Abstract
Isoprostanes are free radical-catalysed PG-like products of unsaturated fatty acids, such as arachidonic acid, which are widely recognized as reliable markers of systemic lipid peroxidation and oxidative stress in vivo. Moreover, activation of enzymes, such as COX-2, may contribute to isoprostane formation. Indeed, formation of isoprostanes is considerably increased in various diseases which have been linked to oxidative stress, such as cardiovascular disease (CVD), and may predict the atherosclerotic burden and the risk of cardiovascular complications in the latter patients. In addition, several isoprostanes may directly contribute to the functional consequences of oxidant stress via activation of the TxA2 prostanoid receptor (TP), for example, by affecting endothelial cell function and regeneration, vascular tone, haemostasis and ischaemia/reperfusion injury. In this context, experimental and clinical data suggest that selected isoprostanes may represent important alternative activators of the TP receptor when endogenous TxA2 levels are low, for example, in aspirin-treated individuals with CVD. In this review, we will summarize the current understanding of isoprostane formation, biochemistry and (patho) physiology in the cardiovascular context.
Keywords: isoprostanes, lipid peroxidation, oxidative stress, cardiovascular disease, TxA2/prostanoid receptor
Introduction
Oxidative stress in biological systems is defined as an imbalance between the generation of reactive oxygen species (ROS) and antioxidant defence mechanisms (Dalle-Donne et al., 2006; Giustarini et al., 2009). In contrast to the physiological condition, during which ROS are only present at moderate levels and play an important role as messengers in redox signalling, in pathological conditions, when the physiological redox state of cells is disturbed, ROS can severely affect cellular signalling and function. Indeed, ROS which are not neutralized or scavenged by antioxidant molecules, such as GSH or superoxide dismutase, may react with nucleic acids and proteins and thereby alter the biochemical and physical properties of these important cellular components. Moreover, exposure of lipids to free radicals induces a non-enzymatic reaction cascade resulting in an increased formation of bioactive molecules named isoprostanes. Consequently, systemic isoprostane formation is significantly increased in a variety of pathological processes associated with oxidative stress, for example, cancer as well as, cardiovascular, metabolic and neurodegenerative diseases, and isoprostanes are increasingly recognized not only as markers of oxidative stress but also as mediators of disease progression (Praticò et al., 1997; Reilly et al., 1998; Davì et al., 1999; Minuz et al., 2002; Vassalle et al., 2003; Schwedhelm et al., 2004, Xia et al., 2005; Montuschi et al., 2007; Schwedhelm et al., 2010; Barocas et al., 2011; Davies and Roberts, 2011; Khadem-Ansari et al., 2011; Montine et al., 2011; Sbardella et al., 2013).
Isoprostanes are PG-like compounds derived from lipid peroxidation of esterified unsaturated fatty acids, for example, arachidonic acid, which are primarily generated in a free radical-dependent and non-enzymatic fashion (Figure 1; nomenclature follows Alexander et al., 2013a). First being described in 1976 as a product of the autoxidation of polyunsaturated fatty acids (PUFA; Pryor et al., 1976), free radical-induced formation of isoprostanes under conditions of oxidative stress has been demonstrated in the 1990s in vitro as well as in vivo (Morrow et al., 1990a,b; 1992). In the following years, the biological activities of isoprostanes have been intensely studied, demonstrating, that is, an isoprostane-mediated modification of platelet aggregation and vascular tone (Yin et al., 1994; Kromer and Tippins, 1996; Möbert et al., 1997; Minuz et al., 1998). These isoprostane-induced effects are mediated via the prostanoid TP receptor, thus pointing to a predominant role of this receptor in isoprostane signal transduction (receptor nomenclature follows Alexander et al., 2013b). Moreover, the close isoprostane–TP receptor interaction may explain why isoprostane levels have been shown to correlate in clinical trials with the extent and severity of, for example, cardiovascular disease (CVD), and why isoprostanes may directly affect prognosis of various pathological processes (Vassalle et al., 2003; Schwedhelm et al., 2004; Di Minno et al., 2012). In this review, we will summarize the current understanding of isoprostane formation, biochemistry and (patho) physiology. In addition, we will give an overview of the TP receptor receptor as the main target of isoprostane-mediated signalling in the cardiovascular system.
Figure 1.
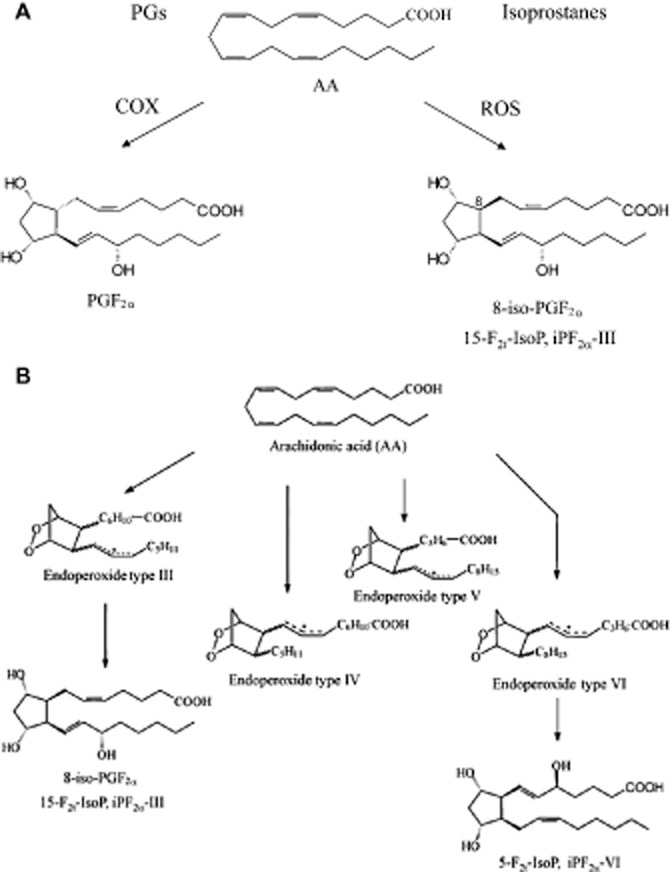
(A) Enzymatic and non-enzymatic formation of PGs and isoprostanes. (B) Non-enzymatic formation of isoprostanes exemplified for 8-iso-PGF2α (15-F2t-IsoP, iPF2α -III) and 5-F2t-IsoP (iPF2α -IV). Arachidonic acid (AA) is released from phospholipids by PLA2 and subsequently converted to PGs by COX. Esterified AA is converted non-enzymatically by ROS to phospholipid-bound isoprostanes. The latter being subsequently released as non-esterified congeners.
Formation of isoprostanes
In contrast to enzymatically formed prostanoids, such as PGE2 or TxA2, isoprostanes are generated in vitro as well as in vivo, primarily independent of COX via free radical-induced peroxidation of unsaturated fatty acids (Figure 1; Morrow et al., 1990a). Under physiological conditions, these PG-like compounds can only be detected as esterified at very low concentrations in the nanomolar range or as free compounds in the picomolar range in biological fluids, for example, plasma and urine (Morrow et al., 1990b). In conditions of oxidative stress, the burst of free radical formation leads to a significant increase in isoprostane levels (Morrow et al., 1990b; 1992). For instance, systemic application of CCl4, a strong inducer of free radical formation, in a rat model of hepatic failure, resulted in a more than 100-fold increase in hepatic isoprostane formation as compared with untreated animals (Morrow et al., 1992). This effect was even more pronounced in rats treated simultaneously with diquat and CCl4 (Morrow et al., 1990b). In contrast, non-selective COX inhibitors do not significantly alter plasma isoprostane levels, thereby supporting the notion of isoprostanes being primarily a product of non-enzymatic lipid modifications (Morrow et al., 1990b). In addition, simple storage of plasma from normal volunteers resulted in a time-dependent ex vivo formation of isoprostanes, underlining the predominant role of enzyme-independent lipid peroxidation in the formation of isoprostanes (Morrow et al., 1990a). Nevertheless, as described afterwards in more detail, COX, in particular the inducible isoform COX-2, may contribute to the generation of isoprostanes in monocytes and in vascular cells in the pulmonary circulation during pathological situations (Praticó and FitzGerald, 1996; Delannoy et al., 2010). In contrast to F2-isoprostanes, D2/E2-isoprostanes are hardly detectable under physiological conditions (Morrow et al., 1994), whereas after induction of lipid peroxidation, the concentrations of D2/E2-isoprostane are increased in the circulation. These data emphasize the role of free radicals and oxidative stress in the formation of isoprostanes and indicate that lipid peroxidation is able to give rise to a variety of heterogeneous yet biologically active isoprostanes (Morrow et al., 1994).
In 1976, Pryor et al. first described the formation of PG-like compounds during autoxidation of PUFA (Pryor et al., 1976). In the 1990s, Morrow et al. could show the formation of isoprostanes from unstable endoperoxide intermediates in vitro as well as in vivo (Morrow et al., 1990a; 1992). As shown in Figure 1, formation of F2-isoprostanes, a group of 64 compounds isomeric to COX-derived PGF2α, was described through intermediates, which undergo endo-cyclization to yield PGG2-like bicyclic endoperoxides, which are then further reduced to form F-ring isoprostanes (Morrow et al., 1990a). The rearrangement of endoperoxide intermediates results in the formation of D2/E2-isoprostanes (Morrow et al., 1994; Chen et al., 1999a). D2/E2-isoprostanes are formed in competition to F2-isoprostanes and the depletion of reducing agents such as α-tocopherol and ascorbic acid favours the formation of D2/E2-isoprostanes over that of F2-isoprostanes (Montine et al., 2003). D2/E2-isoprostanes can undergo further rearrangements generating A/J-isoprostanes, which are known as cyclopentenone isoprostanes (Chen et al., 1999a,b; Brooks et al., 2008; Hardy et al., 2011). Interestingly, degradation of A-isoprostane derivatives has been shown to occur during physiological conditions and has been demonstrated to give rise to biologically active intermediates (Benndorf et al., 2008). Furthermore, the reduction of endoperoxide intermediates from docosahexanoic acid leads to the formation of so-called neuroprostanes in the nervous system (Roberts et al., 1998).
In general, isoprostanes are formed by two routes of lipid peroxidation consisting of the endoperoxide and the dioxetane/endoperoxide mechanism. However, the contribution of the latter route to the generation of isoprostanes in vivo remains unclear (Montuschi et al., 2007). The isoprostane pathway leading to the generation of F2-isoprostanes starts with the formation of three arachidonoyl radicals followed by the formation of four peroxyl radical isomers which subsequently undergo endo cyclization (Morrow, 2006). Four bicycloendoperoxide regioisomers are then reduced to generate F2-isoprostanes (Morrow, 2006). In the dioxetane/endoperoxide route, the formation of the same regioisomers can be observed, but in this cascade, the second and not the first oxygen molecule is incorporated into the PGF ring (Lawson et al., 1999). In contrast to PGs, bioactive compounds generated by COX, isoprostanes have cis- or trans-stereochemistry at the five-membered ring junction as compared to the exclusive trans-ring junction in PGs (Figure 1). Unlike PGs, which are generated from free arachidonic acid, isoprostanes are formed in situ on arachidonoyl-containing lipids and then subsequently released in free form into the circulation via an enzyme-dependent mechanism (Morrow et al., 1992; 1994). This process is dependent on the activity of PLA2 because an incubation of lipid extracts with this enzyme leads to a release of free F2-isoprostanes (Morrow et al., 1992). Furthermore, both plasma and intracellular platelet-activating factor (PAF) acetylhydrolase are able to hydrolyse phospholipids to release esterified F2-isoprostanes increasing free F2-isoprostane concentrations (Stafforini et al., 2006). However, little is known regarding the mechanisms responsible for the extrusion or liberation of isoprostanes from the intracellular space to the extracellular milieu, a process that is likely to significantly affect auto-, para- and endocrine activities of intracellularly formed isoprostanes. Having reached the systemic circulation, isoprostanes, such as 8-iso-PGF2α, are partly metabolized by mechanisms involving, for example, peroxisomal β-oxidation (Schwedhelm et al., 2000). Moreover, direct conjugation to GSH has been described for cyclopentenone isoprostanes, such as 15-A2t-isoprostane, in HepG2 cells indicating that phase II metabolism may also play a role in isoprostane metabolism (Milne et al., 2004). Finally, isoprostanes and isoprostane metabolites are freely filtered in the glomerular apparatus of the kidneys and excreted in urine.
Contribution of enzymatic processes to isoprostane formation
As mentioned previously, enzymatic activity appears to at least partially contribute to the generation of isoprostanes. In human monocytes, LPS induced the formation of PGE2 and the isoprostane 8-iso-PGF2α in a time- and dose-dependent manner, accompanied with the induction of COX-2 (PGHS-2). Incubation with the selective inhibitor of COX-2, L-745,337, decreased the production of PGE2 and 8-iso-PGF2α, indicating that the induction of COX-2 in monocytes is associated with an increased production of isoprostanes (Patrignani et al., 1996). The role of COX-2 in the formation of isoprostanes has been confirmed also by other groups. Stimulation of human monocytes with LPS induced the expression of this enzyme and was accompanied by an increased formation of PGE2, TxB2 and 8-iso-PGF2α (Praticó and FitzGerald, 1996). Furthermore, inhibition of COX-2 as well as pretreatment with superoxide dismutase suppressed the formation of 8-iso-PGF2α, indicating that monocytes may form bioactive 8-iso-PGF2α in an enzyme- and free radical-catalysed pathway (Praticó and FitzGerald, 1996). As mentioned above, COX is the first enzyme catalysing the formation of traditional PGs from arachidonic acid. While COX-1 is expressed constitutively in a variety of different cell types, expression of COX-2 is primarily induced via inflammatory stimuli (Grosser et al., 2010). Human vascular endothelial cells (ECs) treated with pro-inflammatory cytokines, such as IL-1β or TNF-α, showed a significantly increased release of 8-iso-PGF2α, which was blocked by COX-1 and COX-2 inhibitors (Jourdan et al., 1999). In addition, superoxide-producing enzyme xanthine oxidase elevated the release of isoprostanes in these cells, thereby emphasizing the role of oxygen-derived radicals in isoprostane formation (Jourdan et al., 1999). Under hypoxic conditions, an up-regulation of COX-2 in murine pulmonary arteries has been described, which was accompanied by an increase in 8-iso-PGF2α release, suggesting a putative role of COX-2 in the generation of isoprostanes under hypoxic conditions (Delannoy et al., 2010). Furthermore, in a model of renal ischaemia reperfusion injury, accumulation of 8-iso-PGF2α was successfully blocked by administration of acetylsalicylic acid, thereby indicating that COX-dependent generation of isoprostanes may play a role under ischemic conditions (Favreau et al., 2004). In human platelets, a minor role for COX-1 in the production of 8-iso-PGF2α has been shown (Pratico et al., 1995; Pignatelli et al., 2011). In this context, it is proposed that platelet 8-iso-PGF2α formation is mainly associated with NADPH oxidase-dependent superoxide release and only to a minor extent derives from COX-1 activation (Pratico et al., 1995; Pignatelli et al., 2011). In addition, NOS pathways may be involved in the generation and release of isoprostanes (Jourdan et al., 1997). Interestingly, GSH, one of the most important and abundant intracellular antioxidants, has been shown to promote the formation of oxidative stress markers like malondialdehyde and 8-iso-PGF2α from arachidonic acid in a COX-dependent way, indicating that antioxidants may have a paradoxical role in the generation of isoprostanes (Tsikas et al., 2012). In summary, isoprostanes are predominantly generated in the free radical-dependent process of lipid peroxidation, but enzymatic processes may also contribute to the formation of these lipid mediators especially in the context of hypoxia or oxidative burst. Nevertheless, enzyme-dependent isoprostane generation should be analysed in more detail to help to fully elucidate the complex process of isoprostane formation.
Nomenclature of isoprostanes
Currently, two nomenclature systems for isoprostanes are used (Rokach et al., 1997; Taber et al., 1997). The Taber/Roberts nomenclature system has been approved by the IUPAC and Eicosanoid Nomenclature Committee and follows the normal PG conventions. In this system, the different regioisomers are designated by the carbon number of the side chain where the hydroxyl is located, with the carboxyl carbon designated as C-1. Based on this nomenclature, four isoprostane regioisomer classes derived from arachidonic acid are then denoted as either 5, 8, 12 or 15 series (Taber et al., 1997). The abbreviation 2t in the prominent isoprostane (IsoP) 15-F2t-IsoP refers to the number of double bonds (two) and the trans-orientation of the side chains at the five-membered ring. 15-F2t-IsoP is also called 8-iso-PGF2α because the chemical structure of this molecule differs from COX-derived PGF2α only in the stereochemistry of the carbon atom 8. The second nomenclature system was evolved by Rokach et al. creating different regioisomer classes based on the ω-carbon being attacked to form the arachidonoyl radical (Rokach et al., 1997). Free radical attack at carbon ω-8, −11 and −14 leads to the formation of regioisomers type III, IV and VI respectively. The four classes of F2-isoprostanes are designated as type III, IV, V and VI, whereas the oxidation of ω-3 lipids induces the formation of compounds starting with type I.
TP receptors as important mediators of isoprostane-induced signal transduction
As mentioned previously, isoprostanes most likely exert their effects exclusively via activation of the TP receptor (Minuz et al., 1998; Huber et al., 2003; Tang et al., 2005; Benndorf et al., 2008). Thus, the aim of this section is to give an overview of the TP receptor and its main ligand, TxA2, and to briefly outline the role of the TP receptor in the pathogenesis of CVD.
TxA2 is a PG derivative with chemical characteristics of prostanoids but structural differences especially in the ring structure (heterocyclic oxane ring structure vs. 5-carbon ring). It is a short-lived but highly bioactive molecule that mediates its effects via activation of the heptahelical G-protein-coupled TP receptor. TxA2 acts as an autacoid in autocrine or paracrine systems and is involved in a wide variety of physiological and pathophysiological processes, such as vasospasm, hypertension, thrombosis, angiogenesis, inflammation, atherogenesis and myocardial infarction (Palmer et al., 1970; Needleman et al., 1976; Wilson et al., 2005; Nakahata, 2008). Being an unstable intermediate in arachidonate metabolism with a chemical half-life of about 30 s, TxA2 was detected in the conversion of PGG2 into inactive TxB2 in platelets (Hamberg et al., 1975). In a first biosynthetic step, PLA2 catalyses the release of arachidonic acid from membrane phospholipids, which is further converted via COX into the PG endoperoxides PGG2 and PGH2 (Daniel et al., 1999; Nakahata, 2008). Via Tx synthase, an enzyme abundantly expressed in a wide variety of different tissues (Sun et al., 1977), these endoperoxides are then further converted into TxA2 and subsequently non-enzymatically degrade into biologically inactive TxB2 (Needleman et al., 1976).
The TP receptor gene is located at 19p13.3 of human chromosome, spans over 15 kb and contains three exons divided by two introns (Nüsing et al., 1993). The TP receptor protein is widely expressed in different organs and localized on both cell membranes and intracellular structures (Armstrong et al., 1983; Hedberg et al., 1989; Borg et al., 1994; Bowling et al., 1994; Raychowdhury et al., 1994; Fennekohl et al., 1999; Muja et al., 2001). Based on the sequence of the purified protein from human platelets, a GPCR human cDNA was cloned from human placenta, consisting of seven transmembrane spanning regions, three extracellular and three intracellular loops (Ushikubi et al., 1989; Hirata et al., 1991). In mice and rats, TP receptor analogues have been described that are similar to the human TP receptor from placenta (TP-α; Namba et al., 1992). In addition to the TP receptor-α-isoform, a second isoform has been described in ECs, called TP receptor-β (Raychowdhury et al., 1994). TP receptor-β results from alternative splicing of the cytoplasmic carboxyl tail (Raychowdhury et al., 1994). In most cells and tissues, both TP receptor isoforms are expressed, for instance, in vascular smooth muscle cells. However, in most cells, the TP receptor-α dominates over TP receptor-β expression, probably through pronounced constitutive and agonist-induced endocytosis of the TP receptor-β and increased subsequent degradation of this TP receptor isoform by the proteasome (Miggin and Kinsella, 1998; Sasaki et al., 2007). In HUVECs approximately sixfold greater mRNA levels of TP receptor-α than TP receptor-β has been found (Miggin and Kinsella, 1998). Both TP receptor isoforms are able to form homo- and heterodimers via the formation of disulfide bonds (Laroche et al., 2005). Interestingly, formation of (hetero) oligomers of TP receptors is an agonist-independent process regulating both TP receptor-α internalization and TP receptor-mediated signalling (Laroche et al., 2005; Sasaki et al., 2006). In TP receptors, as in many other members of the eicosanoid receptor family, the seventh transmembrane domain is highly critical for ligand binding (Funk et al., 1993). Point mutations in this domain inhibited the binding of the TP receptor antagonist SQ29548 ([1S-[1α,2β (5Z),3β,4α]-7-[3-[[2-[(phenylamino)carbonyl]hydrazino]methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid) to the receptor supporting the role of this structure in ligand binding (Funk et al., 1993). Furthermore, residues in the transmembrane domains 4, 5 and 6 are implicated in ligand binding (Dorn et al., 1997). Various mutational analyses identified critical residues in the first, second and third extracellular loop regulating ligand–TP interaction partially by forming hydrogen bonds (Chiang et al., 1996; D'Angelo et al., 1996; Turek et al., 2002; So et al., 2003). The N-terminal region of TP receptors contains two consensus glycosylation sites, which are supposed to be critical for ligand binding. Inhibition of these N-glycosylations reduced the binding of SQ29548 to TP receptors and affected receptor signalling and efficient transmembrane expression (Walsh et al., 1998). A study from Ruan et al. demonstrated that TP receptor agonists and antagonists share the ligand-binding pocket in general, but the configuration of this binding pocket for the agonist and antagonist are quite different (Ruan et al., 2009). They proposed a model, in which antagonist binding to TP receptors induces an increase in β-sheet and a decrease in α-helical content inducing an unfavourable conformation for G-protein coupling (Ruan et al., 2009). Additional critical residues for antagonist binding to TP receptors have been identified (Khasawneh et al., 2006). The first and third intracellular domains of TP receptors have been shown to mediate the coupling to G-proteins through charge contact and therefore regulating intracellular signalling (D'Angelo et al., 1996; Chung et al., 1999; Geng et al., 2004).
TP receptor signalling
Early studies demonstrated that TP receptor agonists, such as U44069 (9, 11-dideoxy-9α, 11α-epoxymethano-prosta-5Z, 13E-dien-1-oic acid) induced GTPase activity in platelet membranes accompanied by a stimulation of inositol phospholipid metabolism (Houslay et al., 1986). Later it has been shown that TP receptors functionally couple to the Gq-family members Gq/11, G15 and G16 (Figure 2; Offermanns and Simon, 1995; Kinsella et al., 1997). After TP receptor stimulation, these G-proteins mediate the activation of PLC-β, catalysing the conversion from PI-4,5-biphosphate to inositol-1,4,5-trisphosphate and DAG resulting in the release of intracellular Ca2+ stores and activation of PKC (Offermanns and Simon, 1995; Kinsella et al., 1997). In addition to Gq/11-proteins, members of the G12-family are involved in TP receptor-mediated cell signalling (Offermanns et al., 1994; Moers et al., 2003; Miyosawa et al., 2006). A lack of Gα13 reduced the TP receptor-mediated activation of RhoA, significantly decreasing the ability of TxA2 to induce platelet shape changes and aggregation in vivo resulting in severe defects in primary haemostasis and an almost complete protection against arterial thrombosis (Moers et al., 2003). Under pathological conditions, a modulation of TP receptor-coupled G-proteins can be observed. Hypoxia induces actin polymerization of pulmonary arteries independently of RhoA, reflecting a decreased association with G12/13 in favour of Gq (Fediuk et al., 2012).
Figure 2.
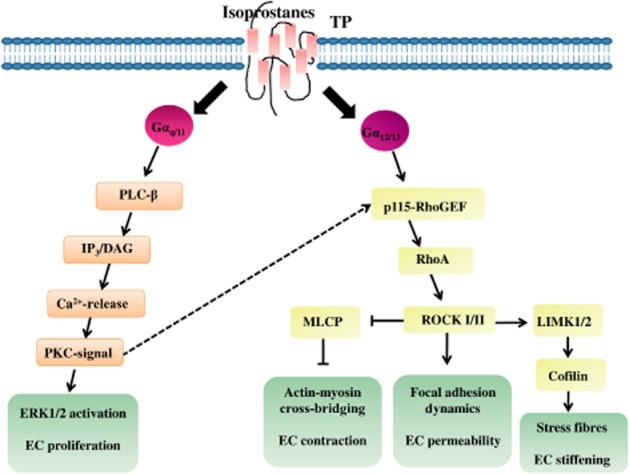
Isoprostane-mediated signalling via TP receptor activation in ECs associated with endothelial function and homeostasis.
Stimulation of TP receptor-α and -β result in differential activation of downstream signalling pathways. Agonist activation of TP receptor-α induced, via the dimeric G-protein Gh, a stimulation of PLC-mediated inositol phosphate production, whereas agonist activation of TP receptor-β had no effect (Vezza et al., 1999). Interestingly, the TP receptor isoforms are differentially regulated in response to the vasorelaxant molecules prostacyclin and NO (Reid and Kinsella, 2003; Wikström et al., 2008). Whereas TP receptor-α undergoes both NO- and prostacyclin-mediated desensitization involving direct PKA and PKG phosphorylation within the C-terminal domain, signalling by TP receptor-β is unaffected by either NO or prostacyclin (Reid and Kinsella, 2003). Furthermore, in human aortic smooth muscle cells, both TP receptor isoforms independently regulate RhoA activation (Wikström et al., 2008). But, although TP receptor-α-mediated RhoA signalling was directly impaired by prostacyclin and NO, TP receptor-β-mediated RhoA signalling was not affected (Wikström et al., 2008).
Relevance of TP receptors in CVD
The role of TxA2 and TP receptors in atherosclerosis and CVD has been investigated in a wide range of experimental and clinical studies. For instance, increased TxA2 biosynthesis has been described in atherosclerosis (Mehta et al., 1988). The initiation and progression of this chronic inflammatory disease and its complications is promoted by TxA2 most likely via regulation of platelet activation, endothelial integrity and leukocyte–EC interaction (Kobayashi et al., 2004). Moreover, the abundance and expression level of TxA2 and TP receptors, respectively, increase during progression of atherogenesis, thereby indicating that TP receptor-dependent signalling pathways become increasingly important in patients with advanced atherosclerotic disease (Cyrus et al., 2010). For instance, in patients with coronary artery disease, an increase in TP receptor expression level was observed in diseased vessels correlating with progression accompanying the increase in endogenous TxA2 levels in CVD (Katugampola and Davenport, 2001; Katugampola et al., 2002). Interestingly, pharmacological inhibition of TP receptors by its antagonist S18886 rather than systemic depletion of TxA2 levels, was effective in reducing the development of atherosclerotic plaques in the ApoE knockout mouse model (Cayatte et al., 2000). These findings indicate that TP receptor agonists other than TxA2, for example, isoprostanes, may be important in the initiation and progression of atherosclerosis.
Moreover, clinical and experimental data point to a critical role of the TP receptor in ischaemia and myocardial infarction. Inhibition of TP receptors by receptor antagonists, for example, SQ29548 or AH-23848 ((4Z)-7-[(rel-1S,2S,5R)-5-((1,1-biphenyl-4-yl)methoxy)-2-(4-morpholinyl)-3-oxocyclopentyl]- 4-heptenoic acid), prevented the extension of ischaemic damage in myocardial ischaemia and improved early survival following permanent coronary artery ligation (Brezinski et al., 1985; 1987; Hock et al., 1986). Furthermore, the application of the TP receptor agonist BAY u3405 ((3R)-3-[[(4-fluorophenyl)sulfonyl]amino]-1,2,3,4-tetrahydro-9H-carbazole-9-propanoic acid), reduced myocardial infarct size as well as myocardial leukocyte accumulation, underlining the relevance of TP receptors and also indicating a role of immune cells in myocardial infarction and ischaemia-reperfusion injury, which has been confirmed by several further studies (Crawford et al., 1988; Squadrito et al., 1993; Vinten-Johansen, 2004).
On platelets from patients with acute myocardial infarction as well as stable and unstable angina pectoris, respectively, an increase in TP receptor expression level has been detected, correlating with the duration of chest pain (Dorn et al., 1990; Modesti et al., 1995). Furthermore, an increase in the maximal velocity of U46619-induced platelet aggregation has been observed in these patients, indicating a significant role of induced TP receptor expression in thrombogenesis (Dorn et al., 1990). Indeed, the role of TxA2 as an important activator of platelet aggregation especially in the context of endothelial dysfunction and CVD has been clearly demonstrated (Ally and Horrobin, 1980; Dorn and DeJesus, 1991). TxA2, an important member of the second-wave agonists of platelet aggregation, is able to alter platelet shape, to amplify integrin activation on adherent platelets and to mediate thrombus growth by recruiting additional platelets via the activation of TP receptor-coupled G-proteins (Moers et al., 2003; Stegner and Nieswandt, 2011). Moreover, the clinical effectiveness of aspirin in preventing thrombotic events in patients with cardiovascular or cerebrovascular disease strongly emphasizes the biological relevance of platelet-derived TxA2 in the pathological interaction of platelets and dysfunctional vascular ECs. Interestingly, during chronic hypoxia, platelet activation is enhanced indicating a correlation between hypoxia and TP receptor expression (Pidgeon et al., 2004). In addition, hypoxia directly affects TP receptor localization, stability and avidity (Valentin et al., 2004; Hinton et al., 2006; 2007). Under normoxic conditions, the TP receptor-β is preferentially located intracellularly, presenting a significant ER-localized population (Valentin et al., 2004). By inducing oxidative stress, an enhanced TP receptor translocation from the ER to the Golgi in COS-7 cells has been observed accompanied by an increased receptor stability and density at the membrane (Valentin et al., 2004). These data indicate that oxidative stress induces maturation and intracellular translocation of TP receptors to increase its functional fraction in the cell membrane (Valentin et al., 2004). In addition to TP receptor maturation and translocation, hypoxia induces an increase in TP receptor ligand binding and avidity (Hinton et al., 2006; 2007). Modified receptor cycling as well as increased Gq coupling seems to be critical in hypoxia-induced TxA2 hypersensitivity in exposed myocytes (Hinton et al., 2006; 2007; Fediuk et al., 2012). In summary, the expression of TP receptors correlates with the extent and the severity of CVD. Conditions of oxidative stress seem to promote the expression, stability and avidity of TP receptors. These phenomena may enhance the biological relevance of endogenous TP receptor agonists, such as isoprostanes, in the context of cardiovascular pathologies.
Functional consequences of isoprostane–TP receptor interaction
Experimental data strongly suggest that isoprostane signalling is exclusively regulated via the interaction with TP receptors. In the context of isoprostane/TP signalling, an association of TP receptors with G-proteins such as Gq, Gi and G11 has been described (Kinsella et al., 1997; Acquaviva et al., 2013). A co-transfection of TP receptor-α with G11 produced greater mobilization of Ca2+ than did co-transfection of Gq in response to 8-iso-PGF2α stimulation, indicating a preferential association of TP receptors with G11 in isoprostane/TP receptor signalling in human platelets (Kinsella et al., 1997). Heterodimerization of TP receptor-α/β not only influences TxA2 signalling, but stimulates isoprostane-mediated inositol phosphate generation thereby enhancing isoprostane-dependent signal transduction (Wilson et al., 2007). Mutagenic analysis revealed that distinct amino acid residues of the TP receptor are responsible for isoprostane/ TP receptor interactions (Khasawneh et al., 2008). 8-iso-PGF2α interacts with two hydrophobic sites (Phe196/184) and one hydrogen binding site (Asp193) residing in transmembrane domain 5 and extracellular loop 2 of TP receptors (Khasawneh et al., 2008). Experimental work of Khasawneh et al. additionally indicated that in human platelets, two separate 8-iso-PGF2α signalling pathways exist, being TP receptor-dependent and TP receptor-independent, possibly mediated via a so far unknown isoprostane receptor bearing close homology to TP receptors (Khasawneh et al., 2008). Moreover, 8-iso-PGE2, an isoprostane generated from the same endoperoxide intermediate as 8-iso-PGF2α, has been proven to be a partial agonist of the TP receptor (Longmire et al., 1994; Audoly et al., 2000; Benndorf et al., 2008; Tables 1 and 2). These results from in vitro studies indicate that isoprostanes are partial agonists at TP receptors and that the biological activity of isoprostanes may be additionally mediated via an isoprostane-specific receptor. However, so far, no molecular evidence has been found for the existence of such an isoprostane-specific receptor. Furthermore, results from our and other groups strongly support the concept that isoprostanes mediate their biological functions exclusively via activation of TP receptors (Audoly et al., 2000; Benndorf et al., 2008).
Table 1.
Overview of TP receptor agonists including ligand-binding capacity and potencies
| TP agonists | ||
|---|---|---|
| Ligand binding (Kd)/potency (pD2 = −log EC50) | References | |
| U-46619 | Kd: 6.19–16 nM | Saussy et al., 1991 |
| Kd: 3.6–18.7 nM | Hedberg et al., 1988 | |
| pD2: 8.34–8.79 | Hou et al., 2000 | |
| U-46609 | Kd: 4.4–7 nM | Hedberg et al., 1988 |
| I-BOP | Kd: 5.5 nM | D'Angelo et al., 1994 |
| Kd: 0.322–7.9 nM | Saussy et al., 1991 | |
| SQ 26655 | Kd: 1.12–3 nM | Saussy et al., 1991 |
| PGF2α | Kd: 17.4 nM | Balapure et al., 1989 |
| pD2: 7.18–7.3 | King et al., 1991; Hou et al., 2000 | |
| PGD2 | King et al., 1991 | |
| 8-iso-PGE1 | pD2: 5.5 | Janssen et al., 2001 |
| pD2: 5.4 | Oliveira et al., 2000 | |
| 8-iso-PGE2 | pD2: 6.7 | Sametz et al., 2000; Janssen et al., 2001 |
| 8-iso-PGF2α | Kd: 31.8 nM | Yura et al., 1999 |
| pD2: 7.41–7.75 | Hou et al., 2000 | |
Table 2.
Overview of TP receptor antagonists including ligand-binding capacity
Role of isoprostanes as modulators of platelet activation
Isoprostanes participate in oxidative injury by modulating platelet activation and adhesion and by reducing the antiplatelet activity of NO (Minuz et al., 1998). A treatment of platelets with 8-iso-PGF2α in the concentration range from 10–1000 nmol·L−1 enhances platelet adhesion to fibrinogen by increasing the functionality of the adhesion molecule glycoprotein IIb/IIIa (Minuz et al., 1998). Furthermore, the anti-aggregatory effect of NO, released by ECs, is reduced by 8-iso-PGF2α (Minuz et al., 1998). All these effects were prevented by the TP receptor antagonist GR32191 ((1R-[1 α(Z),2β,3β,5α]]-(+)-7-[5-([1,1-biphenyl]-4-ylmethoxy)-3-hydroxy-2-(1-piperidinyl) cyclopentyl]-4-heptonic acid), underlining the importance of isoprostane/TP receptor interaction in platelet activation (Minuz et al., 1998). Indeed, 8-iso-PGF2α acts as a partial agonist of TP receptors on platelets (Yin et al., 1994). Whereas 8-iso-PGF2α can cause platelet shape change itself, in the presence of full TP receptor agonists, such as U46619 and I-BOP ([1S- [1α, 2α(Z), 3β(1E, 3S), 4α]]-7-[3-[3-hydroxy-4- (4-iodophenoxy)- 1- butenyl]- 7- oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid), it exhibits anti-aggregatory effects. In contrast, 8-iso-PGF2α may promote ADP-dependent platelet aggregation in a TP receptor-dependent fashion (Yin et al., 1994; Audoly et al., 2000; Schwedhelm et al., 2010).
Isoprostanes regulate immune and EC interaction
In addition to altered platelet behaviour, CVD is characterized by an enhanced interaction of ECs with immune cells such as monocytes and neutrophils (Aukrust et al., 2010). 8-iso-PGE2 and 8-iso-PGF2α may promote atherosclerosis by enhancing the interaction of monocytes with ECs (Leitinger et al., 2001; Huber et al., 2003). This process was mediated via a TP receptor-dependent activation of PKA and p38 (Leitinger et al., 2001; Huber et al., 2003). Interestingly, the effects of 8-iso-PGF2α on the monocyte–endothelial interaction are dependent on the origin of the vascular beds. While inhibiting dose dependently the adhesion to human dermal microvascular ECs in a TP receptor-dependent manner, 8-iso-PGF2α stimulates the binding of monocytes to HUVECs (Kumar et al., 2005). In addition to monocytes, the binding of neutrophils to ECs is regulated by isoprostanes. Whereas 8-iso-PGE2 has no effect on the adhesion of neutrophils, 8-iso-PGF2α enhances adhesion in a TP receptor-dependent as well as a TP receptor-independent manner (Zahler and Becker, 1999; Fontana et al., 2001; 2002; Huber et al., 2003). The capacity of isoprostanes to regulate endothelial/immune cell interaction and thereby affecting the process of atherosclerosis has been confirmed in vivo. Peritoneal injection of 1 μg·kg−1 body weight 8-iso-PGF2α displayed a TP receptor-dependent significant increase in macrophage density and atherosclerotic burden in aortic root sections of mice (Tang et al., 2005). This increased adhesion of macrophages to aortic ECs was accompanied by increased expression of sICAM-1 and CCL2, the latter being a chemokine, crucial in recruiting immune cells to the site of inflammation (Tang et al., 2005). Furthermore, treatment of human macrophages with 8-iso-PGF2α resulted in a NF-κB-independent increase in the expression of the pro-atherogenic molecule IL-8 (Scholz et al., 2003). This underlines the importance of isoprostanes in atherosclerosis and other inflammatory disorders. The effect of isoprostanes on immune cells is not restricted to a modulation of their adhesive properties. Furthermore, 8-iso-PGF2α can induce the activation of CD11b/CD18 and CD11c/CD18 in neutrophils resulting in the activation of NADPH oxidase (Fontana et al., 2001). NADPH oxidase and its catalytic subunit gp91 play an important role in the generation of platelet-derived 8-iso-PGF2α (Pignatelli et al., 2011). Furthermore, it has been demonstrated that functionally active NADPH oxidase in microglial cells generates ROS during inflammation in the CNS, thus exacerbating cerebral injury (Green et al., 2001). These data indicate that isoprostanes can enhance their own generation by activating NADPH oxidase in immune cells. In addition to an increased interaction between immune and ECs, the oxidation of low-density lipoproteins (LDL) plays an important role in the formation of atherosclerotic lesions (Tomkin and Owens, 2001). The incubation of LDL with Cu2+ resulted in a decrease in esterified F2-isoprostane levels and a significant increase in free isoprostane levels (Lynch et al., 1994). This indicates that LDL is a critical source for local isoprostane generation and liberation in the process of atherogenesis.
Isoprostanes act as vasoconstrictors
An increase in vascular tone plays an important role in a variety of pathological processes, such as hypertension and ischaemia. Interestingly, a significant vasoconstrictory potential of isoprostanes has been described. In isolated guinea pig hearts, 8-iso-PGF2α and 8-iso-PGE2 caused a sustained and concentration-dependent coronary vasoconstriction with EC50 values in the range of 10−5 M resulting in a decrease of coronary flow by as much as 50% (Möbert et al., 1997). Simultaneous administration of SQ29548 abolished the vasoconstrictor effect of both isoprostanes indicating a TP receptor-dependent mechanism (Möbert et al., 1997). This pro-vasoconstrictor potential of 8-iso-PGF2α and 8-iso-PGE2 has been confirmed in a wide range of different blood vessels, from human umbilical arteries, chicken embryo ductus arteriosus, pulmonary artery, femoral artery and porcine arteries to bovine coronary arteries, demonstrating the general vasoconstrictor potential of isoprostanes (Kromer and Tippins, 1996; van der Sterren and Villamor, 2011; Sakariassen et al., 2012). Interestingly, no vasoconstriction was induced in bovine coronary arteries indicating a putative species-dependent functionality of 8-iso-PGF2α (Kromer and Tippins, 1996). The vasoconstrictor effect of isoprostanes, such as 8-iso-PGF2α and 8-iso-PGE2, is mainly mediated via TP receptors leading to the release of internally sequestered Ca2+ and activation of the RhoA/Rho kinase 1 and 2 signalling pathway (Kromer and Tippins, 1996; Möbert et al., 1997; Mueed et al., 2008; Sakariassen et al., 2012). On the other hand, bovine aortic ECs possess two distinct binding sites for isoprostanes, indicating that the vasoconstrictor effect of these PG-like compounds may also be mediated by a so far not identified TP receptor-related isoprostane receptor (Yura et al., 1999; van der Sterren and Villamor, 2011). The vasoconstriction of pulmonary vasculature and intestine epithelium mediated via 8-iso-PGE2 was mediated via TP receptors and the PGE receptor, indicating that receptors other than TP receptors may be involved in the mediation of the vasoconstrictor capacity of isoprostanes (Elmhurst et al., 1997; Janssen and Tazzeo, 2002). The modulation of the vascular tone by isoprostanes can also occur in an indirect way. 8-iso-PGF2α concentrations in the range of 10−7 M, stimulate, probably through transcriptional regulation, the production of endothelin-1, a mitogen for ECs with vasoconstrictor potential, thereby inducing strong vasoconstriction (Yura et al., 1999).
Angiogenesis is affected by isoprostanes
Angiogenesis is a central process in several pathological disorders, such as cancer and diabetes (Folkman, 2002; Martin et al., 2003) and is a key event in cardiovascular homeostasis and regeneration, a process often impaired in CVD patients (Griffioen and Molema, 2000; Khurana et al., 2005). Isoprostanes regulate the formation of new blood vessels from pre-existing ones by various mechanisms. First, our group demonstrated that 8-iso-PGF2α and 8-iso-PGA2 synergistically and dose-dependently inhibit the migration and tubule formation of ECs in vitro via the activation of TP receptors (Benndorf et al., 2008). In these studies, we additionally observed that 8-iso-PGA2 can decompose into two biologically active compounds indicating that unstable isoprostanes may exert synergistic effects with endogenous isoprostanes affecting angiogenesis (Benndorf et al., 2008). Moreover, in ex vivo and in vivo assays, the anti-angiogenic effect of 8-iso-PGF2α was confirmed, being again mediated via TP receptors (Benndorf et al., 2008). Interestingly, 8-iso-PGF2α in the presence of VEGF-A induced an enhanced and persistent activation of the small GTPase RhoA in ECs as compared to the transient effect of VEGF-A on RhoA activity in absence of 8-iso-PGF2α (Benndorf et al., 2008). This may be crucial for isoprostane-mediated inhibition of angiogenesis as blockade of RhoA downstream effector Rho kinase completely reversed isoprostane-mediated anti-angiogenic effects in vitro. Indeed, persistent RhoA/Rho kinase activation may inhibit important steps in angiogenesis, such as EC movement and sprouting, via perturbation of cytoskeletal dynamics and focal adhesion turnover and reduces VEGF-induced EC sprouting (Kroll et al., 2009). Moreover, isoprostanes affect biology of further vascular cell types, such as vascular smooth muscle cells (VSMC) and vascular fibroblasts, which have been implicated in vascular maturation and stiffness (Nehls et al., 1994). Isoprostanes induce the proliferation of these cells, an effect that may affect the process of angiogenesis via modified VSMC–endothelial interaction and signalling (Takahashi et al., 1992; Kunapuli et al., 1997). These data indicate that isoprostanes inhibit new blood vessel formation and promote vascular stabilization via activation of TP receptors. However, in this context, it has to be mentioned that the role of TxA2 and TxA2 mimetics in angiogenesis, especially tumour-associated angiogenesis, is not fully elucidated. Synthetic TxA2 mimetics inhibit fibroblast growth factor 2 – and VEGF-induced angiogenesis in vitro as well as in vivo (Ashton and Ware, 2004; Ashton et al., 2004; Pal et al., 2006; Benndorf et al., 2008). In contrast, several studies particularly focusing on tumour-associated angiogenesis demonstrated that TxA2 may also act as a pro-angiogenic factor (Daniel et al., 1999; Nie et al., 2000; Wei et al., 2010). These conflicting results could be interpreted as showing that TP receptor isoforms may contribute, to different extents, to the process of angiogenesis and that TP receptor activation in cancer cells may induce production and release of pro-angiogenic molecules, which induce angiogenesis in a paracrine fashion. Taken together, isoprostanes modulate the process of angiogenesis via TP receptor activation and are likely to affect endothelial homeostasis and regeneration via this route. Nevertheless, further studies are needed to clarify the role of TP receptors and TP receptor agonists especially in the context of tumour-associated angiogenesis.
Role of isoprostanes in cell cycle regulation and cardiac ion channel dysfunction
Under hypoxic conditions, an increase in the generation and release of 8-iso-PGF2α has been observed (Hart et al., 1998). In pulmonary artery ECs, this increase in isoprostane concentration was accompanied by monolayer dysfunction, which was in contrast to the isoprostane-induced apoptosis in ECs, not induced via cell death (Hart et al., 1998; Benndorf et al., 2008). Furthermore, a role of A/J isoprostanes in cell cycle regulation has been shown. These compounds can be incorporated into cells and accumulate in the nucleus, inducing a G1 cell cycle arrest (Chen et al., 1999a; Brooks et al., 2008).
ROS, generated during the process of ischaemia in the mitochondria of cardiomyocytes (Becker et al., 1999) may affect the function of cardiac channel proteins. E2-isoketals, highly reactive products of the isoprostane pathway, are associated with cardiac Na+ channel dysfunction indicating a role of isoprostanes in ischaemia-related conduction abnormalities and arrhythmias (Fukuda et al., 2005). In summary, isoprostanes modulate platelet activation, the initiation of inflammatory processes, vasoconstriction, the disturbance of the vascular endothelial barrier, angiogenesis and EC cell death, indicating a mechanistically relevant role of these oxidative stress markers in the pathogenesis and progression of CVDs.
Relevance of isoprostanes in CVD
Based on the biological activities of isoprostanes discussed above, a role of these compounds in CVD seems to be obvious. Isoprostane-mediated effects with potential relevance for the pathogenesis of CVD are summarized in Figure 3. In apolipoprotein E-deficient mice, overexpression of the hydroperoxide scavenger GSH peroxidase-4 significantly reduced aortic F2-isoprostane levels accompanied by a significant decrease in atherosclerotic lesions sizes (Guo et al., 2008). Furthermore, in patients with coronary heart disease, those with advanced atherosclerotic plaque formation exhibit significantly higher extent of 8-iso-PGF2α accumulation in close proximity to the atherosclerotic lesions (Mehrabi et al., 1999). Levels of isoprostanes correlated with the number of risk factors for coronary artery disease present in patients and significantly increased with the number of diseased vessels thereby confirming the role of oxidative stress in the atherosclerotic process (Schwedhelm et al., 2004; Basarici et al., 2007; 2008). In several studies involving patients with coronary artery disease, up to 2–3-fold higher plasma and urinary levels of 8-iso-PGF2α have been detected as compared with age- and sex-matched healthy individuals, additionally correlating with the extent and the severity of the disease (Schwedhelm et al., 2000; 2004; Vassalle et al., 2003; Wang et al., 2006; Radovanovic et al., 2008; Roest et al., 2008; Di Minno et al., 2012). These studies indicate that isoprostanes are cumulative and independent risk markers in coronary artery diseases.
Figure 3.
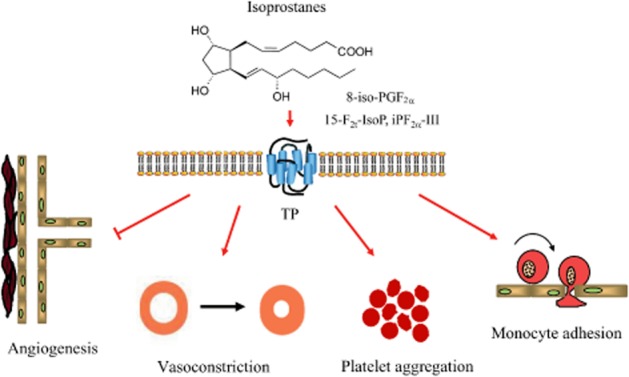
Proposed effects of isoprostanes in the cardiovascular system. Isoprostanes act as partial agonists of the TP receptor and may represent important alternative activators of the TP receptor especially in the context of oxidative stress.
Coronary endothelial dysfunction in humans is characterized by local enhancement of oxidative stress without a decrease in basal NO release (Lavi et al., 2008). Interestingly, isoprostane concentrations measured in the coronary sinus were 29% higher in patients with endothelial dysfunction, emphasizing the role of 8-iso-PGF2α as a marker of regional endothelial dysfunction in humans (Lavi et al., 2008). Furthermore, by investigating changes in coronary artery diameter and coronary flow, a more important role of isoprostanes in epicardial than in microcirculatory endothelial dysfunction has been described (Lavi et al., 2008).
In the effluents of isolated and perfused rat hearts, an increase in 8-iso-PGF2α concentration from only a few pg·mL−1 up to nearly 100 pg·mL−1 during ischaemia has been observed (Xia et al., 2003; 2005). This elevation in isoprostane levels was accompanied by an increased myocardial infarct size and exacerbated post-ischaemic myocardial dysfunction, probably mediated via a stimulated production and release of endothelin-1 during ischaemia (Xia et al., 2005). In a canine model of coronary thrombolysis and in patients with acute myocardial infarction, an increase in urinary 8-iso-PGF2α concentrations of approximately 28 and 300%, respectively, was observed, indicating that coronary reperfusion is associated with an increased generation of isoprostanes, which is likely to reflect oxidant stress in vivo (Delanty et al., 1997). Furthermore, in patients undergoing coronary artery bypass surgery or acute revascularization in the context of myocardial infarction, a 2–3-fold increase in plasma and urinary 8-iso-PGF2α levels has been detected, confirming the association between ischaemia/reperfusion and isoprostane generation (Reilly et al., 1997; Ansley et al., 2003). The generation of isoprostanes resulting from lipid peroxidation seems to occur immediately after reperfusion because no further increase in the isoprostane concentration could be observed in subsequent post-operative period (Ansley et al., 2003; Ulus et al., 2003). In contrast, in clinical ischaemia/reperfusion injury, no increase of 8-iso-PGF2α levels in plasma and urine during early reperfusion of the ischaemic kidney or heart has been described, indicating a highly complex and sensitive process of isoprostane formation under ischaemic conditions (de Vries et al., 2013). Furthermore, in patients with ischaemic chronic heart failure, levels of 8-iso-PGF2α correlated significantly with indices of remodelling (Radovanovic et al., 2008). Here, the authors demonstrated that markers of oxidative stress, such as isoprostanes, are unlikely to play an important role in early stages of chronic heart failure, but might become important in the course of this disease (Radovanovic et al., 2008). In this stage, urinary 8-iso-PGF2α could be used as a reliable indicator of symptomatic chronic heart failure (Radovanovic et al., 2008). Generally, a correlation between oxidative stress, elevated isoprostane concentrations and the severity and outcome of CVD has been demonstrated in animal and human studies. Therefore, a targeted inhibition of isoprostane generation or its interaction with TP receptors could help to improve outcome in patients suffering from CVD.
Outlook
Several cardiovascular pathologies are characterized by elevated isoprostane formation and excretion (Cracowski et al., 2001; Cracowski and Durand, 2006; Schwedhelm et al., 2007). Moreover, isoprostanes are involved in the pathophysiology of CVD by activating the TP receptor (Galano et al., 2013). The inhibition of isoprostane formation or TP receptor activation may therefore represent a valuable clinical strategy in patients at a high cardiovascular risk. Considering a causative role of isoprostanes in CVD, detection of isoprostane concentrations in plasma or further body fluids could help to identify patients at high risk of developing cardiovascular complications. Formation of isoprostanes may then be suppressed by several therapeutic strategies such as up-regulation of antioxidant enzymes, such as SOD and pharmacological inhibition of ROS formation by novel low MW NADPH oxidase inhibitors. Moreover, pharmacological antagonism of TP receptors could represent an alternative therapeutic strategy in patients with extensive isoprostane formation. Several TP receptor antagonists have been developed and used in pre- and clinical testing (Davì et al., 2012), but their clinical impact is still negligible today. In this regard, preclinical and clinical development of TP receptor antagonists may have suffered from insufficient specificity and efficacy or unexpected side effects of drug candidates. Design of more specific TP receptor antagonists and identification of patients who may clearly benefit from additional TP receptor blockade could thus be a rewarding challenge in the near future. So far, the TP receptor has not been crystallized and structural information is still incomplete. Therefore, fully elucidating the molecular structure of the TP receptor may foster the development of more specific and effective antagonists of this receptor, which may help to further reduce cardiovascular complications in high-risk patients.
Acknowledgments
This work was supported by the IZKF Würzburg (E-251), the German Research Foundation [DFG Be 3246/4-1, SFB 688 (S. F.)] and the Bundesministerium für Bildung und Forschung (BMBF01 EO1004) through the Comprehensive Heart Failure Center (S. F.).
Glossary
- CVD
cardiovascular disease
- ECs
endothelial cells
- LDL
low-density lipoproteins
- ROS
reactive oxygen species
- VSMC
vascular smooth muscle cell
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Acquaviva A, Vecchio D, Arezzini B, Comporti M, Gardi C. Signaling pathways involved in isoprostane-mediated fibrogenic effects in rat hepatic stellate cells. Free Radic Biol Med. 2013;65c:201–207. doi: 10.1016/j.freeradbiomed.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013a;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally AI, Horrobin DF. Thromboxane A2 in blood vessel walls and its physiological significance: relevance to thrombosis and hypertension. Prostaglandins Med. 1980;4:431–438. doi: 10.1016/0161-4630(80)90051-8. [DOI] [PubMed] [Google Scholar]
- Ansley DM, Xia Z, Dhaliwal BS. The relationship between plasma free 15-F2t-isoprostane concentration and early postoperative cardiac depression following warm heart surgery. J Thorac Cardiovasc Surg. 2003;126:1222–1223. doi: 10.1016/s0022-5223(03)00794-3. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Jones RL, Wilson NH. Ligand binding to thromboxane receptors on human platelets: correlation with biological activity. Br J Pharmacol. 1983;79:953–964. doi: 10.1111/j.1476-5381.1983.tb10541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004;95:372–379. doi: 10.1161/01.RES.0000138300.41642.15. [DOI] [PubMed] [Google Scholar]
- Ashton AW, Cheng Y, Helisch A, Ware JA. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and alpha(v)beta3. Circ Res. 2004;94:735–742. doi: 10.1161/01.RES.0000122043.11286.57. [DOI] [PubMed] [Google Scholar]
- Audoly LP, Rocca B, Fabre JE, Koller BH, Thomas D, Loeb AL, et al. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation. 2000;101:2833–2840. doi: 10.1161/01.cir.101.24.2833. [DOI] [PubMed] [Google Scholar]
- Aukrust P, Halvorsen B, Ueland T, Michelsen AE, Skjelland M, Gullestad L, et al. Activated platelets and atherosclerosis. Expert Rev Cardiovasc Ther. 2010;8:1297–1307. doi: 10.1586/erc.10.92. [DOI] [PubMed] [Google Scholar]
- Balapure AK, Caicedo IC, Kawada K, Watt DS, Rexroad CE, Jr, Fitz TA. Multiple classes of prostaglandin F2 alpha binding sites in subpopulations of ovine luteal cells. Biol Reprod. 1989;41:385–392. doi: 10.1095/biolreprod41.3.385. [DOI] [PubMed] [Google Scholar]
- Barocas DA, Motley S, Cookson MS, Chang SS, Penson DF, Dai Q, et al. Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J Urol. 2011;185:2102–2107. doi: 10.1016/j.juro.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarici I, Altekin RE, Demir I, Yilmaz H. Associations of isoprostanes-related oxidative stress with surrogate subclinical indices and angiographic measures of atherosclerosis. Coron Artery Dis. 2007;18:615–620. doi: 10.1097/MCA.0b013e3282f0efa5. [DOI] [PubMed] [Google Scholar]
- Basarici I, Altekin RE, Demir I, Yilmaz H. Urinary 8-isoprostane levels can indicate the presence, severity and extent of angiographic coronary artery disease. Acta Cardiol. 2008;63:415–422. doi: 10.2143/AC.63.4.2033038. [DOI] [PubMed] [Google Scholar]
- Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- Benndorf RA, Schwedhelm E, Gnann A, Taheri R, Kom G, Didié M, et al. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ Res. 2008;103:1037–1046. doi: 10.1161/CIRCRESAHA.108.184036. [DOI] [PubMed] [Google Scholar]
- Borg C, Lim CT, Yeomans DC, Dieter JP, Komiotis D, Anderson EG, et al. Purification of rat brain, rabbit aorta, and human platelet thromboxane A2/prostaglandin H2 receptors by immunoaffinity chromatography employing anti-peptide and anti-receptor antibodies. J Biol Chem. 1994;269:6109–6116. [PubMed] [Google Scholar]
- Bowling N, Dubé GP, Kurtz WL, Brune KA, Saussy DL, Jr, Dorn GW, 2nd, et al. Characterization of thromboxane A2/prostaglandin H2 binding sites in guinea pig cardiac membrane preparations. J Mol Cell Cardiol. 1994;26:915–923. doi: 10.1006/jmcc.1994.1109. [DOI] [PubMed] [Google Scholar]
- Brezinski ME, Yanagisawa A, Darius H, Lefer AM. Anti-ischemic actions of a new thromboxane receptor antagonist during acute myocardial ischemia in cats. Am Heart J. 1985;110:1161–1167. doi: 10.1016/0002-8703(85)90006-7. [DOI] [PubMed] [Google Scholar]
- Brezinski ME, Yanagisawa A, Lefer AM. Cardioprotective actions of specific thromboxane receptor antagonist in acute myocardial ischemia. J Cardiovasc Pharmacol. 1987;9:65–71. [PubMed] [Google Scholar]
- Brooks JD, Milne GL, Yin H, Sanchez SC, Porter NA, Morrow JD. Formation of highly reactive cyclopentenone isoprostane compounds (A3/J3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2008;283:12043–12055. doi: 10.1074/jbc.M800122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayatte AJ, Du Y, Oliver-Krasinski J, Lavielle G, Verbeuren TJ, Cohen RA. The thromboxane receptor antagonist S18886 but not aspirin inhibits atherogenesis in apo E-deficient mice: evidence that eicosanoids other than thromboxane contribute to atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:1724–1728. doi: 10.1161/01.atv.20.7.1724. [DOI] [PubMed] [Google Scholar]
- Chen Y, Morrow JD, Roberts LJ., 2nd Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J Biol Chem. 1999a;274:10863–10868. doi: 10.1074/jbc.274.16.10863. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zackert WE, Roberts LJ, 2nd, Morrow JD. Evidence for the formation of a novel cyclopentenone isoprostane, 15-A2t-isoprostane (8-iso-prostaglandin A2) in vivo. Biochim Biophys Acta. 1999b;1436:550–556. doi: 10.1016/s0005-2760(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Chiang N, Kan WM, Tai HH. Site-directed mutagenesis of cysteinyl and serine residues of human thromboxane A2 receptor in insect cells. Arch Biochem Biophys. 1996;334:9–17. doi: 10.1006/abbi.1996.0423. [DOI] [PubMed] [Google Scholar]
- Chung HO, Yang Q, Catt KJ, Arora KK. Expression and function of the gonadotropin-releasing hormone receptor are dependent on a conserved apolar amino acid in the third intracellular loop. J Biol Chem. 1999;274:35756–35762. doi: 10.1074/jbc.274.50.35756. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Durand T. Cardiovascular pharmacology and physiology of the isoprostanes. Fundam Clin Pharmacol. 2006;20:417–427. doi: 10.1111/j.1472-8206.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Devillier P, Durand T, Stanke-Labesque F, Bessard G. Vascular biology of the isoprostanes. J Vasc Res. 2001;38:93–103. doi: 10.1159/000051036. [DOI] [PubMed] [Google Scholar]
- Crawford MH, Grover FL, Kolb WP, McMahan CA, O'Rourke RA, McManus LM, et al. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988;78:1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- Cyrus T, Ding T, Praticò D. Expression of thromboxane synthase, prostacyclin synthase and thromboxane receptor in atherosclerotic lesions: correlation with plaque composition. Atherosclerosis. 2010;208:376–381. doi: 10.1016/j.atherosclerosis.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999;59:4574–4577. [PubMed] [Google Scholar]
- Davì G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- Davì G, Santilli F, Vazzana N. Thromboxane receptors antagonists and/or synthase inhibitors. Handb Exp Pharmacol. 2012;210:261–286. doi: 10.1007/978-3-642-29423-5_11. [DOI] [PubMed] [Google Scholar]
- Davies SS, Roberts LJ., 2nd F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med. 2011;50:559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo DD, Davis MG, Ali S, Dorn GW., 2nd Cloning and pharmacologic characterization of a thromboxane A2 receptor from K562 (human chronic myelogenous leukemia) cells. J Pharmacol Exp Ther. 1994;271:1034–1041. [PubMed] [Google Scholar]
- D'Angelo DD, Eubank JJ, Davis MG, Dorn GW., 2nd Mutagenic analysis of platelet thromboxane receptor cysteines. Roles in ligand binding and receptor-effector coupling. J Biol Chem. 1996;271:6233–6240. doi: 10.1074/jbc.271.11.6233. [DOI] [PubMed] [Google Scholar]
- Delannoy E, Courtois A, Freund-Michel V, Leblais V, Marthan R, Muller B. Hypoxia-induced hyperreactivity of pulmonary arteries: role of cyclooxygenase-2, isoprostanes, and thromboxane receptors. Cardiovasc Res. 2010;85:582–592. doi: 10.1093/cvr/cvp292. [DOI] [PubMed] [Google Scholar]
- Delanty N, Reilly MP, Pratico D, Lawson JA, McCarthy JF, Wood AE, et al. 8-epi PGF2 alpha generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation. 1997;95:2492–2499. doi: 10.1161/01.cir.95.11.2492. [DOI] [PubMed] [Google Scholar]
- Di Minno MN, Cavalca V, D'angelo A, Squellerio I, Coppola A, Tremoli E, et al. Urinary excretion of iPF(2α)-III predicts the risk of future thrombotic events. A 10-year follow-up. Thromb Res. 2012;129:208–211. doi: 10.1016/j.thromres.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, DeJesus A. Human platelet aggregation and shape change are coupled to separate thromboxane A2-prostaglandin H2 receptors. Am J Physiol. 1991;260:H327–H334. doi: 10.1152/ajpheart.1991.260.2.H327. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Liel N, Trask JL, Mais DE, Assey ME, Halushka PV. Increased platelet thromboxane A2/prostaglandin H2 receptors in patients with acute myocardial infarction. Circulation. 1990;81:212–218. doi: 10.1161/01.cir.81.1.212. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Davis MG, D'Angelo DD. Structural determinants for agonist binding affinity to thromboxane/prostaglandin endoperoxide (TP) receptors. Analysis of chimeric rat/human TP receptor receptors. J Biol Chem. 1997;272:12399–12405. doi: 10.1074/jbc.272.19.12399. [DOI] [PubMed] [Google Scholar]
- Elmhurst JL, Betti PA, Rangachari PK. Intestinal effects of isoprostanes: evidence for the involvement of prostanoid EP and TP receptor receptors. J Pharmacol Exp Ther. 1997;282:1198–1205. [PubMed] [Google Scholar]
- Favreau F, Petit-Paris I, Hauet T, Dutheil D, Papet Y, Mauco G, et al. Cyclooxygenase 1-dependent production of F2-isoprostane and changes in redox status during warm renal ischemia-reperfusion. Free Radic Biol Med. 2004;36:1034–1042. doi: 10.1016/j.freeradbiomed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Fediuk J, Gutsol A, Nolette N, Dakshinamurti S. Thromboxane-induced actin polymerization in hypoxic pulmonary artery is independent of rho. Am J Physiol Lung Cell Mol Physiol. 2012;302:L13–L26. doi: 10.1152/ajplung.00016.2011. [DOI] [PubMed] [Google Scholar]
- Fennekohl A, Schieferdecker HL, Jungermann K, Püschel GP. Differential expression of prostanoid receptors in hepatocytes, Kupffer cells, sinusoidal endothelial cells and stellate cells of rat liver. J Hepatol. 1999;30:38–47. doi: 10.1016/s0168-8278(99)80006-3. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Fontana L, Giagulli C, Minuz P, Lechi A, Laudanna C. 8-Iso-PGF2 alpha induces beta 2-integrin-mediated rapid adhesion of human polymorphonuclear neutrophils: a link between oxidative stress and ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2001;21:55–60. doi: 10.1161/01.atv.21.1.55. [DOI] [PubMed] [Google Scholar]
- Fontana L, Giagulli C, Cominacini L, Pasini AF, Minuz P, Lechi A, et al. Beta2 integrin-dependent neutrophil adhesion induced by minimally modified low-density lipoproteins is mainly mediated by F2-isoprostanes. Circulation. 2002;106:2434–2441. doi: 10.1161/01.cir.0000037223.92135.38. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Davies SS, Nakajima T, Ong B-H, Kupershmidt S, Fessel J, et al. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97:1262–1269. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- Funk CD, Furci L, Moran N, Fitzgerald GA. Point mutation in the seventh hydrophobic domain of the human thromboxane A2 receptor allows discrimination between agonist and antagonist binding sites. Mol Pharmacol. 1993;44:934–939. [PubMed] [Google Scholar]
- Galano J-M, Mas E, Barden A, Mori TA, Signorini C, De Felice C, et al. Isoprostanes and neuroprostanes: total synthesis, biological activity and biomarkers of oxidative stress in humans. Prostaglandins Other Lipid Mediat. 2013;107:95–102. doi: 10.1016/j.prostaglandins.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Geng L, Wu J, So S-P, Huang G, Ruan K-H. Structural and functional characterization of the first intracellular loop of human thromboxane A2 receptor. Arch Biochem Biophys. 2004;423:253–265. doi: 10.1016/j.abb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46:241–281. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo JA, Erickson RW, et al. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab Off J. 2001;21:374–384. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52:237–268. [PubMed] [Google Scholar]
- Grosser T, Yu Y, Fitzgerald GA. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu Rev Med. 2010;61:17–33. doi: 10.1146/annurev-med-011209-153129. [DOI] [PubMed] [Google Scholar]
- Guo Z, Ran Q, Roberts LJ, 2nd, Zhou L, Richardson A, Sharan C, et al. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein E-deficient mice. Free Radic Biol Med. 2008;44:343–352. doi: 10.1016/j.freeradbiomed.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy KD, Cox BE, Milne GL, Yin H, Roberts LJ., 2nd Nonenzymic free radical-catalyzed generation of 15-deoxy-Δ(12,14)-prostaglandin J2-like compounds (deoxy-J2-isoprostanes) in vivo. J Lipid Res. 2011;52:113–124. doi: 10.1194/jlr.M010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CM, Karman RJ, Blackburn TL, Gupta MP, Garcia JG, Mohler ER., 3rd Role of 8-epi PGF2alpha, 8-isoprostane, in H2O2-induced derangements of pulmonary artery endothelial cell barrier function. Prostaglandins Leukot Essent Fatty Acids. 1998;58:9–16. doi: 10.1016/s0952-3278(98)90124-7. [DOI] [PubMed] [Google Scholar]
- Hedberg A, Hall SE, Ogletree ML, Harris DN, Liu EC. Characterization of [5,6-3H]SQ 29 548 as a high affinity radioligand, binding to thromboxane A2/prostaglandin H2-receptors in human platelets. J Pharmacol Exp Ther. 1988;245:786–792. [PubMed] [Google Scholar]
- Hedberg A, Mento PF, Liu EC, Hollander AM, Wilkes BM. Evidence for functional thromboxane A2-prostaglandin H2 receptors in human placenta. Am J Physiol. 1989;256:E256–E263. doi: 10.1152/ajpendo.1989.256.2.E256. [DOI] [PubMed] [Google Scholar]
- Hinton M, Mellow L, Halayko AJ, Gutsol A, Dakshinamurti S. Hypoxia induces hypersensitivity and hyperreactivity to thromboxane receptor agonist in neonatal pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;290:L375–L384. doi: 10.1152/ajplung.00307.2005. [DOI] [PubMed] [Google Scholar]
- Hinton M, Gutsol A, Dakshinamurti S. Thromboxane hypersensitivity in hypoxic pulmonary artery myocytes: altered TP receptor receptor localization and kinetics. Am J Physiol Lung Cell Mol Physiol. 2007;292:L654–L663. doi: 10.1152/ajplung.00229.2006. [DOI] [PubMed] [Google Scholar]
- Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, et al. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349:617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- Hock CE, Brezinski ME, Lefer AM. Anti-ischemic actions of a new thromboxane receptor antagonist, SQ-29,548, in acute myocardial ischemia. Eur J Pharmacol. 1986;122:213–219. doi: 10.1016/0014-2999(86)90105-6. [DOI] [PubMed] [Google Scholar]
- Hou X, Gobeil F, Jr, Peri K, Speranza G, Marrache AM, Lachapelle P, et al. Augmented vasoconstriction and thromboxane formation by 15-F(2t)-isoprostane (8-iso-prostaglandin F(2alpha)) in immature pig periventricular brain microvessels. Stroke J Cereb Circ. 2000;31:516–524. doi: 10.1161/01.str.31.2.516. discussion 525. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Bojanic D, Wilson A. Platelet activating factor and U44069 stimulate a GTPase activity in human platelets which is distinct from the guanine nucleotide regulatory proteins, Ns and Ni. Biochem J. 1986;234:737–740. doi: 10.1042/bj2340737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J, Bochkov VN, Binder BR, Leitinger N. The isoprostane 8-iso-PGE2 stimulates endothelial cells to bind monocytes via cyclic AMP- and p38 MAP kinase-dependent signaling pathways. Antioxid Redox Signal. 2003;5:163–169. doi: 10.1089/152308603764816523. [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Tazzeo T. Involvement of TP receptor and EP3 receptors in vasoconstrictor responses to isoprostanes in pulmonary vasculature. J Pharmacol Exp Ther. 2002;301:1060–1066. doi: 10.1124/jpet.301.3.1060. [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Premji M, Netherton S, Coruzzi J, Lu-Chao H, Cox PG. Vasoconstrictor actions of isoprostanes via tyrosine kinase and Rho kinase in human and canine pulmonary vascular smooth muscles. Br J Pharmacol. 2001;132:127–134. doi: 10.1038/sj.bjp.0703784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan KB, Mitchell JA, Evans TW. Release of isoprostanes by human pulmonary artery in organ culture: a cyclo-oxygenase and nitric oxide dependent pathway. Biochem Biophys Res Commun. 1997;233:668–672. doi: 10.1006/bbrc.1997.6523. [DOI] [PubMed] [Google Scholar]
- Jourdan KB, Evans TW, Goldstraw P, Mitchell JA. Isoprostanes and PGE2 production in human isolated pulmonary artery smooth muscle cells: concomitant and differential release. FASEB J. 1999;13:1025–1030. doi: 10.1096/fasebj.13.9.1025. [DOI] [PubMed] [Google Scholar]
- Katugampola SD, Davenport AP. Thromboxane receptor density is increased in human cardiovascular disease with evidence for inhibition at therapeutic concentrations by the AT(1) receptor antagonist losartan. Br J Pharmacol. 2001;134:1385–1392. doi: 10.1038/sj.bjp.0704416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katugampola SD, Kuc RE, Maguire JJ, Davenport AP. G-protein-coupled receptors in human atherosclerosis: comparison of vasoconstrictors (endothelin and thromboxane) with recently de-orphanized (urotensin-II, apelin and ghrelin) receptors. Clin Sci Lond Engl 1979. 2002;103(Suppl. 48):171S–175S. doi: 10.1042/CS103S171S. [DOI] [PubMed] [Google Scholar]
- Khadem-Ansari M-H, Shahsavari Z, Rasmi Y, Mahmoodlo R. Elevated levels of urinary 8-hydroxy-2'-deoxyguanosine and 8-isoprostane in esophageal squamous cell carcinoma. J Carcinog. 2011;10:14. doi: 10.4103/1477-3163.79683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasawneh FT, Huang J-S, Turek JW, Le Breton GC. Differential mapping of the amino acids mediating agonist and antagonist coordination with the human thromboxane A2 receptor protein. J Biol Chem. 2006;281:26951–26965. doi: 10.1074/jbc.M507469200. [DOI] [PubMed] [Google Scholar]
- Khasawneh FT, Huang J-S, Mir F, Srinivasan S, Tiruppathi C, Le Breton GC. Characterization of isoprostane signaling: evidence for a unique coordination profile of 8-iso-PGF(2alpha) with the thromboxane A(2) receptor, and activation of a separate cAMP-dependent inhibitory pathway in human platelets. Biochem Pharmacol. 2008;75:2301–2315. doi: 10.1016/j.bcp.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- King LS, Fukushima M, Banerjee M, Kang KH, Newman JH, Biaggioni I. Pulmonary vascular effects of prostaglandin D2, but not its systemic vascular or airway effects, are mediated through thromboxane receptor activation. Circ Res. 1991;68:352–358. doi: 10.1161/01.res.68.2.352. [DOI] [PubMed] [Google Scholar]
- Kinsella BT, O'Mahony DJ, Fitzgerald GA. The human thromboxane A2 receptor alpha isoform (TP alpha) functionally couples to the G proteins Gq and G11 in vivo and is activated by the isoprostane 8-epi prostaglandin F2 alpha. J Pharmacol Exp Ther. 1997;281:957–964. [PubMed] [Google Scholar]
- Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, et al. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004;114:784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J, Epting D, Kern K, Dietz CT, Feng Y, Hammes H-P, et al. Inhibition of Rho-dependent kinases ROCK I/II activates VEGF-driven retinal neovascularization and sprouting angiogenesis. Am J Physiol Heart Circ Physiol. 2009;296:H893–H899. doi: 10.1152/ajpheart.01038.2008. [DOI] [PubMed] [Google Scholar]
- Kromer BM, Tippins JR. Coronary artery constriction by the isoprostane 8-epi prostaglandin F2 alpha. Br J Pharmacol. 1996;119:1276–1280. doi: 10.1111/j.1476-5381.1996.tb16033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kingdon E, Norman J. The isoprostane 8-iso-PGF2alpha suppresses monocyte adhesion to human microvascular endothelial cells via two independent mechanisms. FASEB J. 2005;19:443–445. doi: 10.1096/fj.03-1364fje. [DOI] [PubMed] [Google Scholar]
- Kunapuli P, Lawson JA, Rokach J, FitzGerald GA. Functional characterization of the ocular prostaglandin f2alpha (PGF2alpha) receptor. Activation by the isoprostane, 12-iso-PGF2alpha. J Biol Chem. 1997;272:27147–27154. doi: 10.1074/jbc.272.43.27147. [DOI] [PubMed] [Google Scholar]
- Laroche G, Lépine M-C, Thériault C, Giguère P, Giguère V, Gallant MA, et al. Oligomerization of the alpha and beta isoforms of the thromboxane A2 receptor: relevance to receptor signaling and endocytosis. Cell Signal. 2005;17:1373–1383. doi: 10.1016/j.cellsig.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, et al. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension. 2008;51:127–133. doi: 10.1161/HYPERTENSIONAHA.107.099986. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Rokach J, FitzGerald GA. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. J Biol Chem. 1999;274:24441–24444. doi: 10.1074/jbc.274.35.24441. [DOI] [PubMed] [Google Scholar]
- Leitinger N, Huber J, Rizza C, Mechtcheriakova D, Bochkov V, Koshelnick Y, et al. The isoprostane 8-iso-PGF(2alpha) stimulates endothelial cells to bind monocytes: differences from thromboxane-mediated endothelial activation. FASEB J. 2001;15:1254–1256. doi: 10.1096/fj.00-0498fje. [DOI] [PubMed] [Google Scholar]
- Longmire AW, Roberts LJ, Morrow JD. Actions of the E2-isoprostane, 8-ISO-PGE2, on the platelet thromboxane/endoperoxide receptor in humans and rats: additional evidence for the existence of a unique isoprostane receptor. Prostaglandins. 1994;48:247–256. doi: 10.1016/0090-6980(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lynch SM, Morrow JD, Roberts LJ, 2nd, Frei B. Formation of non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in plasma and low density lipoprotein exposed to oxidative stress in vitro. J Clin Invest. 1994;93:998–1004. doi: 10.1172/JCI117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- Mehrabi MR, Ekmekcioglu C, Tatzber F, Oguogho A, Ullrich R, Morgan A, et al. The isoprostane, 8-epi-PGF2 alpha, is accumulated in coronary arteries isolated from patients with coronary heart disease. Cardiovasc Res. 1999;43:492–499. doi: 10.1016/s0008-6363(99)00108-x. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Lawson D, Mehta P, Saldeen T. Increased prostacyclin and thromboxane A2 biosynthesis in atherosclerosis. Proc Natl Acad Sci U S A. 1988;85:4511–4515. doi: 10.1073/pnas.85.12.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miggin SM, Kinsella BT. Expression and tissue distribution of the mRNAs encoding the human thromboxane A2 receptor (TP) alpha and beta isoforms. Biochim Biophys Acta. 1998;1425:543–559. doi: 10.1016/s0304-4165(98)00109-3. [DOI] [PubMed] [Google Scholar]
- Milne GL, Zanoni G, Porta A, Sasi S, Vidari G, Musiek ES, et al. The cyclopentenone product of lipid peroxidation, 15-A2t-isoprostane, is efficiently metabolized by HepG2 cells via conjugation with glutathione. Chem Res Toxicol. 2004;17:17–25. doi: 10.1021/tx034213o. [DOI] [PubMed] [Google Scholar]
- Minuz P, Andrioli G, Degan M, Gaino S, Ortolani R, Tommasoli R, et al. The F2-isoprostane 8-epiprostaglandin F2alpha increases platelet adhesion and reduces the antiadhesive and antiaggregatory effects of NO. Arterioscler Thromb Vasc Biol. 1998;18:1248–1256. doi: 10.1161/01.atv.18.8.1248. [DOI] [PubMed] [Google Scholar]
- Minuz P, Patrignani P, Gaino S, Degan M, Menapace L, Tommasoli R, et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation. 2002;106:2800–2805. doi: 10.1161/01.cir.0000039528.49161.e9. [DOI] [PubMed] [Google Scholar]
- Miyosawa K, Sasaki M, Ohkubo S, Nakahata N. Different pathways for activation of extracellular signal-regulated kinase through thromboxane A2 receptor isoforms. Biol Pharm Bull. 2006;29:719–724. doi: 10.1248/bpb.29.719. [DOI] [PubMed] [Google Scholar]
- Modesti PA, Colella A, Cecioni I, Costoli A, Biagini D, Migliorini A, et al. Increased number of thromboxane A2-prostaglandin H2 platelet receptors in active unstable angina and causative role of enhanced thrombin formation. Am Heart J. 1995;129:873–879. doi: 10.1016/0002-8703(95)90106-x. [DOI] [PubMed] [Google Scholar]
- Moers A, Nieswandt B, Massberg S, Wettschureck N, Grüner S, Konrad I, et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003;9:1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Montine KS, Reich EE, Terry ES, Porter NA, Morrow JD. Antioxidants significantly affect the formation of different classes of isoprostanes and neuroprostanes in rat cerebral synaptosomes. Biochem Pharmacol. 2003;65:611–617. doi: 10.1016/s0006-2952(02)01607-6. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Peskind ER, Quinn JF, Wilson AM, Montine KS, Galasko D. Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer's disease as identified by biomarkers. Neuromolecular Med. 2011;13:37–43. doi: 10.1007/s12017-010-8126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi P, Barnes P, Roberts LJ., 2nd Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–717. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- Morrow JD. The isoprostanes – unique products of arachidonate peroxidation: their role as mediators of oxidant stress. Curr Pharm Des. 2006;12:895–902. doi: 10.2174/138161206776055985. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Harris TM, Roberts LJ., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990a;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990b;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ., 2nd Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Minton TA, Mukundan CR, Campbell MD, Zackert WE, Daniel VC, et al. Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J Biol Chem. 1994;269:4317–4326. [PubMed] [Google Scholar]
- Möbert J, Becker BF, Zahler S, Gerlach E. Hemodynamic effects of isoprostanes (8-iso-prostaglandin F2alpha and E2) in isolated guinea pig hearts. J Cardiovasc Pharmacol. 1997;29:789–794. doi: 10.1097/00005344-199706000-00012. [DOI] [PubMed] [Google Scholar]
- Mueed I, Tazzeo T, Liu C, Pertens E, Zhang Y, Cybulski I, et al. Isoprostanes constrict human radial artery by stimulation of thromboxane receptors, Ca2+ release, and RhoA activation. J Thorac Cardiovasc Surg. 2008;135:131–138. doi: 10.1016/j.jtcvs.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Muja N, Blackman SC, Le Breton GC, DeVries GH. Identification and functional characterization of thromboxane A2 receptors in Schwann cells. J Neurochem. 2001;78:446–456. doi: 10.1046/j.1471-4159.2001.00378.x. [DOI] [PubMed] [Google Scholar]
- Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther. 2008;118:18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Namba T, Sugimoto Y, Hirata M, Hayashi Y, Honda A, Watabe A, et al. Mouse thromboxane A2 receptor: cDNA cloning, expression and Northern blot analysis. Biochem Biophys Res Commun. 1992;184:1197–1203. doi: 10.1016/s0006-291x(05)80009-9. [DOI] [PubMed] [Google Scholar]
- Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976;261:558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- Nehls V, Schuchardt E, Drenckhahn D. The effect of fibroblasts, vascular smooth muscle cells, and pericytes on sprout formation of endothelial cells in a fibrin gel angiogenesis system. Microvasc Res. 1994;48:349–363. doi: 10.1006/mvre.1994.1061. [DOI] [PubMed] [Google Scholar]
- Nie D, Lamberti M, Zacharek A, Li L, Szekeres K, Tang K, et al. Thromboxane A(2) regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem Biophys Res Commun. 2000;267:245–251. doi: 10.1006/bbrc.1999.1840. [DOI] [PubMed] [Google Scholar]
- Nüsing RM, Hirata M, Kakizuka A, Eki T, Ozawa K, Narumiya S. Characterization and chromosomal mapping of the human thromboxane A2 receptor gene. J Biol Chem. 1993;268:25253–25259. [PubMed] [Google Scholar]
- Offermanns S, Simon MI. G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem. 1995;270:15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Laugwitz KL, Spicher K, Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc Natl Acad Sci U S A. 1994;91:504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L, Stallwood NA, Crankshaw DJ. Effects of some isoprostanes on the human umbilical artery in vitro. Br J Pharmacol. 2000;129:509–514. doi: 10.1038/sj.bjp.0703083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Wu J, Murray JK, Gellman SH, Wozniak MA, Keely PJ, et al. An antiangiogenic neurokinin-B/thromboxane A2 regulatory axis. J Cell Biol. 2006;174:1047–1058. doi: 10.1083/jcb.200603152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MA, Piper PJ, Vane JR. The release of rabbit aorta contracting substance (RCS) from chopped lung and its antagonism by anti-inflammatory drugs. Br J Pharmacol. 1970;40:581P–582P. [PMC free article] [PubMed] [Google Scholar]
- Patrignani P, Santini G, Panara MR, Sciulli MG, Greco A, Rotondo MT, et al. Induction of prostaglandin endoperoxide synthase-2 in human monocytes associated with cyclo-oxygenase-dependent F2-isoprostane formation. Br J Pharmacol. 1996;118:1285–1293. doi: 10.1111/j.1476-5381.1996.tb15535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidgeon GP, Tamosiuniene R, Chen G, Leonard I, Belton O, Bradford A, et al. Intravascular thrombosis after hypoxia-induced pulmonary hypertension: regulation by cyclooxygenase-2. Circulation. 2004;110:2701–2707. doi: 10.1161/01.CIR.0000145613.01188.0B. [DOI] [PubMed] [Google Scholar]
- Pignatelli P, Carnevale R, Di Santo S, Bartimoccia S, Sanguigni V, Lenti L, et al. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler Thromb Vasc Biol. 2011;31:423–434. doi: 10.1161/ATVBAHA.110.217885. [DOI] [PubMed] [Google Scholar]
- Praticó D, FitzGerald GA. Generation of 8-epiprostaglandin F2alpha by human monocytes. Discriminate production by reactive oxygen species and prostaglandin endoperoxide synthase-2. J Biol Chem. 1996;271:8919–8924. doi: 10.1074/jbc.271.15.8919. [DOI] [PubMed] [Google Scholar]
- Praticò D, Iuliano L, Mauriello A, Spagnoli L, Lawson JA, Rokach J, et al. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest. 1997;100:2028–2034. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D, Reilly M, Lawson J, Delanty N, FitzGerald GA. Formation of 8-iso-prostaglandin F2 alpha by human platelets. Agents Actions Suppl. 1995;45:27–31. [PubMed] [Google Scholar]
- Pryor WA, Stanley JP, Blair E. Autoxidation of polyunsaturated fatty acids: II. A suggested mechanism for the formation of TBA-reactive materials from prostaglandin-like endoperoxides. Lipids. 1976;11:370–379. doi: 10.1007/BF02532843. [DOI] [PubMed] [Google Scholar]
- Radovanovic S, Krotin M, Simic DV, Mimic-Oka J, Savic-Radojevic A, Pljesa-Ercegovac M, et al. Markers of oxidative damage in chronic heart failure: role in disease progression. Redox Rep Commun Free Radic Res. 2008;13:109–116. doi: 10.1179/135100008X259204. [DOI] [PubMed] [Google Scholar]
- Raychowdhury MK, Yukawa M, Collins LJ, McGrail SH, Kent KC, Ware JA. Alternative splicing produces a divergent cytoplasmic tail in the human endothelial thromboxane A2 receptor. J Biol Chem. 1994;269:19256–19261. [PubMed] [Google Scholar]
- Reid HM, Kinsella BT. The alpha, but not the beta, isoform of the human thromboxane A2 receptor is a target for nitric oxide-mediated desensitization. Independent modulation of TP receptor alpha signaling by nitric oxide and prostacyclin. J Biol Chem. 2003;278:51190–51202. doi: 10.1074/jbc.M309314200. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Delanty N, Roy L, Rokach J, Callaghan PO, Crean P, et al. Increased formation of the isoprostanes IPF2alpha-I and 8-epi-prostaglandin F2alpha in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation. 1997;96:3314–3320. doi: 10.1161/01.cir.96.10.3314. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Praticò D, Delanty N, DiMinno G, Tremoli E, Rader D, et al. Increased formation of distinct F2 isoprostanes in hypercholesterolemia. Circulation. 1998;98:2822–2828. doi: 10.1161/01.cir.98.25.2822. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, 2nd, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, et al. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- Roest M, Voorbij HAM, Van der Schouw YT, Peeters PHM, Teerlink T, Scheffer PG. High levels of urinary F2-isoprostanes predict cardiovascular mortality in postmenopausal women. J Clin Lipidol. 2008;2:298–303. doi: 10.1016/j.jacl.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Rokach J, Khanapure SP, Hwang SW, Adiyaman M, Lawson JA, FitzGerald GA. Nomenclature of isoprostanes: a proposal. Prostaglandins. 1997;54:853–873. doi: 10.1016/s0090-6980(97)00184-6. [DOI] [PubMed] [Google Scholar]
- Ruan K-H, Cervantes V, Wu J. Ligand-specific conformation determines agonist activation and antagonist blockade in purified human thromboxane A2 receptor. Biochemistry (Mosc) 2009;48:3157–3165. doi: 10.1021/bi801443g. [DOI] [PubMed] [Google Scholar]
- Sakariassen KS, Femia EA, Daray FM, Podda GM, Razzari C, Pugliano M, et al. EV-077 in vitro inhibits platelet aggregation in type-2 diabetics on aspirin. Thromb Res. 2012;130:746–752. doi: 10.1016/j.thromres.2012.08.309. [DOI] [PubMed] [Google Scholar]
- Sametz W, Hennerbichler S, Glaser S, Wintersteiger R, Juan H. Characterization of prostanoid receptors mediating actions of the isoprostanes, 8-iso-PGE(2) and 8-iso-PGF(2alpha), in some isolated smooth muscle preparations. Br J Pharmacol. 2000;130:1903–1910. doi: 10.1038/sj.bjp.0703522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Miyosawa K, Ohkubo S, Nakahata N. Physiological significance of thromboxane A(2) receptor dimerization. J Pharmacol Sci. 2006;100:263–270. doi: 10.1254/jphs.fp0050839. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Sukegawa J, Miyosawa K, Yanagisawa T, Ohkubo S, Nakahata N. Low expression of cell-surface thromboxane A2 receptor beta-isoform through the negative regulation of its membrane traffic by proteasomes. Prostaglandins Other Lipid Mediat. 2007;83:237–249. doi: 10.1016/j.prostaglandins.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Saussy DL, Jr, Mais DE, Dubé GP, Magee DE, Brune KA, Kurtz WL, et al. Characterization of a thromboxane A2/prostaglandin H2 receptor in guinea pig lung membranes using a radioiodinated thromboxane mimetic. Mol Pharmacol. 1991;39:72–78. [PubMed] [Google Scholar]
- Sbardella E, Greco A, Stromillo ML, Prosperini L, Puopolo M, Cefaro LA, et al. Isoprostanes in clinically isolated syndrome and early multiple sclerosis as biomarkers of tissue damage and predictors of clinical course. Mult Scler. 2013;19:411–417. doi: 10.1177/1352458512457721. [DOI] [PubMed] [Google Scholar]
- Scholz H, Yndestad A, Damås JK, Waehre T, Tonstad S, Aukrust P, et al. 8-isoprostane increases expression of interleukin-8 in human macrophages through activation of mitogen-activated protein kinases. Cardiovasc Res. 2003;59:945–954. doi: 10.1016/s0008-6363(03)00538-8. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Tsikas D, Durand T, Gutzki FM, Guy A, Rossi JC, et al. Tandem mass spectrometric quantification of 8-iso-prostaglandin F2alpha and its metabolite 2,3-dinor-5,6-dihydro-8-iso-prostaglandin F2alpha in human urine. J Chromatogr B Biomed Sci App. 2000;744:99–112. doi: 10.1016/s0378-4347(00)00236-x. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brümmer J, et al. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109:843–848. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Benndorf RA, Böger RH, Tsikas D. Mass spectrometric analysis of F2-isoprostanes: markers and mediators in human disease. Curr Pharm Anal. 2007;3:39–51. [Google Scholar]
- Schwedhelm E, Bierend A, Maas R, Trinks R, Kom GD, Tsikas D, et al. Redox-generated isoprostanes are associated with residual platelet activity in aspirin-treated patients with stable coronary heart disease. J Thromb Haemost, JTH. 2010;8:2662–2670. doi: 10.1111/j.1538-7836.2010.04117.x. [DOI] [PubMed] [Google Scholar]
- So S-P, Wu J, Huang G, Huang A, Li D, Ruan K-H. Identification of residues important for ligand binding of thromboxane A2 receptor in the second extracellular loop using the NMR experiment-guided mutagenesis approach. J Biol Chem. 2003;278:10922–10927. doi: 10.1074/jbc.M209337200. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Ioculano M, Altavilla D, Zingarelli B, Canale P, Campo GM, et al. Reduction of myocardial leukocyte accumulation and myocardial infarct size following administration of BAY u3405, a thromboxane A2 receptor antagonist, in myocardial ischaemia-reperfusion injury. Agents Actions. 1993;39:143–149. doi: 10.1007/BF01998967. [DOI] [PubMed] [Google Scholar]
- Stafforini DM, Sheller JR, Blackwell TS, Sapirstein A, Yull FE, McIntyre TM, et al. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J Biol Chem. 2006;281:4616–4623. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- Stegner D, Nieswandt B. Platelet receptor signaling in thrombus formation. J Mol Med Berl. 2011;89:109–121. doi: 10.1007/s00109-010-0691-5. [DOI] [PubMed] [Google Scholar]
- van der Sterren S, Villamor E. Contractile effects of 15-E2t-isoprostane and 15-F2t-isoprostane on chicken embryo ductus arteriosus. Comp Biochem Physiol A Mol Integr Physiol. 2011;159:436–444. doi: 10.1016/j.cbpa.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Sun FF, Chapman JP, McGuire JC. Metabolism of prostaglandin endoperoxide in animal tissues. Prostaglandins. 1977;14:1055–1074. doi: 10.1016/0090-6980(77)90285-4. [DOI] [PubMed] [Google Scholar]
- Taber DF, Morrow JD, Roberts LJ., 2nd A nomenclature system for the isoprostanes. Prostaglandins. 1997;53:63–67. doi: 10.1016/s0090-6980(97)00005-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nammour TM, Fukunaga M, Ebert J, Morrow JD, Roberts LJ, 2nd, et al. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J Clin Invest. 1992;90:136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Cyrus T, Yao Y, Vocun L, Praticò D. Involvement of thromboxane receptor in the proatherogenic effect of isoprostane F2alpha-III: evidence from apolipoprotein E- and LDL receptor-deficient mice. Circulation. 2005;112:2867–2874. doi: 10.1161/CIRCULATIONAHA105.562223. [DOI] [PubMed] [Google Scholar]
- Tomkin GH, Owens D. Abnormalities in apo B-containing lipoproteins in diabetes and atherosclerosis. Diabetes Metab Res Rev. 2001;17:27–43. doi: 10.1002/dmrr.179. [DOI] [PubMed] [Google Scholar]
- Tsikas D, Suchy M-T, Niemann J, Tossios P, Schneider Y, Rothmann S, et al. Glutathione promotes prostaglandin H synthase (cyclooxygenase)-dependent formation of malondialdehyde and 15(S)-8-iso-prostaglandin F2α. FEBS Lett. 2012;586:3723–3730. doi: 10.1016/j.febslet.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Turek JW, Halmos T, Sullivan NL, Antonakis K, Le Breton GC. Mapping of a ligand-binding site for the human thromboxane A2 receptor protein. J Biol Chem. 2002;277:16791–16797. doi: 10.1074/jbc.M105872200. [DOI] [PubMed] [Google Scholar]
- Ulus AT, Aksoyek A, Ozkan M, Katircioglu SF, Basu S. Cardiopulmonary bypass as a cause of free radical-induced oxidative stress and enhanced blood-borne isoprostanes in humans. Free Radic Biol Med. 2003;34:911–917. doi: 10.1016/s0891-5849(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Ushikubi F, Nakajima M, Hirata M, Okuma M, Fujiwara M, Narumiya S. Purification of the thromboxane A2/prostaglandin H2 receptor from human blood platelets. J Biol Chem. 1989;264:16496–16501. [PubMed] [Google Scholar]
- Valentin F, Field MC, Tippins JR. The mechanism of oxidative stress stabilization of the thromboxane receptor in COS-7 cells. J Biol Chem. 2004;279:8316–8324. doi: 10.1074/jbc.M306761200. [DOI] [PubMed] [Google Scholar]
- Vassalle C, Botto N, Andreassi MG, Berti S, Biagini A. Evidence for enhanced 8-isoprostane plasma levels, as index of oxidative stress in vivo, in patients with coronary artery disease. Coron Artery Dis. 2003;14:213–218. doi: 10.1097/01.mca.0000063504.13456.c3. [DOI] [PubMed] [Google Scholar]
- Vezza R, Habib A, FitzGerald GA. Differential signaling by the thromboxane receptor isoforms via the novel GTP-binding protein, Gh. J Biol Chem. 1999;274:12774–12779. doi: 10.1074/jbc.274.18.12774. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- de Vries DK, Kortekaas KA, Tsikas D, Wijermars LGM, Noorden CJF, van Suchy M-T, et al. Oxidative damage in clinical ischemia/reperfusion injury: a reappraisal. Antioxid Redox Signal. 2013;19:535–545. doi: 10.1089/ars.2012.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MT, Foley JF, Kinsella BT. Characterization of the role of N-linked glycosylation on the cell signaling and expression of the human thromboxane A2 receptor alpha and beta isoforms. J Pharmacol Exp Ther. 1998;286:1026–1036. [PubMed] [Google Scholar]
- Wang B, Pan J, Wang L, Zhu H, Yu R, Zou Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis. 2006;184:425–430. doi: 10.1016/j.atherosclerosis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Wei J, Yan W, Li X, Ding Y, Tai H-H. Thromboxane receptor alpha mediates tumor growth and angiogenesis via induction of vascular endothelial growth factor expression in human lung cancer cells. Lung Cancer. 2010;69:26–32. doi: 10.1016/j.lungcan.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Wikström K, Kavanagh DJ, Reid HM, Kinsella BT. Differential regulation of RhoA-mediated signaling by the TP receptoralpha and TP receptorbeta isoforms of the human thromboxane A2 receptor: independent modulation of TP receptoralpha signaling by prostacyclin and nitric oxide. Cell Signal. 2008;20:1497–1512. doi: 10.1016/j.cellsig.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005;389:763–774. doi: 10.1042/BJ20050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, McGinley K, Huang AJ, Smyth EM. Heterodimerization of the alpha and beta isoforms of the human thromboxane receptor enhances isoprostane signaling. Biochem Biophys Res Commun. 2007;352:397–403. doi: 10.1016/j.bbrc.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Godin DV, Chang TKH, Ansley DM. Dose-dependent protection of cardiac function by propofol during ischemia and early reperfusion in rats: effects on 15-F2t-isoprostane formation. Can J Physiol Pharmacol. 2003;81:14–21. doi: 10.1139/y02-170. [DOI] [PubMed] [Google Scholar]
- Xia Z, Kuo K-H, Godin DV, Walker MJ, Tao MCY, Ansley DM. 15-F(2t)-isoprostane exacerbates myocardial ischemia-reperfusion injury of isolated rat hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1366–H1372. doi: 10.1152/ajpheart.00042.2005. [DOI] [PubMed] [Google Scholar]
- Yin K, Halushka PV, Yan YT, Wong PY. Antiaggregatory activity of 8-epi-prostaglandin F2 alpha and other F-series prostanoids and their binding to thromboxane A2/prostaglandin H2 receptors in human platelets. J Pharmacol Exp Ther. 1994;270:1192–1196. [PubMed] [Google Scholar]
- Yura T, Fukunaga M, Khan R, Nassar GN, Badr KF, Montero A. Free-radical-generated F2-isoprostane stimulates cell proliferation and endothelin-1 expression on endothelial cells. Kidney Int. 1999;56:471–478. doi: 10.1046/j.1523-1755.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- Zahler S, Becker BF. Indirect enhancement of neutrophil activity and adhesion to cultured human umbilical vein endothelial cells by isoprostanes (iPF2alpha-III and iPE2-III) Prostaglandins Other Lipid Mediat. 1999;57:319–331. doi: 10.1016/s0090-6980(98)00079-3. [DOI] [PubMed] [Google Scholar]


