Abstract
Adipose tissue is a dynamic organ that makes up a substantial proportion of the body; in severe obesity it can account for 50% of body mass. Details of the unique immune system resident in human and murine adipose tissue are only recently emerging, and so it has remained a largely unexplored and unappreciated immune site until now. Adipose tissue harbours a unique collection of immune cells, which often display unusual functions compared with their counterparts elsewhere in the body. These resident immune cells are key to maintaining tissue and immune homeostasis, yet in obesity their chronic aberrant stimulation can contribute to the inflammation and pathogenesis associated with obesity. Anti-inflammatory adipose-resident lymphocytes are often depleted in obesity, whereas pro-inflammatory immune cells accumulate, leading to an overall inflammatory state, which is a key step in the development of obesity-induced metabolic disease. A good example is invariant natural killer T (iNKT) cells, which make up a large proportion of lymphocytes in human and murine adipose tissue. Here, they are unusually poised to produce anti-inflammatory or regulatory cytokines, however in obesity, iNKT cells are greatly reduced. As iNKT cells are potent transactivaors of other immune cells, and can act as a bridge between innate and adaptive immunity, their loss in obesity represents the loss of a major regulatory population. Restoring iNKT cells, or activating them in obese mice leads to improved glucose handling, insulin sensitivity, and even weight loss, and hence represents an exciting therapeutic avenue to be explored for restoring homeostasis in obese adipose tissue.
Keywords: adipose tissue, CD1d, lipids, metabolic disorder, natural killer T cells, obesity
Introduction
Adipose tissue is a dynamic tissue serving a primary and essential function in lipid storage, but it also acts as an endocrine organ, producing many adipokines that regulate satiety, storage capacity, insulin sensitivity and glucose handling.1 In addition, human and murine adipose tissue contains a distinct collection of immune cells in the lean steady state. Immune cells reside in the stromovascular fraction of adipose tissue, along with vascular endothelial cells, mesenchymal stem cells and pre-adipocytes, and appear to be in contact with neighbouring adipocytes. This adipose-resident immune system is unique in terms of enrichment of certain otherwise rare cells, and in the phenotype of these cells compared with elsewhere in the body. The immune system resident in adipose tissue plays a key role in maintaining homeostasis and keeping inflammation at bay. Resident alternatively activated macrophages may phagocytose dead cells, adipocytes and their contents, to prevent triggering an immune response by free fatty acid release. Other resident cells like regulatory T cells and eosinophils also prevent an inflammatory environment by producing anti-inflammatory cytokines like interleukin-10 (IL-10) and IL-4 at steady state. However in the obese state, adipocytes are overloaded and stressed, and they release adipokines, which can modulate the immune system. In the state of chronic excess calorie intake and lipid overload in adipose tissue, the resident immune system is aberrantly activated, which has been shown to contribute to the metabolic disorder that ensues in obesity. Hence, the resident immune system in lean adipose tissue is key to maintaining a healthy controlled state of immune tolerance, and at the same time, in obesity, the resident immune system is a key mediator of chronic inflammation at the heart of metabolic disease.
We have discovered the enrichment of one such resident immune cell, the invariant natural killer T (iNKT) cell in human and murine adipose tissue.2,3 Invariant NKT cells represent the lipid-sensing arm of the immune system. Unlike MHC-restricted T cells, iNKT cells recognize lipids presented by CD1d. The iNKT cells can produce various types of cytokines, rapidly and at high levels, which is why they are part of the innate immune system. They are often the first T cells to be activated and their rapid cytokine production means that they potently transactivate other immune cells. Therefore they are an important bridge between the innate and adaptive immune system, and can orchestrate or skew an immune response depending on the array of cytokines that they produce. Importantly, we have identified a striking role for iNKT cells in regulating adipose tissue inflammation, metabolism and weight control. This review will discuss the series of findings on adipose iNKT cells that have emerged in recent years, the controversies in the metabolic phenotype of iNKT-deficient mice, and the exciting potential they may hold for manipulating the adipose immune system in obesity.
Invariant NKT characteristics
Invariant NKT cells are a specialized subset of innate T cells that are highly conserved in mammals.4 Adaptive T cells recognize peptides presented by MHC molecules, but iNKT cells recognize lipids presented by CD1d molecules.5 CD1d is a non-polymorphic MHC class I-like molecule that is expressed on antigen-presenting cells such as dendritic cells, macrophages and B cells. CD1d is also expressed on non-haematopoietic cells including hepatocytes6 and adipocytes.7,8 The iNKT cells recognize their lipid ligands on CD1d through their semi-invariant T-cell receptor (TCR).9–11 In mice, iNKT cells express TCRs comprising a Vα14-Jα18 chain paired with a limited Vβ chain repertoire (Vβ2, Vβ7, Vβ8.1, Vβ8.2 or Vβ8.3).12,13 In humans, iNKT cells express Vα24-Jα18 chain paired almost exclusively with a Vβ11 chain.14 Like iNKT cells, CD1d is highly conserved in mammals.15 There is a large degree of functional and structural similarity between the TCRs that are expressed by human and mouse iNKT cells, to the degree that some lipids presented by human CD1d can be recognized by murine iNKT cells and vice versa. The first lipid to be identified as an antigen for iNKT cells was α-galactosylceramide (αGalCer), which remains the most potent activator of iNKT cells. αGalCer was discovered during a screen of marine sponges for anti-cancer activity in 1997, and is derived from marine sponges, or possibly the microbes that inhabit them, and was synthetically modified to be a potent pharmacalogical activator of iNKT cells. The search for physiologically relevant lipids from pathogens or self-lipids recognized by iNKT cells is under intense investigation, and recently there have been many breakthroughs identifying endogenous and microbial lipid ligands. Endogenous lipids include isoglobotrihexosylceramide,16 glucosylceramide,17 lysophosphatidylcholine18 and ether-bonded phospholipids derived from peroxisomes.19 Lipids derived from Aspergillus fumigatus can also activate iNKT cells, which has been linked to the development of respiratory disease,20 and several bacterial lipids recognized by iNKT cells have also been identified.21–23 To date the endogenous and microbial antigens are weaker activators of iNKT cells, and it is possible that lipids as potent as synthetic αGalCer do not occur in a physiological setting. In addition to recognition of lipids on CD1d through their TCR (Signal 1), iNKT cells can be activated by co-stimulatory signals. However, the co-stimulatory signals for iNKT cells are most often cytokines like IL-12 and IL-18, and these cytokines co-stimulate iNKT cells in many important physiological examples of iNKT cell activation.24,25
Unlike naive adaptive MHC class I and class II restricted T cells, iNKT cells display an effector/memory phenotype and are poised for rapid effector function at steady state.26 Their rapid response, lack of memory and expression of NK receptors have led to them being considered “innate” T cells. Invariant NKT cells characteristically express high levels of the BTB–POZ-ZF family [broad complex, tramtrack, bric-a‘-brac (BTB) or poxvirus and zinc finger (POZ)-zinc finger] transcription factor promyelocytic leukaemia zinc finger (PLZF) encoded by Zbtb16.27,28 PLZF is also expressed by human mucosal-associated invariant T cells, which are another population of invariant T cells, as well a subset of γδ T cells. PLZF is thought to control the innate phenotype and rapid cytokine response of these and forced expression of PLZF on CD4 T cells induced an innate-like iNKT cell phenotype.28
iNKT cell functions
Known functions of iNKT cells are diverse because of their striking ability to kill targets and also produce both T helper type 1 (Th1) and Th2 cytokines upon activation.29,30 A major function of iNKT cells is in transactivating other immune cells through their rapid cytokine production. Therefore they can both kick-start an immune response, and skew the type of response, as well as regulate homeostasis of other cell types. As well as cytokine production, iNKT cells, or at least a subset of iNKT cells, have cytotoxic activity. Indeed, one of the first functions reported for iNKT cells was cytotoxicity again tumour cells. In a B16 model of melanoma with liver metastasis, αGalCer administration completely protected wild-type mice from tumour development, but mice lacking iNKT cells had no protection,31 suggesting that activation of iNKT cells led to their potent cytotoxicity against tumour cells. However, as their role in transactivating other immune cells, like natural killer (NK) cells, through IL-2 or interferon-γ (IFN-γ) production became accepted, it is thought that tumour protection induced by αGalCer could be due to subsequent NK cell activation and cytotoxicity. This scenario seems likely to occur, but in addition, iNKT cells themselves have cytotoxic activity and can also kill tumour cells that express CD1d, but not CD1d-negative tumour cells.32 Nevertheless, the transactivation of NK cells through their rapid copious cytokine production by iNKT cells is a very important example of the potent immunoregulatory potential of iNKT cells, which is now accepted as a major function of these cells. The production of IFN-γ by iNKT cells can quickly transactivate tissue-resident NK cells, γδ T cells and other lymphocytes, like B cells. Invariant NKT cells can also provide help for B cells, by inducing their maturation and increasing their antibody-producing functions.33 Furthermore, interactions of iNKT cells with antigen-presenting cells are bi-directional; when dendritic cells present lipid antigens through CD1d to iNKT cells, this induces IFN-γ production by iNKT cells and also induces further IL-12 production by dendritic cells through CD40–CD40 ligand interactions.25 This interaction is important for dendritic cell maturation,34 and as dendritic cell maturation is important for the initiation of the adaptive immune response, this is another example of how iNKT cells can act as a bridge between the innate and adaptive systems. The potent regulatory potential of iNKT cells is evident in many diseases. Invariant NKT cell defects have been seen in human autoimmune diseases, including type I diabetes, systemic lupus erythematosus and multiple sclerosis, and also in cancer.30,35,36 In humans, cancer and infections are also associated with defects in iNKT cells. As iNKT cells have anti-tumour activity, either through their cytotoxic potential against CD1d on tumour cells, or through their activation of NK cells, they have been shown to be protective against many types of cancer. Many clinical trials in cancer have been designed to target the immunoregulatory potential of iNKT cells by increasing the number of NKT cells or stimulating their production of cytokines so that they might kick-start an immune response against the tumour. More direct evidence of iNKT regulation comes from mice that are completely deficient in iNKT cells or from studies that activate iNKT cells by injecting αGalCer in murine models of disease. Mice lacking iNKT cells (Ja18−/− and CD1d−/−) are generally healthy but are more prone to spontaneously develop autoimmunity and cancer, as well as often having impaired responses to pathogens. Hence, through their regulatory actions on many different immune cells, iNKT cell functions are broad in healthy and disease settings.
Locations of iNKT cells in humans and mice
Invariant NKT cells develop in the thymus from the same precursors as MHC-restricted T cells. They are derived from double-positive thymocytes through stochastic expression of their invariant TCR, followed by positive selection on CD1d expressed by other thhymic double-positive cells, rather than CD1d on epithelial cells.29,37 The iNKT cells then exit the thymus and primarily home to tissues where they complete their maturation. In mice, a large proportion of iNKT cells expressing CXCR6 home to the liver, where they constitute 20–50% of T cells in murine liver, although CXCR6 is important for their survival in, rather than traffic to, the liver.38 The iNKT cells also make up a smaller but substantial population in murine spleen, thymus, blood and bone marrow (0·5–2%). In addition, unlike adaptive MHC-restricted T cells, only a small number of iNKT cells localize to lymph nodes.
Although iNKT cells are highly conserved in mammals, a major difference between human and mouse iNKT cells is their location. Invariant NKT cells are 10–100-fold less frequent at these sites in humans, although frequency of circulating iNKT cells varies greatly between individuals.29 However, in 2009, we reported that iNKT cells are enriched in human omentum, as well as being present at enriched levels in other human adipose sites.2 This represents the highest frequency of iNKT cells in humans, accounting for 8–12% of adipose T cells. The enrichment of iNKT cells in human adipose tissue has been confirmed by several groups.7,39 Since the discovery of iNKT cells in human omentum, it has been reported that iNKT cells are also enriched in murine adipose tissue. Here, they represent 10–25% of adipose T cells, or 2–8% of all adipose lymphocytes.3,7,8,39 Hence, both murine and human adipose tissue harbour a unique population of iNKT cells, which we will describe below.
Location-specific phenotype of iNKT cells
One striking finding concerning iNKT cells in recent years was that, unlike other lymphocytes, iNKT cells are almost exclusively a tissue-resident population. This discovery was found using congenic parabiotic pairs to follow in vivo circulation of lymphocytes.40 Parabiotic pairs of congenic CD45.1 and CD45.2 mice were generated for 20–60 days, which allows for sharing of the circulation within 3 days of parabiosis, and chimerism within organs from 2 weeks onwards. It was shown that iNKT cells did not show significant chimerism between parabiotic pairs in any tissue (with the exception of lymph node, which showed some recirculation of iNKT cells). This was in stark contrast to B cells, CD4 and CD8 T cells and NK cells which recirculated through all tissues (ref. 40 and our unpublished observations). This innovative approach reveals that iNKT cells are uniquely tissue resident with either a very long dwell time, or little to no recirculation through tissues. This fits well with the concept that the iNKT cell phenotype is location dependent, which is especially evident in adipose tissue.
Invariant NKT cells can be divided into functionally distinct subsets, based on localization, the expression of CD4 and NK1.1, transcription factors and cytokine production. Subpopulations of iNKT cells analogous to MHC-restricted CD4+ Th1, Th2 and Th17 have been found. Surface markers such as expression or absence of CD4, NK1.1 and IL-17RB (for IL-25) as well as cytokine receptors are among the most important markers that distinguish Th1-like, Th2-like and Th17-like iNKT cell functional subsets41,26 (Fig. 1). In addition, some of the same transcription factors that are ‘master regulators’ of MHC-restricted T-cell subsets also operate in iNKT cell subsets. For example, T-bet, the transcription factor that controls IFN-γ production,42 is expressed by the majority of iNKT cells. Most of the liver and spleen iNKT cells that are Th1-like express T-bet, are NK1.1+ and produce IFN-γ. The iNKT cells can also express Gata3, which is a major transcription factor involved in inducing Th2 cytokines, especially IL-4, and in suppressing Th1 responses.43 T helper type 2-like iNKT cells express IL-17RB, CD4 and Gata3, and mainly produce IL-13 and Th2 cytokines after stimulation with IL-25.44 However, iNKT cells can simultaneously produce both IFN-γ and IL-4, and can express both T-bet and Gata3. Therefore the ‘master-regulator’ concept in which cells express particular transcription factors that control their Th1 or Th2 polarization is more complicated with iNKT cells, which can be both Th1 and Th2 producers simultaneously. There is also a population of IL-17RB+ iNKT cells that do not express CD4 and primarily produce IL-17 due to their expression of the transcription factor RORγT. These Th17 iNKT cells respond to IL-23 and represent a distinct population in the thymus, and are enriched in lung and skin.41 Other functional differences have been described for iNKT cells based on location. Adoptive transfer of hepatic iNKT cells mediates tumour rejection, whereas thymus-derived iNKT cells do not. Furthermore, this anti-tumour function is unique to hepatic CD4− iNKT cells.45 These studies emphasize the importance of considering the iNKT cell source and phenotype when studying iNKT cells.
Figure 1.
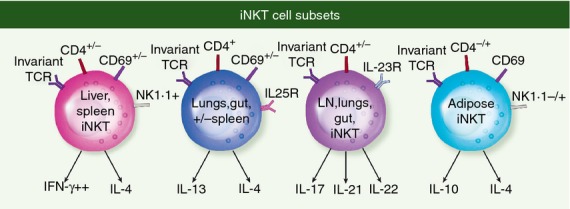
iNKT cell subsets classified by surface receptors and cytokine production are enriched in certain locations in the body.
Adipose iNKT cell phenotype
Invariant NKT cells resident in adipose tissue have a unique phenotype in terms of surface marker expression and function. While the majority of iNKT cells in the periphery are CD4 and have up-regulated NK1.1, adipose iNKT cells are mainly CD4− and a large proportion of adipose iNKT do not express NK1.1.3,7 This could imply that adipose iNKT cells are more immature than iNKT cells in liver and spleen and have yet to up-regulate NK1.1. It could also suggest that adipose iNKT cells are constitutively activated, as NK1.1 is transiently down-regulated following activation.46 The lack of NK1.1 on many adipose iNKT cells also highlights the need to use CD1d-αGalCer tetramers to identify and study adipose iNKT cells, rather than the earlier and less specific method using CD3+ NK1.1+ markers.
Adipose iNKT cells have a different cytokine profile compared with iNKT elsewhere. Although adipose iNKT cells express T-bet (L. Lynch & M. Brenner, unpublished data) and are capable of producing IFN-γ when stimulated with potent activators like PMA and Ionomycin they produce significantly less IFN-γ than iNKT cells elsewhere when activated with lipid antigens.3 They also produce more IL-4 and IL-13 than splenic iNKT cells when stimulated with αGalCer.7 Qi and colleagues reported that a key feature of iNKT regulation of adipose immune and metabolic homeostasis was through IL-4 production/signal transducer and activator of transcription 6 signalling after activation of iNKT cells with αGalCer.39 In addition to IL-4 production, we found that production of IL-10 is a striking feature of adipose iNKT cell activation, not typically observed for iNKT cells in liver or spleen.3 Together, these studies show that iNKT cells are Th2 polarized compared with iNKT elsewhere, and may have regulatory potential based on their IL-10 production.
Adipose iNKT cells in human and murine obesity
It is now clear that inflammation plays an important role in obesity and the metabolic syndrome.47 The resident immune system in adipose tissue is key to this process. The pathological expansion of adipose tissue in obesity is associated with major changes in the adipose immune system, resulting in inflammation, which contributes to local and whole body insulin resistance and type 2 diabetes. Recently, many immunometabolic studies have shed light on key players involved in the transformation from a homeostatic anti-inflammatory environment to a pathogenic pro-inflammatory one in obese adipose tissue. All resident immune cells identified so far have been shown to play some role in either the development or protection from chronic inflammation that drives obesity-induced metabolic disorder.3,7,48–57 This suggests that the adipose immune system is tightly controlled and highly interactive, with any aberrations effecting metabolism, either directly or through the interactions with other immune cells. Recently we, and others, added iNKT cells to the list of key players involved in obesity. In 2009, we reported that iNKT cells were depleted in adipose tissue of obese individuals compared with age-matched lean controls.2 This defect has also been confirmed by other laboratories7,39 and suggests that iNKT cells may play a role in human obesity. There is also a defect in iNKT cells in murine adipose tissue and liver in diet-induced and genetic models of obesity.3 Our study,3 and others39,57,58 have highlighted that adipose tissue iNKT cells protect against diet-induced obesity and glucose intolerance through regulatory cytokine production (Fig. 1). First, it was noted that iNKT-deficient mice on normal diets were heavier than their wild-type counterparts and displayed an increased tendency towards insulin resistance. This association of iNKT cell deficiency and insulin resistance was a trend that was not significant in mice fed a normal chow diet in our study and in a similar study from Qi and colleagues.3,57 However, Boes and colleagues found that this trend was significant, with both CD1d−/− and Ja18−/− mice displaying impaired glucose tolerance and insulin resistance with age on a standard low-fat diet.7 Second, we, and many groups, noted that iNKT cell numbers in adipose tissue fell in mice fed a high-fat diet (HFD), similar to reduction in iNKT cell number in human obesity. Third, restoring iNKT cell levels in obese iNKT-deficient mice (Jα18−/− and CD1d−/−) by adoptive transfer improved glucose handling and induced weight loss.3 Finally, activation of iNKT cells with αGalCer caused rapid weight loss, and reversal of glucose and insulin sensitivity without hypoglycaemia.3,39 Hence, the scenario appears that iNKT cells normally reside in adipose tissue, produce mainly Th2 and regulatory cytokines and positively regulate anti-inflammatory macrophages and adipocyte function. In an obese setting, adipose iNKT cells are depleted, representing the loss of an important regulatory population and at the same time, adipose tissue becomes an inflammatory environment due to an accumulation of pro-inflammatory macrophages (Fig. 2). Although the exact pathway of iNKT cell regulation is not yet clear, it appears that adipose iNKT cells can directly regulate macrophage levels and phenotype, and therefore inflammation. However, the role of iNKT cells in the protection against obesity, weight gain and metabolic disorder has been somewhat controversial. The similarities and differences between these studies are summarized below.
Figure 2.
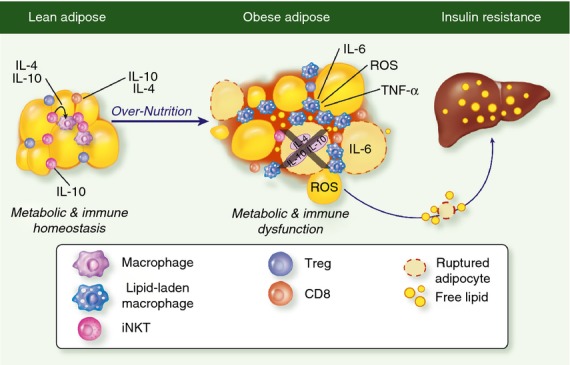
The role of iNKT cells in lipid handling and metabolic regulation. iNKT cells and Tregs are enriched in lean adipose tissue where they produce IL-10. During obesity induced adipose expansion, iNKT cells and Tregs are depleted resulting in less IL-10 and a more inflammatory environment, correlating with increased pro-inflammatory macrophage accumulation. Loss of protective cell subsets like iNKT and Tregs in obese adipose tissue contributes to metabolic dysfunction and eventually systemic inflammation.
To study the effects of iNKT cells on obesity and metabolism control, there are a number of methods that have been applied. Most research groups have used models of iNKT cell deficiency, namely CD1d−/− and Jα18−/− mice. Mice lacking CD1d, which is essential for iNKT cell development, do not develop iNKT cells. However, these mice not only lack type I NKT cells but also type II NKT cells, as well as CD1d itself, which is expressed on adipocytes and other non-hepatopoietic cells and so may be an important molecule in metabolism. Jα18−/− mice have a specific deficiency in the invariant chain of the NKT TCR, and specifically lack iNKT cells, but it has recently come to light that Jα18−/− mice have lower TCR diversity than was first thought,59 which could potentially contribute to any phenotype observed. Loss or gain of function after birth in wild-type mice may be a more appropriate method to study iNKT cell function in obesity. Mice can develop with a normal T-cell repertoire, and then iNKT cells can be depleted or adoptively transferred into mice to measure the effect on weight and metabolism. However, there is currently no way to specifically deplete iNKT cells in vivo. The common method is to use anti-NK1.1 antibody; however, this also depletes NK cells, which often outnumber iNKT cells. This method also would not deplete iNKT cells lacking the NK1.1 receptor, which is a substantial proportion of adipose iNKT cells. We, and others, have performed gain of function studies, by adoptively transferring iNKT cells into obese wild-type and iNKT-deficient mice, as well as specifically activating them by injection of αGalCer.
In the recent studies that aimed to determine the role, if any, for iNKT cells in obesity, the main discrepancies between laboratories were seen in the mouse models of iNKT cell deficiency. On one side of the argument, Ohmura et al. implied that iNKT cells were pathogenic in obesity using β2-microglobulin knockout mice.60 However, these mice also lack CD8 T cells which were shown to be pathogenic in obesity,50 and their loss alone improves obesity, and so clear conclusions could not be drawn. To address this issue, Mantell et al. used CD1d−/− mice with normal CD8 levels to measure the contribution of iNKT cells to obesity.61 Like other studies, Mantell et al. found that iNKT cells were decreased in the liver of obese mice but found an increase in adipose iNKT cells in obesity, unlike the majority of other studies. One caveat is that this study used NK1.1+ CD3+ as surrogate markers for iNKT cells, which also detects other T cells that are not iNKT cells, particularly in adipose tissue where NK1.1 is not expressed on a substantial proportion of iNKT cells. It may also detect non-invariant type II NKT cells, or MHC-restricted T cells, which have been shown to up-regulate NK markers when activated,62 and these could be possible explanations for an increase in NK1.1+ T cells in obesity. Nevertheless, in this study, obese CD1d−/− mice were not significantly different from obese wild-type mice in terms of weight gain and metabolic parameters.61 Boes and colleagues found that iNKT-deficient mice had increased adipocyte size and insulin resistance, and their depletion increased insulin resistance. They also found that CD1d−/− mice on an HFD had increased insulin resistance, but they did not observe any difference in weight gain between wild-type and NKT-deficient mice on an HFD. However, Kotas et al. found that iNKT-deficient mice were metabolically normal on a standard diet, but CD1d−/− mice had mildly but significantly impaired glucose tolerance and increased insulin resistance on an HFD.63 This study particularly focused on hepatic iNKT cells, which they found were reduced in obesity, and mice lacking iNKT cells had increased liver triglycerides and worse hepatic steatosis. However, many of these features were not seen in Ja18−/− mice on HFD, suggesting that either other CD1d-restricted T cells are responsible for the worsened metabolism and steatosis, or that Ja18−/− mice displayed some compensation for the iNKT cell deficiency. Hams et al. also found that while iNKT cells positively regulated obesity and metabolism and were depleted in obese mice, they did not find any differences in weight or glucose homeostasis in CD1d−/− or Ja18−/− mice.64 This series of studies suggests that deficiency in iNKT cells from birth does not alter weight gain and metabolism, yet iNKT deficiency from birth resulted in excess weight and impaired metabolism on a normal diet. On the other hand, our laboratory, Qi and colleagues, and Kim and colleagues all found that mice deficient in iNKT cells had accelerated obesity and had significantly more severe glucose impairment and insulin resistance on an HFD.3,8,39,57 In contrast, Van Kaer and colleagues found that although iNKT cells were depleted in the liver and adipose tissue of obese mice, NKT-deficient mice had slightly worse or no difference in weight gain, worse metabolic control, and more fatty liver deposition on an HFD. The reasons for these divergent results are still unknown but as we understand more about the immune system in adipose tissue one could speculate several explanations for these discrepancies. One possibility is that microbiome differences between laboratories and between wild-type and knockout mice contribute to the difference in weight gain, as the microbiome has been to shown to significantly impact metabolism and the development of obesity,65 as well as iNKT cell development.66 We have exchanged cages between non-littermate wild-type and iNKT knockout mice to reduce the impact of the microbiome. However, the reference standard is to use wild-type and knockout littermates to eliminate the impact of the microbiome, which were used in some,63,67 but not most, of the studies summarized above. Another plausible explanation is the age of mice in each study. In young mice, there is a substantial population of iNKT cells and fewer regulatory T cells in adipose tissue, and at 8–16 weeks, iNKT cells accumulate further but decline in old age, whereas adipose regulatory T cells greatly accumulate in old mice.51 Therefore, it is plausible that iNKT cells may be more influential in younger mice, whereas in older mice it is the regulatory T cells that dominate and the role of iNKT cells, or lack of them may be less dominant. It is also possible that for some reason both wild-type and iNKT-deficient animal colonies in different laboratories have a more Th1 or more Th2 bias among iNKT cells or other lymphocytes, or in some colonies, there is a compensatory mechanism when iNKT cells are absent from birth.
Despite the divergent results using iNKT-deficient mice, other methods to measure the effects of iNKT cells on obesity and metabolism are more consistent. First, over 14 independent studies have shown that iNKT cells (when measured accurately) are depleted in obesity, and all human studies have also found iNKT deficiency associated with obesity. Other immune cells that are shown to be protective in obesity,52 such as regulatory T cells,51 alternatively activated macrophages and eosinophils,54 are depleted in obesity, whereas those that are shown to be pathogenic in obesity like CD8+ T cells50 and classically activated macrophages56 are increased in obese adipose tissue. Based on this comparison, which is not direct evidence and merely an association, iNKT cells appear to be part of the protective anti-inflammatory immune cell group that are lost in obesity as an inflammatory response takes over. More direct evidence comes from gain of function experiments, when iNKT cells are adoptively transferred into wild-type or iNKT-deficient obese mice or activated in wild-type obese mice. The majority of studies have shown that this has a positive impact on metabolic control and on protection against weight gain. In 2006, Margalit et al. reported that administering an iNKT cell agonist glucocerebroside ameliorated metabolic syndrome in severely obese ob/ob mice.68 Similar results were seen by Elinav et al. following adoptive transfer of iNKT cells into ob/ob mice.69 This laboratory also found that improvement in metabolism and non-alcoholic steatosis was associated with increased iNKT cell levels and elevated IL-10 in the serum.70 Ma et al. also found that obesity induced a reduction in hepatic iNKT cells. When obese mice were treated with probiotics, iNKT cells were not depleted, which correlated with improved fatty liver disease in obese mice.71 Our laboratory, Qi and colleagues, and most recently Fallon and colleagues have shown that activation of iNKT cells in vivo with αGalCer injection causes significant weight loss and restoration of glucose homeostasis without hypoglycaemia, and an increase in insulin sensitivity.3,39,64 We, and others, have found that adoptive transfer of iNKT cells into obese mice also induced these effects.3,64 In contrast, Van Kaer and colleagues found that αGalCer injection induced an inflammatory cytokine milieu in obesity, although an increase in anti-inflammatory cytokines was also reported. αGalCer also induced an increase in numbers of many other leucocytes, including macrophages, as would be expected because of the potent transactivatory functions of iNKT cells. However, whether or not the increased macrophages express anti-inflammatory ‘M2’ markers was not tested. The reasons for the somewhat different outcomes of αGalCer treatment in obesity are not fully known, but they could be due to chronic daily treatments, which may cause a cytokine storm, particularly from liver iNKT cells which produce IFN-γ, compared with single or twice weekly treatments, which may allow the anti-inflammatory cytokines produced by iNKT cells in adipose tissue3,39 and elsewhere to dominate.
Harnessing adipose iNKT cells for adipose tissue and metabolic regulation
Great interest exists in how to harness iNKT cells due to their ability to rapidly produce massive amounts of cytokines. This is particularly true in the tissues where they are highly enriched under homeostatic conditions, namely the liver and adipose tissue. Targeting adipose iNKT cells may provide a novel potent therapeutic approach to regulate the inflammatory environment in obese adipose tissue. In 2011, the WHO reported that over 1·4 billion adults and 40 million children under age 5 are overweight or obese worldwide, and obesity is a major risk factor for many serious diseases such as cardiovascular disease, diabetes and cancer. Inflammation is an underlying cause or contributor to many of these diseases,72 and so preventing obesity-induced inflammation should be a key priority in tackling the obesity burden. Resident adipose tissue iNKT cells are unique in terms of their anti-inflammatory phenotype and function. We have recently performed gene expression profiling on adipose iNKT cells; this revealed that adipose iNKT cells express a distinct genetic profile including anti-inflammatory cytokines, including IL-10 and IL-4 (unpublished observations). Interleukin-10 and IL-4 are known to play potent and direct roles in promoting alternatively activated macrophages and suppressing inflammation in macrophages and other cells,73,74 which indirectly influence adipocyte function. However, in obese humans and mice, adipose iNKT cells are greatly reduced, and therefore their protective effects may be blunted.2,3 One potent way to activate iNKT cells in vivo is through αGalCer treatment, which increases iNKT cell levels 10-fold even in obesity.3 We, and others, have shown that adipose iNKT cell activation promotes M2 macrophage polarization as well as inducing weight loss and improved fatty liver and insulin resistance.3,39 Importantly, we did not observe any negative side effects of activating iNKT cells with αGalCer such as hypoglycaemia or cachexia, nor did αGalCer have any effects in mice lacking iNKT cells. While obvious caution needs to be considered given the potential of a cytokine storm, the effects of αGalCer treatment to loss of fat mass but not lean mass in obesity is striking and warrants further study to elucidate the pathway from activation of iNKT cells to weight loss. Also, in obese humans, iNKT cells are found at a much lower frequency in liver and spleen, so administration of αGalCer may not have the potential side effects seen in older mice after repeated injections. Administration of αGalCer to humans has been performed in many different clinical trials for cancer and has proven safe, capable of activating human iNKT cells in vivo, with minimal side effects. However, the effects of chronic iNKT cell activation in humans has not yet been fully studied.
In the case of type 2 diabetes and obesity, an ideal scenario might be to specifically activate anti-inflammatory adipose iNKT cells rather than whole body iNKT cells, which predominantly produce IFN-γ when activated (in mice at least). There is currently no method to specifically target particular populations of iNKT cells, but one may speculate that certain lipids may more potently activate different iNKT cell populations based on TCR affinity and co-stimulatory signals present or enriched in a particular environment. Indeed, indirect but strong evidence suggests that adipose tissue itself may contain an endogenous lipid that activates iNKT cells. First, CD1d is highly expressed in human2 and murine adipose tissue.7,8 Moreover, not only is CD1d expressed on immune cells in the stromovascular fraction of adipose tissue, but CD1d is also expressed by adipocytes themselves.7,8 Furthermore, adipose iNKT cells appear to be constitutively activated in adipose tissue even in lean steady state, as measured by high CD69 expression. Therefore it makes sense that endogenous lipid antigens may be present in the lipid-rich environment of adipose tissue where CD1d is highly expressed. It also makes sense that in the obese state, where the type and quantity of lipids is greatly altered, that this would influence adipose iNKT cells. This may result in their aberrant activation or prevent their survival if their endogenous ligands were no longer present. Therefore identifying such adipose-resident lipid antigens would provide immense insight into the physiological basis of iNKT cell accumulation in adipose tissue and a potential pathway that could be manipulated to prevent their loss in obesity. Whether or not targeting iNKT cells in the clinic would result in meaningful clinical effects on diabetes and weight loss remains to be seen, but pursuing this avenue seems well justified. Lipid antigens that target iNKT cells, as well as other bioactive lipids, have been used clinically to treat patients with cancer. They are also the subject of many clinical trials for various cancers, including melanoma and prostate cancer, as well as autoimmune diseases. Lipid-based drugs for therapeutics for other purposes are also available. Furthermore, studies have shown that lipids given parenterally can activate iNKT cells, making the idea of targeting adipose iNKT cells in obesity a promising and viable strategy.
Conclusion
Adipose iNKT cells represent a unique iNKT cell population, which appear to be poised towards anti-inflammatory cytokine production. Whether anti-inflammatory iNKT cells are destined to migrate to adipose tissue from the thymus, or whether adipose tissue influences their phenotype and function remains to be seen. Nevertheless, the recent surge of reports on adipose iNKT cells have revealed one of the clearest examples of the regulatory function of an iNKT cell population, indicating that they maintain healthy adipose tissue under normal conditions and correct obesity and metabolic disorder when stimulated under high fat diet conditions.3,39,57,58,7 In keeping with their role as a bridge between the innate and adaptive immune systems, iNKT cells seem to be one of the first cells that are affected by obesity, even as early as a few days after commencing an HFD. Therefore, analogous to their key role in autoimmune diseases including type 1 diabetes, multiple sclerosis and systemic lupus erythematosus, and in various cancers, iNKT cells are also early and key players in the immune regulation of metabolism. It is likely that future studies will reveal the mechanism by which iNKT cells are lost in obesity, which may provide insight into how to prevent this loss and a greater understanding of the basis of their accumulation in adipose tissue. It is hoped that adipose lipid antigen(s), if any, will be identified, which would no doubt be very beneficial to answering some of these outstanding questions.
Acknowledgments
We are very grateful to Professors Michael Brenner, Mark Exley, Donal O'Shea and Cliona O'Farrelly for insight and helpful discussions.
Glossary
- αGalCer
α-galactosylceramide
- HFD
high-fat diet
- IFN-γ
interferon-γ
- IL-10
interleukin-10
- iNKT cell
invariant natural killer T cell
- NK
natural killer
- PLZF
promyelocytic leukaemia zinc finger
- TCR
T cell receptor
- Th1
T helper type 1
Disclosures
The author has nothing to disclose.
References
- 1.Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–62. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch L, O'Shea D, Winter DC, Geoghegan J, Doherty DG, O'Farrelly C. Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39:1893–901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 3.Lynch L, Nowak M, Varghese B, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–87. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–8. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–4. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 6.Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990;250:679–82. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- 7.Schipper HS, Rakhshandehroo M, van de Graaf SF, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–54. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh JY, Kim JI, Park YJ, et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol. 2013;33:328–39. doi: 10.1128/MCB.00552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–6. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4– CD8– T cells. J Exp Med. 1997;186:109–20. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 12.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–54. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8– T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dascher CC, Brenner MB. Evolutionary constraints on CD1 structure: insights from comparative genomic analysis. Trends Immunol. 2003;24:412–8. doi: 10.1016/s1471-4906(03)00179-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhou D, Mattner J, Cantu C, 3rd, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 17.Brennan PJ, Tatituri RV, Brigl M, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–11. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox LM, Cox DG, Lockridge JL, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Facciotti F, Ramanjaneyulu GS, Lepore M, et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol. 2012;13:474–80. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- 20.Albacker LA, Chaudhary V, Chang YJ, et al. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med. 2013;19:1297–304. doi: 10.1038/nm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinjo Y, Illarionov P, Vela JL, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011;12:966–74. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 23.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 24.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 25.Brigl M, Tatituri RV, Watts GF, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–77. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–17. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 27.Kovalovsky D, Uche OU, Eladad S, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–64. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 30.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–42. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 31.Kawano T, Cui J, Koezuka Y, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690–3. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, Kronenberg M, Seeger RC. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–22. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 33.Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S, Besra GS, Brenner MB. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008;105:8339–44. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swann J, Crowe NY, Hayakawa Y, Godfrey DI, Smyth MJ. Regulation of antitumour immunity by CD1d-restricted NKT cells. Immunol Cell Biol. 2004;82:323–31. doi: 10.1111/j.0818-9641.2004.01254.x. [DOI] [PubMed] [Google Scholar]
- 36.Bosma A, Abdel-Gadir A, Isenberg DA, Jury EC, Mauri C. Lipid-antigen presentation by CD1d+ B cells is essential for the maintenance of invariant natural killer T cells. Immunity. 2012;36:477–90. doi: 10.1016/j.immuni.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, Bendelac A, Littman DR. Genetic evidence supporting selection of the Vα14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–16. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji Y, Sun S, Xu A, et al. Activation of natural killer T cells promotes M2 macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561–71. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas SY, Scanlon ST, Griewank KG, et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1–ICAM-1 interactions. J Exp Med. 2011;208:1179–88. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watarai H, Sekine-Kondo E, Shigeura T, et al. Development and function of invariant natural killer T cells producing Th2- and Th17-cytokines. PLoS Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 43.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–55. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 44.Terashima A, Watarai H, Inoue S, et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med. 2008;205:2727–33. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–88. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson MT, Johansson C, Olivares-Villagomez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–8. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 48.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 51.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–7. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talukdar S, Oh da Y, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji Y, Sun S, Xia S, Yang L, Li X, Qi L. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J Biol Chem. 2012;287:24378–86. doi: 10.1074/jbc.M112.371807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23:407–15. doi: 10.1016/j.tem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 59.Bedel R, Matsuda JL, Brigl M, White J, Kappler J, Marrack P, Gapin L. Lower TCR repertoire diversity in Traj18-deficient mice. Nat Immunol. 2012;13:705–6. doi: 10.1038/ni.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohmura K, Ishimori N, Ohmura Y, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–9. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 61.Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O'Doherty RM. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS ONE. 2011;6:e19831. doi: 10.1371/journal.pone.0019831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly-Rogers J, Madrigal-Estebas L, O'Connor T, Doherty DG. Activation-induced expression of CD56 by T cells is associated with a reprogramming of cytolytic activity and cytokine secretion profile in vitro. Hum Immunol. 2006;67:863–73. doi: 10.1016/j.humimm.2006.08.292. [DOI] [PubMed] [Google Scholar]
- 63.Kotas ME, Lee HY, Gillum MP, Annicelli C, Guigni BA, Shulman GI, Medzhitov R. Impact of CD1d deficiency on metabolism. PLoS ONE. 2011;6:e25478. doi: 10.1371/journal.pone.0025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–53. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 66.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu L, Parekh VV, Gabriel CL, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci USA. 2012;109:E1143–52. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Margalit M, Shalev Z, Pappo O, et al. Glucocerebroside ameliorates the metabolic syndrome in OB/OB mice. J Pharmacol Exp Ther. 2006;319:105–10. doi: 10.1124/jpet.106.104950. [DOI] [PubMed] [Google Scholar]
- 69.Elinav E, Pappo O, Sklair-Levy M, et al. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J Pathol. 2006;209:121–8. doi: 10.1002/path.1950. [DOI] [PubMed] [Google Scholar]
- 70.Elinav E, Pappo O, Sklair-Levy M, et al. Amelioration of non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice by oral immune regulation towards liver-extracted proteins is associated with elevated intrahepatic NKT lymphocytes and serum IL-10 levels. J Pathol. 2006;208:74–81. doi: 10.1002/path.1869. [DOI] [PubMed] [Google Scholar]
- 71.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–30. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 73.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 74.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]


