical variables, such as side resected (P> .15), pathology (P> .23), mental age (P> .06), AED usage at therapy (P> .07), age at seizure onset (P > .09), seizure duration (P > .30), and gender (P > .080) did not correlate with Fugl-Meyer scores, limb stance times, or normal or fast walking speeds.
Figure 2.
For hemispherectomy patients, line graphs show correlations between limb stance time (seconds) with age at therapy (first row), normal walking speed (m/s) with age at surgery (Surg Age; second row), and fastest walking speed (m/s) with years from surgery to body weight–supported treadmill training (Surg to BWSTT; third row). Pre-BWSTT is in the left column and post-BWSTT in the right column. First row: longer single-limb stance time positively correlated with age at therapy pre- and post-BWSTT (P < .0068). Second row: normal walking speed did not correlate with age at surgery pre-BWSTT (P = .926) but positively correlated post-BWSTT (P = .032). Third row: fast walking speed negatively correlated with years from surgery to BWSTT pre-training (P = .030) but did not correlate posttraining (P = .627).
fMRI for Ankle Dorsiflexion
Nine (75%) hemispherectomy and all control subjects were able to cooperate with fMRI activation procedures for ankle movement. However, only 3 hemispherectomy patients were capable of voluntary ankle dorsiflexion of the paretic foot. These 3 patients had perinatal stroke (n = 1), Rasmussen syndrome (n = 1), and cortical dysplasia (n = 1) as etiologies, and the only clinical variable that trended to be different compared with the 6 with nonvoluntary ankle movement was an earlier age at cerebral injury/surgery (voluntary, n = 3, 0.9 ± 1.0 years, vs nonvoluntary, n = 6, 6.5 ± 4.0; t test, P = .056). The fMRI responses during passive ankle movements were extremely variable even within the same patient in serial scans, mostly due to head movements and limited ability to cooperate when not engaged in an active movement task. As a consequence, these data were excluded from further analysis. Hence, we report the fMRI findings for voluntary ankle dorsiflexion in the 3 hemispherectomy and 2 healthy control subjects who were capable of these studies pre- and posttraining. The fMRI of 2 hemispherectomy patients who had upper extremity but no gait therapy and had voluntary ankle dorsiflexion revealed no differences in spatial extent and intensity of activation in the 4 cortical ROI (data not shown).
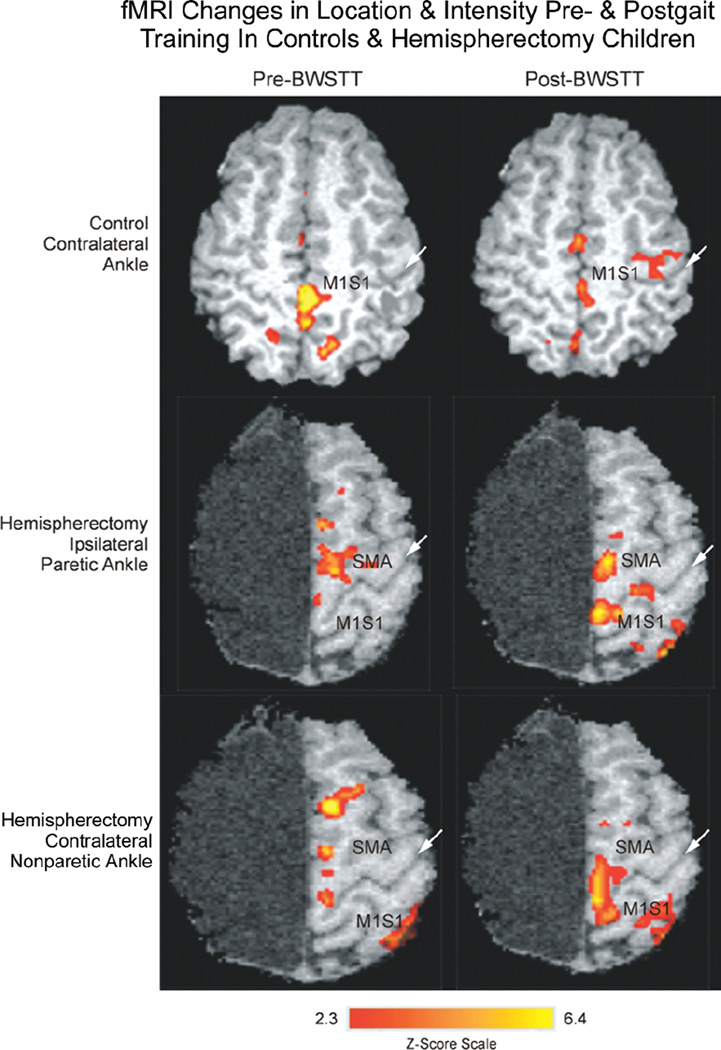
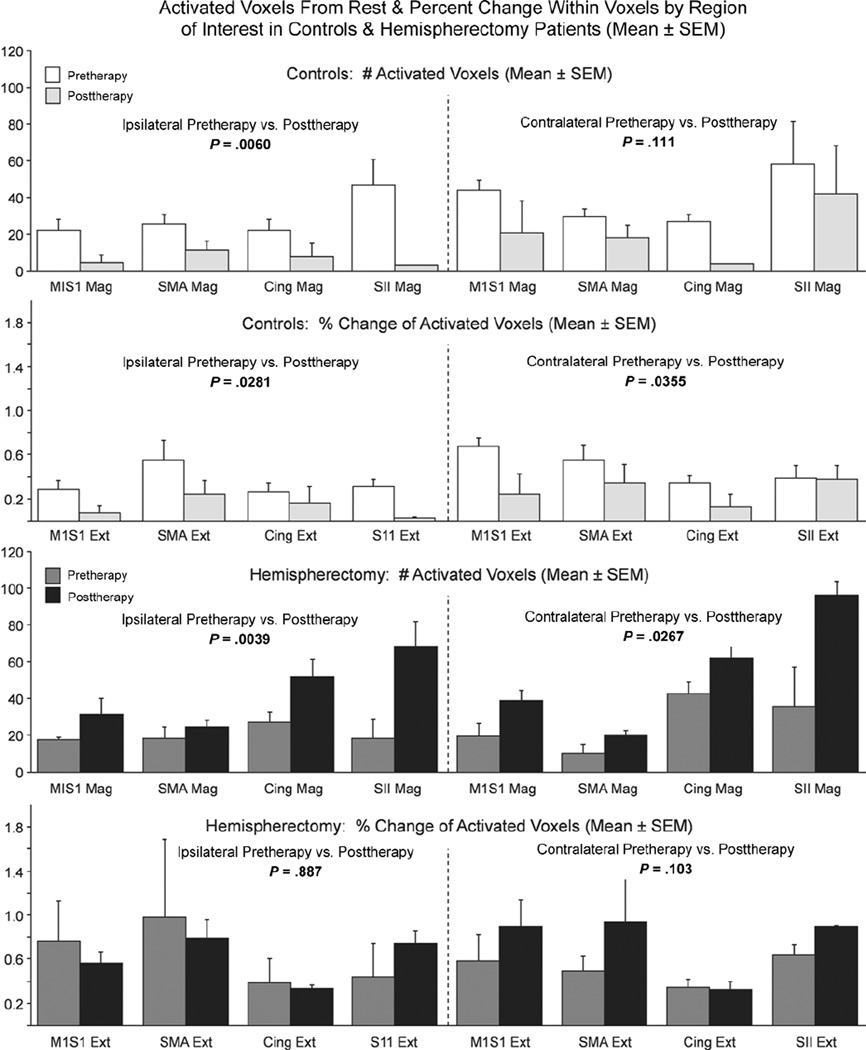
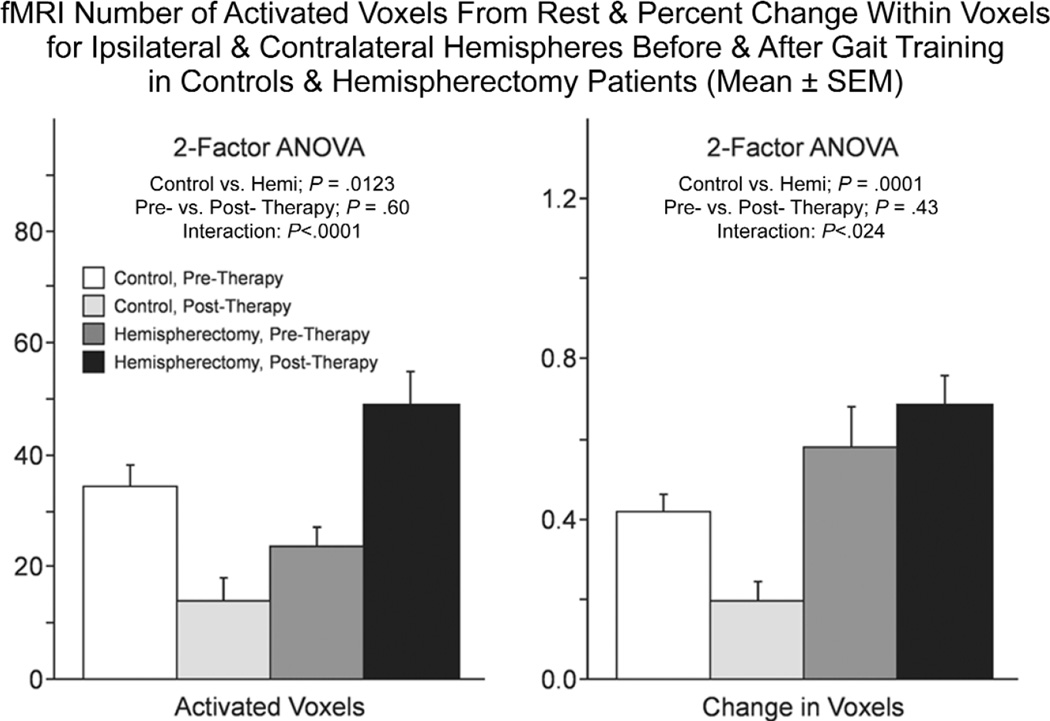
Locomotor training was associated with different effects on fMRI activation with voluntary ankle movements for control and hemispherectomy cases (Figs. 3–6). For controls, posttraining fMRI activations compared with pretraining showed no change or a decreased spatial extent of activated voxels and reduced percent change in intensity for ipsilateral and contralateral ankle movements in M1S1, SMA, cingulate, and SII cortical regions (Fig. 3, first row; Figs. 5 and 6). By comparison, in hemispherectomy children, locomotor training was associated with increased spatial extent and intensity of activated voxels in most of the 4 cortical ROI (Fig. 3, second and third rows). The increase in the number of activated voxels with training resulted in enlarged areas of cortical activation that overlapped between the paretic and nonparetic ankle in the S1M1 and SMA cortex of hemispherectomy patients with voluntary ankle movements (Fig. 4). Using a 2-factor ANOVA (repeated measures) for all ROIs, the fMRI changes were significantly different between control subjects and hemispherectomy patients (Fig. 5, interaction; P < .0001). For hemispherectomy patients, the increase in spatial extent of activated voxels occurred with both ipsi-lateral paretic and contralateral nonparetic voluntary ankle movements (Fig. 6, third row; P < .02). For control subjects, the decrease in activated voxels was observed with ipsilateral (Fig. 6, first row; P= .006) but not contralateral (P= .11) ankle movements. Furthermore, the decrease in magnitude of fMRI cortical activity among control subjects was observed with ipsilateral and contralateral ankle movements (Fig. 6, second row; P < .035), whereas the increase in voxel intensity for hemispherectomy patients was not statistically significant when the 2 limbs were considered separately (Fig. 6, fourth row; P > .10).
Figure 3.
Representative functional magnetic resonance imaging (fMRI) activation scans for a control (first row; 12-year-old) and posthemispherectomy patient (second and third rows; 13-year-old; right hemispherectomy for Rasmussen syndrome 8 years ear-lier) before (left column) and after (right column) body weight–supported treadmill training (BWSTT). White arrow in each panel indicates the central sulcus. Top: in a control patient, voluntary contralateral ankle movement resulted in activation of the M1S1 cortex pre-BWSTT that decreased in area and intensity post-BWSTT. Middle and lower: by contrast, in a hemispherectomy child, voluntary ipsilateral paretic and contralateral nonparetic ankle movement before gait training showed some activation of the sup-plementary motor area (SMA) and M1S1 cortex that increased in area and intensity posttherapy. Z score scale for Figures 4 and 5 is indicated at the bottom of the figure.
Figure 6.
Bar graphs showing the number of activated voxels (top and third row) and percent change in intensity (second and fourth rows) for controls (top and second row) and hemispherectomy patients (third and fourth rows) for each region of interest (ROI) with ipsilateral paretic and contralateral nonparetic voluntary ankle movement. Results of repeated-measures analysis of variance for the 4 ROIs are indicated for ipsilateral and contralateral voluntary ankle movements. SMA, supplementary motor area; SII, secondary somatosensory area.
Figure 5.
Bar graphs showing the number of activated voxels (left) and percent change in intensity (right) between rest and vol-untary ankle movement activations pre- and postgait training for controls and hemispherectomy patients. The mean (± SEM) using repeated measures for all 4 regions of interest (ROIs) and paretic and nonparetic foot movements combined are shown. Statistical results of the repeated measures 2-factor analysis of variance (ANOVA) for all ROIs are indicated above each set of bar graphs.
Figure 4.
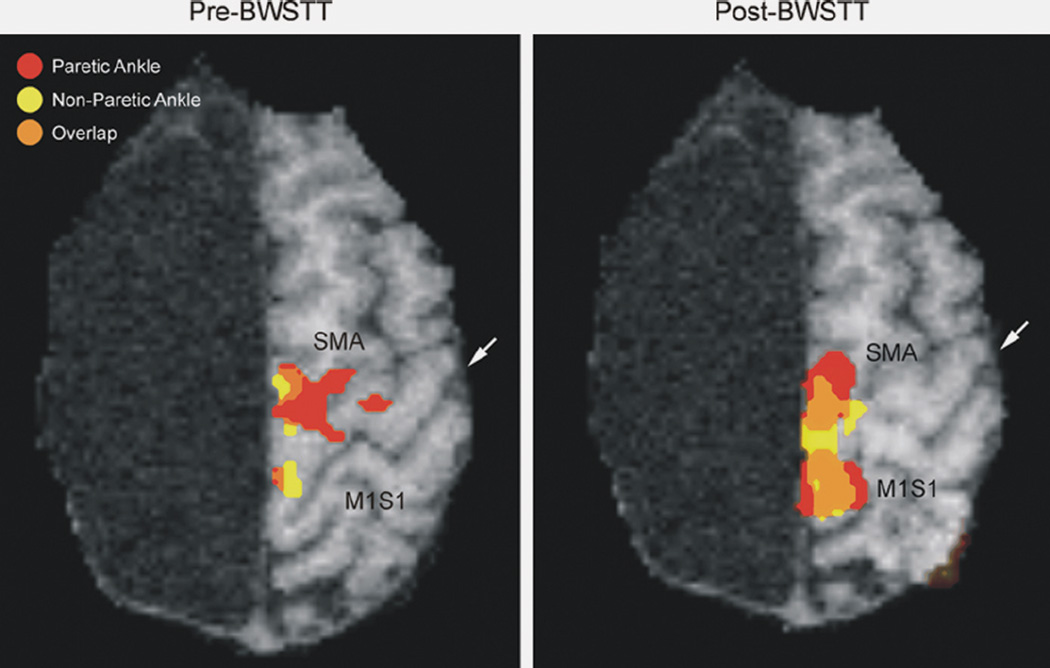
Functional magnetic resonance imaging (fMRI) activation of the ipsilateral paretic ankle (red), contralateral nonparetic ankle (yellow), and areas of overlap (orange) within the supplementary motor area (SMA) and M1S1 cortex with voluntary movement in a hemispherectomy child. Pretraining, only a small area of overlap was present mostly in the SMA region (left). Posttherapy, the areas of activation increased, as did the areas of overlap, in both the SMA and M1S1 cortex. White arrow indicates the central sulcus.
DISCUSSION
In children many years after hemispherectomy, locomotor therapy that included BWSTT and over-ground practice was associated with subjective improvement in walking and altered activation of sensorimotor cortical networks participating in voluntary ankle dorsiflexion by fMRI. In hemispherectomy patients, Fugl-Meyer scores, paretic limb stance times, and normal walking speeds were minimally improved after locomotor training (Table 1). Locomotor therapy in 3 hemispherectomy patients was associated with increased spatial extent and intensity of activation with voluntary ankle movement of both paretic and nonparetic legs in the primary sensorimotor, supplementary motor, cingulate motor, and secondary somatosensory cortex. By comparison, the 2 control subjects showed decreased fMRI activation in the same ROI with treadmill training, which suggested a skills learning effect. No changes in fMRI activation were found in 2 hemispherectomy children who only trained their upper extremity without gain therapy, pointing to the specificity of locomotor training in altering cortical adaptations for ankle representation by fMRI. In hemispherectomy patients, increased spatial extent and intensity of fMRI activations produced greater overlap in S1M1 and the SMA of the remaining hemisphere with paretic and nonparetic foot voluntary ankle movement. Taken together, these findings indicate that locomotor training subjectively improved walking in patients with hemispherectomy in association with physiological adaptations of the residual motor network during voluntary ankle dorsiflexion. The findings are consistent with the notion that cerebral plasticity can be augmented and monitored using fMRI many years after cerebral hemispherectomy through the use of a brief pulse of locomotor training.
This modest-sized rehabilitation study also highlights some of the inherent difficulties and methodological limitations in working with patients after cerebral hemispherectomy. For example, compared with adults with spinal cord injuries and even children with cerebral palsy, all of our children were able to walk independently before they started locomotor training. Thus, their disabilities were probably less severe than other patient groups undergoing gait training. Likewise, locomotor therapy showed only marginal changes in the motor Fugl-Meyer scale, paretic limb stance time, and usual and fast walking speeds. These measurements were initially selected because they were easy to administer, posed little burden on attention span, and have been among the most reliable outcome measures for stroke rehabilitation trials for walking. In retrospect, the ordinal Fugl-Meyer motor assessment for the lower extremities, which appeared to be sensitive to the etiology of seizures in our prior study,2 was not responsive to short-term locomotor therapies that concentrated on stepping. Similarly, walking speed, which was selected as a surrogate marker to assess kinematic and spatiotemporal measures of the gait cycle, turned out to be insensitive in our study. Initial walking speeds were probably too high in our subjects to discern the 20% to 30% increase necessary to reveal a statistically significant gain with therapy. However, it should be noted that our selected measures correlated with other clinical factors, such as age at surgery or therapy, which was unexpected and will require further exploration in future rehabilitation studies. In addition, we found that fMRI studies were only interpretable if the patient was capable of voluntary movement of the paretic foot, which was feasible in only a minority of our patients. Future rehabilitation studies involving hemispherectomy patients will need to consider strategies using passive movement that do not allow head motion artifacts, as well as other procedural difficulties we encountered. However, in this first attempt at studying training-induced cortical plasticity, our study indicates that rehabilitation studies involving task-specific therapies for walking can be performed in patients after cerebral hemispherectomy, therapy seems to induce at least some subjective improvements in walking, and the effects of gait therapy can be monitored with fMRI.
Only a few studies have reported motor function and cortical activation following hemispherectomy or extensive cerebral injury, and they did not include locomotor training.25 For example, Pascual–Leone et al26 recorded motor-evoked potentials (MEPs) for the hand in 7 hemispherectomy patients and reported that the weaker hand’s activation was detected mostly in premotor areas or SMA, 2 to 4 cm anterior to ipsilateral M1. These results were in agreement with an fMRI study in which 2 hemispherectomy patients were scanned during an elbow flexion-extension paradigm. Areas activated included the premotor (not primary motor) cortex for both patients with additional activation in the SMA in one.27 In addition, the area of activation was smaller for the paretic side than for the normal hand. In another study, 2 hemispherectomy subjects demonstrated different patterns of ipsilateral fMRI activation on passive movement of the hemiplegic hand.28 The first patient showed maximum activation in primary S1M1, whereas the other patient had activated the premotor area. Neither patient could voluntarily move the paretic hand. Bernasconi et al29 studied 3 hemispherectomy patients who were capable of opening and closing the contralesional hand. Positron emission tomography activation was found in the premotor area (all patients), the SMA (2 patients), and the secondary sensory area (2 patients), but not M1. The authors concluded that the supplementary and premotor areas might be sufficient to support voluntary motor control of the paretic hand. Similarly, Cohen et al30 reported a patient with congenital hemiparesis and relatively preserved hand function whose paretic hand representation was absent from M1. By contrast, in a paradigm consisting of raising and lowering an extended leg (activation of proximal muscles), a 23-year-old woman with a history of Rasmussen syndrome showed activation in the homologous primary motor cortex for both the strong and weak leg.31 Thus, M1, the premotor cortex, and the SMA in the remaining hemisphere have been reported to be responsible for motor functions after hemispherectomy, but the results have been inconsistent, partly due to differences in activation paradigms and clinical features of the subjects.
Why certain hemispherectomy patients are capable of voluntarily moving distal hand and foot joints whereas others cannot is unknown. One suggested hypothesis is that residual motor functions posthemispherectomy could be due to preservation of developmentally regulated corticospinal tracts.32 In humans, there is anatomical and physiological evidence that ipsilateral and contralateral corticospinal tracts begin to develop at approximately 26 weeks’ gestation and innervate the contralateral and ipsilateral spinal motor neurons equally by birth.33 Transcranial magnetic stimulation of the motor cortex in normal children younger than age 2 years found that the ipsilateral responses regress over the first 18 months of life.34,35 We suggest that the preserved ipsilateral corticospinal tracts or contralateral descending motor tracts that may recross at segmental levels in the spinal cord probably account for better bilateral and distal muscle control of hemispherectomy patients who had their brain injury and surgery at the earliest times after birth. In addition, this small study suggests that the motor control represented within these pathways can be augmented by activity-dependent mechanisms during brief goal-directed rehabilitation even years after hemispherectomy.36–38 In patients with injury at an older age, the ipsilateral tract may have morphologically regressed or have declined in synaptic efficacy. We propose that the more limited distal muscle motor control in older hemispherectomy subjects probably involves other subcortical mechanisms such as the cortico-reticulospinal and propriospinal inputs to the spinal cord that are less efficient than corticospinal tracts.39 On the basis of this anatomical information, one might expect voluntary ankle dorsiflexion of each foot to be accompanied by activation within overlapping portions of S1M1, as well as adaptations induced by skills learning, such as representational expansion or increased overlap during movement of the paretic and nonparetic ankle in patients with a younger age at surgery/injury. Our study seems to have revealed such changes.
Although intriguing, we must emphasize that these concepts will need to be tested in hypothesis-driven future rehabilitation studies. However, our study promotes the possibility that rehabilitation interventions can be designed to optimize motor functions of the residual corticospinal pathway of the developing brain. Functional neuroimaging techniques may reveal whether a therapy engages this network and modulates its activity over the time of training.
ACKNOWLEDGMENTS
Ann Firestine, Richard Dubois, and Robin Gruver assisted with fMRI analyses. This work was supported by the Brain Mapping Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmonton Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, NorthStar Fund, and National Center for Research Resources grants RR 12169, RR13642, and RR08655, as well as the National Institutes of Health (NIH) NRSA in Neurological Rehabilitation (NS 07479) to BD, the NIH RehabNet West grant (R24 HD39629) to S. de Bode, and P01 NS 02808, R01 NS38992, and R21 HD050707 to GWM.
REFERENCES
- 1.Jonas R, Nguyen S, Hu B, et al. Cerebral hemispherectomy: hospital course, developmental, language, and motor outcomes. Neurology. 2004;62:1712–1721. doi: 10.1212/01.wnl.0000127109.14569.c3. [DOI] [PubMed] [Google Scholar]
- 2.de Bode S, Firestine A, Mathern GW, Dobkin B. Residual motor control and cortical representations of function following hemispherectomy: effects of etiology. J Child Neurol. 2005;20:64–75. doi: 10.1177/08830738050200011101. [DOI] [PubMed] [Google Scholar]
- 3.Holthausen H, Strobl K, Pieper T, et al. Prediction of motor functions post hemispherectomy. In: Tuxhorn I, Holthausen H, Boenigk H, editors. Paediatric Epilepsy Syndromes and Their Surgical Treatment. London: John Libbey; 1997. pp. 785–798. [Google Scholar]
- 4.van Empelen R, Jennekens-Schinkel A, Buskens E, et al. Functional consequences of hemispherectomy. Brain. 2004;127(Pt 9):2071–2079. doi: 10.1093/brain/awh224. [DOI] [PubMed] [Google Scholar]
- 5.Ada L, Dean CM, Hall JM, et al. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. Arch Phys Med Rehabil. 2003;84:1486–1491. doi: 10.1016/s0003-9993(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 6.Yagura H, Miyai I, Seike Y, et al. Benefit of inpatient multidisciplinary rehabilitation up to 1 year after stroke. Arch Phys Med Rehabil. 2003;84:1687–1691. doi: 10.1053/s0003-9993(03)00286-7. [DOI] [PubMed] [Google Scholar]
- 7.Green J, Forster A, Bogle S, Young J. Physiotherapy for patients with mobility problems more than 1 year after stroke: a randomised controlled trial. Lancet. 2002;359:199–203. doi: 10.1016/S0140-6736(02)07443-3. [DOI] [PubMed] [Google Scholar]
- 8.Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–884. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–691. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 10.Dobkin BH. Functional rewiring of brain and spinal cord after injury: the three Rs of neural repair and neurological rehabilitation. Curr Opin Neurol. 2000;13:655–659. doi: 10.1097/00019052-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3:528–536. doi: 10.1016/S1474-4422(04)00851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobkin BH, Apple D, Barbeau H, et al. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehabil Neural Repair. 2003;17:153–167. doi: 10.1177/0888439003255508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron JC, Cohen LG, Cramer SC, et al. Neuroimaging in stroke recovery: a position paper from the First International Workshop on Neuroimaging and Stroke Recovery. Cerebrovasc Dis. 2004;18:260–267. doi: 10.1159/000080293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobkin BH, Firestine A, West M, et al. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23:370–381. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Y, Dobkin BH, Cen SY, et al. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–1555. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- 17.Dobkin BH. Rehabilitation and functional neuroimaging dose-response trajectories for clinical trials. Neurorehabil Neural Repair. 2005;19:276–282. doi: 10.1177/1545968305281892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook SW, Nguyen ST, Hu B, et al. Cerebral hemispherectomy for pediatric epilepsy patients: a comparison of three techniques by pathologic substrate in 115 patients. J Neurosurg (Pediatrics 2) 2004;100:125–141. doi: 10.3171/ped.2004.100.2.0125. [DOI] [PubMed] [Google Scholar]
- 19.Mathern GW, Giza CC, Yudovin S, et al. Postoperative seizure control and antiepileptic drug use in pediatric epilepsy surgery patients: the UCLA experience, 1986–1997. Epilepsia. 1999;40:1740–1749. doi: 10.1111/j.1528-1157.1999.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 20.Dunn L. The Peabody Picture Vocabulary Test, PPVT American Guidance Service. Circle Pines, Minn: American Guidance Service; 1981. [Google Scholar]
- 21.Jonas R, Asarnow RF, Lopresti C, et al. Surgery for symptomatic infant-onset epileptic encephalopathy with and without infantile spasms. Neurology. 2005;64:746–750. doi: 10.1212/01.WNL.0000151970.29205.70. [DOI] [PubMed] [Google Scholar]
- 22.Fugl-Meyer AR. Post-stroke hemiplegia assessment of physical properties. Scand J Rehabil Med Suppl. 1980;7:85–93. [PubMed] [Google Scholar]
- 23.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 24.Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. Neuroimage. 2004;21:568–575. doi: 10.1016/j.neuroimage.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 25.Holthausen H, Strobl K. Modes of reorganization of the sensorimotor system in children with infantile hemiplegia and after hemispherectomy. Adv Neurol. 1999;81:201–220. [PubMed] [Google Scholar]
- 26.Pascual-Leone A, Chugani DC, Cohen LG, et al. Reorganization of human motor pathways following hemispherectomy. Ann Neurol. 1992;32:120. [Google Scholar]
- 27.Graveline CJ, Mikulis DJ, Crawley AP, Hwang PA. Regionalized sensorimotor plasticity after hemispherectomy fMRI evaluation. Pediatr Neurol. 1998;19:337–342. doi: 10.1016/s0887-8994(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 28.Holloway V, Gadian DG, Vargha-Khadem F, Porter DA, Boyd SG, Connelly A. The reorganization of sensorimotor function in children after hemispherectomy: a functional MRI and somatosensory evoked potential study. Brain. 2000;123(Pt 12):2432–2444. doi: 10.1093/brain/123.12.2432. [DOI] [PubMed] [Google Scholar]
- 29.Bernasconi A, Bernasconi N, Lassonde M, et al. Sensorimotor organization in patients who have undergone hemispherectomy: a study with (15)O-water PET and somatosensory evoked potentials. Neuroreport. 2000;11:3085–3090. doi: 10.1097/00001756-200009280-00010. [DOI] [PubMed] [Google Scholar]
- 30.Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man: a study with focal magnetic stimulation. Brain. 1991;114(Pt 1B):615–627. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- 31.Wieser HG, Henke K, Zumsteg D, Taub E, Yonekaway Y, Buck A. Activation of the left motor cortex during left leg movements after right central resection. J Neurol Neurosurg Psychiatry. 1999;67:487–491. doi: 10.1136/jnnp.67.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benecke R, Meyer BU, Freund HJ. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp Brain Res. 1991;83:419–426. doi: 10.1007/BF00231167. [DOI] [PubMed] [Google Scholar]
- 33.Eyre JA, Miller S, Clowry GJ, et al. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123(Pt 1):51–64. doi: 10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- 34.Eyre JA, Taylor JP, Villagra F, et al. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–1554. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- 35.Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol. 1997;42:705–711. doi: 10.1002/ana.410420506. [DOI] [PubMed] [Google Scholar]
- 36.Ralston DD, Ralston HJIII. The terminations of corticospinal tract axons in the macaque monkey. J Comp Neurol. 1985;242:325–337. doi: 10.1002/cne.902420303. [DOI] [PubMed] [Google Scholar]
- 37.Donkelaar HJ. Development and regenerative capacity of descending supraspinal pathways in tetrapods: a comparative approach. Adv Anat Embryol Cell Biol. 2000;154:1–145. doi: 10.1007/978-3-642-57125-1. ii-ix. [DOI] [PubMed] [Google Scholar]
- 38.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 39.Maegaki Y, Maeoka Y, Ishii S, et al. Mechanisms of central motor reorganization in pediatric hemiplegic patients. Neuropediatrics. 1997;28:168–174. doi: 10.1055/s-2007-973695. [DOI] [PubMed] [Google Scholar]