Abstract
Elevations of cardiomyocyte apoptosis and fibrotic deposition are major characteristics of the ageing heart. Resveratrol, a polyphenol in grapes and red wine, is known to improve insulin resistance and increase mitochondrial biogenesis through the SIRT1–PGC-1α signalling axis. Recent studies attempted to relate SIRT1 activation by resveratrol to the regulation of apoptosis in various disease models of cardiac muscle. In the present study, we tested the hypothesis that long-term (8-month) treatment of resveratrol would activate SIRT1 and improve the cardiac function of senescent mice through suppression of Foxo1-associated pro-apoptotic signalling. Our echocardiographic measurements indicated that the cardiac systolic function measured as fractional shortening and ejection fraction was significantly reduced in aged mice when compared with the young mice. These reductions, however, were not observed in resveratrol-treated hearts. Ageing significantly reduced the deacetylase activity, but not the protein abundance of SIRT1 in the heart. This reduction was accompanied by increased acetylation of the Foxo1 transcription factor and transactivation of its target, pro-apoptotic Bim. Subsequent analyses indicated that pro-apoptotic signalling measured as p53, Bax and apoptotic DNA fragmentation was up-regulated in the heart of aged mice. In contrast, resveratrol restored SIRT1 activity and suppressed elevations of Foxo1 acetylation, Bim and pro-apoptotic signalling in the aged heart. In parallel, resveratrol also attenuated the ageing-induced elevations of fibrotic collagen deposition and markers of oxidative damage including 4HNE and nitrotyrosine. In conclusion, these novel data demonstrate that resveratrol mitigates pro-apoptotic signalling in senescent heart through a deacetylation mechanism of SIRT1 that represses the Foxo1–Bim-associated pro-apoptotic signalling axis.
Key points
Cardiac function is impaired and Foxo1/Bim-related apoptotic signalling is up-regulated in senescent heart
Activation of SIRT1 deacetylase activity by resveratrol attenuates the Foxo1/Bim signalling axis in senescent heart
Introduction
Sirtuin 1 (SIRT1) protein is classified as a class III NAD+-dependent histone deacetylase. Activation of the non-mammalian orthologue Sir2 by resveratrol, a natural polyphenolic antioxidant in grapes and red wine, in yeast has been shown to remarkably extend the yeast lifespan by 70% (Howitz et al. 2003). Although resveratrol supplemented at a later life-stage was found to be unsuccessful in extending the lifespan of mice (Miller et al. 2011), it has been shown to promote mitochondrial biogenesis and improve insulin sensitivity through the deacetylation mechanism of SIRT1 (Lagouge et al. 2006). Intriguingly, resveratrol mimics the transcriptional effects of calorie restriction and attenuates various signs of ageing in different tissues including the aorta, bone, eye and skeletal muscle (Pearson et al. 2008). Calorie restriction, which involves a reduction of energy intake by ∼30%, is known to improve mitochondrial bioenergetics in the heart through the up-regulation of SIRT1, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), which is the master regulator of mitochondrial metabolism, and COXIV, which is a subunit of complex IV of the electron transport chain (Nisoli et al. 2005). Although acute treatments with whole grape powder and resveratrol were shown to prevent the suppression of PGC-1α transcription cascade in hearts of hypertensive rats (Seymour et al. 2010; Rimbaud et al. 2011), whether chronic resveratrol intervention might confer protective effects on the ageing heart in a similar manner or in concert with other signalling mechanisms is largely unclear.
Cardiac ageing is characterised by increased deposition of collagen fibres (Gazoti Debessa et al. 2001). This fibrotic process is considered as a pathogenic reparative response of the myocardium to compensate for the considerable loss of cardiomyocytes (Biernacka & Frangogiannis, 2011). The Foxo transcription factors have been implicated in the regulation of oxidative defence and apoptosis (Van Der Heide et al. 2004). The role of Foxo1 in cardiac pathologies, however, remains a subject of on-going debate. For example, over-expression of Foxo1 has been demonstrated to result in transcriptional activation of the anti-oxidative enzyme catalase (Alcendor et al. 2007), whereas in rejecting allografts the protein content of Foxo1 was reported to be reduced with a concomitant increase in cardiac fibrosis (Wei et al. 2012). In experimental models of diabetic cardiomyopathy induced by high-fat diet and leptin receptor deficiency, the level of nuclear Foxo1 was increased in conjunction with up-regulation of the atrophic factors atrogin and MuRF (Battiprolu et al. 2012). However, knock-out experiments indicated that compared with wild type mice, Foxo1-deficient mice were observed to exhibit better cardiac function and lower mortality when challenged with high-fat diet (Battiprolu et al. 2012). In cardiomyocytes, resveratrol, being a potent SIRT1 agonist, has been found to reduce the expression and nuclear localization of Foxo1 in hypoxic H9c2 cardiomyocyte cultures (Chen et al. 2009).
The activity of Foxo proteins is not solely dependent on the protein abundance but is also governed by post-translational modifications. It is well-established that Akt phosphorylates and inhibits the activity of Foxo proteins (Zhu et al. 2004; Shen et al. 2010; Jothi et al. 2013). The regulatory roles of acetylating/deacetylating mechanisms in Foxo1 activity, particularly in response to ageing and resveratrol treatment, are largely unresolved. It has been demonstrated that SIRT1 deacetylated Foxo1 to mediate the starvation-induced autophagy in cardiomyocytes (Hariharan et al. 2010) whereas the SIRT1 inhibitor nicotinamide was found to increase acetylation of Foxo1 and expression of pro-apoptotic Bim in human lung cancer cells (Yang et al. 2009). Provided that 35% calorie restriction for 6 months improves myocardial ischaemic tolerance in aged F344 rats (Shinmura et al. 2005) through increasing the level of nuclear SIRT1 to reduce activation of caspase 3 (Shinmura et al. 2008) whereas functional recovery of post-ischaemic hearts requires inhibition of Foxo1 acetylation by SIRT1 (Yang et al. 2013), it is plausible to postulate that SIRT1, as a deacetylase, might reduce apoptosis in the senescent heart through deacetylation and inhibition of Foxo1. Furthermore, the reduction of SIRT1 activity in the aged myocardium was accompanied by elevation of oxidative stress (Gu et al. 2013) whereas 30% calorie restriction for 5 weeks reduces lipid peroxidation and induces the activities of different superoxide dismutase isoforms in the heart of ethanol-treated rats (Vucevic et al. 2013).
In the present study, using SAMP8 mice (a mouse model for accelerated ageing), we tested the hypothesis that long-term resveratrol treatment would reduce acetylation of Foxo1, protein abundance of pro-apoptotic Bim, fibrotic collagen deposition and oxidative damage through the restoration of SIRT1 deacetylase activity, which would result in improvement of cardiac systolic function in ageing heart. The effects of ageing and long-term resveratrol feeding on mitochondrial energy production machinery were also examined.
Methods
Animals and experimental design
Male senescence accelerated mice prone (SAMP8) mice obtained from the Chinese University of Hong Kong were used in this study. Two-month-old mice were randomly assigned to a young group (Y), an aged group (AG) and an aged with resveratrol treatment group (AR) (N = 10 mice in each group). Resveratrol was incorporated in laboratory chow at a concentration of 167 mg kg−1 (Research Diets, New Brunswick, NJ, USA). For the AG group, mice were housed until the age of 10 months. For the AR group, two-month-old mice were housed likewise and were subject to dietary supplementation of 4.9 mg kg−1 day−1 resveratrol for a period of 8 months. The dosage of resveratrol was based on previous findings that demonstrated long-term administration of 4.9 mg kg−1 day−1 resveratrol improves the cardiac function of aged mice (Barger et al. 2008). All animals were housed in pathogen-free conditions at an ambient temperature of ∼20°C in the Centralised Animal Facilities of The Hong Kong Polytechnic University. The animals were exposed to light-controlled environment with a 12:12 h of light:dark cycle each day. All mice were fed with standard nutrient diet and water ad libitum throughout the study period. All experimental procedures in the present study were carried out with approval from the Animal Subjects Ethics Subcommittee of The Hong Kong Polytechnic University. Mice reaching the respective ages (2-month-old for mice in the Y group and 10-month-old for mice in AG and AR groups) were subject to non-invasive echocardiography. Mice were then sacrificed by overdose of ketamine and xylazine and the heart tissues were rapidly excised, quickly frozen and stored at −80°C for further analyses.
Echocardiographic assessment
Mice were anaesthetised with intraperitoneal injection of 10 mg kg−1 ketamine Alfasan, Woerden, the Netherlands. The ventral throax region was then shaved followed by application of the ultrasound conduction gel. Echocardiography was performed in the prone decubitus position with an Esaote MyLab 70 X-Vision Ultrasound System (Esaote, Genova, Italy). Cardiac structures were assessed by two-dimensional grey-scale ultrasound scanning in the parasternal short-axis view at the mid-papillary level. The grey-scale echocardiographic view was used to position the M-mode echocardiographic line. Parameters including left ventricle end-systolic dimension (LVESD), left ventricle end-diastolic dimension (LVEDD), anterior wall thickness (AWT), posterior wall thickness (PWT) and heart rate (HR) were measured under M-mode scanning according to the leading-edge method of the American Society of Echocardiography (Lang et al. 2006). Systolic function measured as fractional shortening (FS) and ejection fraction (EF) was calculated by the equations for FS and EF:
where Y = [(LVEDD2 − LVESD2)/LVEDD2] × 100. All results were presented as the averaged values over three consecutive cardiac cycles.
Western blotting
Cytoplasmic protein fractions were extracted by following the protocol as described by Sin et al. 2013. Protein concentration was determined in duplicate by the Bradford assay (Coomassie Protein Assay, Pierce, Rockford, IL, USA) with bovine serum albumin (BSA) as the standard. Protein extracts were boiled at 95°C for 5 min in Laemmli buffer with 5% β-mercaptoethanol. Forty micrograms of protein were loaded on 10% polyacrylamide gel and subject to electrophoretic separation by SDS–PAGE. The proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon P, Millipore, Billerica, MA, USA) at 300 mA for 2 h in 1× transfer buffer containing 20% methanol except for COL1A1 in 1× transfer buffer containing 5% methanol. Equal loading and transfer efficiency were verified by staining gels with Coomassie blue and membranes with Ponceau S red. After the transfer, the membranes were blocked in 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20 (TBST) for 1 h at room temperature followed by overnight incubation at 4°C with the corresponding primary antibodies: anti-SIRT1 rabbit polyclonal antibody (1:500 dilution; 15404, Santa Cruz, CA, USA), anti-Ac-FKHR rabbit polyclonal antibody (1:200 dilution; 49437, Santa Cruz), anti-FKHR rabbit polyclonal antibody (1:500 dilution; 11350, Santa Cruz), anti-Bim rabbit polyclonal antibody (1:500 dilution; 11425, Santa Cruz), anti-p53 mouse monoclonal antibody (1:200 dilution; 56179, Santa Cruz), anti-Bax rabbit polyclonal antibody (1:200 dilution; 493, Santa Cruz), anti-Bcl2 rabbit monoclonal antibody (1:1000 dilution; 2870, Cell Signalling, Danvers, MA, USA), anti-COL1A1 goat polyclonal antibody (1:100 dilution; 8784, Santa Cruz), anti-4HNE mouse monoclonal antibody (1:1000 dilution; 24327, Oxis, Portland, OR, USA), anti-nitrotyrosine mouse monoclonal antibody (1:200 dilution; 32731, Santa Cruz), anti-PGC1 rabbit polyclonal antibody (1:500 dilution; 13067, Santa Cruz) or anti-OXPHOS mouse antibody cocktail (1:1000 dilution; 604, MitoSciences, Eugene, OR, USA) diluted in TBST with 2% bovine serum albumin (BSA). The membranes were washed three times in TBST prior to incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 1 h (1:3000 dilution; 7076 for anti-mouse antibody and 7074 for anti-rabbit antibody, Cell Signalling; 2020 for anti-goat antibody, Santa Cruz). Luminol reagent (NEL103001EA, Perkin Elmer, Waltham, MA, USA) for chemiluminescent detection of HRP was then applied. The chemiluminescent signal was captured with a Kodak 4000R Pro camera. The resulting bands were quantified as optical density (OD) × band area and expressed as arbitrary units. β-Tubulin (1:2000 dilution; T0198, Sigma Aldrich, St Louis, MO, USA) was probed and used as the reference of internal control. Data were expressed by normalising to the signal of β-tubulin.
SIRT1 deacetylation assay
Deacetylase activity of SIRT1 was assessed by a fluorometric assay (Cyclex, Nagoya, Aichi, Japan) in accordance to the manufacturer's instructions. Briefly, a reaction mixture containing 1 mm fluoro-substrate peptide, 5 mAU lysylendopeptidase, 2 mm NAD+, 50 mm Tris-HCl–0.5 mm DTT-containing SIRT1 assay buffer, 1 μm Trichostatin A (a NAD+-independent histone deacetylase inhibitor) was prepared. The reaction was initiated by adding 5 μl protease inhibitor-free cardiac protein extracts under thorough mixing. Fluorescence intensity (excitation 340 nm, emission 460 nm) was measured by a microplate fluorometer (Infinite F200, Tecan, Mannedorf, Switzerland) immediately and at 1 min intervals. All readings were normalised to the protein contents of the respective samples.
Complex IV activity assay
The activity of complex IV was measured by a colorimetric cytochrome oxidase activity assay kit (BioVision, Milpitas, CA, USA) following the manufacturer's recommendations. A working solution of reduced cytochrome c was prepared at room temperature and was added to the protein samples without prior treatment with protease inhibitor. The working compounds were mixed thoroughly and subject to an immediate measurement at 550 nm using a microplate reader (Infinite F200, Tecan). Subsequent readings were obtained at regular 30 s intervals. All results were normalised to the protein concentrations of the respective samples.
Cell death ELISA assay
Apoptotic DNA fragmentation was assessed using the Cell Death ELISA Assay (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer's instructions. In brief, muscle homogenates were added in duplicate after coating the wells of a 96-well plate with primary anti-histone mouse monoclonal antibody. The wells were then subject to incubation with a peroxidase-linked secondary anti-DNA mouse monoclonal antibody. The respective immunocomplex peroxidase activity in each well was measured at 405 nm by a fluorometric microplate reader (Infinite F200, Tecan) following the addition of 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate) (ABTS) substrate.
Masson's trichrome staining
Cardiac fibrosis was assessed by using a Masson's trichrome staining kit (Sigma Aldrich) according to the manufacturer's instructions. Frozen left ventricle cross-sections 10 μm thick were prepared in a freezing cryostat at −20°C. The sections were fixed in Bouin's solution overnight and incubated with Weigert's Haematoxylin solution for 10 min at room temperature. After a quick wash in distilled water, sections were subject to incubation with Biebrich scarlet-acid fuchsin solution for 5 min. Collagen staining was performed by adding Aniline Blue solution following thorough washing. After incubation with 1% glacial acetic acid, the sections were then washed, dehydrated and mounted. Slides were then examined by light microscopy (Biological Research Microscope 80i, Nikon Melville, NY, USA) equipped with digital camera (DXM 1200c, Nikon). All histological analyses were conducted in four random, non-overlapping image fields captured using the 20× objective and the average results were reported. All areas with blue stain, which indicate the presence of collagen deposition, were quantified by using the histogram function of Photoshop software (Adobe system, San Jose, CA, USA) to obtain the number of pixels within the range of blue colour. The total number of pixels in each image was also counted. The level of collagen deposition was calculated by (number of pixels within the blue colour range/total number of pixels) × 100. All results were expressed as the percentage of fibrotic area relative to the total image area.
Statistical analysis
Statistical analyses were performed using the SPSS 18.0 software package (IBM, Chicago, IL, USA). A normality test was performed to examine data distributions. All data were expressed as means ± standard error of the mean (SEM). Comparisons were made by one-way ANOVA followed by Tukey's post hoc test. Relationships between given variables were examined by computing the Pearson product-moment correlation coefficient (r). Statistical differences were considered significant at P < 0.05.
Results
Cardiac systolic function
Representative echocardiographic images obtained in young (Y), aged (AG) and resveratrol-treated aged (AR) mice are shown in Fig. 1. Left ventricular end-systolic dimension (LVESD) was increased significantly in aged mice relative to young mice whereas resveratrol was observed to abolish this elevation (Table 1). Besides, resveratrol abrogated the ageing-induced reduction in systolic function measured as fractional shortening (FS) and ejection fraction (EF) (Y vs. AG vs. AR: FS, 66.3% vs. 43.3% vs. 64.1% and EF, 90.3% vs. 72.4% vs. 88.2%; Table 1). Measurements of LVEDD, AWT, PWT and HR were not found to be significantly different among groups (Table 1).
Figure 1. Changes in contractile parameters with ageing and resveratrol treatment in the heart.
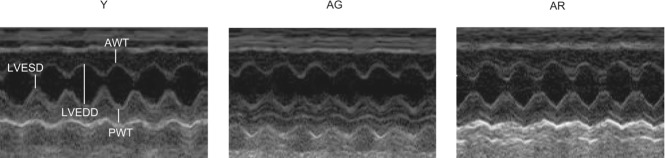
SAMP8 mice at respective ages were subject to non-invasive echocardiographic measurements. Fractional shortening (FS) and ejection fraction (EF) were significantly reduced in aged hearts relative to young hearts whereas these ageing-associated reductions were not observed in resveratrol-treated mice. Y, young mice; AG, aged mice; AR, aged mice with resveratrol treatment.
Table 1.
Echocardiographic parameters
| Y | AG | AR | |
|---|---|---|---|
| FS (%) | 66.3 ± 0.95 | 43.3 ± 1.83* | 64.1 ± 3.07# |
| EF (%) | 90.3 ± 0.54 | 72.4 ± 1.74* | 88.2 ± 2.17# |
| LVESD (cm) | 0.098 ± 0.003 | 0.154 ± 0.002* | 0.098 ± 0.006# |
| LVEED (cm) | 0.291 ± 0.006 | 0.279 ± 0.011 | 0.279 ± 0.011 |
| AWT (cm) | 0.123 ± 0.002 | 0.123 ± 0.003 | 0.122 ± 0.003 |
| PWT (cm) | 0.112 ± 0.003 | 0.132 ± 0.004 | 0.112 ± 0.002 |
| HR (beats min–1) | 551.9 ± 8.07 | 558.8 ± 8.91 | 551.9 ± 7.32 |
Non-invasive echocardiography was performed to assess the effects of ageing and resveratrol treatment on cardiac contractile performance in SAMP8 mice. Parameters including left ventricle end-systolic dimension (LVESD), left ventricle end-diastolic dimension (LVEDD), anterior wall thickness (AWT), posterior wall thickness (PWT) and heart rate (HR) were measured.
P < 0.05, aged group relative to young group;
P < 0.05, aged group treated with resveratrol compared to aged group. Y, young mice; AG, aged mice; AR, aged mice with resveratrol treatment.
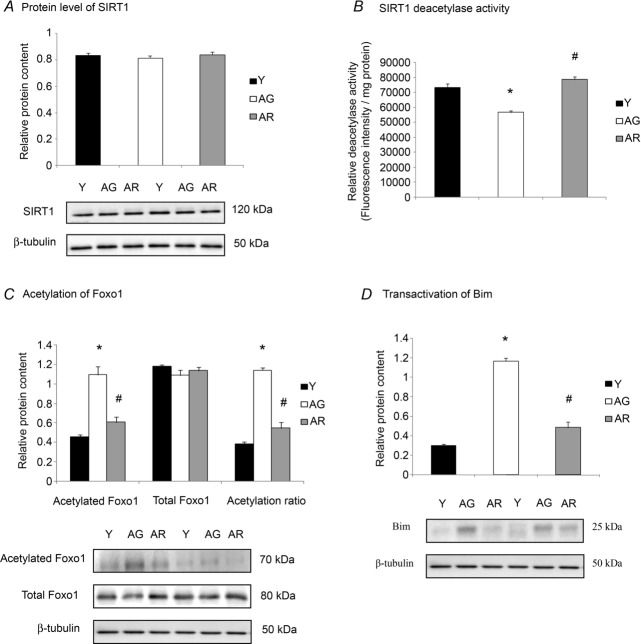
SIRT1 and Foxo1 signalling
The protein abundance of SIRT1 was not significantly different between the hearts of young and aged groups (Fig. 2A). The protein level of SIRT1 was not found to be altered significantly in resveratrol-treated aged mice when compared with untreated aged mice (Fig. 2A). However, ageing reduced significantly the deacetylase activity of SIRT1 by 21% whereas this reduction was not observed in resveratrol-treated aged mice (Fig. 2B). In support of the reduction of SIRT1 deacetylase activity in the aged heart, ageing increased significantly the acetylation of Foxo1, indicated by the 140% increase in acetylated Foxo1 content and 196% elevation of the ratio of acetylated Foxo1 to total Foxo1 (Fig. 2C). This age-associated elevation of Foxo1 acetylation was coincident with a significant increase in pro-apoptotic Bim, which is a transcriptional target of Foxo1, by 289% (Fig. 2D). By comparing resveratrol-treated aged mice with untreated aged mice, resveratrol was observed to reduce the acetylation of Foxo1 and protein abundance of Bim (Fig. 2C and 2D).
Figure 2. Modulation of SIRT1–Foxo1 signalling by ageing and resveratrol treatment in the heart.
A, the level of SIRT1 protein was not significantly altered in response to ageing and resveratrol treatment. B, the ageing-associated reduction of SIRT1 deacetylase activity, as assessed by a commercially available fluorometric assay was prevented by resveratrol supplementation. C and D, Foxo1 signalling was studied by measuring its acetylation status (C) and the expression of its transcriptional target, Bim (D). *P < 0.05, aged group relative to young group; #P < 0.05, aged group treated with resveratrol compared to aged group. Y, young mice; AG, aged mice; AR, aged mice with resveratrol treatment.
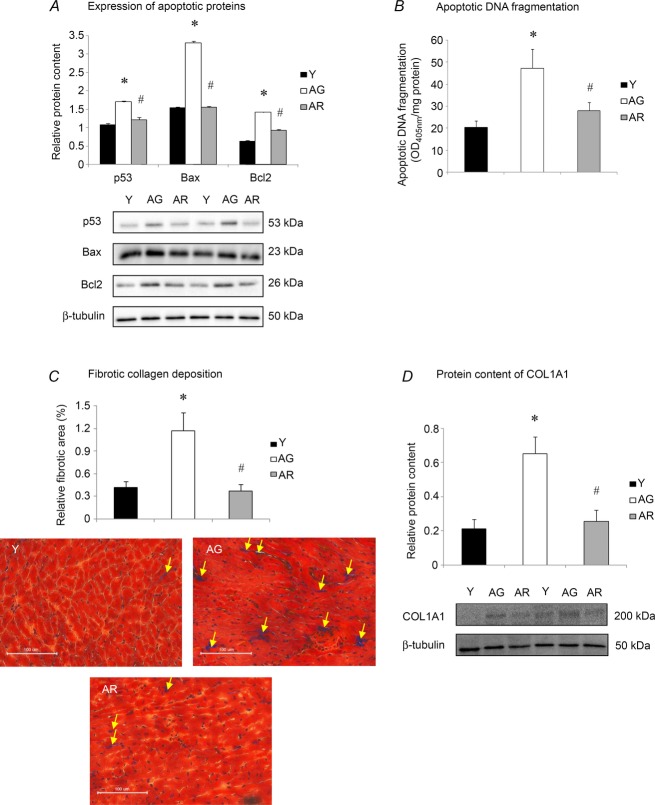
Cardiac apoptosis and fibrosis
Our immunoblot analysis revealed that ageing increased significantly the protein content of p53 by 58% whereas this elevation was attenuated by resveratrol treatment (Fig. 3A). The protein expression of the apoptotic regulators Bax and Bcl2 were observed to exhibit a similar change in pattern. The protein levels of Bax and Bcl2 were elevated significantly by 114% and 126%, respectively, in response to ageing but these increases were observed to be ameliorated in resveratrol-treated aged mice (Fig. 3A). Moreover, ageing increased significantly apoptotic DNA fragmentation by 132% (Fig. 3B) and this elevation was abolished by resveratrol treatment (Fig. 3B). To assess cardiac fibrosis, the content of collagen was measured by Masson's trichrome staining and Western blotting using an anti-COL1A1 antibody. Fibrotic collagen deposition was increased significantly in the heart of aged mice relative to young mice (Fig. 3C). However, resveratrol reduced significantly collagen deposition measured as fibrotic area in the aged heart (Fig. 3C). These observations were in agreement with our immunoblot analysis demonstrating that the protein content of COL1A1 was elevated significantly by 206% in aged hearts relative to their young counterparts whereas this increase was abolished by resveratrol treatment (Fig. 3D).
Figure 3. Changes of apoptotic signalling and cardiac fibrosis with ageing and resveratrol.
A, immunoblotting was performed to study the protein abundances of p53, Bax and Bcl2. B, the activation of pro-apoptotic signalling was assessed by determining apoptotic DNA fragmentation. C, cardiac fibrotic collagen deposition was examined by Masson's trichrome staining. D, the protein level of COL1A1 was measured by Western blotting. *P < 0.05, aged group relative to young group; #P < 0.05, aged group treated with resveratrol compared to aged group. Y, young mice; AG, aged mice; AR, aged mice with resveratrol treatment.
Oxidative and nitrosative stress
The level of 4HNE, which is a lipid peroxidation product indicative of elevated oxidative stress, was found to be elevated significantly, by 191%, in the aged heart when compared to young heart (Fig. 4A). Resveratrol alleviated significantly the ageing-induced increase of 4HNE in resveratrol-treated aged heart relative to the untreated aged heart (Fig. 4A). Similarly, the level of nitrotyrosine, which is a product formed from the nitrosylation reaction of protein tyrosine residues indicative of nitrosative stress, was observed to be increased by 114% in cardiac muscle of aged mice relative to the young controls (Fig. 4B); however, this elevation was found to be reduced significantly in resveratrol-treated aged mice (Fig. 4B).
Figure 4. Effects of ageing and resveratrol treatment on oxidative and nitrosative stress in the heart.
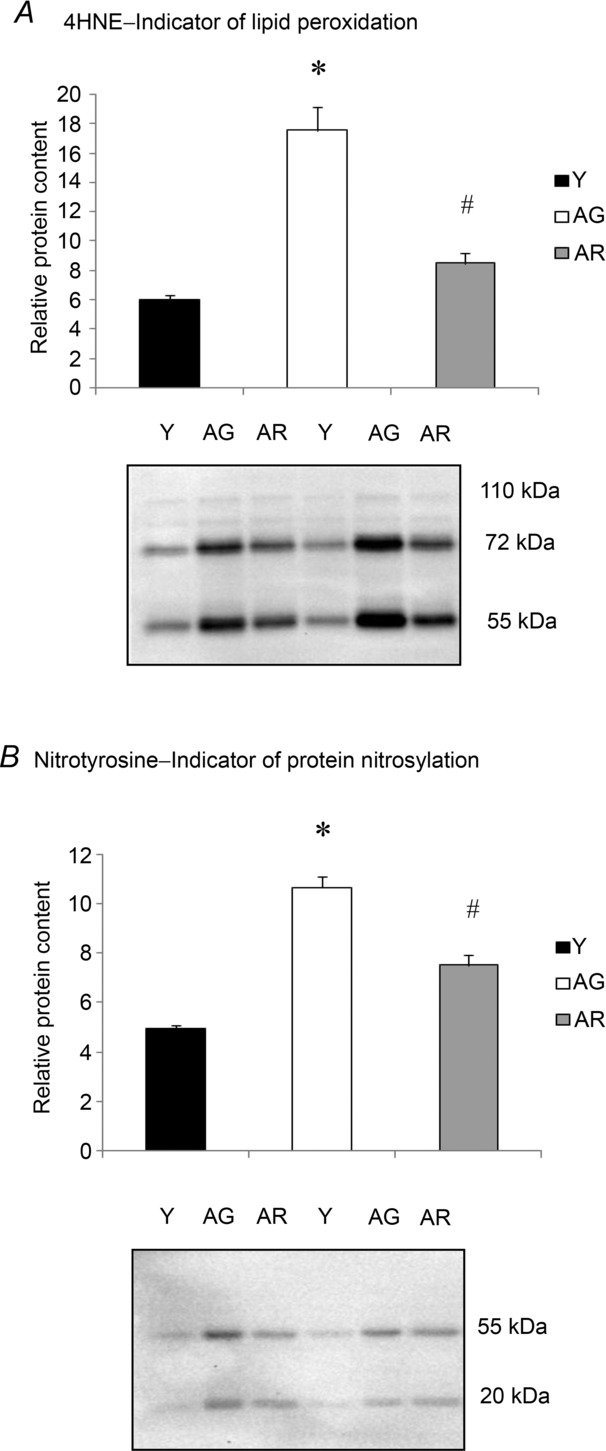
Western blot analyses indicated that resveratrol treatment significantly reduced the ageing-associated increases in lipid peroxidation measured as 4-HNE (A) and protein nitrosylation measured as nitrotyrosine (B) in the aged heart. *P < 0.05, aged group relative to young group; # P < 0.05, aged group treated with resveratrol compared to aged group. Y, young mice; AG, aged mice; AR, aged mice with resveratrol treatment.
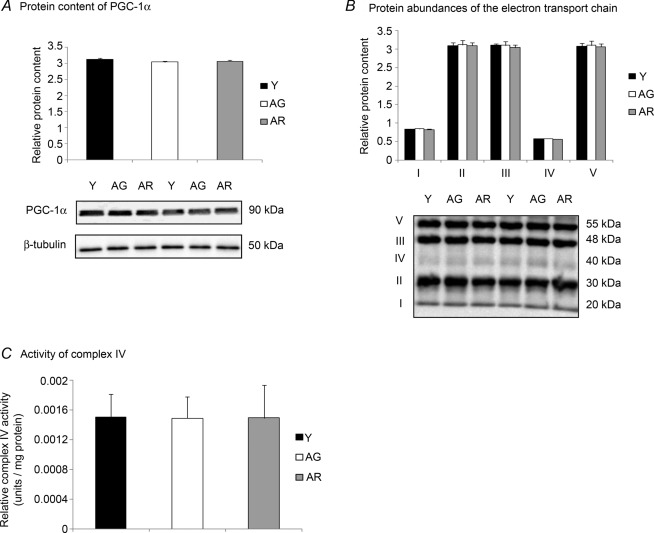
Mitochondrial energy production machinery
Despite our data indicating the activation of SIRT1 deacetylase activity by resveratrol in aged mice, our subsequent analyses did not suggest any modulating effects of ageing or resveratrol treatment on the signalling markers of energy metabolism. The protein level of PGC-1α was not significantly different between the hearts of young and aged mice (Fig. 5A). No significant alteration of the protein abundance of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) was found between resveratrol-treated aged mice and untreated aged mice (Fig. 5A). Consistently, our immunoblot analyses revealed that protein levels of all five complexes of the electron transport chain, namely complex I, II, III, IV and V, were not significantly different among all groups (Fig. 5B). To further confirm our findings that the mitochondrial energy production machinery was not affected by ageing and resveratrol treatment, complex IV activity was also examined. There was no effect of ageing on complex IV activity in the heart, regardless of resveratrol treatment (Fig. 5C).
Figure 5. Mitochondrial energy production machinery unchanged with ageing and resveratrol treatment in the heart.
A, the protein content of PGC-1α was examined by Western blotting. B, the protein levels of all 5 complexes of the electron transport chain were not significantly changed in all groups. C, the activity of complex IV was measured using a colorimetric assay. Y, young mice; AG, aged mice; AR, aged mice with resveratrol treatment.
Correlation analyses
The level of SIRT1 deacetylase activity was found to be correlated significantly with the acetylation level of Foxo1 (r = −0.669, P < 0.001; Fig. 6A), acetylation ratio of Foxo1 (r = −0.625, P < 0.001; Fig. 6B), protein expression of the Foxo1 transcriptional target Bim (r = −0.729, P < 0.001; Fig. 6C), pro-apoptotic proteins including p53 (r = −0.670, P < 0.001; Fig. 6D) and Bax (r = −0.823, P < 0.001; Fig. 6E) and markers of oxidative damage including 4HNE (r = −0.717, P < 0.001; Fig. 6F) and nitrotyrosine (r = −0.564, P = 0.003; Fig. 6G). Moreover, the level of acetylated Foxo1 was observed to be correlated significantly with the protein level of pro-apoptotic factors Bim (r = 0.820, P < 0.001; Fig. 6H), p53 (r = 0.793, P < 0.001; Fig. 6I) and Bax (r = 0.821, P < 0.001; Fig. 6J).
Figure 6. Correlation analyses of SIRT1 deacetylase activity and Foxo1 acetylation.
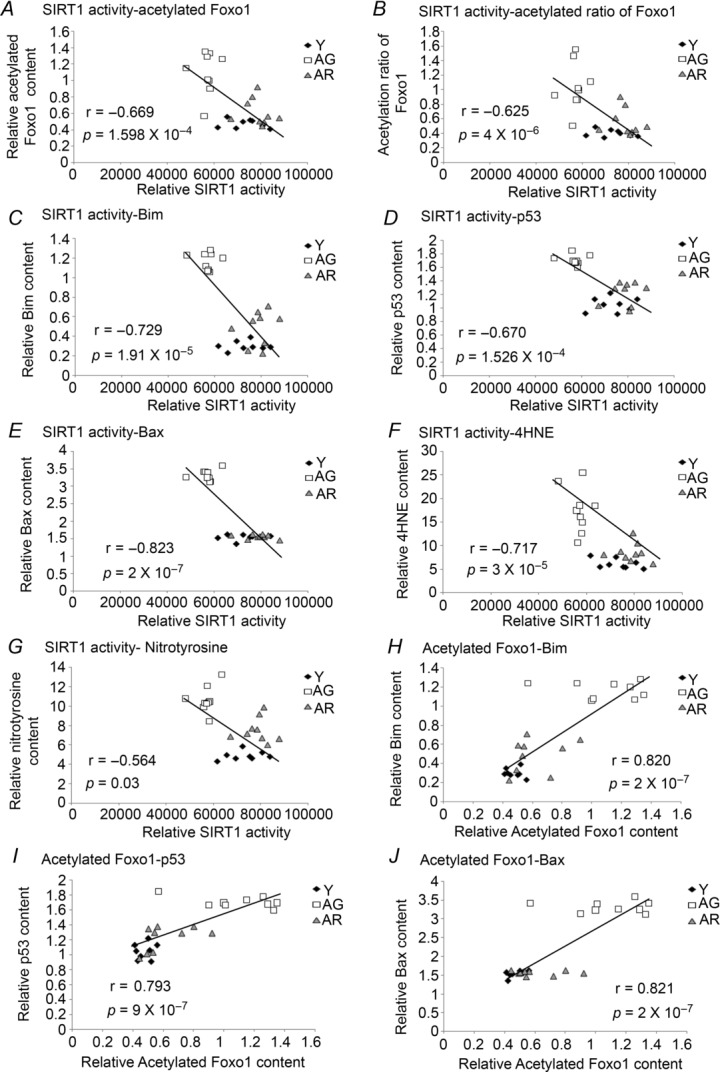
A–G, the deacetylase activity of SIRT1 was significantly negatively correlated with markers of Foxo1-associated pro-apoptotic signalling including acetylation of Foxo1 (A), ratio of acetylated Foxo1-to-total Foxo1 (B), Bim (C), p53 (D) and Bax (E) and oxidative stress including 4HNE (F) and nitrotyrosine (G). H–J, the level of acetylated Foxo1 was positively correlated with the expression of its transcriptional target, Bim (H) and the contents of pro-apoptotic proteins including p53 (I) and Bax (J). Y, young mice; AG, aged mice; AR, aged mice with resveratrol treatment.
Discussion
Resveratrol protects against the age-related reduction of cardiac function
Acute treatment with low dose resveratrol has been shown to preserve systolic function of the heart in various disease models. It has been demonstrated that 10 weeks of oral gavage with 2.5 mg kg−1 day−1 resveratrol alleviated the aberrant cardiac morphologies and prevented the decrease in ejection fraction in spontaneous hypertensive rats (Thandapilly et al. 2010) and a 5 day administration of 1 mg kg−1 day−1 resveratrol protected the diabetic heart from myocardial injury-induced reduction in dP/dt (maximal slope of systolic pressure increment; Huang et al. 2010). Furthermore, longer feeding with resveratrol (i.e. 4 months) was also shown to confer protection against the increase in ventricular wall thickness induced by high-fat diet (Qin et al. 2012). It has been revealed by serial echocardiography that fractional shortening and ejection fraction were reduced in aged mice (Boyle et al. 2011). However, whether long-term treatment with low dose resveratrol would improve systolic contractile parameters of the aged heart remains elusive. Here, we demonstrated that an 8 month intervention with 4.9 mg kg−1 day−1 resveratrol attenuates the ageing-induced reduction of fractional shortening and ejection fraction in a mouse model of accelerated ageing. However, whether this also applies to animals with natural ageing process remains to be determined.
Resveratrol activates SIRT1 and suppresses Foxo1-associated apoptotic signalling in senescent hearts
We tested the hypothesis that resveratrol would improve the cardiac function in a model of aged mice through activated SIRT1-mediated suppression of pro-apoptotic signalling. Although the expression of SIRT1 in the heart was neither affected by ageing nor resveratrol, the protein level of SIRT1 has been shown to be increased in the heart of aged Wistar rats (Braidy et al. 2011) and increased by resveratrol gavage to protect against the doxorubicin-induced cardiotoxicity (Zhang et al. 2011). In this study, we not only replicated the results of a recent study showing that the activity of SIRT1 was reduced in aged hearts (Braidy et al. 2011), but our data also demonstrated that this ageing-associated reduction (by 1.4-fold) was abrogated by resveratrol treatment. It is thought that the level of SIRT1 activity needs to be delicately regulated, based on previous studies showing that over-activation of SIRT1 (by 12.5-fold) can elevate cardiomyocyte apoptosis (Alcendor et al. 2007). In contrast, moderate induction of SIRT1 activity (by 3.5- to 4-fold) has been shown to beneficially reduce left ventricular systolic pressure in aged Wistar rats following 8 weeks of treadmill training (Ferrara et al. 2008) and SIRT1 activation induced by 3 months of resveratrol feeding was demonstrated to protect against streptozotocin-induced diabetic cardiomyopathy (Sulaiman et al. 2010). Findings of studies conducted in proliferating cell lines also demonstrated that transfection with SIRT1 down-regulates Bim, a pro-apoptotic protein induced by Foxo3a (Motta et al. 2004) whereas pharmacological inhibition of SIRT1 by nicotinamide increases Foxo1 acetylation and Bim mRNA (Yang et al. 2009). The regulatory role of resveratrol in pro-apoptotic signalling in the ageing heart through the SIRT1–Foxo1 axis has yet to be determined, despite resveratrol having been shown to reduce the protein abundances of Foxo1 and Bim in H9c2 cardiomyocytes challenged with hypoxia (Chen et al. 2009), and resveratrol was observed to suppress the dexamethasone-induced elevations of Foxo1 acetylation and protein degradation in C2C12 myotubes (Alamdari et al. 2012). Our findings extend previous findings of the effects of resveratrol and SIRT1 by demonstrating the restoration of SIRT1 deacetylase activity after the treatment with resveratrol in the senescent heart, accompanied by concurrent alleviations in Foxo1 acetylation and protein content of Bim.
Resveratrol down-regulates apoptosis and oxidative stress in aged heart
We speculated that SIRT1 activation induced by resveratrol would reduce apoptosis and oxidative damages in the senescent heart. Our previous investigations indicated that acute resveratrol treatment abolished the elevations of p53 and Bax in skeletal muscle in a rat model of pressure compression injury (Sin et al. 2013). Despite the fact that the present study involves the use of a mouse model of accelerated ageing, aged hearts from these animals also exhibited pro-oxidative, pro-apoptotic status as induced by natural ageing and ischaemic conditions. Consistent with other findings showing that over-expression of SIRT1 in the heart prevents the increase in Bax protein following reperfusion injury (Hsu et al. 2010), our data revealed that resveratrol activates SIRT1 deacetylase activity and reduces pro-apoptotic signalling measured as p53, Bax and apoptotic DNA fragmentation in the aged heart. Curcumin, an antioxidant that mimics the vast effects of resveratrol has also been reported to reduce acetylation of Foxo1, apoptotic TUNEL index and infarction size in a model of ischaemia-reperfusion injury, whereas all these effects were abolished by the administration of the SIRT1 inhibitor sirtinol (Yang et al. 2013). It has recently been shown that SIRT1 activity was reduced with concomitant increases in 4HNE and carbonylated products in the aged heart, suggesting that there is a relationship between SIRT1 and cellular oxidative stress (Gu et al. 2013). In agreement with this speculation, we further demonstrated that resveratrol restores the deacetylase activity of SIRT1 and reduces the content of 4HNE and nitrotyrosine in the senescent heart.
Resveratrol may repress cardiac fibrosis through multiple signalling pathways
Our observations that resveratrol reduces fibrotic collagen deposition in ageing hearts are consistent with the reports that demonstrated ageing increases the collagen content in human hearts (Gazoti Debessa et al. 2001), whereas resveratrol has been shown to inhibit the growth of fibroblasts in response to angiotensin-II stimulation (Olson et al. 2005). Although the anti-fibrotic mechanisms of resveratrol are not well understood, it has been observed that oral administration of resveratrol up-regulates the anti-oxidative enzyme SOD2 and alleviates fibronectin immunoreactivity in failing hearts of TO-2 hamsters (Tanno et al. 2010). It has also been revealed recently that resveratrol inhibited cigarette smoke-induced elevations of cardiomyocyte apoptosis and fibrotic area through up-regulation of SIRT1 (Hu et al. 2013). Collectively, these data suggest that the anti-fibrotic effects of resveratrol can be attributed, at least in part, to the anti-oxidative, anti-apoptotic properties of SIRT1. Indeed, treating diabetic kidneys with resveratrol was found to reduce expression of collagen IV and fibronectin through suppression of Smad phosphorylation, which is an important regulatory mechanism of the fibrotic response (Chen et al. 2011). Furthermore, it has been demonstrated that signal transduction of Smad requires co-activation by p300 acetylase, an enzyme exhibiting opposing effects to the deacetylating mechanism of SIRT1 (Chan et al. 2010), whereas resveratrol has been shown to repress the increase in the protein level of p300 and reduce the mRNA contents of several collagen isoforms and fibronectin in dystrophin-deficient hearts (Kuno et al. 2013). However, whether p300 would reduce cardiac function in ageing mice through the classic Smad–CTGF fibrosis axis and thus the therapeutic potential of resveratrol to repress the pro-fibrotic effects of p300 through activation of the SIRT1 deacetylase activity requires further research.
Resveratrol is not observed to affect the PGC-1α-associated energy production machinery
We evaluated the effects of resveratrol on the mitochondrial metabolism machinery in aged mice. It is known that resveratrol activates the SIRT1–PGC-1α axis to increase mitochondrial density and oxidative capacity in liver (Baur et al. 2006) and skeletal muscle (Lagouge et al. 2006). However, we did not observe any alteration of the protein level of PGC-1α in the resveratrol-treated hearts. This discrepancy could be due to the fact that we employed a ∼80-fold lower, but physiologically equivalent dose of resveratrol (i.e. 4.9 mg kg−1 day−1), compared to the previous study that reported significant changes of PGC-1α (Lagouge et al. 2006). Indeed, the resveratrol dosage used in this study has been shown to abolish the elevation of myocardial performance index, an indicator of reduced cardiac function without inducing PGC-1α (Barger et al. 2008). It has also been reported that the PGC-1α content in gastrocnemius muscle was unaffected following 10 months of resveratrol treatment (Jackson et al. 2011) and SIRT1 deficiency did not abrogate the exercise-induced elevations of both the protein and activity levels of the electron transport chain (Philp et al. 2011). An earlier study where concomitant elevations of PGC-1α protein and subsequent complex IV promoter activity were observed in triceps muscle as soon as 18 h following a single bout of swimming exercise (Baar et al. 2002) led us to speculate that SIRT1 deacetylase activation by long-term resveratrol treatment might not induce the energy production machinery. In agreement with our PGC-1α data, the protein abundance of all five electron transport chain complexes were not found to be altered by resveratrol treatment in the present study. These observations are consistent with previous findings demonstrating that neither 30% calorie restriction nor an 8 week high-dose resveratrol intervention affects the protein levels of PGC-1α, complex IV subunits and energy metabolism markers including LCAD and citrate synthase in the heart (Hancock et al. 2011) and triceps muscle (Higashida et al. 2013).
In conclusion, we have demonstrated that resveratrol treatment activates SIRT1 deacetylase activity and improves contractile parameters in the aged heart through mitigation of apoptosis and oxidative damage. Notably, the present study is the first attempt to reveal that resveratrol confers anti-apoptotic effects through the restoration of SIRT1 deacetylase activity, which represses the acetylation of Foxo1 and the expression of its transcriptional target, Bim, in the senescent heart. These new findings hence suggest that the SIRT1–Foxo1 signalling axis may serve as potential therapeutic target for future development of anti-apoptotic, anti-ageing regimens in the heart. It is also worth-noting that resveratrol reduces fibrotic collagen deposition in the aged heart and this merits further investigation of how the modulating mechanisms of resveratrol relate to SIRT1 activation and fibrotic signalling in response to ageing. However, it should be taken into account that certain features of the accelerated ageing model may not fully replicate the actual ageing process, although the hearts of SAMP8 mice exhibit similar molecular and physiological profiles to their naturally aged counterparts.
Acknowledgments
The authors acknowledge the animal husbandry support received from the Centralised Animal Facilities of The Hong Kong Polytechnic University.
Glossary
- AG
aged (mice)
- AR
resveratrol-treated aged (mice)
- AWT
anterior wall thickness
- EF
ejection fraction
- FS
fractional shortening
- LVEDD
left ventricle end-diastolic dimension
- LVESD
left ventricular end-systolic dimension
- PWT
posterior wall thickness
- SIRT1
sirtuin 1 (protein)
- Y
young (mice)
Additional information
Competing interests
None declared.
Author contributions
Experiments were conducted in the Centralised Animal Facilities of The Hong Kong Polytechnic University for housing the animals and the dissection of the animals; Room Y1001 for all the biochemical and molecular analyses. Conception and design of experiments: T.K.S., A.P.Y., B.Y.Y., S.P.Y., L.W.C., C.S.W. and P.M.S. Collection, data analysis and interpretation of data: T.K.S., A.P.Y., M.Y., J.A.R. and P.M.S. Drafting the article or revising it critically for important intellectual content: T.K.S. and P.M.S. All authors approved the final version for publication.
Funding
This study was supported by The Hong Kong Polytechnic University (RPTL).
References
- Alamdari N, Aversa Z, Castillero E, Gurav A, Petkova V, Tizio S, Hasselgren PO. Resveratrol prevents dexamethasone-induced expression of the muscle atrophy-related ubiquitin ligases atrogin-1 and MuRF1 in cultured myotubes through a SIRT1-dependent mechanism. Biochem Biophys Res Commun. 2012;417:528–533. doi: 10.1016/j.bbrc.2011.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- Boyle AJ, Shih H, Hwang J, Ye J, Lee B, Zhang Y, Kwon D, Jun K, Zheng D, Sievers R, Angeli F, Yeghiazarians Y, Lee R. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol. 2011;46:549–559. doi: 10.1016/j.exger.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EC, Dusting GJ, Guo N, Peshavariya HM, Taylor CJ, Dilley R, Narumiya S, Jiang F. Prostacyclin receptor suppresses cardiac fibrosis: role of CREB phosphorylation. J Mol Cell Cardiol. 2010;49:176–185. doi: 10.1016/j.yjmcc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res Commun. 2009;378:389–393. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- Chen KH, Hung CC, Hsu HH, Jing YH, Yang CW, Chen JK. Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF-β/smad and ERK1/2 signaling in streptozotocin-induced diabetic rats. Chem Biol Interact. 2011;190:45–53. doi: 10.1016/j.cbi.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Gu C, Xing Y, Jiang L, Chen M, Xu M, Yin Y, Li C, Yang Z, Yu L, Ma H. Impaired cardiac SIRT1 activity by carbonyl stress contributes to aging-related ischemic intolerance. PLoS One. 2013;8:e74050. doi: 10.1371/journal.pone.0074050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CR, Han DH, Higashida K, Kim SH, Holloszy JO. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J. 2011;25:785–791. doi: 10.1096/fj.10-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YX, Cui H, Fan L, Pan XJ, Wu JH, Shi SZ, Cui SY, Wei ZM, Liu L. Resveratrol attenuates left ventricular remodeling in old rats with COPD induced by cigarette smoke exposure and LPS instillation. Can J Physiol Pharmacol. 2013;91:1044–1054. doi: 10.1139/cjpp-2012-0464. [DOI] [PubMed] [Google Scholar]
- Huang JP, Huang SS, Deng JY, Chang CC, Day YJ, Hung LM. Insulin and resveratrol act synergistically, preventing cardiac dysfunction in diabetes, but the advantage of resveratrol in diabetics with acute heart attack is antagonized by insulin. Free Radic Biol Med. 2010;49:1710–1721. doi: 10.1016/j.freeradbiomed.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011;66:751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothi M, Mal M, Keller C, Mal AK. Small molecule inhibition of PAX3-FOXO1 through AKT activation suppresses malignant phenotypes of alveolar rhabdomyosarcoma. Mol Cancer Ther. 2013;12:2663–2674. doi: 10.1158/1535-7163.MCT-13-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno A, Hori YS, Hosoda R, Tanno M, Miura T, Shimamoto K, Horio Y. Resveratrol improves cardiomyopathy in dystrophin-deficient mice through SIRT1 protein-mediated modulation of p300 protein. J Biol Chem. 2013;288:5963–5972. doi: 10.1074/jbc.M112.392050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John SM, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol. 2005;288:H1131–H1138. doi: 10.1152/ajpheart.00763.2004. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp A, Chen A, Lan D, Meyer GA, Murphy AN, Knapp AE, Olfert IM, McCurdy CE, Marcotte GR, Hogan MC, Baar K, Schenk S. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation following endurance exercise. J Biol Chem. 2011;286:30561–30570. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Siwik DA, Luptak I, Hou X, Wang L, Higuchi A, Weisbrod RM, Ouchi N, Tu VH, Calamaras TD, Miller EJ, Verbeuren TJ, Walsh K, Cohen RA, Colucci WS. The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1764. doi: 10.1161/CIRCULATIONAHA.111.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbaud S, Ruiz M, Piquereau J, Mateo P, Fortin D, Veksler V, Garnier A, Ventura-Clapier R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One. 2011;6:e26391. doi: 10.1371/journal.pone.0026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour EM, Bennink MR, Watts SW, Bolling SF. Whole grape intake impacts cardiac peroxisome proliferator-activated receptor and nuclear factor kappaB activity and cytokine expression in rats with diastolic dysfunction. Hypertension. 2010;55:1179–1185. doi: 10.1161/HYPERTENSIONAHA.109.149393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Gao L, Hsu YT, Bledsoe G, Hagiwara M, Chao L, Chao J. Kallistatin attenuates endothelial apoptosis through inhibition of oxidative stress and activation of Akt-eNOS signaling. Am J Physiol Heart Circ Physiol. 2010;299:H1419–H1427. doi: 10.1152/ajpheart.00591.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin TK, Pei XM, Teng BT, Tam EW, Yung BY, Siu PM. Oxidative stress and DNA damage signalling in skeletal muscle in pressure-induced deep tissue injury. Pflugers Arch. 2013;465:295–317. doi: 10.1007/s00424-012-1205-9. [DOI] [PubMed] [Google Scholar]
- Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2010;298:H833–H843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, Yu L, Juric D, Kopilas MA, Anderson HD, Netticadan T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens. 2010;23:192–196. doi: 10.1038/ajh.2009.228. [DOI] [PubMed] [Google Scholar]
- Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucevic D, Mladenovic D, Ninkovic M, Aleksic V, Stankovic MN, Stankovic M, Jorgacevic B, Vukicevic RJ, Radosavljevic T. The effects of caloric restriction against ethanol-induced oxidative and nitrosative cardiotoxicity and plasma lipids in rats. Exp Biol Med (Maywood) 2013;238:1396–1405. doi: 10.1177/1535370213506806. [DOI] [PubMed] [Google Scholar]
- Wei L, Wang M, Qu X, Mah A, Xiong X, Harris AG, Phillips LK, Martinez OM, Krams SM. Differential expression of microRNAs during allograft rejection. Am J Transplant. 2012;12:1113–1123. doi: 10.1111/j.1600-6143.2011.03958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J, Wang N, Deng C, Zhang S, Li Y, Chen W, Yu S, Yi D, Jin Z. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med. 2013;65:667–679. doi: 10.1016/j.freeradbiomed.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhao Y, Liao W, Yang J, Wu L, Zheng Z, Yu Y, Zhou W, Li L, Feng J, Wang H, Zhu WG. Acetylation of FoxO1 activates Bim expression to induce apoptosis in response to histone deacetylase inhibitor depsipeptide treatment. Neoplasia. 2009;11:313–324. doi: 10.1593/neo.81358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, Liu M, Chen G, Xiao X. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res. 2011;90:538–545. doi: 10.1093/cvr/cvr022. [DOI] [PubMed] [Google Scholar]
- Zhu W, Bijur GN, Styles NA, Li X. Regulation of FOXO3a by brain-derived neurotrophic factor in differentiated human SH-SY5Y neuroblastoma cells. Brain Res Mol Brain Res. 2004;126:45–56. doi: 10.1016/j.molbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]