Abstract
The transport of fluid, nutrients and electrolytes to and from the intestinal lumen is a primary function of epithelial cells. Normally, the intestine absorbs approximately 9 l of fluid and 1 kg of nutrients daily, driven by epithelial transport processes that consume large amounts of cellular energy and O2. The epithelium exists at the interface of the richly vascularised mucosa, and the anoxic luminal environment and this steep O2 gradient play a key role in determining the expression pattern of proteins involved in fluid, nutrient and electrolyte transport. However, the dynamic nature of the splanchnic circulation necessitates that the epithelium can evoke co-ordinated responses to fluctuations in O2 availability, which occur either as a part of the normal digestive process or as a consequence of several pathophysiological conditions. While it is known that hypoxia-responsive signals, such as reactive oxygen species, AMP-activated kinase, hypoxia-inducible factors, and prolyl hydroxylases are all important in regulating epithelial responses to altered O2 supply, our understanding of the molecular mechanisms involved is still limited. Here, we aim to review the current literature regarding the role that O2 plays in regulating intestinal transport processes and to highlight areas of research that still need to be addressed.
 |
Stephen Keely received his BSc and PhD degrees from the Department of Pharmacology, University College Dublin, before travelling to the United States to continue his training at the Department of Medicine, University of California, San Diego. He returned to Dublin in 2005 to become the Associate Director of the Molecular Medicine Laboratories at the Royal College of Surgeons in Ireland. His research focuses primarily on the cellular and molecular physiology of intestinal epithelial cells and how these cells can be targeted to treat common intestinal disorders. Joseph Ward obtained his BSc and MSc degrees in biochemistry from the National University of Ireland, Galway. Following this, he worked in diagnostics for 2 years before completing a PhD in physiology at the Royal College of Surgeons in Ireland in 2011. Since then, his work as a postdoctoral researcher has focused on the transport function and immunology of the gastrointestinal epithelium.
Introduction
The entire surface of the gastrointestinal (GI) tract is lined by the epithelium, a single layer of cells that separates us from the harsh and changeable environment of the intestinal lumen. The primary role of the epithelium is to recognise nutrients and other useful substances in the lumen and to transport them into the body, while at the same time excluding potentially harmful substances and acting as a barrier against the entry of pathogens. Under physiological conditions the intestinal epithelium is exposed to one of the highest oxygen gradients in the body. Although the serosa is supplied with amply oxygenated blood by the splanchnic circulation, the epithelium is invariably exposed to the anoxic environment of the lumen (Taylor & Colgan, 2007). Add to this the highly dynamic nature of the splanchnic circulation, and the result is a hugely fluctuating oxygen supply to the intestinal mucosa, which at times may not meet the demands of the tissue, leading to a state of hypoxia. While pathophysiological conditions, such as intestinal inflammation or splanchnic hypoperfusion, are known to be associated with mucosal hypoxia, recent studies highlight that even in normal conditions the intestinal epithelium exists in a low-level state of hypoxia, which has come to be referred to as ‘physiological hypoxia’ (Karhausen et al. 2004; Mastrogiannaki et al. 2009; Colgan & Taylor, 2010). We propose that this state of physiological hypoxia is responsible for homeostatic regulation of intestinal transport. This is achieved by maintaining a state of low energy consumption during fasting conditions, thereby reducing the activity of ATP-dependent transporters, such as Na+/K+-ATPase pumps, which generate the electrochemical gradients that drive the secondary active transport of fluid, nutrients and electrolytes. Conversely, ATP-independent transport remains unaffected and crucial processes, such as iron transport, appear to be facilitated by hypoxia while others, such as glutamine absorption (Kles et al. 2001), appear to be independent of hypoxic regulation. Although O2 supply to the intestine clearly has important implications in physiological regulation of epithelial transport function, there is still a poor understanding of the molecular mechanisms involved. Here, we aim to review the relevant literature regarding the role of O2 in regulating intestinal epithelial transport, and how such processes can become dysregulated in conditions of hypoxic disease.
Intestinal epithelial transport
Two important characteristics of epithelial cells that enable them to transport substances to and from the gut lumen are their ability to form tight junctions with one another and their functional polarity (Keely et al. 2009). Tight junctions are complex structures located towards the apical pole of the cell through which adjacent cells make contact with one another (Shen et al. 2011). Tight junctions also constitute a physical barrier that separates epithelial cells into distinct apical and basolateral domains. It is this functional polarity that enables epithelial cells to differentially express channels, pumps, exchangers and cotransporters in the apical and basolateral domains, thereby enabling the vectorial transport of nutrients, ions and fluid to occur (Barrett & Keely, 2000).
Nutrient transport
Through the actions of proteases and peptidases, proteins are degraded into amino acids and short peptides (2–6 amino acids), which are then absorbed by distinct amino acid and peptide transporters. Di- and tripeptides are absorbed by a single broad-specificity H+-coupled co-transporter, called peptide transporter 1 (PepT1) (Thwaites & Anderson, 2007), while a number of different transport proteins, such as system B(0) neutral amino acid transporter 1 (B0AT1), sodium-dependent neutral amino acid transporter (ATB0) and excitatory amino acid transporter 3, exist for the uptake of neutral, cationic and anionic amino acids (Broer, 2008). A separate set of transporters exists on the basolateral membrane that mediates the export of amino acids into the portal circulation.
Glucose and galactose are the main products of carbohydrate digestion by amylases and membrane-bound saccharidases and these are transported across the apical membrane via the sodium glucose co-transporter (SGLT) 1. The Na+ electrochemical gradient generated by the basolateral Na+/K+-ATPase pump is the key value that drives SGLT1 activity (Wright et al. 2011). Another product of carbohydrate digestion, fructose, is taken up by a Na+-independent mechanism through the glucose transporter (GLUT) 5 and the exit of these monosaccharides across the basolateral membrane is mediated by GLUT2.
Dietary lipids are digested in the stomach and small intestine by lipases to yield fatty acids and 2–monoacylglycerol. These molecules are incorporated into mixed micelles with bile acids, cholesterol, fat-soluble vitamins and phospholipids. Micelles can then diffuse to the epithelium where the fatty acids can enter epithelial cells by passive diffusion or through the activity of specific transporters in the small intestine and colon (Stahl et al. 1999; Sun et al. 2009).
The only mechanism for iron uptake is in the intestine and dysregulated iron homeostasis can lead to conditions of iron deficiency, such as anaemia, or iron overload, such as haemochromatosis, the most common genetic disorder in man. Ferrous iron (Fe2+) is co-transported with H+ across the intestinal epithelium by divalent metal transporter 1 (DMT1). The co-transport with H+ down its electrochemical gradient ensures a concentrative uptake of divalent cations (Gunshin et al. 1997). There can be slippage of the ratio of Fe2+ and H+ uptake by DMT1 at low luminal pH, such that Fe2+ uptake may also cause a mild acidification of the cells requiring efflux of H+ (Mackenzie et al. 2006). Fe2+ is then either stored in the enterocyte bound to ferritin, or transported to the blood via the sole basolateral transporter, ferroportin. DMT1 is also responsible for the transport of an array of other divalent cations such as Zn2+, Co2+ and Cd2+ amongst others (Illing et al. 2012).
Fluid and electrolyte transport
Between digestive secretions and ingested water, approximately 9 l of fluid enters the proximal intestine on a daily basis, and of this only ∼200 ml is lost in the faeces. All of this water movement occurs passively in response to ionic gradients established by active transport processes (Fig. 1). Absorption normally predominates and is driven mainly by the absorption of cations, most notably Na+, with several mechanisms for Na+ absorption being present along the intestine (Keely et al. 2009). In the small intestine, SGLT1 accounts for approximately 6 l of absorbed fluid each day (Wright et al. 2011), while another critical mechanism driving Na+ and fluid absorption in the intestine is that of Na+/H+ exchange (Zachos et al. 2009; Kato & Romero, 2011). Sodium hydrogen exchanger (NHE) 3, the most important NHE for Na+ absorption across the apical membrane in the intestine, extrudes H+ in exchange for Na+ into the cell. The activity of NHE3 is coupled to that of Cl−/HCO3− exchangers in the apical membrane, of which multiple isoforms exist, including down regulated in adenoma (DRA) and proton amino acid transporter 1 (PAT-1) (Soleimani, 2006). These proteins extrude HCO3− in exchange for Cl− and so the net activity of the two transport mechanisms serves to absorb NaCl from the lumen, with H2O following by passive diffusion. A third mechanism for Na+ absorption in the intestine is that of electrogenic Na+ absorption and in humans this process occurs predominantly in the distal colon and rectum through channels in the apical membrane known as epithelial sodium channels (ENaCs) (Kashlan & Kleyman, 2012).
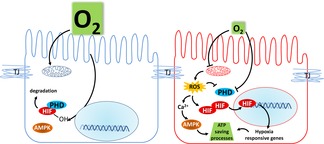
Figure 1. Schematic representation of intestinal absorptive and secretory mechanisms.
A, transport processes occur in a spatially distinct manner along the crypt–villus axis. While transporters responsible for nutrient and fluid absorption are primarily expressed in villus cells and upper crypt cells, Cl− and fluid secretion occur primarily from the base of the crypt. B: upper panel, intestinal epithelial cell with a complement of transporters involved in nutrient absorption; middle panel, cell expressing transporters responsible for fluid absorption; lower panel, a crypt cell expressing the transport proteins involved in Cl− and fluid secretion. aa, amino acid; VIP, vasoactive intestinal polypeptide. It should be noted that these figures are purely schematic representations, designed to summarise various transport mechanisms that can be expressed in intestinal epithelial cells. They do not take into account that transport proteins are differentially expressed along the crypt villus axis and in different intestinal sections.
The two main anions driving intestinal fluid secretion in the intestine are HCO3− and Cl− and there is an aboral gradient in their relative contributions. In the duodenum, HCO3− secretion predominates in order to afford protection against acid-induced mucosal ulceration (Akiba & Kaunitz, 2011). HCO3− enters duodenocytes through a Na+–HCO3− cotransporter on the basolateral side of the cell, or is generated intracellularly by the activity of carbonic anhydrase, and is secreted apically by predominantly two mechanisms: an electrogenic pathway mediated by cystic fibrosis transmembrane conductance regulator (CFTR) channels, and an electroneutral pathway involving DRA and PAT-1 (Seidler et al. 2011). Cl− enters the cell through the Na+–K+–2Cl− cotransporter (NKCC1) on the basolateral side and exits through channels in the apical membrane. CFTR is the predominant pathway for Cl− exit across the apical membrane but other channels, such as the Ca2+-dependent Cl− channel transmembrane protein 16A (TMEM16A), are also expressed (Barrett & Keely, 2000; Flores et al. 2009).
The balance between absorptive and secretory processes in the intestine is highly dynamic, with absorption normally predominating in order to conserve the large volumes of fluid passing through the intestine each day. However, many pathological conditions exist that can disrupt this finely tuned balance so that there is an overexpression of secretion, an underexpression of absorption, or vice versa, leading to the clinical manifestation of diarrhoea or constipation.
O2 and the Na+/K+-ATPase
One protein that is common to all of the transport processes described above is the Na+/K+-ATPase pump. This protein can be considered as the ‘engine’ that drives all epithelial transport mechanisms. When one considers that the intestinal epithelium covers an area equivalent to a tennis court and that it must actively pull 9 l of fluid and approximately 1 kg of nutrients from the lumen each day, one can get an appreciation of the energy requirement for the Na+/K+-ATPase to perform its functions. Thus, transepithelial transport processes are critically dependent on the availability of O2 to generate ATP by oxidative phosphorylation. Hence, there is an increase in blood flow through the splanchnic circulation after ingestion of a meal, when energy requirements to drive epithelial transport processes are at their highest (Nowicki & Granger, 2009). Conversely, in the fasting state, energy requirements of the epithelium are at their lowest and mucosal blood flow is diminished. With this in mind, it is not hard to envision how disruptions in mucosal blood flow, and O2 supply, can have drastic consequences for the ability of the intestine to appropriately handle fluid, electrolytes and nutrients.
While aerobic conditions facilitate ATP generation by oxidative phosphorylation, hypoxic conditions result in considerably less ATP generation and this is exacerbated by the reversal of ATP synthase activity in order to maintain mitochondrial membrane potential (Jennings et al. 1991; Fig. 2). Thus, as a consequence of the diminished supply of molecular energy in hypoxia, cells must regulate their metabolism accordingly. Consequently, Na+/K+-ATPase activity is reduced and non-essential transport processes are shut down.
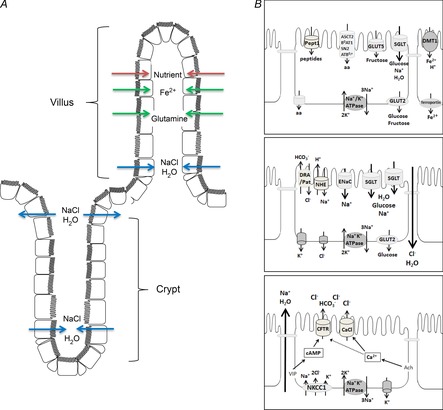
Figure 2. ATP generation in normoxic and hypoxic conditions.
When oxygen supply is sufficient to meet cellular demands, pyruvate formed by glycolysis enters the tricarboxylic acid (TCA) cycle which yields 2 molecules of ATP and NADH. NADH is then used in the process of oxidative phosphorylation, yielding 32 molecules of ATP. In normoxia there is sufficient ATP available to drive Na+/K+-ATPase activity. However, in hypoxic conditions lower levels of ATP production result in reduced Na+/K+-ATPase activity. Arrows in green only occur in normoxic conditions.
Physiology and pathophysiology of splanchnic circulation
The splanchnic circulation perfuses the organs of the GI tract and stems from the anterior aspect of the abdominal aorta where it diverges into three main arteries, the coeliac, superior mesenteric and inferior mesenteric arteries, that together perfuse the liver, stomach, pancreas and intestines (Takala, 1996; Green & Tendler, 2005; Mensink et al. 2011; van Wijck et al. 2012). The intestines are supported by a vast network of interconnected collateral blood vessels within the splanchnic circulation, which imparts redundancy and thus protection from vascular occlusion and ischaemic insults (Mensink et al. 2011). However, a more global reduction in splanchnic flow can be caused by events ranging from strenuous exercise to superior mesenteric artery embolism (Alhan et al. 2012). Disruptions in flow can result in intestinal ischaemia and hypoxia, with sometimes fatal outcomes resulting from intestinal gangrene, sepsis and multi-organ failure (Wyers, 2010). Such is the case in acute mesenteric ischaemia (AMI) where mortality rates are as high as 71% (McKinsey & Gewertz, 1997; Brandt & Boley, 2000). Since, under normal circumstances the colonic epithelium already exists in a state of physiological hypoxia, such global reductions in splanchnic perfusion pose a significant threat to intestinal epithelial barrier integrity and transport function (Karhausen et al. 2004; Mastrogiannaki et al. 2009; Colgan & Taylor, 2010).
Physiological regulation
At rest, the intestines are supplied by approximately 20% of the total cardiac output, from which they consume 10–20% of the available oxygen (Rowell et al. 1964; ter Steege & Kolkman, 2012). In healthy individuals the volume of the splanchnic circulation changes throughout the day depending on various factors, most notably, feeding. It is well established that GI blood flow increases after meals, a phenomenon known as postprandial hyperaemia which has two phases. Firstly, the anticipatory phase occurs prior to ingestion and is regulated by the sympathetic nervous system (Vatner et al. 1970). The anticipatory phase is transient, lasting up to 30 min and causes only minor changes in GI vascular resistance (Vatner et al. 1970; Nowicki & Granger, 2009). On the other hand, the digestive/absorptive phase is characterised by GI hyperaemia that progresses along the GI tract in association with the aboral movement of food (Nowicki & Granger, 2009). Mesenteric blood flow increases rapidly within 5–15 min of ingestion, reaching a maximum level between 30 and 90 min, and returning to pre-fed states within several hours. The duration of postprandial hyperaemia depends on the food type ingested (Vatner et al. 1970; Gallavan et al. 1980; Qamar & Read, 1988; Sieber et al. 1991; Sidery et al. 1994; Kozar et al. 2002a; Mensink et al. 2011). Increased blood flow to the proximal intestine precedes that of the ileum, whereas colonic postprandial hyperaemia does not appear to occur (Bond et al. 1979; Gallavan et al. 1980). The biochemical stimuli that induce postprandial hyperaemia are not well understood, but are known to involve several factors that act in an orchestrated, region-specific, and synergistic fashion. Constituents of chyme, such as luminal bile, are known to be potent vasodilators and play a key role in intestinal hyperaemia. While bile alone can stimulate ileal hyperaemia without having direct vasoactive effects in the jejunum, at concentrations of up to 33% of gallbladder bile, it also has the ability to render food molecules vasoactive. For example, at normal postprandial luminal concentrations in the proximal bowel, bile can render long chain fatty acids and amino acids vasoactive and it can potentiate the vasoactive effects of luminal glucose (Chou et al. 1978; Kvietys et al. 1980, 1981). The mechanisms of tissue hyperaemia induced by bile are poorly understood but with respect to fatty acids they are thought to be due to increased bioavailability as a consequence of their solubilisation in the aqueous phase (Gallavan et al. 1985). Other studies have shown that intra-ileal bile administration causes an increase in plasma vasoactive intestinal polypeptide (VIP), an effect that can be blocked by atropine and is thus suggestive of either an indirect neuronally mediated effect or a direct effect of bile on muscarinic receptors (Chijiiwa et al. 1986). Tissue metabolic activity also plays an important role in regulating postprandial vasoactivity. Postprandial hyperaemia is accompanied by enhanced intestinal O2 uptake (Madsen et al. 2006), presumably to fuel the increased activity of processes involved in digestion and absorption. In fact, nutrient absorption from the colon has been shown to increase O2 uptake in a manner that is mediated entirely by enhanced O2 extraction from the blood, as opposed to increased O2 supply (Kvietys & Granger, 1981). Such increases in O2 extraction from the available blood supply can be achieved by increasing capillary density and modulation of precapillary sphincters, events that have been postulated to occur postprandially (Shepherd, 1982a, b1982b).
Other factors can also affect splanchnic perfusion, such as exercise (Qamar & Read, 1987; Perko et al. 1998), stress (Veenstra et al. 2007), medication (Stadeager et al. 1989) and recreational drug abuse (Elramah et al. 2012). For example during exercise, splanchnic vasoconstriction rapidly redistributes blood to where it is required in the lungs, muscle and skin (Perko et al. 1998; van Wijck et al. 2012), with splanchnic blood flow dropping to as low as 4% of cardiac output (Rowell et al. 1964). Although the intestines are able to utilise oxygen more efficiently during hypoperfusion, strenuous exercise has been associated with gut dysfunction in healthy individuals (van Wijck et al. 2011, 2012; ter Steege & Kolkman, 2012). It should also be noted that the intestinal villi are more susceptible than crypts to alterations in splanchnic perfusion. Villus atrophy can ensue with a concomitant reduction in the absorptive function of the gut (Niinikoski et al. 2004).
Pathophysiological regulation
Regulation of the splanchnic circulation is highly dynamic and it can become disrupted in many pathophysiological conditions, such as AMI, chronic mesenteric ischaemia, colonic ischaemia, necrotising enterocolitis (NEC), hypertension (King et al. 2007; Osborn et al. 2011), vasculitis (Geboes & Dalle, 2002), thrombosis (De Stefano & Martinelli, 2010), and surgical procedures, such as cardiopulmonary bypass surgery (Wilmore et al. 1988). Pathophysiological reductions in intestinal perfusion lead to ischaemia, the most common form of which is ischaemic colitis (IC), which is reported to be responsible for 50–60% of all GI ischaemic episodes (Brandt & Boley, 2000; Green & Tendler, 2005; Zou et al. 2009). IC is most frequent in the elderly due to low flow states associated with cardiac dysfunction or hypovolaemia, but is also evident when other risk factors are present, such as the use of medications (Seon et al. 2011; Ajani et al. 2012) and recreational drugs, such as cocaine (Elramah et al. 2012). A deficit in O2 availability is thought to be a major risk factor in the development of NEC, which is the most frequent life-threatening GI emergency of neonatal intensive care units (Kafetzis et al. 2003; Hunter et al. 2008). The diminished O2 availability associated with NEC is thought to arise from both splanchnic hypoperfusion and increased intestinal epithelial metabolic demand.
While it is clear that dramatic alterations in the flow of the splanchnic circulation can occur under both physiological and pathophysiological circumstances, there are also mechanisms in place to prevent the occurrence of mucosal hypoxia. For example, there is considerable redundancy in the vascular network, which helps to prevent the loss of mucosal perfusion when blood vessels become occluded. Furthermore, in order to cope with the dynamic nature of the splanchnic circulation, cells and tissues in the intestine have evolved mechanisms that enable them to survive under hypoxic conditions. This ability for adaptation to fluctuating levels of available O2 is facilitated by a complex interplay of intracellular signalling pathways and intricate molecular interactions.
Epithelial responses to hypoxia
Hypoxia is defined as a state of cellular oxygen deprivation whereby the O2 requirements of a cell exceed that of available O2. Cellular energy status is a key factor in cell survival and metabolic stresses, such as hypoxia, which deplete intracellular ATP trigger adaptive responses (Laderoute et al. 2006). Epithelial responses to oxidative stress elicited by hypoxia can have biochemical consequences, including generation of reactive oxygen species (ROS), or can induce compensatory protective mechanisms, such as activation of AMP-activated protein kinase (AMPK) 1, inhibition of hypoxia-inducible factor (HIF) prolyl hydroxylases (PHDs) and activation of the master regulators of transcription, HIF and nuclear factor κB (NFκB) (Fig. 3).
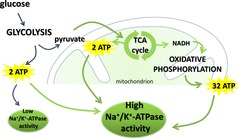
Figure 3. Effects of hypoxia on epithelial signalling pathways.
In conditions where oxygen supply meets demand, PHDs can hydroxylate target proteins, such as HIF-α, which is subsequently targeted for proteasomal degradation. However, in hypoxic conditions, insufficient oxygen availability prevents PHDs from hydroxylating HIFα, leading to its stabilisation, nuclear translocation, dimerisation with HIFβ and ultimately, the transcription of HIF-responsive genes. Hypoxia also induces mitochondrial ROS production which can regulate transport protein expression and activity in several ways, including elevation of intracellular Ca2+ and AMPK activation. TJ, tight junction.
Biochemical responses to hypoxia
One of the most rapid cellular responses to hypoxia is the generation of ROS due to the incomplete reduction of O2 at the Q0 site of complex III within mitochondria (Bell et al. 2007). ROS induces elevations in intracellular Ca2+, which in turn lead to the activation of AMPK (Mungai et al. 2011). ROS also activates other pathways associated with hypoxia, such as those involved in promoting energy and redox homeostasis and enhancing cellular survival (Liu et al. 2008).
Compensatory responses to hypoxia
The key role of AMPK is to act as a cellular energy sensor and in conditions of hypoxia it becomes rapidly activated due to increases in cellular levels of AMP, oxidants and ROS. ROS exerts its actions through increases in intracellular Ca2+ levels, and activation of Ca2+ release-activated Ca2+ channels, with subsequent activation of the upstream Ca2+-dependent regulator of AMPK, Ca2+–calmodulin-dependent kinase kinase (CaMKK) β (Dyck et al. 1996; Mungai et al. 2011). Upon its activation, AMPK induces a cellular switch from ATP-consuming anabolic pathways to ATP-generating catabolic pathways as an efficient means to conserve cellular ATP at times of cellular stress (Mungai et al. 2011; S. Wang et al. 2012).
Another important consequence of cellular hypoxia is that it leads to the induction of an array of genes that are pivotal to the ability of cells to survive and function in low O2 environments (Ratcliffe et al. 1998; Semenza, 1998). Many of these genes are regulated by the HIF proteins (G. L. Wang et al. 1995; G. L. Wang & Semenza, 1995). Known for their importance in carcinogenesis (Semenza, 2000), HIFs also have protective roles to play against inflammatory diseases (Semenza et al. 2000). Under conditions of normal cellular O2 supply, HIF levels are constitutively suppressed by proteasomal degradation. The initiating step in this process is the iron-dependent proline hydroxylation of HIF-1α (Huang et al. 1998) by the HIF PHDs (Epstein et al. 2001). This then mediates the association of the von Hipple–Lindau E3 ligase complex, culminating in the proteasomal degradation of HIF-α (Epstein et al. 2001; Jaakkola et al. 2001). However, when cellular O2 is depleted, PHDs become inactive with a consequent rise in cytosolic HIF-α levels. Hypoxic stabilisation of HIF-α in this way initiates a multi-step pathway of activation that includes nuclear translocation and dimerisation with HIF-β subunits. The HIF complex then interacts with cognate hypoxia-response elements on the promoters of target genes, to induce transcriptional responses (Semenza, 2001).
Interestingly, there is also a complex interplay between HIF and other mediators of the hypoxic responses that allow for fine-tuning of cellular responses to reduced O2 availability. Thus, while PHDs are known to be responsible for stabilisation of cytosolic HIF levels, they have also been implicated in regulation of AMPK activity (Yan et al. 2012). Furthermore, AMPK is essential for the transcriptional activity of HIF and for HIF-induced responses to occur (Lee et al. 2003). PHDs have also been shown to be important in regulating cellular expression of NFκB. During hypoxia, NFκB becomes activated and this is due to an inability of PHDs to hydroxylate and inhibit the activity of IκB kinase, an activating kinase of NFκB. Given that NFκB is a master regulator of epithelial responses to inflammation, it is not surprising that its activation in conditions of hypoxia has important consequences for intestinal barrier and transport function.
Regulation of epithelial transport processes in hypoxia
Given the adaptable nature of intestinal epithelial cells and their inherent ability to evoke homeostatic responses to changes in their environment, it is not surprising that alterations in O2 supply induce dramatic changes in transport function. Indeed, it is well documented that intestinal disorders that cause hypoxia are often associated with the onset of diarrhoea. At their most basic level, changes in intestinal fluid movement occur either as a consequence of a loss of epithelial barrier function or changes in transepithelial osmotic gradients. Reduced absorption (Kles & Tappenden, 2002) and/or increased Cl− secretion (Matthews et al. 1995) is disturbed and as a consequence can result in a net efflux of fluid as seen in diarrhoea. Likewise, any change in motility of the GI tract causing a reduction in transit time and subsequent reduction in the intestinal epithelial capacity for absorption could also result in diarrhoea (Spiller, 2006). The effects of hypoxia on intestinal epithelial barrier function are complex, depend on the duration of exposure to hypoxia, and are mediated by numerous factors. Loss of epithelial barrier function during hypoxia, such as that of tight junction dysregulation (Matthews et al. 1994), is also an important cause of diarrhoea and can be exacerbated by subsequent inflammatory responses (Binder, 2009). While the actions of hypoxia on epithelial barrier function have been reviewed elsewhere (Karhausen et al. 2003; Glover & Colgan, 2011), here we focus on its consequences for epithelial transport function.
Impact of hypoxia on Na+/K+-ATPase function
Many of the transporting functions of the intestinal epithelium are highly energy-demanding, none more so than that of the Na+/K+-ATPase pump. The oxygen demand of the Na+/K+-ATPase is such that in the sigmoid colon, it is reported to consume 26% of available oxygen under basal conditions (Carra et al. 2011), whereas it can demand 20% of available ATP in resting duodenum (Milligan & McBride, 1985). Despite the fact that the pump is the primary driving force for intestinal epithelial transport processes, there is still little known of its regulation in conditions associated with intestinal hypoxia. However, studies in a variety of models have shown that Na+/K+-ATPase activity becomes downregulated when O2 supply is reduced. For example, studies in rats have shown that fasting for 48 h is associated with a 33% reduction in jejunal Na+/K+-ATPase activity, with activity of the pump increasing when feeding is restored (Murray & Wild, 1980). This is likely to be an important regulatory mechanism under physiological circumstances that prevents excessive consumption of cellular energy by the pump in the interdigestive period when intestinal blood supply is at its lowest (Fronek & Stahlgren, 1968). Similarly, pathological conditions associated with mucosal hypoxia are also associated with decreased Na+/K+-ATPase activity (Berant et al. 1986; DuVall et al. 1998; Barrett & Keely, 2000). In this context, inhibition of pump activity might represent a homeostatic response whereby cellular energy is directed away from transport processes to those that are more important for cell survival.
The molecular mechanisms by which cellular hypoxia regulates intestinal epithelial Na+/K+-ATPase activity are poorly defined. Studies in animal models and cultured colonic epithelia suggest that the generation of ROS with subsequent decreases in cellular levels of ATP or downregulation in expression of the catalytic α subunit of the pump may be involved (DuVall et al. 1998; Orsenigo et al. 2007). Mechanisms by which ROS inhibit Na+/K+-ATPase activity in the intestine have not been defined but studies from airway epithelial cells suggest that AMPK may have a role to play (Gusarova et al. 2009). In these cells, ROS produced during hypoxia activate a signalling cascade involving CaMKKβ, AMPK1 and protein kinase C ζ (Gusarova et al. 2009), which then phosphorylates Na+/K+-ATPase α1 causing its clathrin-dependent endocytosis (Dada et al. 2003; Chen et al. 2006). However, it remains to be determined if intestinal epithelial Na+/K+-ATPase pumps are also regulated in such a fashion.
Studies of a potential role for HIF in regulating intestinal epithelial Na+/K+-ATPase activity are also lacking, although studies from other systems suggest it may have a role to play in endocytosis and degradation of the pump under hypoxic conditions (Comellas et al. 2006; Zhou et al. 2008). Interestingly, studies from our own laboratory have shown that pharmacological inhibition of HIF PHDs reduces Na+/K+-ATPase activity in colonic epithelial cells (Ward et al. 2011). However, in this case the response did not involve alterations in the expression or cellular location of the pump. These experiments suggest that while PHD-dependent activation of HIF may be sufficient to inhibit Na+/K+-ATPase activity, additional hypoxia-induced signals are necessary for internalisation and degradation of the pump.
Overall, while it seems clear that hypoxic signals downregulate epithelial Na+/K+-ATPase activity in the intestine, there is still much work to be done to elucidate the molecular mechanisms involved. Nevertheless, given its central role as the driving force for intestinal transport, such negative effects on pump activity would be expected to have significant consequences for fluid and nutrient movement in conditions of both health and disease.
Impact of hypoxia on fluid and electrolyte transport
As discussed above, fluid movement in the intestine is driven by osmotic gradients, which are in turn established by active transepithelial ion transport. Fluid secretion into the lumen is primarily driven by the secretion of Cl− ions and studies from several models have shown that this process is attenuated in conditions of hypoxia (Taylor et al. 1998; Ibla et al. 2006; Thwaites & Anderson, 2007; Keely et al. 2009; Sun et al. 2009; Zheng et al. 2009). While inhibition of Na+/K+-ATPase pump activity is likely to play an important role in this effect, several studies indicate that other transport proteins that comprise the secretory pathway are also affected. For example, hypoxia has been shown to inhibit colonic epithelial CFTR (Zheng et al. 2009) and NKCC1 function (Ibla et al. 2006), thereby preventing both the entry and exit of Cl− ions from epithelial cells. Both of these effects appear to be mediated by HIF-dependent transcriptional repression of the transport proteins. However, it should be noted that our studies using the HIF PHD inhibitor dimethyloxalylglycine revealed no effects on the activity of CFTR in colonic epithelial cells (Ward et al. 2011), suggesting that while HIF-1 is required for hypoxia-induced downregulation of the channel, other hydroxylase-independent signals are also likely to be involved. Interestingly, studies from rat small intestine and cultured colonic epithelia suggest that cAMP-dependent secretory responses are more sensitive to downregulation by hypoxia than those stimulated by Ca2+-dependent agonists, although the physiological/pathophysiological significance of this remains to be defined (Taylor et al. 1998; Thwaites & Anderson, 2007).
In addition to transcriptional regulation by HIF-1, it is likely that ROS and AMPK also have important roles to play in mediating the effects of hypoxia on intestinal secretion. In support of this idea, many previous studies have established ROS as important regulators of epithelial Cl− secretion. While exposure of voltage-clamped colonic epithelial cells to ROS can rapidly and transiently induce Cl− secretion, their more long-term (>30 min) actions appear to be to render the epithelium refractory to subsequent stimulation with secretagogues (DuVall et al. 1998). The antisecretory effects of ROS appear to be mediated by inhibition of the activity of multiple transport proteins (DuVall et al. 1998; Chappell et al. 2008). It is interesting to note that, similar to hypoxia, responses to cAMP-dependent secretagogues appear to be more sensitive to ROS than those stimulated by Ca2+-dependent agonists. Several studies suggest that such selectivity may be conferred through activation of AMPK, which has been shown to function downstream of ROS generation. Activation of AMPK exerts antisecretory effects on intestinal epithelial cells, at least in part, by directly phosphorylating and inhibiting CFTR channels (Hallows et al. 2000; Kongsuphol et al. 2009a; King et al. 2012), thereby attenuating cAMP-dependent Cl− secretory responses (Hallows et al. 2003; Walker et al. 2003; Kongsuphol et al. 2009b). Furthermore, hypoxia-induced inhibition of Cl− secretion in human colon can be reversed by AMPK inhibitors (Collins et al. 2011). Whether AMPK regulates other components of the secretory pathway in intestinal epithelia is not known, although studies from airway and renal epithelia suggest it can regulate the activity of the calcium-activated K+ channel KCNN4, and the voltage-gated K+ channel KCNQ1 (Alzamora et al. 2010).
In addition to the direct mechanisms described above, intestinal epithelial secretory function could also be regulated by hypoxia in a more indirect fashion. For example, a recent study has shown that hypoxia and ischaemia–reperfusion (I/R) induce apoptosis of intestinal Paneth cells, important producers of anti-microbial defensins (Grootjans et al. 2011; van Wijck et al. 2012). Defensins have the ability to form apical Cl− conductive channels in colonic epithelial cells (Lencer et al. 1997; Kunzelmann & Mall, 2002), while also being capable of regulating cholinergic responses in the colon (Himmerkus et al. 2010). Thus, the possibility that hypoxia-induced reductions in defensin production may have an indirect role in regulating intestinal secretory function warrants further investigation.
While Cl− secretion is the primary driving force for fluid secretion in the distal intestine, in the proximal small intestine, HCO3− is the main driving force for secretion. This process is necessary for alkalisation of the duodenal mucosa and protection against acid from the stomach. Similar to Cl− secretion, this process also appears to be dependent on cellular O2 availability, since it has been shown that decreased oxygenation of human duodenal biopsies in vitro significantly inhibits HCO3− secretion (Pratha et al. 1998). Such an effect probably explains the propensity of patients with mesenteric ischaemia to develop ulceration of the duodenal mucosa (Gomez-Rubio et al. 1995). However, there is a disconnect between the production and the secretion of HCO3−, since carbonic anhydrase IX is expressed in the GI tract and is highly inducible by hypoxia (Wykoff et al. 2000). Molecular mechanisms underlying attenuated duodenal HCO3− secretion under conditions of low O2 availability remain to be determined, but could conceivably involve similar pathways to those described above for hypoxic regulation of CFTR activity, since this channel is a primary exit pathway for HCO3− in epithelial cells (Clarke & Harline, 1998).
As described earlier, fluid absorption in the intestine is driven by electrogenic and electroneutral Na+ absorption, through SGLT1, ENaCs and NHEs. Although early studies in rat ileum in vitro showed that reduced O2 supply inhibits intestinal salt and water absorption (Curran, 1960), how absorptive processes are regulated under conditions of physiological or pathophysiological hypoxia have since received scant research attention. One study shows that Na+ absorption is decreased after 2 h of hypoxia in rat jejunum (Berant et al. 1986), while another shows that jejunal SGLT1 activity is attenuated by a mechanism involving altered membrane trafficking of the protein (Kles & Tappenden, 2002). Other studies show that hypoxia-induced signalling intermediates can also attenuate intestinal absorptive function. For example, ROS generated by H2O2 treatment of Caco-2 colonic epithelial cells, inhibits Cl−/OH− exchange, a component of the electroneutral absorptive pathway (Saksena et al. 2008). Some additional clues may come from studies of hypoxia, and hypoxia-induced signalling mechanisms, in other tissues. For example, hypoxia and AMPK activation have been shown to downregulate ENaC activity in airway epithelia by mechanisms that are independent of changes in gene expression (Carattino et al. 2005; Bhalla et al. 2006), while generation of ROS has been reported to upregulate ENaC activity in airway and renal epithelia (Ma, 2011; Downs et al. 2013). Whether such effects occur in the hypoxic intestine is not yet known and is an area requiring a great deal of study in the future.
Finally, when considering the impact of compromised O2 availability on epithelial fluid and electrolyte transport, one must also consider the potential contribution of altered transport protein activity to the propagation of cellular responses to hypoxia. For example, it has been recently shown in renal epithelial cells that CFTR regulates HIF expression. Under hypoxic conditions, CFTR facilitates the exit of glutathione, a ROS scavenger, from the cell, thus leading to an accumulation of ROS within the cell (Duranton et al. 2012). ROS has been shown to cause stabilisation of HIF-1α expression through transcriptional and translational mechanisms that are activated via phosphorylation of p70S6K, 4E-BP1 and eIF-4E, in addition to reducing its degradation by preventing its association with pVHL (Sasabe et al. 2010). Since HIF can, in turn, regulate the expression of CFTR, this mechanism may constitute a feedback loop, enabling fine-tuning of hypoxic and transport responses within epithelial cells. Again, whether such mechanisms are present in the intestine remains to be determined.
In summary, it is apparent that a primary response of intestinal epithelial cells to hypoxic stress is to downregulate the energy-consuming activities of transport proteins that control fluid and electrolyte movement to and from the gut. While such a response may allow the cells to redirect available cellular energy to survival processes, inhibition of absorptive function is likely to be a major contributing factor to the diarrhoea that is associated with conditions of mucosal hypoxia. On the other hand, it has been proposed that inhibition of Cl− secretion under such conditions may be a beneficial protective mechanism that prevents excessive fluid loss from the intestine.
Impact of hypoxia on nutrient absorption
Decreased O2 supply to the intestinal epithelium also impacts its capacity to absorb nutrients. However, in contrast to most other transport processes which are suppressed by hypoxia, nutrient absorption persists in order to maintain barrier integrity. The physiology of nutrient absorption under hypoxic conditions has not been comprehensively studied, but there does appear to be cross talk between regulatory processes during normoxic and hypoxic states.
During hypoxic episodes, generation of ATP switches from the highly efficient process of oxidative phosphorylation, yielding 32 ATP per glucose molecule, to that of the poorly efficient process of glycolysis which yields just 2 ATP per glucose molecule. To facilitate further glucose uptake to meet cellular energy demand during hypoxia, HIF-dependent generation of transporters involved in glucose uptake and glycolysis occurs. Regulation of intestinal SGLT1 in response to hypoxia is likely to be complex, with post-translational regulation of SGLT1 in jejunal epithelial cells causing its rapid endocytosis within 1 h from the plasma membrane, coinciding with a reduction in glucose uptake (Kles & Tappenden, 2002).
Investigations of the effects of AMPK activation on sugar transporters in rat small intestine show that both GLUT2 and GLUT5 are inserted into the intestinal epithelial brush border membrane leading to enhanced galactose and fructose transport, respectively (Sakar et al. 2009). Studies of AMPK in mice have shown that, within 20 min of its activation, AMPK increases jejunal GLUT2-mediated glucose absorption, a facilitative transport process that does not require ATP (Walker et al. 2005), while on the other hand reducing the activity and expression of the ATP-dependent transporter SGLT1. Again, these data suggest that in conditions where O2 and ATP availability are limited, the intestinal epithelium undergoes adaptation so that it favours energy-independent mechanisms of sugar transport. Studies from normoxic gut show that GLUT2 can partially compensate for a lack of SGLT1-mediated glucose absorption (Chaudhry et al. 2012). However, despite this compensatory mechanism, higher luminal glucose concentrations would be likely to enter the colon and alter microbial metabolism during hypoxia. Studies in rats and humans have found that in the presence of increased colonic glucose, resident bacteria anaerobically metabolise the glucose to short-chain fatty acids, which are then absorbed and oxidised by the host (Bond & Levitt, 1976). Such an effect would be expected to protect the host from the onset of diarrhoea by preventing free luminal glucose from creating a massive osmotic gradient.
While maintenance of glucose absorption is an important facet of hypoxic responses, so too is that of amino acid absorption. The activity and expression of amino acid transporters has been shown to be regulated under conditions of hypoxia and oxidative stress in several systems, including intestinal epithelial cells (Wasa et al. 2004; Huang et al. 2008). Inhibition of glutamine transport in intestinal epithelial cells under ischaemic conditions appears to involve downregulation of sodium-dependent neutral amino acid transporter type 2 (ASCT2) expression at the level of gene transcription (Huang et al. 2008). Reports have also shown that the apical abundance and activity of the intestinal epithelial peptide transporter PepT1 is attenuated by AMPK activation. Interestingly, although PepT1 activity is usually coupled to that of NHE3 in the intestine, AMPK was not found to alter NHE3 activity in these studies (Pieri et al. 2010). This suggests that an uncoupling of ion absorption from nutrient absorption may occur under conditions of hypoxia.
Glutamine, a primary metabolic fuel of enterocytes, has long been known to exert protective effects on the intestinal mucosa and its levels become depleted during periods of prolonged stress and intestinal hypoperfusion (Plauth et al. 1999; Ban & Kozar, 2010). The amount of energy glutamine provides to epithelial cells is similar to that provided by glucose (Mastrogiannaki et al. 2012) and is readily metabolised, whereas other amino acids, such as the non-metabolisable arginine can exacerbate intestinal hypoxic injury (Kozar et al. 2004b). Cell culture models have shown the glutamine transporters ASCT2 and B (ATB) to be negatively regulated by hypoxia and ischaemia (Wasa et al. 2004; Huang et al. 2008). However, this is in contrast to in vivo models showing intestinal absorption of glutamine to be unaffected by hypoxia (Kles & Tappenden, 2002; Li et al. 2008; Saha et al. 2012). Protective effects of luminal glutamine have also been demonstrated in intestinal I/R injury models (Kozar et al. 2002b, 2004a) through induction of anti-inflammatory molecules, such as peroxisome proliferator-activated receptor γ (PPARγ) (Ban & Kozar, 2008), and by inhibiting apoptosis (Ban & Kozar, 2010), effects that are likely to be mediated by HIF-2. HIF-2α has been shown to upregulate the metabolism of glutamine in a c-Myc-dependent manner (Gordan et al. 2007; Gao et al. 2009). Despite many other ATP-dependent transport processes being suppressed during hypoxic episodes, the intestinal absorption of glutamine, for the most part, appears to be maintained and is facilitated by several transporters. Together, these studies indicate that the hypoxic intestine preferentially utilises readily metabolisable amino acids, such as glutamine, to provide energy and to protect barrier function. The apparent discrepancies that have been observed between in vitro and in vivo models may be due to the simplicity of in vitro models compared to the more adaptive nature of whole intestinal tissues (Saha et al. 2012).
Another source of energy particularly important to the colonic epithelium is fatty acids. Following a hypoxic switch to glycolytic metabolism, the energy required to convert fatty acids to triglycerides and glycerol would increase thus imparting a higher energy burden on the enterocyte. The monocarboxylate or short-chain fatty acid, butyrate, is produced by bacteria in the colonic lumen and is transported into the epithelium via the monocarboxylate transporters MCT1 and MCT4 (Goncalves et al. 2011; Kekuda et al. 2013). MCT4 is perhaps better known for mediating efflux of lactate from glycolytic cells, but it has also been shown to be transcriptionally promoted by HIF-1 under hypoxic conditions (Ullah et al. 2006). Conversely, other reports have shown that under such conditions, butyrate suppresses HIF-1 transcriptional activity in what may constitute a negative feedback loop (Miki et al. 2004). Furthermore, AMPK may also have the capacity to alter the abundance of MCT1 and 4 mRNA (Takimoto et al. 2013); however, to date no studies have been published of such effects in intestinal epithelia.
The intestinal epithelium is the only site of iron uptake into the body and transporters involved in iron homeostasis are known to be regulated by hypoxia. In fact, the activity of iron transport proteins has been shown to be dependent on the activity of HIFs (Shah et al. 2009; Romney et al. 2011). In an elegant study employing a conditional knockdown of HIF-2α in murine intestinal epithelium it was demonstrated that lack of HIF activity resulted in impaired iron uptake and subsequent iron deficiency (Mastrogiannaki et al. 2009). Conversely, a model of haemochromatosis employing hepcidin knockout mice has shown that HIF-2 ablation in the enterocytes decreases the severity of tissue iron loading (Mastrogiannaki et al. 2012). Using the Caenorhabditis elegans model, under conditions of either iron depletion or hypoxia, it has been shown that the intracellular iron-sequestering protein ferritin is transcriptionally repressed, thus releasing intracellular Fe2+. Furthermore, the expression of both the apical iron transporter DMT1 (Romney et al. 2011) and the basolateral transporter, ferroportin, are rapidly increased through HIF-dependent transcriptional pathways (Taylor et al. 2011). Thus, physiological hypoxia appears to be an important factor in the regulation of iron homeostasis.
In summary, exposure of epithelial cells to hypoxia triggers rapid adaptive responses that diminish the activity of many ATP-consuming transporters, while maintaining the activity of ATP-independent transporters, such as the GLUTs. Many of these rapid responses persist until O2 levels normalise. However, additional signalling pathways, many of which involve HIF-mediated suppression of transporter expression and activity, can also be recruited to mediate additional adaptive responses (Table 1).
Table 1.
Effects of hypoxia on epithelial transport protein activity
| Substrate | Transporter | Response to hypoxia | Mechanisms involved | Effect on protein | Source |
|---|---|---|---|---|---|
| Cl− | CFTR NKCC1 | ↓ ↓ | HIF-1/AMPK HIF-1 | Transcription/phosphorylation Transcription | (Zheng et al. 2009)/(Kongsuphol et al. 2009a) (Ibla et al. 2006) |
| Na+ | ENaC SGLT1 Na+/K+-ATPase NKCC1 | ↓↑ ↓ ↓ ↓ | AMPK Hypoxia ROS/AMPK /PHDs HIF-1 | Endocytosis Endocytosis Endocytosis/endocytosis /activity Transcription | (Carattino et al. 2005; Bhalla et al. 2006) (Kles & Tappenden, 2002) (DuVall et al. 1998; Dada et al. 2003; Orsenigo et al. 2007) (Ibla et al. 2006) |
| K+ | KCNQ1 Na+/K+-ATPase NKCC1 | ↓ ↓ ↓ | AMPK ROS/AMPK /HIF HIF-1 | Ubiquitination Endocytosis/endocytosis /activity Transcription | (Alzamora et al. 2010) (DuVall et al. 1998; Dada et al. 2003; Orsenigo et al. 2007) (Ibla et al. 2006) |
| Fe2+ | DMT1 Ferroportin | ↑ ↑ | HIF/HIF-2 HIF-2 | Transcription/transcription Transcription | (Romney et al. 2011)/(Mastrogiannaki et al. 2009) (Taylor et al. 2011) |
| Glucose | SGLT1 GLUT2 GLUT5 | ↓ ↑ ↑ | Hypoxia Hypoxia Hypoxia | Endocytosis Localisation Localisation | (Kles & Tappenden, 2002) (Sakar et al. 2009) (Sakar et al. 2009) |
| Glutamine | B0AT1 SN2 ASCT2 ATB0 | ↓ ↑ ↓ ↓ | Inflammation Inflammation Ischaemia Ischaemia | Abundance Affinity for substrate mRNA expression mRNA expression | (Saha et al. 2012) (Saha et al. 2012) (Huang et al. 2008) (Wasa et al. 2004) |
| Peptides | PepT1 | ↓ | AMPK | Localisation | (Pieri et al. 2010) |
| Water | SGLT1 Aquaporin 4 | ↓ ↑ | Hypoxia Traumatic brain injury | Endocytosis mRNA expression | (Kles & Tappenden, 2002) (Duan et al. 2013) |
Hypoxia differentially affects the activity of proteins involved in the transport of electrolytes, fluid and nutrients. While transport protein activity can be either increased or decreased during periods of hypoxia, in many cases the molecular mechanisms involved are not understood.
Summary and conclusion
There is now considerable evidence in the literature to suggest that fluctuating levels of O2 in the splanchnic circulation play a critical role in regulating intestinal epithelial transport function. In response to altered O2 availability, the epithelium is capable of evoking orchestrated responses which enable it to appropriately direct cellular energy consumption to, or from, costly transport processes. Here, we have highlighted how such dynamic changes in epithelial function are likely to be necessary in order to maintain intestinal homeostasis and to facilitate digestive function under normal conditions. On the other hand, pathophysiological states, such as AMI, IC or NEC, illustrate the detrimental effects that hypoxia can have on epithelial function, leading to dysregulated fluid, nutrient and electrolyte transport, and ultimately to the onset of diarrhoea. However, while it is clear that dramatic alterations in epithelial transport protein activity and expression occur in response to changes in O2 availability, the molecular pathways involved still remain poorly understood. An important focus for future research in intestinal physiology should be on elucidating molecular mechanisms underlying hypoxia-induced regulation of transport protein expression and activity. In particular, given its central role as the driving force for transepithelial transport in the intestine, a greater understanding of how fluctuations in mucosal O2 supply regulate the Na+/K+-ATPase pump is required. This is an area that is ripe for research and future studies investigating transcriptional, post-transcriptional, and post-translational mechanisms by which altered O2 supply regulates epithelial transport function will be of great benefit in developing our understanding of the role of hypoxia in intestinal disease. Another aspect of intestinal biology that is currently receiving a great deal of research interest is that of epithelial/bacterial interactions in regulating intestinal function in health and disease. As a part of this growing field of research, it is important to also develop our understanding of how mucosal hypoxia impacts on the intestinal microbiome and its production of metabolites, such as short-chain fatty acids, that regulate epithelial function. Overall, a more complete understanding of how mucosal O2 supply regulates epithelial transport function will lead to a greater understanding of the pathogenesis of intestinal diseases and to the identification of new targets for their treatment.
Glossary
- AMI
acute mesenteric ischaemia
- AMPK
AMP kinase
- ASCT2
sodium-dependent neutral amino acid transporter type 2
- B0AT1
system B(0) neutral amino acid transporter 1
- CaMKK
calcium–calmodulin-dependent kinase kinase
- CFTR
cystic fibrosis transmembrane conductance regulator
- DMT1
divalent metal transporter 1
- DRA
down regulated in adenoma
- ENaC
epithelial sodium channel
- GI
gastrointestinal
- GLUT
glucose transporter
- HIF
hypoxia-inducible factor
- IC
ischaemic colitis
- I/R
ischaemia–reperfusion
- KCNN4
potassium intermediate/small conductance calcium-activated channel, subfamily N, member 4
- KCNQ1
potassium voltage-gated channel, KQT-like subfamily, member 1
- MCT
monocarboxylate transporter
- NEC
necrotising enterocolitis
- NFκB
nuclear factor κB
- NHE
sodium hydrogen exchanger
- NKCC1
sodium potassium chloride cotransporter 1
- PAT–1
proton amino acid transporter 1
- PepT1
peptide transporter 1
- PHD
prolyl hydroxylase
- ROS
reactive oxygen species
- SGLT1
sodium glucose cotransporter 1
Additional information
Competing interests
The authors have no conflicts of interest to declare.
Funding
The authors are supported by a Science Foundation Ireland Principal Investigator award to S.J.K.
References
- Ajani S, Hurt RT, Teeters DA, Bellmore LR. Ischaemic colitis associated with oral contraceptive and bisacodyl use. BMJ Case Rep. 2012;2012:bcr1220115451. doi: 10.1136/bcr-12-2011-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba Y, Kaunitz JD. Duodenal chemosensing and mucosal defenses. Digestion. 2011;83(Suppl. 1):25–31. doi: 10.1159/000323401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhan E, Usta A, Cekic A, Saglam K, Turkyilmaz S, Cinel A. A study on 107 patients with acute mesenteric ischemia over 30 years. Int J Surg. 2012;10:510–513. doi: 10.1016/j.ijsu.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Alzamora R, Gong F, Rondanino C, Lee JK, Smolak C, Pastor-Soler NM, Hallows KR. AMP-activated protein kinase inhibits KCNQ1 channels through regulation of the ubiquitin ligase Nedd4-2 in renal epithelial cells. Am J Physiol Renal Physiol. 2010;299:F1308–F1319. doi: 10.1152/ajprenal.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban K, Kozar RA. Enteral glutamine: a novel mediator of PPARγ in the postischemic gut. J Leukoc Biol. 2008;84:595–599. doi: 10.1189/jlb.1107764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban K, Kozar RA. Glutamine protects against apoptosis via downregulation of Sp3 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1344–G1353. doi: 10.1152/ajpgi.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berant M, Alon U, Antebi D, Diamond E, Koerner H, Mordechovitz D. Effects of nonischemic hypoxia on jejunal mucosal structure and function: study of an experimental model in dogs. Pediatr Res. 1986;20:1143–1146. doi: 10.1203/00006450-198611000-00016. [DOI] [PubMed] [Google Scholar]
- Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem. 2006;281:26159–26169. doi: 10.1074/jbc.M606045200. [DOI] [PubMed] [Google Scholar]
- Binder HJ. Mechanisms of diarrhea in inflammatory bowel diseases. Ann N Y Acad Sci. 2009;1165:285–293. doi: 10.1111/j.1749-6632.2009.04039.x. [DOI] [PubMed] [Google Scholar]
- Bond JH, Levitt MD. Fate of soluble carbohydrate in the colon of rats and man. J Clin Invest. 1976;57:1158–1164. doi: 10.1172/JCI108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond JH, Prentiss RA, Levitt MD. The effects of feeding on blood flow to the stomach, small bowel, and colon of the conscious dog. J Lab Clin Med. 1979;93:594–599. [PubMed] [Google Scholar]
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954–968. doi: 10.1016/s0016-5085(00)70183-1. [DOI] [PubMed] [Google Scholar]
- Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem. 2005;280:17608–17616. doi: 10.1074/jbc.M501770200. [DOI] [PubMed] [Google Scholar]
- Carra GE, Ibanez JE, Saravi FD. Electrogenic transport, oxygen consumption, and sensitivity to acute hypoxia of human colonic epithelium. Int J Colorectal Dis. 2011;26:1205–1210. doi: 10.1007/s00384-011-1215-7. [DOI] [PubMed] [Google Scholar]
- Chappell AE, Bunz M, Smoll E, Dong H, Lytle C, Barrett KE, McCole DF. Hydrogen peroxide inhibits Ca2+-dependent chloride secretion across colonic epithelial cells via distinct kinase signaling pathways and ion transport proteins. FASEB J. 2008;22:2023–2036. doi: 10.1096/fj.07-099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry RM, Scow JS, Madhavan S, Duenes JA, Sarr MG. Acute enterocyte adaptation to luminal glucose: A posttranslational mechanism for rapid apical recruitment of the transporter GLUT2. J Gastrointest Surg. 2012;16:312–319. doi: 10.1007/s11605-011-1752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Krmar RT, Dada L, Efendiev R, Leibiger IB, Pedemonte CH, Katz AI, Sznajder JI, Bertorello AM. Phosphorylation of adaptor protein-2 μ2 is essential for Na+,K+-ATPase endocytosis in response to either G protein-coupled receptor or reactive oxygen species. Am J Respir Cell Mol Biol. 2006;35:127–132. doi: 10.1165/rcmb.2006-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiiwa Y, Misawa T, Ibayashi H. Evidence of local mechanism involvement in vasoactive intestinal polypeptide release from canine small intestine. Gastroenterology. 1986;90:1877–1881. doi: 10.1016/0016-5085(86)90256-8. [DOI] [PubMed] [Google Scholar]
- Chou CC, Kvietys P, Post J, Sit SP. Constituents of chyme responsible for postprandial intestinal hyperemia. Am J Physiol Heart Circ Physiol. 1978;235:H677–H682. doi: 10.1152/ajpheart.1978.235.6.H677. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol Gastrointest Liver Physiol. 1998;274:G718–G726. doi: 10.1152/ajpgi.1998.274.4.G718. [DOI] [PubMed] [Google Scholar]
- Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Kopic S, Bachlechner J, Ritter M, Winter DC, Geibel JP. Hypoxia inhibits colonic ion transport via activation of AMP kinase. Ann Surg. 2011;254:957–963. doi: 10.1097/SLA.0b013e31821d477f. [DOI] [PubMed] [Google Scholar]
- Comellas AP, Dada LA, Lecuona E, Pesce LM, Chandel NS, Quesada N, Budinger GR, Strous GJ, Ciechanover A, Sznajder JI. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ Res. 2006;98:1314–1322. doi: 10.1161/01.RES.0000222418.99976.1d. [DOI] [PubMed] [Google Scholar]
- Curran PF. Na, Cl, and water transport by rat ileum in vitro. J Gen Physiol. 1960;43:1137–1148. doi: 10.1085/jgp.43.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-ζ. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano V, Martinelli I. Splanchnic vein thrombosis: clinical presentation, risk factors and treatment. Intern Emerg Med. 2010;5:487–494. doi: 10.1007/s11739-010-0413-6. [DOI] [PubMed] [Google Scholar]
- Downs CA, Kumar A, Kreiner LH, Johnson NM, Helms MN. H2O2 regulates lung epithelial sodium channel (ENaC) via ubiquitin-like protein Nedd8. J Biol Chem. 2013;288:8136–8145. doi: 10.1074/jbc.M112.389536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Hao C, Fan Y, Wang H, Liu Y, Hao J, Xu C, Liu X, Zhang H. The role of neuropeptide Y and aquaporin 4 in the pathogenesis of intestinal dysfunction caused by traumatic brain injury. J Surg Res. 2013;184:1006–1012. doi: 10.1016/j.jss.2013.03.096. [DOI] [PubMed] [Google Scholar]
- Duranton C, Rubera I, Cougnon M, Melis N, Chargui A, Mograbi B, Tauc M. CFTR is involved in the fine tuning of intracellular redox status: physiological implications in cystic fibrosis. Am J Pathol. 2012;181:1367–1377. doi: 10.1016/j.ajpath.2012.06.017. [DOI] [PubMed] [Google Scholar]
- DuVall MD, Guo Y, Matalon S. Hydrogen peroxide inhibits cAMP-induced Cl− secretion across colonic epithelial cells. Am J Physiol Cell Physiol. 1998;275:C1313–C1322. doi: 10.1152/ajpcell.1998.275.5.C1313. [DOI] [PubMed] [Google Scholar]
- Dyck JR, Gao G, Widmer J, Stapleton D, Fernandez CS, Kemp BE, Witters LA. Regulation of 5′-AMP-activated protein kinase activity by the noncatalytic β and γ subunits. J Biol Chem. 1996;271:17798–17803. doi: 10.1074/jbc.271.30.17798. [DOI] [PubMed] [Google Scholar]
- Elramah M, Einstein M, Mori N, Vakil N. High mortality of cocaine-related ischemic colitis: a hybrid cohort/case-control study. Gastrointest Endosc. 2012;75:1226–1232. doi: 10.1016/j.gie.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Flores CA, Cid LP, Sepulveda FV, Niemeyer MI. TMEM16 proteins: the long awaited calcium-activated chloride channels. Braz J Med Biol Res. 2009;42:993–1001. doi: 10.1590/s0100-879x2009005000028. [DOI] [PubMed] [Google Scholar]
- Fronek K, Stahlgren LH. Systemic and regional hemodynamic changes during food intake and digestion in nonanesthetized dogs. Circ Res. 1968;23:687–692. doi: 10.1161/01.res.23.6.687. [DOI] [PubMed] [Google Scholar]
- Gallavan RH, Jr, Chen MH, Joffe SN, Jacobson ED. Vasoactive intestinal polypeptide, cholecystokinin, glucagon, and bile-oleate-induced jejunal hyperemia. Am J Physiol Gastrointest Liver Physiol. 1985;248:G208–G215. doi: 10.1152/ajpgi.1985.248.2.G208. [DOI] [PubMed] [Google Scholar]
- Gallavan RH, Jr, Chou CC, Kvietys PR, Sit SP. Regional blood flow during digestion in the conscious dog. Am J Physiol Heart Circ Physiol. 1980;238:H220–H225. doi: 10.1152/ajpheart.1980.238.2.H220. [DOI] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geboes K, Dalle I. Vasculitis and the gastrointestinal tract. Acta Gastroenterol Belg. 2002;65:204–212. [PubMed] [Google Scholar]
- Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140:1748–1755. doi: 10.1053/j.gastro.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rubio M, Opio V, Acin F, Guilleuma J, Moyano E, Garcia J. Chronic mesenteric ischemia: a cause of refractory duodenal ulcer. Am J Med. 1995;98:308–310. doi: 10.1016/S0002-9343(99)80381-7. [DOI] [PubMed] [Google Scholar]
- Goncalves P, Araujo JR, Martel F. Characterization of butyrate uptake by nontransformed intestinal epithelial cell lines. J Membr Biol. 2011;240:35–46. doi: 10.1007/s00232-011-9340-3. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J. 2005;98:217–222. doi: 10.1097/01.SMJ.0000145399.35851.10. [DOI] [PubMed] [Google Scholar]
- Grootjans J, Hodin CM, de Haan JJ, Derikx JP, Rouschop KM, Verheyen FK, van Dam RM, Dejong CH, Buurman WA, Lenaerts K. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology. 2011;140:529–539.e3. doi: 10.1053/j.gastro.2010.10.040. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. α1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase Cζ. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol. 2003;284:C1297–C1308. doi: 10.1152/ajpcell.00227.2002. [DOI] [PubMed] [Google Scholar]
- Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest. 2000;105:1711–1721. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmerkus N, Vassen V, Sievers B, Goerke B, Shan Q, Harder J, Schroder JM, Bleich M. Human β-defensin-2 increases cholinergic response in colon epithelium. Pflugers Arch. 2010;460:177–186. doi: 10.1007/s00424-009-0780-x. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Li N, Zhang W, Zhu W, Li Q, Wang B, Li J. Na+-dependent neutral amino acid transporter ASCT2 is downregulated in seriously traumatized human intestinal epithelial cells. J Pediatr Gastroenterol Nutr. 2008;46:71–79. doi: 10.1097/01.mpg.0000304457.22670.6f. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC) Pediatr Res. 2008;63:117–123. doi: 10.1203/PDR.0b013e31815ed64c. [DOI] [PubMed] [Google Scholar]
- Ibla JC, Khoury J, Kong T, Robinson A, Colgan SP. Transcriptional repression of Na-K-2Cl cotransporter NKCC1 by hypoxia-inducible factor-1. Am J Physiol Cell Physiol. 2006;291:C282–C289. doi: 10.1152/ajpcell.00564.2005. [DOI] [PubMed] [Google Scholar]
- Illing AC, Shawki A, Cunningham CL, Mackenzie B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J Biol Chem. 2012;287:30485–30496. doi: 10.1074/jbc.M112.364208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jennings RB, Reimer KA, Steenbergen C. Effect of inhibition of the mitochondrial ATPase on net myocardial ATP in total ischemia. J Mol Cell Cardiol. 1991;23:1383–1395. doi: 10.1016/0022-2828(91)90185-o. [DOI] [PubMed] [Google Scholar]
- Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Curr Opin Infect Dis. 2003;16:349–355. doi: 10.1097/00001432-200308000-00007. [DOI] [PubMed] [Google Scholar]
- Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhausen J, Ibla JC, Colgan SP. Implications of hypoxia on mucosal barrier function. Cell Mol Biol (Noisy-le-grand) 2003;49:77–87. [PubMed] [Google Scholar]
- Kashlan OB, Kleyman TR. Epithelial Na+ channel regulation by cytoplasmic and extracellular factors. Exp Cell Res. 2012;318:1011–1019. doi: 10.1016/j.yexcr.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Romero MF. Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol. 2011;73:261–281. doi: 10.1146/annurev-physiol-012110-142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely SJ, Montrose MH, Barrett KE. Electrolyte secretion and absorption: Small intestine and colon. In: Yamada T, editor. Textbook of Gastroenterology. 5th edn. Oxford: Wiley-Blackwell; 2009. pp. 330–367. [Google Scholar]
- Kekuda R, Manoharan P, Baseler W, Sundaram U. Monocarboxylate 4 mediated butyrate transport in a rat intestinal epithelial cell line. Dig Dis Sci. 2013;58:660–667. doi: 10.1007/s10620-012-2407-x. [DOI] [PubMed] [Google Scholar]
- King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- King JD, Jr, Lee J, Riemen CE, Neumann D, Xiong S, Foskett JK, Mehta A, Muimo R, Hallows KR. Role of binding and nucleoside diphosphate kinase A in the regulation of the cystic fibrosis transmembrane conductance regulator by AMP-activated protein kinase. J Biol Chem. 2012;287:33389–33400. doi: 10.1074/jbc.M112.396036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kles KA, Tappenden KA. Hypoxia differentially regulates nutrient transport in rat jejunum regardless of luminal nutrient present. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1336–G1342. doi: 10.1152/ajpgi.00055.2002. [DOI] [PubMed] [Google Scholar]
- Kles KA, Wallig MA, Tappenden KA. Luminal nutrients exacerbate intestinal hypoxia in the hypoperfused jejunum. JPEN J Parenter Enteral Nutr. 2001;25:246–253. doi: 10.1177/0148607101025005246. [DOI] [PubMed] [Google Scholar]
- Kongsuphol P, Cassidy D, Hieke B, Treharne KJ, Schreiber R, Mehta A, Kunzelmann K. Mechanistic insight into control of CFTR by AMPK. J Biol Chem. 2009a;284:5645–5653. doi: 10.1074/jbc.M806780200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuphol P, Hieke B, Ousingsawat J, Almaca J, Viollet B, Schreiber R, Kunzelmann K. Regulation of Cl− secretion by AMPK in vivo. Pflugers Arch. 2009b;457:1071–1078. doi: 10.1007/s00424-008-0577-3. [DOI] [PubMed] [Google Scholar]
- Kozar RA, Hu S, Hassoun HT, DeSoignie R, Moore FA. Specific intraluminal nutrients alter mucosal blood flow during gut ischemia/reperfusion. JPEN J Parenter Enteral Nutr. 2002a;26:226–229. doi: 10.1177/0148607102026004226. [DOI] [PubMed] [Google Scholar]
- Kozar RA, Schultz SG, Bick RJ, Poindexter BJ, DeSoignie R, Moore FA. Enteral glutamine but not alanine maintains small bowel barrier function after ischemia/reperfusion injury in rats. Shock. 2004a;21:433–437. doi: 10.1097/00024382-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Kozar RA, Schultz SG, Hassoun HT, Desoignie R, Weisbrodt NW, Haber MM, Moore FA. The type of sodium-coupled solute modulates small bowel mucosal injury, transport function, and ATP after ischemia/reperfusion injury in rats. Gastroenterology. 2002b;123:810–816. doi: 10.1053/gast.2002.35389. [DOI] [PubMed] [Google Scholar]
- Kozar RA, Verner-Cole E, Schultz SG, Sato N, Bick RJ, Desoignie R, Poindexter BJ, Moore FA. The immune-enhancing enteral agents arginine and glutamine differentially modulate gut barrier function following mesenteric ischemia/reperfusion. J Trauma. 2004b;57:1150–1156. doi: 10.1097/01.ta.0000151273.01810.e9. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- Kvietys PR, Gallavan RH, Chou CC. Contribution of bile to postprandial intestinal hyperemia. Am J Physiol Gastrointest Liver Physiol. 1980;238:G284–G288. doi: 10.1152/ajpgi.1980.238.4.G284. [DOI] [PubMed] [Google Scholar]
- Kvietys PR, Granger DN. Effects of solute-coupled fluid absorption on blood flow and oxygen uptake in the dog colon. Gastroenterology. 1981;81:450–457. [PubMed] [Google Scholar]
- Kvietys PR, McLendon JM, Granger DN. Postprandial intestinal hyperemia: role of bile salts in the ileum. Am J Physiol Gastrointest Liver Physiol. 1981;241:G469–G477. doi: 10.1152/ajpgi.1981.241.6.G469. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, Kim SS, Ha J. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003;278:39653–39661. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Cheung G, Strohmeier GR, Currie MG, Ouellette AJ, Selsted ME, Madara JL. Induction of epithelial chloride secretion by channel-forming cryptdins 2 and 3. Proc Natl Acad Sci U S A. 1997;94:8585–8589. doi: 10.1073/pnas.94.16.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Huang Q, Niu LY, Zhu WM. Study of enteral nutrients transport in intestinal hypoperfused rat model. Zhonghua Wei Chang Wai Ke Za Zhi. 2008;11:343–347. [PubMed] [Google Scholar]
- Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HP. Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J Biol Chem. 2011;286:32444–32453. doi: 10.1074/jbc.M111.254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie B, Ujwal ML, Chang MH, Romero MF, Hediger MA. Divalent metal-ion transporter DMT1 mediates both H+-coupled Fe2+ transport and uncoupled fluxes. Pflugers Arch. 2006;451:544–558. doi: 10.1007/s00424-005-1494-3. [DOI] [PubMed] [Google Scholar]
- McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77:307–318. doi: 10.1016/s0039-6109(05)70550-8. [DOI] [PubMed] [Google Scholar]
- Madsen JL, Søndergaard SB, Møller S. Meal-induced changes in splanchnic blood flow and oxygen uptake in middle-aged healthy humans. Scand J Gastroenterol. 2006;41:87–92. doi: 10.1080/00365520510023882. [DOI] [PubMed] [Google Scholar]
- Mastrogiannaki M, Matak P, Delga S, Deschemin JC, Vaulont S, Peyssonnaux C. Deletion of HIF-2α in the enterocytes decreases the severity of tissue iron loading in hepcidin knockout mice. Blood. 2012;119:587–590. doi: 10.1182/blood-2011-09-380337. [DOI] [PubMed] [Google Scholar]
- Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JB, Smith JA, Tally KJ, Menconi MJ, Nguyen H, Fink MP. Chemical hypoxia increases junctional permeability and activates electrogenic ion transport in human intestinal epithelial monolayers. Surgery. 1994;116:150–157. [PubMed] [Google Scholar]
- Matthews JB, Tally KJ, Smith JA, Zeind AJ, Hrnjez BJ. Activation of Cl secretion during chemical hypoxia by endogenous release of adenosine in intestinal epithelial monolayers. J Clin Invest. 1995;1:117–125. doi: 10.1172/JCI118010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensink PB, Moons LM, Kuipers EJ. Chronic gastrointestinal ischaemia: shifting paradigms. Gut. 2011;60:722–737. doi: 10.1136/gut.2009.199695. [DOI] [PubMed] [Google Scholar]
- Miki K, Unno N, Nagata T, Uchijima M, Konno H, Koide Y, Nakamura S. Butyrate suppresses hypoxia-inducible factor-1 activity in intestinal epithelial cells under hypoxic conditions. Shock. 2004;22:446–452. doi: 10.1097/01.shk.0000140664.80530.bd. [DOI] [PubMed] [Google Scholar]
- Milligan LP, McBride BW. Energy costs of ion pumping by animal tissues. J Nutr. 1985;115:1374–1382. doi: 10.1093/jn/115.10.1374. [DOI] [PubMed] [Google Scholar]
- Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D, Wild GE. Effect of fasting on Na-K-ATPase activity in rat small intestinal mucosa. Can J Physiol Pharmacol. 1980;6:634–639. doi: 10.1139/y80-106. [DOI] [PubMed] [Google Scholar]
- Niinikoski H, Stoll B, Guan X, Kansagra K, Lambert BD, Stephens J, Hartmann B, Holst JJ, Burrin DG. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr. 2004;134:1467–1474. doi: 10.1093/jn/134.6.1467. [DOI] [PubMed] [Google Scholar]
- Nowicki PT, Granger DN. Miscellaneous. In: Yamada T, editor. Textbook of Gastroenterology. 5th edn. Oxford: Wiley-Blackwell; 2009. pp. 548–550. [Google Scholar]
- Orsenigo MN, Faelli A, Porta C, Sironi C, Laforenza U, Paulmichl M, Tosco M. Oxidative stress reduces transintestinal transports and (Na+, K+)-ATPase activity in rat jejunum. Arch Biochem Biophys. 2007;466:300–307. doi: 10.1016/j.abb.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Fink GD, Kuroki MT. Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep. 2011;13:221–228. doi: 10.1007/s11906-011-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perko MJ, Nielsen HB, Skak C, Clemmesen JO, Schroeder TV, Secher NH. Mesenteric, coeliac and splanchnic blood flow in humans during exercise. J Physiol. 1998;513:907–913. doi: 10.1111/j.1469-7793.1998.907ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri M, Christian HC, Wilkins RJ, Boyd CA, Meredith D. The apical (hPepT1) and basolateral peptide transport systems of Caco-2 cells are regulated by AMP-activated protein kinase. Am J Physiol Gastrointest Liver Physiol. 2010;299:G136–G143. doi: 10.1152/ajpgi.00014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plauth M, Raible A, Vieillard-Baron D, Bauder-Gross D, Hartmann F. Is glutamine essential for the maintenance of intestinal function? A study in the isolated perfused rat small intestine. Int J Colorectal Dis. 1999;14:86–94. doi: 10.1007/s003840050191. [DOI] [PubMed] [Google Scholar]
- Pratha VS, Thompson SM, Hogan DL, Paulus P, Dreilinger AD, Barrett KE, Isenberg JI. Utility of endoscopic biopsy samples to quantitate human duodenal ion transport. J Lab Clin Med. 1998;132:512–518. doi: 10.1016/s0022-2143(98)90130-5. [DOI] [PubMed] [Google Scholar]
- Qamar MI, Read AE. Effects of exercise on mesenteric blood flow in man. Gut. 1987;28:583–587. doi: 10.1136/gut.28.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar MI, Read AE. Effects of ingestion of carbohydrate, fat, protein, and water on the mesenteric blood flow in man. Scand J Gastroenterol. 1988;23:26–30. doi: 10.3109/00365528809093842. [DOI] [PubMed] [Google Scholar]
- Ratcliffe PJ, O'Rourke JF, Maxwell PH, Pugh CW. Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J Exp Biol. 1998;201:1153–1162. doi: 10.1242/jeb.201.8.1153. [DOI] [PubMed] [Google Scholar]
- Romney SJ, Newman BS, Thacker C, Leibold EA. HIF-1 regulates iron homeostasis in Caenorhabditis elegans by activation and inhibition of genes involved in iron uptake and storage. PLoS Genet. 2011;7:e1002394. doi: 10.1371/journal.pgen.1002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB, Blackmon JR, Bruce RA. Indocyanine green clearance and estimated hepatic blood flow during mild to maximal exercise in upright man. J Clin Invest. 1964;43:1677–1690. doi: 10.1172/JCI105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P, Arthur S, Kekuda R, Sundaram U. Na-glutamine co-transporters B0AT1 in villus and SN2 in crypts are differentially altered in chronically inflamed rabbit intestine. Biochim Biophys Acta. 2012;1818:434–442. doi: 10.1016/j.bbamem.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Sakar Y, Nazaret C, Letteron P, Ait Omar A, Avenati M, Viollet B, Ducroc R, Bado A. Positive regulatory control loop between gut leptin and intestinal GLUT2/GLUT5 transporters links to hepatic metabolic functions in rodents. PLoS One. 2009;4:e7935. doi: 10.1371/journal.pone.0007935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksena S, Gill RK, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Role of Fyn and PI3K in H2O2-induced inhibition of apical Cl−/OH− exchange activity in human intestinal epithelial cells. Biochem J. 2008;416:99–108. doi: 10.1042/BJ20070960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe E, Yang Z, Ohno S, Yamamoto T. Reactive oxygen species produced by the knockdown of manganese-superoxide dismutase up-regulate hypoxia-inducible factor-1alpha expression in oral squamous cell carcinoma cells. Free Radic Biol Med. 2010;48:1321–1329. doi: 10.1016/j.freeradbiomed.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Seidler U, Song P, Xiao F, Riederer B, Bachmann O, Chen M. Recent advances in the molecular and functional characterization of acid/base and electrolyte transporters in the basolateral membranes of gastric and duodenal epithelial cells. Acta Physiol (Oxf) 2011;201:3–20. doi: 10.1111/j.1748-1716.2010.02107.x. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- Seon CS, Park YS, Park SH, Ryu SR, Jo YJ, Kim SH, Son BK, Ahn SB. A case of oral-contraceptive related ischemic colitis in young woman. Clin Endosc. 2011;44:129–132. doi: 10.5946/ce.2011.44.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AP. Metabolic control of intestinal oxygenation and blood flow. Fed Proc. 1982a;41:2084–2089. [PubMed] [Google Scholar]
- Shepherd AP. Role of capillary recruitment in the regulation of intestinal oxygenation. Am J Physiol Gastrointest Liver Physiol. 1982b;242:G435–G441. doi: 10.1152/ajpgi.1982.242.5.G435. [DOI] [PubMed] [Google Scholar]
- Sidery MB, Macdonald IA, Blackshaw PE. Superior mesenteric artery blood flow and gastric emptying in humans and the differential effects of high fat and high carbohydrate meals. Gut. 1994;35:186–190. doi: 10.1136/gut.35.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber C, Beglinger C, Jaeger K, Hildebrand P, Stalder GA. Regulation of postprandial mesenteric blood flow in humans: evidence for a cholinergic nervous reflex. Gut. 1991;32:361–366. doi: 10.1136/gut.32.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M. Expression, regulation and the role of SLC26 Cl−/HCO3− exchangers in kidney and gastrointestinal tract. Novartis Found Symp. 2006;273:91–102. discussion 103–106, 261–104. [PubMed] [Google Scholar]
- Spiller R. Role of motility in chronic diarrhoea. Neurogastroenterol Motil. 2006;18:1045–1055. doi: 10.1111/j.1365-2982.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- Stadeager C, Hesse B, Henriksen O, Christensen NJ, Bonde-Petersen F, Mehlsen J, Giese J. Effects of angiotensin blockade on the splanchnic circulation in normotensive humans. J Appl Physiol. 1989;67:786–791. doi: 10.1152/jappl.1989.67.2.786. [DOI] [PubMed] [Google Scholar]
- Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- Sun W, Lo C, Tso P. Intestinal lipid absorption. In: Yamada T, editor. Textbook of Gastroenterology. 5th edn. Oxford: Wiley-Blackwell; 2009. pp. 445–463. [Google Scholar]
- Takala J. Determinants of splanchnic blood flow. Br J Anaesth. 1996;77:50–58. doi: 10.1093/bja/77.1.50. [DOI] [PubMed] [Google Scholar]
- Takimoto M, Takeyama M, Hamada T. Possible involvement of AMPK in acute exercise-induced expression of monocarboxylate transporters MCT1 and MCT4 mRNA in fast-twitch skeletal muscle. Metabolism. 2013;62:1633–1640. doi: 10.1016/j.metabol.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med (Berl) 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Lisco SJ, Awtrey CS, Colgan SP. Hypoxia inhibits cyclic nucleotide-stimulated epithelial ion transport: role for nucleotide cyclases as oxygen sensors. J Pharmacol Exp Ther. 1998;284:568–575. [PubMed] [Google Scholar]
- Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140:2044–2055. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Steege RW, Kolkman JJ. Review article: the pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow. Aliment Pharmacol Ther. 2012;35:516–528. doi: 10.1111/j.1365-2036.2011.04980.x. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CM. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol. 2007;92:603–619. doi: 10.1113/expphysiol.2005.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J Biol Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- van Wijck K, Lenaerts K, Grootjans J, Wijnands KA, Poeze M, van Loon LJ, Dejong CH, Buurman WA. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol. 2012;303:G155–G168. doi: 10.1152/ajpgi.00066.2012. [DOI] [PubMed] [Google Scholar]
- van Wijck K, Lenaerts K, van Loon LJ, Peters WH, Buurman WA, Dejong CH. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PloS One. 2011;6:e22366. doi: 10.1371/journal.pone.0022366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner SF, Franklin D, Van Citters RL. Mesenteric vasoactivity associated with eating and digestion in the conscious dog. Am J Physiol. 1970;219:170–174. doi: 10.1152/ajplegacy.1970.219.1.170. [DOI] [PubMed] [Google Scholar]
- Veenstra RP, Geelkerken RH, Verhorst PM, Huisman AB, Kolkman JJ. Acute stress-related gastrointestinal ischemia. Digestion. 2007;75:205–207. doi: 10.1159/000109373. [DOI] [PubMed] [Google Scholar]
- Walker J, Jijon HB, Churchill T, Kulka M, Madsen KL. Activation of AMP-activated protein kinase reduces cAMP-mediated epithelial chloride secretion. Am J Physiol Gastrointest Liver Physiol. 2003;285:G850–G860. doi: 10.1152/ajpgi.00077.2003. [DOI] [PubMed] [Google Scholar]
- Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. 5-Aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2005;385:485–491. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic–helix–loop–helix–PAS heterodimer regulated by cellular oxygen tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Wang S, Song P, Zou MH. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci (Lond) 2012;122:555–573. doi: 10.1042/CS20110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JB, Lawler K, Amu S, Taylor CT, Fallon PG, Keely SJ. Hydroxylase inhibition attenuates colonic epithelial secretory function and ameliorates experimental diarrhea. FASEB J. 2011;25:535–543. doi: 10.1096/fj.10-166983. [DOI] [PubMed] [Google Scholar]
- Wasa M, Wang HS, Shimizu Y, Okada A. Amino acid transport is down-regulated in ischemic human intestinal epithelial cells. Biochim Biophys Acta. 2004;1670:49–55. doi: 10.1016/j.bbagen.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wilmore DW, Smith RJ, O'Dwyer ST, Jacobs DO, Ziegler TR, Wang XD. The gut: a central organ after surgical stress. Surgery. 1988;104:917–923. [PubMed] [Google Scholar]
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- Wyers MC. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23:9–20. doi: 10.1053/j.semvascsurg.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- Yan H, Zhang DX, Shi X, Zhang Q, Huang YS. Activation of the prolyl-hydroxylase oxygen-sensing signal cascade leads to AMPK activation in cardiomyocytes. J Cell Mol Med. 2012;16:2049–2059. doi: 10.1111/j.1582-4934.2011.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos NC, Kovbasnjuk O, Donowitz M. Regulation of intestinal electroneutral sodium absorption and the brush border Na+/H+ exchanger by intracellular calcium. Ann N Y Acad Sci. 2009;1165:240–248. doi: 10.1111/j.1749-6632.2009.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Kuhlicke J, Jäckel K, Eltzschig HK, Singh A, Sjöblom M, Riederer B, Weinhold C, Seidler U, Colgan SP, Karhausen J. Hypoxia inducible factor-1 (HIF-1)-mediated repression of cystic fibrosis transmembrane conductance regulator (CFTR) in the intestinal epithelium. FASEB J. 2009;23:204–213. doi: 10.1096/fj.08-110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Dada LA, Chandel NS, Iwai K, Lecuona E, Ciechanover A, Sznajder JI. Hypoxia-mediated Na-K-ATPase degradation requires von Hippel Lindau protein. FASEB J. 2008;22:1335–1342. doi: 10.1096/fj.07-8369com. [DOI] [PubMed] [Google Scholar]
- Zou X, Cao J, Yao Y, Liu W, Chen L. Endoscopic findings and clinicopathologic characteristics of ischemic colitis: a report of 85 cases. Dig Dis Sci. 2009;54:2009–2015. doi: 10.1007/s10620-008-0579-1. [DOI] [PubMed] [Google Scholar]


