Abstract
Intrauterine growth restriction (IUGR) is associated with impaired cardiac function in childhood and is linked to short- and long-term morbidities. Placental dysfunction underlies most IUGR, and causes fetal oxidative stress which may impact on cardiac development. Accordingly, we investigated whether antenatal melatonin treatment, which possesses antioxidant properties, may afford cardiovascular protection in these vulnerable fetuses. IUGR was induced in sheep fetuses using single umbilical artery ligation on day 105–110 of pregnancy (term 147). Study 1: melatonin (2 mg h−1) was administered i.v. to ewes on days 5 and 6 after surgery. On day 7 fetal heart function was assessed using a Langendorff apparatus. Study 2: a lower dose of melatonin (0.25 mg h−1) was administered continuously following IUGR induction and the ewes gave birth normally at term. Lambs were killed when 24 h old and coronary vessels studied. Melatonin significantly improved fetal oxygenation in vivo. Contractile function in the right ventricle and coronary flow were enhanced by melatonin. Ischaemia–reperfusion-induced infarct area was 3-fold greater in IUGR hearts than in controls and this increase was prevented by melatonin. In isolated neonatal coronary arteries, endothelium-dependent nitric oxide (NO) bioavailability was reduced in IUGR, and was rescued by modest melatonin treatment. Melatonin exposure also induced the emergence of an indomethacin-sensitive vasodilation. IUGR caused marked stiffening of the coronary artery and this was prevented by melatonin. Maternal melatonin treatment reduces fetal hypoxaemia, improves heart function and coronary blood flow and rescues cardio-coronary deficit induced by IUGR.
Key points
Failure of the placenta to develop and perform gives rise to intrauterine growth restriction (IUGR) in the fetus. IUGR is associated with impaired heart function in childhood and can even persist long term.
Oxidative stress is increased in IUGR and we asked if an antioxidant could reduce this. Melatonin is a well-known and well-studied hormone, present in all of us, and it has potent antioxidant properties.
In this study we administered melatonin to pregnant ewes carrying twins, one with IUGR.
Maternal melatonin improved oxygen delivery to the IUGR fetus and strengthened and protected its heart against infarct. After birth, the poor function and stiffness in the coronary arteries of IUGR lambs were entirely prevented by melatonin.
Our results demonstrate that administration of melatonin to a mother carrying an IUGR fetus can markedly dampen the adverse heart and artery effects in the offspring following birth.
Introduction
Failure of normal placental development and function is the most common cause of fetal intrauterine growth restriction (IUGR). IUGR is associated with increased risk of acute adverse outcomes such as preterm birth, perinatal morbidity and mortality, and of longer term consequences such as increased risk of neurodevelopmental impairment and cardiovascular disease (Hallows et al. 2012; Longo et al. 2012). It is becoming apparent that some of the cardiovascular morbidity in childhood is secondary to the dramatic cardiovascular adaptations of the IUGR fetus in utero in response to impaired placentation and reduced oxygen availability (Bahtiyar & Copel, 2008; Crispi et al. 2010). This leads to vasodilator dysfunction (Norman, 2008) and to increased cardiac infarct susceptibility in response to ischaemic-reperfusion insult (Li et al. 2003; Rueda-Clausen et al. 2011; Tare et al. 2012a). Currently, neither the placental dysfunction causing intrauterine hypoxaemia nor the fetal adaptive responses are treated by therapeutic intervention that might offer cardiovascular protection. Human IUGR is associated with enhanced oxidative stress in both the placenta and the fetal circulation (Lian et al. 2011). We speculated that targeting fetal oxidative stress may offer an opportunity to improve fetal cardio-coronary health in the growth restricted neonate.
While the role of the hormone melatonin in regulating circadian and circannual rhythms has long been established, melatonin is also a potent antioxidant and anti-inflammatory agent (Hardeland et al. 2011). Melatonin scavenges hydroxyl (Poeggeler et al. 1993), carbonate (Hardeland et al. 2003) and peroxynitrite (Hardeland et al. 2007) radicals by receptor-independent mechanisms, probably involving its aromatic indole ring structure. Classical G-protein-coupled melatonin receptors occur in the cardiovascular system (Dubocovich & Markowska, 2005; Paulis et al. 2012) and these are involved in the reduction by melatonin of free radical formation by mitochondria (Acuna-Castroviejo et al. 2007), cyclooxygenase (COX) during prostaglandin synthesis (Cardinali & Ritta, 1983), and uncoupled nitric oxide synthase (NOS) (Tapias et al. 2009). Melatonin also acts via its receptors to stimulate production of free radical scavengers such as superoxide dismutase, catalase and glutathione peroxidase and reductase (López et al. 2006). In adult rat heart, melatonin prevents the adverse effects of acute hypoxia–reperfusion on contractile function by protecting mitochondria (Petrosillo et al. 2006), and prevents disruption of cardiomyocyte calcium handling in chronically hypoxic animals (Yeung et al. 2008). In fetal sheep, melatonin increases umbilical blood flow by mechanisms that involve NO (Thakor et al. 2010). Melatonin also prevents the reduction in umbilical blood flow and enlargement of fetal cardiomyocytes in a restricted food intake model of ovine IUGR (Lemley et al. 2012).
In this study we tested the hypothesis that melatonin would prevent the suppression of cardiac contractility induced by IUGR, increase coronary blood flow, reduce vascular contractility and reduce cardiac susceptibility to ischaemia–reperfusion injury. Vascular function was investigated in isolated coronary arteries. We used sheep because induction of IUGR using single umbilical artery ligation (SUAL) is an established, proven technique producing placental insufficiency and fetal growth restriction (Miller et al. 2009). We have recently reported that this model is associated with an increase in oxidative stress, as indicated by 4–hydroxynonenal levels, in the neonatal brain that is ameliorated by maternal melatonin administration (Miller et al. 2014).
Methods
Ethical approval
Prior to commencement of the studies, ethical approval was obtained from Monash University School of Biomedical Sciences Ethics Committee A, in accordance with the National Health and Medical Research Council of Australia guidelines for the treatment and management of experimental animals. Time-mated ewes were obtained from Monash Animal Services (Monash University). Directly following removal of the fetuses/lambs, the ewes were euthanized with 20 ml intravenous pentabarbital (7g, Letabarb, Virbac PTL, Australia).
Experimental design
Singleton and twin-bearing ewes underwent surgery for the procedure of SUAL to induce placental insufficiency and IUGR, as described previously (Miller et al. 2007). The maturational profile of fetal lamb hearts in late gestation (Burrell et al. 2003) resembles that which occurs in human hearts (Mayhew et al. 1997). Heart function was studied in preterm fetuses at days 112–117 of gestation, the equivalent of 30–32 weeks of pregnancy in humans, which are therefore truly preterm, strengthening the clinical usefulness of the study. Of further clinical relevance, coronary artery function was also assessed in neonates. In brief, on day 105–110 of pregnancy (term ∼147 days), anaesthesia was induced in the ewes with intravenously administered 20 mg/Kg of thiopentone (Thiobarb, Jurox Pty Ltd, Australia), followed by intubation and anaesthetic maintenance with isoflurane (1–2%) in oxygen: nitrous oxide (50:50, volume/volume). The uterus was exposed and each fetus was fitted with a femoral artery catheter for the determination of blood gases and recording of blood pressure and heart rate. A blood sample was taken and basal blood gases measured. In twin pregnancies, the umbilical arteries were identified within the cord of one fetus and one umbilical artery (randomly selected) was ligated using two ties (SUAL-IUGR fetus); in the second fetus the umbilical cord was handled but not ligated (sham-SUAL control fetus). The fetuses were resettled in the uterus, an open catheter was placed in the amniotic cavity for correction of blood pressure readings and the incision was repaired. In singleton fetuses used in Study 2, a catheter was inserted into the femoral artery and either the SUAL or sham-SUAL procedure was undertaken. The catheters were exteriorized through the flank of the ewe, and the wound secured. To minimize pain, Xylazil (0.2 mg, Troy Laboratories, Australia) was administered intramuscularly and a transdermal fentanyl patch (Durogesic 75 μg/h, Janssen-Cilag Pty Ltd, Australia) was positioned in the inguinal region for two days. A catheter was placed in a maternal jugular vein. Intravenous ampicillin was administered to the ewe before surgery and into the amniotic fluid for 3 days following surgery. The fetal catheter was flushed daily with heparinized saline and arterial blood gases (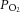 ,
, 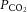 ), oxygen saturation and pH were monitored (ABL 700 blood gas analyser; Radiometer, Copenhagen, Denmark).
), oxygen saturation and pH were monitored (ABL 700 blood gas analyser; Radiometer, Copenhagen, Denmark).
Study 1
Twelve ewes carrying twins were used in this study. On day 1 (day 105–110 of pregnancy) SUAL was performed on one twin to induce IUGR. The umbilical cord of the other twin, control, was handled but not ligated. At 09.00 h on day 5, a bolus dose of melatonin (2 mg) was administered i.v. to six of the ewes followed immediately by a continuous melatonin infusion (2 mg h−1). The remaining six control ewes received saline. Thus, there were four groups: control, IUGR, control + melatonin and IUGR +melatonin.
Cardiac contractile function
On day 7, anaesthesia was induced in the ewe and her fetuses by infusion of thiopentone into the ewe followed by maintenance with isoflurane, as described above. The fetuses were lifted and removed from the uterus. The chests of the fetuses were opened by a midline incision and the hearts removed to ice-cold physiological saline solution (PSS) and mounted on a Langendorff apparatus as previously described (Tare et al. 2012a). Despite the replacement of blood with PSS, we used the Langendorff heart approach because it permits interrogation of cardiac effects without complications of changes in pre- and after-load or complex reflex events. In brief, a latex balloon filled with saline solution was inserted into each ventricle of both hearts and diastolic pressure was set at 5 mmHg. The balloons were connected to a pressure transducer (MLT0699, ADInstruments, BellaVista, NSW, Australia) and bridge amplifier. Data were acquired using a PowerLab 16/30 and displayed and analysed using Labchart 6.0 (ADInstruments). The hearts were perfused with PSS via the aortae, at a constant pressure of 35 mmHg and 35°C, with a heated jacket raised to enclose each beating heart, ensuring optimal temperature and humidity. The hearts were allowed to equilibrate for 20 min. PSS contained (in mm): 127 NaCl, 4 KCl, 2 MgSO4, 2 KH2PO4, 10 glucose, 10 Hepes, 1.5 CaCl2, pre-warmed and gassed with oxygen. Heart function was tested in terms of basal contractile responses and responses to β-adrenoceptor stimulation using isoprenaline, as described previously (Tare et al. 2012a). Isoprenaline was delivered in increasing concentrations via a thin tube (0.2 mm diameter) at a location immediately before entry of PSS into the heart. Full recovery from each concentration of isoprenaline occurred before the next concentration was administered.
Stop-flow reperfusion injury
On recovery from the last dose of isoprenaline the flow of PSS into the heart was stopped for 20 min. Reperfusion was then continued for 1 h, following which the heart was removed from the apparatus, weighed and the left ventricle sliced into 2 mm slices. Slices were incubated in 1% 2,3,5-triphenyltetrazolium (TTZ, 10 mg ml−1) in phosphate-buffered saline for 15 min at 37°C, to develop infarcted areas, and photographed within 24 h (Li et al. 2003). Infarct area was determined using ImageJ software (Centre for Information Technology, NIH, Bethesda, MA, USA). Infarct area was calculated as:
Study 2
Using ewes carrying a single fetus, IUGR or control was induced on days 105–110 of pregnancy. Melatonin (0.25 mg h−1 i.v.) was administered continuously to six ewes carrying an IUGR fetus and to six ewes carrying a control fetus (n = 6 in each group), until term birth. Another six ewes, each carrying an IUGR fetus, and nine ewes, each with a control fetus, were not treated with melatonin. Fetal arterial blood (5 ml) was collected at 110, 115, 120, 125, 130, 135 and 140 days of gestation for melatonin assay, pH and blood gas measurements. Lambs were born spontaneously at term (∼145 days gestation) and remained with their mother to feed.
The lambs were killed when 24 h old using pentobarbitone (Lethabarb, 0.5 g in 2 ml via the jugular vein) and the heart removed. Segments of branches of the left anterior inter-ventricular coronary artery (250–300 μm outside diameter and about 1–2 mm in length) were mounted on a wire myograph for isometric tension recording as described previously (Bubb et al. 2007; Mazzuca et al. 2010). The segments were continuously superfused with Kreb's solution containing (mN): NaCl 120, KCl 5, NaHCO3 25, KH2PO4 1, MgSO4 1.2, glucose 11, CaCl2 2.5, and bubbled with carbogen (95% O2 and 5% CO2) at 35°C. Smooth muscle and endothelial integrity were tested using the thromboxane mimetic U46619 and bradykinin, respectively. Smooth muscle contractile function was tested using cumulative concentrations of U46619. Following 30 min rest, the segment was submaximally constricted (∼60% of maximal) with U46619 and endothelial vasodilator release was induced by cumulative addition of bradykinin. Following 30 min rest, endothelium-derived NO release was blocked by including NG-nitro-l-arginine methyl ester (l-NAME, 2 × 10−4 m) in the bathing solution. The process was repeated in the presence of l–NAME plus indomethacin (2 × 10−6 m), to block cyclooxygenase and hence prostanoid production. Relaxations persisting in the presence of l–NAME and indomethacin were attributed to endothelium-derived hyperpolarizing factor (EDHF) (Bubb et al. 2007). Endothelium-independent smooth muscle relaxation was tested using sodium nitroprusside (SNP) (Mazzuca et al. 2010).
Arterial stiffness was tested by mounting leak-free segments of coronary artery in a pressure myograph (Living Systems Instrumentation, Burlington, VT, USA) with no luminal flow (Wigg et al. 2004). The segments were continuously superfused at 15 ml min−1 with Kreb's containing 2 mm EGTA but no added calcium. Pressure was increased in 10 mmHg increments from 0 to 150 mmHg and changes in outside diameter, wall thickness and length in response to the pressure increases were measured. Stress/strain values were derived (Bubb et al. 2007; Mazzuca et al. 2010) (see below).
All reagents were purchased from Sigma-Aldrich (St Louis, MO, USA).
Melatonin assay
Melatonin concentration was measured in the fetal circulation using a commercial kit (RK-MEL2; Buhlmann Laboratories, Schonenbuch, Switzerland) with a limit of detection of 0.3 pg ml−1. Intra-assay and inter-assay coefficients of variation were 8% and 18%, respectively.
mRNA determination
Total RNA was isolated from blood vessels and DNase treatment was performed on-column using the RNAeasy micro kit (QIAGEN, Melbourne, Australia). cDNA conversion was performed on 20–45 μg RNA using the Superscript VILO cDNA synthesis kit (Invitrogen, Life Technologies, Melbourne, Australia). Quantitative PCR was performed on Rotorgene (QIAGEN) with the following cycling conditions: 95°C for 2 min, 35 cycles of 95°C for 20 s, melting temperature (Tm) for 20 s, 72°C for 20 s. Details of primer sequences and annealing temperatures are given in Table 1. mRNA levels were normalized against the housekeeping gene β–actin and analysed using the ΔCT (cycle threshold) method. For each gene, data are expressed relative to the mean mRNA levels obtained for samples of the control animals.
Table 1.
Primer sequences and annealing temperatures (Tm)
| Gene | Sequence | Tm (°C) | GenBank Accession |
|---|---|---|---|
| COX-1 | F 5'-ATGAGTACCGCAAGAGGTTTGG-3' R 5'-ACGTGGAAGGAGACATAGG-3' | 57 | NM_001009476 |
| COX-2 | F 5'-CAGAGCTCTTCCTCCTGTGC-3' R 5'-CAAAAGGCGACGGTTATGC-3' | 58 | NM_001009432 |
| Collagen Iα1 | F 5'-AAGACATCCCACCAGTCACC-3' R 5'-CAGATCACGTCATCGCACA-3' | 60 | AF129287 |
| Collagen Iα2 | F 5'-GGCTCAACCTGAAGACATCC-3' R 5'-TCTCCTACCCAGACATGCTTC-3' | 59 | AF129287 |
| Collagen III | F 5'-CTGCTGGAAAGAATGGTGAG-3' R 5'-GTCACCAGAAGGCCCAGTA-3' | 58 | AF129287 |
| eNOS | F 5'-GATCAGCAACGCTATCACGA-3' R 5'-ATACGGCTTGTCACCTCCTG-3' | 54 | NM_001129901 |
| Tropoelastin | F 5'-ATCTCTCAGTCAGGCACCAG-3' R 5'-GTTTGTTGGGAAAGAAAGCA-3' | 54 | NM_175772 |
| β-Actin | F 5'-CATCCTGACCCTCAAGTACCC-3' R 5'-GTGGTGGTGAAGCTGTAGCC-3' | 58 | U39357 |
Data analysis and statistical testing
Data were analysed using GraphPad Prism and GraphPad InStat (GraphPad Software Inc., San Diego, CA, USA). For all data sets, equality of standard deviations and Gaussian distribution, using the Kolmogorov–Smirnov method, were tested. Data are expressed as mean and standard error of the mean (SEM). Throughout, n represents the number of animals studied and P < 0.05 was accepted as statistically significant.
Data for fetal blood pressure and heart rate were collected every 30 min from post-surgery day 4 to day 7 and analysed by repeated measures ANOVA. Repeated measures ANOVA was used for blood gas analyses (GraphPad). Melatonin concentrations were not normally distributed, and between-group differences were analysed by one-way ANOVA using a Kruskal–Wallis test for non-parametric data.
Ventricular performance was analysed using ADInstruments analytical software (LabChart). Peak developed pressure, and the maximum rate of rise (+dP/dt) and maximum rate of fall (−dP/dt) were determined after 20 min of baseline recording, and immediately before and during the peak response to each dose of isoprenaline (Tare et al. 2012a). Due to the slight decline in performance with time in each heart, responses to isoprenaline were calculated as the differences between the values immediately before versus those following drug application. Two-way ANOVA was used to determine the effects of IUGR and melatonin followed by Tukey's post hoc testing.
Responses of isolated coronary arteries to vasoconstrictors and vasodilators were analysed as described previously (Bubb et al. 2007; Mazzuca et al. 2010). Sigmoidal curves were fitted to the concentration–response data using the least squares method (Prism). From these, the concentration of drug that evoked a half-maximal response (EC50), pD2 (−log EC50) and maximal response were determined. Area-under-the-curve for endothelium-dependent relaxation was also calculated (Herrera et al. 2010) and analysed using 2-way ANOVA.
Stress–strain values were determined for each arterial segment:
 |
An exponential function was fitted to the data and tangential elastic modulus (Etang) was determined from:
(k is the slope of the curve). Thus, Etang is proportional to k (Izzard et al. 2006). Data were analysed using one-way ANOVA and Tukey's post hoc testing.
Results
Melatonin restores fetal oxygenation in IUGR
Study 1
All four experimental groups, control, IUGR, control + melatonin and IUGR + melatonin, had similar pre-treatment baseline fetal arterial melatonin concentrations. Administration of melatonin significantly increased fetal melatonin levels, >30-fold, in control + melatonin and IUGR + melatonin fetuses (Table 2). There were no differences in either fetal mean arterial blood pressure (P = 0.8) or heart rate (P = 0.9) between groups over the course of the experiment. IUGR was associated with lower basal fetal arterial oxygen saturation pre-treatment (Table 2) and, whilst not significant, the only group to show improved oxygen saturation over time was IUGR fetuses exposed to maternal melatonin (P = 0.06 versus basal; Table 2). Fetal arterial pH, 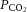 and
and 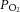 levels were within normal range for preterm fetuses. Oxygen saturation and
levels were within normal range for preterm fetuses. Oxygen saturation and 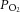 were significantly reduced in IUGR fetuses and these measures were entirely rescued by melatonin exposure (Table 2).
were significantly reduced in IUGR fetuses and these measures were entirely rescued by melatonin exposure (Table 2).
Table 2.
Arterial melatonin (MT) levels and oxygen partial pressure and saturation in control, control+MT, IUGR and IUGR+MT fetuses
| Group/measure | Control | Control + MT | IUGR | IUGR + MT |
|---|---|---|---|---|
| Study 1 | ||||
| MT – basal (pg ml–1) | 235 ± 117 | 456 ± 241 | 146 ± 64 | 214 ± 51 |
| – post treatment (pg ml–1) | 208 ± 69 | 14288 ± 2648† | 171 ± 93 | 9440 ± 2339† |
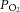 – basal (mmHg) – basal (mmHg) |
22.5 ± 0.4 | 18.1 ± 1.1 | 20.4 ± 1.3 | 18.8 ± 1.7 |
| – post treatment (mmHg) | 20.7 ± 1.4 | 18.7 ± 0.7 | 17.5.0 ± 1.3* | 19.2 ± 0.6† |
| O2 saturation – basal (%) | 62.6 ± 1.7 | 53.1 ± 4.6 | 55.4 ± 2.3 | 47.4 ± 5.4 |
| – post treatment (%) | 55.8 ± 4.5 | 52.8 ± 4.6 | 47.1 ± 3.3* | 52.3 ± 3.0† |
| Study 2 | ||||
| MT (pg ml–1) | 244 ± 93 | 1045 ± 183† | 215 ± 71 | 1223 ± 126† |
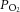 (mmHg) (mmHg) |
22.6 ± 1.8 | 20.7 ± 0.1 | 17.3 ± 1.2* | 19.5 ± 0.6 |
| O2 saturation (%) | 60.7 ± 5.9 | 54.1 ± 1.4 | 44.0 ± 2.0* | 51.8 ± 2.8 |
IUGR induced significant hypoxaemia (*). Maternal MT administration increased circulating fetal MT levels and returned oxygen partial pressure (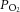 ) and saturation to control values (†).
) and saturation to control values (†).
Study 2
The success of melatonin treatment in Study 1 with regard to fetal oxygenation and heart function (see below) prompted us to administer a lower concentration of melatonin over a longer period of time to the mother (as might be required in a clinical setting), and to test the effects after birth. There was no difference in mean fetal melatonin concentrations in control and IUGR fetuses over the course of late gestation. Control + melatonin and IUGR + melatonin fetuses had significant 4- to 5-fold elevated circulating melatonin levels (Table 2). IUGR fetuses were significantly hypoxaemic in late gestation, between 110 and 145 days, compared with control fetuses. Melatonin treatment significantly improved fetal oxygenation in terms of 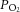 and O2 saturation (Table 2). Fetal arterial pH and
and O2 saturation (Table 2). Fetal arterial pH and 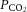 were within the normal physiological range and were not different between groups.
were within the normal physiological range and were not different between groups.
Single umbilical artery ligation induced IUGR
SUAL resulted in significant IUGR (to 75% of control fetal body weight, Study 1, Fig. 1A). In Study 1, growth restriction was significantly mitigated by the administration of melatonin to the ewe (to 93% of control, P = 0.024, Fig. 1A). Melatonin exposure did not affect fetal growth in control lambs. Heart weight, as a proportion of body weight, was not affected by either IUGR or by melatonin treatment (Fig. 1B).
Figure 1. Effect of IUGR and melatonin on fetal lambs.
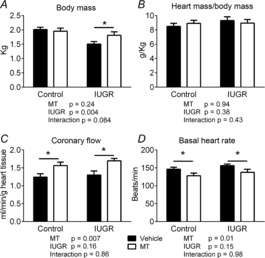
A, body weight. B, heart weight corrected for body weight. C, coronary flow. D, basal heart rate in isolated Langendorff hearts. Two-way ANOVA was used to obtain P values for IUGR and melatonin (MT) effects. Control vehicle n = 6; all other groups n = 5. *Significant effect of MT.
Melatonin increased coronary blood flow
Under ex vivo Langendorff experimental conditions, basal coronary flow was not affected by IUGR but flow was significantly increased in the hearts of control (by 37%, P = 0.03) and IUGR (by 21%, P = 0.02) lambs treated with melatonin (Fig. 1C). Basal heart rate was significantly lower in hearts pre-exposed to melatonin (Fig. 1D, P = 0.04 for controls, P = 0.03 for IUGR).
Melatonin on basal ventricular contractile function
In the left ventricle, maximum developed pressure and rates of rise (+dP/dt) and fall (−dP/dt) in pressure were significantly increased in hearts of IUGR fetuses (Fig. 2, P = 0.02). Melatonin treatment alone also resulted in larger left ventricular developed pressure, +dP/dt and −dP/dt in hearts of control fetuses (P = 0.004). There was no additional effect of melatonin on left ventricular function in IUGR hearts (Fig. 2).
Figure 2. Effect of IUGR and melatonin on heart function in fetal lambs.
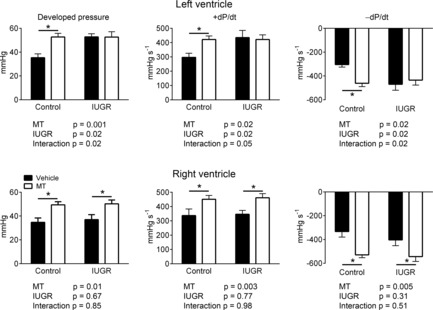
Peak ventricular pressure development, maximum rate of contraction (+dP/dt), and relaxation (−dP/dt) in fetal hearts. Two-way ANOVA used to obtain P values for IUGR and melatonin (MT) effects in the left and right ventricles. Control vehicle n = 6; all other groups n = 5. *Significant effect of MT.
In the right ventricle of control and IUGR (untreated) fetuses, maximum developed pressure, +dP/dt and −dP/dt were similar, but melatonin treatment resulted in a significant increase in these measures in control (developed pressure P = 0.009, +dP/dt P = 0.05, −dP/dt P = 0.004) and IUGR ventricles (developed pressure P = 0.03, +dP/dt P = 0.01, −dP/dt P = 0.05) (Fig. 2, P = 0.006).
Response to β-adrenoceptor stimulation
Isoprenaline, a β-adrenoceptor agonist, increased ventricular developed pressure and heart rate in all four groups in a dose-dependent manner. There was no significant effect of IUGR or melatonin treatment on these responses (Fig. 3).
Figure 3. Effects of IUGR and melatonin on isoprenaline response in fetal lamb heart.
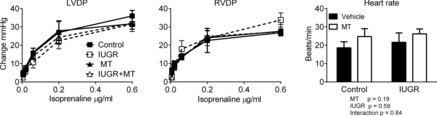
Neither IUGR nor melatonin (MT) influenced the response to bolus (10 s) application of increasing doses of isoprenaline with respect to left ventricular or right ventricular peak pressure development, or heart rate (isoprenaline, 0.6 μg ml−1 response shown) (2–way ANOVA). LVDP and RVDP, left and right ventricle developed pressure, respectively. Control vehicle n = 6; all other groups n = 5.
Melatonin curtailed ischaemia–reperfusion damage
Global ischaemia for 20 min, followed by 1 h reperfusion induced an infarct area of 2.4% in control hearts and this was significantly increased to 7.4% in hearts from IUGR fetuses (Fig. 4, P = 0.0005). Melatonin exposure was without effect in hearts of normally grown fetuses but it prevented the increase in infarct area in response to IUGR (2.9%) (Fig. 4, P = 0.003 IUGR vehicle versus melatonin treated).
Figure 4. Ischemia-reperfusion-induced infarct area increased in IUGR prevented by melatonin.
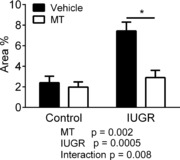
Stop-flow reperfusion resulted in the development of a markedly larger infarct area in hearts from IUGR fetuses. This increase was prevented by melatonin (MT) exposure. Control vehicle n = 6; all other groups n = 5. *Significant effect of MT.
Melatonin enhanced coronary endothelium-dependent vasodilator function
Segments of coronary arteries were isolated from hearts of lambs 24 h after birth, submaximally constricted with thromboxane analogue U46619 and the endothelium stimulated using bradykinin (BK). Arteries from all groups relaxed fully in response to maximal stimulation with BK (Fig. 5A). Sensitivity to BK was significantly reduced 2.3-fold in arteries from IUGR arteries versus controls (Table 3). In coronary arteries of both control and IUGR lambs treated with melatonin, sensitivity to BK was significantly enhanced. Coronary arteries from IUGR + melatonin lambs were more than 10-fold more sensitive to the vasodilator effects of BK versus coronaries from IUGR lambs alone and 5–fold more sensitive to BK than arteries from control fetuses (Table 3).
Figure 5. Endothelium-dependent vasorelaxation in coronary arteries from control, IUGR, melatonin and IUGR + melatonin animals.
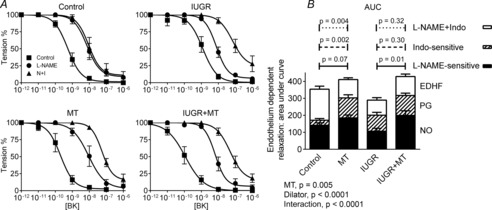
A, The endothelium was stimulated using bradykinin (BK) in arteries submaximally preconstricted with U46619. Nitric oxide (NO) and prostanoid (PG) production were blocked using l-NAME (N) and indomethacin (I, Indo), respectively. pD2 values for these curves shown in Table 3. B, Vasorelaxation attributable to NO, PG and endothelium-derived hyperpolarizing factor (EDHF) for each animal were derived and analysed using ANOVA. There were highly significant effects of treatment (IUGR and/or melatonin, MT) and dilator, with a significant interaction, indicating different contributions of dilator/treatment in the different treatment groups. Tukey's post hoc test P values for l-NAME and Indo-sensitive responses and the responses remaining in the presence of l–NAME + Indo, n = 6 per group.
Table 3.
pD2 values for endothelium-dependent relaxation in response to bradykinin stimulation in the four treatment groups
| No blocker | l-NAME | l-NAME + Indo | ||||
|---|---|---|---|---|---|---|
| pD2 | P value | pD2 | P value | pD2 | P value | |
| Control | 9.273 ± 0.086 | 8.136 ± 0.123 | 8.041 ± 0.147 | |||
| MT | 9.689 ± 0.142 | 0.025* | 8.049 ± 0.108 | NS | 7.255 ± 0.095 | 0.001* |
| IUGR | 8.916 ± 0.103 | 0.021* | 8.120 ± 0.060 | NS | 7.167 ± 0.200 | 0.006* |
| IUGR + MT | 9.943 ± 0.099 | 0.0001† | 8.188 ± 0.099 | NS | 7.307 ± 0.144 | 0.58 |
Treatment groups are: control, melatonin (MT) treatment, IUGR and IUGR + MT before (no blocker) and following blockade of NO and prostanoid production with l–NAME and indomethacin (Indo), respectively. n = 6 per group. P values:
significantly different from control vehicle;
significantly different from IUGR treatment. NS, P > 0.05.
Blockade of NO production with l–NAME shifted the concentration–relaxation curves to the right in coronary arteries from all groups (Fig. 5A). The shift was greater (14-fold) in control than in IUGR (6-fold), suggesting reduced NO bioavailability (rates of production and/or degradation) in IUGR. The effectiveness of l–NAME was increased 44-fold for control + melatonin, and 57-fold for IUGR + melatonin arteries. Of particular interest, pD2 was the same in all groups in the presence of l–NAME (Table 3).
With NO production blocked, additional blockade of COX enzymes, and hence prostanoid production, with indomethacin was without effect in coronary arteries of control lambs (Fig. 5A, Table 3). The effects of an endothelium-dependent dilator prostanoid (PG) were apparent in coronary arteries of IUGR, IUGR + melatonin and control + melatonin lambs, as indicated by the significant rightward shift in the concentration–relaxation curve in the presence of indomethacin (Fig. 5A, Table 3).
The area under each relaxation curve for coronary arteries from each lamb was determined and the data pooled within the four experimental groups and across the three endothelium-derived vasodilators (Fig. 5B). While NO-induced relaxation was not affected by IUGR per se (P = 0.09), melatonin enhanced NO bioavailability in IUGR coronaries (P = 0.01). The induction of an indomethacin-sensitive vasorelaxation in IUGR (P = 0.01) and melatonin-exposed (P = 0.002) coronary arteries was also evident. The relaxation remaining when the production of NO and prostanoid were blocked, and attributed to EDHF, was significantly reduced in IUGR (P = 0.003) and melatonin-treated coronaries (P = 0.004) versus controls (Fig. 5B).
Contractile responses and endothelium-independent relaxation in coronary artery
U46619 evoked concentration-dependent contraction in all groups. The maximum response was significantly greater in vessels from IUGR lambs versus controls and this was mitigated by melatonin exposure (Fig. 6A). Maximal U46619 contractions were significantly smaller in arteries of IUGR neonates antenatally exposed to melatonin (P = 0.02) (Fig. 6B). Sensitivity to U46619 (pD2) was not different between groups.
Figure 6. Effects of IUGR and melatonin on coronary artery smooth muscle contraction and relaxation.
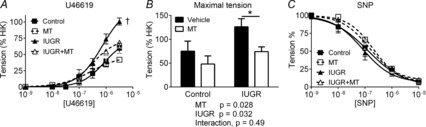
A, contractile responses to U46619 (expressed as % of contraction to high K PSS) in coronary arteries from control, IUGR, melatonin (MT) and IUGR + MT-treated animals (n = 6 per group). B, two-way ANOVA of the maximal response to U46619, effect of IUGR and melatonin. C, endothelium-independent relaxation to sodium nitroprusside (SNP) in coronary arteries from control, IUGR, MT and IUGR + MT-treated animals. †Significantly different from the other 3 groups. *Significant effect of MT.
SNP evoked endothelium-independent and complete relaxations in arteries from all groups (Fig. 6C). The responses were not different in arteries from the four experimental groups.
Coronary stiffness increased in IUGR, restored by melatonin
Increasing the pressure in closed segments of coronary arteries induced an increase in segment outside diameter. The increase was greatest in vessels from melatonin-treated lambs and least in vessels from IUGR lambs (Fig. 7A). Taking diameter and wall thickness into account, the passive stress–strain relationship was shifted significantly to the left in arteries from IUGR lambs (Fig. 7B), demonstrating enhanced wall stiffness. The data were well fitted by a single exponential function (r2 = 0.79–0.88) (Fig. 7B). The k value for stress = stressk strain was significantly larger in IUGR arteries (Fig. 7C), indicating changes in wall properties. Melatonin treatment significantly shifted the stress–strain relationship to the right in control + melatonin and IUGR + melatonin coronary arteries (Fig. 7B), without changing k (Fig. 7C).
Figure 7. Passive mechanical properties of isolated coronary arteries.
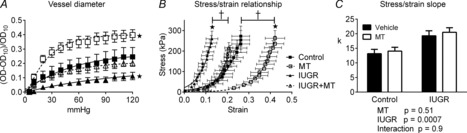
A, Increasing intraluminal pressure induced an increase in outside diameter, corrected for initial diameter. B, Stress–strain relationships for control, IUGR, melatonin (MT) and IUGR + MT in coronary artery segments. (Note: the Control and IUGR + MT overlap significantly.) C, Two-way ANOVA of the k constant for the stress–strain relationships revealed a significant effect of IUGR but no effect of MT treatment. *Significantly different from control. †Significant effect of MT, n = 6 per group.
mRNA in coronary artery
Endothelial NOS (eNOS) was significantly increased in coronary arteries of IUGR lambs, an effect mitigated by exposure to melatonin (P = 0.001) (Fig. 8). COX–2 mRNA expression was significantly increased in response to IUGR, an effect that did not occur in IUGR + melatonin lambs (P = 0.01). COX–2 expression was also reduced by melatonin in control arteries (P = 0.005). Collagen 2 mRNA expression was increased by IUGR. Melatonin treatment reduced levels of collagen 2 and 3 in control (P = 0.02 and 0.03, respectively) and IUGR coronary arteries (collagen 3, P = 0.04) (Fig. 8). Tropoelastin mRNA expression was increased in IUGR arteries and was increased by melatonin in the control group.
Figure 8. mRNA expression in neonatal coronary arteries.
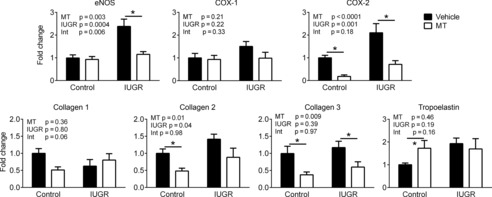
eNOS, cyclooxygenase (COX) 1 and 2, collagen 1, 2 and 3, and tropoelastin mRNA expression in control or IUGR neonatal coronary arteries following maternal treatment with saline or melatonin (MT). Two-way ANOVA P values for IUGR or MT effects and interaction (Int). Control saline n = 6; IUGR n = 5; MT n = 5; IUGR + MT, n = 5. *Significant effect of MT.
Discussion
We investigated whether administering melatonin to pregnant sheep would prevent the deleterious effects of IUGR on heart and coronary function in the preterm fetus equivalent to 30–32 weeks of human gestation, and in newborn lambs 24 h after term birth. Melatonin improved fetal oxygenation, enhanced right ventricular contractile performance and reduced infarct area following global ischaemia–reperfusion. Melatonin treatment rescued coronary endothelium-dependent vasodilator dysfunction induced by IUGR by enhancing NO bioavailability and harnessing additional prostanoid vasodilator mechanisms. Melatonin also reversed the enhanced coronary reactivity to vasoconstrictor. Passive coronary arterial stiffness was increased in IUGR and this was abrogated by melatonin, associated with a marked reduction in collagen 2 and 3 expression.
A striking effect of maternal melatonin administration was an increase in blood flow in the intact heart that was underpinned by marked enhancement of endothelium-dependent vasodilator function, revealed in isolated coronary arteries. Melatonin receptors MT1 and MT2 have been identified in human myocardium and coronary arteries (Ekmekcioglu et al. 2003; Paulis et al. 2012) and acutely administered melatonin acts on these receptors to induce NO-derived vasodilation in pigs (Thakor et al. 2010). The NO component of endothelial vasodilation was impaired in IUGR coronaries, despite an increase in plasma nitrite (Pisaneschi et al. 2012) and a marked increase in eNOS mRNA expression (present study). Reduced NO bioavailability in IUGR is most likely due to enhanced oxidative stress (Mert et al. 2012). Oxidative imbalance can lead to the synthesis of eNOS as a compensatory mechanism, typically observed in conditions of increased oxidative stress, such as diabetes (Hink et al. 2001), with the eNOS invariably being uncoupled and producing superoxide rather than NO. Melatonin alone increased NO bioavailability, as shown previously (Thakor et al. 2010), which may occur as a consequence of melatonin-induced reduced oxidative stress. Here we showed that melatonin exposure prevented the impairment of NO bioavailability in IUGR, leading to increased sensitivity to endothelium-dependent vasodilation. We also found enhanced contractile responses to the stable thromboxane analogue U46619 in IUGR coronaries, demonstrating further dysfunction. This potentiation of the contractile response was abrogated following melatonin treatment. Melatonin has previously been shown to suppress contraction in middle cerebral artery in fetal sheep (Torres-Farfan et al. 2008). Thus, an increase in NO bioavailability following melatonin treatment may account for the blunting of responses to constrictors.
In this study, the induction of an additional indomethacin-sensitive component to endothelium-dependent vasodilation was observed in coronary arteries of untreated IUGR and control melatonin-treated arteries. IUGR is associated with an increase in oxidative stress, and the oxidant peroxynitrite enhances the activities of COX and thromboxane synthase, while suppressing prostacyclin synthase, actions that interfere with the constrictor/dilator balance (Xu & Zou, 2009). The doubling in COX-2 mRNA expression in our IUGR coronary arteries is consistent with this. On the other hand, melatonin exposure alone resulted in enhancement of the indomethacin-sensitive vasodilation component, which was accompanied by a marked reduction in COX-2 mRNA expression. Enhanced vasodilation could reflect a reduction in constrictor (thromboxane) and an increase in dilator (prostacyclin) produced following reduction of the effects of oxidative stress, mediated by melatonin, on prostacyclin synthase in the endothelial cells. In contrast, in rat femoral arteries melatonin has been reported to increase COX-sensitive endothelium-dependent constriction (Paulis et al. 2010). The interactions between the NO and PG pathways are complex and the mechanisms incompletely understood (see Kim, 2011 for review). The complexity of the system occurring in our study did not emerge until the completion of data analysis. Clearly, a more detailed understanding will require further detailed studies involving manipulation of the enzymes COX, NOS and the various PG synthases separately and determination of protein levels using western blotting, perhaps even using cultured endothelial cells.
The vasodilation that persisted in the presence of NO blockade and indomethacin is attributable to EDHF, an important mechanism of dilation in the smallest vessels. This dilation was reduced in IUGR coronaries. Unlike the response to NO, vasorelaxation resistant to l–NAME and indomethacin was not rescued by melatonin treatment. The mechanisms underlying EDHF can differ in different arterial beds (Sandow & Tare, 2007), and some of the ion channels involved in EDHF can either be upregulated or be vulnerable to oxidative stress and disease states (Giles et al. 2012), with responses varying with species. This issue requires further study.
In our model of IUGR, ischaemia–reperfusion gave rise to a 3-fold increase in infarct area above levels in control hearts, and melatonin prevented this. This result extends to the fetus that which has previously been observed in adult hearts exposed to melatonin (Petrosillo et al. 2006; Sallinen et al. 2008; Yeung et al. 2008; Hardeland et al. 2011). Contractile performance in both ventricles of control as well as IUGR fetuses was enhanced following melatonin exposure. We believe this reflects the significant increase in coronary flow. In an elegant study, Grossini and colleagues demonstrated in adult pigs that acute melatonin increased heart contractility via an action on β1-adrenoceptors, while the increase in coronary flow resulted from NO release via β2-adrenoceptor activity (Grossini et al. 2011). In our studies we found no evidence for long-term effects of melatonin on β-adrenoceptor regulation of heart function in sheep fetuses. However, additional direct mechanisms could also be involved (Paulis et al. 2012), in view of the likelihood of some degree of basal oxidative stress, even in healthy fetuses.
Arterial stiffness is an important indicator of cardiovascular disease risk (Laurent et al. 2001). As distensibility declines, the smoothing out of the pressure pulse from the left ventricle is impaired, giving rise to an increase in pulse pressure and pulse wave velocity. The consequent increase in cardiac after-load can result in left ventricular hypertrophy. IUGR is associated with enhanced arterial stiffness in human adolescents (Zieman et al. 2005; Chan et al. 2010; Rossi et al. 2011) and in animal models during fetal development (Thompson et al. 2011b), and in adults (Thompson et al. 2011a; Tare et al. 2012b), with collagen content increased. In the present study we found that passive coronary arterial stiffness was markedly increased in IUGR, associated with an increase in collagen–2 mRNA expression. Melatonin had a dramatic effect, reducing stiffness in both normally grown and in IUGR coronaries, associated with decreases in collagen 2 and 3. Suppression of inflammatory cytokine production by melatonin, again linked to its antioxidative stress role (Rezzani et al. 2010), leads to a decrease in nuclear factor κB and matrix metalloproteinase expression (Qin et al. 2012). There was an increase in the elastic modulus (k) in coronary arteries of IUGR lambs, irrespective of melatonin exposure. This indicates that IUGR induced fundamental structural changes, such as in the arrangement of the collagen (e.g. differences in fibril thickness; Mazzuca et al. 2010) and proteoglycan components, rather than a mere change in the concentration of similar elements (Zieman et al. 2005; Izzard et al. 2006; Voges et al. 2012).
As mentioned above, melatonin has potent antioxidant properties and is anti-inflammatory in adults (Hardeland et al. 2011); the ewes in the present study thrived, gave birth without difficulty and fed and cared for their lambs in a normal manner. In a recent study we have shown that the lipid peroxidation product 4–hydroxynonenal is significantly decreased within the brain of IUGR lambs exposed to antenatal melatonin (see Fig. 4 in Miller et al. 2014). Furthermore, malondialdehyde, an end-stage marker of oxidative stress, is reduced in the placenta of human pregnancies with an IUGR fetus treated with maternal melatonin (Miller et al. 2014). Evidence of a more direct effect of melatonin, involving its G protein-coupled receptors, would require the use of blockers, such as luzindole, which completely prevented direct release of NO by melatonin infusion in pigs (Grossini et al. 2011).
In conclusion, IUGR imposes a significant burden on oxidative balance in the fetus with consequences for fetal organ and vascular development, including neurological deficits and cardiac and coronary function. Here we show that antenatal administration of melatonin to ewes with a growth restricted fetus has direct protective effects on the developing heart and its circulation. Improvements in heart function will have short- and long-term positive outcomes for perfusion of the brain and the preservation of neurological function, in addition to ameliorating cardio-coronary dysfunction that may underlie long-term cardiovascular disease. While the NIH points out that insufficient evidence warrants caution in the unprescribed administration of melatonin to children, IUGR can have devastating effects on the fetus in utero that can persist postnatally and long term. Hence we have recently commenced a clinical trial of oral administration of melatonin to women whose pregnancy is complicated by IUGR (Alers et al. 2013).
Acknowledgments
We thank Ms Jan Loose for technical support in this project.
Glossary
- BK
bradykinin
- COX
cyclooxygenase
- EDHF
endothelium-derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- IUGR
intrauterine growth restriction
- k strain
slope of the stress/strain curve
- l-NAME
NG-nitro-l-arginine methyl ester
- MT
melatonin
- NO
nitric oxide
- NOS
nitric oxide synthase
- pD2
−log EC50, the concentration of a drug or hormone required to elicit a half-maximal response
- SNP
sodium nitroprusside
- SUAL
single umbilical artery ligation
- U46619
a thromboxane receptor agonist
Additional information
Competing interests
None declared.
Author contributions
Experiments were carried out in the Department of Physiology, Monash University. Study conception and design: S.L.M., H.C.P., M.T., E.M.W. and G.J. Data collection and analysis: H.C.P., M.T., A.E.S., R.L., T.Y. and H.A.C. Interpretation of data: all authors. Manuscript drafting: H.C.P. and M.T. Manuscript revision and important intellectual input: H.C.P., M.T., S.L.M., E.M.W., H.A.C. and G.J. All authors approved the final submitted version[LJ1].
Funding
This work was supported by the National Health and Medical Research Council of Australia and by the Victorian Government's Operational Infrastructure Program.
References
- Acuna-Castroviejo D, Escames G, Rodriguez MI, Lopez LC. Melatonin role in the mitochondrial function. Front Biosci. 2007;12:947–963. doi: 10.2741/2116. [DOI] [PubMed] [Google Scholar]
- Alers NO, Jenkin G, Miller SL, Wallace EM. Antenatal melatonin as an antioxidant in human pregnancies complicated by fetal growth restriction–a phase I pilot clinical trial: study protocol. BMJ Open. 2013;3:e004141. doi: 10.1136/bmjopen-2013-004141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahtiyar MO, Copel JA. Cardiac changes in the intrauterine growth-restricted fetus. Semin Perinatol. 2008;32:190–193. doi: 10.1053/j.semperi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Bubb KJ, Cock ML, Black MJ, Dodic M, Boon W-M, Parkington HC, Harding R, Tare M. Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol. 2007;578:871–881. doi: 10.1113/jphysiol.2006.121160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI, Lumbers ER. Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol. 2003;274:952–961. doi: 10.1002/ar.a.10110. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Ritta MN. The role of prostaglandins in neuroendocrine junctions: studies in the pineal gland and the hypothalamus. Neuroendocrinology. 1983;36:152–160. doi: 10.1159/000123452. [DOI] [PubMed] [Google Scholar]
- Chan PY, Morris JM, Leslie GI, Kelly PJ, Gallery ED. The long-term effects of prematurity and intrauterine growth restriction on cardiovascular, renal, and metabolic function. Int J Pediatr. 2010;2010:280402. doi: 10.1155/2010/280402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, Ahmed A, Gratacos E. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation. 2010;121:2427–2436. doi: 10.1161/CIRCULATIONAHA.110.937995. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Holzenbein T, Markovic O, Leibetseder VJ, Strauss-Blasche G, Marktl W. The melatonin receptor subtype MT2 is present in the human cardiovascular system. J Pineal Res. 2003;35:40–44. doi: 10.1034/j.1600-079x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Giles TD, Sander GE, Nossaman BD, Kadowitz PJ. Impaired vasodilation in the pathogenesis of hypertension: focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J Clin Hypertens. 2012;14:198–205. doi: 10.1111/j.1751-7176.2012.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossini E, Molinari C, Uberti F, Mary DA, Vacca G, Caimmi PP. Intracoronary melatonin increases coronary blood flow and cardiac function through β–adrenoreceptors, MT1/MT2 receptors, and nitric oxide in anaesthetized pigs. J Pineal Res. 2011;51:246–257. doi: 10.1111/j.1600-079X.2011.00886.x. [DOI] [PubMed] [Google Scholar]
- Hallows SE, Regnault TR, Betts DH. The long and short of it: the role of telomeres in fetal origins of adult disease. J Pregnancy. 2012;2012:638476. doi: 10.1155/2012/638476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, Backhaus C, Fadavi A. Reactions of the NO redox forms NO+•NO and HNO (protonated NO–) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. J Pineal Res. 2007;43:382–388. doi: 10.1111/j.1600-079X.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin–a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Poeggeler B, Niebergall R, Zelosko V. Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. J Pineal Res. 2003;34:17–25. doi: 10.1034/j.1600-079x.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Verkerk MM, Derks JB, Giussani DA. Antioxidant treatment alters peripheral vascular dysfunction induced by postnatal glucocorticoid therapy in rats. PLoS One. 2010;5:e9250. doi: 10.1371/journal.pone.0009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- Izzard AS, Horton S, Heerkens EH, Shaw L, Heagerty AM. Middle cerebral artery structure and distensibility during developing and established phases of hypertension in the spontaneously hypertensive rat. J Hypertens. 2006;24:875–880. doi: 10.1097/01.hjh.0000222757.54111.06. [DOI] [PubMed] [Google Scholar]
- Kim SF. The role of nitric oxide in prostaglandin biology; update. Nitric Oxide. 2011;25:255–264. doi: 10.1016/j.niox.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Lemley CO, Meyer AM, Camacho LE, Neville TL, Newman DJ, Caton JS, Vonnahme KA. Melatonin supplementation alters uteroplacental hemodynamics and fetal development in an ovine model of intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2012;302:R454–R467. doi: 10.1152/ajpregu.00407.2011. [DOI] [PubMed] [Google Scholar]
- Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Invest. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Lian IA, Loset M, Mundal SB, Fenstad MH, Johnson MP, Eide IP, Bjorge L, Freed KA, Moses EK, Austgulen R. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta. 2011;32:823–829. doi: 10.1016/j.placenta.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo S, Bollani L, Decembrino L, Comite AD, Angelini M, Stronati M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR) J Matern Fetal Neonatal Med. 2012;26:222–225. doi: 10.3109/14767058.2012.715006. [DOI] [PubMed] [Google Scholar]
- López LC, Escames G, Tapias V, Utrilla P, León J, Acuña-Castroviejo D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: its relation with mitochondrial dysfunction and prevention by melatonin. Int J Biochem Cell Biol. 2006;38:267–278. doi: 10.1016/j.biocel.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Pharaoh A, Austin A, Fagan DG. Stereological estimates of nuclear number in human ventricular cardiomyocytes before and after birth obtained using physical disectors. J Anat. 1997;191:107–115. doi: 10.1046/j.1469-7580.1997.19110107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuca MQ, Wlodek ME, Dragomir NM, Parkington HC, Tare M. Uteroplacental insufficiency programs regional vascular dysfunction and alters arterial stiffness in female offspring. J Physiol. 2010;588:1997–2010. doi: 10.1113/jphysiol.2010.187849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert I, Oruc AS, Yuksel S, Cakar ES, Buyukkagnici U, Karaer A, Danisman N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J Obstet Gynaecol Res. 2012;38:658–664. doi: 10.1111/j.1447-0756.2011.01771.x. [DOI] [PubMed] [Google Scholar]
- Miller SL, Chai M, Loose J, Castillo-Melendez M, Walker DW, Jenkin G, Wallace EM. The effects of maternal betamethasone administration on the intrauterine growth-restricted fetus. Endocrinology. 2007;148:1288–1295. doi: 10.1210/en.2006-1058. [DOI] [PubMed] [Google Scholar]
- Miller SL, Supramaniam VG, Jenkin G, Walker DW, Wallace EM. Cardiovascular responses to maternal betamethasone administration in the intrauterine growth-restricted ovine fetus. Am J Obstet Gynecol. 2009;201:613 e611–618. doi: 10.1016/j.ajog.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Miller SL, Yawno T, Alers NO, Castillo-Melendez M, Supramaniam VG, Vanzyl N, Sabaretnam T, Loose JM, Drummond GR, Walker DW, Jenkin G, Wallace EM. Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J Pineal Res. 2014;56:283–294. doi: 10.1111/jpi.12121. [DOI] [PubMed] [Google Scholar]
- Norman M. Low birth weight and the developing vascular tree: a systematic review. Acta Paediatrica. 2008;97:1165–1172. doi: 10.1111/j.1651-2227.2008.00904.x. [DOI] [PubMed] [Google Scholar]
- Paulis L, Pechanova O, Zicha J, Barta A, Gardlik R, Celec P, Kunes J, Simko F. Melatonin interactions with blood pressure and vascular function during l–NAME-induced hypertension. J Pineal Res. 2010;48:102–108. doi: 10.1111/j.1600-079X.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- Paulis L, Simko F, Laudon M. Cardiovascular effects of melatonin receptor agonists. Expert Opin Investig Drugs. 2012;21:1661–1678. doi: 10.1517/13543784.2012.714771. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Di Venosa N, Pistolese M, Casanova G, Tiravanti E, Colantuono G, Federici A, Paradies G, Ruggiero FM. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia-reperfusion: role of cardiolipin. FASEB J. 2006;20:269–276. doi: 10.1096/fj.05-4692com. [DOI] [PubMed] [Google Scholar]
- Pisaneschi S, Strigini FA, Sanchez AM, Begliuomini S, Casarosa E, Ripoli A, Ghirri P, Boldrini A, Fink B, Genazzani AR, Coceani F, Simoncini T. Compensatory feto-placental upregulation of the nitric oxide system during fetal growth restriction. PLoS One. 2012;7:e45294. doi: 10.1371/journal.pone.0045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res. 1993;14:151–168. doi: 10.1111/j.1600-079x.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Qin W, Lu W, Li H, Yuan X, Li B, Zhang Q, Xiu R. Melatonin inhibits IL1β-induced MMP9 expression and activity in human umbilical vein endothelial cells by suppressing NF-κB activation. J Endocrinol. 2012;214:145–153. doi: 10.1530/JOE-12-0147. [DOI] [PubMed] [Google Scholar]
- Rezzani R, Porteri E, De Ciuceis C, Bonomini F, Rodella LF, Paiardi S, Boari GE, Platto C, Pilu A, Avanzi D, Rizzoni D, Agabiti Rosei E. Effects of melatonin and Pycnogenol on small artery structure and function in spontaneously hypertensive rats. Hypertension. 2010;55:1373–1380. doi: 10.1161/HYPERTENSIONAHA.109.148254. [DOI] [PubMed] [Google Scholar]
- Rossi P, Tauzin L, Marchand E, Boussuges A, Gaudart J, Frances Y. Respective roles of preterm birth and fetal growth restriction in blood pressure and arterial stiffness in adolescence. J Adolesc Health. 2011;48:520–522. doi: 10.1016/j.jadohealth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res. 2011;90:285–294. doi: 10.1093/cvr/cvq363. [DOI] [PubMed] [Google Scholar]
- Sallinen P, Mänttäri S, Leskinen H, Vakkuri O, Ruskoaho H, Saarela S. Long-term postinfarction melatonin administration alters the expression of DHPR, RyR2, SERCA2, and MT2 and elevates the ANP level in the rat left ventricle. J Pineal Res. 2008;45:61–69. doi: 10.1111/j.1600-079X.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci. 2007;28:61–67. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Tapias V, Escames G, López LC, López A, Camacho E, Carrión MD, Entrena A, Gallo MA, Espinosa A, Acuña-Castroviejo D. Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J Neurosci Res. 2009;87:3002–3010. doi: 10.1002/jnr.22123. [DOI] [PubMed] [Google Scholar]
- Tare M, Miller SL, Wallace EM, Sutherland AE, Yawno T, Coleman HA, Jenkin G, Parkington HC. Glucocorticoid treatment does not alter early cardiac adaptations to growth restriction in preterm sheep fetuses. BJOG. 2012a;119:906–914. doi: 10.1111/j.1471-0528.2012.03309.x. [DOI] [PubMed] [Google Scholar]
- Tare M, Parkington HC, Bubb KJ, Wlodek ME. Uteroplacental insufficiency and lactational environment separately influence arterial stiffness and vascular function in adult male rats. Hypertension. 2012b;60:378–386. doi: 10.1161/HYPERTENSIONAHA.112.190876. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Herrera EA, Seron-Ferre M, Giussani DA. Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. J Pineal Res. 2010;49:399–406. doi: 10.1111/j.1600-079X.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Gros R, Richardson BS, Piorkowska K, Regnault TR. Central stiffening in adulthood linked to aberrant aortic remodeling under suboptimal intrauterine conditions. Am J Physiol Regul Integr Comp Physiol. 2011a;301:R1731–R1737. doi: 10.1152/ajpregu.00274.2011. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Richardson BS, Gagnon R, Regnault TR. Chronic intrauterine hypoxia interferes with aortic development in the late gestation ovine fetus. J Physiol. 2011b;589:3319–3332. doi: 10.1113/jphysiol.2011.210625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Farfan C, Valenzuela FJ, Mondaca M, Valenzuela GJ, Krause B, Herrera EA, Riquelme R, Llanos AJ, Seron-Ferre M. Evidence of a role for melatonin in fetal sheep physiology: direct actions of melatonin on fetal cerebral artery, brown adipose tissue and adrenal gland. J Physiol. 2008;586:4017–4027. doi: 10.1113/jphysiol.2008.154351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges I, Jerosch-Herold M, Hedderich J, Pardun E, Hart C, Gabbert DD, Hansen JH, Petko C, Kramer HH, Rickers C. Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: a cross-sectional study. J Cardiovasc Magn Reson. 2012;14:77. doi: 10.1186/1532-429X-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigg SJ, Tare M, Forbes J, Cooper ME, Thomas MC, Coleman HA, Parkington HC, O'Brien RC. Early vitamin E supplementation attenuates diabetes-associated vascular dysfunction and the rise in protein kinase C-β in mesenteric artery and ameliorates wall stiffness in femoral artery in rats. Diabetologia. 2004;47:1038–1046. doi: 10.1007/s00125-004-1411-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung HM, Hung MW, Fung ML. Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J Pineal Res. 2008;45:373–382. doi: 10.1111/j.1600-079X.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]


