Abstract
The accepted model of autonomic control of heart rate (HR) during dynamic exercise indicates that the initial increase is entirely attributable to the withdrawal of parasympathetic nervous system (PSNS) activity and that subsequent increases in HR are entirely attributable to increases in cardiac sympathetic activity. In the present review, we sought to re-evaluate the model of autonomic neural control of HR in humans during progressive increases in dynamic exercise workload. We analysed data from both new and previously published studies involving baroreflex stimulation and pharmacological blockade of the autonomic nervous system. Results indicate that the PSNS remains functionally active throughout exercise and that increases in HR from rest to maximal exercise result from an increasing workload-related transition from a 4 : 1 vagal–sympathetic balance to a 4 : 1 sympatho–vagal balance. Furthermore, the beat-to-beat autonomic reflex control of HR was found to be dependent on the ability of the PSNS to modulate the HR as it was progressively restrained by increasing workload-related sympathetic nerve activity. In conclusion: (i) increases in exercise workload-related HR are not caused by a total withdrawal of the PSNS followed by an increase in sympathetic tone; (ii) reciprocal antagonism is key to the transition from vagal to sympathetic dominance, and (iii) resetting of the arterial baroreflex causes immediate exercise-onset reflexive increases in HR, which are parasympathetically mediated, followed by slower increases in sympathetic tone as workloads are increased.
Daniel W. White is a veteran of the United States Marine Corps. He received his PhD at the University of North Texas Health Science Center (UNTHSC) at Fort Worth under the mentorship of Peter B. Raven. Dr White is currently a postdoctoral researcher at the University of Illinois at Chicago in the Integrative Physiology Laboratory under Bo Fernhall and is studying the autonomic nervous system and vascular adaptations in veterans. Peter B. Raven, PhD, Professor, Department of Integrative Physiology, UNTHSC has 41 years of experience in directing human integrative physiological research. He received his PhD in exercise physiology in 1969 under the mentorship of Eugene Evonuk at the University of Oregon in Eugene, OR. He was awarded a National Institutes of Health post-doctoral fellowship in 1969 and rose to an associate professorship in 1975 at the Institute of Environmental Stress at the University of California Santa Barbara, where he worked with Steven M. Horvath, PhD. In 1977 he joined UNTHSC in Fort Worth as a faculty member and has been a principal investigator funded by the National Institute of Environmental Health Sciences, National Institute for Occupational Safety and Health, National Heart, Lung and Blood Institute, US Air Force and NASA. Since 1984 Dr Raven has focused his investigations on arterial baroreflex control of blood pressure during exercise. With the help of many PhD mentees and a cadre of renowned scientists, arterial baroreflex regulation of blood pressure during dynamic exercise has been defined.
Introduction
The increases in heart rate (HR) that occur from rest to maximal dynamic exercise rely on a balance between the respective influences of the parasympathetic and sympathetic branches of the autonomic nervous system (ANS). An early investigation into the autonomic control of HR examined five supine human subjects performing dynamic exercise from rest to maximal oxygen uptake (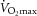 ) during: control; complete β-adrenergic receptor blockade of the heart with propranolol; complete parasympathetic blockade of the heart with atropine, and a complete combined autonomic blockade of the heart (i.e. intrinsic HR) (Robinson et al. 1966). Later, mathematical models were used in determining autonomic influences on the heart at rest (Katona et al. 1976, 1982). However, the authors cautioned the reader to consider that the modelling data developed at rest might not be confidently extrapolated to exercise (Katona et al. 1982). As a result, the data provided by Robinson et al. (1966) were reinterpreted and simplified to convey the finding that increases in HR from rest to mild exercise workloads are primarily a result of decreased parasympathetic nervous activity (PSNA), later termed ‘parasympathetic (vagal) withdrawal’ (Rowell, 1986, 1993). Furthermore, subsequent workload-related increases in HR above 100 beats min−1 (at which norepinephrine spillover into the plasma occurs) up to the individual's HR maximum result from increasing sympathetic nervous activity (SNA) (Rowell, 1986).
) during: control; complete β-adrenergic receptor blockade of the heart with propranolol; complete parasympathetic blockade of the heart with atropine, and a complete combined autonomic blockade of the heart (i.e. intrinsic HR) (Robinson et al. 1966). Later, mathematical models were used in determining autonomic influences on the heart at rest (Katona et al. 1976, 1982). However, the authors cautioned the reader to consider that the modelling data developed at rest might not be confidently extrapolated to exercise (Katona et al. 1982). As a result, the data provided by Robinson et al. (1966) were reinterpreted and simplified to convey the finding that increases in HR from rest to mild exercise workloads are primarily a result of decreased parasympathetic nervous activity (PSNA), later termed ‘parasympathetic (vagal) withdrawal’ (Rowell, 1986, 1993). Furthermore, subsequent workload-related increases in HR above 100 beats min−1 (at which norepinephrine spillover into the plasma occurs) up to the individual's HR maximum result from increasing sympathetic nervous activity (SNA) (Rowell, 1986).
Unfortunately, this nuanced interpretation of the mechanisms involved in exercise-induced increases in HR has resulted in a generalized acceptance of the view that increases in HR occurring from rest to an exercise workload of 100 beats min−1 result exclusively from parasympathetic withdrawal and that increases above 100 beats min−1 result from increasing SNA (Rowell, 1993). Although Rowell (1993) probably did not intend his interpretation to be taken so rigidly, an unintended consequence of this interpretation is that it is generally accepted, by beginning students of the topic, that SNA has very little influence on HR increases from rest to 100 beats min−1 and that PSNA has no influence on HR increases above 100 beats min−1. It should be noted that the original diagram (Fig. 5–4) in Rowell (1993) is a schematic representation that uses the arbitrary rate of 100 beats min−1 as a correlated estimate of exercise workload, whereas this will differ among subpopulations of subjects.
Figure 5. Modified version of the diagram proposed by Rowell (1993).
The modified diagram (Fig. 5–4, Rowell 1993) depicts the reflex continuum of autonomic influence from both branches of the autonomic nervous system throughout exercise. The shaded area under the central line represents the sympathetic influence at all exercise workloads. The dotted area represents the functional parasympathetic modulation of heart rate (HR) at all exercise workloads. As the area of the sympathetic portion of the graph increases, the area of the parasympathetic modulation portion of the graph decreases to show the inverse relationship between sympathetic tone and parasympathetic modulation. The centre line indicates the relative HR (i.e. dynamic sympatho–vagal balance).
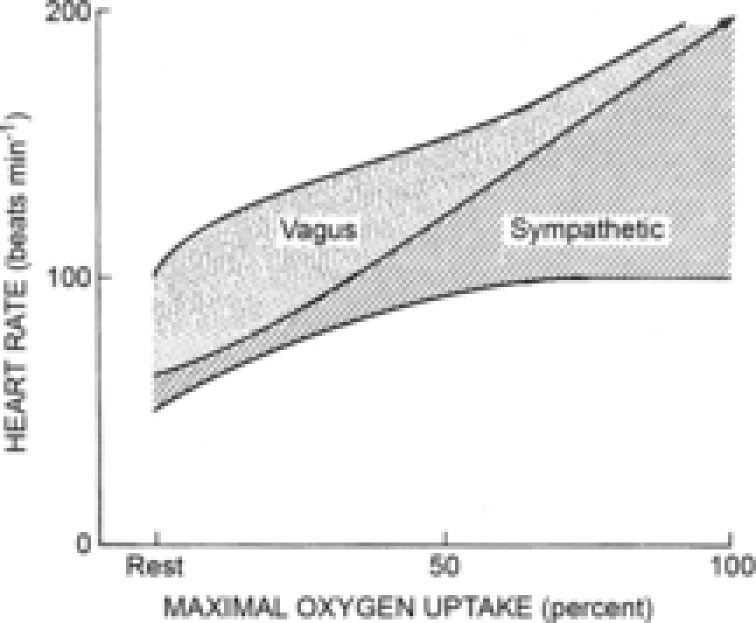
Figure 4. Modified version of the diagram proposed by Rowell (1993.
The modified diagram (Fig. 5–4, Rowell 1993) depicts the steady state continuum of autonomic influence from both branches of the autonomic nervous system throughout exercise. The shaded area under the central line represents the sympathetic influence at all exercise workloads. The dotted area represents the parasympathetic influence of heart rate (HR) at all exercise workloads. The centre line indicates the relative HR (i.e. the dynamic sympatho–vagal balance).
However, historical and recent investigations into the autonomic neural control of HR and arterial blood pressure (ABP) during dynamic exercise in dogs (O'Leary et al. 1997) and humans (Ogoh et al. 2005) raise questions as to whether: (i) PSNA is withdrawn in the transition from rest to exercise and continues to be withdrawn until it has negligible effect on further HR increases, at approximately 100 beats min−1 (Matsukawa, 2012); (ii) reciprocal antagonism can explain the changes in the sympatho–vagal balance resulting in exercise workload-related increases in HR (Levy, 1971), and (iii) the exercise pressor reflex and the arterial and cardiopulmonary baroreflexes’ control of ABP play a role in resetting the operating point (OP) pressure of the HR reflex to a point of reduced gain (Fadel & Raven, 2012; Fisher, 2013). Hence, the purpose of this presentation is to revisit and provide a reinterpretation of the well-accepted model of the autonomic control of HR that occurs during dynamic exercise from rest to maximum workload.
Methods
Experiments used to obtain HR modelling data
In 2012 in Fort Worth, we addressed the repeatability of the methods used to obtain the data necessary to model the carotid baroreflex (CBR) function curves by recruiting nine healthy human subjects to repeat exactly the same control resting and exercise protocols that had been performed in Copenhagen in 2004 by eight different human subjects (Ogoh et al. 2005). There was no statistically significant difference in HR between the Copenhagen data and the Fort Worth data when the steady state means and reflex responses at each exercise workload were compared between the studies; therefore the control data were considered as if derived from one population.
Hence, the data utilized in the current presentation for modelling the HR response from rest to progressive increases in dynamic exercise workloads in the control condition were obtained from 17 subjects. However, the autonomic blockade studies performed in Copenhagen involved data for only eight subjects.
All subjects were free of any known cardiovascular or pulmonary disorder and were not using prescribed or over-the-counter medications. Each subject provided written informed consent as approved by the Ethics Committee of Copenhagen (KF01-369/97) or the Institutional Review Board at the University of North Texas Health Science Center (IRB 2010-058). All experiments were performed in accordance with the Declaration of Helsinki. In both studies, all subjects were requested to abstain from caffeinated beverages for 12 h and to refrain from strenuous physical activity and alcohol for at least 1 day prior to the experiments. Subjects performed a progressively graded increase in workload exercise test on a cycle ergometer to determine workloads at HRs of 90 beats min−1, 120 beats min−1 and 150 beats min−1 to be used during the experimental treatments.
On experiment days and after 30 min of resting data collection, each subject performed 25 min bouts of exercise at steady state HRs of 90 beats min−1 (EX90), 120 beats min−1 (EX120) and 150 beats min−1 (EX150). Beat-to-beat HR and mean arterial pressure (MAP) responses to the neck pressure/neck suction (NP/NS) protocol during steady state rest, EX90, EX120 and EX150 during the control, complete metoprolol (β1-adrenergic receptor) blockade and complete glycopyrrolate (peripheral muscarinic receptor) blockade experiments were recorded. Details of establishing the autonomic blockades were reported previously by Ogoh et al. (2005). The data were used to assess the carotid–HR baroreflex at each stage of the protocol.
Results
Construction of the model of HR increases from rest to maximal exercise
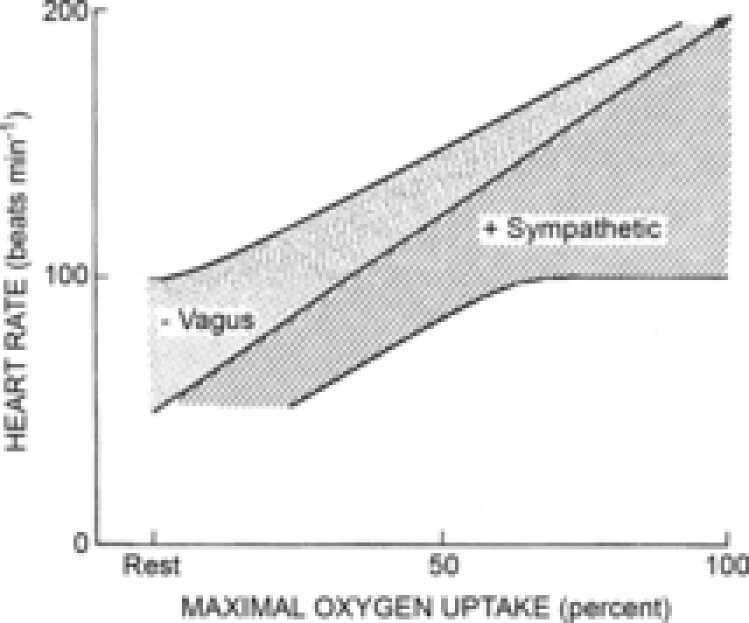
Control (sympatho–vagal balanced) HR response to exercise and NP/NS. Specific details of the data analyses and the construction of the carotid–HR baroreflex function curves have been published previously (Ogoh et al. 2005). In the present review, the data were analysed as described below. Heart rates at the OP pressures at rest, EX90, EX120 and EX150 without autonomic blockade were plotted against their related exercise workload target HRs and fit to a linear regression line that included an extrapolated HR at a calculated maximal workload (r2 = 0.99).This HR/workload target HR regression line is representative of the changes in the sympatho–vagal balance without autonomic blockade (Fig. 1A).
Figure 1. Autonomic reflex and steady-state influence on heart rate with increasing exercise.
A, modelling of the reflexive control of heart rate (HR) by the carotid baroreflex (CBR). Steady state HR at each workload target HR (Workload) represents the sympatho–vagal balance (red line). Increases and decreases in HR by simulated hypo- and hypertension induced by neck pressure/suction (green and blue lines, respectively) are plotted against workload. These data indicate that the parasympathetic influence on the reflex control of HR is diminished but never absent as the exercise workload increases from rest to maximal exercise. B, modelling of pharmacological blockade studies of each branch of the autonomic nervous system from rest to maximal exercise showing the steady state HR at each workload target HR (Workload) without blockade (solid red line). Steady state HRs at each workload with muscarinic blockade by glycopyrrolate (green dashed line) and β1-adrenergic blockade by metoprolol (blue dashed line) represent the un-opposed sympathetic HR and un-opposed parasympathetic HR, respectively. As HR increases with workload, the sympatho–vagal balance shows greater sympathetic dominance. PSNS, parasympathetic nervous system; SNS, sympathetic nervous system.
Heart rates at carotid–HR baroreflex threshold and saturation pressures were plotted against rest, EX90, EX120 and EX150 workloads. A linear regression relationship between HRs and related workload target HRs existed for the threshold point pressures (r2 = 0.97) (Fig. 1A). A non-linear best-fit regression relationship existed between HRs and related workload target HRs at the saturation point pressures. This required the use of non-linear modelling analyses to identify that the line of best fit was a third-order polynomial regression (r2 = 0.96) (Fig. 1A).
Autonomic neural influences on HR response to exercise and NP/NS
Control condition (without autonomic blockade). The difference between the OP regression line (i.e. the sympatho–vagal balance line) and the saturation point regression line (i.e. the maximum mechanically stimulated decrease in HR) is the NS-induced effective parasympathetic reserve at any given workload (Fig. 1A). The difference between the OP regression line and the threshold point regression line (i.e. the maximum mechanically stimulated increase in HR by NP) is effective parasympathetic tone at any given workload (Fig. 1A). The difference between the saturation line and threshold line represents the overall NP/NS-induced functional reflexive influence of the parasympathetic nervous system (PSNS) on HR (Fig. 1A).
Cardiac muscarinic receptor blockade condition
Heart rates recorded during parasympathetic cardiac blockade by glycopyrrolate were plotted against their related workloads (dashed green line) and exhibited a linear relationship (r2 = 0.99) (Fig. 1B). The difference between the OP regression line and the maximal vagal inhibition line represents the PSNS contribution to the steady state HR from rest to maximal exercise.
Cardiac β1-adrenergic receptor blockade condition
Heart rates during maximal cardiac β1-adrenergic receptor blockade with metoprolol were plotted against their related workloads and exhibited a linear regression line of best fit (r2 = 0.99) (Fig. 1B). The difference between the sympatho–vagal balance line and the maximal sympathetic inhibition line represents the sympathetic nervous system (SNS) contribution to the steady state HR from rest to maximal exercise (Fig. 1B).
Summary of modelling outcomes
The steady state HR becomes closer to the unopposed sympathetic HR as the workload increases, indicating a stronger influence of the SNS at higher workloads. However, because it never reaches the same value, there is never a complete absence of parasympathetic influence (Fig. 1B).
The relative influence of each branch of the ANS starts with a parasympathetic dominant (PSNS to SNS ratio of 4 : 1) influence at rest. As exercise workload increases there is a shift to a more sympathetic (PSNS to SNS ratio of 1 : 4) influence at 175 beats min−1 (Fig. 2).
According to the selective PSNS and SNS blockade studies, the PSNS and SNS efferent activity at the sinoatrial node reach an equal beats min−1 influence on HR at approximately 140 beats min−1. There is a linear increase in SNS influence from 9 beats min−1 to 14 beats min−1 from rest to maximum exercise and a linear decrease in PSNS influence from 35 beats min−1 to 3 beats min−1 from rest to maximum exercise. However, the change in the PSNS : SNS ratio mainly reflects a greater linear decrease in parasympathetic influence on steady state HR at exercising workloads.
Figure 2. Contributions to heart rate by each branch of the autonomic nervous system determined by autonomic blockade studies.
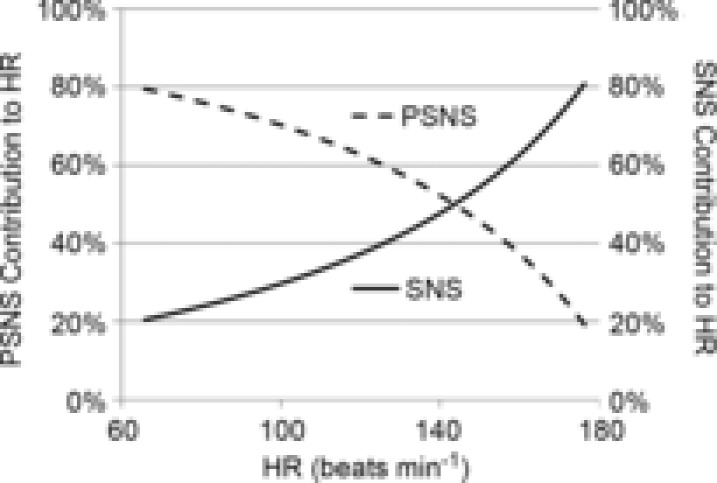
The parasympathetic nervous system (PSNS) contributes 80% influence to resting heart rate (HR) and the sympathetic nervous system (SNS) contributes the other 20%. Both branches make an equal contribution at close to 140 beats min−1, after which the ratio changes quickly to a more sympathetically dominant system. The respective lines indicate the change in HR from a single branch's selective autonomic blockade as a percentage change in HR from the sum of the absolute values of HR change of both branches.
It should be noted that these outcomes reflect the individuals studied and may change in other subjects according to age and individual fitness.
Discussion
Over the years, a large number of animal and human investigations have established the involvement of three integrated autonomic neural mechanisms, central command (CC), the exercise pressor reflex (EPR) and the arterial baroreflex (ABR), in the regulation of ABP during dynamic exercise (Raven, 2012). The specific investigations are documented in a number of comprehensive reviews which show that an exercise workload-related activation of CC and EPR from both central and peripheral neural inputs results in appropriate workload-related efferent cardiorespiratory responses (Shepherd, 1981; Mitchell, 1990; Rowell, 1993; Raven et al. 2006; Fadel & Raven, 2012). During progressive increases in exercise workload from exercise onset to maximal exercise, haemodynamic responses are directly related to the workloads and the individual's rating of perception of effort (RPE) (Williamson et al. 2001; Williamson, 2010). For many years a simple concept of PSNA (vagal) withdrawal was proposed to be the mechanism by which HR rises at exercise onset up to a rate of 100 beats min−1 (Rowell, 1986, 1993), after which increases in HR to >100 beats min−1 were considered to be directly related to increases in SNA.
In the present review, we add more relevant published findings and a reanalysis of previously published data (Ogoh et al. 2005) in order to re-examine Rowell's (1993) simplified representation of Robinson et al.'s (1966) initial study. In this earlier work selective pharmacologic blockade and double blockade of the parasympathetic and sympathetic arms of the autonomic control of HR were accomplished. The new information resulting from our analyses indicates that a balance between the sympathetic and parasympathetic control of HR with progressive increases in exercise workload remains viable and effective from rest to maximum workload (Fig. 3). In addition, the data from the blockade studies indicate that the PSNS influence on HR is necessary for reflex control, whereas the SNS is required to set the workload-related steady state HR. Fisher et al. (2010) recently reported that PSNS blockade during post-exercise muscle ischaemia following handgrip exercise in humans allowed exercise-induced elevations in HR to remain, whereas blockade of the SNS did not, a finding identified earlier in dogs during dynamic exercise (O'Leary & Seamans, 1993; O'Leary et al. 1997). Our additional data analyses identify a more refined model of the progressive exercise workload/HR-related changes in the sympatho–vagal balance.
Figure 3. See-saw model of autonomic beat-to-beat reflex control of heart rate.
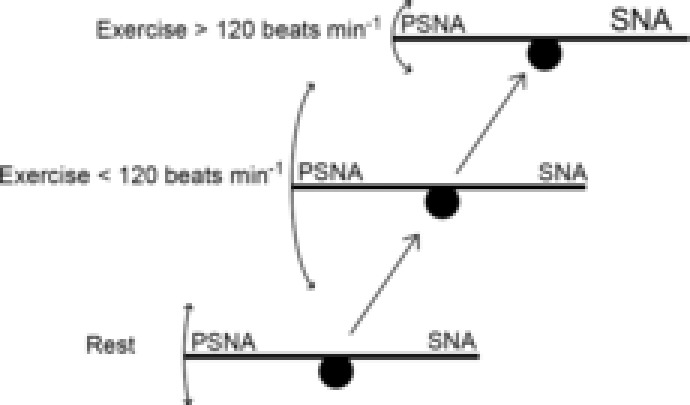
At rest as well as throughout exercise, there is a sympatho–vagal balance. The parasympathetic functional control is related to the sympathetic tone and maintains a balance. The relative amount of sympathetic nervous activity (SNA) on the right side of the see-saw affects the reflexive control of heart rate (HR) by the parasympathetic nervous activity (PSNA) on the left side as depicted by the size of the arrows. As exercise is initiated, there is a slight decrease in sympathetic tone caused by the loading of the cardiopulmonary baroreceptors, which allows for changes in the parasympathetic nervous outflow to exert more influence on the heart. Along with this initial decrease in sympathetic tone is a transient decrease in blood pressure so that HR is increased reflexively by the lessening of parasympathetic tone to maintain and increase cardiac output. As exercise workload increases, central command and exercise pressor reflexes force increases in sympathetic tone, causing a further rise in HR and a depression of parasympathetically mediated HR reflex response.
By contrast with the simple concept of vagal withdrawal at exercise onset, we propose that HR increases at the onset of exercise and with increases in exercise workload are related to an ABP regulatory mechanism (Rowell, 1986, 1993). The proposed ABP regulatory mechanism requires CC to actively reset the ‘set point’ of the ABR to allow concurrent increases in the ABP and HR in order to increase cardiac output (Williamson et al. 2001; Williamson, 2010). During progressive increases in dynamic exercise workload, ABR resetting is linearly related to the increase in exercise workload (Fadel & Raven, 2012). At exercise onset the immediate act of resetting the ‘set point’ reference pressure of the ABR results in negative feedback from the arterial baroreceptors, indicating that the operating pressure of the reflex is too low, which initiates the rapid responding inter-beat withdrawal of the parasympathetic hold on HR (DiCarlo & Bishop, 1992). In addition, at exercise onset the increased venous return associated with activation of the muscle pump increases right atrial blood volume and the resultant stretch of atrial type A and B receptors (Paintal, 1973) is transmitted via afferent fibres of the vagus and reflexively increases HR in a process known as the ‘Bainbridge reflex’ (Bainbridge, 1914). At the same time the slower responding increase (sec) in central SNA outflow progressively inhibits parasympathetic influence (Ogoh et al. 2005) by reciprocal antagonism (Levy, 1971; Uijtdehaage & Thayer, 2000), and the increased SNA becomes the major driver of increases in HR (Fig. 4). As the exercise workload progressively rises, the increases in SNA (Hartley et al. 1972; Savard et al. 1989) directly excite the cardiac pacemaker cells and progressively inhibit parasympathetic modulation of the HR up to maximum workloads. The exercise workload-related increases in SNA are directly related to the increases in CC (McIlveen et al. 2001; Fadel & Raven, 2012) and the EPR (McIlveen et al. 2001; Fisher, 2013). However, in dynamic leg cycling or supine exercise, workload-related increases in SNA are also inhibited by the increased central blood volume (CBV) occurring in Phase 1 of the venous return (Wasserman, 1994), which loads the cardiopulmonary baroreceptors (CPBRs) (Fadel & Raven, 2012).
Reciprocal antagonism, a proposed mechanism by which the continuous increase in workload-related HR is enabled, works through the opposition of one branch of the ANS to the effect of the other branch of the ANS in the central nervous system (CNS) and at the end organ in the peripheral nervous system (PNS). Ostensibly the inhibition in the periphery is required to prevent competitive effects taking place within an organ with dual innervations (Levy, 1971). At the sinoatrial node, reciprocal antagonism requires that a portion of the noradrenaline released at the sympathetic nerve terminals binds to the inhibitory adrenoreceptors on the post-ganglionic parasympathetic neurons inhibiting the release of acetylcholine (ACh), thereby accentuating the increase in HR caused by direct excitatory action of SNA. Conversely, ACh binds muscarinic receptors on the sympathetic post-ganglionic neurons to inhibit the release of noradrenaline (Uijtdehaage & Thayer, 2000) and accentuates the decrease in HR for a given amount of PSNA. This mechanism is useful in reflexive actions, such as the ABR, in which changes in HR and cardiac output are adjusted on a beat-to-beat basis (Levy, 1971).
In the transition from rest to progressive increases in exercise workload, our analysis identifies that the dynamic (reflex-mediated) influence on HR of the sympatho–vagal balance progresses to a sympatho-dominant balance but not before an initial increase in a parasympathetic dominance results from CPBR inhibition of SNA (Figs 3 and 5). The occurrence of this SNA inhibition following the onset of exercise was confirmed by reports that muscle sympathetic nerve activity briefly decreased at the onset of leg exercise, and returned to and then exceeded baseline at approximately 140 beats min−1 (Ichinose et al. 2008). In addition, the CBR–HR response range is increased at lower exercise workloads (Fig. 1A), which supports the claim that reciprocal antagonism is a dominant mechanism in the sympatho–vagal reflex control of HR throughout progressive increases in exercise workload. Bevegard & Shepherd (1966) reported similar results when they stimulated the carotid baroreceptors using NS and elicited the same decrease in HR from rest to exercising HRs of 120 beats min−1, after which responses were diminished. These earlier results confirm the present Fig. 3 as a valid representation of the sympatho–vagal balance in the control of HR during progressive increases in dynamic exercise workload.
Sympatho–vagal balance has traditionally been thought of as akin to a see-saw in a playground. When one side goes up, the other comes down. During exercise the fulcrum of that see-saw is elevated (resetting) so that the blood pressures (BPs) eliciting the baroreflexes are allowed to be higher for increased steady state conditions with the ability to produce similar reflexive magnitude changes in HR (Fig. 3). This is evident in the data of Potts et al. (1993), Fadel et al. (2001), Volianitis et al. (2004) and Ogoh et al. (2005), all of whom showed that ABR is reset to higher BPs as exercise workload increases. The mechanism for the resetting of the baroreflexes has not been completely identified. It is postulated that CC has a significant role in the fulcrum of the see-saw's resetting to a higher OP pressure, resulting in an increase in sympathetic dominance of the system and a subsequent decrease in the ability of the PSNS to modulate HR (Fig. 3). As Ogoh et al. (2005) reported, baroreflex changes in beat-to-beat HR are attributable to the PSNS and the shift to a more sympathetically dominant interaction at higher exercise workloads would explain the decrease in the response range of the carotid–HR baroreflex.
More recently, other neuromodulators, such as nitric oxide (NO), neuropeptide Y and natriuretic peptides within the heart, have been found to act as co-transmitters and to interact in the neuronal cyclic nucleotide-dependent pathways to modulate PSNA and SNA locally (Mohan et al. 2000). It has been reported that SNA-mediated HR changes are dependent on a pre-ganglionic mechanism involving NO (Mohan et al. 2000). This mechanism was found to be enhanced after exercise training and to cause a reduction in the cardiac sympathetic response; however, the NO influence on chronotropic response after exercise training was found to be probably minimal because a significant decrease in training response occurred even with NO inhibition (Mohan et al. 2000). Whether or not local reactive oxygen/nitrogen species (RONS) at the sinoatrial node scavenge the local NO, allowing increased SNA and resulting in a greater antagonism of the PSNS, remains to be established. Another set of neuromodulators with the mechanistic potential to inhibit PSNA refer to the rapid change in opioid receptor populations and locally produced enkaphalins on the sinoatrial node (Napier et al. 1998; Farias et al. 2001; Jackson et al. 2001). However, the roles of opioid neuromodulators in further accentuating the SNA/PSNA antagonism have yet to be determined.
Data providing additional support of an active sympatho–vagal balance at exercise onset were reported by Takahashi et al. (2004), who found that voluntary static arm exercise in tetraplegic subjects, who lack supraspinal sympathoadrenal control but have intact vagal control, does not induce the increases in HR associated with static exercise in healthy subjects. Therefore, if vagal withdrawal were the primary mechanism by which HR increased at exercise onset, the increase in HR at the onset of static exercise in tetraplegic subjects with an intact PSNS would be the same as in normal subjects. The fact that the HR response in tetraplegic subjects was lower than in healthy normal subjects indicates that increases in SNA and the presence of a sympatho–vagal balance are required for the physiological HR response of healthy subjects to occur (Takahashi et al. 2004).
However, using the fictive exercising decerebrate cat model, it was recently demonstrated that both cardiac sympathetic and parasympathetic neuron activity were increased at the onset of spontaneous fictive movement (Kadowaki et al. 2011; Matsukawa, 2012). One explanation for this paradoxical increase in both branches of the ANS at exercise onset is that it is a redundant mechanism to protect against an exacerbated increase in HR caused by immediate CC-induced increases in cardiac SNA. Unfortunately, without measurements of neurotransmitter release at the end organ, the assumption of the presence of redundant mechanisms cannot be confirmed. Furthermore, it has been reported that muscle sympathetic nerve activity decreases at the onset of dynamic leg cycling (Saito et al. 1993; Ichinose et al. 2008) because the muscle pump increases venous return and loads the CPBRs (Saito et al. 1993). Hence, it is possible that the findings of Kadowaki et al. (2011) and Matsukawa (2012) indicate the presence of a differential control of muscle and cardiac SNA. Another possible explanation is that in the non-physiological decerebrate cat studies resulting in fictive movements, the muscle pump activity may not have been of sufficient strength to increase venous return to load the CPBRs in a manner similar to that observed in exercising humans. The lack of CPBR loading would account for the increases in cardiac SNA at the start of fictive movement in cats (Matsukawa, 2012). However, the increase in PSNA at the onset of fictive movement identified by Kadowaki et al. (2011) and Matsukawa (2012) is contrary to the concept of CC-related ABR resetting to an increased ‘set point’ pressure and the vagal withdrawal-mediated increase in HR, which have been shown in similar preparations by McMahon & McWilliam (1992). The decerebrate cat studies should be interpreted with caution because the nature of the preparation may not be physiologically relevant as many autonomic control regions were removed.
In humans, the autonomic control of the cardiovascular system involves a number of cerebral cortical and subcortical structures. Integration of afferent pain signals has been indicated in the sensorimotor cortex, anterior cingulate cortex and the thalamus (Kropotov et al. 1997; Rainville et al. 1997). The insular cortex has received much attention as a result of its many subcortical neural connections, which have been implicated in BP regulation and baroreflex control (Ruggiero et al. 1987; Saleh & Connell, 1998). Williamson et al. (2001) reported increased activity in the insular cortex, thalamus and anterior cingulate cortex during a hypnotically suggested increase in cycling workload. The increased cortical activity was mirrored by increases in BP and HR. These effects were said to be related to ‘effort sense’, which may be part of the mechanism for CC-activated increases in cardiovascular variables. Further studies into the cortical pathways eliciting the cardiovascular responses have utilized deep brain stimulation. Thornton et al. (2002) identified that electrical stimulation of the thalamus, subthalamic nucleus and substantia nigra all elicit increases in HR and BP similar to those induced by exercise. These increases were accompanied by the facilitation of movement alluding to these subcortical structures as part of the CC pathway. A key location in the brain for anticipatory cardiorespiratory responses prior to the onset of exercise might be the periaquaductal grey (PAG) region (Green et al. 2007). This region is also likely to be integral in the transduction of the EPR (Basnayake et al. 2011). Given that the PAG is involved in the EPR and anticipation of exercise, it may prove to be a key location in the central resetting of the ABRs at the onset of exercise.
Another issue that confounds investigations into whether the parasympathetic influence on exercising HR is withdrawn or inhibited is the large number of published studies on HR variability (HRV) which conclude that the exercise-induced reduction in power spectral high-frequency (HF) peaks indicates parasympathetic withdrawal with increasing exercise workload. Because there is no parasympathetic neuron that is safe and accessible for nerve recordings in human studies, the measure of HRV is often used as an indicator of PSNA. It is well known that in healthy and diseased subjects, increased parasympathetic control of the heart at rest can be detected by time and frequency domain indices of HRV, especially in the HF peaks (Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology, 1996). However, a common error of interpretation is that the HF peaks in HRV spectra are indicators of ‘parasympathetic tone’, when in fact it is more accurate to say they describe ‘parasympathetic modulation’ (Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology, 1996). For example, drugs that inhibit cardiac muscarinic receptors on the sinoatrial node (atropine or glycopyrrolate) cause the HF peak to disappear as if in response to a ‘withdrawal of parasympathetic tone’. When the opposite is caused by pharmacological or electrical stimulation of the vagus nerve to increase parasympathetic tone, the HF peak also disappears. Changes in HF peaks do not indicate changes in parasympathetic tone (Napier et al. 1998) and what is actually identified by the change in the HF peak is parasympathetic modulation caused mostly by respiratory modulation of central vagal neurons. Furthermore, during exercise, increases in breathing frequency shift the HF peak to the frequency of breathing, thereby potentially eliminating the expected HF peak and leading to false conclusions about decreased parasympathetic activity. Current perspectives and animal data suggest there is a decrease in the modulating effect of the PSNS on HR during exercise caused by inhibition by increased SNA.
Conclusion
Our proposed models (Figs 4 and 5) depict the reflex and steady state roles, respectively, of the parasympathetic and sympathetic nervous systems throughout all exercise workloads. The research points to a system of control that does not have any clear on/off thresholds, but represents a continuum of balanced sympatho–vagal control (Fig. 4). It is important to think of sympatho–vagal balance not as representing the equal influence of two branches of the ANS, but as representing the interplay between the two for the short- and longterm modulation of HR, and to bear in mind that the PSNS is quick and short-lived, whereas the SNS can be tonically activated and has the ability to attenuate the PSNS influence.
As exercise is initiated, CC resets the ABR, which is immediately met by decreased PSNA and a slight decrease in SNA caused by increased venous return in phase 1 (Wasserman, 1994) and loading of the CPBRs, which enables the PSNS to exert greater modulation on the heart, resulting in a greater reflexive range during the initial HR increase. As exercise workload increases, the parallel increases in CC and EPR increase the ABR resetting, which, in turn, augments the SNA increases in HR and depresses the PSNA HR reflex response. The involvement of CC and EPR in ABR resetting during dynamic exercise was clearly identified by Strange et al. (1993), who established that combined blockade of input from CC and EPR resulted in the performance of exercise without increases in BP.
Further research will be required to elucidate the nervous system outputs versus functional contributions to changes in HR during exercise. A particular confounding factor will be the multiple linkages within the intrathoracic ganglia, which exert an effect on cardiodynamics that render the sum greater than the individual parts, making in situ examination essential (Armour, 2004). In situ examination of PSNA in humans will remain difficult until better methods of obtaining direct nerve recordings are developed.
Perspective
It is well established that age-related reductions in maximal HR and β1-adrenergic receptor blockade resulting in reduced maximal and sub-maximal HR in cardiac patients are symptoms of chronotropic incompetence (CI). In addition, we noted in our discussion that tetraplegic subjects, who lack supraspinal sympathoadrenal control but have intact vagal control, also exhibit CI. More recently, Mendonca et al. (2011) identified that individuals with Down's syndrome exhibit CI and autonomic dysfunction. Our reanalysis of recently published data (Ogoh et al. 2005), in conjunction with a review of the previously published data, identifies that CI may be related to functional inhibition of the PSNA by the SNA mechanism of reciprocal antagonism (Uijtdehaage & Thayer, 2000). Other interesting targets for CI are cellular mechanisms which affect neuronal cyclic nucleotide-dependent pathways involving NO, neuropeptide Y, natriuretic peptides and opioid receptors in autonomic neurons at the sinoatrial node (Mohan et al. 2000; Farias et al. 2001; Jackson et al. 2001; Adlam et al. 2012).
Acknowledgments
The authors thank all subjects and collaborating investigators for their time and cooperation in the initial studies in which original data were collected.
Glossary
- ABP
arterial blood pressure
- ABR
arterial baroreflex
- ANS
autonomic nervous system
- CBR
carotid baroreflex
- CBV
central blood volume
- CC
central command
- CI
chronotropic incompetence
- CNS
central nervous system
- CPBR
cardiopulmonary baroreceptors
- EPR
exercise pressor reflex
- HF
high frequency
- HR
heart rate
- HRV
heart rate variability
- NO
nitric oxide
- NP
neck pressure
- NS
neck suction
- OP
operating point
- PAG
periaquaductal grey
- PNS
peripheral nervous system
- PSNA
parasympathetic nervous activity
- PSNS
parasympathetic nervous system
- RONS
reactive oxygen/nitrogen species
- SNA
sympathetic nervous activity
- SNS
sympathetic nervous system
Additional information
Competing interests
None declared.
Author contributions
D.W.W. analyzed data, calculated models, and prepared figures. D.W.W. and P.B.R. prepared the manuscript. Both authors approved the final version of the manuscript.
Funding
The studies from which the data were reanalysed were supported in part by funds from the National Heart, Lung and Blood Institute (grant HL-045547) to P.B.R., the Texas Chapter of the American College of Sports Medicine Student Research Development Award 2012 to D.W.W., and the Cardiovascular Research Institute and Department of Integrative Physiology at the University of North Texas Health Science Center, Fort Worth, TX, USA.
References
- Adlam D, Herring N, Douglas G, De Bono JP, Li D, Danson EJ, Tatham A, Lu CJ, Jennings KA, Cragg SJ, Casadei B, Paterson DJ, Channon KM. Regulation of β-adrenergic control of heart rate by GTP-cyclohydrolase 1 (GCH1) and tetrahydrobiopterin. Cardiovasc Res. 2012;93:694–701. doi: 10.1093/cvr/cvs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol. 2004;287:R262–R271. doi: 10.1152/ajpregu.00183.2004. [DOI] [PubMed] [Google Scholar]
- Bainbridge FA. On some cardiac reflexes. J Physiol. 1914;48:332–340. doi: 10.1113/jphysiol.1914.sp001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basnayake SD, Hyam JA, Pereira EA, Schweder PM, Brittain JS, Aziz TZ, Green AL, Paterson DJ. Identifying cardiovascular neurocircuitry involved in the exercise pressor reflex in humans using functional neurosurgery. J Appl Physiol (1985) 2011;110:881–891. doi: 10.1152/japplphysiol.00639.2010. [DOI] [PubMed] [Google Scholar]
- Bevegard BS, Shepherd JT. Circulatory effects of stimulating the carotid arterial stretch receptors in man at rest and during exercise. J Clin Invest. 1966;45:132–142. doi: 10.1172/JCI105317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo SE, Bishop VS. Onset of exercise shifts operating point of arterial baroreflex to higher pressures. Am J Physiol Heart Circ Physiol. 1992;262:H303–H307. doi: 10.1152/ajpheart.1992.262.1.H303. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2001;280:H1383–H1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol. 2012;97:39–50. doi: 10.1113/expphysiol.2011.057554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias M, Jackson K, Stanfill A, Caffrey JL. Local opiate receptors in the sinoatrial node moderate vagal bradycardia. Auton Neurosci. 2001;87:9–15. doi: 10.1016/S1566-0702(00)00244-7. [DOI] [PubMed] [Google Scholar]
- Fisher JP. Autonomic control of the heart during exercise in humans: role of skeletal muscle afferents. Exp Physiol. 2013;99:300–305. doi: 10.1113/expphysiol.2013.074377. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH, Fadel PJ. Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol. 2010;588:1117–1127. doi: 10.1113/jphysiol.2009.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL, Wang S, Purvis S, Owen SL, Bain PG, Stein JF, Guz A, Aziz TZ, Paterson DJ. Identifying cardiorespiratory neurocircuitry involved in central command during exercise in humans. J Physiol. 2007;578:605–612. doi: 10.1113/jphysiol.2006.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley LH, Mason JW, Hogan RP, Jones LG, Kotchen TA, Mougey EH, Wherry FE, Pennington LL, Ricketts PT. Multiple hormonal responses to graded exercise in relation to physical training. J Appl Physiol. 1972;33:602–606. doi: 10.1152/jappl.1972.33.5.602. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol. 2008;586:2753–2766. doi: 10.1113/jphysiol.2007.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KE, Farias M, Stanfill A, Caffrey JL. Delta opioid receptors inhibit vagal bradycardia in the sinoatrial node. J Cardiovasc Pharmacol Ther. 2001;6:385–393. doi: 10.1177/107424840100600408. [DOI] [PubMed] [Google Scholar]
- Kadowaki A, Matsukawa K, Wakasugi R, Nakamoto T, Liang N. Central command does not decrease cardiac parasympathetic efferent nerve activity during spontaneous fictive motor activity in decerebrate cats. Am J Physiol Heart Circ Physiol. 2011;300:H1373–H1385. doi: 10.1152/ajpheart.01296.2010. [DOI] [PubMed] [Google Scholar]
- Katona PG, Martin PJ, Jih F. Neural control of heart rate: a conciliation of models. IEEE Trans Biomed Eng. 1976;23:164–166. doi: 10.1109/tbme.1976.324579. [DOI] [PubMed] [Google Scholar]
- Katona PG, McLean M, Dighton DH, Guz A. Sympathetic and parasympathetic cardiac control in athletes and nonathletes at rest. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:1652–1657. doi: 10.1152/jappl.1982.52.6.1652. [DOI] [PubMed] [Google Scholar]
- Kropotov JD, Crawford HJ, Polyakov YI. Somatosensory event-related potential changes to painful stimuli during hypnotic analgesia: anterior cingulate cortex and anterior temporal cortex intracranial recordings. Int J Psychophysiol. 1997;27:1–8. doi: 10.1016/s0167-8760(97)00785-x. [DOI] [PubMed] [Google Scholar]
- Levy MN. Sympathetic–parasympathetic interactions in the heart. Circ Res. 1971;29:437–445. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- Matsukawa K. Central command: control of cardiac sympathetic and vagal efferent nerve activity and the arterial baroreflex during spontaneous motor behaviour in animals. Exp Physiol. 2012;97:20–28. doi: 10.1113/expphysiol.2011.057661. [DOI] [PubMed] [Google Scholar]
- McIlveen SA, Hayes SG, Kaufman MP. Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol Heart Circ Physiol. 2001;280:H1454–H1463. doi: 10.1152/ajpheart.2001.280.4.H1454. [DOI] [PubMed] [Google Scholar]
- McMahon SE, McWilliam PN. Changes in R–R interval at the start of muscle contraction in the decerebrate cat. J Physiol. 1992;447:549–562. doi: 10.1113/jphysiol.1992.sp019017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca GV, Pereira FD, Fernhall B. Cardiac autonomic function during submaximal treadmill exercise in adults with Down syndrome. Res Dev Disabil. 2011;32:532–539. doi: 10.1016/j.ridd.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Mitchell JH. J B Wolffe Memorial Lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22:141–154. [PubMed] [Google Scholar]
- Mohan RM, Choate JK, Golding S, Herring N, Casadei B, Paterson DJ. Peripheral pre-synaptic pathway reduces the heart rate response to sympathetic activation following exercise training: role of NO. Cardiovasc Res. 2000;47:90–98. doi: 10.1016/s0008-6363(00)00066-3. [DOI] [PubMed] [Google Scholar]
- Napier LD, Stanfill A, Yoshishige DA, Jackson KE, Barron BA, Caffrey JL. Autonomic control of heart rate in dogs treated chronically with morphine. Am J Physiol Heart Circ Physiol. 1998;275:H2199–H2210. doi: 10.1152/ajpheart.1998.275.6.H2199. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Rossi NF, Churchill PC. Substantial cardiac parasympathetic activity exists during heavy dynamic exercise in dogs. Am J Physiol Heart Circ Physiol. 1997;273:H2135–H2140. doi: 10.1152/ajpheart.1997.273.5.H2135. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Seamans DP. Effect of exercise on autonomic mechanisms of baroreflex control of heart rate. J Appl Physiol (1985) 1993;75:2251–2257. doi: 10.1152/jappl.1993.75.5.2251. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Dawson EA, White MJ, Secher NH, Raven PB. Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J Physiol. 2005;566:599–611. doi: 10.1113/jphysiol.2005.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973;53:159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 1993;265:H1928–H1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Raven PB. Neural control of the circulation: exercise. Exp Physiol. 2012;97:10–13. doi: 10.1113/expphysiol.2011.057406. [DOI] [PubMed] [Google Scholar]
- Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol. 2006;91:37–49. doi: 10.1113/expphysiol.2005.032250. [DOI] [PubMed] [Google Scholar]
- Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res. 1966;19:400–411. doi: 10.1161/01.res.19.2.400. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation Regulation during Physical Stress. New York, NY; Oxford: Oxford University Press; 1986. [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York, NY; Oxford: Oxford University Press; 1993. [Google Scholar]
- Ruggiero DA, Mraovitch S, Granata AR, Anwar M, Reis DJ. A role of insular cortex in cardiovascular function. J Comp Neurol. 1987;257:189–207. doi: 10.1002/cne.902570206. [DOI] [PubMed] [Google Scholar]
- Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ. Role of the insular cortex in the modulation of baroreflex sensitivity. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1417–R1424. doi: 10.1152/ajpregu.1998.274.5.R1417. [DOI] [PubMed] [Google Scholar]
- Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise in humans: role of muscle mass. Am J Physiol Heart Circ Physiol. 1989;257:H1812–H1818. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- Shepherd JT. The lungs as receptor sites for cardiovascular regulation. Circulation. 1981;63:1–10. doi: 10.1161/01.cir.63.1.1. [DOI] [PubMed] [Google Scholar]
- Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol. 1993;470:693–704. doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Sakaguchi A, Matsukawa K, Komine H, Kawaguchi K, Onari K. Cardiovascular control during voluntary static exercise in humans with tetraplegia. J Appl Physiol (1985) 2004;97:2077–2082. doi: 10.1152/japplphysiol.00546.2004. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thornton JM, Aziz T, Schlugman D, Paterson DJ. Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J Physiol. 2002;539:615–621. doi: 10.1113/jphysiol.2001.014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uijtdehaage SH, Thayer JF. Accentuated antagonism in the control of human heart rate. Clin Auton Res. 2000;10:107–110. doi: 10.1007/BF02278013. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Yoshiga CC, Vogelsang T, Secher NH. Arterial blood pressure and carotid baroreflex function during arm and combined arm and leg exercise in humans. Acta Physiol Scand. 2004;181:289–295. doi: 10.1111/j.1365-201X.2004.01292.x. [DOI] [PubMed] [Google Scholar]
- Wasserman K. Coupling of external to cellular respiration during exercise: the wisdom of the body revisited. Am J Physiol Endocrinol Metab. 1994;266:E519–E539. doi: 10.1152/ajpendo.1994.266.4.E519. [DOI] [PubMed] [Google Scholar]
- Williamson JW. The relevance of central command for the neural cardiovascular control of exercise. Exp Physiol. 2010;95:1043–1048. doi: 10.1113/expphysiol.2009.051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation. J Appl Physiol (1985) 2001;90:1392–1399. doi: 10.1152/jappl.2001.90.4.1392. [DOI] [PubMed] [Google Scholar]


