Abstract
In the present study, we sought to determine the effect of a traditional, 12 week aerobic training protocol on skeletal muscle fibre type distribution and satellite cell content in sedentary subjects. Muscle biopsies were obtained from the vastus lateralis [n = 23 subjects (six male and 17 female); body mass index 30.7 ± 1.2 kg m−2] before and after 12 weeks of aerobic training performed on a cycle ergometer. Immunohistochemical analyses were used to quantify myosin heavy chain (MyHC) isoform expression, cross-sectional area and satellite cell and myonuclear content. Following training, a decrease in MyHC hybrid type IIa/IIx fibre frequency occurred, with a concomitant increase in pure MyHC type IIa fibres. Pretraining fibre type correlated with body mass index, and the change in fibre type following training was associated with improvements in maximal oxygen consumption. Twelve weeks of aerobic training also induced increases in mean cross-sectional area in both MyHC type I and type IIa fibres. Satellite cell content was also increased following training, specifically in MyHC type I fibres, with no change in the number of satellite cells associated with MyHC type II fibres. With the increased satellite cell content following training, an increase in myonuclear number per fibre also occurred in MyHC type I fibres. Hypertrophy of MyHC type II fibres occurred without detectable myonuclear addition, suggesting that the mechanisms underlying growth in fast and slow fibres differ. These data provide intriguing evidence for a fibre type-specific role of satellite cells in muscle adaptation following aerobic training.
Key points
Satellite cell activation and fusion accompany resistance exercise training.
Aerobic exercise training is capable of inducing subtle muscle fibre hypertrophy; however, the role of satellite cell activation during aerobic exercise-induced muscle adaptation is unknown.
Twelve weeks of aerobic training in sedentary subjects yielded an increase in myosin heavy chain type I and type II muscle fibre cross-sectional area.
Satellite cell activation and myonuclear addition occurred only in myosin heavy chain type I fibres, with no change in myosin heavy chain type II fibres.
These results help us better understand the role of satellite cells in muscle fibre adaptation to aerobic exercise, and suggest differential fibre type regulation of the myonuclear domain.
Introduction
While the necessity of satellite cells, or muscle stem cells, in the regenerative response following skeletal muscle injury has been well demonstrated (Lepper et al 2011; McCarthy et al 2011; Murphy et al 2011; Sambasivan et al 2011), their role in response to exercise remains equivocal. For instance, resistance training studies in humans have shown a concomitant increase in satellite cell content and myonuclear number with muscle hypertrophy, presumed to be the result of satellite cell fusion (Kadi & Thornell, 2000; Roth et al 2001; Petrella et al 2006, 2008; Verdijk et al 2009). From this association it is often assumed that satellite cell fusion is a requirement for muscle hypertrophy. This assumption is further reinforced by rodent irradiation studies that demonstrate a necessity for satellite cells during overload-induced hypertrophy (Rosenblatt & Parry, 1992; Rosenblatt et al 1994; Adams et al 2002). However, more recent research has challenged this assumption, where several groups using transgenic mouse models have shown robust muscle growth in the absence of satellite cell fusion (Amthor et al 2009; Blaauw et al 2009; McCarthy et al 2011). While debate exists about the necessity of satellite cells during muscle hypertrophy, in typical conditions satellite cell activation/fusion and myonuclear accretion accompany muscle growth.
A lack of consensus exists for the role of satellite cells during aerobic exercise. Previous work in rodent models using both voluntary wheel running and forced treadmill running have shown increased satellite cell content (Kurosaka et al 2009; Shefer et al 2010). Certain aerobic training studies in human subjects have demonstrated increases in the satellite cell pool (Charifi et al 2003; Verney et al 2008), while others failed to do so (Snijders et al 2011). With high-intensity interval training, evidence from human studies supports a role for satellite cell activation in muscle adaptation and remodelling, independent of myofibre hypertrophy (Joanisse et al 2013).
Satellite cells may contribute differentially to muscle adaptation to training based on fibre type. Historical studies in rodents have shown higher satellite cell content in muscles with higher type I than type II myosin heavy chain (MyHC) expression (Schmalbruch & Hellhammer, 1977; Gibson & Schultz, 1983). Human skeletal muscle displays a more ‘mixed’ fibre type distribution and does not appear to demonstrate fibre type-specific satellite cell content at baseline (Kadi et al 2006; Verdijk et al 2009; Mackey et al 2014). In contrast, data from humans suggest satellite cell activation specifically in hybrid I/IIa fibres following high-intensity interval training (Joanisse et al 2013) and in type II fibres following resistance training (Verdijk et al 2009). These data provide evidence for fibre type-specific activation of satellite cells; however, the role of satellite cell activation in adaptation following traditional aerobic exercise in humans is unknown.
In the present study, we sought to determine the effect of a 12 week aerobic training protocol on skeletal muscle fibre type distribution and satellite cell content in sedentary subjects. We hypothesized that the aerobic training would induce an increase in MyHC type I satellite cell content, contributing to fibre type-specific adaptation to exercise.
Methods
Ethical approval
The participants signed consent forms approved by the Institutional Review Board from the University of Kentucky which is in accordance with the standards set by the latest revision of the Declaration of Helsinki.
Human subjects
Twenty-three sedentary subjects (six male and 17 female) were studied, with an average age of 47.6 years and body mass index (BMI) of 30.7 kg m−2. Subjects were classified as non-diabetic from results of an oral glucose tolerance test. Subject characteristics can be found in Table 1. Subjects were deemed sedentary based on physical activity self-reports of fewer than two sessions of structured exercise per week lasting <30 min. Subjects were not taking any medications known to affect muscle, such as angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, statins, steroids or antidiabetic drugs. Subjects treating mild hypertension with β-blockers or diuretics were allowed to participate in the study.
Table 1.
Subject characteristics
| Characteristic | Mean ± SEM | Range |
|---|---|---|
| n | 23 (6 male, 17 female) | |
| Age (years) | 47.6 ± 2.7 | 26–68 |
| Body mass index (kg m−2) | 30.7 ± 1.2 | 23.3–41.8 |
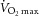 pretraining (ml kg−1 min−1) pretraining (ml kg−1 min−1) |
30.2 ± 2.2 | 19.4–53.2 |
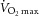 post-training (ml kg−1 min−1) post-training (ml kg−1 min−1) |
32.4 ± 2.8* | 17.4–53.7 |
| Fasting glucose (mg dl−1) | 84.8 ± 1.3 | 73–100 |
| 2 h glucose (mg dl−1) | 121.7 ± 5.7 | 70–187 |
Abbreviation: 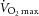 , maximal oxygen uptake.
, maximal oxygen uptake.
P = 0.06 from pretraining value.
Study design
Following screening, subjects underwent a basal dual energy X-ray absorptiometry (DEXA) scan and maximal oxygen consumption (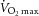 ) testing to assess pretraining values. During
) testing to assess pretraining values. During 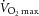 testing, subjects’ heart rate, blood pressure and rating of perceived exertion were monitored, and ventilation and expired air samples were measured by a metabolic cart (Vmax Encore Metabolic Cart; CareFusion, San Diego, CA, USA) for the determination of O2 uptake. Percutaneous muscle biopsies were then sampled from the vastus lateralis using a Bergström 5 mm muscle biopsy needle with suction. Following the collection of the muscle biopsy (pretraining), subjects underwent a 12 week aerobic training programme consisting of three sessions per week of 45 min on a cycle ergometer (Monark Sports & Medical, Stockholm, Sweden) at 70% of their heart rate reserve. Following successful completion of the training programme (no more than six missed sessions), a second muscle biopsy (post-training) was collected, and the subjects repeated DEXA and
testing, subjects’ heart rate, blood pressure and rating of perceived exertion were monitored, and ventilation and expired air samples were measured by a metabolic cart (Vmax Encore Metabolic Cart; CareFusion, San Diego, CA, USA) for the determination of O2 uptake. Percutaneous muscle biopsies were then sampled from the vastus lateralis using a Bergström 5 mm muscle biopsy needle with suction. Following the collection of the muscle biopsy (pretraining), subjects underwent a 12 week aerobic training programme consisting of three sessions per week of 45 min on a cycle ergometer (Monark Sports & Medical, Stockholm, Sweden) at 70% of their heart rate reserve. Following successful completion of the training programme (no more than six missed sessions), a second muscle biopsy (post-training) was collected, and the subjects repeated DEXA and 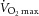 testing. Post-training biopsies and testing were performed within 48 h following completion of the last training session. The design of the study can be seen in Fig. 1.
testing. Post-training biopsies and testing were performed within 48 h following completion of the last training session. The design of the study can be seen in Fig. 1.
Figure 1. Study design.
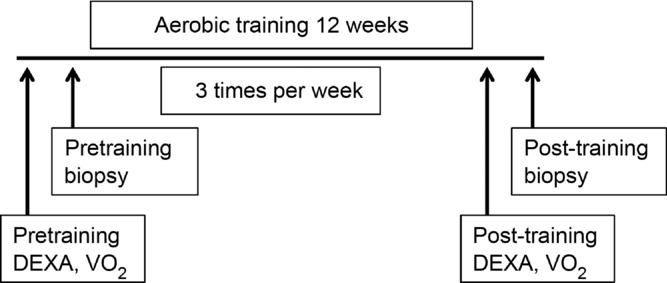
Subjects underwent pretraining testing prior to collection of a muscle biopsy from the vastus lateralis before undergoing a 12 week aerobic training programme. Following training, a second muscle biopsy was collected, and testing was repeated.
Immunohistochemistry
Muscle tissue samples (∼50 mg) were mounted in tragacanth gum on cork immediately after biopsy and frozen in liquid nitrogen-cooled isopentane to be processed for immunohistochemical analysis. Seven-micrometre-thick sections were cut in a cryostat, and sections were allowed to air dry for 1 h. For Pax7/MyHC type I/laminin staining, slides were fixed for 10 min in ice-cold acetone, endogenous peroxidases were blocked with 3% H2O2, and then slides were incubated overnight in anti-laminin (#ab14055; Abcam, Cambridge, MA, USA) and anti-MyHC I antibodies [BA.D5; IgG2b; Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA] at 4°C. The next day, slides were incubated in goat anti-chicken IgG1 AF488 (#A11039; Invitrogen, Grand Island, NY, USA) and goat anti-mouse IgG2b, AF647 (#A21242; Invitrogen) for 1 h and then blocked for 1 h in 2.5% normal horse serum. Slides were incubated overnight at 4°C in anti-Pax7 antibody (DSHB), followed by goat anti-mouse biotin secondary antibody (#115-065-205; Jackson Immuno Research, West Grove, PA, USA) for 1 h, and reacted with streptavidin–horseradish peroxidase included with the TSA kit (#T20935; Invitrogen). Slides were co-stained with 4′,6-diamidino-2-phenylindole (DAPI, #D35471; Invitrogen) prior to being mounted with fluorescent mounting media (Vectashield, #H-1000; Vector Laboratories Inc., Burlingame, CA, USA). In a subset of subjects (n = 9), the relative frequency of satellite cells associated with MyHC type IIx was determined as described above for MyHC type I, except that anti-MyHC IIx (6H1; IgM; DSHB) was used, detected using goat anti-rabbit IgM, AF647 (#A21245; Invitrogen). Relative frequencies of satellite cells associated with MyHC type I and IIa/IIx fibres were determined by quantifying both stains and merging results, with the remaining unstained fibres counted as pure type IIa fibres and associated satellite cells.
For fibre typing, unfixed slides were incubated overnight at room temperature with antibodies against MyHC isoforms type I, type IIa (SC.71; IgG1) and type IIx from DSHB. The next day, slides were incubated with immunoglobulin-specific secondary antibodies, namely goat anti-mouse IgG2b AF647 (#A21242), goat anti-mouse IgG1 AF488 (#A21121) and goat anti-mouse IgM biotin (#626840), all from Invitrogen, and then 15 min in streptavidin–Texas Red (#SA-5006; Vector Laboratories Inc.). Slides were postfixed in methanol prior to mounting with fluorescent mounting media.
Image acquisition and analysis
Images were captured at ×20 magnification at room temperature with a Zeiss upright microscope (AxioImager M1; Zeiss, Oberkochen, Germany) and analysis carried out using the AxioVision Rel software (v4.8). To capture the entire muscle cross-section required between five and 15 images (pretraining biopsy, 282 ± 48 fibres per subject; post-training biopsy, 198 ± 24 fibres per subject) to be analysed. Fibre type-specific satellite cell abundance was assessed using Pax7 staining in conjunction with MyHC type I/laminin, or MyHC type IIx/laminin, and only those loci that were scored as Pax7+ and DAPI+ within the laminin border were counted. The DAPI+/Pax7− nuclei residing within the laminin border were counted as myonuclei and identified with their associated fibre type (MyHC type I or II).
Statistical analysis
Student's paired t tests were performed to compare each dependent variable pre- and post-training, with significance set at P ≤ 0.05. Where appropriate, correlations were tested by assessing the existence of a linear fit between the various outcome measures, with significance set at P ≤ 0.05. All analyses were done with SigmaStat 12.0 (Systat Software Inc., San Jose, CA, USA).
Results
Training induced improvements in oxidative capacity, correlated with changes in fibre type composition
Following the 12 week aerobic training protocol, the mean 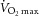 tended to increase in subjects (Table 1). This was associated with a fibre type shift, assessed on sections with MyHC isoform-specific antibodies (Fig. 2). Type I (Fig. 2A), type IIa (Fig. 2B) and type IIx fibres (Fig. 2C), overlaid in Fig. 2D (enabling identification of hybrid fibres), were counted. As shown in Fig. 3A, there was an increase in the relative proportion of MyHC type IIa fibres and a decrease in the proportion of MyHC type IIa/IIx hybrid fibres. No change was observed in the frequency of MyHC type I fibres (P > 0.05). A positive correlation existed between the relative proportion of MyHC type IIa fibres gained and the increase in
tended to increase in subjects (Table 1). This was associated with a fibre type shift, assessed on sections with MyHC isoform-specific antibodies (Fig. 2). Type I (Fig. 2A), type IIa (Fig. 2B) and type IIx fibres (Fig. 2C), overlaid in Fig. 2D (enabling identification of hybrid fibres), were counted. As shown in Fig. 3A, there was an increase in the relative proportion of MyHC type IIa fibres and a decrease in the proportion of MyHC type IIa/IIx hybrid fibres. No change was observed in the frequency of MyHC type I fibres (P > 0.05). A positive correlation existed between the relative proportion of MyHC type IIa fibres gained and the increase in 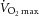 that occurred with training (P < 0.05; Fig. 3B). Prior to beginning the training protocol, a positive correlation between BMI and relative frequency of MyHC type IIa/IIx hybrid fibres existed (P < 0.05; Fig. 3C); in all circumstances, all MyHC type IIx-expressing fibres (Fig. 2C) co-expressed MyHC type IIa (Fig. 2B), with the shift to a pure MyHC type IIa phenotype associated with increased oxidative capacity. No effect of age or gender was observed in the fibre type frequency. Whole-body lean mass and leg lean mass were unchanged with training, as was whole-body fat mass. Leg fat mass decreased following the training period (P < 0.05).
that occurred with training (P < 0.05; Fig. 3B). Prior to beginning the training protocol, a positive correlation between BMI and relative frequency of MyHC type IIa/IIx hybrid fibres existed (P < 0.05; Fig. 3C); in all circumstances, all MyHC type IIx-expressing fibres (Fig. 2C) co-expressed MyHC type IIa (Fig. 2B), with the shift to a pure MyHC type IIa phenotype associated with increased oxidative capacity. No effect of age or gender was observed in the fibre type frequency. Whole-body lean mass and leg lean mass were unchanged with training, as was whole-body fat mass. Leg fat mass decreased following the training period (P < 0.05).
Figure 2. Representative immunohistochemical image of myosin heavy chain (MyHC) fibre type identification.
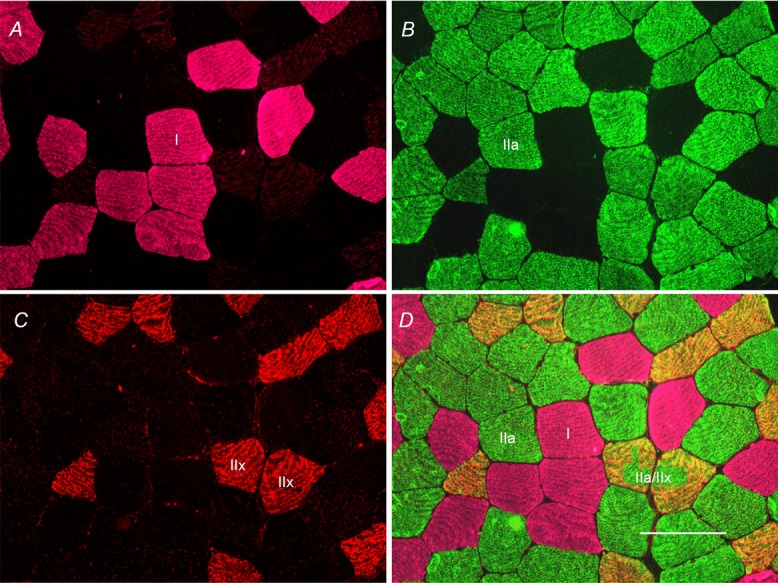
Panels are MyHC type I (pink; A), MyHC type IIa (green; B), MyHC type IIx (red; C) and the fused image (D). Scale bar represents 100 μm.
Figure 3. Twelve weeks of aerobic training yields a decrease in MyHC hybrid IIa/IIx fibre frequency and an increase in MyHC type IIa fibre frequency.
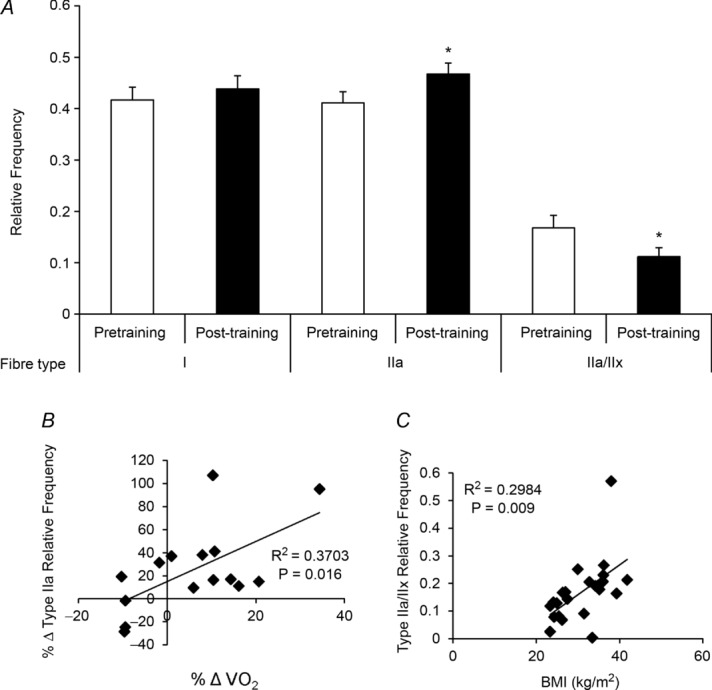
A, quantification of MyHC fibre type from immunohistochemical analysis, presented as mean fibre type frequency + SEM. B, correlation of the change in relative frequency of MyHC type IIa fibres with the change in maximal oxygen consumption (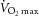 ) following 12 weeks of aerobic training. C, correlation of pretraining MyHC hybrid IIa/IIx fibre frequency and body mass index (BMI). *Significantly different from pretraining value (P < 0.05).
) following 12 weeks of aerobic training. C, correlation of pretraining MyHC hybrid IIa/IIx fibre frequency and body mass index (BMI). *Significantly different from pretraining value (P < 0.05).
Training induced increases in mean fibre cross-sectional area (CSA) in both MyHC type I and type IIa fibres
The mean CSA of MyHC type IIa fibres increased following the 12 weeks of aerobic training, as did the CSA of MyHC type I fibres (Fig. 4A). Mean CSA of MyHC type IIa/Iix fibres showed a trend to increase as well, but did not reach statistical significance (P > 0.05). Pooled CSA also demonstrated an increase (Fig. 4B). When presented as a binned histogram, a clear rightward shift in fibre size distribution was observed (Fig. 4C; shown by fibre type in Fig. 5A–C). No effect of age or gender was observed in the change in fibre CSA with training.
Figure 4. Twelve weeks of aerobic training yields an increase in mean fibre cross-sectional area (CSA) and in mean fibre CSA of MyHC types I and IIa.
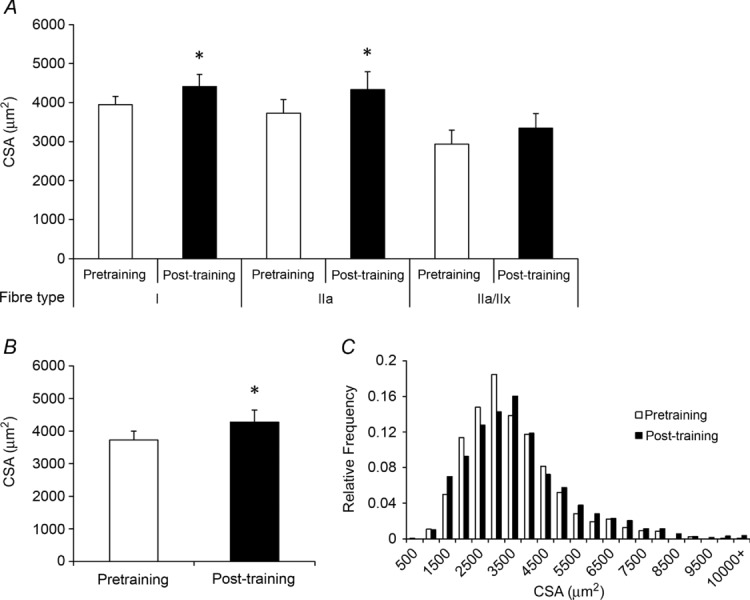
A, mean fibre CSA (in square micrometres) of MyHC type I, IIa and IIa/IIx fibres presented as means + SEM. B, pooled mean fibre CSA presented as means + SEM. C, histogram distributions of pooled fibre CSA. *Significantly different from pretraining value (P < 0.05).
Figure 5. Histogram distributions of individual MyHC fibre CSA following 12 weeks of aerobic training demonstrate rightward shifts.
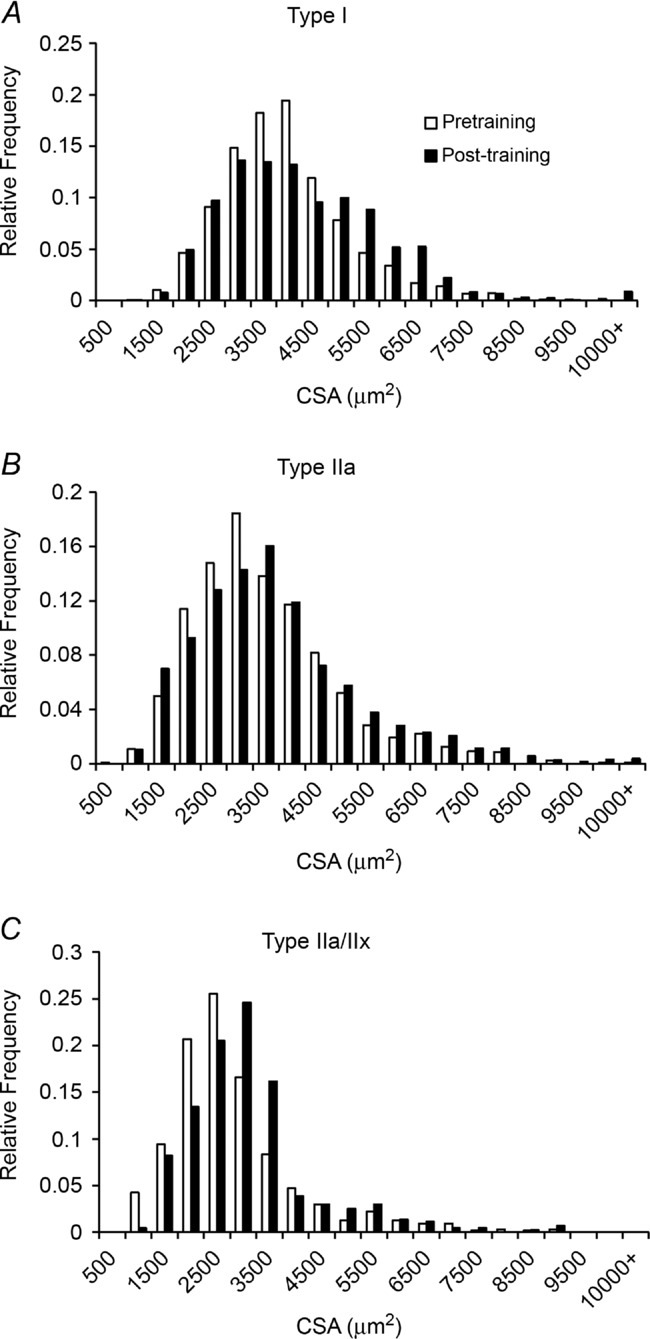
Myosin heavy chain type I CSA presented as a binned histogram (A), MyHC type IIa CSA presented as a binned histogram (B) and MyHC type IIa/IIx CSA presented as a binned histogram (C).
Increased satellite cell content and increased myonuclear number with training occurs only in MyHC type I fibres
Figure 6 shows fibre type-specific satellite cell content, assessed immunohistochemically with antibodies against MyHC type I (Fig. 6C), laminin (Fig. 6D) and Pax7 (Fig. 6E), co-stained with DAPI (Fig. 6F). The location of satellite cells underneath the basal lamina allows for their identification with the associated fibre (MyHC type I or II), as seen in the fused images in Fig. 6A and B.
Figure 6. Representative immunohistochemical image of fibre type-specific satellite cell identification.
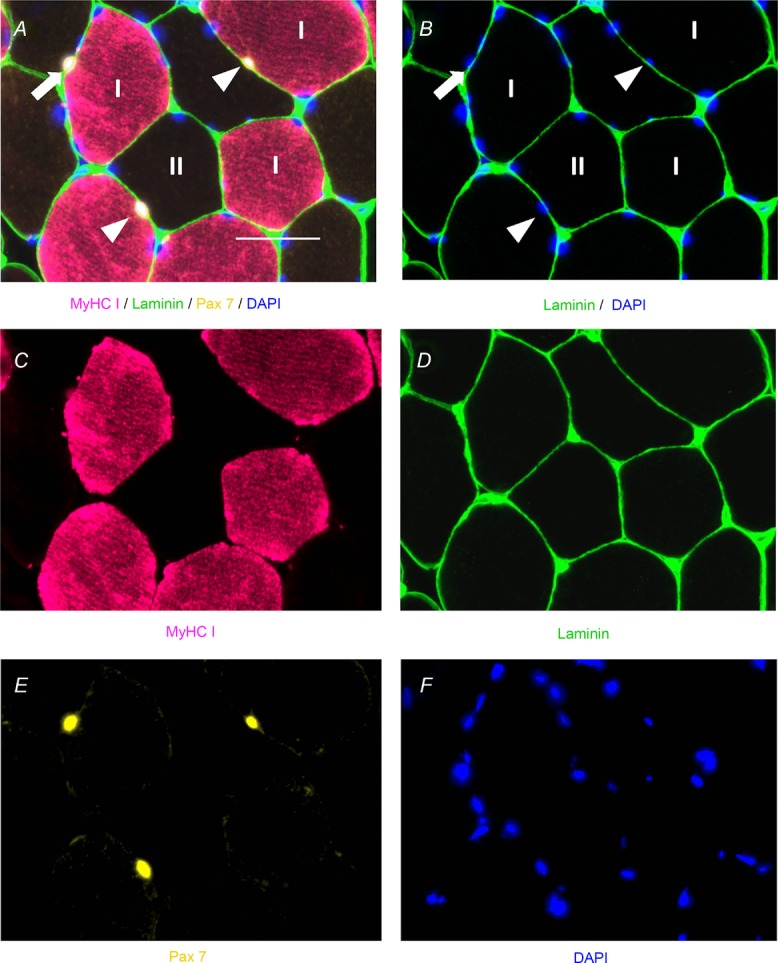
A, fused image demonstrating laminin (green), Pax7 (yellow), MyHC type I (pink) and 4′,6-diamidino-2-phenylindole (DAPI; blue) with MyHC type I satellite cells denoted by white arrowheads and a MyHC type II satellite cell denoted by a white arrow. Scale bar represents 50 μm. B, fused image demonstrating satellite cell location within laminin (green), costained with DAPI (blue), and MyHC type I satellite cells denoted by white arrowheads and a MyHC type II satellite cell denoted by a white arrow. C, single-channel image demonstrating MyHC type I (pink). D, single-channel image demonstrating laminin (green). E, single-channel image demonstrating Pax7 (yellow). F, single-channel image demonstrating DAPI (blue).
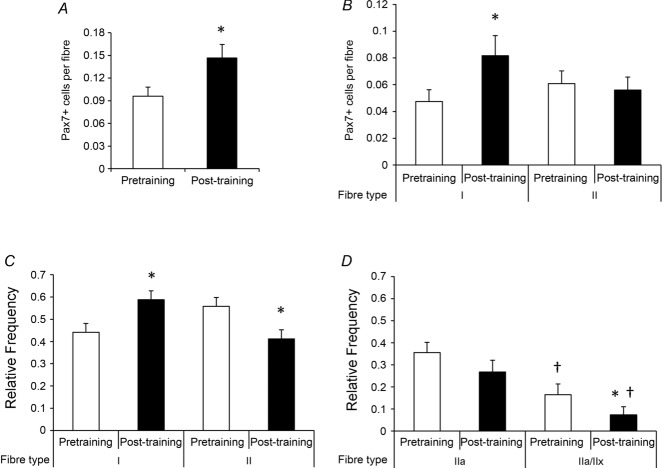
No significant difference in satellite cells associated with MyHC type I and II fibres was apparent pretraining (Fig. 7A). Following training, a clear increase in satellite cell content occurred exclusively in MyHC type I fibres (Fig. 7B). This increase resulted in an alteration in the relative distribution of satellite cells so that a greater proportion of satellite cells were associated with MyHC type I than with type II fibres after the 12 weeks of aerobic training (Fig. 7C). Although the overall abundance of type II fibre-associated satellite cells was unaffected by training (Fig. 7B), there was a shift in the relative distribution of satellite cells in MyHC type IIa compared with type IIa/IIx fibres; abundance was relatively unchanged in pure type IIa fibres and decreased in type IIa/IIx fibres with training (Fig. 7D). No effect of age or gender was observed in satellite cell content.
Figure 7. Following 12 weeks of aerobic training, an increase in satellite cell content associated with MyHC type I fibres occurred.
A, quantification of satellite cell content, expressed as mean satellite cells per fibre + SEM. B, quantification of fibre type-specific satellite cell content, expressed as mean satellite cells per fibre + SE. C, relative frequency of satellite cells associated with MyHC type I and II fibres, expressed as mean frequency + SEM. D, relative frequency of satellite cells associated with MyHC type IIa and IIa/IIx fibres in a subset of subjects (n = 9), expressed as mean frequency + SEM. *Significantly different from pretraining value (P < 0.05). †Significantly different from MyHC type IIa value (P < 0.05).
Myonuclear content was quantified by counting DAPI+/Pax7− nuclei residing within the laminin border (Fig. 8A). Myonuclei were localized to either MyHC type I or II fibres using an antibody against MyHC type I. Supportive of the MyHC type I-dependent increase in satellite cell content following training, myonuclear accretion occurred in only MyHC type I fibres (Fig. 8B).
Figure 8. Following 12 weeks of aerobic training, an increase in myonuclear number in MyHC type I fibres occurred.
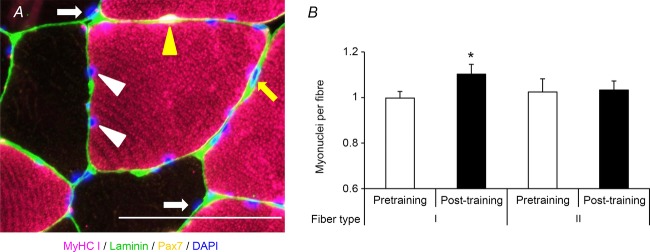
A, representative image demonstrating MyHC type I (pink), laminin (green), Pax7 (yellow) and DAPI (blue). Myonuclei associated with MyHC type I fibres are denoted by white arrowheads; myonuclei associated with MyHC type II fibres are denoted by white arrows; a satellite cell is denoted by a yellow arrowhead; and an interstitial nucleus is denoted by a yellow arrow. Scale bar: 100 μm. B, quantification of myonuclei in MyHC type I and II fibres, expressed as mean myonuclei per fibre + SEM. *Significantly different from pretraining value (P < 0.05).
Discussion
The purpose of this study was to define the adaptations in satellite cell content and muscle fibre type distribution that occur following a 12 week traditional aerobic exercise programme in sedentary individuals. We found a shift in relative fibre type distribution, with a decrease in hybrid MyHC type IIa/IIx fibres and a concomitant increase in pure MyHC type IIa fibres and no change in the frequency of type I fibres. However, following training we found a significant increase in the number of satellite cells, specifically associated with MyHC type I fibres, which was accompanied by an increase in myonuclear content in MyHC type I fibres and type I fibre hypertrophy. Surprisingly, we also found an increase in mean CSA of MyHC type IIa fibres, which was not associated with increased satellite cell abundance or myonuclear number. These results provide intriguing evidence for a potential fibre type-specific role of satellite cells during aerobic training and for different mechanisms underlying fast and slow myofibre growth.
Overall increases in satellite cell content following traditional aerobic training have previously been observed in older men (Charifi et al 2003), but no changes were observed following 6 months of aerobic training in subjects with type 2 diabetes (Snijders et al 2011). While subjects in the study by Snijders et al (2011) had similar BMI to those in the present study, subjects in the present study were younger, did not have type 2 diabetes and participated in a more controlled aerobic training programme. Snijders et al (2011) used a variety of aerobic exercise modalities, including walking, cycling and skiing during training sessions. In the present study, the use of cycling alone may have led to greater stimulation of leg extensors than a programme involving walking, leading to enhanced recruitment of satellite cells.
We report, for the first time, an increase in MyHC type I fibre satellite cell content following aerobic training. In comparison, a recent study by Joanisse et al (2013), using a 6 week high-intensity interval training programme, did not find an increase in MyHC type I satellite cell content, but rather an increase in satellite content in hybrid type I/IIa fibres. Unlike the study by Joanisse et al (2013), the intensity of our training protocol was insufficient to promote transition of type IIa fibres to type I/IIa hybrids. The increase in MyHC type I satellite cell content observed in the present study was accompanied by myonuclear accretion and hypertrophy of MyHC type I fibres. It has been shown that MyHC type I fibres are more insulin sensitive than their type II counterparts (Henriksen et al 1990; He et al 2001), and interventions aimed at inducing positive adaptations in type I fibres are likely to serve to improve substrate utilization.
Following 12 weeks of aerobic training, we found an increase in overall mean fibre CSA, which included an increase in the CSA of both MyHC type I and IIa fibres. Aerobic training in humans is not traditionally associated with muscle fibre hypertrophy; however, several studies have demonstrated increased fibre CSA in adults of varying ages (Harber et al 2009b; Hudelmaier et al 2010; Konopka et al 2010; McPhee et al 2010; Harber et al 2012). The study by Harber et al (2012) employed a similar training regimen to the one used in the present study, which resulted in a comparable increase in the CSA of MyHC type I fibres. The use of a cycle ergometer, or a similar cycling exercise, during aerobic training may facilitate greater recruitment of muscle fibres than other forms of aerobic exercise, contributing to fibre hypertrophy. An emergent body of literature also exists demonstrating increased muscle protein synthesis following an acute bout of aerobic exercise (Sheffield-Moore et al 2004; Harber et al 2009a; Durham et al 2010). This increase in protein synthesis, if extrapolated over the course of a training protocol, could help explain the increase in muscle fibre size observed in the present study.
Following training, a reduction in the frequency of hybrid fibres occurred along with an increase in MyHC type IIa fibre frequency. The frequency of MyHC type IIa/IIx hybrid fibres was strongly correlated with subject BMI pretraining. Muscle fibre type is known to correlate with obesity (Tanner et al 2002), and the greater frequency of MyHC type IIa/IIx hybrid fibres associated with higher BMI is indicative of the detrained state of the subjects (Larsson & Ansved, 1985). The frequency of hybrid fibres was significantly reduced with training, and the relative increase in MyHC type IIa frequency was correlated with improvements in maximal oxygen consumption. While the frequency of MyHC type I fibres remained relatively unchanged with training, the decrease in hybrid IIa/IIx fibres and subsequent increase in pure MyHC type IIa fibres could contribute to the positive changes in oxygen consumption observed following training. Similar fibre type distribution changes have been seen in other studies of younger adults (Harber et al 2012) and provide important evidence for the effect of aerobic training on muscle health. Several rodent studies have established that fibre type transitions can occur in the absence of satellite cell activation (Rosenblatt & Parry, 1993; Adams et al 2002; Fry et al 2014), suggesting that the fibre type-specific increase in satellite cell content/myonuclear addition are not involved in a MyHC isoform shift in the present study. However, recent work by Joanisse et al (2013) provides evidence for satellite cell mediation of non-hypertrophic remodelling of hybrid muscle fibres, potentially contributing to fibre type transitions independent of fusion.
Both MyHC type I and IIa fibres had significant increases in mean CSA following 12 weeks of aerobic training, yet satellite cell activation and myonuclear accretion were observed only in MyHC type I fibres, suggesting that type II fibre growth in response to aerobic training is less reliant on satellite cell fusion. In contrast, following resistance training in humans, satellite cell activation and/or myonuclear addition have been reported in MyHC type II fibres (Petrella et al 2006, 2008; Verdijk et al 2013). The increase in MyHC type II fibre CSA in those studies (>20%) was more robust than in the present study (12%), potentially necessitating satellite cell fusion. However, our results suggest that the type II fibre myonuclear domain is somewhat flexible, able to expand to support modest growth. The myonuclear domain theory posits that each myonucleus in a fibre is responsible for governing a defined volume of cytoplasm, and during fibre hypertrophy, the expansion of cytoplasmic volume necessitates the addition of new myounclei (from satellite cells) to maintain a relatively constant myonuclear domain. Increases in mean MyHC type I and IIa CSA were similar with training, yet the increase in myonuclei per fibre was over 3-fold higher in MyHC type I fibres (10 and 3%, respectively). These data imply that regulation of the myonuclear domain during adaptation following aerobic training may occur in a fibre type-specific fashion, where remodelling and growth of MyHC type I fibres necessitates fusion of satellite cells, whereas the myonuclear domain of MyHC type IIa fibres is less fixed. Early work in spinal cord-injured rats suggested that myonuclear domain size is more tightly regulated in predominantly MyHC type I versus MyHC type II muscles (Dupont-Versteegden et al 2000). The differential expansion of the satellite cell pool during fibre hypertrophy following aerobic training provides intriguing evidence for fibre type specificity of satellite cell function in humans during muscle adaptation and warrants further study.
Acknowledgments
We would like to thank Douglas Long, Stacie BeBout, Kimberly Fairbrother and Marilyn Campbell for their technical assistance during the screening, testing and training of the subjects. We wish to thank Tamara Bennett, PA-C, for help with the biopsies.
Glossary
- BMI
body mass index
- CSA
cross-sectional area
- DAPI
4′,6-diamidino-2-phenylindole
- DEXA
dual energy X-ray absorptiometry
- MyHC
myosin heavy chain
- TSA
tyramide signal amplification
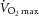
maximal oxygen consumption
Additional information
Competing interests
None declared.
Author contributions
Experiments were performed in the laboratory of C.A.P. C.S.F., B.N., P.A.K. and C.A.P. were involved with conception and design of the experiments; C.S.F., B.N., J.M., M.F.U. and P.M.W. collected, analysed and interpreted the data; C.S.F., B.N., P.A.K. and C.A.P. were involved with drafting the article or revising it critically for important intellectual content. All authors read and approved the final version of the manuscript.
Funding
Research was supported by the Jeane B. Kempner Postdoctoral Scholar Award to C.S.F.; and NIH grants AR062069 to B.N., AR60701 to C.A.P. and DK071349 to C.A.P. and P.A.K. The project described was also partially supported by the National Center for Advancing Translational Sciences, NIH grant UL1TR000117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002;283:C1182–C1195. doi: 10.1152/ajpcell.00173.2002. [DOI] [PubMed] [Google Scholar]
- Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, Hourdé C, Macharia R, Friedrichs M, Relaix F, Zammit PS, Matsakas A, Patel K, Partridge T. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci U S A. 2009;106:7479–7484. doi: 10.1073/pnas.0811129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S, Reggiani C. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009;23:3896–3905. doi: 10.1096/fj.09-131870. [DOI] [PubMed] [Google Scholar]
- Charifi N, Kadi F, Feasson L, Denis C. Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve. 2003;28:87–92. doi: 10.1002/mus.10394. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Murphy RJL, Houle JD, Gurley CM, Peterson CA. Mechanisms leading to restoration of muscle size with exercise and transplantation after spinal cord injury. Am J Physiol Cell Physiol. 2000;279:C1677–C1684. doi: 10.1152/ajpcell.2000.279.6.C1677. [DOI] [PubMed] [Google Scholar]
- Durham WJ, Casperson SL, Dillon EL, Keske MA, Paddon-Jones D, Sanford AP, Hickner RC, Grady JJ, Sheffield-Moore M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 2010;24:4117–4127. doi: 10.1096/fj.09-150177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SR, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28:1654–1665. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MC, Schultz E. Age-related differences in absolute numbers of skeletal-muscle satellite cells. Muscle Nerve. 1983;6:574–580. doi: 10.1002/mus.880060807. [DOI] [PubMed] [Google Scholar]
- Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol. 2009a;296:R708–R714. doi: 10.1152/ajpregu.90906.2008. [DOI] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009b;297:R1452–R1459. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol. 2012;113:1495–1504. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–823. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein-content and glucose-transport capacity in rat skeletal-muscles. Am J Physiol Endocrinol Metab. 1990;259:E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- Hudelmaier M, Wirth W, Himmer M, Ring-Dimitriou S, Sänger A, Eckstein F. Effect of exercise intervention on thigh muscle volume and anatomical cross-sectional areas—quantitative assessment using MRI. Magn Reson Med. 2010;64:1713–1720. doi: 10.1002/mrm.22550. [DOI] [PubMed] [Google Scholar]
- Joanisse S, Gillen JB, Bellamy LM, McKay BR, Tarnopolsky MA, Gibala MJ, Parise G. Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J. 2013;27:4596–4605. doi: 10.1096/fj.13-229799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Henriksson J. The number of satellite cells in slow and fast fibres from human vastus lateralis muscle. Histochem Cell Biol. 2006;126:83–87. doi: 10.1007/s00418-005-0102-0. [DOI] [PubMed] [Google Scholar]
- Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113:99–103. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- Konopka AR, Douglass MD, Kaminsky LA, Jemiolo B, Trappe TA, Trappe S, Harber MP. Molecular Adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci. 2010;65:1201–1207. doi: 10.1093/gerona/glq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka M, Naito H, Ogura Y, Kojima A, Goto K, Katamoto S. Effects of voluntary wheel running on satellite cells in the rat plantaris muscle. J Sports Sci Med. 2009;8:51–57. [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Ansved T. Effects of long-term physical training and detraining on enzyme histochemical and funcational skeletal muscle characteristic in man. Muscle Nerve. 1985;8:714–722. doi: 10.1002/mus.880080815. [DOI] [PubMed] [Google Scholar]
- Lepper C, Partridge TA, Fan C-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Karlsen A, Couppe C, Mikkelsen UR, Nielsen RH, Magnusson SP, Kjaer M. Differential satellite cell density of type I and II fibres with lifelong endurance running in old men. Acta Physiol (Oxf) 2014;214:612–627. doi: 10.1111/apha.12195. [DOI] [PubMed] [Google Scholar]
- McPhee JS, Williams AG, Degens H, Jones DA. Inter-individual variability in adaptation of the leg muscles following a standardised endurance training programme in young women. Eur J Appl Physiol. 2010;109:1111–1118. doi: 10.1007/s00421-010-1454-2. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291:E937–E946. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim J-S, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol. 1992;73:2538–2543. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Parry DJ. Adaptation of rat extensor digitorum longus muscle to gamma-irradiation and overload. Pflugers Arch. 1993;423:255–264. doi: 10.1007/BF00374404. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2001;56:B240–B247. doi: 10.1093/gerona/56.6.b240. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H, Hellhammer U. Number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec. 1977;189:169–175. doi: 10.1002/ar.1091890204. [DOI] [PubMed] [Google Scholar]
- Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS One. 2010;5:e13307. doi: 10.1371/journal.pone.0013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287:E513–E522. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- Snijders T, Verdijk LB, Hansen D, Dendale P, van Loon LJC. Continuous endurance-type exercise training does not modulate satellite cell content in obese type 2 diabetes patients. Muscle Nerve. 2011;43:393–401. doi: 10.1002/mus.21891. [DOI] [PubMed] [Google Scholar]
- Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PRG, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–E1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Gleeson BG, Jonkers RAM, Meijer K, Savelberg H, Dendale P, van Loon LJC. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64:332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 2013 doi: 10.1007/s11357-013-9583-2. DOI 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney J, Kadi F, Charifi N, Feasson L, Saafi MA, Castells J, Piehl-Aulin K, Denis C. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve. 2008;38:1147–1154. doi: 10.1002/mus.21054. [DOI] [PubMed] [Google Scholar]