Abstract
While the impact of alcohol consumption by pregnant women on fetal neurodevelopment has received much attention, the effects on the cardiovascular system are not well understood. We hypothesised that repeated exposure to alcohol (ethanol) in utero would alter fetal arterial reactivity and wall stiffness, key mechanisms leading to cardiovascular disease in adulthood. Ethanol (0.75 g (kg body weight)–1) was infused intravenously into ewes over 1 h daily for 39 days in late pregnancy (days 95–133 of pregnancy, term ∼147 days). Maternal and fetal plasma ethanol concentrations at the end of the hour were ∼115 mg dl−1, and then declined to apparent zero over 8 h. At necropsy (day 134), fetal body weight and fetal brain–body weight ratio were not affected by alcohol infusion. Small arteries (250–300 μm outside diameter) from coronary, renal, mesenteric, femoral (psoas) and cerebral beds were isolated. Endothelium-dependent vasodilatation sensitivity was reduced 10-fold in coronary resistance arteries, associated with a reduction in endothelial nitric oxide synthase mRNA (P = 0.008). Conversely, vasodilatation sensitivity was enhanced 10-fold in mesenteric and renal resistance arteries. Arterial stiffness was markedly increased (P = 0.0001) in all five vascular beds associated with an increase in elastic modulus and, in cerebral vessels, with an increase in collagen Iα mRNA. Thus, we show for the first time that fetal arteries undergo marked and regionally variable adaptations as a consequence of repeated alcohol exposure. These alcohol-induced vascular effects occurred in the apparent absence of fetal physical abnormalities or fetal growth restriction.
Key points
To date, the effects of maternal alcohol consumption in pregnancy have focused on neurodevelopmental outcomes, with the impact on the arterial system poorly understood.
In this study we investigated the effects of moderate maternal alcohol consumption (∼3 standard drinks per day) in late pregnancy on the ability of arteries in the fetus to relax, and hence deliver blood, and on arterial stiffness in five important organs.
Maternal alcohol consumption in late pregnancy resulted in a marked increase in stiffness in arteries of the heart, kidney, gut, leg muscle and brain in the fetus, and this could slow contraction and relaxation and overall blood delivery.
Coronary artery endothelial vasodilator function was severely blunted as a result of alcohol exposure, while in contrast endothelium-dependent relaxation in renal and mesenteric arteries was enhanced.
Together, the results of this study provide a warning for pregnant women and their carers that maternal consumption of ∼3 standard drinks per day, levels that do not evoke physical abnormalities or growth restriction, can dramatically alter arterial function in the fetus.
Introduction
Alcohol use by pregnant women is 10–20% according to the 2010 US Centers for Disease Control and Prevention report (Prevention, 2010), and in Australia, 30–60% of women reported consuming one to seven alcoholic drinks per week in the 2nd and 3rd trimesters of pregnancy (Colvin 2007; Morley et al. 2010). There is unimpeded distribution of alcohol (ethanol) across the placenta, and the maternal and fetal blood ethanol concentration–time curves are virtually identical (Brien et al. 1985; Cudd et al. 2001). It is evident that heavy episodic (binge) drinking can significantly impact maternal endocrine and cardiovascular systems (Ramadoss & Magness, 2012). Thus, as a result of maternal alcohol consumption, the fetus is subjected both to the direct effects of alcohol and the consequences of changes in maternal physiology.
A well-studied consequence of substantial maternal alcohol consumption, called fetal alcohol syndrome (FAS), includes a characteristic facial dysmorphology, growth restriction and neurodevelopmental delay resulting in intellectual impairment (Pruett et al. 2013). In contrast to the major focus on neurological outcomes, the impact of fetal alcohol exposure during pregnancy on the cardiovascular system has received scant attention. It is well established that alcohol consumption in adults is causally linked with cardiovascular abnormalities (Kurihara et al. 2004; Fatjo et al. 2007; Fenelon et al. 2007; Djousse et al. 2009; Klatsky, 2009). Long-term alcohol consumption in adult rats induces dilated cardiomyopathy (Piano et al. 2007). The incidence of cardiac malformations in the offspring is as high as 20% consequent to heavy maternal alcohol consumption in early pregnancy, compared with 1.2% in control children (Tikkanen & Heinonen, 1991; Kvigne et al. 2004). Even moderate alcohol consumption is associated with a lower Apgar score at birth (Iveli et al. 2007), and maternal alcohol consumption at any stage in pregnancy resulted in a halving of the capacity of the umbilical cord to contract (Iveli et al. 2007). While arterial pressure was normal in 9-year-old children of women who had consumed alcohol in pregnancy, pulse-wave velocity was increased, suggesting enhanced vascular stiffness (Morley et al. 2010).
In experimental models of FAS, the incidence of kidney defects, urethral obstruction, renal hypoplasia and hydronephrosis is increased (Assadi, 1990; Taylor et al. 1994). Even with more modest alcohol exposure, nephron endowment is reduced in fetal sheep (Gray et al. 2008) and in rat offspring as adults (Gray et al. 2010). In utero alcohol exposure led to increased arterial pressure in adult rats (Turcotte et al. 2002; Gray et al. 2010), but it was without effect on blood pressure in sheep fetuses (Gray et al. 2008). In another study of sheep fetuses, cerebral blood flow was enhanced, but only in the cerebellum (Parnell et al. 2007) and the vasodilator response to hypoxia was reduced (Gleason et al. 1997) following alcohol exposure in utero. Endothelium-dependent relaxation in aorta was reduced in 25-week-old rat offspring from alcohol-affected pregnancies (Turcotte et al. 2002). These results suggest that the impact of prenatal alcohol exposure varies between vascular beds.
Owing to the limited understanding of the effects of prenatal alcohol exposure on the developing vasculature, our objective was to determine the effects of maternal alcohol consumption on endothelial vasodilator function and arterial stiffness in the fetus; these are both key vascular variables that, when perturbed, are an important basis for cardiovascular disease. The levels attained here, ∼3 standard drinks per day, approximate those measured in young women during social drinking (Moore et al. 2007). We focused on resistance arteries and determined the effects of alcohol exposure on mechanisms of arterial contraction and endothelium-dependent and -independent relaxation in major vascular beds in fetal sheep, namely, the coronary, renal, mesenteric, skeletal muscle (psoas), and cerebral circulations, at a time of peak structural and functional maturation of these organs.
Methods
Animals
All procedures were completed at Monash University and were approved by Monash University School of Biomedical Sciences Animal Ethics Committee A and were in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. On day 92 of pregnancy (term ∼147 days), twelve Border-Leicester × Merino crossbred ewes, each carrying a single fetus, underwent isoflurane anaesthesia (Bubb et al. 2007) and catheters were implanted chronically into a jugular vein and carotid artery of the ewe. Starting on day 95 of pregnancy, ethanol was infused into the jugular vein at 0.75 g (kg body weight-1) for 1 h (09.00 h to 10.00 h) per day to six of the ewes. The infused solution consisted of four parts absolute ethanol diluted with six parts of isotonic saline. Six control ewes received an equivalent volume of saline (∼120 ml in a 50 kg sheep) over 1 h. Ewes were weighed every 2 weeks to adjust the alcohol dose for body weight gain. Food intake was monitored and control ewes were given extra Lucerne chaff to provide a total of 20 kJ per day to compensate for the additional calories contained in the alcohol dose that was infused.
Alcohol was not administered on day 125, and on day 126 each ewe was again anaesthetised, the fetal head and a forelimb were exposed, and a catheter was implanted into a brachial artery for blood sampling and recording of arterial pressure. Another catheter was placed in the amniotic cavity to measure amniotic fluid pressure. The ewes were allowed one day to recover and daily alcohol infusion was resumed on day 128 until day 133 and necropsy was performed on day 134. Heart rate and blood pressure were measured from days 131–133, using the analysis program Lab Chart 6 ADInstruments, Bellvista, NSW).
For the purpose of determining the effects of short-term alcohol exposure, an additional three ewes underwent surgery on day 126 and were infused with alcohol on days 131–133 of pregnancy, with necropsy on day 134.
Blood vessel function testing
On day 134 of pregnancy, 24 h after the last alcohol infusion, each ewe was killed with pentobarbitone (Lethabarb, 120 mg kg−1), the fetus was removed, weighed and measured, and the fetal heart, brain, kidneys, psoas muscle and small intestines were removed and placed in ice-cold physiological saline solution (PSS) containing (in mm): 120 NaCl, 5 KCl, 1.2 MgSO4, 25 NaHCO3, 1 KH2PO4, 2.5 CaCl2, 11 glucose, gassed with 95% O2–5%CO2 (Carbogen). Arteries were cleared of surrounding tissue and fat, segments were taken for wire and pressure myography, and the remaining arteries were snap frozen in liquid nitrogen for later molecular analysis. Segments of arteries (250–300 μm outside diameter and 1–2 mm in length) were mounted on a 4-channel wire myograph for isometric tension recording as described previously (Bubb et al. 2007; Mazzuca et al. 2010). The segments were bathed with PSS at 36°C and smooth muscle and endothelial integrity were tested using the agent that provided the greatest contractile response and also provided optimal endothelial stimulation, as determined for each vessel type in preliminary studies. These were: the thromboxane mimetic U46619 to constrict the coronary artery, phenylephrine (PE) for the mesenteric and renal arteries, and 5-hydroxytryptamine (5-HT) for the cerebral and femoral (from the psoas muscle) arteries. Smooth muscle contractile function was tested using cumulative concentrations of these constrictor agents. Following 30 min rest, the arterial segment was submaximally constricted (∼60% of maximal) and endothelial vasodilator release was induced by cumulative addition of the endothelial agonist bradykinin (BK) in all arteries except the femoral, for which acetylcholine (ACh) was used. Following 30 min rest, endothelium-derived nitric oxide (NO) synthase was inhibited by including Nϖ-nitro-l-arginine methyl ester (l-NAME, 2 × 10−4 m) in the PSS. The process was repeated in the presence of indomethacin (2 × 10−6 m) to block cyclooxygenase (COX) and hence prostanoid (PG) production. Arterial relaxation persisting in the presence of l-NAME and indomethacin was attributed to endothelium-derived hyperpolarising factor (EDHF; Bubb et al. 2007). Endothelium-independent smooth muscle relaxation was tested using the NO donor sodium nitroprusside (SNP; Mazzuca et al. 2010). At the end of each experiment the blood vessel was exposed to high-K+ PSS (isotonic replacement of NaCl with 100 mm KCl) to elicit a standard contraction.
Passive mechanical wall properties – arterial stiffness
Arterial stiffness was tested by mounting leak-free segments of artery in a pressure myograph (Living Systems Instrumentation, Burlington, VT, USA) with no luminal flow (Wigg et al. 2004). The segments were continuously superfused at 15 ml min−1 with PSS containing 2 mm EGTA but no added calcium. Pressure was increased in 10 mmHg increments from 0 to 120 mmHg and changes in outside diameter and wall thickness in response to the pressure increases were measured.
Gene expression analysis
Fetal coronary, mesenteric, renal, femoral and cerebral arterial tissue that was frozen at −80°C at necropsy was used for RNA extraction using an RNeasy mini kit (Qiagen, Australia), and relative mRNA levels of the following genes were measured using quantitative real-time PCR (qPCR): collagens Ia1, Ia2 and III, elastase 2, tropoelastin, endothelial nitric oxide synthase (eNOS) and transforming growth factor beta 1 (TGFβ1). RNA samples were treated with DNase (Qiagen) and were reverse-transcribed into cDNA (Promega); qPCR was performed using the Realplex Real-Time Multiplexing System (Eppendorf, Germany) or the Stratagene MX3000P qPCR machine (Agilent Technologies, USA). Primer sequences (Table 1) were designed using the nucleotide sequences of the genes of interest obtained from the Genbank database (http://www.ncbi.nlm.nih.gov). Primer and cDNA concentrations and annealing temperatures were optimised (Table 1), and dissociation curves were conducted for each gene to ensure optimal amplification. Samples were analysed in triplicate, with a negative control that did not have template DNA included in each PCR analysis. mRNA levels were normalised against the housekeeping genes 18S rRNA, ribosomal protein S29 or beta-actin and analysed using the delta CT (cycle threshold) method, which did not change with alcohol treatment. For each gene, data are expressed relative to the mean mRNA levels obtained for samples of the control animals.
Table 1.
qPCR primers
| Gene | Primer sequence | Primer concentration | Annealing temperature | |
|---|---|---|---|---|
| Collagen I α1 | Forward | AAGACATCCCACCAGTCACC | 10 μm | 60°C |
| Reverse | CAGATCACGTCATCGCACA | |||
| Collagen I α2 | Forward | GGCTCAACCTGAAGACATCC | 4 μm | 59°C |
| Reverse | TCTCCTACCCAGACATGCTTC | |||
| Collagen III | Forward | CTGCTGGAAAGAATGGTGAG | 10 μm | 59°C |
| Reverse | GTCACCAGAAGGCCCAGTA | |||
| Elastase 2 | Forward | AGACTCCTTTGCCTCTGTCG | 10 μm | 58°C |
| Reverse | AGCCTTTCTAGTGGGTCCTG | |||
| Tropoelastin | Forward | ATCTCTCAGTCAGGCACCAG | 10 μm | 58°C |
| Reverse | GTTTGTTGGGAAAGAAAGCA | |||
| TGF-β1 | Forward | GCTGACCCACAGAGAGGAAA | 10 μm | 60°C |
| Reverse | AACTGAACCCGTTGATGTCC | |||
| eNOS | Forward | GATCAGCAACGCTATCACGA | 10 μm | 60°C |
| Reverse | ATACGGCTTGTCACCTCCTG | |||
| 18S rRNA | Forward | GTCTGTGATGCCCTTAGATGTC | 10 μm | 58–60°C |
| Reverse | AAGCTTATGACCCGCACTTAC | |||
| Ribosomal protein S29 | Forward | CAGGGTTCTCGCTCTTGC | 10 μm | 60°C |
| Reverse | ACTGGCGGCACATATTGAG | |||
| beta-actin | Forward | TGTTACCAACTGGGACGACA | 10 μm | 60°C |
| Reverse | GGGGTGTTGAAGGTCTCAAA |
Forward and reverse primer sequences (5′–3′) used for qPCR to amplify collagen Iα1, collagen Iα2, collagen III, elastase 2, tropoelastin, transforming growth factor beta 1 (TGFβ1), endothelial nitric oxide synthase (eNOS), 18S rRNA, ribosomal protein S29 and beta-actin.
Data analysis
Responses of isolated arterial segments to vaso-constrictors and -dilators were analysed as described previously (Bubb et al. 2007; Mazzuca et al. 2010). Sigmoidal curves were fitted to the concentration–response data using the least squares methods (GraphPad Prism, GraphPad Software, San Diego, CA, USA) (Bubb et al. 2007; Mazzuca et al. 2010). From these curves, the concentration of drug that evoked a half-maximal response (EC50), pD2 (−log EC50) and maximal response (Emax) were determined. For endothelium-dependent relaxation, the area-under-the-curve (AUC) was also calculated (Herrera et al. 2010) and analysed using 2-way ANOVA (maternal treatment and vasodilator), followed by Tukey's post hoc testing. Stress–strain values were derived as described previously (Bubb et al. 2007; Mazzuca et al. 2010). For normalisation of internal and outside arterial diameters, values were expressed in relation to the diameter at 10 mmHg. An exponential function was fitted to the data and tangential elastic modulus (Etang) was determined from circumferential stress = circumferential stress at 10 mmHgk.strain (k is the slope of the curve). Thus, Etang is proportional to k (Izzard et al. 2006). N is the number of animals and statistical significance was accepted as P < 0.05. Data are expressed as mean ± SEM and were analysed using one-and two-way ANOVA and unpaired Student's t test (GraphPad Prism).
Results
General effects of maternal ethanol infusion
At the end of the hour of maternal ethanol infusion, ethanol concentration in the maternal and fetal plasma was 117 ± 5 mg dl−1 and 108 ± 6 mg dl−1, respectively; these peak ethanol concentrations in the ewes and fetuses were not significantly different. Ethanol concentrations declined in both ewes and fetuses over 8 h to apparent baseline. Maternal infusion of ethanol had no effect on fetal arterial pressure (P = 0.93) or heart rate (P = 0.53).
Alcohol exposure had no significant effect on mean arterial pressure (control 38 ± 2 mmHg, alcohol 40 ± 2 mmHg), fetal body weight, on weight of the fetal heart (P = 0.15), brain (P = 0.34), or kidney (P = 0.66), determined at necropsy (data previously reported) (Kenna et al. 2011).
Vascular smooth muscle reactivity
Contractile response
We first determined the effects of alcohol on the ability of the fetal arteries to contract upon exposure to high-K+ PSS. Absolute contraction (tension mN mm−1) to high-K+ PSS was not different between saline and alcohol groups for fetal coronary, mesenteric, femoral, renal and cerebral arteries.
In coronary artery, U46619 evoked concentration-dependent constriction and the maximum constriction was significantly larger in arteries obtained from alcohol-exposed fetuses (Fig. 1). In contrast, alcohol exposure resulted in a decrease in the responsiveness of cerebral artery to 5-HT (maximal constriction, P = 0.001) and renal artery to PE (pD2 shifted from 6.16 ± 0.07 to 5.56 ± 0.08, n = 6, P = 0.001). There was no effect of alcohol exposure on the concentration-constriction curves to PE or 5-HT in mesenteric or femoral vessels (Fig. 1).
Figure 1. Effects of alcohol exposure in late pregnancy on fetal vascular smooth muscle contraction.
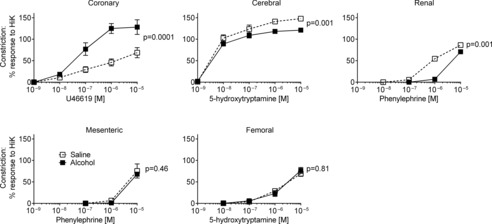
Constriction (relative to high-K+ PSS) was enhanced in coronary artery, suppressed in cerebral and renal segments, while mesenteric and femoral arteries were unaffected. Fetuses from 6 control ewes (saline) and 6 alcohol-treated ewes. Values of P refer to Emax.
Relaxation
Endothelium-independent relaxation was tested using SNP. In pre-constricted fetal arteries SNP induced maximal relaxation in all but the coronary artery, in which 10–20% tension remained. Relaxation in segments of coronary, mesenteric, femoral, and cerebral arteries was not affected following alcohol exposure (Fig. 2). However, the sensitivity of the renal artery to SNP was significantly reduced 10-fold following alcohol exposure (pD2 6.80 ± 0.11 vs. 7.90 ± 0.09 in control, n = 6, P < 0.0003; Fig. 2).
Figure 2. Endothelium-independent relaxation in fetal arteries.
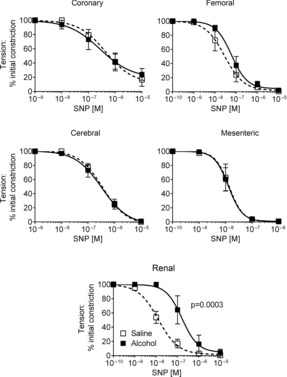
Alcohol exposure in late pregnancy significantly reduced the pD2 to nitroprusside in fetal renal artery but was without effect in the other arteries. N = 6 in each group. The P value refers to pD2.
Endothelium-dependent vasodilation
Endothelial stimulation
In the coronary artery, alcohol exposure blunted total endothelium-dependent relaxation induced by BK, with a significant 3-fold shift to the right in the concentration–relaxation curve (Fig. 3, Table 2). Following blockade of NO production, the capacity for maximal dilation was markedly reduced, and inclusion of both l-NAME and indomethacin in the bathing solution revealed that the response attributed to EDHF was significantly attenuated in alcohol-exposed coronary artery, with a significant reduction in maximal relaxation (Fig. 3 and Table 2). Thus, the area-under-curve (AUC) was significantly reduced for NO (P = 0.02) and EDHF (P = 0.03) in coronary artery (Fig. 4A).
Figure 3. Endothelium-dependent relaxation in fetal arteries.
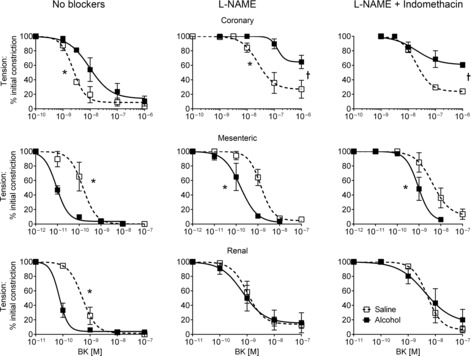
Endothelium-dependent relaxation was significantly reduced in fetal coronary artery, while relaxation was enhanced in mesenteric and renal arteries exposed to alcohol. N = 6 in each group. *Significant difference in pD2, †significant difference in Emax.
Table 2.
Values of pD2 (−log EC50) and Emax (% maximal constriction) responses to endothelial stimulation in small coronary, cerebral, renal, mesenteric and femoral arteries of fetal sheep following alcohol exposure
| pD2 | Emax (% maximal constriction) | |||||
|---|---|---|---|---|---|---|
| Saline | Alcohol | P | Saline | Alcohol | P | |
| No blocker | ||||||
| Coronary | 8.63 ± 0.08 | 8.02 ± 0.18* | 0.01 | 9 ± 4 | 14 ± 9 | 0.62 |
| Mesenteric | 9.82 ± 0.17 | 11.05 ± 0.04* | 0.0001 | 0 ± 0 | 4 ± 2 | 0.21 |
| Renal | 9.29 ± 0.10 | 10.14 ± 0.22* | 0.006 | 1 ± 3 | 4 ± 3 | 0.50 |
| Femoral | 7.45 ± 0.09 | 7.53 ± 0.12 | 0.61 | 1 ± 3 | 4 ± 4 | 0.56 |
| Cerebral | 9.66 ± 0.18 | 9.48 ± 0.08 | 0.38 | 8 ± 7 | 7 ± 4 | 0.90 |
| + l-NAME | ||||||
| Coronary | 7.60 ± 0.23 | 6.88 ± 0.09* | 0.02 | 27 ± 9 | 65 ± 6* | 0.005 |
| Mesenteric | 8.86 ± 0.12 | 9.80 ± 0.16* | 0.0008 | 5 ± 6 | 2 ± 6 | 0.73 |
| Renal | 8.98 ± 0.12 | 9.15 ± 0.25 | 0.55 | 14 ± 8 | 15 ± 10 | 0.96 |
| Femoral | 6.75 ± 0.10 | 6.67 ± 0.11 | 0.56 | 3 ± 4 | 11 ± 5 | 0.24 |
| Cerebral | 8.79 ± 0.31 | 8.73 ± 0.45 | 0.94 | 57 ± 11 | 63 ± 10 | 0.70 |
| + l-NAME + Indo | ||||||
| Coronary | 7.70 ± 0.03 | 7.70 ± 0.40 | 0.99 | 24± 10 | 61 ± 8* | 0.02 |
| Mesenteric | 8.37 ± 0.13 | 9.04 ± 0.11* | 0.003 | 11 ± 10 | 4 ± 8 | 0.60 |
| Renal | 8.30 ± 0.14 | 8.38 ± 0.38 | 0.85 | 6 ± 8 | 16 ± 6 | 0.34 |
| Femoral | 5.97 ± 0.40 | 6.00 ± 0.41 | 0.96 | 19 ± 18 | 32 ± 20 | 0.64 |
| Cerebral | 8.43 ± 0.56 | 6.61 ± 0.67 | 0.06 | 71 ± 10 | 39 ± 21 | 0.20 |
Daily maternal infusion for 1 h of saline (n = 6) or alcohol (n = 6). Concentration–relaxation curves were first obtained in control solution and then in the presence of l-NAME to block NO production, and in l-NAME + indomethacin (Indo) to additionally block prostanoid production. The statistical significance of the data is indicated by P;
significant difference between saline and alcohol treatment.
Figure 4. Integrated endothelium-dependent relaxation and tone development in fetal arteries.
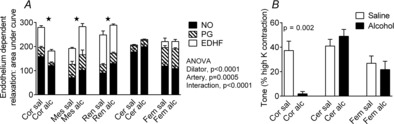
A, area under the endothelium-dependent relaxation curves for NO, PG and EDHF in the five fetal arteries studied. Alcohol exposure during late pregnancy changed NO and/or EDHF contraction in coronary, mesenteric and renal, but not in cerebral or femoral arteries. B, basal tone development following inclusion of l-NAME in the superfusing solution. N = 6 per group. *Significant difference in overall AUC between saline and alcohol exposure.
In contrast, in mesenteric segments, exposure to alcohol resulted in a marked increase in overall endothelium-dependent vasodilation, with a 10-fold left shift in the curve and a significant increase in pD2, and this persisted in the presence of l-NAME and in l-NAME plus indomethacin (Fig. 3 and Table 2). Overall vasodilator capacity was also increased by alcohol in the renal artery, but this reflected a 7-fold enhancement of NO bioavailability alone, as there was no difference between treatments in the presence of blockers of NO and prostanoid (Fig. 3). AUC showed up-regulation of NO-mediated relaxation for mesenteric (P = 0.04) and renal (P = 0.04) arteries and also for EDHF in mesenteric (P = 0.03) artery (Fig. 4A).
Endothelium-dependent relaxations in cerebral and femoral arteries induced by BK and ACh, respectively, were unaffected by alcohol exposure (Fig. 4A, Table 2).
Tone development
When l-NAME was added to the superfusing solution, control coronary, cerebral and femoral segments developed substantial tone (∼40% of a high potassium contraction). This tone was similar in cerebral and femoral arteries from saline- and alcohol-exposed fetuses. However, l-NAME-induced tone did not develop in coronary artery from alcohol-exposed fetuses (Fig. 4B).
Passive mechanical wall properties
Increasing the pressure in segments of fetal coronary, mesenteric, femoral, renal and cerebral arteries induced a progressive increase in vessel diameter. For all arteries tested, the increase was significantly greater in control vessels versus arteries from alcohol-exposed fetuses (Fig. 5A). Stress–strain curves for arteries from alcohol-exposed fetuses were shifted to the left, and k was significantly increased, an indication of increased stiffness, in all arterial segments following exposure to alcohol (Fig. 5B and Table 3). Wall thickness and lumen diameter were determined at 50 mmHg, and there was no effect of alcohol on these dimensions or on lumen diameter:wall thickness ratio in any of the arteries studied (data not shown).
Figure 5. Effect of alcohol on arterial stiffness in the fetus.
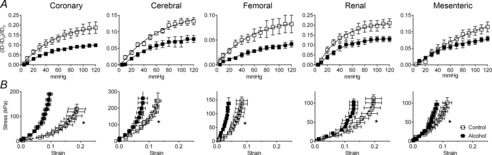
A, increasing pressure in segments of fetal artery induced an increase in lumen diameter. This response was blunted in arteries from alcohol-exposed fetuses. Alcohol versus control was significantly different (P < 0.0001 for all arteries). B, segments from all 5 vascular beds were significantly stiffer following alcohol exposure. N = 6 per group. *Significant difference in k between control and alcohol-exposed fetuses.
Table 3.
Elastic modulus, k, for small coronary, cerebral, renal, mesenteric and femoral arteries of fetal sheep following daily maternal infusion for 1 h of saline (n = 6) or alcohol (n = 6)
| Saline | Alcohol | P | |
|---|---|---|---|
| Coronary | 16.4 ± 1.7 | 31.4 ± 3.8* | 0.005 |
| Cerebral | 19.7 ± 2.3 | 33.5 ± 4.8* | 0.002 |
| Renal | 15.7 ± 1.2 | 26.6 ± 1.9* | 0.0007 |
| Mesenteric | 21.3 ± 1.9 | 33.7 ± 2.3* | 0.002 |
| Femoral | 35.4 ± 2.5 | 63.3 ± 7.1* | 0.004 |
The statistical significance of the data is indicated by P;
significant difference between saline and alcohol treatment.
Short-term alcohol treatment
Maternal infusion of alcohol for 3 days between 131 and 133 days of gestation had no effect on smooth muscle contraction, endothelium-independent relaxation, endothelium-dependent relaxation or stiffness in fetal coronary artery (data not shown).
Quantitative real-time PCR analysis for selected gene expression
Relative collagen Ia1 mRNA level was significantly increased in the cerebral artery (P = 0.02) of alcohol-exposed fetuses (Fig. 6), while collagen Ia2 and collagen III mRNA levels in the five selected fetal arteries were unaffected. Relative tropoelastin mRNA level was increased only in cerebral artery (P = 0.0003), and eNOS mRNA level was decreased in coronary artery (P = 0.002) of alcohol-exposed fetuses compared with controls (Fig. 6). Relative TGF-β1 and elastase 2 mRNA levels in the fetal arteries were not altered by alcohol exposure (Fig. 6).
Figure 6. mRNA gene expression in arteries of fetuses exposed to alcohol during pregnancy.
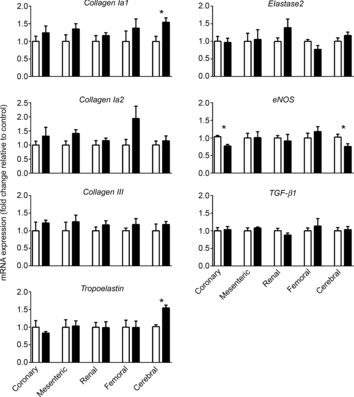
Prenatal alcohol exposure increased collagen Ia1 mRNA level in fetal cerebral artery, but there was no change in collagen Ia2 or collagen III. Prenatal alcohol exposure increased tropoelastin mRNA expression in cerebral artery, while decreasing eNOS mRNA expression in coronary arteries. There was no difference in TGFβ1 or elastase 2 mRNA level between alcohol and control groups. Open bars, saline (n = 4–5); filled bars, alcohol (n = 4–5). *Significant difference between saline and alcohol exposure.
Discussion
This study demonstrates significant widespread changes in vasodilator function and arterial stiffness in the fetus following daily alcohol exposure during late pregnancy. Effects on vasodilator function were region dependent. Endothelium-dependent vasodilation was markedly reduced in coronary artery, accompanied by a decrease in eNOS mRNA. In contrast, vasodilation was enhanced in mesenteric and renal resistance arteries. There was a striking and widespread increase in stiffness in all arteries studied, which was accompanied by an increase in elastic modulus, suggesting changes in vessel wall composition. These changes were the result of relatively long-term (30 days) exposure to alcohol, and were not produced by short (3 day) exposure in late pregnancy.
Endothelium-dependent vasodilator function is critical for the maintenance of tissue perfusion and for matching blood flow with organ activity. Endothelial dysfunction precedes the development of hypertension, atherosclerosis, stroke and is prominent in inflammatory diseases such as diabetes (Vanhoutte et al. 2009). Endothelial vasodilator function is affected in many forms of intrauterine insult (Poston, 2007), to which prenatal alcohol exposure can now be added. Several effects of alcohol consumption on endothelial function in the adult cardiovascular system have been reported. Thus, while alcohol directly constricts canine aorta denuded of endothelium (Yang et al. 2001), it normally produces relaxation of rat aorta by inducing endothelial NO production (Turcotte et al. 2002). In rat mesenteric artery, alcohol increased endothelin receptor A (ETA) expression and enhanced constriction, while in the kidney, alcohol decreased endothelial ETB expression with a consequent increase in NO production and renal vessel dilation (Tirapelli et al. 2006). Alcohol can also increase endothelin production by human endothelial cells (Yeligar et al. 2009). Thus, the effect of alcohol depends on the vascular bed. It must also be borne in mind that, while the initial endothelial response to a variety of stresses such as oxidative stress or inflammation is a compensatory increase of NO production, this does not always persist with chronic stress. These issues may explain the differences in endothelial responses observed in different arteries of the alcohol-exposed fetus, as in the present study. Whereas NO and EDHF functions were markedly reduced in coronary artery, the function of these dilators was enhanced in mesenteric and renal arteries. The endothelium is well known to modulate sensitivity to vasoconstrictors. It may also be that the increase in sensitivity of coronary artery to the contractile effect of U46619 and the marked reduction in the constrictor effect of phenylephrine in the renal artery are related to fundamental functional changes in the endothelium as a result of alcohol exposure.
The small vessels derived from the fetal cerebral and femoral arteries were remarkably resistant to alcohol exposure, in terms of endothelium-dependent or -independent changes in vascular tone. Cerebral blood vessels have been the focus of most studies targeting the effects of intrauterine alcohol exposure on vascular function to date and the findings have been variable. An elegant study by Parnell and colleagues in a sheep model similar to the one used in our study found that plasma alcohol concentration of ∼185 mg dl−1 increased global cerebral blood flow, but there was significant variability between different regions of the brain (Parnell et al. 2007). In another sheep study in which alcohol was infused into ewes in early pregnancy and the lambs were studied postnatally, alcohol had no effect on cerebral blood flow but the response to hypoxia was blunted (Gleason et al. 1997). Clearly, the effects of alcohol on autoregulatory processes in the brain require further study.
The kidney has a central role in cardiovascular and blood pressure control. Feeding pregnant rat dams with alcohol-containing food resulted in a significant increase in blood pressure in the offspring (Turcotte et al. 2002; Gray et al. 2010), although there was no effect on this variable in our fetal sheep (Gray et al. 2008), or in children of mothers who consumed alcohol during pregnancy (Morley et al. 2010). Nephron endowment was reduced in postnatal rats (Gray et al. 2010) and in our fetal sheep (Gray et al. 2008) following intrauterine alcohol exposure. In the present study smooth muscle responsiveness to SNP in the renal artery was reduced by an order of magnitude following alcohol exposure. This was an unexpected finding, as this variable is usually preserved following intrauterine compromise of fetal development (Payne et al. 2003; Bubb et al. 2007; Mazzuca et al. 2010). However, impaired responsiveness to SNP in cerebral artery of offspring exposed to maternal low protein diet is underpinned by reduced levels of guanylate cyclase and cGMP (Lamireau et al. 2002). Remarkably, in our study, despite impaired smooth muscle responsiveness to NO in fetal renal artery, total endothelial vasodilator function was significantly enhanced by alcohol exposure, which can be attributed to an increased contribution of NO. The enhanced endothelium-derived NO response was probably underestimated in our study, as the functional response must overcome the reduced sensitivity of the muscle to this vasodilator. Alternatively, up-regulation of the l-NAME-sensitive component may be underpinned by an additional endothelial vasodilator, namely, nitroxyl (HNO). HNO is a one-electron reduced and protonated form of NO, can target signalling pathways distinct from NO, and is resistant to scavenging by superoxides (Andrews et al. 2009; Bullen et al. 2011).
Arterial stiffness was significantly increased in fetal resistance arteries of all five beds studied. The increase was most marked in coronary, renal and cerebral vessels. This is the first study to show that alcohol exposure potently increases arterial stiffness in the fetus. In adult males, progressive alcohol consumption is associated with an increase in pulse-wave velocity (PWV) (Kurihara et al. 2004) and augmentation index (van Trijp et al. 2005), reliable indicators of decreased arterial compliance (DeLoach & Townsend, 2008). Alcohol consumption by women at any stage of pregnancy was associated with a persistent increase in PWV in their children at 9 years of age (Morley et al. 2010). In the present study, collagen Ia1 mRNA expression was increased following alcohol exposure in fetal cerebral artery. Elastin is laid down extensively during the perinatal period of development (Martyn & Greenwald, 1997), making it especially vulnerable to intrauterine insult. Elastin has a very long half-life, up to 40 years in humans, indicating that perturbations in this extracellular matrix protein in early life are likely to have long lasting effects (Martyn & Greenwald, 2001). Tropoelastin mRNA was increased, but only in alcohol-exposed cerebral artery. The distensibility and stiffness of the arterial wall depends not only on the amount of collagen and elastin, but on the relative thickness of the fibres and their organisation (Zieman et al. 2005). While direct determination of collagen and elastin protein content would be of interest, the small size of these vessels precluded direct measurement here. None-the-less, our observations are important because a reduction in arterial compliance is associated with an increased risk of cardiovascular disease (Laurent et al. 2001).
Structural and functional maturation of many organs is high in late pregnancy. DNA replication and cell proliferation are vulnerable to disruptions in methionine metabolism and protein and DNA methylation. Men consuming 24 g of alcohol for 2 weeks had a reduction in folate and vitamin B12 and an increase in homocysteine (Gibson et al. 2008). Homocysteine is critically important in methionine metabolism and protein and DNA methylation (Lutz, 2008). Epigenetic modifications predispose to life-long cardiovascular disease and maternal hyperhomocysteinemia is associated with an increased risk of congenital heart disease in the offspring (van Driel et al. 2008). Homocysteine can also inhibit vascular endothelial cell growth (Chang et al. 2008), and is involved in the methylation of arginine, giving rise to asymmetric dimethylargine (ADMA) production, an endogenous inhibitor of NOS. In addition, alcohol can increase oxidative stress (Altura & Gebrewold, 2002), which also reduces NO bioavailability and endothelium-dependent vasodilator function. On the other hand, modest alcohol consumption can reduce cardiovascular risk as a result of enhanced NOS synthesis (Di Castelnuovo et al. 2006). This varied palette of possibilities forms the basis for the wide range of effects observed in the present study.
We conclude that daily maternal alcohol administration during the last trimester equivalent induces widespread vascular adaptations in the fetus, with increased arterial stiffness, present in all five vascular beds under study. In contrast, endothelium-dependent vasodilator responses to fetal alcohol exposure are not uniform across different vascular beds, with suppressed (coronary) or enhanced (renal and mesenteric) dilatation shown here for the first time. Maladaptive changes in the fetal vasculature in response to alcohol exposure may persist postnatally, thereby increasing the risk of cardiovascular disease in adulthood. Of alarming concern is that these marked changes in vascular function occur at levels of exposure that are not associated with overt signs of FAS.
Acknowledgments
We acknowledge the expert technical assistance of Ms Judy Ng.
Glossary
- 5-HT
5-hydroxytryptamine
- ACh
acetylcholine
- alcohol
ethanol
- AUC
area-under-curve
- BK
bradykinin
- COX
cyclooxygenase
- EDHF
endothelium-derived hyperpolarising factor
- ETR
endothelin receptor
- FAS
fetal alcohol syndrome
- l-NAME
Nϖ-nitro-l-arginine methyl ester
- NOS
nitric oxide synthase
- PE
phenylephrine
- PG
prostaglandin
- SNP
sodium nitroprusside
Additional information
Competing interests
None declared.
Author contributions
Study conception and design: H.C.P., M.T., H.A.C., D.W.W., R.H. Data collection, analysis and interpretation: H.C.P., M.T., K.K., F.S., H.A.C. Manuscript preparation and intellectual input: H.C.P., M.T., J.F.B., R.H., D.W.W., H.A.C., K.K., F.S., A.B. All authors have approved the final version.
Funding
This work was funded by the Canadian Institutes of Health Research, NET-54014, the National Heart Foundation of Australia, and ANZ Trustees (Australia) Ltd.
References
- Altura BM, Gebrewold A. Inhibitor of nuclear factor-Kappa B activation attenuates venular constriction, leukocyte rolling-adhesion and microvessel rupture induced by ethanol in intact rat brain microcirculation: relation to ethanol-induced brain injury. Neurosci Lett. 2002;334:21–24. doi: 10.1016/s0304-3940(02)01061-3. [DOI] [PubMed] [Google Scholar]
- Andrews KL, Irvine JC, Tare M, Apostolopoulos J, Favaloro JL, Triggle CR, Kemp-Harper BK. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br J Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assadi FK. Renal tubular dysfunction in fetal alcohol syndrome. Pediatr Nephrol. 1990;4:48–51. doi: 10.1007/BF00858439. [DOI] [PubMed] [Google Scholar]
- Brien JF, Clarke DW, Richardson B, Patrick J. Disposition of ethanol in maternal blood, fetal blood, and amniotic fluid of third-trimester pregnant ewes. Am J Obstet Gynecol. 1985;152:583–590. doi: 10.1016/0002-9378(85)90632-5. [DOI] [PubMed] [Google Scholar]
- Bubb KJ, Cock ML, Black MJ, Dodic M, Boon W-M, Parkington HC, Harding R, Tare M. Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol. 2007;578:871–881. doi: 10.1113/jphysiol.2006.121160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen ML, Miller AA, Andrews KL, Irvine JC, Ritchie RH, Sobey CG, Kemp-Harper BK. Nitroxyl (HNO) as a vasoprotective signaling molecule. Antioxid Redox Signal. 2011;14:1675–1686. doi: 10.1089/ars.2010.3327. [DOI] [PubMed] [Google Scholar]
- Chang PY, Lu SC, Lee CM, Chen YJ, Dugan TA, Huang WH, Chang SF, Liao WS, Chen CH, Lee YT. Homocysteine inhibits arterial endothelial cell growth through transcriptional downregulation of fibroblast growth factor-2 involving G protein and DNA methylation. Circ Res. 2008;102:933–941. doi: 10.1161/CIRCRESAHA.108.171082. [DOI] [PubMed] [Google Scholar]
- Colvin L, Payne J, Parsons D, Kurinczuk JJ, Bower C. Alcohol consumption during pregnancy in nonindigenous west Australian women. Alcohol Clin Exp Res. 2007;31:276–284. doi: 10.1111/j.1530-0277.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- Cudd TA, Chen WJ, Parnell SE, West JR. Third trimester binge ethanol exposure results in fetal hypercapnea and acidemia but not hypoxemia in pregnant sheep. Alcohol Clin Exp Res. 2001;25:269–276. [PubMed] [Google Scholar]
- DeLoach SS, Townsend RR. Vascular stiffness: its measurement and significance for epidemiologic and outcome studies. Clin J Am Soc Nephrol. 2008;3:184–192. doi: 10.2215/CJN.03340807. [DOI] [PubMed] [Google Scholar]
- Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- Djousse L, Lee IM, Buring JE, Gaziano JM. Alcohol consumption and risk of cardiovascular disease and death in women: potential mediating mechanisms. Circulation. 2009;120:237–244. doi: 10.1161/CIRCULATIONAHA.108.832360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatjo F, Sancho-Bru P, Fernandez-Sola J, Sacanella E, Estruch R, Bataller R, Nicolas JM. Up-regulation of myocardial L-type Ca2+ channel in chronic alcoholic subjects without cardiomyopathy. Alcohol Clin Exp Res. 2007;31:1099–1105. doi: 10.1111/j.1530-0277.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- Fenelon G, Balbao CE, Fernandes R, Arfelli E, Landim P, Ayres O, Paola AA. Characterization of the acute cardiac electrophysiologic effects of ethanol in dogs. Alcohol Clin Exp Res. 2007;31:1574–1580. doi: 10.1111/j.1530-0277.2007.00451.x. [DOI] [PubMed] [Google Scholar]
- Gibson A, Woodside JV, Young IS, Sharpe PC, Mercer C, Patterson CC, McKinley MC, Kluijtmans LA, Whitehead AS, Evans A. Alcohol increases homocysteine and reduces B vitamin concentration in healthy male volunteers – a randomized, crossover intervention study. QJM. 2008;101:881–887. doi: 10.1093/qjmed/hcn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CA, Iida H, Hotchkiss KJ, Northington FJ, Traystman RJ. Newborn cerebrovascular responses after first trimester moderate maternal ethanol exposure in sheep. Pediatr Res. 1997;42:39–45. doi: 10.1203/00006450-199707000-00007. [DOI] [PubMed] [Google Scholar]
- Gray SP, Denton KM, Cullen-McEwen L, Bertram JF, Moritz KM. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J Am Soc Nephrol. 2010;21:1891–1902. doi: 10.1681/ASN.2010040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SP, Kenna K, Bertram JF, Hoy WE, Yan EB, Bocking AD, Brien JF, Walker DW, Harding R, Moritz KM. Repeated ethanol exposure during late gestation decreases nephron endowment in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2008;295:R568–R574. doi: 10.1152/ajpregu.90316.2008. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Verkerk MM, Derks JB, Giussani DA. Antioxidant treatment alters peripheral vascular dysfunction induced by postnatal glucocorticoid therapy in rats. PLoS One. 2010;5:e9250. doi: 10.1371/journal.pone.0009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iveli MF, Morales S, Rebolledo A, Savietto V, Salemme S, Apezteguia M, Cecotti N, Drut R, Milesi V. Effects of light ethanol consumption during pregnancy: increased frequency of minor anomalies in the newborn and altered contractility of umbilical cord artery. Pediatr Res. 2007;61:456–461. doi: 10.1203/pdr.0b013e3180332c59. [DOI] [PubMed] [Google Scholar]
- Izzard AS, Horton S, Heerkens EH, Shaw L, Heagerty AM. Middle cerebral artery structure and distensibility during developing and established phases of hypertension in the spontaneously hypertensive rat. J Hypertens. 2006;24:875–880. doi: 10.1097/01.hjh.0000222757.54111.06. [DOI] [PubMed] [Google Scholar]
- Kenna K, De Matteo R, Hanita T, Rees S, Sozo F, Stokes V, Walker D, Bocking A, Brien J, Harding R. Daily ethanol exposure during late ovine pregnancy: physiological effects in the mother and fetus in the apparent absence of overt fetal cerebral dysmorphology. Am J Physiol Regul Integr Comp Physiol. 2011;301:R926–R936. doi: 10.1152/ajpregu.00711.2010. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Alcohol and cardiovascular diseases. Expert Rev Cardiovasc Ther. 2009;7:499–506. doi: 10.1586/erc.09.22. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Tomiyama H, Hashimoto H, Yamamoto Y, Yano E, Yamashina A. Excessive alcohol intake increases the risk of arterial stiffening in men with normal blood pressure. Hypertens Res. 2004;27:669–673. doi: 10.1291/hypres.27.669. [DOI] [PubMed] [Google Scholar]
- Kvigne VL, Leonardson GR, Neff-Smith M, Brock E, Borzelleca J, Welty TK. Characteristics of children who have full or incomplete fetal alcohol syndrome. J Pediatr. 2004;145:635–640. doi: 10.1016/j.jpeds.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Lamireau D, Nuyt AM, Hou X, Bernier S, Beauchamp M, Gobeil F, Jr, Lahaie I, Varma DR, Chemtob S. Altered vascular function in fetal programming of hypertension. Stroke. 2002;33:2992–2998. doi: 10.1161/01.str.0000039340.62995.f2. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Lutz UC. Alterations in homocysteine metabolism among alcohol dependent patients – clinical, pathobiochemical and genetic aspects. Curr Drug Abuse Rev. 2008;1:47–55. doi: 10.2174/1874473710801010047. [DOI] [PubMed] [Google Scholar]
- Martyn CN, Greenwald SE. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997;350:953–955. doi: 10.1016/s0140-6736(96)10508-0. [DOI] [PubMed] [Google Scholar]
- Martyn CN, Greenwald SE. A hypothesis about a mechanism for the programming of blood pressure and vascular disease in early life. Clin Exp Pharmacol Physiol. 2001;28:948–951. doi: 10.1046/j.1440-1681.2001.03555.x. [DOI] [PubMed] [Google Scholar]
- Mazzuca MQ, Wlodek ME, Dragomir NM, Parkington HC, Tare M. Uteroplacental insufficiency programs regional vascular dysfunction and alters arterial stiffness in female offspring. J Physiol. 2010;588:1997–2010. doi: 10.1113/jphysiol.2010.187849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Shepherd J, Perham N, Cusens B. The prevalence of alcohol intoxication in the night-time economy. Alcohol Alcohol. 2007;42:629–634. doi: 10.1093/alcalc/agm054. [DOI] [PubMed] [Google Scholar]
- Morley R, Dwyer T, Hynes KL, Cochrane J, Ponsonby AL, Parkington HC, Carlin JB. Maternal alcohol intake and offspring pulse wave velocity. Neonatology. 2010;97:204–211. doi: 10.1159/000252973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell SE, Ramadoss J, Delp MD, Ramsey MW, Chen WJ, West JR, Cudd TA. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: ovine model. Exp Physiol. 2007;92:933–943. doi: 10.1113/expphysiol.2007.038091. [DOI] [PubMed] [Google Scholar]
- Payne JA, Alexander BT, Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension. 2003;42:768–774. doi: 10.1161/01.HYP.0000084990.88147.0C. [DOI] [PubMed] [Google Scholar]
- Piano MR, Geenen DL, Schwertz DW, Chowdhury SA, Yuzhakova M. Long-term effects of alcohol consumption in male and female rats. Cardiovasc Toxicol. 2007;7:247–254. doi: 10.1007/s12012-007-9002-y. [DOI] [PubMed] [Google Scholar]
- Poston L. Influences of maternal nutritional status on vascular function in the offspring. Curr Drug Targets. 2007;8:914–922. doi: 10.2174/138945007781386910. [DOI] [PubMed] [Google Scholar]
- Prevention CfDCa. Fetal alcohol spectrum disorders. 2010. http://www.cdc.gov/ncbddd/fasd/data.html.
- Pruett D, Waterman EH, Caughey AB. Fetal alcohol exposure: consequences, diagnosis, and treatment. Obstet Gynecol Surv. 2013;68:62–69. doi: 10.1097/OGX.0b013e31827f238f. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Vascular effects of maternal alcohol consumption. Am J Physiol Heart Circ Physiol. 2012;303:H414–H421. doi: 10.1152/ajpheart.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CL, Jones KL, Jones MC, Kaplan GW. Incidence of renal anomalies in children prenatally exposed to ethanol. Pediatrics. 1994;94:209–212. [PubMed] [Google Scholar]
- Tikkanen J, Heinonen OP. Maternal exposure to chemical and physical factors during pregnancy and cardiovascular malformations in the offspring. Teratology. 1991;43:591–600. doi: 10.1002/tera.1420430614. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Casolari DA, Montezano AC, Yogi A, Tostes RC, Legros E, D'Orleans-Juste P, Lanchote VL, Uyemura SA, de Oliveira AM. Ethanol consumption enhances endothelin-1-induced contraction in the isolated rat carotid. J Pharmacol Exp Ther. 2006;318:819–827. doi: 10.1124/jpet.106.103010. [DOI] [PubMed] [Google Scholar]
- Turcotte LA, Aberle NS, Norby FL, Wang GJ, Ren J. Influence of prenatal ethanol exposure on vascular contractile response in rat thoracic aorta. Alcohol. 2002;26:75–81. doi: 10.1016/s0741-8329(01)00198-7. [DOI] [PubMed] [Google Scholar]
- van Driel LM, de Jonge R, Helbing WA, van Zelst BD, Ottenkamp J, Steegers EA, Steegers-Theunissen RP. Maternal global methylation status and risk of congenital heart diseases. Obstet Gynecol. 2008;112:277–283. doi: 10.1097/AOG.0b013e31817dd058. [DOI] [PubMed] [Google Scholar]
- van Trijp MJ, Beulens JW, Bos WJ, Uiterwaal CS, Grobbee DE, Hendriks HF, Bots ML. Alcohol consumption and augmentation index in healthy young men: the ARYA study. Am J Hypertens. 2005;18:792–796. doi: 10.1016/j.amjhyper.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Wigg SJ, Tare M, Forbes J, Cooper ME, Thomas MC, Coleman HA, Parkington HC, O'Brien RC. Early vitamin E supplementation attenuates diabetes-associated vascular dysfunction and the rise in protein kinase C-β in mesenteric artery and ameliorates wall stiffness in femoral artery in rats. Diabetologia. 2004;47:1038–1046. doi: 10.1007/s00125-004-1411-x. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Ethanol-induced contractions in cerebral arteries: role of tyrosine and mitogen-activated protein kinases. Stroke. 2001;32:249–257. doi: 10.1161/01.str.32.1.249. [DOI] [PubMed] [Google Scholar]
- Yeligar S, Tsukamoto H, Kalra VK. Ethanol-induced expression of ET-1 and ET-BR in liver sinusoidal endothelial cells and human endothelial cells involves hypoxia-inducible factor-1alpha and microrNA-199. J Immunol. 2009;183:5232–5243. doi: 10.4049/jimmunol.0901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]


