Abstract
BACKGROUND AND PURPOSE
Appetite suppression induced by amphetamine has been attributed to its inhibition of neuropeptide Y (NPY) neurons and activation of pro-opiomelanocortin (POMC) neurons in the hypothalamus. This study examined whether STAT3 was involved in these actions of amphetamine.
EXPERIMENTAL APPROACH
Rats were given amphetamine daily for 4 days. Changes in the expression of NPY, POMC, melanocortin MC3 receptors, PI3K and STAT3 in the hypothalamus were assessed by RT-PCR and Western blotting. Antisense oligonucleotides to STAT3 were also used.
KEY RESULTS
Expression of NPY decreased with a maximum effect day 2 of amphetamine treatment. Expression of POMC, MC3 receptors, PI3K and STAT3 increased with a maximum response on day 2. Moreover, phosphorylation of STAT3 and its DNA binding activity increased and was expressed in a similar pattern. Infusion (i.c.v.) of STAT3 antisense at 60 min before amphetamine treatment, partly blocked amphetamine-induced anorexia and modulated expression of NPY, POMC, MC3 receptors and PI3K, indicating the involvement of STAT3 in amphetamine-treated rats.
CONCLUSIONS AND IMPLICATIONS
Hypothalamic PI3K–STAT3 signalling participated in the regulation of NPY- and POMC-mediated appetite suppression. These findings may contribute to a better understanding of anorectic drugs.
Keywords: STAT3, PI3K, NPY, POMC, appetite, brain
Introduction
The mechanism underlying the response of appetite suppression in amphetamine-treated rats is relevant to the action of dopamine in the brain. Dopamine regulates feeding via hypothalamic circuits in the brain and dopaminergic neurons project to the arcuate nucleus and to the median eminence in the hypothalamus. These neurons may influence the function of neuropeptide Y (NPY) and pro-opiomelanocortin (POMC) neurons involved in ingestive behaviours (Kuo, 2006; Kim et al., 2010; nomenclature follows Alexander et al., 2013). Dopamine may decrease NPY but increase POMC in the hypothalamus, resulting in decreased food intake in amphetamine-treated rats (Chen et al., 2001; Kuo et al., 2011; 2012a). Hypothalamic NPY is a highly conserved neuropeptide that regulates feeding behaviour, energy balance (Mercer et al., 2011) and stress response (Morales-Medina et al., 2010). POMC neurons are essential regulators of food intake, energy expenditure and glucose homeostasis. Thus, POMC neurons integrate several key metabolic signals that include neurotransmitters and hormones, such as 5-HT, NPY, leptin and insulin (Sohn and Williams, 2012).
STAT3 was first identified and cloned from mouse liver cDNA library in the study of IL-6 signalling (Zhong et al., 1994). Of all the STAT family members, STAT3 seems to be specifically linked to neuronal survival during development and after injury, as STAT3 mediates a wide variety of biological functions in the central and peripheral nervous systems; therefore, injury to neural tissue induces STAT3 activation and STAT3 is increasingly recognized as having a role in neuronal survival (Dziennis and Alkayed, 2008; Rajan, 2011). STAT3 in NPY neurons is required for normal energy homeostasis (Gong et al., 2008) and mice without STAT3 in NPY neurons are mildly hyperphagic, and brain-specific depletion of STAT3 in NPY neurons results in increased expression of NPY mRNA, which leads to comparable obese phenotypes. STAT3 activation requires phosphorylation of a critical tyrosine residue (Tyr705), which mediates its dimerization, essential for its entry into the nucleus and DNA binding (Zhong et al., 1994). The phosphorylation of STAT3 (pSTAT3) at Tyr705 is most commonly mediated by JAKs, especially JAK2, but its activity is also subject to fine-tuning by other mechanisms, including serine (Ser727) phosphorylation (Wen et al., 1995). As amphetamine treatment in rats could modulate NPY and endogenous antioxidant expression (Kuo et al., 2012a), we hypothesized that pSTAT3 (Tyr705), pSTAT3 (Ser727) and pSTAT3/DNA binding activity might be involved in regulating NPY-mediated appetite control in amphetamine-treated rats.
The signalling kinase, PI3K, appears to play a role in the regulation of food intake and energy balance through hypothalamic neurons as pretreatment with PI3K inhibitors blunt the anorectic effect of i.c.v. administered leptin (Niswender et al., 2001; Sahu, 2011). Earlier work had revealed that the fasted rats showed increased expression of NPY and decreased expression of PI3K in the arcuate nucleus of the hypothalamus (Schwartz, 2006) and that pretreatment with a PI3K inhibitor blunted leptin-mediated inhibition of hypothalamic NPY gene expression (Morrison et al., 2005). These results revealed the close relationship between NPY and PI3K in the regulation of food intake. The PI3K activates downstream targets, such as Akt, which in turn may convey and coordinate the STAT3 to induce NPY or POMC gene expression (Roman et al., 2010; Sahu, 2011). Thus, we tested the prediction that PI3K–STAT3 signalling was involved in regulating the NPY- and POMC-mediated appetite suppression in amphetamine-treated rats.
Methods
Animal treatments
All animal care and experimental procedures complied with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health and were approved and reviewed by the National Science Council, Taiwan, Republic of China. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 250 animals were used in the experiments described here.
Male Wistar rats weighing 200–300 g were obtained from the National Laboratory Animal Center (Taipei, Taiwan). The animals were housed individually in cages and maintained at a temperature of 22 ± 2°C in a room with a 12 h light–dark cycle (light on at 0600 h). The rats were also habituated to frequent handling. Drugs were administered and food intake was determined every day at the beginning of the dark phase (1800 h). Water and chow (LabDiet; PMI Nutrition International, Brentwood, MO, USA) were freely available throughout the experiment. Food intake data points above 35 g·day–1 were discarded because they indicated food spillage.
Experimental procedures
To examine the effect of amphetamine on feeding behaviour and on the expression of NPY, POMC, PI3K and STAT3 mRNA levels, rats (N = 8 for each group) were injected i.p. with amphetamine at a dose of 2 or 4 mg·kg–1 daily for 4 days. Amphetamine was first injected at the end of day 0 (i.e. at 1800 h or at the beginning of day 1), and the daily food intake data were calculated with respect to the food amount in the cage, that is, the food amount on the measuring day minus the food amount on the previous day. Rats were anaesthetized (pentobarbital, 30–40 mg·kg–1, i.p.) and decapitated. The hypothalamus was immediately removed from the brain and used for determinations of mRNA levels or stored at −80°C until its use for other analyses.
To determine the effect of amphetamine (2 mg·kg–1, i.p.) on changes in hypothalamic expression of NPY, melanocortin MC3 receptors, PI3K, total STAT3 (t-STAT3), pSTAT3 (Tyr705) and pSTAT3 (Ser727), rats were injected with amphetamine (at 1800 h) once a day for 1, 2, 3 or 4 days, depending on the group. On the last rats received a treatment of 2 mg·kg–1 amphetamine 40 min before being killed to enhance the effects of the drug. It is because amphetamine-induced anorexia showed marked response in the first 6 h and a gradual restoration over the subsequent 18 h over a 24 h testing period (Kuo, 2005).
To examine the effect of amphetamine on STAT3 and DNA binding activity, rats were given amphetamine (2 mg·kg–1, i.p.; N = 6 per group) daily for four consecutive days. At 40 min after the last daily treatment, the rats were killed and the hypothalamus was removed to determine the STAT3 and DNA binding activity by an EMSA.
To assess the effect of pretreatment with STAT3 antisense oligodeoxynucleotides (ODN) on the anorectic response to amphetamine, rats (N = 8 per group) were given i.c.v. STAT3 antisense ODN (20 μg in 10 μL vehicle), 1 h before amphetamine (4 mg·kg–1, i.p.) treatment daily for 1, 2, 3 or 4 days, depending on the group. Before amphetamine treatment, rats were given a similar dose of STAT3 antisense (or missense) i.c.v. daily for 2–3 days until the feeding response was slightly reduced. This regimen was chosen because either continuous or repeated i.c.v. injections of antisense may be necessary to maximize behavioural effects and importantly to block the synthesis of a constitutively active gene product (Zhang and Creese, 1993; Ogawa and Pfaff, 1998). A full description of the i.c.v. cannulation and of the antisense is given below.
A further experiment was designed to determine the effect of pretreatment with STAT3 antisense (or missense) before daily amphetamine on NPY, POMC, PI3K and STAT3 mRNA levels. Before starting amphetamine treatment, rats (N = 6–8 per group) were infused daily with antisense or missense (20 μg in a 10 μL vehicle, i.c.v.) daily for 2–3 days until the feeding behaviour response was reduced slightly in the antisense group, as above. Then the same dose of antisense ODN was givne i.c.v. 1 h before amphetamine (4 mg·kg–1, i.p.) injections for 4 days. At 40 min after the last amphetamine treatment, rats were anaesthetized and the hypothalamus of each rat was removed and their NPY, POMC, PI3K and STAT3 mRNA levels were determined by RT-PCR. Similar experiments were undertaken to determine the effect of pretreatment with STAT3 antisense (or missense) before daily amphetamine on the expression of pSTAT3 (Tyr705) and pSTAT3 (Ser727). by Western blot.
RNA extraction
Hypothalamic NPY, POMC, PI3K and STAT3 mRNA were extracted in a block of mediobasal hypothalamic tissue as described previously (Magni and Barnea, 1992). In brief, total RNA was isolated from this block using the modified guanidinium thiocyanate-phenol-chloroform method (Chomczynski and Sacchi, 1987). Each hypothalamic block was homogenized in 1 mL of TRIZOL reagent (Life Technologies, Inc.) using an Ultrasonic Processor (Vibra Cell, Model CV17; Sonics & Materials, Inc., Danbury, CT, USA). After incubation at 22°C for 5 min, 0.2 mL of chloroform was added to each sample, shaken vigorously for 15 s, incubated at 22°C for 3 min and then centrifuged at 12 000× g for 15 min at 4°C. After removal of the aqueous phase and precipitation with 0.5 mL isopropanol, samples were incubated at 22°C for 10 min and centrifuged at 12 000× g for 15 min at 4°C. The gel-like RNA pellets were washed with 75% ethanol by vortexing and centrifugation at 7500× g for 5 min at 4°C. Thereafter, RNA pellets were dried briefly, dissolved in RNase-free water and stored at −80°C. The content of RNA was determined spectrophotometrically at 260 nm (Hitachi U-3210, Tokyo, Japan).
RT-PCR
Using the First Strand cDNA Synthesis Kit (Boehringer Mannheim GmbH, D-6800 Mannheim, Germany), RNA was reversely transcribed into single-stranded cDNA. For each sample, 8 μL of sterile diethyl pyrocarbonate (DEPC) water containing 2 μg of RNA was added to oligo-p(dT)15 primer (0.8 μg·μL–1) followed by heating at 65°C for 15 min, cooling at 25°C for 10 min and then adding to a reaction mixture consisting of 10× reaction buffer (100 mM Tris, 500 mM KCl; pH 8.3), deoxynucleotide mix (10 mM each), MgCl2 (25 mM), RNase inhibitor (40 U·μL–1) and AMV reverse transcriptase (25 U·μL–1). Reaction mixtures were incubated at 42°C for 2 h and then brought to 95°C for 5 min to terminate the reaction followed by soaking at 16°C. PCR was subsequently carried out by mixing 3 μL of cDNA product with mastermix solution consisting of DEPC water, 10× reaction buffer, MgCl2 (25 mM), deoxynucleotide mix (10 mM each), P1 and P2 primers (1 μg·μL–1 each), and Taq polymerase (5 U·μL–1). GAPDH was used as the internal standard calibrator. PCRs for NPY were carried out on a PCR thermocycler (Perkin-Elmer GeneAmp 2400, Applied Biosystem, Foster City, CA, USA) for 28 cycles with the following steps: 91°C for 1 min (denaturing), 60°C for 1 min (annealing) and 72°C for 30 s (extension), followed by a final elongation step at 72°C for 7 min, and finally the PCR products were soaked at 16°C. PCR reactions for other molecules analysed were carried out in steps similar to those described above except the changes of two steps (annealing and cycles) that were described as follows: POMC (55°C, 25 cycles), PI3K (52°C, 25 cycles), STAT3 (55°C, 25 cycles) and GAPDH (52°C, 25 cycles). The sequences of primers used in RT-PCR are shown in Table 1.
Table 1.
Summary of the RT-PCR primer sequences
| Genes | GenBank accession no. | Primer | Sequence 5′→3′ | Size of product (base pairs) |
|---|---|---|---|---|
| NPY | NM_012614 | Forward | GGGCTGTGTGGACTGACC | 264 |
| Reverse | GGAAGGGTCTTCAAGCCT | |||
| POMC | NM_139326 | Forward | GAGATTCTGCTACAGTCGCTC | 678 |
| Reverse | TTGATGATGGCGTTCTTGAA | |||
| PI3K | NM_133399 | Forward | TAGGGAAATTCTGGGCTCGC | 471 |
| Reverse | AGATGGGTGTGCAATGAGGG | |||
| STAT3 | NM_012747 | Forward | ATCCTAAGCACAAAGCCCCC | 491 |
| Reverse | TCCATGTCAAACGTGAGCGA | |||
| GAPDH | NM_017008 | Forward | TCCCTCAAGATTGTCAGCAA | 309 |
| Reverse | AGATCCACAACGGATACATT |
Gel electrophoresis
At the completion of RT-PCR, 8 μL of each PCR product was subsequently separated by flat bed gel electrophoresis on a 3% agarose gel. Gels stained with ethidium bromide (0.5 μg·mL–1, Sigma-Aldrich) were visualized under UV light, photographed and then scanned densitometrically. Ratios of NPY and GAPDH mRNA were calculated to determine relative NPY mRNA levels. Contents of NPY mRNA in Amphetamine-treated group were indicated as the percentage of control group. The ratio of NPY/GAPDH mRNA was measured by digital densitometry (Hoefer, San Francisco, CA, USA). Similar steps were used to determine the levels of POMC, PI3K and STAT3 mRNA.
STAT3/DNA binding assay
Binding of STAT3 to DNA in nuclear extracts was assessed by EMSA with double-stranded deoxyoligonucleotides specific for STAT3 consensus sequence 5′-GATCCTTCTGGGAATTCCTAGATC-3′, which was labelled on the 3′ end with biotin (Ju et al., 2011). EMSA was carried out using the Lightshift kit (Thermo Fisher Scientific Inc., Rockford City, IL, USA). Briefly, 10 μg of nuclear protein was preincubated with 10 mM Tris, 50 mM KC1, 1 mM DTT, 5 mM MgCl2, 2 μg poly (dI × dC) and 2 pmol of oligonucleotide probe for 20 min at room temperature. Specific binding was confirmed using a 200-fold excess of unlabelled probes as specific competitor. Protein–DNA complexes were separated by a 6% non-denaturing acrylamide gel electrophoresis. Complexes were transferred to positively charged nylon membranes and UV cross-linked in a cross-linker. Gel shifts were visualized with a streptavidin–HRP followed by chemiluminescent detection (Chen et al., 2006).
Lateral ventricular cannulation
Stereotaxic surgery (Kopf Model 900, Tujunga, CA, USA) was performed on each rat under pentobarbital anaesthesia (30 mg·kg–1, i.p.). The target of cannulation was near the junction of the right lateral ventricle and the third ventricle (coordinates: 0.8 mm posterior to the Bregma, 1.5 mm from the midline and 3.5–4.0 mm below the dura) (Paxinos and Watson, 1986). A stainless steel guide cannula (23G) was implanted and secured to the skull using stainless steel screws and dental cement. The correct placement was confirmed by observing the transient and rapid inflow of the vehicle in polyethylene tubing connected to a injector cannula (28G). The cannula was then occluded with a 28 G stylet. For the infusion of antisense, the stylet was replaced with a 28 G injector cannula extending 0.5 mm below the tip of the guide cannula. For all experiments, the cannula placement was verified by histochemistry of brain section and by the administration of angiotensin II (100 ng per rat). Angiotensin II reliably induced water drinking in non-deprived rats when administered into the cerebral ventricles (Ritter et al., 1981). Only data from rats that drank more than 10 mL of water in 30 min were included in this study. Behavioural testing of drinking began about 1 week after the cannulation surgery and the restoration of feeding behaviour, and then angiotensin II was administered to confirm the cannula placement. About 2 days after the treatment with angiotensin II normal drinking behaviour was restored and then we started the amphetamine treatment (day 0).
i.c.v. administration of STAT3 antisense ODN
The sequence of the STAT3 antisense ODN was directed against the rat STAT3 transcript corresponding to base pair 61–78: 5′-GCCAGGAACTGCCGCAGC-3′. Control (missense) ODN consisted of a randomized sequence of a similar nucleotide composition: 5′-AGTCCAGGCCCAGGTCCG-3′. This STAT3 antisense ODN has been used to inhibit STAT3 expression in astroglial cells (Aberg et al., 2001). The missense sequence did not show significant matches in the database. We used ODNs that were phosphorothioate-modified (S-ODNs) only on the three terminal bases of both the 5′ and 3′ ends, because these S-ODNs can improve hybridization affinity and nuclease resistance and were regarded as a well-established agent in several vertebrate systems (Ogawa and Pfaff, 1998) and rat brain (Kuo et al., 2012b). Both antisense and missense S-ODNs were dissolved in aCSF containing 140 mM NaCl, 3.35 mM KCl, 1.15 mM MgCl2, 1.26 mM CaCl2, 1.2 mM Na2HPO4 and 0.3 mM NaH2PO4; pH 7.4.
Western blotting
Hypothalamus tissue extracts were analysed by electrophoresis. Proteins were separated on a 12.5% polyacrylamide gel, transferred onto a nitrocellulose membrane and incubated with specific antibodies against NPY, PI3K, MC3 receptors, STAT3 and β-actin. After incubation with HRP goat anti-rabbit IgG, the colour signal was developed using 4-chloro-1-naphthol/3,3′-diaminobenzidine and 0.9% (w/v) NaCl in Tris-HCl. The relative photographic density was quantified by scanning the photographic negative film on a Gel Documentation and Analysis System (AlphaImager 2000; Alpha Innotech Corporation, San Leandro, CA, USA).
Statistical analysis
Data are presented as the means ± SEM. A two-way or one-way anova followed by Dunnett's test was used to detect significant differences between the groups. Statistical significance was set at P < 0.05.
Materials
Chow (LabDiet) was purchased from PMI Nutrition International. d-amphetamine sodium salt, angiotensin II, pentobarbital sodium salt and Tris-HCl were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against NPY, STAT3, C23 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). MC3 receptor antibody was from Gibco BRL, Life Technologies, Inc. (Rockville, MD, USA), p-STAT3 (Ser 727) and p-STAT3 (Tyr705) antibodies were from Cell Signaling Technology (Dancers, MA), and PI3K antibody was from BD Biosciences (Bedford, MA, USA). Semi-quantitative real-time PCR analysis was performed using a Taqman one-step PCR Master Mix (Applied Biosystems, Foster City, CA, USA). TRIZOL reagent (Life Technologies, Inc., Grand Island, NY, USA) was used in tissue homogenization. Antisense and DNA primer were synthesized by Proligo Singapore Pty Ltd. (Singapore, Singapore). d-Amphetamine and pentobarbital were dissolved in water, and angiotensin II and the STAT3 antisense ODN were dissolved in artificial cerebrospinal fluid (aCSF).
Results
The effect of amphetamine and/or STAT3 antisense on feeding behaviour
Changes of feeding behaviour in rats receiving amphetamine (2, 4 mg·kg–1, i.p.) treatment and/or pretreatment with STAT3 antisense are shown in the upper panel of Figure 1. Results revealed a significant dose-dependent [F(2,20) = 4.15] and time-dependent effect [F(4,35) = 5.17], but no significant interaction. Amphetamine (2 mg·kg–1) reduced the food intake from day 1 to day 3, and the higher dose (4 mg·kg–1) reduced food intake from day 1 to day 4, compared with the controls. Moreover, the effect of 2 mg·kg–1 amphetamine on day 4 was significantly higher than that on day 2, revealing that tolerance to amphetamine was induced in response to this dose. The daily changes of body weight during the 4 days of amphetamine treatment were similar to those of daily food intake, as described in our previous report (Kuo et al., 2012a). Therefore, amphetamine at dose of 2 mg·kg–1 was employed for most of the subsequent experiments as, after 2 days, there was a restoration of food intake, while the higher dose of 4 mg·kg–1 amphetamine was used for STAT3 antisense studies as this exerted a greater anorexic effect and was more appropriate for assessing examine the attenuation of amphetamine-induced anorexia by antisense pretreatment.
Figure 1.

Upper panel: the effect of daily amphetamine on food intake over a 4 day period. Amphetamine (AMPH; 2 or 4 mg·kg–1) was given i.p. to rats at 1800 h. The first injection of amphetamine was made at the end of day 0. Each point represents the mean ± SEM of eight rats. *P < 0.05 versus control group of each treatment day. #P < 0.05 versus the 2 mg·kg–1 amphetamine-treated group on day 2. Lower panel: the effect of STAT3 antisense (or missense) pretreatment on daily amphetamine-mediated food intake over a 4 day period. Daily missense or antisense treatment (20 μg per 10 μL·day–1, i.c.v.) was administered 1 h before daily amphetamine treatment. *P < 0.05 versus the missense groups of each treatment day. Data shown are the means ± SEM. N = 8 per group. #P < 0.05 versus the amphetamine -treated groups of each treatment day.
As shown in the lower panel of Figure 1, pretreatment with STAT3 antisense partly blocked the anorectic response to amphetamine, indicating the involvement of STAT3 signalling in amphetamine anorexia. There was significant dose-dependency [F(3,28) = 4.55] and time-dependency [F(4,35) = 5.78], but no interaction, in the effects of STAT3 antisense on amphetamine-induced anorexia. Food intake was significantly different between STAT3 antisense/amphetamine-treated and amphetamine-treated groups, from day 1 to day 4. There was also a significant difference was also observed from day 1 to day 4 between STAT3 antisense/ amphetamine-treated and missense-treated (control) groups. The feeding response in missense-treated rats was similar to that in saline-treated rats over the 4 day period. Moreover, the anorectic response in missense/ amphetamine-treated rats (lower panel of Figure 1) was not significantly changed compared with that in amphetamine-treated rats (upper panel of Figure 1). These results indicated that inhibition of STAT3 could partly reverse the feeding responses to repeated amphetamine treatments.
The effect of amphetamine on NPY, POMC, PI3K and STAT3 mRNA levels
Daily treatment with amphetamine (Figure 2) decreased NPY mRNA levels from day 1 to day 3 [F(4,25) = 2.75], but increased POMC mRNA from day 1 to day 3 [F(4,25) = 3.18], increased PI3K mRNA from day 1 to day 4 [F(4,25) = 2.77] and increased in STAT3 mRNA from day 1 to day 3 [F(4,25) = 3.53], compared with the control values. Moreover, these experiments also revealed that the maximum response of POMC, PI3K and STAT3 mRNA levels was observed on day 2 of amphetamine treatment.
Figure 2.
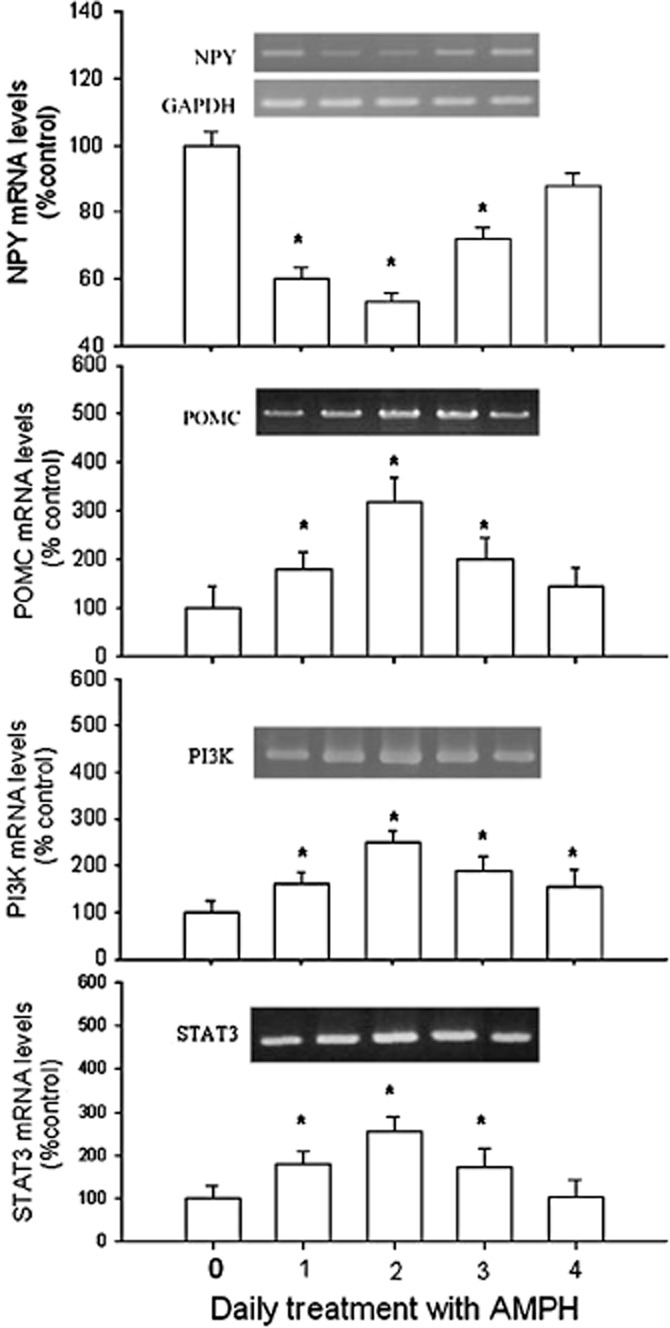
The effect of amphetamine (AMPH; 2 mg·kg–1, i.p.) on changes of NPY, POMC, PI3K and STAT3 mRNA levels. Results showed the relative densitometric value for RT-PCR products. Inserted images: the results of mRNA levels in stained gels. Contents of mRNA were assessed relative to that of GAPDH and are shown as the percentage of the control group. Data shown are means ± SEM. N = 6 each group. *P < 0.05 versus control.
The effect of amphetamine on the expression of NPY, MC3 receptors and PI3K
As shown in Figure 3, daily amphetamine treatment decreased NPY from day 1 to day 3 [F(4,25) = 3.12], but increased MC3 receptor expression from day 1 to day 3 [F(4,25) = 3.25], PI3K expression from day 1 to day 4 [F(4,25) = 2.85] and STAT3 expression from day 1 to day 4 [F(4,25) = 3.69], compared with the control. The maximum response of MC3 receptor and PI3K expression occurred on day 2 of amphetamine treatment.
Figure 3.
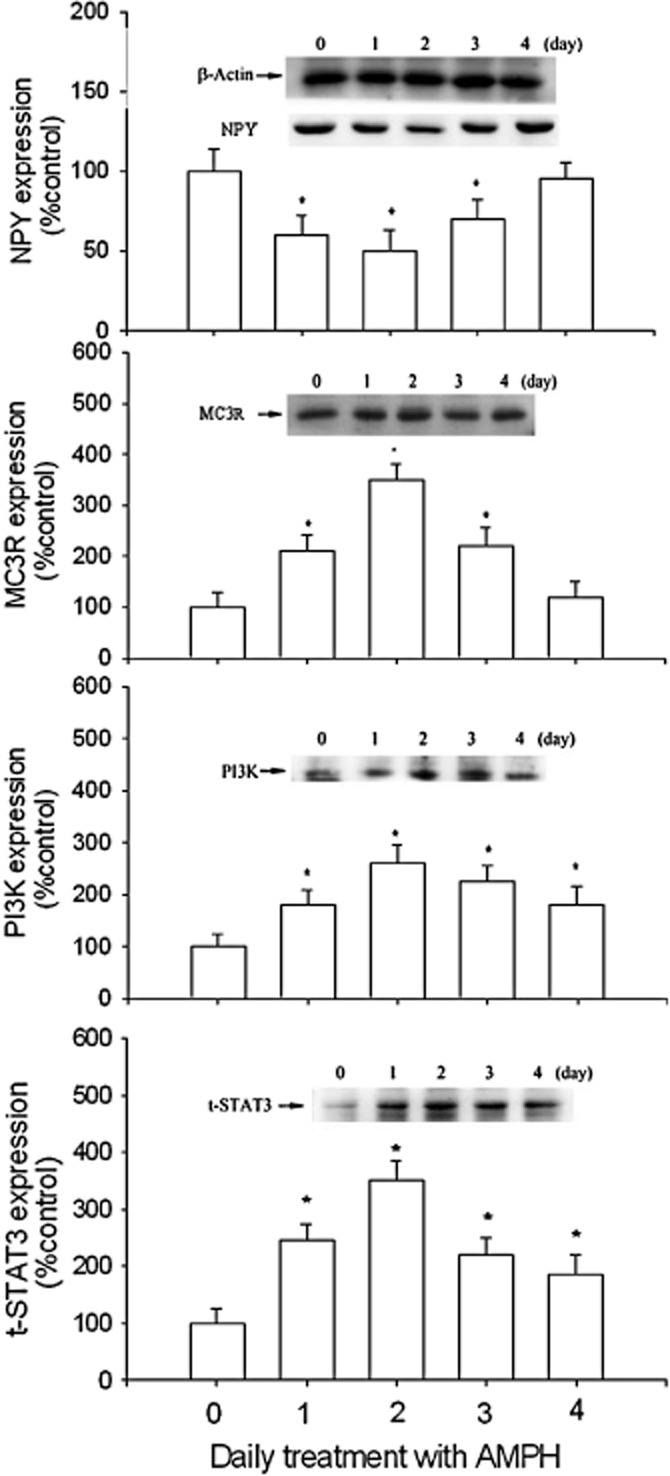
The effect of daily amphetamine (AMPH) on the expression of NPY, MC3 receptors, PI3K and t-STAT3 over a 4 day period. Results showed the relative densitometric values for Western blots inamphetamine- and saline-treated groups. β-Actin was used as an internal standard. Content of NPY, MC3 receptors, PI3K and t-STAT3 in the 2 mg·kg–1 amphetamine-treated group was indicated as the percentage of the control (day 0). Inserted images: results of Western blot analysing hypothalamic protein levels. Data shown are means ± SEM. N = 6 each group. *P < 0.05 versus control.
Effects of amphetamine on pSTAT3 expression and pSTAT3/DNA binding activity
Results in the upper panel of Figure 4 showed that daily amphetamine treatment increased pSTAT3 (Tyr705) [F(4,25) = 3.42] and pSTAT3 (Ser727) [F(4,25) = 3.86] from day 1 to day 3, compared with the control. The maximum responses were observed on day 2. As shown in the lower panel of Figure 4, daily treatment with amphetamine also increased hypothalamic STAT3/DNA binding activity [F(4,25) = 3.89, P < 0.05], with the maximum response on day 2, a finding was consistent with the patterns of response for pSTAT3, PI3K and MC3 receptors during amphetamine treatment.
Figure 4.
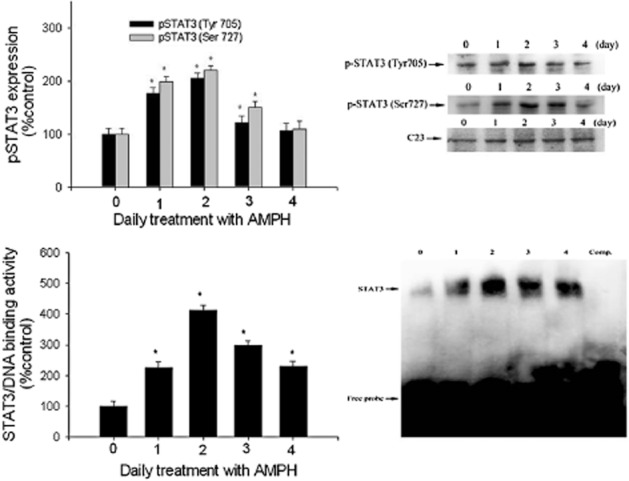
Upper panel: the effect of daily amphetamine (AMPH) on pSTAT3 expression over a 4 day period. Results show the relative densitometric values for Western blots in pSTAT3 (Tyr705) and pSTAT3 (Ser727) expression in amphetamine-treated rats. C23 is a nuclear protein used as an internal standard. Contents of pSTAT3 were indicated as the percentage of the control group. Lower panel: the effect of daily amphetamine on STAT3/DNA binding ability over a 4 day period. Results show the relative densitometric values for EMSA assay. Contents of STAT3/DNA binding ability were indicated as the percentage of the control group. Lane 6 represented nuclear extracts incubated with unlabelled ODN (competitive control; Comp) to confirm the specificity of binding. Data shown are means ± SEM. N = 6 each group. *P < 0.05 versus control.
The effect of pretreatment with STAT3 antisense on hypothalamic mRNA levels
Pretreatment with STAT3 antisense (Figure 5) partly reversed the effects of amphetamine. Thus, in amphetamine-treated rats, the levels of mRNAs for NPY, POMC, PI3K and STAT3 were returned towards normal by 40-60%, following STAT3 knockdown. Note that changes in STAT3 mRNA induced by amphetamine treatment were totally blocked after STAT3 knockdown.
Figure 5.
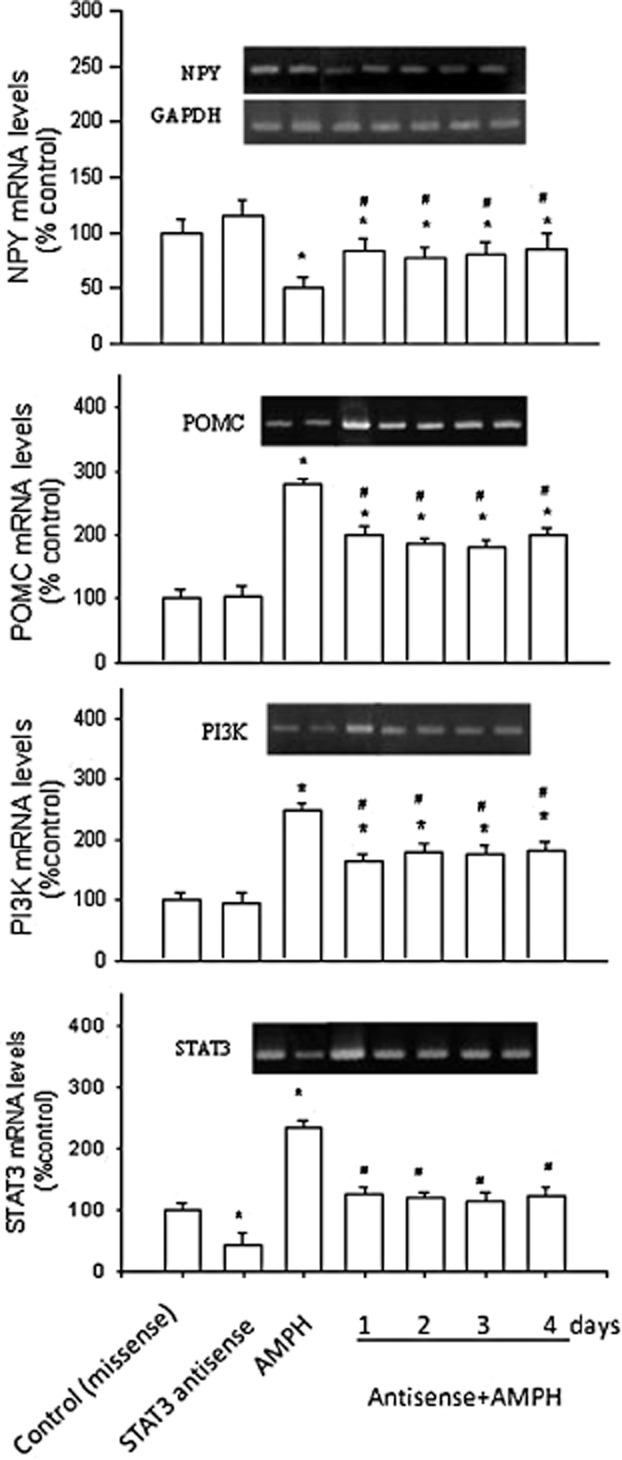
Effects of pretreatment with STAT3 antisense (or missense; i.c.v.) on the expression of NPY, POMC, PI3K and STAT3 mRNA levels in amphetamine (AMPH)-treated rats. Results show the relative densitometric values for RT-PCR in antisense STAT3- and/or amphetamine-treated groups. Expression of hypothalamic mRNA levels in the 2 mg·kg–1 amphetamine-treated group was indicated as the percentage of the control (day 0). Inserted images: the results of mRNA levels in stained gels. Data shown are means ± SEM. N = 6 per group. *P < 0.05 versus control (missense treated); the statistical data shown in control-, antisense- and amphetamine-treated groups were analysed from the samples on day 1.
The effect of pretreatment with STAT3 antisense on pSTAT3 expression
As shown in Figure 6, rats given daily amphetamine had increased expression of both pSTAT3 (Tyr705) [F(6,35) = 4.49] and pSTAT3 (Ser727) [F(6,35) = 5.12], compared with the control. However, pretreatment with the STAT3 antisense of these rats reduced both pSTAT3 (Tyr705) and pSTAT3 (Ser727) expression by about 50%.
Figure 6.
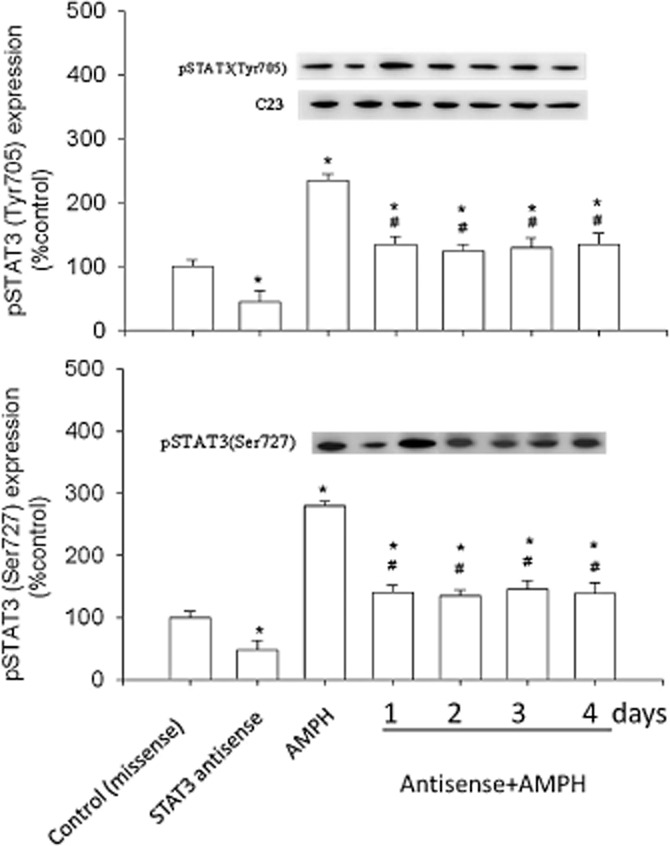
Effects of STAT3 antisense (or missense) pretreatment on the expression of pSTAT3 (Tyr705) and pSTAT3 (Ser727) in amphetamine (AMPH)-treated rats. Results show the relative densitometric values for Western blots in antisense STAT3- and/or amphetamine-treated groups. Content of pSTAT3 (Tyr705) and pSTAT3 (Ser727) in the 2 mg·kg–1 amphetamine-treated group was indicated as the percentage of the control (day 0). Inserted images: results of Western blot analysing hypothalamic protein levels. Data shown are means ± SEM. N = 6 each group. *P < 0.05 versus control (missense treated; i.c.v.). #P < 0.05 versus the amphetamine-treated groups; the statistical data shown in control-, antisense- and amphetamine-treated groups were analysed from the samples on day 1.
Discussion
The main findings of the present study are that hypothalamic PI3K–STAT3 signalling participates in the regulation of NPY- and POMC-mediated appetite control in amphetamine-treated rats. The current results showed that NPY decreased but POMC increased during amphetamine treatment. Additionally, PI3K, STAT3 and MC3 receptors increased with a time course comparable to that of the increase of POMC, with the maximum increase on day 2. This pattern, however, contrasted with that of changes in NPY which decreased and show its maximal fall also on day 2 of amphetamine treatment. This result implies that the increase in PI3K and STAT3 expression is likely to occur in POMC-containing neurons, but this result needs to be confirmed by showing the co-localization of PI3K and p-STAT3 in POMC neurons. However, a further experiment supported this finding where pretreatment with STAT3 antisense (i.e. STAT3 knockdown) in amphetamine-treated rats modulated the feeding behaviour, attenuated the NPY decrease and partly reversed the changes in expression of MC3 receptors and PI3K. These results suggest that PI3K–STAT3 signalling plays a functional role in the control of NPY- and POMC-mediated appetite control in amphetamine-treated rats.
In accordance with an earlier report (Kuo et al., 2009), the present results showed that the alteration in feeding behaviour during amphetamine treatment was consistent with the change in NPY expression, revealing that NPY was involved in the regulation of both amphetamine-induced anorexia (on day 1 and day 2) and amphetamine tolerance (from day 2 to day 4). Moreover, the changes in POMC (or MC3 receptor) expression were opposite to those in NPY expression, suggesting that POMC and NPY could function reciprocally in regulating anorexia and tolerance to amphetamine (Kuo et al., 2011). In the present study, STAT3 expression was consistent with POMC expression but opposite that of NPY during amphetamine treatment, implying that STAT3 could play a functional role in the inhibition of NPY and the activation of POMC expression. The reciprocal expression of NPY and POMC reflects inhibition by NPY of POMC-containing neurons, via unidirectional input from NPY to POMC neurons (Horvath et al., 1992; Chee et al., 2010). Several reports have revealed that STAT3 was involved in regulating hypothalamic NPY and POMC gene expression. For example, depletion of STAT3 in NPY neurons increased expression of NPY mRNA, leading to mild hyperphagia and a comparable obese phenotype (Gong et al., 2008). STAT3 activation in hypothalamic POMC-expressing neurons plays an important role in the maintenance of energy homeostasis (Xu et al., 2007). Additionally, enhanced STAT3 activation in POMC neurons may provoke a negative feedback inhibition of leptin and insulin signalling in obese animals (Ernst et al., 2009). Thus, we suggest that STAT3 activation in hypothalamic POMC neurons played a functional role in regulating the amphetamine-induced anorectic response. Our findings are in accord with an earlier report that bacterial endotoxin LPS-induced anorexia is linked with the activation of intracellular STAT3 signalling. Moreover, tolerance to LPS-induced anorexia is accompanied by a blunted STAT3 phosphorylation in the hypothalamic arcuate nucleus (Riediger et al., 2010).
As described previously, the decrease of NPY following amphetamine treatment is located in hypothalamic arcuate nucleus and paraventricular nucleus (Hsieh et al., 2004; 2005). Thus, the activation of PI3K signalling during amphetamine treatment might be located in hypothalamic POMC-containing neurons. This is because PI3K was increased and expressed in a pattern similar to POMC expression during amphetamine treatment. Previous evidence revealed that hypothalamic PI3K signalling participates in the control of energy balance and may be regarded as a mediator of leptin's effects of on hypothalamic neurons (Donato et al., 2010). Moreover, PI3K signalling in hypothalamic POMC neurons is essential for leptin-induced activation and insulin-induced inhibition in those neurons (Hill et al., 2008). Thus, PI3K–Akt signalling may activate downstream targets through pSTAT3 in NPY- or POMC-containing neurons (Sahu, 2011). The interaction between the dopaminergic system and leptin signalling in the hypothalamus is important in the control of energy homeostasis and treatment with dopamine D2 receptor agonists and antagonists modulated leptin-induced food intake, STAT3 phosphorylation and nuclear trans-localization of pSTAT3 in the hypothalamus (Kim et al., 2010). In the present study, amphetamine, which is known to release dopamine, increased PI3K and pSTAT3 expression, a result similar to the action of leptin (Sahu, 2011). It is suggested that the hypothalamic PI3K–STAT3 pathway is involved in the regulation of dopamine-evoked anorexia via the modulation of POMC neurons.
Most of the currently available STAT3 inhibitors target the conventional STAT3 pathway (i.e. STAT3 tyrosine phosphorylation, dimerization and DNA binding). Use of the STAT3 antisense ODN markedly reduced STAT3 protein amounts and inhibited cell proliferation in rodents (Li et al., 2006; He and Karin, 2011). The present results showed that daily pretreatment with STAT3 antisense before and during amphetamine treatment reversed pSTAT3 expression to almost normal levels in the hypothalamus. However, the same treatment only partly (45–60%) reversed expression of NPY, PI3K and POMC. This result implies that PI3K–STAT3 signalling might not be the only pathway regulating NPY and POMC expression in amphetamine-treated rats. Several other signal pathways, such as c-fos/c-jun signalling (Hsieh et al., 2006), PKA signalling (Hsieh et al., 2007) and PKCλ signalling (Hsieh et al., 2011), are also involved in regulating NPY and POMC gene expression.
Moreover, in addition to STAT3, the transcription factor NF-κB might also be a downstream regulator of PI3K–Akt signalling, and participate in the control of NPY and POMC gene expression (Morrison et al., 2005). Our previous report revealed that NF-κB participated in regulating NPY and POMC gene expression in amphetamine-treated rats (Kuo et al., 2012b; Hsieh et al., 2013). The current results showed that the increase of PI3K–STAT3 signalling was expressed in a pattern consistent with that of NF-κB with the maximum increase on day 2 during a 4 day period of amphetamine treatment. STAT3 may be co-activated with NF-κB to prevent neurotoxicity in the brain (Kuo et al., 2012b). Moreover, previous reports reveal that PI3K–Akt signalling mediates the neuroprotective effect against methamphetamine-induced damage in the rat brain (Rau et al., 2011). Furthermore, the NF-κB pathway was involved in the reduction of methamphetamine-mediated increase of oxidative stress in the brain (Lee et al., 2002; Kuo et al., 2012b). Thus, we suggest that the hypothalamic PI3K/STAT3 and PI3K/NF-κB pathways might be co-activated to regulate NPY and POMC gene expression in amphetamine-treated rats.
In the present study, changes of food intake during daily amphetamine treatment were associated with the changes in hypothalamic NPY, but showed less strong association with the effect of amphetamine-induced hyperlocomotion. Previous reports indicated that daily amphetamine can induce hyperlocomotion during an 8 day testing period, which was related to increased dopamine release and activation of dopamine transporters (Carboni et al., 2001; Spielewoy et al., 2001). Thus, if amphetamine-induced hyperlocomotion played a major role in the decrease of food intake, then daily amphetamine (from day 2 to day 4) would not induce a reversal of food intake and of hypothalamic NPY expression to baseline level.
The therapeutic potential of compounds targeted at the PI3K/STAT3 pathway has recently attracted attention. In the CNS, STAT3 is a potential target in the prevention and the treatment of medulloblastoma (Ajeawung et al., 2012) and glioblastoma multiforme, the most common and aggressive primary brain tumour (Luwor et al., 2013). STAT3 inhibition also prevents ischaemic brain injury and has a therapeutic potential for ischaemic strokes (Yu et al., 2013). Additionally, the PI3K inhibitor blocked the effect of amphetamine psychosis and reversed schizophrenia-related phenotypes (Law et al., 2012). In spite of these advances in the clinical uses of PI3K and STAT3 inhibitors, little is known about the possible role of PI3K–STAT3 signalling in the regulation of amphetamine-induced anorexia. The present study provides evidence that PI3K–STAT3 signalling plays an essential role in the control of dopamine-, NPY- and POMC-mediated appetite suppression in amphetamine-treated rats. These data may be helpful for the improvement of anorectic and anti-obesity drugs. Moreover, as the activation of hypothalamic POMC contributes to the protection of brain against oxidative stress, as described in our previous reports (Hsieh et al., 2011; Kuo et al., 2011) and STAT3 is sensitive to redox stress and is a logical candidate for redox modification by oxidants and antioxidants (Zgheib et al., 2012), compounds that could activate POMC and PI3K/STAT3 signalling might be applied, as therapeutic agents, to the improvement of oxidative damage induced by amphetamine.
In conclusion, our present results suggest that the hypothalamic PI3K–STAT3 signal pathway is involved in regulating NPY- and POMC-mediated appetite suppression in amphetamine-treated rats. These results may add to our understanding of the molecular mechanisms responsible for the appetite-suppressing effect of amphetamine.
Acknowledgments
This study was supported by a grant from the National Science Council (NSC- 101-2320-B-040-002-MY3) in Taiwan, Republic Of China.
Glossary
- aCSF
artificial corticospinal fluid
- DEPC
diethyl pyrocarbonate
- MC3R
melanocortin receptor 3
- NPY
neuropeptide Y
- ODN
oligodeoxynucleotides
- POMC
pro-opiomelanocortin
- RT-PCR
reverse transcription-PCR
- S-ODN
phosphorothioate ODN
Conflict of interest
There are no conflicts of interest for this manuscript.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg MA, Ryttsén F, Hellgren G, Lindell K, Rosengren LE, MacLennan AJ, et al. Selective introduction of antisense oligonucleotides into single adult CNS progenitor cells using electroporation demonstrates the requirement of STAT3 activation for CNTF-induced gliogenesis. Mol Cell Neurosci. 2001;17:426–443. doi: 10.1006/mcne.2000.0947. [DOI] [PubMed] [Google Scholar]
- Ajeawung NF, Wang HY, Gould P, Kamnasaran D. Advances in molecular targets for the treatment of medulloblastomas. Clin Invest Med. 2012;35:E246. doi: 10.25011/cim.v35i5.18697. [DOI] [PubMed] [Google Scholar]
- Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-09-j0001.2001. RC141: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MJ, Myers MG, Jr, Price CJ, Colmers WF. Neuropeptide Y suppresses anorexigenic output from the ventromedial nucleus of the hypothalamus. J Neurosci. 2010;30:3380–3390. doi: 10.1523/JNEUROSCI.4031-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang CL, Hsieh YS. Mulberry anthocyanins, cyanidin 3-rutinoside and cyaniding 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006;235:248–259. doi: 10.1016/j.canlet.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Chen TY, Duh SL, Huang CC, Lin TB, Kuo DY. Evidence for the involvement of dopamine D1 and D2 receptors in mediating the decrease of food intake during repeated treatment with amphetamine. J Biomed Sci. 2001;8:462–466. doi: 10.1007/BF02256608. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid quanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Donato J, Jr, Frazão R, Elias CF. The PI3K signaling pathway mediates the biological effects of leptin. Arq Bras Endocrinol Metabol. 2010;54:591–602. doi: 10.1590/s0004-27302010000700002. [DOI] [PubMed] [Google Scholar]
- Dziennis S, Alkayed NJ. Role of signal transducer and activator of transcription 3 in neuronal survival and regeneration. Rev Neurosci. 2008;19:341–361. doi: 10.1515/revneuro.2008.19.4-5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, et al. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Yao F, Hockman K, Heng HH, Morton GJ, Takeda K, et al. Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide Y neurons for normal energy homeostasis. Endocrinology. 2008;149:3346–3354. doi: 10.1210/en.2007-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Karin M. NF-κB and STAT3 – key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Naftolin F, Kalra SP, Leranth C. Neuropeptide-Y innervation of beta endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology. 1992;131:2461–2467. doi: 10.1210/endo.131.5.1425443. [DOI] [PubMed] [Google Scholar]
- Hsieh YH, Chen PN, Yu CH, Liao JM, Kuo DY. Inhibiting neuropeptide Y Y1 receptor modulates melanocortin receptor- and NFkB-mediated feeding behavior in phenylpropanolamine-treated rats. Horm Behav. 2013;64:95–102. doi: 10.1016/j.yhbeh.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Hsu JD, Yang SF, Kuo DY. Immunohistochemical and genomic evidence for the involvement of hypothalamic neuropeptide Y in phenylpropranolamine-mediated appetite suppression. Peptides. 2004;25:2155–2161. doi: 10.1016/j.peptides.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Yang SF, Kuo DY. Amphetamine, an appetite suppressant, decreases neuropeptide Y immunoreactivity in rat hypothalamic paraventriculum. Regul Pept. 2005;127:169–176. doi: 10.1016/j.regpep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Yang SF, Chiou HL, Kuo DY. Activations of c-fos/c-jun signaling are involved in the modulation of hypothalamic superoxide dismutase (SOD) and neuropeptide Y (NPY) gene expression in amphetamine-mediated appetite suppression. Toxicol Appl Pharmacol. 2006;212:99–109. doi: 10.1016/j.taap.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Yang SF, Kuo DY. Intracerebral administration of protein kinase A (PKA) or c-AMP response element binding protein (CREB) antisense oligonucleiotide can modulate amphetamine-mediated appetite suppression in free-moving rats. Am J Physiol Endocrinol Metab. 2007;292:123–131. doi: 10.1152/ajpendo.00195.2006. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Yang SF, Chen PN, Chu SC, Chen CH, Kuo DY. Knocking down the transcript of protein kinase C-lambda modulates hypothalamic glutathione peroxidase, melanocortin receptor and neuropeptide Y gene expression in amphetamine-treated rats. J Psychopharmacol. 2011;25:982–994. doi: 10.1177/0269881110376692. [DOI] [PubMed] [Google Scholar]
- Ju KD, Lim JW, Kim KH, Kim H. Potential role of NADPH oxidase-mediated activation of Jak2/Stat3 and mitogen-activated protein kinases and expression of TGF-β1 in the pathophysiology of acute pancreatitis. Inflamm Res. 2011;60:791–800. doi: 10.1007/s00011-011-0335-4. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Yoon YR, Lee HJ, Yoon S, Kim SY, Shin SW, et al. Enhanced hypothalamic leptin signaling in mice lacking dopamine D2 receptors. J Biol Chem. 2010;285:8905–8917. doi: 10.1074/jbc.M109.079590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DY. Involvement of hypothalamic neuropeptide Y in regulating the amphetamine-induced appetite suppression in streptozotocin diabetic rats. Regul Pept. 2005;127:19–26. doi: 10.1016/j.regpep.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Kuo DY. Hypothalamic neuropeptide Y (NPY) and the attenuation of hyperphagia in streptozotocin diabetic rats treated with dopamine D1/D2 agonists. Br J Pharmacol. 2006;148:640–647. doi: 10.1038/sj.bjp.0706754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DY, Yang SF, Chu SC, Chen CH, Hsieh YS. Amphetamine-evoked changes of oxidative stress and neuropeptide Y gene expression in hypothalamus: regulation by the protein kinase C-delta signaling. Chem Biol Interact. 2009;180:193–201. doi: 10.1016/j.cbi.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Kuo DY, Chen PN, Yang SF, Chu SC, Chen CH, Kuo MS, et al. Role of reactive oxygen species-related enzymes in neuropeptide Y and proopiomelanocortin-mediated appetite control: a study using atypical protein kinase C knockdown. Antioxid Redox Signal. 2011;15:2147–2159. doi: 10.1089/ars.2010.3738. [DOI] [PubMed] [Google Scholar]
- Kuo DY, Chen PN, Yu CH, Kuo MH, Hsieh YS, Chu SC. Involvement of neuropeptide Y Y1 receptor in the regulation of amphetamine-mediated appetite suppression. Neuropharmacology. 2012a;63:842–850. doi: 10.1016/j.neuropharm.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Kuo DY, Chen PN, Kuo MH, Chen CH, Hsieh YS, Chu SC. NF-kappaB knockdown can modulate amphetamine-mediated feeding response. Neuropharmacology. 2012b;62:1684–1694. doi: 10.1016/j.neuropharm.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Law AJ, Wang Y, Sei Y, O′Donnell P, Piantadosi P, Papaleo F, et al. Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110δ inhibition as a potential therapeutic strategy. Proc Natl Acad Sci U S A. 2012;109:12165–12170. doi: 10.1073/pnas.1206118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Son KW, Flora G, Hennig B, Nath A, Toborek M. Methamphetamine activates DNA binding of specific redox-responsive transcription factors in mouse brain. J Neurosci Res. 2002;70:82–89. doi: 10.1002/jnr.10370. [DOI] [PubMed] [Google Scholar]
- Li WC, Ye SL, Sun RX, Liu YK, Tang ZY, Kim Y, et al. Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res. 2006;12:7140–7148. doi: 10.1158/1078-0432.CCR-06-0484. [DOI] [PubMed] [Google Scholar]
- Luwor RB, Stylli SS, Kaye AH. The role of Stat3 in glioblastoma multiforme. J Clin Neurosci. 2013;20:907–911. doi: 10.1016/j.jocn.2013.03.006. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni P, Barnea A. Forskolin and phorbol ester stimulation of neuropeptide Y (NPY) production and secretion by aggregating fetal brain cells in culture: evidence for regulation of NPY biosynthesis at transcriptional and posttranscriptional levels. Endocrinology. 1992;130:976–984. doi: 10.1210/endo.130.2.1370798. [DOI] [PubMed] [Google Scholar]
- Mercer RE, Chee MJ, Colmers WF. The role of NPY in hypothalamic mediated food intake. Front Neuroendocrinol. 2011;32:398–415. doi: 10.1016/j.yfrne.2011.06.001. (Review). [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab. 2005;289:E1051–E1057. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Pfaff DW. Current status of antisense DNA methods in behavioral studies. Chem Senses. 1998;23:249–255. doi: 10.1093/chemse/23.2.249. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd edn. Sydney: Academic Press; 1986. [Google Scholar]
- Rajan P. STATus and context within the mammalian nervous system. Mol Med. 2011;17:965–973. doi: 10.2119/molmed.2010.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau TF, Kothiwal A, Zhang L, Ulatowski S, Jacobson S, Brooks DM, et al. Low dose methamphetamine mediates neuroprotection through a PI3K–AKT pathway. Neuropharmacology. 2011;61:677–686. doi: 10.1016/j.neuropharm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Riediger T, Cordani C, Potes CS, Lutz TA. Involvement of nitric oxide in lipopolysaccharide induced anorexia. Pharmacol Biochem Behav. 2010;97:112–120. doi: 10.1016/j.pbb.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Roman EAFR, Reis D, Romanatto T, Maimoni D, Ferreira EA, Santos GA, et al. Central leptin action improves skeletal muscle AKT, AMPK, and PGC1[alpha] activation by hypothalamic PI3K-dependent mechanism. Mol Cell Endocrinol. 2010;314:62–69. doi: 10.1016/j.mce.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Sahu A. Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology. 2011;93:201–210. doi: 10.1159/000326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14(Suppl. 1):1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Williams KW. Functional heterogeneity of arcuate nucleus pro-opiomelanocortin neurons: implications for diverging melanocortin pathways. Mol Neurobiol. 2012;45:225–233. doi: 10.1007/s12035-012-8240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielewoy C, Biala G, Roubert C, Hamon M, Betancur C, Giros B. Hypolocomotor effects of acute and daily d-amphetamine in mice lacking the dopamine transporter. Psychopharmacology (Berl) 2001;159:2–9. doi: 10.1007/s002130100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148:72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- Yu L, Chen C, Wang LF, Kuang X, Liu K, Zhang H, et al. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-κB and STAT3 in transient focal stroke. PLoS ONE. 2013;8:e55839. doi: 10.1371/journal.pone.0055839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgheib C, Kurdi M, Zouein FA, Gunter BW, Stanley BA, Zgheib J, et al. Acyloxy nitroso compounds inhibit LIF signaling in endothelial cells and cardiac myocytes: evidence that STAT3 signaling is redox-sensitive. PLoS ONE. 2012;7:e43313. doi: 10.1371/journal.pone.0043313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Creese I. Antisense oligodeoxynucleotide reduces brain dopamine D2 receptors: behavioral correlates. Neurosci Lett. 1993;161:223–226. doi: 10.1016/0304-3940(93)90299-z. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]


