Abstract
The histamine 4 receptor (H4R) is expressed primarily on cells involved in inflammation and immune responses. Despite much research into inflammatory diseases, no drugs with favourable safety profiles are yet available for their treatment. The aim of the present study was to determine the potential anti-inflammatory effect of 4-methylhistamine (4-MeH) or JNJ77777120 (JNJ) and to explore the role of H4R in a mouse model of carrageenan (Cg) -induced pleurisy. A single dose of 4-MeH or JNJ (30 mg/kg) was administered intraperitoneally 1 hr before Cg administration. The results illustrate that both the numbers of CD4+, CD25+, CD4+ CD25+, GITR+, GITR+ IL-17A+-expressing T cells and the levels of T helper type 1 (Th1)/Th17 cytokines were markedly increased in both the Cg-treated and 4-MeH-treated groups, whereas the cytokines produced by Th2 cells were significantly decreased in the same groups. However, JNJ treatment significantly decreased both the number of T-cell subsets and GITR+, GITR+ IL-17A+-expressing T cells, and the production of Th1/Th17 cytokines. Further, JNJ up-regulated the expression of the Th2 cytokines. RT-PCR analysis revealed an increased expression of interleukin-1β, tumour necrosis factor-α, monocyte chemoattractant protein-1 and intercellular adhesion molecule-1 in the Cg-treated and 4-MeH-treated groups, which was reduced by treatment with JNJ in lung tissues. Moreover, histological examinations revealed anti-inflammatory effects of JNJ, whereas 4-MeH worsened Cg-induced inflammation. In conclusion, the results of the present work clearly indicate that JNJ possesses important anti-inflammatory properties that are increased in 4-MeH-treated mice, suggesting that H4R are involved in pleurisy and that JNJ has an anti-inflammatory effect in associated disease conditions.
Keywords: λ-Carrageenan-induced inflammation, cytokines, GITR-expressing cells, histamine 4 receptor, JNJ77777120, 4-methylhistamine dihydrochloride
Introduction
Histamine is a biogenic amine that affects a variety of functions in the human body. Histamine has been shown to play a role in inflammation, gastric acid secretion and neurotransmission.1 Multiple receptors exist for histamine in mammalian tissues, and these receptors have been classified into four distinct receptor types (H1R, H2R, H3R and H4R), all of which are G-protein-coupled receptors.2 The H4R subtypes are distinct in terms of their pharmacology and molecular biology. In addition, these receptors have been implicated in different biological effects of histamine.3 The H4R, identified in 2000, mediates its effects by coupling to Gαi/o G-proteins and has low homology with the other histamine receptors, sharing only 35% amino acid identity with H3R and a much lower homology with H1R and H2R.4 This receptor has a distinct expression profile on immune cells, including mast cells, eosinophils, dendritic cells and T cells; in these cells, it modulates functions such as activation, migration and the production of cytokines and chemokines,5 suggesting that H4R plays a role in inflammatory and immune responses. Some studies have reported H4R expression on neutrophils and monocytes, but functional data are lacking.6 Additionally, H4R appears to play a role in pruritus and inflammatory bowel disease.7 A previous study suggested that H4R may be involved in the early phase of the acute inflammation induced by carrageenan (Cg) in rats.8
Few studies have examined the biological role of the H4R in intact animals by using the first selective H4R antagonist, JNJ7777120 (JNJ).9 In rats, a protective effect of JNJ was reported in a model of intestinal inflammation.10 Despite much research into inflammatory diseases, no drugs with favourable safety profiles are yet available for their treatment. For these reasons, the present study used a potent and selective H4 antagonist, JNJ, as well as a selective agonist, 4-methylhistamine (4-MeH), to explore the role of the H4R in pre-clinical inflammatory models.
There is no doubt that tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) are key cytokines in the development of pleural inflammation because they act to enhance IL-8 and monocyte chemoattractant protein-1 (MCP-1) production from mesothelial cells.11 In addition, studies using function-blocking antibodies suggest that activated resident macrophage could be responsible for this TNF-α and IL-1β secretion.11 T helper type 17 (Th17) cells secrete IL-17A, a pro-inflammatory cytokine that has been linked to the pathogenesis of autoimmune diseases and to immune responses to bacterial and fungal infections.12 Monocyte chemoattractant protein-1 is a chemokine that attracts monocytes and neutrophils both in vitro and in vivo, and it appears to play a key role in leucocyte migration by promoting the transition from leucocyte rolling to adhesion on the endothelial surface.13 The glucocorticoid-induced tumour necrosis factor receptor (GITR) is a co-activating receptor expressed in many components of the immune system, and its expression is increased following cellular activation.14
Carrageenan-induced pleurisy is a well-established model of acute inflammation15 and is characterized by a rapid influx of polymorphonuclear cells followed by mononuclear cell infiltration.16 This model is often used to assess the anti-inflammatory effects of pharmaceutical agents17 and to assess the in vivo importance of established inflammatory mediators.18 The aim of this study is to evaluate the effects of the H4R agonist 4-MeH and the H4R antagonist JNJ on Cg-induced pleurisy in mice. To characterize the role of H4R receptors in this model of acute inflammation, we assessed several end-points of the inflammatory response. Cells expressing CD4+ or CD25+ receptors (or both), expressing GITR+ or producing GITR+ IL-17A+ were analysed by flow cytometry in pleural exudate. Moreover, the levels of cytokines produced by IL-17A, Th1 and Th2 were analysed by an ELISA in pleural fluid following intrapleural Cg administration. We also studied the effects of 4-MeH and JNJ on IL-1β, TNF-α, intercellular adhesion molecule-1 (ICAM-1) and MCP-1 mRNA expression in lung tissues by RT-PCR. Moreover, a histological examination of lung tissues was performed.
Materials and methods
Chemicals
The 4-MeH and JNJ were obtained from Axon Medchem BV, Postbus, Groningen (the Netherlands). The FITC-labelled CD4 and IL-17A anti-mouse monoclonal antibody, allophycocyanin-labelled CD25 and phycoerythrin-labelled anti-GITR anti-mouse monoclonal antibody, FcR blocking reagent, FACS lysing and fixation/permeabilization and permeabilizing solutions were obtained from Miltenyi Biotec (Bergisch Gladbach, Germany). Heparin and Cg were obtained from Sigma Aldrich (St. Louis, MO), and the mouse Th1/Th2 and Th17 ELISA kit was obtained from Ray Biotech, Inc. (Norcross, GA). The primers used in the current study were obtained from Applied Biosystems (Paisley, UK). JNJ was dissolved in DMSO whereas 4-MeH and Cg were dissolved in saline. Control animals received equal amounts of DMSO in saline and the administered volumes were 0·01 ml per 1 g body weight.
Animals
Female adult BALB/c mice that were 6–7 weeks old and that weighed 20–22 g were obtained from the animal house of the College of Pharmacy in King Saud University, Riyadh. The mice were maintained at a room temperature of 22 ± 2° on a 12-hr/12-hr light/dark cycle and were housed in a specific pathogen-free environment and fed standard rodent chow and water ad libitum. All procedures were performed with the approval of the Institutional Animal Care and Use Committee.
Experimental design
The mice were acclimatized for 7 days and divided into four groups of six mice each, as follows: Control group, which received vehicles only [intraperitoneally (i.p.)]. Cg group, mice were subjected to Cg-induced pleural and lung inflammation. Cg + 4-MeH group, mice received a single dose of 4-MeH (30 mg/kg, i.p.) 1 hr before Cg-induced pleurisy. Cg + JNJ group, mice received a single dose of JNJ (30 mg/kg, i.p.) 1 hr before Cg-induced pleurisy. The doses of JNJ and 4-MeH were selected based on the results of the previous in vivo study.8
Carrageenan-induced pleurisy
Pleurisy was induced using λ-Cg as previously described.19 Pleurisy was induced by a single intrapleural injection of 0·1 ml sterile saline (NaCl 0·9%) containing λ-Cg (1%) on the right side of the chest. After pleurisy induction (4 hr), the animals were anaesthetized with an overdose of ether, the thorax was opened, and the pleural cavity was washed with 1·0 ml of sterile PBS (pH 7·6; composition: 130 mm NaCl, 5 mm Na2HPO4, 1 mm KH2PO4 and 1000 ml distilled water containing 20 IU/ml heparin). The fluid leakage and lung samples were collected for further determination of the T-cell subsets, GITR+, GITR+ IL-17A+, Th1/Th2 and the Th17 cytokine levels. The mRNA expression of IL-1β, TNF-α, ICAM-1 and MCP-1 was assessed in the lung tissues. All four parameters were analysed after the injection of Cg.
Determination of the number of CD4+, CD25+ and CD4+ CD25+ T cells in pleural exudate
After isolation, the pleural exudates were washed twice with PBS (pH = 7·4), and the number of cells in the suspension was standardized to 10 × 106 cells/ml. To stain for the superficial markers CD4 and CD25, 20 μl of the corresponding fluorescently labelled monoclonal antibodies was added to the cell suspension (CD4 FITC-labelled or CD25 allophycocyanin-labelled) and then incubated at room temperature for 20 min in the dark. The cells were washed twice with 2 ml PBS (pH = 7·4). The measurements were carried out on a Cytomics FC500 (Beckman Coulter, Brea, CA), which has a 488-nm wavelength laser and standard filters. The analysis of the obtained data was performed using Beckman Coulter CXP software.
Determination of the number of GITR+ and GITR+ IL-17A+ cells in pleural exudate
Samples of the pleural exudate (200 μl) were pipetted directly into a 12 × 75 mm fluorescence-activated cell-sorting tube containing 20 μl of monoclonal phycoerythrin-labelled anti-GITR and incubated at room temperature in the dark for 10 min. Then, 1% paraformaldehyde (0·5 ml) was added for 8 min to stabilize the monoclonal antibody–surface antigen complex. The red blood cells were lysed using 3 ml of FACS lysing solution (Miltenyi Biotech) for 8 min. After centrifugation at 300 g for 5 min, the supernatant was aspirated and a 1 × permeabilizing solution (500 μl; Miltenyi Biotec) was added to the pellet and incubated for 10 min at room temperature in the dark. After washing with 3 ml washing buffer, cytokine-specific antibodies (20 μl IL-17A FITC; Miltenyi Biotech) were added to the cells and incubated for 30 min at room temperature in the dark. After one final wash, the cells were resuspended in 1% paraformaldehyde (500 μl) and stored at 4° until flow cytometric analysis.
Quantification of Th1/Th2 and Th17 produced cytokines in pleural exudate
The pleural exudate samples obtained from all animals including the normal control were collected and immediately prepared for the analysis of cytokine levels. In this protocol, commercially available kits were used with monoclonal antibodies specific for each cytokine. The cytokine levels were measured with an ELISA kit according to the manufacturer's instructions (Ray Biotech, Inc.). Briefly, individual samples were added in triplicate to 96-well plates that had been coated with an anti-cytokine capture antibody. After reaction overnight, the plates were washed extensively, and the biotinylated anti-cytokine detection antibodies were then added followed by incubation at room temperature for 2 hr. The bound antibodies were detected using horseradish peroxidase-conjugated streptavidin and visualized using tetramethylbenzidine substrate followed by measuring the absorbance at 450 nm. The individual recombinant cytokines provided in the kits were used to establish standard curves.
RNA extraction and cDNA synthesis
All of the extraction procedures were performed on ice using ice-cold reagents. The total RNA from lung tissues from each mouse homogenate was isolated using TRIzol reagent (Invitrogen, Carslbad, CA). The isolation method was performed according to the manufacturer's instructions and RNA was quantified by measuring the absorbance at 260 nm. The RNA quality was determined by measuring the 260/280 ratio. The cDNA synthesis was performed using the high-capacity cDNA reverse transcription kit (Applied Biosystems) according to the manufacturer's instructions. Briefly, 1·5 μg of total RNA from each sample was added to a mixture of 2·0 μl of 10 × reverse transcriptase buffer, 0·8 μl of 25 × dNTP mix (l00 mm), 2·0 μl of l0 × reverse transcriptase random primers, 1·0 μl of multi-scribe reverse transcriptase and 3·2 μl of nuclease-free water. The final reaction mixture was kept at 25° for 10 min, heated to 37° for 120 min, heated to 85° for 5 seconds and finally cooled to 4°.
Quantification of mRNA expression in lung tissue by RT-PCR
The quantitative analysis of mRNA expression of the target genes was performed by RT-PCR by subjecting the cDNA generated by the above preparation to PCR amplification using 96-well optical reaction plates in the ABI Prism 7500 System (Applied Biosystems). The 25-μl reaction mixture contained 0·1 μl of 10 μm forward primer and 0·1 μl of 10 μm reverse primer (40 μm final concentration of each primer), 12·5 μl of SYBR Green Universal Mastermix, 11·05 μl of nuclease-free water and 1·25 μl of the cDNA sample. The primers used in the current study were chosen from the PubMed database and are listed in Table 1. No template controls were incorporated onto the same plate to test for the contamination of any assay reagents. The real-time PCR data were analysed using the relative gene expression (i.e. ΔΔCt) method, as described in Applied Biosystems User Bulletin No. 2. Briefly, the data are presented as the fold change in gene expression normalized to the endogenous reference gene (glyceraldehyde 3-phosphate dehydrogenase) and relative to a calibrator.
Table 1.
Primer sequences
| Gene | Direction and sequence |
|---|---|
| IL-1β | F:5′-TCGTGCTGTCGGACCCATAT-3′ R: 5′-GTCGTTGCTTGGTTCTCCTTGT-3′ |
| TNF-α | F: 5′-GCGGAGTCCGGGCAGGTCTA-3′ R: 5′-GGGGGCTGGCTCTGTGAGGA-3′ |
| ICAM-1 | F: 5′-GCGGAGTCCGGGCAGGTCTA-3′ R: 5′-GGGGGCTGGCTCTGTGAGGA-3′ |
| MCP-1 | F:5′-ACCACAGTCCATGCCATCAC-3′ R: 5′-TTGAGGTGGTTGTGGAAAAG-3′ |
| GAPDH | F: 5′-CCCAGCAAGGACACTGAGCAAG-3′ R: 5′-GGTCTGGGATGGAAATTGTGAGGG-3′ |
IL-1β, interleukin-1β; TNF-α, tumour necrosis factor-α; ICAM-1, intracellular cell adhesion molecule-1; MCP-1, monocyte chemotactic protein-1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Histological examination in lung tissues
Lung tissue samples were taken 4 hr after the injection of Cg. The samples were fixed for 1 week in buffered formaldehyde solution (10% in PBS) at room temperature, dehydrated with graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ). Tissue sections (thickness 7 μm) were deparaffinized with xylene, stained with haematoxylin and eosin and studied using light microscopy (Dialux 22 Leitz, Milan, Italy). The following morphological criteria were used for scoring: 0, normal lung; grade 3, minimal oedema or infiltration of alveolar or bronchiolar walls; grade 5, inflammatory cell infiltration with lobar lung pneumonia, alveolar septum and occasional obliterated alveoli with obvious damage to lung architecture; grade 7, severe inflammatory cell infiltration with obvious damage to lung architecture. All the histological studies were performed in a blinded fashion on a 0–10 scale to avoid scoring biases.
Data analysis
All data are presented as the mean ± SEM, and six animals are included in each group. The results were analysed by analysis of variance, followed by the Tukey–Kramer test. The level of statistical significance was set at P < 0·05 for differences between the pairs of compared groups.
Results
Effects of 4-MeH and JNJ on the flow cytometric analysis of T-cell subsets
The results revealed that both Cg and 4-MeH significantly increased the percentage of CD4+, CD25+ and CD4+ CD25+ T-cell subsets compared with the control group. JNJ significantly decreased all CD4+, CD25+ and CD4+ CD25+ T-cell subsets compared with either Cg or 4-MeH, but the values of the T-cell subsets were higher than those of the control group (Fig. 1). Representative dot plots for CD4+, CD25+ and CD4+ CD25+ are shown in the exudate from a mouse from each group at 4 hr (Fig. 2).
Figure 1.

Effect of 4-MeH and JNJ on CD4+, CD25+ and CD4+CD25+ levels. The levels were evaluated by flow cytometry in the exudate cells at 4 after the induction of pleurisy via Cg injection. Statistical analysis was performed using a one-way anova followed by the Tukey-Kramer post-test. Each value indicates the mean ± SEM of six animals. P < 0.05 is accepted as the level of significance; *P < 0.05 compared to the control group; aP < 0.05 compared to the Cg group; bP < 0.05 compared to the 4-MeH group.
Figure 2.
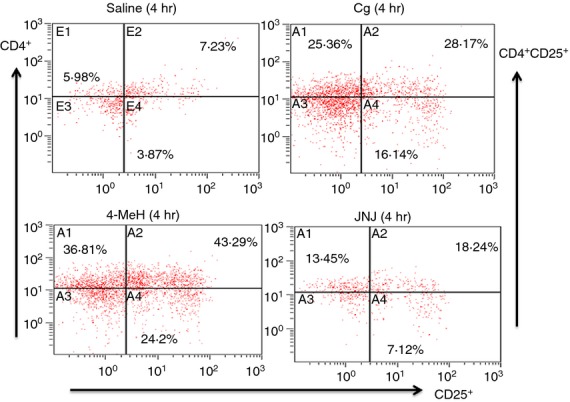
Representative dot plots for CD4+, CD25+ and CD4+ CD25+ in the exudate from a mouse from each group at 4 h.
Both Cg and 4-MeH significantly increased the total percentage of GITR+ and IL-GITR+ IL-17A+ expressing cells compared with the control group (Fig. 3). In contrast, JNJ caused a marked reduction in the total percentage of GITR+ and GITR+ IL-17A+-expressing cells compared with either Cg or 4-MeH (Fig. 3a,b). Representative dot plots are shown for GITR+ and GITR+ IL-17A+-expressing cells in the exudate from a mouse from each group at 4 hr (Fig. 4).
Figure 3.
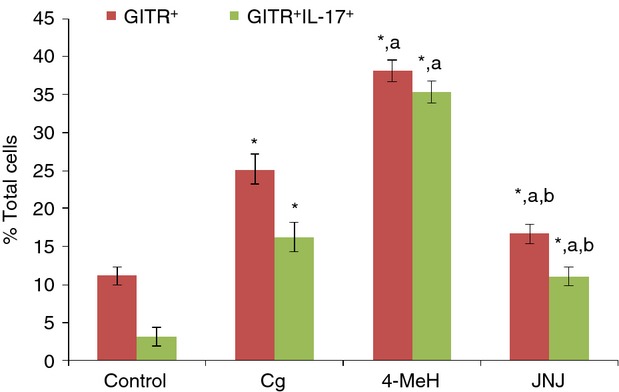
Effect of 4-MeH and JNJ on GITR+ and GITR+IL-17A+ levels. The levels were evaluated by flow cytometry using the exudate cells at 4 after the induction of pleurisy via Cg injection. Statistical analysis was performed using a one-way anova followed by the Tukey-Kramer post-test. Each value indicates the mean ± SEM of six animals. P < 0.05 is accepted as the level of significance; *P < 0.05 compared to the control group; aP < 0.05 compared to the Cg group; bP < 0.05 compared to the 4-MeH group.
Figure 4.

Representative dot plots for GITR+ and GITR+IL-17A+ cells in the exudate from a mouse from each group at 4 h.
Effects of 4-MeH and JNJ on Th1/Th2 and Th17 cytokine levels
An increase in the levels of IL-2, IL-6, IL-12, IL-17, IL-23 and interferon-γ (IFN-γ) in the exudates of animals following the induction of Cg-induced pleurisy was observed compared with the corresponding normal values in the control group. JNJ significantly decreased IL-2, IL-6 and IFN-γ cytokine levels; moreover, JNJ slightly reduced IL-12, IL-17 and IL-23 cytokine levels compared with Cg or 4-MeH, but their values were still higher than in the control group (Fig. 5a,b). Administration of Cg alone or before 4-MeH (to induce pleurisy) resulted in a significant decrease in transforming growth factor-β1 (TGF-β1) and IL-10 levels compared with the control group. JNJ increased both TGF-β1 and IL-10 cytokine levels compared with Cg or 4-MeH, and the levels were significantly higher than those of the control group (Fig. 6).
Figure 5.

Effect of 4-MeH and JNJ on the levels of pro-inflammatory cytokines (a) IL-2, IL-6, IL-12 and (b) IL-17A, IL-23, IFN-γ. The levels were evaluated by ELISA from the exudates at 4 after the induction of pleurisy by Cg injection. Statistical analysis was performed using a one-way ANOVA followed by the Tukey-Kramer post-test. Each value indicates the mean ± SEM of six animals. P < 0.05 is accepted as the level of significance; *P < 0.05 compared to the control group; aP < 0.05 compared to the Cg group; bP < 0.05 compared to the 4-MeH group.
Figure 6.

Effect of 4-MeH and JNJ on TGF-β1 and IL-10 anti-inflammatory cytokine levels. The levels were evaluated by ELISA in the exudates at 4 after the induction of pleurisy by Cg injection. Statistical analysis was performed using a one-way anova followed by the Tukey-Kramer post-test. Each value indicates the mean ± SEM of six animals. P < 0.05 is accepted as the level of significance; *P < 0.05 compared to the control group; aP < 0.05 compared to the Cg group; bP < 0.05 compared to the 4-MeH group.
Effect of 4-MeH and JNJ on mRNA expression
Figure 7(a,b) shows that Cg significantly increased IL-1β and TNF-α mRNA expression levels in lung tissues compared with the control group. 4-MeH treatment significantly increased the expression level of TNF-α mRNA compared with the Cg group. Treatment with JNJ decreased this expression. Furthermore, 4-MeH to some extent up-regulated IL-1β mRNA expression levels compared with the Cg group. The IL-1β mRNA expression levels exhibited a substantial decrease in the JNJ-treated group compared with the Cg- and 4-MeH-treated groups (Fig. 7a,b).
Figure 7.

Effect of 4-MeH and JNJ on the gene expressions of pro-inflammatory mediators (a) IL-1β and (b) TNF-α. mRNA expression was measured by quantitative RT-PCR in the lung tissue at 4 after the induction of pleurisy by Cg injection. Statistical analysis was performed using a one-way anova followed by the Tukey-Kramer post-test. Each value indicates the mean ± S.E.M. of six animals. P < 0.05 is accepted as the level of significance; *P < 0.05 compared to the control group; aP < 0.05 compared to the Cg group; bP < 0.05 compared to the 4-MeH group.
MCP-1 and ICAM-I chemokine expression was significantly induced following either Cg or 4-MeH treatment, but the signal was higher for 4-MeH than Cg (Fig. 8). JNJ completely prevented this induction by down-regulating MCP-1 and ICAM-I expression compared with the Cg- and 4-MeH-treated groups (Fig. 8).
Figure 8.
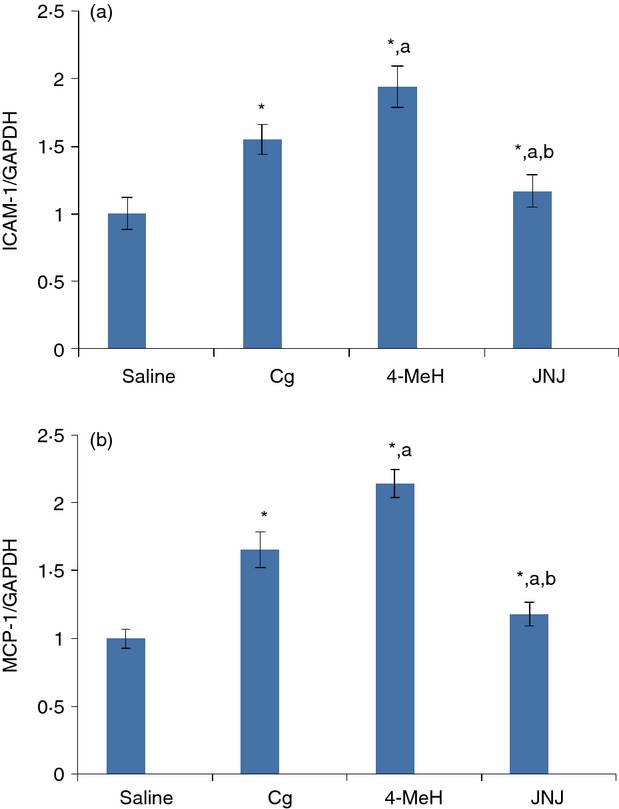
Effect of 4-MeH and JNJ on gene expression of the (a) ICAM-1 and (b) MCP-1 chemokines. mRNA expression was measured by quantitative RT-PCR in the lung tissue at 4 the induction of pleurisy by Cg injection. Statistical analysis was performed using a one-way anova followed by the Tukey-Kramer post-test. Each value indicates the mean ± SEM of six animals. P < 0.05 is accepted as the level of significance; *P < 0.05 compared to the control group; aP < 0.05 compared to the Cg group; bP < 0.05 compared to the 4-MeH group.
Effects of 4-MeH and JNJ on histological examination
Figure 9(a) shows a lung section of a normal mouse with a well-formed, patent and opened alveolar lumen with a normal looking alveolar septum. Figure 9(b, c) shows lung sections from a Cg-treated mouse with lobar lung pneumonia, a thickened alveolar septum and occasional obliterated alveoli. These sections also contain acute and inflammatory cells, interalveolar eosinophilic secretions, fibrils and extravagated red blood cells. Figure 9(d–f) shows lung sections from a mouse treated with 4-MeH after 4 hr. The overall tissue examination shows a worsened inflammatory process with larger areas of the lung tissues reflecting the features of red hepatization and congestive stages of pneumonia with dense scattered inflammation and limited areas of grey hepatization. Figure 9(g,h,j) shows sections from a mouse treated with JNJ after 4 hr. Figure 9(k) shows that JNJ reduced the degree of lung injury. The overall tissue examination shows a significant reduction in the inflammatory process, as illustrated by a marked decrease in the inflammatory areas that reflect the red hepatization or congestive stages of pneumonia with some dilated congested blood vessels, whereas the examination also revealed wider areas of mild inflammation and more areas with patent, opened alveoli. The histological score for Fig. 9(k) was made by an independent observer.
Figure 9.
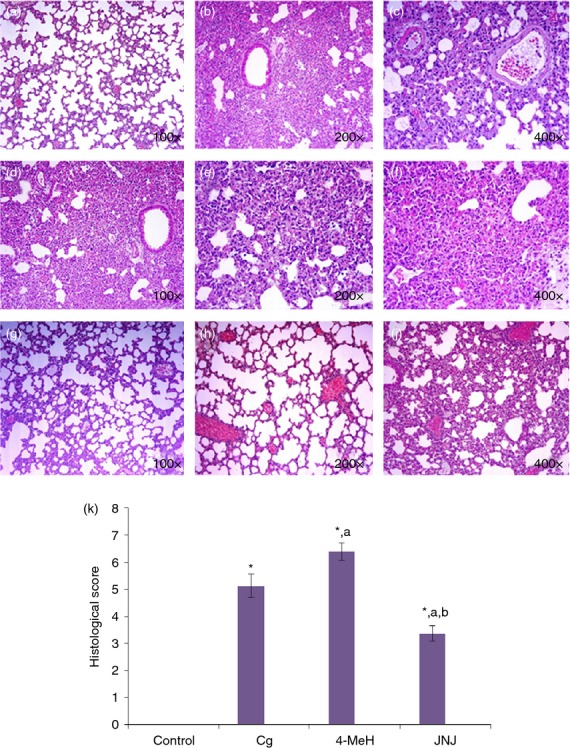
The effect of 4-MeH and JNJ on lung histology following 4 h of Cg administration in a mouse pleurisy model. (a) Shows a lung section of a normal mouse. (b,c) shows lung sections from a Cg treated mouse. (d,e,f) shows lung sections from a mouse treated with 4-MeH 4 h before Cg treatment. (g, h, j) shows sections from a mouse treated with JNJ 4 h before Cg treatment. The histological score (k) was made by an independent observer. Statistical analysis was performed using a one-way anova followed by the Tukey-Kramer post-test. Each value indicates the mean ± SEM of six animals. P < 0.05 is accepted as the level of significance; *P < 0.05 compared to the control group; aP < 0.05 compared to the Cg group; bP < 0.05 compared to the 4-MeH group.
Discussion
Histamine has long been known to mediate inflammatory and allergic responses by acting predominately through H1R, and H1R antagonists have been used to treat allergies for many years.20 Accumulating evidence from diverse in vivo and in vitro studies using animal models of diseases and from human biological samples substantiates the fundamental role of H4R in the histamine-induced chemotaxis of mast cells, eosinophils and other immune cells.21 In addition, the presence of the H4R in immune system organs and their immunomodulatory role in cytokine production22 argue for the pathophysiological significance of this receptor in inflammatory conditions that are characterized by an increase in immune cell numbers, such as asthma, allergic disorders and autoimmune diseases. This relationship suggests that the H4R contributes not only in the histamine-mediated initial inflammatory signal but also in the maintenance of inflammation.21,23 Consequently, the H4R is currently an attractive target for the pharmacological modulation of histamine signals in inflammatory conditions24 and for the development of beneficial therapeutic strategies for these conditions.25
Furthermore, the use of H4R targeting agents, including JNJ, the first highly selective H4R antagonist to be developed,26 and the potent selective agonist 4-MeH,27 has provided useful information on the physiological role of histamine receptors in inflammatory conditions, the amplification of allergic symptoms and maintaining chronic inflammation.26 In addition, JNJ is able to block histamine-induced leucocyte morphological changes as well as the up-regulation of the adhesion molecules, whereas H1, H2 and H3 selective receptor antagonists are not effective.28
Recently, Pini et al.29 have reported that histamine H4R antagonists protect against Cg-induced acute inflammation in rats by reduction of inducible nitric oxide synthase, cyclo-oxygenase-2, nitric oxide and pro-inflammatory prostaglandins. However, in our study, we evaluated the role that H4R can play in Cg-induced pleurisy using both agonist and antagonists of the receptor. In addition, our study investigates the immunological aspect of the modulation of Cg-induced pleurisy by H4R. Specifically, we showed that Cg caused an intense recruitment of T-cell subsets, GITR+ and GITR+ IL-17A+ cells and production of IL-17A and Th1 cytokines in pleural exudates. In addition, we demonstrated that Cg increased the mRNA expression of pro-inflammatory mediators in lung tissues. We also showed that the H4R antagonist JNJ markedly reduced all the above-mentioned parameters, revealing that H4R plays a critical role in Cg-induced pleurisy. This clearly suggests that H4R antagonists have anti-inflammatory effects and could have potential therapeutic applications for the treatment of inflammatory diseases.
Functionally, CD25 acts as the IL-2Rα chain;30 its expression is not specific to activated T cells, and it is also expressed by T regulatory cells and on activated B cells.31 It is well known that GITR plays a co-accessory role in effector T-cell activation, which is further potentiated by the inhibition of regulatory T-cell function.32 Interleukin-17A has been postulated to play a pivotal role in the inflammatory process because it is able to stimulate the synthesis of other pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and prostaglandins.33 Furthermore, IL-17A has been suggested as a critical mediator for neutrophil recruitment and migration.34
The present results demonstrate that the inflammatory process caused by the administration of Cg into the pleural cavity led to a substantial increase in the levels of Th1 (IL-2, IL-6, IL-12, IL-17A, IL-23, IFN-γ) and Th17 cytokines in the pleural exudate, and these levels were significantly lower in the exudates obtained from JNJ-treated animals, whereas JNJ stimulated the secretion of Th2 (TGF-β1 and IL-10) cytokines in the pleural exudates compared with the Cg- or 4-MeH-treated groups. The present study shows that the anti-inflammatory action of JNJ in the mouse model of pleurisy can be explained by the reduction of Th1 and Th17A levels concomitant with the increase in Th2 released cytokines at the site of inflammation.
To further clarify the mechanism of the preventive action of JNJ or 4-MeH on lung tissues, we examined the mRNA expression of inflammatory mediators in the lung tissue using RT-PCR. The mRNA expression levels of inflammatory mediators, such as IL-1β and TNF-α, were significantly increased in the presence of Cg or 4-MeH, whereas treatment with JNJ attenuated the mRNA transcription of IL-1β and TNF-α. The mRNA expression levels of cytokines and chemokines were increased after Cg challenge but were significantly inhibited in the group treated with JNJ compared with the Cg- or 4-MeH-treated groups. These results suggest that JNJ effectively modulates anti-inflammatory cytokines to accelerate the repair process from lung inflammation and inhibits the infiltration of inflammatory cells to the damaged area through the down-regulation of chemokines and cytokines. Our results show that JNJ not only induces its anti-inflammatory effect by readjusting the delicate balance among the pro-inflammatory and anti-inflammatory cytokines at the releasing level but also affects this cytokine balance at the gene expression level.
The results of our study highlight the complex network of cytokines that contribute to the initiation and maintenance of inflammation. Pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IFN-γ, are the key mediators of the inflammatory process, and their activation leads to inflammatory impairment.35 There is evidence that the pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and IFN-γ help to propagate the extension of a local or systemic inflammatory process.36These pro-inflammatory cytokines are related to the stimulation of cellular chemotaxis and induce the expression of adhesion molecules.37 Moreover, TNF-α, IL-1β, IL-6 and IFN-γ can also release pro-inflammatory mediators, such as nitric oxide, bradykinin, histamine and/or substance P, at the site of the inflammatory process.38 Activated inflammatory cells synthesize and secrete pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-17A.39 The suppression of these pro-inflammatory mediators has been found to reduce the severity of the inflammatory reaction.40 MCP-1 is a monocyte chemotactic factor that exerts potent and specific chemoattractant activity on both monocytes and neutrophils.13 Monocytes and, to a lesser extent, granulocytes secrete MCP-1 in response to cytokines, viruses, bacterial endotoxins and mitogens.41 Studies have also shown that cell influx into the pleural cavity occurs after the release of chemotactic cytokines by pleural mesothelial cells in response to other cytokines.42
Interleukin-10 is an anti-inflammatory cytokine with potent immunosuppressive properties. It also has strong immunosuppressive actions mediated through the down-regulation of pro-inflammatory cytokines, MHC class II molecules and T-cell-mediated inflammatory responses, including delayed hypersensitivity and Th2-driven allergic responses.42 It is likely that IL-10 plays a role during the early phases of pleural inflammation by mediating cell trafficking to the pleura and vascular leak.43 Intrapleural IL-10 inhibits the early phase of Cg-induced pleural inflammation in a murine model.43 Transforming growth factor-β1 plays a critical role in immune regulation. Typically, TGF-β1 behaves as a potent anti-inflammatory cytokine44 and suppresses the production of TNF-α and IL-1β.45 Moreover, our histological examination revealed anti-inflammatory effects of JNJ, whereas 4-MeH worsened the Cg-induced inflammation. Evidence is accumulating to suggest that H4R has a role in Cg-induced inflammation in the pleural and lung tissues.
Conclusion
In conclusion, this study suggests that the H4R may be involved in the early phase of acute inflammation induced by Cg in mice. Our results also highlight that histamine maintains inflammation in Cg-induced pleurisy through H4R activation, and blocking H4 with JNJ resulted in a marked anti-inflammatory effect.
Acknowledgments
The authors are grateful to the College of Pharmacy Research Centre and the Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia for financial support.
Disclosures
The authors declare that there is no conflict of interest.
References
- 1.Passani MB, Giannoni P, Bucherelli C, Baldi E, Blandina P. Histamine in the brain: beyond sleep and memory. Biochem Pharmacol. 2007;73:1113–22. doi: 10.1016/j.bcp.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Schneider E, Rolli-Derkinderen M, Arock M, Dy M. Trends in histamine research: new functions during immune responses and hematopoiesis. Trends Immunol. 2002;23:255–63. doi: 10.1016/s1471-4906(02)02215-9. [DOI] [PubMed] [Google Scholar]
- 3.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Wilson SJ, Kuei C, Lovenberg TW. Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther. 2001;299:121–30. [PubMed] [Google Scholar]
- 5.De Esch IJ, Thurmond RL, Jongejan A, Leurs R. The histamine H4 receptor as a new therapeutic target for inflammation. Trends Pharmacol Sci. 2005;26:462–9. doi: 10.1016/j.tips.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Morse KL, Behan J, Laz TM, West RE, Jr, Greenfeder SA, Anthes JC. Cloning and characterization of a novel human histamine receptor. J Pharmacol Exp Ther. 2001;296:1058–66. [PubMed] [Google Scholar]
- 7.Dunford PJ, Williams KN, Desai PJ, Karlsson L, McQueen D, Thurmond RL. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119:176–83. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Coruzzi G, Adami M, Guaita E, de Esch IJ, Leurs R. Anti-inflammatory and antinociceptive effects of the selective histamine H4-receptor antagonists JNJ7777120 and VUF6002 in a rat model of carrageenan-induced acute inflammation. Eur J Pharmacol. 2007;563:240–4. doi: 10.1016/j.ejphar.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Jablonowski JA, Grice CA, Chai W, Dvorak CA, Venable JD, Kwok AK. The first potent and selective non-imidazole human histamine H4 receptor antagonists. J Med Chem. 2003;46:3957–60. doi: 10.1021/jm0341047. [DOI] [PubMed] [Google Scholar]
- 10.Varga C, Horvath K, Berko A, Thurmond RL, Dunford PJ, Whittle BJ. Inhibitory effects of histamine H4 receptor antagonists on experimental colitis in the rat. Eur J Pharmacol. 2005;522:130–8. doi: 10.1016/j.ejphar.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 11.Park JS, Kim YS, Jee YK, Myong NH, Lee KY. Interleukin-8 production in tuberculous pleurisy: role of mesothelial cells stimulated by cytokine network involving tumour necrosis factor-α and interleukin-1β. Scand J Immunol. 2003;57:463–9. doi: 10.1046/j.1365-3083.2003.01201.x. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of Th17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christopherson K, Hromas R. Chemokine regulation of normal and pathologic immune responses. Stem Cells. 2001;19:388–96. doi: 10.1634/stemcells.19-5-388. [DOI] [PubMed] [Google Scholar]
- 14.Hanabuchi S, Watanabe N, Wang YH, et al. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL) Blood. 2006;107:3617–23. doi: 10.1182/blood-2005-08-3419. [DOI] [PubMed] [Google Scholar]
- 15.Murai N, Nagai K, Fujisawa H, Hatanaka K, Kawamura M, Harada Y. Concurrent evolution and resolution in an acute inflammatory model of rat carrageenan-induced pleurisy. J Leukoc Biol. 2003;73:456–63. doi: 10.1189/jlb.1002502. [DOI] [PubMed] [Google Scholar]
- 16.Harada Y, Hatanaka K, Kawamura M, et al. Role of prostaglandin H synthase-2 in prostaglandin E2 formation in rat carrageenan-induced pleurisy. Prostaglandins. 1996;51:19–33. doi: 10.1016/0090-6980(95)00168-9. [DOI] [PubMed] [Google Scholar]
- 17.Cuzzocrea S, Pisano B, Dugo L, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-γ, reduces acute inflammation. Eur J Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 18.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 2004;18:489–98. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 19.Talero E, Di Paola R, Mazzon E, Esposito E, Motilva V, Cuzzocrea S. Anti-inflammatory effects of adrenomedullin on acute lung injury induced by Carrageenan in mice. Mediators Inflamm. 2012;2012:717851. doi: 10.1155/2012/717851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–78. [PubMed] [Google Scholar]
- 21.Dunford PJ, O'Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176:7062–70. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- 22.Gutzmer R, Diestel C, Mommert S, Kother B, Stark H, Wittman M. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol. 2005;174:5224–32. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Thurmond RL, Dunford PJ. The histamine H4 receptor: a novel modulator of inflammatory and immune disorders. Pharmacol Ther. 2007;113:594–606. doi: 10.1016/j.pharmthera.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Bäumer W, Wendorff S, Gutzmer R, Werfel T, Dijkstra D, Chazot P. Histamine H4 receptors modulate dendritic cell migration through skin-immunomodulatory role of histamine. Allergy. 2008;63:1387–94. doi: 10.1111/j.1398-9995.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 25.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 26.Thurmond RL, Desai PJ, Dunford PJ, et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J Pharmacol Exp Ther. 2004;309:404–13. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- 27.Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther. 2005;314:1310–21. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- 28.Ling P, Ngo K, Nguyen S, Thurmond RL, Edwards JP, Karlsson L, Fung-Leung WP. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br J Pharmacol. 2004;142:161–71. doi: 10.1038/sj.bjp.0705729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pini A, Somma T, Formicola G, Lucarini L, Bani D, Thurmond R, Masini E. Effects of a selective histamine H4R antagonist on inflammation in a model of carrageenan-induced pleurisy in the rat. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990553. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Ferenczi K, Burack L, Pope M, Krueger JG, Austin LM. CD69, HLA-DR and the IL-2R identify persistently activated T cells in psoriasis vulgaris lesional skin: blood and skin comparisons by flow cytometry. J Autoimmun. 2000;14:63–78. doi: 10.1006/jaut.1999.0343. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 32.Nocentini G, Riccardi C. A multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005;35:1016–22. doi: 10.1002/eji.200425818. [DOI] [PubMed] [Google Scholar]
- 33.Hurst SD, Muchamuel T, Gorman DM, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Huang AL, Vergis H, et al. IL-17 produced by neutrophils regulates IFN-γ-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–42. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao L, Tao JY, Zhang SL, Pang R, Jin F, Dong JH, Guo YJ. Inner anti-inflammatory mechanisms of petroleum ether extract from Melilotus suaveolens Ledeb. Inflammation. 2007;30:213–23. doi: 10.1007/s10753-007-9039-x. [DOI] [PubMed] [Google Scholar]
- 36.Alonzi T, Fattori E, Cappelletti M, Ciliberto G, Poli V. Impaired STAT3 activation following localized inflammatory stimulus in IL-6 deficient mice. Cytokine. 1998;10:13–8. doi: 10.1006/cyto.1997.0250. [DOI] [PubMed] [Google Scholar]
- 37.Borish LC, Steinke JW. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:460–75. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 38.Menegazzi M, Di Paola R, Mazzon E, Genovese T, Crisafulli C. Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol Res. 2008;58:22–31. doi: 10.1016/j.phrs.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 40.Stow JL, Low PC, Offenhauser C, Sangermani D. Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology. 2009;214:601–12. doi: 10.1016/j.imbio.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Wuyts A, Proost P, Put JP, Lenaerts L, Paemen L, van Damme J. Leukocyte recruitment by monocyte W. chemotactic proteins (MCPs) secreted by human phagocytes. J Immunol Methods. 1994;174:237–47. doi: 10.1016/0022-1759(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 42.Cuzzocrea S, Mazzon E, Calabro G, Dugo L, Sarro A, Loo FAJV, Achille PC. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am J Respir Crit Care Med. 2000;162:1859–66. doi: 10.1164/ajrccm.162.5.9912125. [DOI] [PubMed] [Google Scholar]
- 43.Oberholzer A, Oberholze C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30(Suppl. 1):S58–63. [PubMed] [Google Scholar]
- 44.Frode TS, Souza GE, Calixto JB. The effects of IL-6 and IL-10 and their specific antibodies in the acute inflammatory responses induced by carrageenan in the mouse model of pleurisy. Cytokine. 2002;17:149–56. doi: 10.1006/cyto.2001.0980. [DOI] [PubMed] [Google Scholar]
- 45.Kelley J. Transforming growth factor-β. In: Kelley J, editor. Cytokines of the Lung. New York: Marcel Dekker; 1993. pp. 101–37. [Google Scholar]


