Abstract
Murine embryonic stem (ES) cell-derived haematopoietic progenitor cells (HPCs) engraft and populate lymphoid organs. In vivo, HPCs engraft across MHC barriers protecting donor-type allografts from rejection. However, the underlying phenomenon remains elusive. Here, we sought to determine the mechanism by which ES cell-derived HPCs regulate alloreactive T cells. We used the 2C mouse, which expresses a transgenic T-cell receptor against H2-Ld to determine whether HPCs are deleted by cytotoxic T lymphocytes (CTLs). Previously, we reported that HPCs express MHC class I antigens poorly and do not express class II antigens. In vitro stimulated 2C CTLs failed to lyse H2-Ld HPCs in a standard 4-hr 51chromium release assay. Similarly, when the HPCs were tested in an ELISPOT assay measuring the release of interferon-γ by CTLs, HPCs failed to induce CTL degranulation. In addition, mice that were injected with HPCs showed a marked decrease in T-cell responses to alloantigen and CD3 stimulation, but showed a normal response to PMA/ionomycin, suggesting that HPCs impaired T-cell signalling through the T-cell receptor/CD3 complex. Here, we show that HPCs secrete arginase, an enzyme that scavenges l-arginine, leading to metabolites that down-regulate CD3 ζ chain. Indeed an arginase inhibitor partially restored expression of the CD3 ζ chain, implicating arginase 1 in the down-regulation of T cells. This previously unrecognized property of ES cell-derived HPCs could positively enhance the engraftment of ES cell-derived HPCs across MHC barriers by preventing rejection.
Keywords: arginase, embryonic stem cells, T-cell down-regulation
Introduction
About a decade ago, we and colleagues made the seminal observation that non-differentiated rat embryonic stem (ES) cells induce tolerance to cardiac allografts.1 These ES cells were not differentiated, begging the question as to whether they would maintain this property after differentiation into haematopoietic progenitor cells (HPCs). Indeed, very recently, we showed that murine ES-cell-derived HPCs protect cardiac allografts from immunological rejection,2,3 an observation that has consequences for the use of HPCs in transplantation. Interestingly, the mechanisms by which ES cells or HPCs induce tolerance have remained elusive. One aspect to consider is that ES cells and their HPCs poorly express MHC antigens.4 Taking advantage of this poor immunogenicity, we were able to induce mixed chimerism across MHC barriers after sub-lethal irradiation of recipient mice. Transplantation of donor cardiac allografts into these chimeric mice showed that the mice were tolerant to donor antigen.2 One possible explanation for these remarkable results is that the HPCs lack robust MHC expression and co-stimulatory molecules at the time of transplantation, allowing their engraftment in allogeneic recipients. We showed that the impact of HPCs on T cells was universally shared by HPCs from different mouse strains, and therefore required further investigation.
Recently, Yachimovich-Cohen et al. reported that human ES cells secrete arginase 1 (ARG), an enzyme that metabolizes l-arginase into ornithine and urea.5 Ornithine has been showed to down-regulate the CD3 ξ chain, rendering T cells non-responsive.5 This is an interesting observation because it has been reported that suppressor myeloid cells infiltrate tumours and secrete ARG, which is thought to down-regulate tumour infiltrating T cells in the local environment.6 In a recent paper, it was noted that ocular immune privilege is maintained by ARG, suggesting that this enzyme's role in immune tolerance might be broader than previously thought.7 Further, ARG is secreted by placental villi8 and might be involved in maintaining non-responsiveness of the mother's T cells to the fetus, avoiding immunological rejection of the fetus.
Others have suggested that lack of lysis of HPCs by natural killer (NK) cells was due to the expression of Serpin 6 by ES cells.9 However, knockdown experiments of this protein would be necessary to substantiate this claim. Alternatively, it was shown that undifferentiated or differentiated ES cells lacked ligands for human NK cells, which led to poor lysis of these cells by NK cells.10 In contrast to human HPCs, we recently reported robust expression of NK cell ligands on murine ES-cell-derived HPCs.11 Although these HPCs were not susceptible to NK cell killing in vitro, they were readily deleted by NK cells in vivo. Altogether, there might be multiple mechanisms contributing to the lack of killing by NK cells and alloreactive T cells. The Lin-Sca-1+ and the Lin– c-kit+ progenitor cells are targeted by NK cells, preventing long-term engraftment of HPCs. Lineage-committed cells appeared to be resistant to NK cells in vivo. This could be because the lineage-committed progenitors express higher levels of MHC class I antigens than freshly isolated HPCs. This property is, however, highly dependent on the culture conditions used to maintain the HPCs.
Here, we sought to investigate the mechanisms by which ES-cell-derived HPCs avoid immunological rejection. We and colleagues previously noted that ES cells poorly express MHC antigens.4,10–12 Class I expression is low but can be up-regulated by interferon-γ (IFN-γ).13 In contrast, ES cells do not express class II molecules, which could not be up-regulated by IFN-γ stimulation, suggesting that the class II assembly machinery was probably not developed in HPCs as suggested by others.14 Here, we decided to examine whether alloreactive cytotoxic T-lymphocytes (CTLs) can lyse ES-cell-derived HPCs. Using a cytotoxicity assay and the ELISPOT assay, we failed to observe any target cell killing.
Materials and methods
Mice
The 2C mice were a generous gift from Dr H. Schreiber (University of Chicago, IL). This mouse expresses a transgenic T-cell receptor (TCR) directed against H2-Ld that is expressed by BALB/c cells. C57BL/6, BALB/c, 129SvJ and MRL mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in the animal facility at the VA Medical Center, Iowa City, IA. Animal procedures were conducted under IACUC approved protocols.
Generation of HPCs and induction of mixed chimerism
BALB/c × 129SvJ ES cells were transduced with HoxB4-green fluorescent protein (GFP) retroviral particles as previously reported4 and allowed to form embryoid bodies. Embryoid bodies were dissociated and cultured in serum-free haematopoietic differentiation medium containing StemPro34 plus nutrient supplements (Invitrogen, Carlsbad, CA) and murine stem cell factor (100 ng/ml, R&D Systems, Minneapolis, MN), murine interleukin-6 (mIL-6; 5 ng/ml, Peprotech, Rocky Hill, NJ), Flt3-L (10 ng/ml, Peprotech), insulin-like growth factor 1 (40 ng/ml, Promega, Madison, WI), dexamethasone (1 μm, Sigma, St Louis, MO) over a period of 26 days. Half of the haematopoietic progenitor medium was changed every other day.
To induce mixed chimerism using HPCs, BALB/c or 129SvJ mice were sublethally irradiated and injected with 2–3 million 129SvJ ES-derived HPCs. To prevent NK-cell-mediated rejection of HPCs, recipient mice were treated with the anti-asialo-GM1 antibody once a week. Chimerism was monitored by flow cytometry to determine the percentage of GFP-positive cells.
Colony-forming unit assay
To confirm whether BALB/c × 129SvJ F1 ES-cell-derived HPCs differentiate into the haematopoietic cells, HPCs were plated onto 35-mm dishes with methylcellulose colony-forming assay medium containing stem cell factor, granulocyte–macrophage colony-stimulating factor, IL-3 and erythropoietin (R&D Systems). After 10–14 days, colony-forming units were plated onto slides using a Cytospin and subsequently stained with Giemsa–Wright solution.
Flow cytometry
To determine MHC I expression on HPCs and BALB/c splenocytes the cells were stained with an anti-H2-Ld antibody (BD Bioscience, Franklin Lakes, NJ) and analysed by flow cytometry. Briefly, the harvested single cells were washed with cold FACS buffer (PBS containing 1% fetal bovine serum and 0·1% NaN3) and stained with the phycoerythrin (PE) -conjugated anti-H2-Ld antibody. After washing, twice with cold FACS buffer, the cells were analysed for MHC class I molecule expression using an LSRII flow cytometer. For data analysis, flow jo software was used (Treestar Inc., Ashland, OR).
Peptides
QL9 (QLSPFPFDL) and OVA (SIINFEKL) peptides were purchased from AnaSpec, Inc. (Fremont, CA) The HPCs were pulsed with QL9 or OVA peptides (100 μm) for 4 hr at 37°. These peptide-treated HPCs were stained with PE-conjugated anti-H2-Ld to verify the up-regulation of MHC class I molecules. Furthermore, these pulsed HPCs were also used as target cells for cytotoxicity assays.
Cytotoxicity assay
To generate the effector cells, 2C mouse splenocytes were incubated with γ-irradiated BALB/c splenocytes for 5 days at 37° in recombinant IL-2 (40 U/ml, R&D Systems). BALB/c, 129SvJ splenocytes activated with or without concanavalin A (Con A; 5 μg/ml) for 48 hr, HPCs pulsed with QL9, OVA or QL9 plus IFN-γ were used for target cells. Effector cells were incubated with 51Cr-labelled target cells (4 × 104 cells) at various effector : target ratios. After 4 hr, released 51Cr was measured using a γ-scintillation counter. Specific lysis killing was calculated using the following formula: % lysis = [(experimental release – spontaneous release) / (maximum release – spontaneous release)] × 100.
IFN-γ ELISPOT assay
BALB/c splenocytes (1 × 107 cells) or HPCs (1 × 107 cells) were injected into 2C mice intraperitoneally. After 7 days, sensitized 2C mice were killed and their spleens were harvested. Sensitized 2C splenocytes were co-cultured with γ-irradiated BALB/c splenocytes, respectively. After 48 hr, CD8+ cytotoxic T cells were sorted using PE-conjugated anti-CD8 antibody and anti-PE MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) from each culture. CD8+ CTLs (2 × 105 cells) were plated onto the IFN-γ capture antibody-coated (1 : 200, BD Biosciences) 96-well multiscreen filter plates (Millipore, Billerica, MA) with the same number of stimulator cells namely BALB/c splenocytes or HPCs with or without IL-2 (40 U/ml, R&D Systems). For activation of CTLs, anti-CD3 (10 μg/ml)/CD28 (5 μg/ml) antibody or ionomycin (1 μg/ml) was added to CD8+ CTLs. After 24 hr, IFN-γ production was determined using the ELISPOT assay (BD Biosciences) according to the manufacturer's instructions. Developed plates were dried overnight and analysed using immunospot software (Cellular Technology Limited, Shaker Heights, OH).
Western blotting
To confirm the expression of ARG on HPCs, cell lysates were isolated from the HPCs of the ES lines CCE, R1 and BALB/c × 129SvJ using RIPA lysis buffer (Millipore) and loaded onto 10% SDS–PAGE gels. After running the gels, proteins were transferred onto the PVDF membrane for 30 min using the transfer kit (Bio-Rad, Hercules, CA). The PVDF membrane was incubated with purified mouse anti-ARG antibody (BD Biosciences, 1 : 4000) in blocking buffer (5% skim milk and 1% BSA) contained overnight. After three washing steps, anti-mouse immunoglobulin horseradish peroxidase-conjugated antibody was used as secondary antibody (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA). Horseradish peroxidase-conjugated mouse anti-mouse β-actin (1:4000; Santa Cruz Biotechnology) was used as a normalization control.
CD3 ζ chain down-regulation
To investigate whether HPCs down-regulate CD3 ζ chain expression on the CTLs, CD3 ζ chain expression was measured on T cells by flow cytometry analysis. Briefly, C57BL/6 mice were sensitized with γ-irradiated BALB/c splenocytes (1 × 107 cells) or 107 BALB/c × 129SvJ HPCs. After 8 days, these C57BL/6 mice were killed and the harvested splenocytes from each of the sensitized mice were co-cultured with γ-irradiated BALB/c splenocytes or BALB/c × 129SvJ HPCs with or without arginase inhibitor (Nω-hydroxy-nor-l-arginine (NoHa), 100 μm, Calbiochem, San Diego, CA) treatment, for 3 days. To perform flow cytometry for CD3 ζ chain expression, these cells were intracellularly stained with PE-conjugated anti-CD3 ζ chain antibody (Abcam, Cambridge, UK) according to the manufacturer's instructions after surface staining of CD3 molecules using an allophycocyanin-conjugated anti-CD3 antibody (BD Pharmingen, San Diego, CA). The cells were analysed by flow cytometry.
Histological sections of the sternum
The HPCs (2·5 × 106 cells) were injected intravenously into irradiated Rag2−/− mice (400 CGy). An hour before mice were killed, Dextran Texas Red (10 000 molecular weight, Vector Laboratories, Burlingame, CA) was injected and the sternums were removed 24, 48 and 72 hr after cell injection. The sternums were surgically cut to expose the bone marrow cells. Cells were visualized using two-photon excitation microscopy.
Results
ES-cell-derived HPCs induce multi-lineage chimerism and are detectable in the bone marrow early after transplantation
Haematopoietic progenitor cells were derived from mouse ES cells as previously described.4 To determine whether the cells are capable of haematopoiesis, we used a standard colony-forming unit assay. Our results show that ES-cell-derived HPCs form colonies of haematopoietic cells (Fig. 1a). To investigate the migration of HPCs into lymphoid tissues, irradiated Rag2−/− γc−/− mice were transplanted with 2 × 106 BALB/c × 129SvJ HPCs. Mice were killed after 24, 48 or 72 hr, for analysis. Frozen sections of the sternums were histologically cut and studied by confocal microscopy. The HPCs express GFP and are therefore easy to distinguish from the mouse's own cells. A gradual increase of the HPCs locating in the bone marrow was observed (Fig. 1b). However, after counting the HPCs in the bone marrow, it appeared there was no significant difference between the HPC levels in the bone marrow between 48 and 72 hr, as visualized by two-photon microscopy. There appeared to be a polarization of the HPCs towards the dorsal side of the sternums (Fig. 1c). When mice were injected with Dextran Texas Red, the HPCs appeared to locate within the sinusoids and blood vessels (Fig. 1d).
Figure 1.
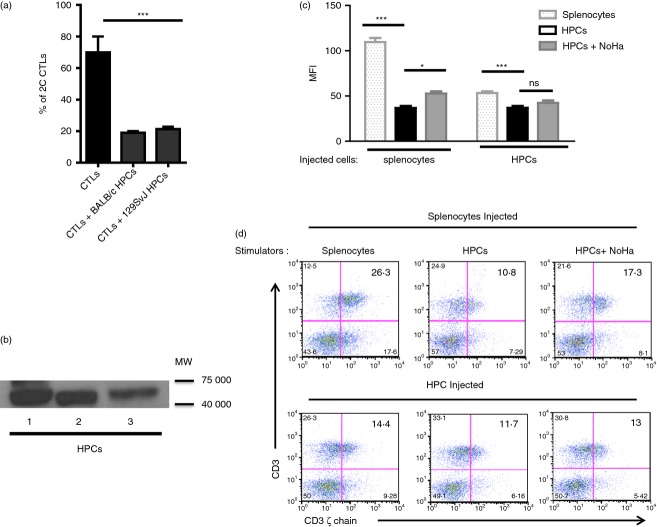
Embryonic stem (ES) cell-derived haematopoietic progenitor cells (HPCs) form colony-forming units (CFUs) and engraft into the bone marrow. (a) HPCs were derived from the 129SvJ ES cells and used to form CFUs. The cells formed multi-lineage haematopoietic cells (upper row) which were stained by the Giemsa stain (lower panel). (b) Engraftment of the HPCs into the bone marrow was studied by two-photon excitation microscopy 24, 48 and 72 hr post-transplantation. Sternums of transplanted mice were surgically cut open and subsequently imaged. After 24 hr, the population of HPCs was quite low. The density of the HPCs in the bone marrow increased with time. These experiments were repeated three times. (c) The transplanted HPCs were polarized towards the dorsal side of the sternum; however, it is still unclear why this is the case. (d) Two hours before mice were killed, they were intravenously injected with Dextran Texas Red to visualize blood vessels. Interestingly, HPCs were detected perivascularly and within the sinusoids (arrow head) of the bone marrow. (e) To investigate the distribution of the HPCs in lymphoid tissues post transplantation, mice were killed 24, 48 or 72 hr post transplantation and the spleen, thymus and bone marrow cells were recovered. The cells were analysed by flow cytometry for green fluorescent cells. Initially, most HPCs were in the spleen, but had drastically redistributed to other organs after 24 hr.
In addition, splenic cells, bone marrow cells and thymic cells of these mice were processed and analysed by flow cytometry to determine the relative numbers of the HPCs at the different times. After 24 hr, most of the HPCs were detectable in the spleen but had significantly redistributed into other lymphoid organs after 48 hr (Fig. 1e), suggesting a redistribution to other organs and into the circulation. Consequently, the numbers of HPCs gradually increased in the thymus and bone marrow. The number of HPCs entering the bone marrow had doubled after 72 hr, clearly indicating that counting the cells was less accurate than flow cytometry. These data show the dynamics of HPC migration and population of lymphoid organs with time post-transplantation. In a recent manuscript, the Dunbar group introduced 4D imaging of bone marrow cells using cells that were marked with five different colours.15 This technique allowed the study of how competitively different haematopoietic cell types populate lymphoid organs post transplantation. Although we did not use multicolour imaging, our data in the sternum, are similar to theirs.
Alloreactive T cells fail to lyse HPCs in vitro
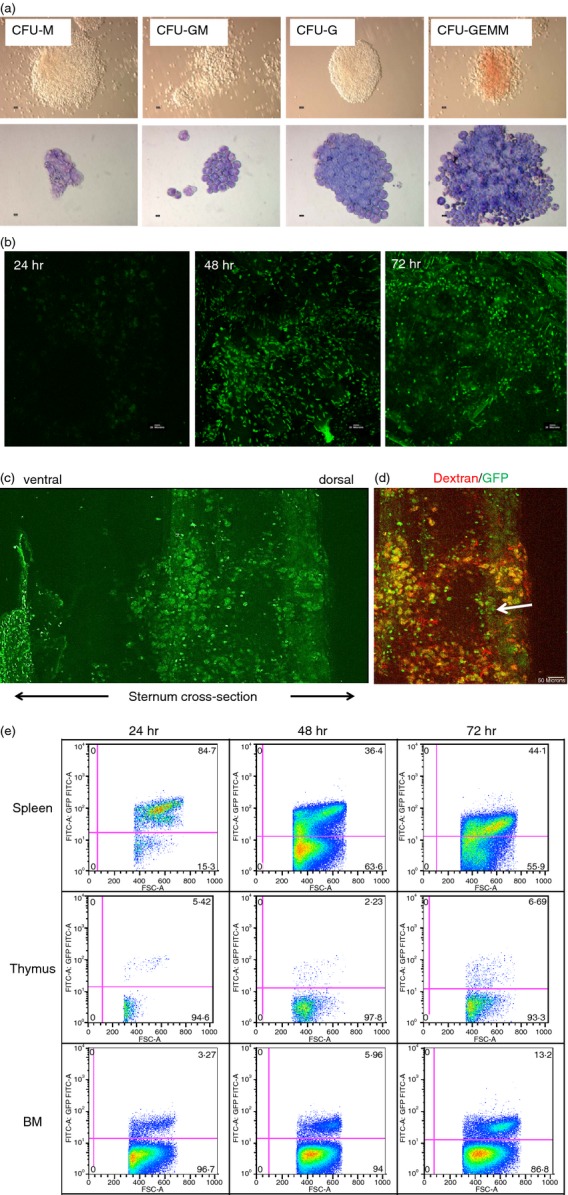
An important objective of these studies was to determine the antigenicity of ES-cell-derived HPCs. To accomplish that goal, we determined the levels of MHC class I expression on the progenitor cells using flow cytometry. Since the HPCs are from the BALB/c × 129SvJ strain, we used an antibody specific for H2-Ld. Class I expression on the HPCs was quite low, but could be strongly elevated by IFN-γ stimulation (Fig. 2a). In contrast, HPCs hardly express MHC class II antigens, which were not elevated by IFN-γ stimulation. We previously reported that ES-cell-derived HPCs are not susceptible to NK cell killing in vitro, but are readily lysed by NK cells in vivo.3 Here, we focused on alloreactive T cells. To determine the susceptibility of these HPCs to alloreactive CTLs, we raised anti-H2-Ld CTLs by immunizing the 2C mice using BALB/c splenocytes. The 2C mouse is transgenic for a TCR specific for an epitope QL9 (QLSPFPFDL) presented by H2-Ld.16,17 The 129SvJ × BALB/c HPCs or BALB/c Con A blasts were used as target cells against the alloreactive CTLs in a cytotoxicity assay. Third-party 129SvJ Con A blasts were used as negative controls. Using a 4-hr cytotoxicity assay, we showed that the BALB/c HPCs were not susceptible to alloreactive CTLs (Fig. 2c). Next, we wondered whether the HPCs were not lysed by the CTLs because of their low MHC expression. Despite up-regulation of MHC class I antigens using IFN-γ (Fig. 2a–b), the HPCs were again not sensitive to CTL killing (Fig. 2c). We speculated that despite class I up-regulation, these MHCs might not be properly peptide loaded. Therefore we pulsed the HPCs with the synthetic QL9 peptide.17 The peptide-pulsed HPCs were subsequently used as target cells in a cytotoxicity assay. A scrambled OVA peptide was used as control (Fig. 2d). None of these target cells were lysed, except for the BALB/c Con A blasts that were used as positive controls. Hence, HPCs are not susceptible to CTL killing in vitro even after peptide pulsing.
Figure 2.
(a, b) Haematopoietic progenitor cells (HPCs) poorly express MHC antigens. (a) To determine MHC expression by HPCs, cells were stained with either an anti-class I or an anti-class II antibody. In addition, HPCs that had been previously treated with interferon-γ (IFN-γ) were also included. Class I expression by HPCs was greatly increased by IFN-γ, but not that of MHC class II antigens. These results are also displayed as dot plots in (b). (c) HPCs were used as target cells against anti-H2-Ld alloreactive T cells. BALB/c concanavalin A (Con A) blasts were readily lysed, but not the BALB/c HPCs (n = 5). The 129SvJ HPCs were used as controls. (d) Since the HPCs were not lysed by cytotoxic T lymphocytes, we pulsed the HPCs with either the H2-Ld-specific QL9 peptide or with a scrambled OVA peptide and used these HPCs as target cells. None of these cell types was lysed by the HPCs (n = 5). All experiments were performed in triplicate. Con A blasts were again used as positive controls.
HPCs down-regulate T-cell responses in vivo
To investigate the interaction between alloreactive T cells and HPCs in vivo, we immunized 2C mice with either BALB/c splenocytes or with the 129 SvJ × BALB/c HPCs. After 7 days, the mice were injected again with splenocytes only and the spleens were harvested after another 2 days. The experimental design is depicted in (Fig. 3a). CD8+ T cells were isolated from the spleens by immunomagnetic bead separation. In both groups of mice, the T cells were re-stimulated with either BALB/c splenocytes, HPCs or by mitogens and evaluated on ELISPOTS for IFN-γ secretion. T cells derived from mice that had been stimulated with splenocytes responded strongly to splenic cell stimulation, as expected (Fig. 3b). In contrast, there was no T-cell response to HPC stimulation. However, T cells highly responded to the anti-CD3/CD28 antibodies and to PMA/Ionomycin, which were used as positive controls. On the other hand, T cells derived from mice that had been immunized with HPCs hardly secreted any IFN-γ on stimulation with either splenocytes or HPCs (Fig. 3c). Interestingly, these T cells responded poorly to CD3 stimulation, although their response to PMA/Ionomycin stimulation was very robust. This finding suggested that signalling through the TCR/CD3 complex was impaired in T cells that had been previously exposed to HPCs. These results are of interest because they suggest that HPCs down-regulate T-cell responses by skewing signalling through the TCR/CD3 complex.
Figure 3.
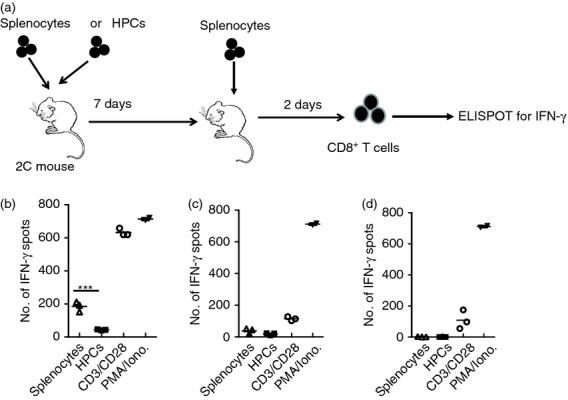
Haematopoietic progenitor cells (HPCs) down-regulate alloreactive cytotoxic T lymphocytes. (a) Our experimental design of these experiments was to either inject 2C mice with BALB/c splenocytes or with HPCs. The injection was repeated 7 days later and mice were killed after another 2 days. CD8+ T cells were sorted and used in ELISPOTs as responder cells. (b) Splenocytes from mice sensitized with BALB/c splenocytes were used as responder cells in ELISPOTs. BALB/c splenocytes used as stimulator cells induced a strong response that was significantly higher (P < 0·001) than that of T cells stimulated with HPCs. As expected, the T cells had a robust response to CD3 and PMA/Ionomycin stimulation. These experiments were repeated five times. (c) T cells from mice sensitized with HPCs in the first injection responded poorly to stimulation by BALB/c splenocytes, HPCs or by an anti- CD3 antibody. In contrast, the T cells responded normally to PMA/Ionomycin stimulation. (d) To determine whether T-cell response could be rescued by interleukin-2 (IL-2), T cells were again stimulated by either splenocytes or HPCs in the presence of recombinant IL-2. We observed no T-cell recovery. Stimulation with PMA/Ionomycin resulted in a robust T-cell response. All experiments were performed in triplicates.
Abrogation of T-cell responses by HPCs is not restored by IL-2
Next, we wondered whether the down-regulation of the T cells could be restored by recombinant IL-2. The rationale for these experiments was that if HPCs induce T-cell anergy, it would be expected that IL-2 restores T-cell reactivity. T cells from mice that had been immunized with HPCs were tested for their ability to release IFN-γ. The T cells failed to respond to either splenocytes or HPCs despite treatment with recombinant IL-2 (Fig. 3d). This result suggested that the HPCs were not rendering T cells non-responsive through T-cell anergy but rather through T-cell exhaustion. Indeed, the spleens of mice that had been immunized with HPCs were noticeably smaller than those of naive mice. Although the results varied, in general the splenocytes shrank by at least 30%. No particular cell type was targeted, but rather there was a global decrease in the total number of splenocytes, suggesting involvement of HPC-produced metabolites that impacted the haematopoietic compartment.
Next, we set up a transwell experiment whereby the 2C CTLs were put in the lower chamber of the transwell. In addition either the allospecific BALB/c HPCs or the 129SvJ third-party HPCs were put into the upper chambers. If HPCs secrete molecules that might perturb T-cell function without direct contact to the T cells we should observe T-cell down-regulation. In both cases only about 20% of the CTLs survived (Fig. 4a), suggesting that the effect was not allospecific.
Figure 4.
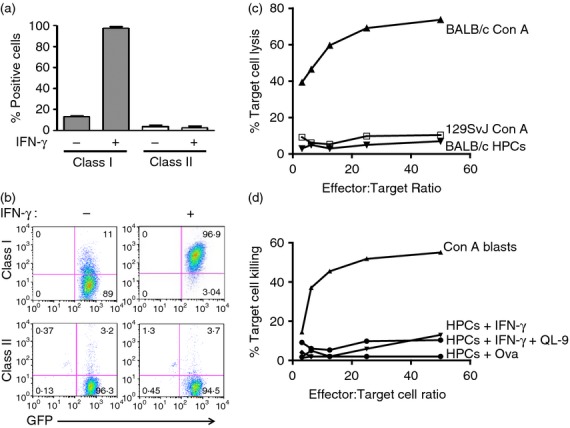
Haematopoietic progenitor cells (HPCs) and embryonic stem (ES) cells secrete arginase 1 (ARG), which results in the down-regulation of the CD3 ζ chain. (a) Soluble factors produced by HPCs down-regulate T cells. To determine whether direct contact of T cells with HPCs was required for HPCs to affect T cells, we used transwell plates, whereby the alloreactive T cells were placed in the lower chamber while the allogeneic or third party HPCs were placed in the upper chamber. The number of T cells was counted after 48 hr. Indeed the number of viable T cells was equally diminished (P < 0·001) independent of the strain of the HPCs, suggesting that the effect was not allospecific. These experiments were repeated three times. (b) Cell lysates of ES-cell-derived HPCs were run on Western blots and probed with an anti-ARG antibody. All samples tested positive for ARG. (c) HPCs down-regulate the CD3 ζ chain. Splenocytes from mice that had been injected with BALB/c splenocytes were re-stimulated in vitro with either BALB/c splenocytes or HPCs. Re-stimulation with splenocytes showed that the T cells highly expressed the ζ chain, as assessed by the high MFI. In contrast when the same splenocytes were stimulated with HPCs, the MFI was significantly lower than that observed in T cells stimulated with splenocytes, P < 0·001. To inhibit ARG, we used the inhibitor NoHa and observed a significant recovery of the ζ chain, P < 0·05. In contrast, the ζ chain was significantly reduced in T cells that had been recovered from mice that were initially injected with BALB/c HPCs. These T cells showed further diminished expression of the ζ chain after in vitro stimulation with HPCs, P < 0·001. Interestingly, T cells from these mice showed no recovery of the ζ chain when the ARG inhibitor NoHa was added, suggesting that the HPCs were very effective at down-regulating T cells. All experiments were repeated three times and performed in triplicates. (d) Representative dot blots of the CD3ζ chain down-regulation. T cells from mice injected with HPCs had lower CD3ζ chain expression. The CD3ζ chain expression could be partially restored by the arginase inhibitor NoHa.
Arginase down-regulates the CD3 ζ chain
To further investigate the mechanism by which HPCs regulate T cells, we wondered whether HPCs express ARG, which has been shown to down-regulate T cells, particularly in tumour biology.5 Cell lysates of three different ES-cell-derived HPCs were run on Western blots to determine whether they express ARG. All three batches of HPCs strongly expressed ARG (Fig. 4b), which is known to scavenge arginine, converting it into urea and ornithine, starving T cells of arginine. In addition, ornithine reduces the expression of the ζ chain on T cells, making T cells less responsive to stimulation.5
Finally, we studied the impact of ARG on the expression of the ζ chain on CD3. To do this, 2C mice were injected with HPCs or with splenocytes as described above. The splenocytes were prepared and stimulated with either BALB/c splenocytes, HPCs or HPCs supplemented with the ARG inhibitor NoHa. The CD3 ζ chain was analysed by flow cytometry after 3 days. T cells from mice that had been sensitized with splenocytes showed high MFI of the CD ζ chain, suggesting that they were highly stimulated (Fig. 4c). However, T cells re-stimulated with HPCs, showed a base MFI significantly lower than that of T cells stimulated with splenocytes. Treatment with the ARG inhibitor partially rescued the ζ chain expression. In contrast, the expression level of the ζ chain in T cells derived from animals that had been sensitized with HPCs were about half of those stimulated with splenocytes in vivo. Re-stimulation of the T cells that had been previously exposed to HPCs with splenocytes instead of HPCs did not recover ζ chain expression. When these T cells were stimulated with HPCs, the ζ chain expression was lower and partially improved after addition of the ARG inhibitor NoHa (Fig. 4c). These results are further represented as dot blots (Fig. 4d). Our results demonstrate that HPCs significantly down-regulate the CD3 ζ chain rendering T cells non-responsive. These data suggest that transplantation of ES-cell-derived HPCs in the allogeneic setting could lead to T-cell down-regulation and protection against alloreactive T cells.
Finally, to test whether HPCs down-regulated T cells in vivo, we generated HPCs from 129SvJ ES cells. For these experiments, we used the HM1 ES cell line. BALB/c allogeneic mice were sublethally irradiated and transplanted with 2–3 million HPCs. To protect HPCs from NK cell deletion, mice were treated weekly with the anti-asialo-GM1 antibody. As controls, 129SvJ HPCs were transplanted into the autologous 129SvJ mice in parallel. Interestingly, HPCs engrafted in both mouse strains without any further immunosuppressive therapy (Fig. 5a). Chimerism was monitored for up to 35 days. Donor cells were further displayed as a distinct cell population using flow cytometry (Fig. 5b). Hence, here we show that ES-cell-derived HPCs down-regulate alloreactive T cells allowing them to engraft across MHC barriers without the requirement for drug-related medical intervention. Hence, HPCs strongly down-regulate alloreactive T cells in vitro and escape T-cell depletion in vivo.
Figure 5.
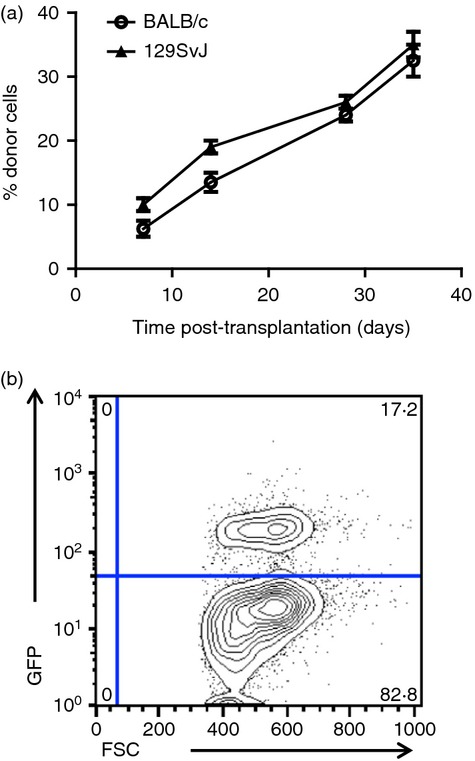
(a) Haematopoietic progenitor cells (HPCs) engraft across MHC barriers with minimal pre-conditioning. 129SvJ HPCs were prepared and injected into sub-lethally irradiated BALB/c mice or 129SvJ autologous mice. Mixed chimerism was monitored by flow cytometry over 35 days. More than 20 animals were transplanted in each setting. Mixed chimerism was detected at about the same levels in both strains suggesting that these HPCs did not evoke T-cell-related immune rejection. (b) Representative dot plot showing peripheral blood chimerism.
Discussion
The derivation of HPCs from ES cells in the mouse model has now been well established allowing important studies on the functionality of the haematopoietic cells into which they develop.4,18,19 Basically there are two protocols that have been successfully used by different groups, namely, the embryoid body formation assay and the OP9 stromal cell approach.19,20 Both methods yield high numbers of HPCs that can be transplanted. Here, we used the embryoid body formation assay to generate HPCs.4 The ability of the HPCs generated by these two approaches to form multi-lineage haematopoiesis can vary. Our data here show that HPCs engraft well and establish mixed chimerism. When the HPCs were transplanted, the cells rapidly populated bone marrow. The spleen harbours most HPCs soon after transplantation because they begin to migrate into other lymphoid organs. The HPCs increasingly enter the thymus and bone marrow in a more gradual fashion. In our previous work, we showed that the majority of the cells that locate to the bone marrow are lin– c-kit+ sca-1+. This is the first time that ES-cell-derived HPCs have been tracked in real time in vivo.
The surprising finding that ES-cell-derived HPCs poorly express MHC, as we previously reported,4 is intriguing for allogeneic transplantation. We initially speculated that their MHC expression might be low because of a poor MHC peptide loading machinery. To test this, we cultivated the HPCs in the QL9 peptide that specifically binds to H2-Ld. Although the MHC expression went up, it was not significantly higher than in the control cells that were co-cultured with a scrambled control OVA peptide. This observation suggested that the low MHC expression on the HPCs was not due to a lack of endogenous peptides. More interestingly, these HPCs were not susceptible to alloreactive T cells. Interferon-γ up-regulation of the MHC antigens on the HPCs did not confer susceptibility to CTL killing. The observation that these HPCs are not susceptible to CTL killing suggested that these cells might more successfully engraft across MHC barriers than bone marrow cells, which are highly immunogenic.
We recently reported that HPCs poorly express MHC antigens and no co-stimulatory molecules.4 Here, we wondered whether HPCs stimulate T cells. When allogeneic mice were sensitized in vivo with HPCs, we made two important observations. The total number of splenocytes shrank significantly. Secondly, the overall response of the T cells to alloantigen and to CD3 stimulation was significantly impaired. However, the T cells responded normally to PMA/ionomycin stimulation. This finding clearly demonstrates that HPCs skewed the TCR/CD3 signalling pathway without globally impairing T cells, because the T cells responded well to PMA/ionomycin stimulation. This is a new finding that suggested that HPCs were down-regulating T cells by abrogating the TCR/CD3 pathway. Further, IL-2 failed to restore T-cell responses in our in vitro assays. ARG has been described as an enzyme used by cancer cells to down-regulate T cells. We speculated whether HPCs and ES cells secrete this enzyme, which hydrolyses arginine. Once arginine has been broken down, ornithine and urea accumulate.7,21 These metabolic products down-regulate the CD3 ζ chain, thereby rendering T cells non-responsive to stimulation. The ARG inhibitor NoHa led to partial recovery of the ζ chain expression, but was not sufficient to fully restore normal CD3 ζ chain expression. Overall, our data show that ES cell-derived HPCs down-regulate T cells, a characteristic that could be exploited for successful allogeneic transplantation. This mechanism could be exploited for the transplantation of HPCs across MHC barriers with minimal requirement for immunosuppression. Indeed, 129SvJ-derived HPCs engrafted in BALB/c mice after sublethal irradiation and NK cell deletion. The degree of chimerism in these mice was comparable to that in the autologous 129SvJ mice.
These findings provide a better understanding of our earlier observations that transplantation of non-differentiated ES cells protected cardiac allografts from rejection.1 When non-differentiated ES cells were used as stimulator cells in mixed lymphocyte reactions, we observed total inhibition of T-cell proliferation.13 Interestingly, ES-cell-derived HPCs inhibited T-cell proliferation in a similar fashion, suggesting that non-differentiated ES cells and ES-cell-derived HPCs share the same characteristic of inhibiting T cells.
We further speculated that HPCs failed to stimulate alloreactive T cells because of their inability to express endogenous peptides. However, when we pulsed HPCs with an appropriate allele-specific synthetic peptide, they remained non-susceptible to alloreactive T-cell killing. This finding bolstered our finding that HPCs express a protein that globally leads to T-cell suppression. Indeed that protein is ARG. Although we did not pursue transforming growth factor-β in these studies, ES cells and their progenitors secrete transforming growth factor-β,22 which might contribute to the down-regulation of T cells as well. Since the down-regulation of the ζ chain by the HPCs could not be fully recovered by recombinant IL-2, we anticipate that HPCs may induce T-cell exhaustion rather than T-cell anergy. These findings suggest that transplantation of ES-cell-derived HPCs across MHC barriers could be successful long-term by abrogating alloreactive T cells.
Acknowledgments
This material is based upon work supported in part by the Department of Veterans' Affairs, Veterans' Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, 1I01BX001125-01A1, by NIH/NHLBI Grants 5R01HL073015-09 and 3R01HL073015-04A1S1 and by a Career Development Award to EMK (14SDG18690008) The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies. The authors have no conflicts of interest to report. We acknowledge the technical help afforded by Chantal Allermargot at the Central Microscopy Research Facility of the University of Iowa for technical help with imaging of the bone marrow. We also thank the Flow Cytometry Core at the University of Iowa for all the flow cytometry analyses that we were able to perform at the Core Facility. We thank Dr Elizabeth Field for making her ELISPOT equipment available to us and we thank Gohar Manzar for constructive discussions and for technical assistance.
Glossary
- ARG
arginase 1
- Con A
concanavalin A
- CTL
cytotoxic T lymphocytes
- ES
embryonic stem
- HPC
haematopoietic progenitor cells
- IFN-γ
interferon-γ
- mIL-6
murine interleukin-6
- NK
natural killer
- NoHa
α-amino acid Nω- hydroxy-nor-l-arginine
- TCR
T-cell receptor
Authorship
EM Kim was responsible for the differentiation of the ES cells, using a method that she designed. B Miyake performed ELISPOTs and the in vivo experiments. M Aggarwal performed some of the ELISPOTs. M Griffith performed the bone dissection and confocal microscopy. R Voetlause performed the bone dissection and confocal microscopy. N Zavazava designed this study, wrote the paper and performed data analysis.
Disclosures
The authors declare no conflict of interest.
References
- 1.Faendrich F, Lin X, Chai GX, et al. Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nat Med. 2002;8:171–8. doi: 10.1038/nm0202-171. [DOI] [PubMed] [Google Scholar]
- 2.Bonde S, Chan KM, Zavazava N. ES-cell derived hematopoietic cells induce transplantation tolerance. PLoS ONE. 2008;3:e3212. doi: 10.1371/journal.pone.0003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonde S, Dowden AM, Chan KM, Tabayoyong WB, Zavazava N. HOXB4 but not BMP4 confers self-renewal properties to ES-derived hematopoietic progenitor cells. Transplantation. 2008;86:1803–9. doi: 10.1097/TP.0b013e31818fe741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan KM, Bonde S, Klump H, Zavazava N. Hematopoiesis and immunity of HOXB4-transduced embryonic stem cell-derived hematopoietic progenitor cells. Blood. 2008;111:2953–61. doi: 10.1182/blood-2007-10-117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yachimovich-Cohen N, Even-Ram S, Shufaro Y, Rachmilewitz J, Reubinoff B. Human embryonic stem cells suppress T cell responses via Arginase I-dependent mechanism. J Immunol. 2010;184:1300–8. doi: 10.4049/jimmunol.0804261. [DOI] [PubMed] [Google Scholar]
- 6.Du C, Wang Y. The immunoregulatory mechanisms of carcinoma for its survival and development. J Exp Clin Cancer Res. 2011;30:12. doi: 10.1186/1756-9966-30-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu H, Khan A, Coe D, et al. Arginine depletion as a mechanism for the immune privilege of corneal allografts. Eur J Immunol. 2011;41:2997–3005. doi: 10.1002/eji.201141683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa T, Harada T, Koi H, Kubota T, Azuma H, Aso T. Identification of arginase in human placenta villi. Placenta. 2007;28:133–8. doi: 10.1016/j.placenta.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Abdullah Z, Saric T, Kashkar H, et al. Serpin-6 expression protects embryonic stem cells from lysis by antigen-specific CTL. J Immunol. 2007;178:3390–9. doi: 10.4049/jimmunol.178.6.3390. [DOI] [PubMed] [Google Scholar]
- 10.Drukker M, Katz G, Urbach A, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:9864–9. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabayoyong WB, Salas JG, Bonde S, Zavazava N. HOXB4-transduced embryonic stem cell-derived Lin-c-kit+ and Lin-Sca-1+ hematopoietic progenitors express H60 and are targeted by NK cells. J Immunol. 2009;183:5449–57. doi: 10.4049/jimmunol.0901807. [DOI] [PubMed] [Google Scholar]
- 12.Drukker M, Katchman H, Katz G, et al. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–9. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 13.Bonde S, Zavazava N. Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells. 2006;24:2192–201. doi: 10.1634/stemcells.2006-0022. [DOI] [PubMed] [Google Scholar]
- 14.Ladhoff J, Bader M, Brosel S, Effenberger E, Westermann D, Volk HD, Seifert M. Low immunogenicity of endothelial derivatives from rat embryonic stem cell-like cells. Cell Res. 2009;19:507–18. doi: 10.1038/cr.2009.21. [DOI] [PubMed] [Google Scholar]
- 15.Malide D, Métais J-Y, Dunbar CE. Dynamic clonal analysis of murine hematopoietic stem and progenitor cells marked by 5 fluorescent proteins using confocal and multiphoton microscopy. Blood. 2012;120:e105–16. doi: 10.1182/blood-2012-06-440636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo H, Chen H, Qi S, Loh D, Daloze P, Veillette A, Xu D, Wu J. De novo-developed T cells have compromised response to existing alloantigens: using Ld-specific transgenic 2C T cells as tracers in a mouse heart transplantation model. J Immunol. 1998;161:73–82. [PubMed] [Google Scholar]
- 17.Hornell TMC, Martin SM, Myers NB, Connolly JM. Peptide length variants p2Ca and QL9 present distinct conformations to Ld-specific T cells. J Immunol. 2001;167:4207–14. doi: 10.4049/jimmunol.167.8.4207. [DOI] [PubMed] [Google Scholar]
- 18.Pilat S, Carotta S, Schiedlmeier B, et al. HOXB4 enforces equivalent fates of ES-cell-derived and adult hematopoietic cells. Proc Natl Acad Sci USA. 2005;102:12101–6. doi: 10.1073/pnas.0505624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk-sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 20.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–26. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor CD3ζ chain expression byl-Arginine. J Biol Chem. 2002;277:21123–9. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 22.Oshimori N, Fuchs E. The harmonies played by TGF-β in stem cell biology. Cell Stem Cell. 2012;11:751–64. doi: 10.1016/j.stem.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]