Abstract
Peroxisome proliferator-activated receptor gamma (PPARγ) agonists are known to have many immunomodulatory effects. We have previously shown that the PPARγ agonist rosiglitazone is beneficial when used early in prevention of disease in murine models of systemic lupus erythematosus (SLE) and SLE-related atherosclerosis. In this report, we demonstrate that another PPARγ agonist, pioglitazone is also beneficial as a treatment for early murine lupus, indicating that this is a class effect and not agent-specific. We further attempt to define the ability of PPARγ agonists to ameliorate established or severe autoimmune disease using two mouse models: the MRL.lpr SLE model and the gld.apoE−/− model of accelerated atherosclerosis and SLE. We demonstrate that, in contrast to the marked amelioration of disease seen when PPARγ agonist treatment was started before disease onset, treatment with rosiglitazone after disease onset in MRL.lpr or gld.apoE−/− mice had minimal beneficial effect on the development of the autoimmune phenotype; however, rosiglitazone treatment remained highly effective at reducing lupus-associated atherosclerosis in gld.apoE−/− mice after disease onset or when mice were maintained on a high cholesterol Western diet. These results suggest that beneficial effects of PPARγ agonists on the development of autoimmunity might be limited to the early stages of disease, but that atherosclerosis, a major cause of death in SLE patients, may be ameliorated even in established or severe disease.
Keywords: animal models, atherosclerosis, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease that results from a complex and incompletely understood interaction between diverse genetic and environmental factors.1,2 Non-specific immunosuppressants are used to lessen disease activity, but treatment is not always effective and is often complicated by drug-related adverse events.3
Thiazolidinediones are peroxisome proliferator-activated receptor γ (PPARγ) agonists that have been used clinically for treating type 2 diabetes because of their ability to enhance insulin sensitivity.4 More recently, it has been recognized that PPARγ agonists also possess anti-inflammatory and immunomodulatory properties.5–7 Recent studies have evaluated the effects of PPARγ agonists on inflammatory diseases such as SLE. We found that the PPARγ agonist rosiglitazone reduced disease progression in two different mouse models of SLE and SLE-associated atherosclerosis.8 Venegas-Pont et al.9 showed that rosiglitazone treatment is reno-protective and decreases hypertension in the NZBWF1 lupus-prone mouse strain. It is important to note that this study was designed to begin treatment before overt nephritis onset in mice showing no evidence of proteinuria. Similarly, a second PPARγ agonist, pioglitazone, was shown to ameliorate renal inflammation as well as improve endothelial function and insulin sensitivity in 10-week-old pre-nephritic NZBWF1 mice.10 Macrophage-specific deletion of PPARγ leads to anti-nuclear antibody (ANA) production and glomerulonephritis.11 More recently, a small cohort of human SLE patients received a 4-week pioglizatone treatment in a randomized, double-blind clinical trial. The results showed that pioglitazone reduced C-reactive protein, increased high-density lipoprotein cholesterol levels, and improved insulin sensitivity, suggesting a beneficial effect on markers of cardiovascular disease risk associated with SLE.12 Like the animal studies, these patients were in clinical remission with no apparent active renal disease at the start of pioglitazone treatment. Taken together, these data suggest that thiazolidinediones are an important class of drugs to study as possible therapeutics for human SLE.
In the current study, we expanded on our previous rosiglitazone study8 by treating murine lupus with pioglitazone to determine if the beneficial effects of PPARγ agonists are generalizable as a class. In addition, we used mouse models of SLE to assess whether the PPARγ agonist rosiglitazone would ameliorate disease parameters if treatment was started either after the onset of disease or early in mice with more severe disease. The gld.apoE−/− mouse is deficient in both Fas ligand and apolipoprotein E and develops a lupus-like autoimmune disease and accelerated atherosclerosis.13 This models the higher incidence of accelerated atherosclerosis observed in human lupus patients that is associated with increased morbidity and mortality due to cardiovascular events such as stroke or myocardial infarction.14 The MRL.lpr mouse is a well-established model that develops a lupus-like disease characterized by ANA accumulation and lupus nephritis. Our previous study showing beneficial effects of rosiglitazone via an adiponectin-dependent mechanism,8 used an MRL.lpr strain that was subsequently reported by Jackson Laboratories (Bar Harbor, ME) to have a progressive loss of phenotype resulting in a ‘mild’ lupus-like disease. The original MRL.lpr strain was subsequently re-derived by Jackson Laboratories from cryopreserved embryo archives, and exhibits the original ‘severe’ lupus phenotype in females.15 Therefore, for this current study, we refer to both the ‘mild’ and ‘severe’ lines of MRL.lpr mice in addition to the gld.apoE−/− model. We present data to determine whether the effects of PPARγ agonists on murine lupus are agent-specific or generalizable to PPARγ agonists as a class, and to determine if the beneficial effects of rosiglitazone are limited to early or mild disease.
Materials and methods
Animals and drug treatment
Gld.apoE−/− mice on a C57BL/6 background have been previously described.13 Two MRL.lpr mouse strains were purchased from Jackson Laboratories: #006825, developing a ‘mild’ lupus phenotype, and #000485, developing a ‘severe’ lupus phenotype at an earlier age. The ‘mild’ phenotype, MRL/MpJ-Faslpr/2J (#006825), was reported to have a progressive loss of phenotype described as an approximate fivefold reduction in lymph node size, as well as a threefold reduction in splenomegaly compared with the ‘severe’ phenotype, MRL/MpJ-Faslpr/J (#000485). In addition, survival was reported to be much longer than expected in the ‘mild’ phenotype, compared with that originally described in the ‘severe’ phenotype, in which female mice have an average lifespan of 17 weeks (http://jaxmice.jax.org/strain/000485.html). Six-week-old ‘mild’ MRL.lpr mice received either normal diet or normal diet supplemented with pioglitazone (GlaxoSmithKline, Brentford, UK) at a dosage of 30 mg/kg per day for a period of 12 weeks. Thirteen-week-old gld.apoE−/− mice, 12-week-old ‘mild’ MRL.lpr mice and 6-week-old ‘severe’ MRL.lpr mice received either normal diet or normal diet supplemented with rosiglitazone (GlaxoSmithKline) at a dosage of 10 mg/kg per day for a period of 12 weeks. The dose of rosiglitazone and the total duration of treatment were the same as we had used in our previous studies.8 Another cohort of gld.apoE−/− mice received high-cholesterol Western diet (Harlan-Teklad Special Diets #88137) or Western diet supplemented with rosiglitazone starting at 7 weeks of age at a dosage of 10 mg/kg per day for a period of 12 weeks. All diets were designed and prepared by Harlan Teklad for the purposes of these studies. Body weight and food intake were assessed weekly. Study protocols were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine.
Tissue analysis and histology
After 12 weeks on the diet, mice were weighed and blood was drawn by cardiac puncture. Sub-mandibular lymph nodes and spleens were excised and weighed. Portions of the kidneys from each mouse were either immediately frozen in optimal cutting temperature compound or fixed in 10% neutral-buffered formalin overnight and processed for paraffin embedding. Paraffin blocks were sectioned (7 μm thick) and slides were stained with haematoxylin and eosin. Cross-sectional areas of at least 25 glomeruli were measured in each animal using computer-assisted pixel counting (Photoshop CS3; Adobe). Glomerular cell count was determined using the same photographs of sections stained with haematoxylin and eosin. The blue-stained nuclei in the glomerular tuft were counted in at least 25 glomeruli per animal. For IgG and C3 immunofluorescence, frozen kidney tissue was sectioned (7 μm thick) and fixed with a chilled methanol : acetone mixture. After blocking, samples were incubated with anti-IgG (Sigma, St Louis, MO, #F-2883) or anti-C3 (Cappel, #55500) used at a dilution of 1 : 150 or 1 : 100, respectively. Immunofluorescent images were obtained using a Keyence microscope system. Slides were examined blindly by two investigators.
Analysis of atherosclerotic lesion
The vasculature was perfused, removed, cleaned of adventitia, opened longitudinally and fixed in 10% neutral buffered formalin for 24 hr. The extent of lesion area in the aorta was analysed as previously described.13 Briefly, the aortas were stained with Oil Red O and photographed under a stereomicroscope with an Olympus imaging system. Atherosclerotic lesions were quantified using Adobe Photoshop and are expressed as the amount of atherosclerosis relative to total aortic area.
Serum measurements
Circulating ANA levels were measured by immunofluorescence using HEp-2-coated slides (The Binding Site Inc., San Diego, CA). Slides were incubated for 1 hr with serial log-scale dilutions of mouse serum as previously described,16 washed in PBS, and then incubated with FITC-labelled goat anti-mouse IgG (whole molecule; Sigma-Aldrich, St Louis, MO). Slides were viewed using fluorescence microscopy. To detect autoantibodies that have bound to dsDNA, diluted serum was incubated on glass slides coated with Crithidia luciliae (Antibodies Incorporated, Davis, CA).17 Slides were visualized by fluorescence microscopy after incubation with FITC-conjugated anti-mouse IgG, and fluorescence luminosity was quantified using Adobe Photoshop. Circulating adiponectin levels were determined using a mouse adiponectin ELISA (B-Bridge International, Cupertino, CA).
Statistical analysis
Results are shown as the mean ± SEM. Differences between groups were determined by Student's t-test. Results were considered statistically significant for P < 0·05.
Results
Treatment of mild lupus with the PPARγ agonist pioglitazone ameliorates disease
To expand on our previous data and confirm that the therapeutic effects of rosiglitazone are applicable to PPARγ agonists as a class, we tested the effect of pioglitazone on disease manifestations in ‘mild’ MRL.lpr mice. No statistically significant difference was observed in body weight or food intake regardless of treatment (Table 1). PPARγ agonists induce adiponectin production and we have used this previously as a marker for adequate PPARγ agonist ingestion.8,18 As expected, serum adiponectin levels were increased after 12 weeks on a normal diet in the MRL.lpr mice taking pioglitazone (Fig. 1a). Although no significant change in spleen weight was observed (Fig. 1b), treatment with pioglitazone significantly decreased lymphadenopathy (Fig. 1c), serum ANA titre (Fig. 1d) and anti-dsDNA antibody levels (Fig. 1e). Kidney disease was assessed by quantification of glomerular tuft size and glomerular cell number, both of which were significantly decreased by pioglitazone treatment (Fig. 1f,g). In addition, treatment with pioglitazone decreases IgG deposition, and complement C3 deposition within the glomeruli (Fig. 1h).
Table 1.
Body weight and food intake
| Model | Disease state | Diet/treatment | Body weight (g) | Food intake (g/mouse per day) |
|---|---|---|---|---|
| MRL-lpr – ‘mild’ | Early | Normal chow | 45·6 ± 1·73 | 4·31 ± 1·02 |
| NC/Pio. | 48·3 ± 1·21 | 3·96 ± 0·87 | ||
| MRL-lpr – ‘mild’ | Established | Normal chow | 43·5 ± 2·11 | 4·27 ± 0·45 |
| NC/Rosi. | 48·1 ± 1·03 | 4·73 ± 0·69 | ||
| MRL-lpr – ‘severe’ | Severe | Normal chow | 39·6 ± 1·30 | 3·81 ± 0·72 |
| NC/Rosi. | 40·0 ± 1·23 | 3·96 ± 0·65 | ||
| gld.apoE−/− | Established | Normal chow | 39·3 ± 1·44 | 3·89 ± 0·91 |
| NC/Rosi. | 36·5 ± 0·62 | 3·77 ± 0·56 | ||
| gld.apoE−/− | Severe | Western diet | 32·1 ± 2·13 | 3·24 ± 0·83 |
| WD/Rosi. | 34·7 ± 1·99 | 3·39 ± 0·64 |
NC, normal chow; Pio., Pioglitazone; Rosi., rosiglitazone; WD, Western diet.
Figure 1.
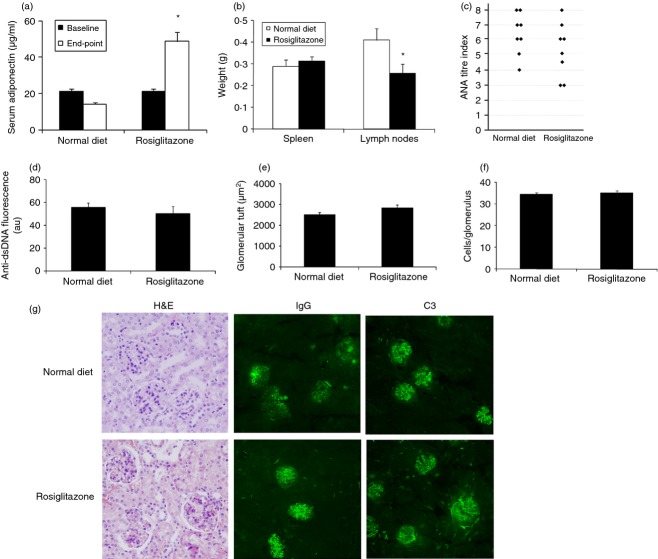
‘Mild’ phenotype MRL.lpr mice treated with pioglitazone before disease onset have less severe disease. MRL.lpr female mice were maintained on a normal diet (n = 13) or normal diet supplemented with 30 mg/kg per day pioglitazone (n = 15) for 12 weeks starting at 6 weeks of age (baseline) and then disease parameters were measured (end-point; 18 weeks of age). (a) Circulating levels of adiponectin were quantified by ELISA. (b) Spleen and (c) sub-mandibular lymph nodes were harvested and weighed. (d) Serum anti-nuclear antibody (ANA) titre was determined by HEp2 immunofluorescence. ANA titre index is a log scale of staining intensity.16 (e) Circulating levels of anti-dsDNA antibodies were examined by analysis of serial serum dilutions on Crithidia luciliae. Intensity of fluorescence is reported as arbitrary units (au). Kidney sections stained with haematoxylin and eosin (H&E; 40×) were used to quantify (f) glomerular tuft area and (g) glomerular cell count. (h) Representative photomicrographs of kidney sections stained with H&E, and for IgG and complement C3 deposition. **P < 0·01; ***P < 0·001.
MRL.lpr mice with established disease do not respond to rosiglitazone if treatment is started after disease onset
Having demonstrated efficacy of both pioglitazone and rosiglitazone in the ‘mild’ MRL.lpr model when treatment is started before disease onset (Fig. 1 and ref. 8), we sought to determine if treatment would still be beneficial in established disease. This portion of the study was performed using the ‘mild’ MRL.lpr phenotype. We previously demonstrated beneficial effects of rosiglitazone on ameliorating disease in MRL.lpr mice when treatment began at 6 weeks of age.8 Here, we administered rosiglitazone to 12-week-old mice displaying detectable ANA titres. As expected, circulating adiponectin levels were significantly increased in the rosiglitazone-treated group compared with untreated mice indicating that the mice ingested sufficient rosiglitazone to induce PPARγ-responsive gene expression (Fig. 2a). No statistically significant difference was observed in body weight or food intake regardless of treatment (Table 1). Splenomegaly, characteristic of MRL.lpr mice, was not significantly altered; however, lymphadenopathy was lessened by rosiglitazone administration (Fig. 2b). Anti-nuclear antibody and anti-dsDNA levels were similar between the two groups (Fig. 2c, d) and the pattern of immunofluorescent staining remained unchanged regardless of treatment. Autoimmune renal disease as measured by glomerular tuft size and the number of cells within the glomerulus was unaffected by rosiglitazone treatment (Fig. 2e, f). Mild kidney disease was not affected by rosiglitazone treatment as demonstrated by similar glomerular morphology and immune complex and complement deposition within the glomeruli (Fig. 2g). Hence, MRL.lpr mice with the ‘mild’ phenotype did not receive striking benefits from rosiglitazone treatment when it was started after disease onset, in contrast to the beneficial effects observed when treatment was started before disease onset.
Figure 2.
‘Mild’ phenotype MRL.lpr mice are unaffected by rosiglitazone treatment started after onset of disease. Twelve-week-old female MRL.lpr mice received normal diet (n = 8) or normal diet containing rosiglitazone at a dose of 10 mg/kg per day (n = 8) for 12 weeks. (a) Circulating adiponectin levels were measured by ELISA. (b) Lymph node and spleen were weighed after tissue harvest. (c) Serum anti-nuclear antibody (ANA) titre as measured by staining intensity of HEp-2 immunofluorescence. (d) Circulating levels of anti-dsDNA antibodies were examined by analysis of serial serum dilutions on Crithidia luciliae. Intensity of fluorescence is reported as arbitrary units (au). Kidney sections stained with haematoxylin and eosin (H&E) were used to quantify (e) glomerular tuft area and (f) glomerular cell count. (g) Representative photomicrographs of kidney sections stained with H&E, and for IgG and complement C3 deposition *P < 0·05.
Severe lupus disease in MRL.lpr mice is not ameliorated by rosiglitazone treatment
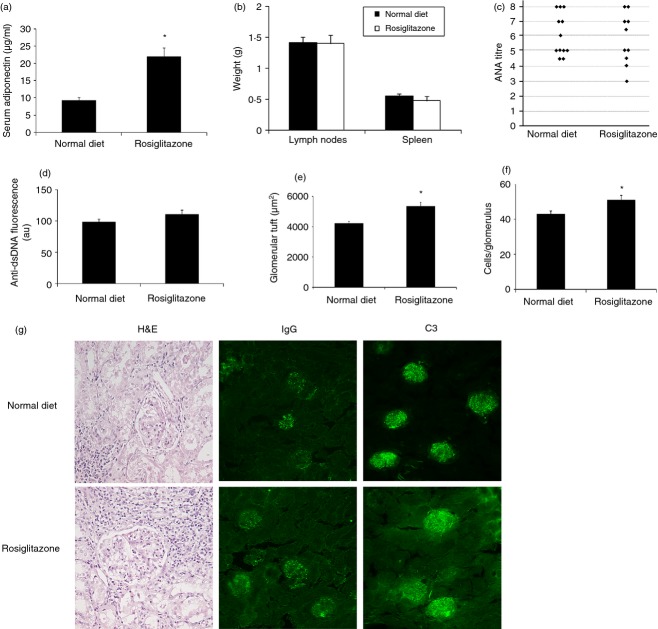
Female MRL.lpr mice with the ‘severe’ disease phenotype have an average lifespan of 17 weeks.15,19 To determine whether early treatment with rosiglitazone would be effective in a severe lupus model, 6-week-old ‘severe’ MRL.lpr mice were treated with rosiglitazone for 12 weeks. Two mice in our experiment died before completing the course of treatment because of the severity of the phenotype. Analysis of adiponectin levels after 12 weeks of rosiglitazone treatment revealed a significant increase compared with untreated mice (Fig. 3a). No statistically significant difference was observed in body weight or food intake regardless of treatment (Table 1). Spleen and lymph node weight did not differ whether the mice were treated with rosiglitazone or not (Fig. 3b); however, as expected, these weights were significantly increased compared with the mild phenotype MRL.lpr mice (Fig. 2b). Rosiglitazone treatment had no effect on the elevated ANA titres or staining pattern (Fig. 3c), nor on anti-dsDNA analysis in this severe mouse model of SLE (Fig. 3d). Surprisingly, treatment with rosiglitazone was not beneficial, but instead seemed to significantly worsen kidney disease as assessed by glomerular tuft size and glomerular cell count (Fig. 3e, f). Further evidence of kidney disease was observed by the presence of IgG and C3 deposition within the glomeruli, and these observations remained unchanged regardless of treatment (Fig. 3g).
Figure 3.
‘Severe’ phenotype MRL.lpr mice do not benefit from rosiglitazone treatment. Six-week-old female mice with the ‘severe’ lupus-like phenotype received either normal diet (n = 12) or normal diet supplemented with 10 mg/kg per day rosiglitazone (n = 10). (a) Serum adiponectin levels after 12 weeks of diet with rosiglitazone treatment. (b) Average weight of lymph nodes or spleen. (c) Anti-nuclear antibodies (ANA). (d) Circulating levels of anti-dsDNA antibodies were examined by analysis of serial serum dilutions on Crithidia luciliae. Intensity of fluorescence is reported as arbitrary units (au). Kidney sections stained with haematoxylin and eosin (H&E) were used to quantify (e) glomerular tuft area and (f) glomerular cell count. (g) Representative photomicrographs of kidney sections stained with H&E, and for IgG and complement C3 deposition. Error bars represent mean ± SEM. *P < 0·05.
Rosiglitazone treatment ameliorates atherosclerosis in gld.apoE−/− mice with established disease
To determine whether the observed lack of rosiglitazone effect if started after disease onset in the ‘mild’ MRL.lpr lupus model would apply to other models of SLE, we studied the gld.apoE−/− model. We had previously found a reduction in disease when rosiglitazone treatment was started before disease onset.8 We therefore started rosiglitazone in the gld.apoE−/− mice after disease onset by administering a 12-week treatment of rosiglitazone to gld.apoE−/− mice starting at 13 weeks of age, a time-point at which they are already ANA positive (data not shown). Serum adiponectin levels were measured and found to be increased in the mice treated with rosiglitazone, indicating that sufficient rosiglitazone was being ingested to induce effective PPARγ signalling in vivo (Fig. 4a). No statistically significant difference was observed in body weight or food intake regardless of treatment (Table 1). Splenomegaly and lymphadenopathy are hallmarks of the gld.apoE−/− autoimmune phenotype,13 and although there was a trend of reduction, rosiglitazone treatment did not significantly reduce spleen or lymph node size (Fig. 4b). Similarly, although a trend towards reduced ANA titres was seen with rosiglitazone treatment (Fig. 4c), this was not statistically significant and the ANA expression pattern was not different. In addition, analysis of circulating anti-dsDNA revealed similar titres regardless of treatment (Fig. 4d). Assessment of renal disease demonstrated no significant effect of rosiglitazone on glomerular tuft size or glomerular cell count (Fig. 4e, f). Deposition of IgG and C3 within the glomeruli was observed to a similar extent in treated or untreated mice (Fig. 4g). In contrast to the autoimmune phenotype, analysis of atherosclerosis revealed a marked and statistically significant decrease in lesion area with rosiglitazone treatment as evaluated by oil red O staining (Fig. 4h, i). Therefore, when treatment was started in gld.apoE−/− mice with measureable ANA titres, rosiglitazone markedly inhibited the progression of atherosclerosis. However, measures of autoimmune activity were not significantly affected, although a possible modest beneficial effect on lymphadenopathy and ANA titre cannot be excluded.
Figure 4.
Effect of rosiglitazone on disease manifestations in gld.apoE−/− mice when treatment is started after onset of disease. Gld.apoE−/− mice were maintained on a normal diet (n = 8) or normal diet supplemented with 10 mg/kg per day rosiglitazone (n = 9) for 10 weeks starting at 13 weeks of age (baseline), and then disease parameters were measured (end-point; 23 weeks of age). (a) Serum adiponectin levels. (b) Lymph node and spleen weights. (c) Serum anti-nuclear antibody (ANA) titre as determined by HEp2 immunofluorescence. (d) Circulating levels of anti-dsDNA antibodies were examined by analysis of serial serum dilutions on Crithidia luciliae. Intensity of fluorescence is reported as arbitrary units (au). Kidney sections stained with haematoxylin and eosin (H&E) were used to quantify (e) glomerular tuft area and (f) glomerular cell count. (g) Representative photomicrographs of kidney sections stained with H&E, and for IgG and complement C3 deposition. (h) Aortic atherosclerosis lesion area. (i) Representative photomicrographs of en face aortas stained with oil red O to detect lesion deposition. **P < 0·01.
Accelerated atherosclerosis in mice with exacerbated autoimmunity is retarded by rosiglitazone treatment
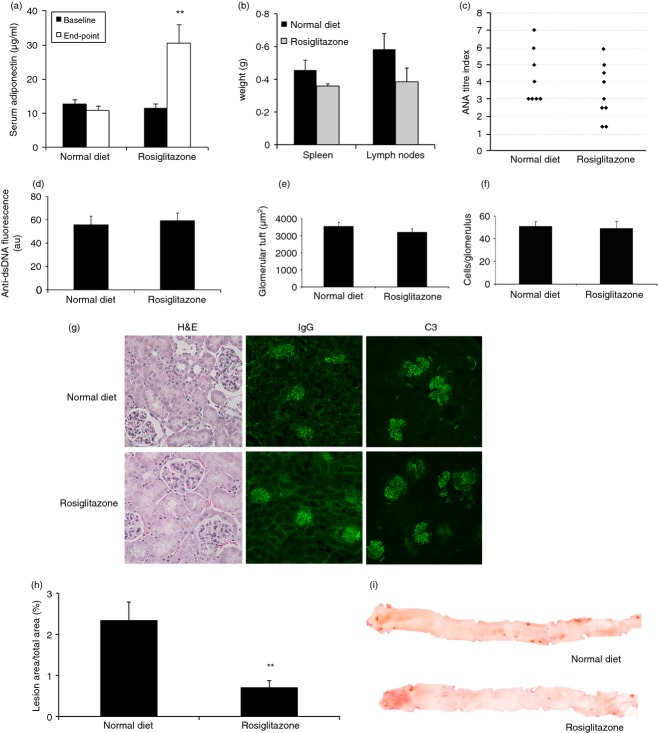
The high-cholesterol Western diet markedly enhances the severity of the disease phenotype in the gld.apoE−/− mouse model, with the development of exacerbated atherosclerosis and autoimmunity compared with gld.apoE−/− mice on normal chow.13 To examine the effects of rosiglitazone on the more severe disease phenotype, we concomitantly began feeding of Western diet and rosiglitazone to 7-week-old gld.apoE−/− mice. No statistically significant difference was observed in body weight or food intake regardless of treatment (Table 1). We observed that circulating adiponectin levels rose in the treated group compared with those not receiving treatment, indicating that the mice given rosiglitazone were ingesting the diet (Fig. 5a). Despite a rise in adiponectin levels, rosiglitazone treatment had no effect on lymph node or spleen weight (Fig. 5b). Serum levels of ANA and anti-dsDNA antibodies were measured using immunofluorescence and revealed similar titres in both the treated and untreated groups (Fig. 5c, d). Quantification of glomerular tuft size revealed a significant decrease by rosiglitazone treatment compared with untreated mice and a trend towards decreased glomerular cell count (Fig. 5e, f). To further determine the extent of kidney disease, fluorescent immunostaining was performed that revealed significant IgG and C3 deposition within glomeruli (Fig. 5g). With regard to atherosclerosis, this mouse model with exacerbated disease receives striking beneficial effects from rosiglitazone in terms of reducing atherosclerotic lesion area (Fig. 5h, i). These data provide further evidence that rosiglitazone is effective in inhibiting the progression of atherosclerosis, and partially ameliorates renal disease, even in mice on a Western diet.
Figure 5.
Rosiglitazone ameliorates atherosclerosis in gld.apoE−/− mice maintained on a high-cholesterol Western diet. Seven-week-old gld.apoE−/− mice were fed a Western diet (n = 12) or Western diet supplemented with 10 mg/kg per day rosiglitazone (n = 10) for 12 weeks. (a) Quantification of serum adiponectin levels at baseline (7 weeks old) and end-point (19 weeks of age). (b) Lymph node and spleen weights. (c) Analysis of anti-nuclear antibody (ANA) titre was determined by HEp2 immunofluorescence. (d) Circulating levels of anti-dsDNA antibodies were examined by analysis of serial serum dilutions on Crithidia luciliae. Intensity of fluorescence is reported as arbitrary units (au). Kidney sections stained with haematoxylin and eosin (H&E) were used to quantify (e) glomerular tuft area and (f) glomerular cell count. (g) Representative photomicrographs of kidney sections stained with H&E, and for IgG and complement C3 deposition. (h) Total atherosclerotic lesion area was quantified after oil red O staining. (i) Representative photographs of aortas opened longitudinally and stained with oil red O. *P < 0·05; **P < 0·01.
Discussion
In a previous study, we showed that the PPARγ agonist rosiglitazone reduced disease in the ‘mild’ phenotype MRL.lpr model, an effect mediated at least in part through the induction of adiponectin.8 In the current study, we have shown that early treatment with the PPARγ agonist pioglitazone also reduces lupus disease in the ‘mild’ phenotype MRL.lpr model demonstrating that this is probably a class-effect of PPARγ agonists. In addition, we show, in a number of lupus models and conditions, that rosiglitazone is not able to consistently ameliorate disease progression in models of severe SLE or if treatment is started beyond the early stage of disease (characterized by the appearance of ANA). This expands on our previous knowledge about the beneficial effects of rosiglitazone on SLE and SLE-related atherosclerosis and may guide us in selecting appropriate patient populations to consider for clinical trials of PPARγ agonist therapy.
In all experiments in this report, the experimental groups received treatment for a period of 12 weeks so differences observed were not the result of different lengths of treatment. We observed that rosiglitazone treatment of 12-week-old ‘mild’ MRL.lpr mice or 6-week-old ‘severe’ MRL.lpr mice (both demonstrating the presence of serum ANA before initiation of treatment) did not ameliorate the lupus phenotype. Similarly, 13-week-old gld.apoE−/− mice on normal chow did not receive any beneficial effects from rosiglitazone on lupus disease, whereas treatment induced a reduction in atherosclerosis. When gld.apoE−/− mice were fed a high-cholesterol Western-type diet, which is known to exacerbate disease progression,13 rosiglitazone treatment decreased atherosclerosis as well as glomerular tuft size. This somewhat surprising finding of reduced glomerular tuft size may be explained by the fact that glomerular hypertrophy is associated with a high-fat diet.20,21 In our model, we observed a much larger glomerular tuft size when mice were maintained on a Western diet (Fig. 5g) compared with the normal diet cohort (Fig. 4g). Hence, glomerular tuft size may be reduced as a consequence of rosiglitazone effects on lipid metabolism rather than on lupus per se. Together with our previous data showing that rosiglitazone has beneficial effects in mouse models of SLE,8 these new data suggest that optimal treatment of SLE with PPARγ agonists may be achieved if they are used as a preventive treatment, rather than as treatment for active disease.
Accelerated atherosclerosis and an increased incidence of cardiovascular disease are observed in SLE patients.22 A recent study has linked arterial stiffness to metabolic syndrome in SLE patients, suggesting that sub-clinical atherosclerosis may be affected by metabolic syndrome in patients with SLE.23 In addition, there is evidence of metabolic dysfunction, even when maintained on a low-fat diet, in a mouse model susceptible to lupus.24 This extends to humans, where there is an increased prevalence of metabolic syndrome and insulin resistance in patients with SLE.25–27 A recently published study involving patients with SLE receiving pioglitazone demonstrated a reduction in C-reactive protein and an increase in high-density lipoprotein cholesterol after 4 weeks of treatment, suggesting a beneficial effect on cardiovascular disease risk factors associated with SLE.12 Although several years ago, concern was raised about the association of thiazolidinediones with increased risk for heart failure in patients with type 2 diabetes,28 certain restrictions placed on these medications have recently been removed by the US Food and Drug Administration after re-evaluating cardiovascular outcomes.29 More importantly, novel synthetic compounds that bind PPARγ have anti-diabetic effects in obese mice without weight gain or fluid retention (a known side-effect of PPARγ agonists), but whether these drugs have an associated risk of heart failure is unknown.30 Nevertheless, these data suggest that novel compounds targeting the PPARγ pathway could be potentially valuable therapeutic agents to curtail the incidence and interactions of cardiovascular disease and metabolic dysfunction in SLE patients.
The data presented here also demonstrate that pioglitazone treatment reduces disease manifestations in the MRL.lpr model to a similar extent as rosiglitazone treatment.8 This amelioration of disease provides evidence that the effects of PPARγ agonists on lupus pathogenesis are a class effect and are not agent specific. Further evidence towards this is demonstrated in two independent studies, showing similar effects of rosiglitazone and pioglitazone on renal disease in NZB/W mice.9,31 In addition, there is some evidence that pioglitazone has a more favourable effect than rosiglitazone on cardiovascular outcomes and lipid profiles in human clinical trials,32,33 therefore, it would be of interest to perform more advanced studies using pioglitazone treatment in SLE patients.
We have previously demonstrated that induction of adiponectin is a major mechanism underlying the immunomodulatory effects of PPARγ agonists.8 There are a number of possible explanations for the lack of efficacy of rosiglitazone on ANA levels and renal disease when administered after disease onset, despite an effective induction of adiponectin expression. It might be that severe active disease simply overwhelms the protective effects of PPARγ agonists and the increased adiponectin levels. Alternatively, it might be that more advanced disease specifically impairs adiponectin's protective effects by, for example, down-regulating adiponectin receptor expression. This latter possibility is suggested by work from other groups showing that inflammatory stimuli are able to down-regulate the expression of adiponectin receptors.34,35
Our previous studies demonstrated that rosiglitazone can ameliorate mild disease in the MRL.lpr and gld.apoE−/− models if treatment is initiated at a relatively early point in disease development,8 and here we have shown that PPARγ agonists do not exhibit such beneficial effects if treatment is started during active or severe disease (summarized in Table 2). Early disease in the murine models might be analogous to SLE patients with quiescent clinical disease, rather than to those with active disease. It is possible that PPARγ agonists could play a useful role in this large sub-group of patients to potentially serve to maintain remission, allow steroid use to be reduced, and prevent long-term sequelae – in particular, cardiovascular complications. The data we obtained support consideration of PPARγ agonists as a potential therapy in this sub-group of patients.
Table 2.
Experimental conditions and outcomes
| Model | Disease state | Diet/treatment | ANA | Renal disease | Atherosclerosis. |
|---|---|---|---|---|---|
| MRL-lpr – ‘mild’ 1 | Early | NC/Rosi. | ↓ | ↓ | n/a |
| gld.apoE−/−1 | Early | NC/Rosi. | ↓ | ↓ | ↓ |
| MRL-lpr – ‘mild’ | Early | NC/Pio. | ↓ | ↓ | n/a |
| MRL-lpr – ‘mild’ | Established | NC/Rosi. | No change | No change | n/a |
| MRL-lpr – ‘severe’ | Severe | NC/Rosi. | No change | No change | n/a |
| gld.apoE−/− | Established | NC/Rosi. | No change | No change | ↓ |
| gld.apoE−/− | Severe | WD/Rosi. | No change | ↓ | ↓ |
ANA, anti-nuclear antibodies; NC, Normal chow; WD, Western diet.
8.
Acknowledgments
The authors would like to thank Rocco Richards, Guneet Kochar, Alyssa Brown and Parth Desai for technical assistance. TA and IRR designed the study. TA and ZW performed the experiments. TA, RGB, and IRR wrote the paper, This work was supported by a grant from the Alliance for Lupus Research (IRR), and the following grants from the NIH/NIAMS: K01 AR055965-02 (TA) and P01 AR050256 (IRR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH.
Disclosures
There are no financial conflicts of interest to declare.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Davidson A. Taming lupus – a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18:871–82. doi: 10.1038/nm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merrill JT, Erkan D, Buyon JP. Challenges in bringing the bench to bedside in drug development for SLE. Nat Rev Drug Discov. 2004;3:1036–46. doi: 10.1038/nrd1577. [DOI] [PubMed] [Google Scholar]
- 4.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 5.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–59. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Plutzky J. Inflammation in diabetes mellitus: role of peroxisome proliferator-activated receptor-α and peroxisome proliferator-activated receptor-γ agonists. Am J Cardiol. 2007;99:27B–40B. doi: 10.1016/j.amjcard.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–8. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Aprahamian T, Bonegio RG, Richez C, Yasuda K, Chiang LK, Sato K, Walsh K, Rifkin IR. The peroxisome proliferator-activated receptor γ agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J Immunol. 2009;182:340–6. doi: 10.4049/jimmunol.182.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venegas-Pont M, Sartori-Valinotti JC, Maric C, et al. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1282–9. doi: 10.1152/ajpregu.90992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao W, Thacker SG, Hodgin JB, et al. The peroxisome proliferator-activated receptor γ agonist pioglitazone improves cardiometabolic risk and renal inflammation in murine lupus. J Immunol. 2009;183:2729–40. doi: 10.4049/jimmunol.0804341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roszer T, Menendez-Gutierrez MP, Lefterova MI, Alameda D, Nunez V, Lazar MA, Fischer T, Ricote M. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor γ or retinoid X receptor α deficiency. J Immunol. 2011;186:621–31. doi: 10.4049/jimmunol.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juarez-Rojas JG, Medina-Urrutia AX, Jorge-Galarza E, et al. Pioglitazone improves the cardiovascular profile in patients with uncomplicated systemic lupus erythematosus: a double-blind randomized clinical trial. Lupus. 2012;21:27–35. doi: 10.1177/0961203311422096. [DOI] [PubMed] [Google Scholar]
- 13.Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet JM, Sato K, Castellot JJ, Jr, Walsh K. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med. 2004;199:1121–31. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikpour M, Urowitz MB, Gladman DD. Premature atherosclerosis in systemic lupus erythematosus. Rheum Dis Clin North Am. 2005;31:329–54. doi: 10.1016/j.rdc.2005.01.001. vii-viii. [DOI] [PubMed] [Google Scholar]
- 15.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–69. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 16.Komori H, Furukawa H, Mori S, et al. A signal adaptor SLAM-associated protein regulates spontaneous autoimmunity and Fas-dependent lymphoproliferation in MRL-Faslpr lupus mice. J Immunol. 2006;176:395–400. doi: 10.4049/jimmunol.176.1.395. [DOI] [PubMed] [Google Scholar]
- 17.Slater NG, Cameron JS, Lessof MH. The Crithidia luciliae kinetoplast immunofluorescence test in systemic lupus erythematosus. Clin Exp Immunol. 1976;25:480–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Maeda N, Takahashi M, Funahashi T, et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 19.Andrews BS, Eisenberg RA, Theofilopoulos AN, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–7. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 21.Ruggiero C, Ehrenshaft M, Cleland E, Stadler K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am J Physiol Endocrinol Metab. 2011;300:E1047–58. doi: 10.1152/ajpendo.00666.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE – mechanisms and management. Nat Rev Rheumatol. 2012;8:214–23. doi: 10.1038/nrrheum.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valero-Gonzalez S, Castejon R, Jimenez-Ortiz C, Rosado S, Tutor-Ureta P, Vargas JA, Yebra-Bango M. Increased arterial stiffness is independently associated with metabolic syndrome and damage index in systemic lupus erythematosus patients. Scand J Rheumatol. 2013;43:54–8. doi: 10.3109/03009742.2013.803150. [DOI] [PubMed] [Google Scholar]
- 24.Gabriel CL, Smith PB, Mendez-Fernandez YV, Wilhelm AJ, Ye AM, Major AS. Autoimmune-mediated glucose intolerance in a mouse model of systemic lupus erythematosus. Am J Physiol Endocrinol Metab. 2012;303:E1313–24. doi: 10.1152/ajpendo.00665.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker B, Bruce I. SLE and metabolic syndrome. Lupus. 2013;22:1259–66. doi: 10.1177/0961203313502570. [DOI] [PubMed] [Google Scholar]
- 26.Parker B, Urowitz MB, Gladman DD, et al. Clinical associations of the metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Ann Rheum Dis. 2013;72:1308–14. doi: 10.1136/annrheumdis-2012-202106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung CP, Avalos I, Oeser A, Gebretsadik T, Shintani A, Raggi P, Michael Stein C. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: association with disease characteristics and cardiovascular risk factors. Ann Rheum Dis. 2007;66:208–14. doi: 10.1136/ard.2006.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 29.Mahaffey KW, Hafley G, Dickerson S, et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J. 2013;166:240–9. doi: 10.1016/j.ahj.2013.05.004. e1. [DOI] [PubMed] [Google Scholar]
- 30.Choi JH, Banks AS, Kamenecka TM, et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–81. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao W, Thacker SG, Hodgin JB, et al. The peroxisome proliferator-activated receptor γ agonist pioglitazone improves cardiometabolic risk and renal inflammation in murine lupus. J Immunol. 2009;183:2729–40. doi: 10.4049/jimmunol.0804341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 33.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 34.Ajuwon KM, Banz W, Winters TA. Stimulation with Peptidoglycan induces interleukin 6 and TLR2 expression and a concomitant downregulation of expression of adiponectin receptors 1 and 2 in 3T3-L1 adipocytes. J Inflamm (Lond) 2009;6:8. doi: 10.1186/1476-9255-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito Y, Fujioka D, Kawabata K, et al. Statin reverses reduction of adiponectin receptor expression in infarcted heart and in TNF-α-treated cardiomyocytes in association with improved glucose uptake. Am J Physiol Heart Circ Physiol. 2007;293:H3490–7. doi: 10.1152/ajpheart.00310.2007. [DOI] [PubMed] [Google Scholar]