Abstract
The in vivo or in vitro formation of IgG4 hybrid molecules, wherein the immunoglobulins have exchanged half molecules, has previously been reported under experimental conditions. Here we estimate the incidence of polyclonal IgG4 hybrids in normal human serum and comment on the existence of IgG4 molecules with different immunoglobulin light chains. Polyclonal IgG4 was purified from pooled or individual donor human sera and sequentially fractionated using light-chain affinity and size exclusion chromatography. Fractions were analysed by SDS–PAGE, immunoblotting, ELISA, immunodiffusion and matrix-assisted laser-desorption mass spectrometry. Polyclonal IgG4 purified from normal serum contained IgG4κ, IgG4λ and IgG4κ/λ molecules. Size exclusion chromatography showed that IgG4 was principally present in monomeric form (150 000 MW). SDS–PAGE, immunoblotting and ELISA showed the purity of the three IgG4 samples. Immunodiffusion, light-chain sandwich ELISA and mass spectrometry demonstrated that both κ and λ light chains were present on only the IgG4κ/λ molecules. The amounts of IgG4κ/λ hybrid molecules ranged from 21 to 33% from the five sera analysed. Based on the molecular weight these molecules were formed of two IgG4 heavy chains plus one κ and one λ light chain. Polyclonal IgG (IgG4-depleted) was similarly fractionated according to light-chain specificity. No evidence of hybrid IgG κ/λ antibodies was observed. These results indicate that hybrid IgG4κ/λ antibodies compose a substantial portion of IgG4 from normal human serum.
Keywords: Fab-arm exchange, IgG4, kappa, lambda, polyclonal
Introduction
Immunoglobulin G4 is the least abundant IgG subclass in human plasma (approximately 5%) but has a 100-fold normal concentration range (0·01–2·1 g/l).1 IgG4 constant regions show 95% amino acid sequence similarity to the other IgG subclasses. However, they have very weak affinity to bind to Fcγ receptors, activate complement or cross-link antigens in vitro.2–4 IgG4 has been implicated in a range of pathological conditions4 including pemphigus and autoimmune pancreatitis,3 where raised serum levels are helpful for diagnosis.5
The classic antibody paradigm is that a single mature plasma cell produces one type of immunoglobulin heavy chain (G, A, M, D and E in humans) and one type of immunoglobulin light chain (κ or λ). These are combined within the cell to produce a tetrameric molecule composed of two identical heavy chains and two identical light chains. Within the last 10 years it has been shown that human IgG4 molecules are dynamic and can exchange half molecules in vitro to become bi-specific (monovalent) antibodies.2,6–11 This process involves the swapping of a heavy–light chain pair (half antibody) between different IgG4 molecules, possibly reforming new disulphides in the hinge region but without disruption of the heavy–light chain disulphide bond.12 Hence, an asymmetrical or hybrid immunoglobulin with two different antigen-binding domains can be produced.13 A serine residue at position 228 in the core hinge of IgG4 that allows the formation of intra-chain rather than inter-chain disulphides,12,14 and an arginine at position 409 within the CH3 domain,15–17 have been implicated in the control of this mechanism. Alternative amino acids at both of these positions, as found in other IgG subclasses and in low abundance in IgG4 isoallotype molecules,18 abolish or reduce the exchange process.9,16 Glutathione reduction in vitro can catalyse this process,7,10,12 which has perhaps controversially been termed Fab-arm rather than half-molecule exchange.19,20 It has been proposed that half-molecule exchange may have a physiological role as naturally produced bi-specific molecules cannot cross-link antigen or elicit lymphoid responses, and this may dampen inflammatory responses.7,21 The in vivo location of the half-molecule exchange process has not been determined, but it is likely to be related to local redox conditions in the blood or cell surfaces.7,12
The majority of the previous work on IgG4-exchange has been obtained with purified monoclonal or antigen-specific IgG4 molecules spiked into human or animal plasma. The aim of this work was to discover the extent of polyclonal IgG4 which undergoes half-molecule exchange and whether this process is immunoglobulin light-chain independent.
Materials and methods
Analytical purification and fractionation of polyclonal IgG4
Human serum from pooled or single healthy donors containing 30 mg IgG4 was loaded onto a 10 ml Capture Select IgG4 (Hu) Affinity Matrix (Life Technologies, Paisley, UK) column with a linear flow rate of 15 cm/hr in fully preserved PBS (25 mm NaH2PO4, 150 mm NaCl, 5 mm EDTA, 0·1% NaN3, 0·1% εACA, 0.02% benzamidine; pH 7). The column was eluted with 0·1 m glycine pH 3 at 30 cm/hr, and the fractions were immediately neutralized with 1/10 volume 2 m Tris–HCl, 1·5 m NaCl, pH 9·0. Eluted material (pure IgG4) was buffer exchanged into fully preserved PBS. The pure IgG4 was loaded onto a 5 ml Capture Select LC-λ (Hu) Affinity Matrix (Life Technologies) column under the same conditions. The unbound material was collected. The eluted material was neutralized and buffer exchanged into fully preserved PBS by dialysis, before loading onto a 5-ml Capture Select LC-κ (Hu) Affinity Matrix (Life Technologies) column under the same conditions as described above. The eluted material containing both IgG4κ and IgG4λ was buffer exchanged into fully preserved PBS by dialysis. Chromatography was performed using automated AKTA Purifier or AKTA Prime Plus systems and XK series columns (GE Healthcare, Chalfont St Giles, UK). All buffers were filtered off-line using 0·45-μm filters (Millipore, Watford, UK).
High-performance liquid chromatography size-exclusion chromatography
IgG4κ/λ was fractionated on a TSK Gel G3000SW column (7·5 × 600 mm, 10 μm; Tosoh Bioscience, Redditch, UK) equilibrated: with 50 mm NaH2PO4, 300 mm NaCl, 0·1% NaN3; pH 7 at 0·5 ml/min. Fractions were collected and analysed by SDS–PAGE and Coomassie Brilliant Blue staining. Fractions containing monomeric IgG4 were pooled. Chromatography was performed using a GILSON HPLC system with UV detection, controlled by clarity software.
Sandwich ELISA analysis
Ninety-six-well microplates (Greiner Bio-One, Stonehouse, UK) were coated with 100 μl per well of either anti-total κ light-chain polyclonal antibody or anti-total λ light-chain polyclonal antibody (The Binding Site, Birmingham, UK), both at 5 μg/ml in PBS-azide (25 mm NaH2PO4, 150 mm NaCl, 0·1% NaN3; pH 7). Plates were incubated overnight at 4° in a moist box. After washing three times with TBS-Tween (20 mm Trizma Base, 500 mm NaCl, 0·05% v/v Tween-20, pH 7·5), the plates were blocked with 300 μl per well BSA-TBS-Tween (1% w/v BSA in TBS-Tween) for 1·5 hr at 37°. The plates were washed with TBS-Tween and probed with 100 μl per well of a tripling serial dilution of the appropriate immunoglobulin sample for 1 hr at 37°. Top concentration of the sample was 1000 ng/ml. Dilutions were made in 1% BSA-TBS-Tween. After washing, the plates were re-probed with 100 μl per well of either sheep anti-κ or anti-λ light-chain horseradish peroxidase (HRP) -conjugated antibodies (1 : 2500; The Binding Site) for 1 hr at 37°. The plates were washed once more and developed with 100 μl per well TMB substrate (BioFX; SurModics, Eden Prairie, MN). After 10 min of development, the reaction was stopped with 50 μl per well H3PO4. Absorbance was read at 450 nm using a Thermo Multiskan EX plate reader.
Direct ELISA
ELISA 96-well microplates (Greiner Bio-One) were coated with 100 μl per well of the protein of interest at 1 μg/ml in PBS-azide. Plates were incubated overnight at 4°. After washing three times with TBS-Tween, the plates were blocked with 300 μl per well BSA-TBS-Tween for 1 hr at 37°. The plates were washed and probed with 100 μl per well of a tripling serial dilution (0–1000 ng/ml) of the appropriate HRP-conjugated antibody. Dilutions were made in BSA-TBS-Tween. After washing with TBS-Tween, the plates were developed with 100 μl per well TMB substrate, as described above. The IgG capture ELISA was performed to essentially the same protocol, except serially diluted IgG4 proteins were incubated in microplates pre-coated with anti-total IgG antisera (1 μg/ml; The Binding Site), followed by detection with HRP-labelled antibodies against human IgG4, κ or λ light chains.
Analytical procedures
Protein concentration
Protein concentration determination was measured by absorbance at 280 nm (using E0·1% = 1·45; Jenway 7305 UV/Vis spectrophotometer; Bibby-Scientific, Stone, UK) or by the Bicinchoninic acid assay procedure (Gamma globulin std.; Thermo-Pierce, Cranlington, UK). Total IgG, IgG4, Hevylite IgGκ and IgGλ concentrations were determined using commercially available turbimetric immunoassays (Binding Site Group Limited) on an SPA+ analyser. These assays were calibrated against the plasma protein reference standard CRM470 or Da470k.
Electrophoresis and blotting
SDS–PAGE was performed using NuPAGE Novex 10% Bis-Tris Gels and MOPS running buffers as per the manufacturer's instructions (Life Technologies). Samples were prepared in NuPAGE LDS Sample Buffer (Life Technologies). For Western blotting, 500 ng total protein was loaded per lane. For Coomassie Brilliant Blue staining, 1 μg total protein was loaded per lane. Gel staining was performed by fixing gels for 1 hr at room temperature (40% v/v ethanol, 10% v/v acetic acid, 50% distilled H2O) followed by staining for 1 hr at room temperature (0·1% w/v Coomassie Brilliant Blue, 20% v/v ethanol, 10% v/v acetic acid, 70% distilled H2O). Excess stain was removed by incubating for 1 hr at room temperature in de-stain solution (15%v/v industrial methylated spirit, 7·5% v/v acetic acid, 77·5% v/v distilled H2O). Polypeptides were Western blotted onto nitrocellulose membranes (Whatman Protran BA85; GE Healthcare), air dried, and subsequently blocked with 5% milk powder or 2% BSA in TBS-Tween. Western blots were probed with sheep anti-κ or anti-λ light-chain HRP-conjugated antibodies or HRP-conjugated mouse anti-IgG4 monoclonal antibody (clone HP6025;The Binding Site). All antibodies were diluted 1 : 5000 in 2% BSA in TBS-Tween. Western blots were probed for 90 min at room temperature before being developed using a standard AEC (3-amino-9-ethylcarbazole; Sigma, Poole, UK) method.22
Ouchterlony double diffusion was performed as described by Bailey.23 Equal amounts of IgG4 samples were diffused against titre-matched monospecific light-chain antisera (Dako, Ely, UK). Mass-spectrometric analysis was performed using 1 μl of sample (0·5–1 mg/ml concentration) by matrix-assisted laser-desorption time of flight mass spectrometry (MALDI-TOF MS) using a Bruker Ultraflextreme instrument in linear mode using sinapinic acid as the matrix. Samples were either analysed native or reduced with 10 mm dithiothreitol (DTT) for 5 min at room temperature.
Results
Immunoglobulin IgG4 was purified from a pooled normal human serum by affinity chromatography and sequentially fractionated into IgG4κ or IgG4λ (Fig. 1). Some of this IgG4 bound to both light-chain affinity columns. Repeated chromatography using the light-chain affinity columns did not correct this, indicating that column capacity was not responsible for this observation. Subsequent fractionation by HPLC size-exclusion chromatography identified principally immunoglobulin monomers (70–80%) and a lower level of aggregates of higher molecular weight (> 150 000) in this IgG4κ/λ fraction.
Figure 1.
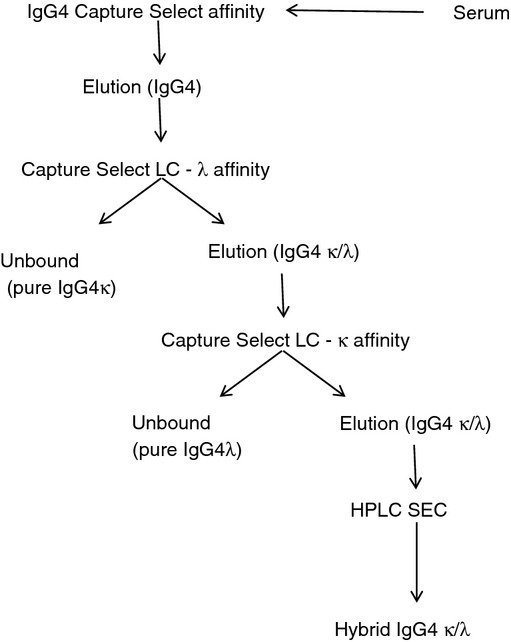
Fractionation of serum polyclonal IgG4 into IgG4κ, IgG4λ and hybrid IgG4κ/λ monomers. Polyclonal IgG4 was purified from a pooled human plasma source by affinity chromatography and sequentially fractionated into either IgG4κ or IgG4λ. A substantial portion of purified IgG4 possessed both κ and λ light chains and was further fractionated by HPLC-size-exclusion chromatography.
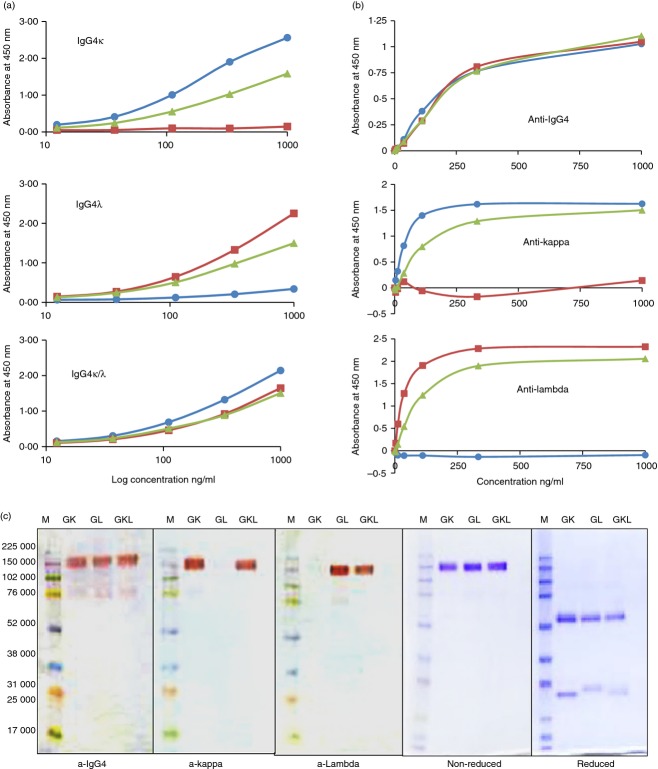
The purity of the three different IgG4 samples (IgG4κ, IgG4λ, IgG4κ/λ) was assessed by ELISA and SDS–PAGE immunoblotting. Monospecific antibodies different to those used during the purification process were employed to rule out idiotypic false positives. Direct antigen-binding (Fig. 2a) and IgG-capture ELISAs (Fig. 2b) both showed that only the IgG4κ/λ hybrid reacts with anti-κ, anti-λ and anti-IgG4 antisera. Whereas no contamination of opposing light-chain molecules were detectable in either the IgG4κ or IgG4λ preparations. Coomassie blue-stained SDS–PAGE gels showed that each sample from the IgG4κ/λ fraction was composed of only monomeric immunoglobulin of 150 000 that could be separated into only heavy and light chains upon reduction (Fig. 2c). The different mobility of reduced κ and λ light chains from the IgG4κ/λ sample indicated that both κ and λ were present. Interestingly only minor amounts of 75 000 IgG4 half-molecules were observed on non-reduced gels (Fig. 2c). This indicates that the inter-heavy-chain disulphides were probably intact and only minor amounts of non-disulphide-linked IgG4 molecules were present.
Figure 2.
Purity and light-chain specificity of purified polyclonal IgG4κ, IgG4λ and IgG4κ/λ hybrids. (a) Direct ELISA; purified IgG4 protein were bound to ELISA plates and probed with serially diluted antisera against IgG4 (▲), total light chain κ (•) or λ (▪).(b) IgG-capture ELISA; serially diluted purified IgG4κ (•), IgG4λ (▪) and IgG4κ/λ (▲) were captured by anti-IgG-coated microplates and probed with antisera against IgG4, total light-chain κ or λ, as indicated. These graphs are representative figures for all the different IgG4 pools. (c) IgG4 purity analysed by Western blotting and Coomassie stain. 0·5 μg protein/lane was analysed non-reduced by SDS–PAGE and immunoblotted with antisera against IgG4, total light-chain κ or λ as indicated. One microgram was loaded either reduced or non-reduced and stained with Coomassie blue.
The light-chain capture sandwich ELISA showed that only the IgG4κ/λ hybrid molecules could be both captured and detected by antisera against both light chains. In contrast, polyclonal IgGκ and IgGλ (IgG4-depleted) could only be captured and detected by antisera against the same light chain (Fig. 3a). A similar result was observed with IgG4κ and IgG4λ (data not shown). When both the capture and detection antibodies had the same light-chain specificity a lower signal strength was observed with the IgG4κ/λ hybrid molecules. When the capture antibody was different to the detecting antibody the signal strength was higher.
Figure 3.
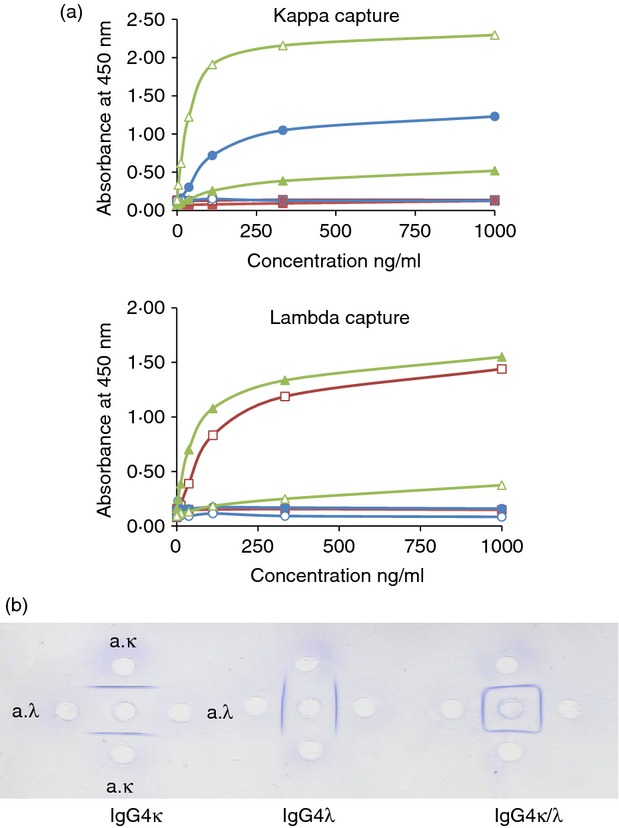
(a) Sandwich-ELISA of IgGκ (•, ○), IgGλ (▪, □) and IgG4κ/λ hybrids (▲, △). Serially diluted and purified IgG immunoglobulins were captured by ELISA plates coated with either anti-total κ or anti-λ antisera. The bound proteins were then detected with horseradish peroxidase-labelled antisera against the same or opposite light chain (anti-κ, •▪▲; anti-λ ○□△). (b) Ouchtolony double-immunodiffusion. Equal amounts of IgG4 antigens (centre well) were diffused against titre-matched monospecific antisera against κ (upper and lower wells) and λ (left and right wells) for 24 hr. Precipitation arcs were visualized with Coomassie blue. These figures are representative of those obtained for all the different IgG4 pools.
Ouchterlony double immunodiffusion can be used to compare different antigen preparations. If several different antigens are present in a solution then multiple precipitation lines will result. A single line of precipitation or identity was observed when IgG4κ or IgG4λ was precipitated by anti-κ or anti-λ antisera, respectively (Fig. 3b). In contrast, a square pattern of identity was seen when the IgG4 hybrid sample was similarly analysed (Fig. 3b). This indicated that the hybrid IgG4 molecule is immunologically identical to both light-chain antisera.
The turbimetric Hevylite™ immunoassay (Binding Site, Birmingham, UK) is designed to recognize the junctional epitopes between an immunoglobulin heavy and light chain, so can accurately distinguish between human immunoglobulins with different light-chain usage.24 The Hevylite assay gives a positive value with both Gκ and Gλ assays for only the IgG4 hybrid molecules (see Supporting information, Table S1).
Mass spectrometric analysis of the native proteins showed a slight difference in the average molecular mass between the three IgG4 preparations (Fig. 4a). These were 148 093 and 150 029 MW for IgG4λ and IgG4κ, respectively. The IgG4κ/λ hybrid possessed an average m/z of 148 995 MW, which was approximately equidistant from the other two. Intact IgG is a large molecule to analyse by mass spectrometry and because polyclonal and glycosylated forms of IgG4 were used the mass ranges obtained are quite broad. Reduction with DTT split the molecule into its constituent heavy and light chains. MALDI-TOF MS of the resultant light chains distinguished clearly between IgG4κ and IgG4λ (Fig. 4b). Both κ and λ light chains were present in the IgG4κ/λ hybrid sample, but only κ or λ were found in IgG4κ and IgG4λ.
Figure 4.
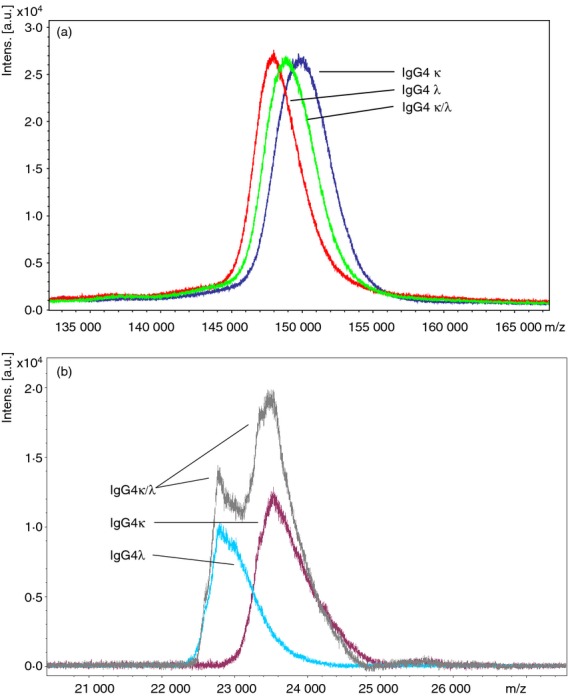
Matrix-assisted laser-desorption time of flight mass spectrometry (MALDI-TOF MS) analysis of serum polyclonal IgG4κ, IgG4λ and IgG4κ/λ. Immunoglobulins were analysed native and intact (a) or following reduction with 10 mm dithiothreitol (DTT), (b). The proteins were analysed by MALDI-TOF-MS using a Bruker Ultraflextreme instrument in linear mode and using sinapinic acid as the matrix. These figures are representative for all the different IgG4 pools.
The analytical extraction procedure (Fig. 1) was repeated with serum from another donor pool and a further three individual healthy donors. In all these sera the polyclonal IgG4 could be split into three fractions based on light-chain composition (Table 1). These samples were essentially pure (Fig. 2c), so a total protein assay was employed to determine their relative composition. The percentage composition of IgG4κ/λ chain hybrids ranged from 21·6 to 32·7% (median 30·12%), with approximately 5–15% IgG4λ and 55–75% IgG4κ (Table 1). To show that these results were not due to the particular immuno-affinity (light chain) columns employed, another sample of serum Pool 1 (Pool 1-L) was analytically fractionated using an alternative κ-binding matrix (Hi-Trap-Protein L). A very similar IgG4 fractionation composition was obtained (Table 1). Polyclonal IgG depleted of IgG4 was also purified and fractionated according to light-chain specific chromatography. No evidence of light-chain hybrid immunoglobulins was observed either during the process (data not shown) or when these were analysed by the light-chain capture ELISA (Fig. 3a).
Table 1.
Composition of polyclonal IgG4κ, IgG4λ and hybrid light-chain IgG4 in normal human sera
| Percentage composition (%) | ||||
|---|---|---|---|---|
| Sample | IgG4 concentration (mg/l) | IgG4κ | IgG4λ | IgG4 κ/λ hybrid |
| Pool 1 | 1494 | 58·6 | 12·9 | 28·6 |
| Pool 1 (L) | 1494 | 58·54 | 14·25 | 27·12 |
| Pool 2 | 284 | 57·40 | 10·97 | 31·63 |
| Single donor 3 | 729 | 56·25 | 11·74 | 32·01 |
| Single donor 4 | 527 | 73·65 | 4·7 | 21·65 |
| Single donor 5 | 534 | 55·54 | 11·78 | 32·68 |
| Median | N/A | 57·97 | 11·76 | 30·12 |
The analytical results indicated that IgG4 proteins containing different light chains on the same molecule were present. Based on molecular weight, these molecules were formed of two IgG4 heavy chains (presumably non-identical) plus one κ and one λ light chain (Fig. 5).
Figure 5.
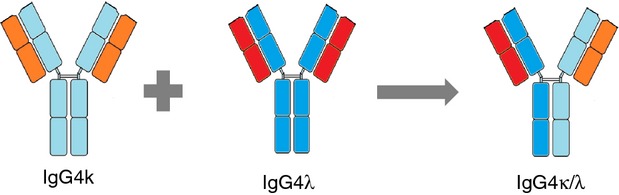
Schematic cartoon of IgG4 half-molecule exchange.
Discussion
We have shown that hybrid IgG4κ/λ antibodies are present in normal serum alongside IgG4 molecules with only a single light-chain type. There is currently no serological assay to differentiate between mixed and single light-chain IgG4 molecules in serum. We developed an analytical–extraction procedure to overcome this discrepancy. The extraction process reported here produced very pure samples of IgG4 without any other plasma proteins present. The sequential binding of IgG4 molecules to both anti-κ and anti-λ affinity columns could be due to aggregation of IgG4κ and IgG4λ, or because half-molecule exchange has resulted in hybrid IgG4κ/λ molecules. Immunoglobulin aggregation can be induced during an affinity-protein purification process by the elution conditions employed.25 We observed such aggregates but they comprised only a minor component of the hybrid IgG4κ/λ preparations and were removed by size-exclusion chromatography. Only monomeric immunoglobulins were indicated by the SDS–PAGE analysis (Fig. 2c). This suggested that the monomeric IgG4 immunoglobulins probably possessed both κ and λ light chains on the same molecule. A combination of ELISA, immunoprecipitation and Hevylite immunoassay (Figs 2 and 3, Table S1) supported this conclusion. These immunoassays used two different sources of polyclonal antisera (from Dako and Binding Site), to ensure that the results were not limited to the peculiarity of one antisera batch or host species. In contrast the IgG4 proteins were purified using affinity chromatography columns possessing single heavy-chain variable fragment llama monoclonal antibodies.
The light-chain capture ELISA resulted in a different signal strength dependent on whether the same or opposite light-chain antisera was employed (Fig. 3a). This was presumably due to a different orientation of the hybrid IgG4κ/λ arms for binding the specific conjugates. For example if the IgG4κ/λ molecule was captured by an anti-κ antibody then its other light chain is orientated away from the plate surface and is more accessible to an anti-λ detection antibody. Alternatively the binding of the sample to the capture antibody could reduce the number of epitopes available for binding of the conjugate antibody, because both antibodies would be competing for the same sites on the light chain.
The Hevylite immunoassay is calibrated by using immunoglobulins containing only single light chains and the effect of hybrid light-chain molecules on the calibration is unknown. This could explain the discrepancy between Hevylite Gκ and Gλ values observed for the hybrid IgG4κ/λ molecules (Table S1). Nevertheless, the Hevylite assay has clearly shown that only the IgG4 hybrid samples are positive for both assays.
The MALDI-TOF MS produced mean molecular masses of 148 000–150 000. This was exactly the correct size for monomeric immunoglobulins composed of two heavy chain and two light chains. An m/z difference of 2000 MW was observed between polyclonal IgG4κ and IgG4λ and the hybrid IgG4κ/λ was roughly in the middle (Fig. 4a). Since the released polyclonal κ and λ light chains also had a 1000 mass difference between them (Fig. 4b), it is very likely that the 149 000 hybrid molecule has one κ and one λ light chain. The difference in MALDI signal intensity (peak heights) for reduced κ and λ light chains (Fig. 4b) could possibly be due to different ionization efficiencies as a result of sequence and structural variations.
Serum polyclonal IgG4 molecules that are composed of two different light chains (κ and λ) are likely to have been produced by the process of half-molecule exchange (Fig. 5). Assuming that these have not been created during the analytical extraction procedure, the amounts of these hybrid IgG4κ/λ antibodies can indicate the minimum extent of half-molecules exchanged in normal healthy serum. As the IgG4 preparations were essentially pure, a generic total protein assay was used to unambiguously determine their relative composition. The percentage of hybrid IgG4κ/λ antibodies within the total serum purified polyclonal IgG4 fraction ranged from 21 to 33% (Table 1). With the exception of donor 4 the percentage compositions of IgG4κ, IgG4λ and IgG4κ/λ are remarkably similar. Although this is a substantial proportion, it is likely to represent only a minimum estimation of the amounts of polyclonal IgG4 that have undergone the exchange process. Presumably some or all of the remaining polyclonal IgG4 with the same light chain on both Fab-arms (67–79%) may also have undergone half-molecule exchange at some point, but swapped half IgG4 molecules of the same light-chain type. Curiously we noticed an average κ to λ light-chain ratio in the non-hybrid light-chain IgG4 of 6·5 : 1, and a ratio of 3·1 : 1 when the hybrids were included. This may indicate a bias to κ light-chain usage in IgG4 greater than the observed 2 : 1 ratio for total polyclonal IgG.26
Previously published work obtained by the introduction of monoclonal or monospecific IgG4 into humans or monkeys and following the extent of IgG4 exchange produced comparable values to ours.7–9,27 Shapiro et al.28 showed that when the humanized IgG4 monoclonal therapeutic antibody Natalizumab was introduced into rats, 66% had undergone exchange in 6 hr and 87% after 24 hr. This suggests that bi-specific (monovalent) IgG4 molecules are widespread in normal serum and possibly represents the typical configuration.
It should be considered that the high levels of IgG4 hybrid molecules identified in vivo in our study could have been enhanced by hybrid formation during the analytical extraction process. However, this is unlikely for several reasons. First, a variety of studies have shown that a reducing agent such as DTT or glutathione is required to induce half-molecule exchange in vitro7,8,21 but reducing agents were absent during our extraction process. Second, acidic pH conditions similar to those employed here to elute proteins from the affinity column have been shown to induce G4 exchange but only in the presence of disulphide-reduced proteins or reducing agents.28 When polyclonal IgG depleted for IgG4 was fractionated following the same affinity chromatography process no evidence of IgGκ/λ hybrid molecules were observed, demonstrating that this phenomenon is restricted to the IgG4 subclass only. Finally, submitting an artificial mix of purified polyclonal IgG4κ and λ to the affinity chromatography process resulted in only a tiny amount of hybrid molecule formation that could not be quantified accurately.
The protein structural features that allow half-molecule exchange to occur specifically in human IgG4 but not in the other IgG subclasses have been previously described.13,15–17 Furthermore, methods to limit undesirable half-molecule exchange in therapeutic IgG4 monoclonals have been developed.8,9,27 However, the site and mechanism of half-molecule exchange in vivo have yet to be established. Van der Neut Kolfschoten et al.7 showed that the exchange in human serum required the presence of erythrocytes or a small molecule such as glutathione. Glutathione concentrations of between 1 and 5 mm have proved sufficient to instigate half-molecule exchange in vitro.7,8,21 The intracellular level of glutathione in mammalian cells is between 0·5–10 mm and, approximately 1 mm in whole blood.29 This would appear to be sufficient to promote IgG4 exchange in vivo.
Data presented here strongly support the existence of IgG4 molecules with different κ and λ light chains on the same molecule. When this is taken in context with the supporting literature on dynamic Fab-Arm exchange IgG4 in vivo,8,9 it is very likely that polyclonal IgG4κ/λ hybrid molecules are present in normal human serum. However, further studies are required to understand the clinical relevance of such molecules.
Authors' contributions
GW designed the study and co-wrote the manuscript with EY and SH. EY, EL, DG and AC performed the experiments. EY, EL, AC, SH and GW are full-time employees of the Binding Site Group Ltd.
Glossary
- DTT
dithiothreitol
- HRP
horse radish peroxidase
- MALDI-TOF-MS
matrix-assisted laser-desorption time of flight mass spectrometry
Disclosures
The authors declare no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Immunoassay quantification of the purified polyclonal IgG4κ, IgG4λ and mixed light-chain IgG4 hybrids from normal human sera.
References
- 1.Aucouturier P, Danon F, Daveau M, Guillou B, Sabbah A, Besson J, Preudohomme JL. Measurement of serum IgG4 levels by a competitive immunoenzymatic assay with monoclonal antibodies. J Immunol Methods. 1984;74:151–62. doi: 10.1016/0022-1759(84)90376-4. [DOI] [PubMed] [Google Scholar]
- 2.Salfeld JG. Isotype selection in antibody engineering. Nat Biotechnol. 2007;25:1369–72. doi: 10.1038/nbt1207-1369. [DOI] [PubMed] [Google Scholar]
- 3.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–77. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 4.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–51. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 5.Takuma K, Kamisawa T, Igarashi Y. Autoimmune pancreatitis and IgG4-related sclerosing cholangitis. Curr Opin Rheumatol. 2011;23:80–7. doi: 10.1097/BOR.0b013e3283412f60. [DOI] [PubMed] [Google Scholar]
- 6.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–7. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 8.Labrijn AF, Buijsse AO, van den Bremer ET, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol. 2009;27:767–71. doi: 10.1038/nbt.1553. [DOI] [PubMed] [Google Scholar]
- 9.Lewis KB, Meengs B, Bondensgaard K, Chin L, Hughes SD, Kjaer B, Lund S, Wang L. Comparison of the ability of wild type and stabilized human IgG4 to undergo Fab arm exchange with endogenous IgG4 in vitro and in vivo. Mol Immunol. 2009;46:3488–94. doi: 10.1016/j.molimm.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Rispens T, den Bleker TH, Aalberse RC. Hybrid IgG4/IgG4 Fc antibodies form upon ‘Fab-arm’ exchange as demonstrated by SDS–PAGE or size-exclusion chromatography. Mol Immunol. 2010;47:1592–994. doi: 10.1016/j.molimm.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Nirula A, Glaser SM, Kalled SL, Taylora FR. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2011;23:119–24. doi: 10.1097/BOR.0b013e3283412fd4. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. MAbs. 2012;4:17–23. doi: 10.4161/mabs.4.1.18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rispens T, Ooijevaar-de Heer P, Bende O, Aalberse RC. Mechanism of immunoglobulin G4 Fab-arm exchange. J Am Chem Soc. 2011;133:10302–11. doi: 10.1021/ja203638y. [DOI] [PubMed] [Google Scholar]
- 14.Schuurman J, Perdok GJ, Gorter AD, Aalberse RC. The inter-heavy chain disulphide bonds of IgG4 are in equilibrium with intrachain disulphide bonds. Mol Immunol. 2001;38:1–8. doi: 10.1016/s0161-5890(01)00050-5. [DOI] [PubMed] [Google Scholar]
- 15.Labrijn AF, Rispens T, Meesters J, et al. Species-specific determinants in the IgG CH3 domain enable Fab-Arm exchange by affecting the noncovalent CH3-CH3 Interaction Strength. J Immunol. 2011;187:3238–46. doi: 10.4049/jimmunol.1003336. [DOI] [PubMed] [Google Scholar]
- 16.Rispens T, Meesters J, den Bleker TH, Ooijevaar-de Heer P, Schuurman J, Parren PW, Labrijn A, Aalberse RC. Fc-Fc interactions of human IgG4 require dissociation of heavy chains and are formed predominantly by the intra-chain hinge isomer. Mol Immunol. 2013;53:35–42. doi: 10.1016/j.molimm.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Davies AM, Rispens T, den Bleker TH, McDonnell JM, Gould HJ, Aalberse RC, Sutton BJ. Crystal structure of the human IgG4 CH3 dimer reveals the role of Arg409 in the mechanism of Fab-arm exchange. Mol Immunol. 2013;54:1–7. doi: 10.1016/j.molimm.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Brusco A, Saviozzi S, Cinque F, DeMarchi M, Boccazzi C, de Lange G, van Leeuwen AM, Caronara AO. Molecular characterization of immunoglobulin G4 gene isoallotypes. Eur J Immunogenet. 1998;25:349–55. doi: 10.1046/j.1365-2370.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 19.Pandey JP. Fab-arm exchange is a misnomer. MAbs. 2012;4:635. doi: 10.4161/mabs.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuurman J, Labrijn AF, Parren PW. Fab-arm exchange: what's in a name? MAbs. 2012;4:636. doi: 10.4161/mabs.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen K, Ruttekolk IR, Glauner H, Becker F, Brock R, Hannus S. The in vitro biological activity of the HLA-DR-binding clinical IgG4 antibody 1D09C3 is a consequence of the disruption of cell aggregates and can be abrogated by Fab arm exchange. Mol Immunol. 2009;46:3269–77. doi: 10.1016/j.molimm.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Harlow E, Lane D. Antibodies – A Laboratory Manual. New York, NY: Cold Spring Harbor; 1988. [Google Scholar]
- 23.Bailey GS. Ouchterlony Double diffusion. In: Walker JM, editor. The Protein Protocols Handbook. Totowa, NJ: Humana Press; 1996. pp. 749–52. [Google Scholar]
- 24.Bradwell AR, Harding SJ, Fourrier NJ, Wallis GLF, Drayson MT, Carr-Smith HD, Mead GP. Assessment of monoclonal gammopathies by nephelometric measurement of individual immunoglobulin κλ ratios. Clin Chem. 2009;55:1646–55. doi: 10.1373/clinchem.2009.123828. [DOI] [PubMed] [Google Scholar]
- 25.Arakawa T, Kita Y, Sato H, Ejima D. Stress-free chromatography: affinity chromatography. Curr Pharm Biotechnol. 2009;10:456–60. doi: 10.2174/138920109788488969. [DOI] [PubMed] [Google Scholar]
- 26.Lievens MM. Medical and technical usefulness of measurement of κ and λ immunoglobulin light chains in serum with an M-component. J Clin Chem Clin Biochem. 1989;27:519–23. [PubMed] [Google Scholar]
- 27.Stubenrauch K, Wessels U, Regula JT, Kettenberger H, Schleypen J, Kohnert U. Impact of molecular processing in the hinge region of therapeutic IgG4 antibodies on disposition profiles in cynomolgus monkeys. Drug Metab Dispos. 2010;38:84–91. doi: 10.1124/dmd.109.029751. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro RI, Plavina T, Schlain BR, et al. Development and validation of immunoassays to quantify the half-antibody exchange of an IgG4 antibody, natalizumab (Tysabri®) with endogenous IgG4. J Pharm Biomed Anal. 2011;55:168–75. doi: 10.1016/j.jpba.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Pastore A, Federici G, Betini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333:19–39. doi: 10.1016/s0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.