Abstract
BACKGROUND AND PURPOSE
Uncoupling protein-2 (UCP2) may regulate glucose-stimulated insulin secretion. The current study investigated the effects of berberine, an alkaloid found in many medicinal plants, on oxidative stress and insulin secretion through restoration of UCP2 expression in high glucose (HG)-treated INS-1E cells and rat islets or in db/db mouse islets.
EXPERIMENTAL APPROACH
Mouse and rat pancreatic islets were isolated. Nitrotyrosine, superoxide dismutase (SOD)-1 and UCP2 expression and AMPK phosphorylation were examined by Western blotting. Insulin secretion was measured by elisa. Mitochondrial reactive oxygen species (ROS) production was detected by confocal microscopy.
KEY RESULTS
Incubation of INS-1E cells and rat islets with HG (30 mmol·L−1; 8 h) elevated nitrotyrosine level, reduced SOD-1 and UCP2 expression and AMPK phosphorylation, and inhibited glucose-stimulated insulin secretion. HG also increased mitochondrial ROS in INS-1E cells. Co-treatment with berberine inhibited such effects. The AMPK inhibitor compound C, the UCP2 inhibitor genipin and adenovirus ucp2 shRNA inhibited these protective effects of berberine. Furthermore, compound C normalized berberine-stimulated UCP2 expression but genipin did not affect AMPK phosphorylation. Islets from db/db mice exhibited elevated nitrotyrosine levels, reduced expression of SOD-1 and UCP2 and AMPK phosphorylation, and decreased insulin secretion compared with those from db/m+ mice. Berberine also improved these defects in diabetic islets and genipin blocked the effects of berberine.
CONCLUSIONS AND IMPLICATIONS
Berberine inhibited oxidative stress and restored insulin secretion in HG-treated INS-IE cells and diabetic mouse islets by activating AMPK and UCP2. UCP2 is an important signalling molecule in mediating anti-diabetic effects of berberine.
Keywords: berberine, uncoupling protein-2, oxidative stress, insulin secretion, INS-1E cells, islets
Introduction
Type 2 diabetes is characterized by failures to maintain glucose homeostasis due to impaired insulin secretion and insulin resistance (King, 2012). Chronic exposure of beta cells to high glucose (HG) causes detrimental effects on expression of genes related to insulin production and content, glucose-stimulated insulin secretion (GSIS), and mitochondrial function (Wallace et al., 2013). In addition, oxidative stress may contribute to chronic hyperglycaemia-induced beta cell dysfunction. Acute and transient glucose-dependent reactive oxygen species (ROS) contributes to normal GSIS in beta cells (Pi and Collins, 2010). However, chronic and persistent elevation of ROS acts as a negative modulator of GSIS (Li et al., 2012). The mitochondrion is one major source of intracellular ROS and the uncoupling proteins (UCPs), a superfamily of mitochondrial anion transporters, are important natural antioxidants in controlling cellular ROS homeostasis (Chan et al., 2010). Among them, UCP2 contributes to the control of mitochondrial ROS production, thus preventing oxidative stress (Robson-Doucette et al., 2011). Enhanced GSIS and increased intracellular ROS have been found in both islet cells of UCP2-deficient mice (Zhang et al., 2001; Lee et al., 2009) and INS-1E cells (Affourtit et al., 2011) and lean mouse islets (Saleh et al., 2006) with UCP2 knockdown. However, backcrossing UCP2-deficient mice for several generations onto highly congenic background strains exhibited impaired GSIS (Pi et al., 2009).
Berberine ([C20H18NO4]+), a constituent of many medicinal plant extracts, improves metabolic conditions in dyslipidaemia, obesity and type 2 diabetes (Kong et al., 2004; Jeong et al., 2009). Berberine reduces body weight and improves glucose tolerance and insulin action in obese and/or diabetic mice by activating the AMP-activated protein kinase (AMPK) (Lee et al., 2006; Kim et al., 2009). Berberine increases AMPK1α and UCP2 mRNA in visceral adipose tissues and livers of high-fat diet-fed mice (Xie et al., 2011). Moreover, AMPK stimulates UCP2 activation in endothelial cells (Xu et al., 2011; Liu et al., 2014). Recent studies however show contradictory results regarding the effects of berberine on insulin secretion. Berberine enhanced insulin secretion (Ko et al., 2005) or attenuated cAMP elevator-augmented insulin secretion (Zhou et al., 2008) in the mouse insulinoma cell line MIN6. Such discrepancies may be partly attributed to variations in experimental protocols, cell types and stimuli under study. Nevertheless, the role of UCP2 in berberine-induced beneficial effects in beta cells remains unexplored. The present study thus aimed to investigate the mechanisms by which berberine promoted insulin secretion in INS-1E cells and islets, involving UCP2, under hyperglycaemic and diabetic conditions.
Methods
Cell culture
The rat insulinoma cell line (INS-1E) was a highly differentiated and glucose-sensitive clone of parental INS-1 cells. INS-1E cells were cultured in RPMI-1640 supplemented with 10% FBS, 25 mmol·L−1 HEPES, 2 mmol·L−1 L-glutamine, 100 U·mL−1 penicillin, 100 μg·mL−1 streptomycin, 1 mmol·L−1 sodium pyruvate and 50 μmol·L−1 β-mercaptoethanol at 37°C and 5% CO2. Standard medium contains 11.1 mmol·L−1 glucose. Cells were seeded at 2 × 105 per well in 1 mL complete RPMI-1640 in a 24-well plate for secretion assay and 1 × 106 per well in a 6-well plate for Western blotting. Before the experiment, the medium was replaced with serum-free RPMI-1640 containing 0.1% BSA to starve cells. To determine the optimal concentration of berberine, INS-1E cells were seeded in 6-well plates, grown for 48 h and then incubated for 8 h with HG (30 mmol·L−1) in control and in the presence of berberine (0.1, 1, 5 and 10 μmol·L−1).
Animals and islet isolation
All animal care and experimental procedures in this investigation were approved by Animal Experimentation Ethics Committee of Chinese University of Hong Kong (CUHK) and Peking University Health Science Center and complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1996). Male db/m+ mice (12 weeks old) and db/db mice (12 weeks old) were supplied by CUHK Laboratory Animal Service Center. Male Sprague-Dawley rats (10 weeks old) were supplied by Laboratory Animal Service Center of Peking University Health Science Center. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). Four Sprague–Dawley rats, 6 db/m+ mice and six db/db mice were used in the experiments described here.
Islets from mice and rats were isolated by distending the pancreatic duct with collagenase. After digestion, the islets were separated on a Histopaque density gradient and further purified by handpicking under a stereomicroscope. Islets were cultured in RPMI-1640 medium supplemented with 10% FBS, penicillin (100 U·mL−1) and streptomycin (100 μg·mL−1) in standard humidified culture conditions of 5% CO2 and 95% O2 air at 37°C (Xu et al., 2007). After tissue culture, batches of 100 islets were further cultured in 6-well plates for 8 h under various pharmacological conditions.
Adenoviral infection
pAd-rat UCP2 shRNA was designed and synthesized by D&H Biosciences, Inc. (Peking, China). The target sequence for rat UCP2 was CGTAGTAATGTTTGTCACCTA; scrambled sequence was GCGCGCTTTGTAGGATTCG. Recombinant virus was produced in HEK 293A cells. INS-1E cells were infected with pAd-rat UCP2 shRNA (with red fluorescent protein) or scramble control, using a protocol of 4 h exposure to 6 μL of adenovirus to 6-well plate or 1.5 μL of adenovirus to 24-well plate (1 × 108 plaque-forming units·mL−1). Four hours after infection, cells were cultured for 36 h in RPMI-1640 medium and then treated with HG (30 mmol·L−1) with or without berberine for additional 8 h. Thereafter, cells were collected for Western blotting and insulin assay. The knockdown efficiency of UCP2 in INS-1E cells after 48 hours of transfection was verified nearly 70%.
Western blot analysis
After treatment, INS-1E cells or islets were homogenized in RIPA lysis buffer containing 1 μg·mL−1 leupeptin, 5 μg·mL−1 aprotinin, 100 μg·mL−1 PMSF, 1 mmol·L−1 sodium orthovanadate, 1 mmol·L−1 EDTA, 1 mmol·L−1 EGTA, 1 mmol·L−1 sodium fluoride, and 2 μg·mL−1 β-glycerophosphate, and centrifuged at 20 000× g for 20 min at 4°C. Protein lysates (10 μg for cells, 15 μg for islets) were separated by electrophoresis and transferred onto immobilon-P PVDF membrane. Blots were blocked with 1% BSA or 5% non-fat milk for 1 h and incubated overnight at 4°C with antibodies against superoxide dismutase 1 (SOD1), SOD2, nitrotyrosine, phosphorylated AMPKα, total AMPKα, UCP2 and GAPDH. After wash, blots were incubated with HRP-conjugated swine anti-rabbit, anti-mouse or anti-goat IgG. Immunoreactive bands were visualized by chemiluminescence and exposed to Kodak Image Station 440 (Brunswick, OH, USA) for densitometric analysis.
Cell viability assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) was used to quantify cell viability. INS-1E cells were seeded onto a 96-well plate at 2 × 104 cells per well in 100 μL of culture medium for 24 h, and then incubated for 8 h subjected to different pharmacological treatments. After the incubation period, 10 μL of the MTT labelling reagent (0.5 mg·mL−1) was added to each well. The microplate was incubated in a humidified atmosphere for 4 h and then 100 μL of the solubilization solution was added into each well. After incubation overnight, the ODs in the 96-well plates were determined using a microplate reader/spectrophotometer (iMark™ Microplate Reader from Bio-Rad, Philadelphia, PA, USA) at a wavelength of 595 nm.
Measurement of insulin secretion
INS-1E cells were seeded in 24-well plates at a density of 2 × 105 cells, grown for 48 h and then incubated with HG (30 mmol·L−1) with and without berberine (5 μmol·L−1). Both AMPK inhibitor compound C (10 μmol·L−1) and UCP2 inhibitor genipin (1 μmol·L−1) were tested for their action on berberine-induced effect on stimulated insulin release by co-incubation for 8 h. In addition, INS-1E cells were infected with scrambled and pAd-rat UCP2 shRNA. Thereafter, cells were incubated for 1 h in KRB solution without glucose, supplemented with 0.1% albumin and finally stimulated for 1 h with 11.1 mmol·L−1 glucose. After the experiment, medium was vacated and gently centrifuged at 3000 r.p.m. for 10 min at 4°C to discard the detached cells.
The rat islets were incubated with HG (30 mmol·L−1) in the presence or absence of berberine (5 μmol·L−1) and genipin (1 μmol·L−1) for 8 h. The mouse islets were treated for 8 h with berberine (5 μmol·L−1) or genipin (1 μmol·L−1). After treatment, islets from rats and mice were also incubated for 1 h in KRB solution without glucose, supplemented with 0.1% albumin and finally stimulated for 1 h with 11.1 mmol·L−1 glucose. Then the islets were centrifuged at 3000 r.p.m. for 10 min at 4°C to collect the supernatant for insulin assay.
The level of secreted insulin in the supernatant fraction was determined by insulin elisa kit using rat and mouse insulin as standard, and the resulting values were normalized to the protein content.
Mitochondrial ROS measurement
INS-1E cells seeded on glass coverslips were incubated for 8 h with HG (30 mmol·L−1) in the presence or absence of berberine (5 μmol·L−1) and individual inhibitors. They were then incubated with a fluorescent ROS indicator MitoSOX™ (5 μmol·L−1) for 10 min at 37°C in a chamber designed for fluorescence imaging. Production of mitochondrial ROS stimulated by angiotensin II (Ang II; 100 nmol·L−1) was measured by a confocal scanning unit (FV1000, Olympus, Tokyo, Japan) at excitation 405 nm and emission 585 nm (Robinson et al., 2008). Data were expressed as percentage change before (F0) and after (F1) the addition of Ang II.
Data analysis
Results are shown as means ± SEM of n experiments. For statistical analysis, Student's t-test or two-way anova followed by Bonferroni post hoc tests were used when more than two treatments were compared (GraphPad Software, San Diego, CA, USA). P < 0.05 was considered as significantly different.
Materials
Cell Proliferation Kit I (Roche Applied Science, Penzberg, Germany); Rat and Mouse Insulin elisa Kit (ALPCO Diagnostics, Salem, NH, USA); anti-phospho-AMPKα (Thr172) and anti-AMPKα (Cell Signaling Technology, Beverly, MA, USA); anti-UCP2 antibody (R&D Systems, Minneapolis, MN, USA); antibody against GAPDH (Ambion, Austin, TX, USA); HRP-conjugated swine anti-goat, anti-rabbit or anti-mouse IgG (DakoCytomation, Carpinteria, CA, USA); immobilon-P PVDF membrane (Millipore, Billerica, MA, USA); chemiluminescence (ECL reagents, Amersham Pharmacia, GE Healthcare Life Sciences, Buckinghamshire, UK); histopaque, collagenase, berberine and compound C (Sigma-Aldrich Chemical, St. Louis, MO, USA); Ang II (Tocris Bioscience, Bristol, UK); RPMI-1640, FBS and MitoSOX™ (Invitrogen, Carlsbad, CA, USA). Berberine, genipin, compound C and MitoSOX™ were dissolved in DMSO. Other drugs were dissolved in distilled water.
Results
HG-induced oxidative stress in INS-1E cells
Hyperglycaemia increases intracellular ROS formation and causes oxidative stress (Giacco and Brownlee, 2010). The levels of nitrotyrosine, SOD1 and SOD2 were first detected to reflect HG-induced oxidative stress in INS-1E cells. Western blot analysis revealed that 8 h exposure of INS-1E cells to HG (30 mmol·L−1) elevated the nitrotyrosine content (Figures 1A and C and 2C) and reduced the SOD1 expression (Figures 1D) without modifying SOD2 level (Supporting Information Figure S1). By contrast, mannitol as the osmotic control did not affect the expression of nitrotyrosine and SOD1 (Figure 1C and D). In addition, HG treatment reduced AMPK phosphorylation and UCP2 expression (Figure 2A and B).
Figure 1.
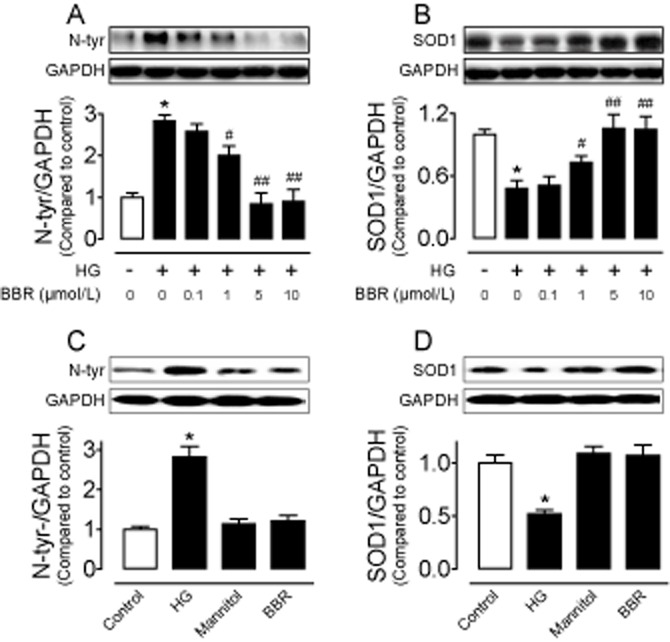
Eight hour treatment of INS-1E cells with HG (30 mmol·L−1) elevated nitrotyrosine (N-tyr) levels (A and C) and reduced SOD1 expression (B and D); such effects were reversed by co-incubation with berberine (BBR ;0.1, 1, 5 and 10 μmol·L−1) (A and B). Mannitol or BBR alone did not affect N-tyr levels (C) and SOD1 expression (D). Results are means ± SEM of four to six experiments. *P < 0.001 versus control; #P < 0.05 versus HG; ##P < 0.001 versus HG.
Figure 2.
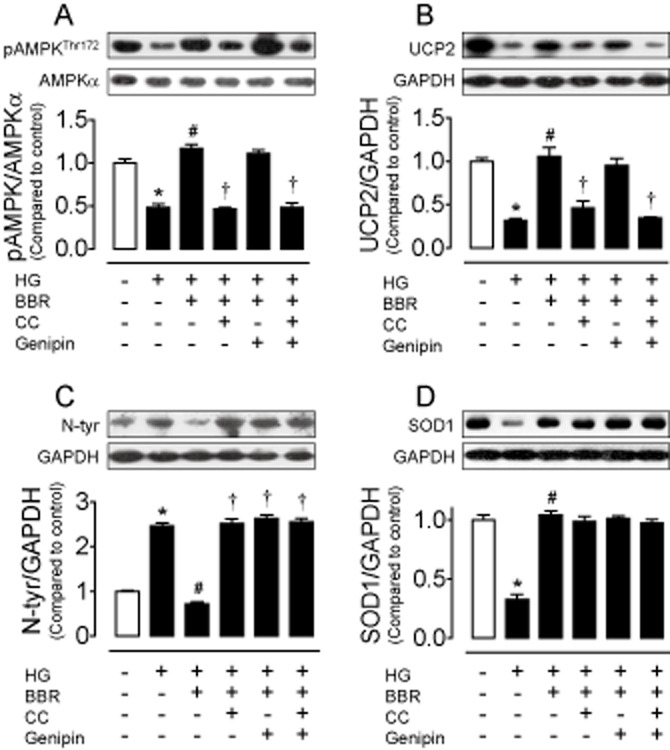
Eight hour treatment of INS-1E cells with HG (30 mmol·L−1) reduced AMPK phosphorylation (A) and UCP2 expression (B), elevated nitrotyrosine (N-tyr) level (C) and decreased SOD1 expression (D), which were all reversed by co-treatment with berberine (BBR; 5 μmol·L−1). Compound C (CC; 10 μmol·L−1) abolished (1) the increased AMPK phosphorylation, (2) the up-regulated UCP2 expression and (3) the reduced N-tyr level in BBR-treated cells (A–C). UCP2 inhibitor genipin (1 μmol·L−1) prevented the inhibitory effect of BBR on N-tyr content (C). By contrast, CC and genipin did not affect SOD1 expression (D). CC plus genipin did not cause additive benefit (A–D). Results are means ± SEM of four to six experiments. *P < 0.001 versus control; #P < 0.001 versus HG; †P < 0.01 versus HG + BBR.
Berberine reduced oxidative stress via AMPK/UCP2 cascade in INS-1E cells
Berberine increases the AMPK activity in liver and muscle of db/db mice (Kim et al., 2009), in mouse insulinoma NIT-1 cells (Shen et al., 2012) or in the H9c2 rat cardiomyoblast cells (Chang et al., 2013). Moreover, UCP2 activation is stimulated by AMPK in endothelial cells (Xu et al., 2011). Incubation with berberine (0.1, 1, 5 and 10 μmol·L−1) concentration-dependently reduced nitrotyrosine level and restored SOD1 expression in HG-treated cells (Figure 1A and B). Based on the above observations, we chose 5 μmol·L−1 berberine for the subsequent experiments. Berberine (5 μmol·L−1) alone did not affect the levels of nitrotyrosine and SOD1 (Figure 1C and D), but it normalized the HG-induced reductions in AMPK phosphorylation and UCP2 expression (Figure 2A and B). To elucidate the role of both AMPK and UCP2 in berberine-induced inhibition of HG-stimulated oxidative stress, we first tested the effects of AMPK inhibitor compound C and UCP2 inhibitor genipin. Both compound C (10 μmol·L−1) and genipin (1 μmol·L−1) reversed the ability of berberine to suppress nitrotyrosine and to increase SOD1 expression in INS-1E cells (Figure 2C and D). Furthermore, compound C inhibited the elevated UCP2 expression (Figure 2B) while genipin did not affect AMPK phosphorylation in berberine-treated cells (Figure 2A), indicating that berberine attenuates oxidative stress likely through stimulating AMPK and subsequently activating UCP2. We next confirmed the critical role of UCP2 by silencing UCP2 with adenovirus ucp2 short hairpin RNA transduction (Figure 3A). Western blot analysis shows that adenovirus ucp2 short hairpin RNA abolished the ability of berberine to lower nitrotyrosine levels in the transfected cells exposed to HG (Figure 3B).
Figure 3.
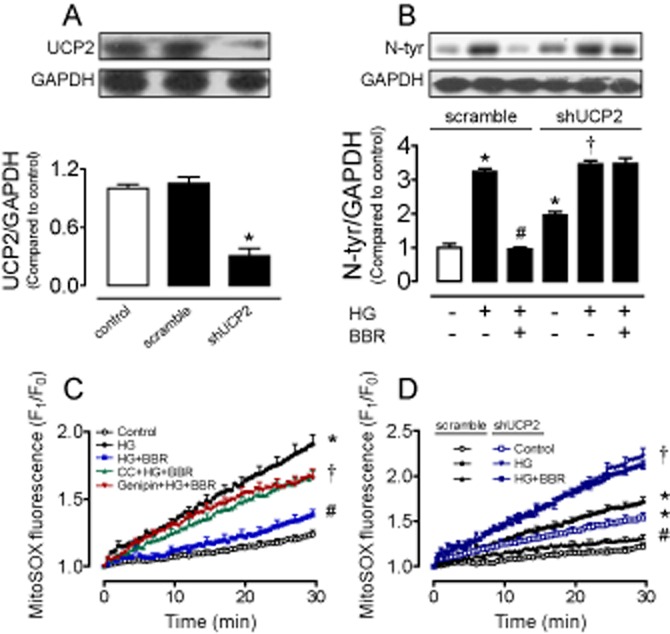
Silencing UCP2 with adenovirus ucp2 short hairpin RNA reduced the UCP2 expression (A), elevated nitrotyrosine (N-tyr) level (B) and abolished the ability of berberine (BBR) to suppress N-tyr contents (B) in transfected INS-1E cells as compared with scramble control. (C) Increased Ang II-stimulated mitochondrial ROS production following 8 h exposure to HG (30 mmol·L−1) was reversed by BBR (5 μmol·L−1), whereas the effect of BBR was inhibited by Compound C (CC; 10 μmol·L−1) or genipin (1 μmol·L−1). (D) Silencing UCP2 with adenovirus ucp2 short hairpin RNA increased Ang II-stimulated mitochondrial ROS production compared with scramble control. HG-induced further elevation in mitochondrial ROS production in transfected INS-1E cells was unaffected by berberine (5 μmol·L−1). Results are means ± SEM of four to six experiments. *P < 0.001 versus control or control (scramble); #P < 0.001 versus HG or HG (scramble); †P < 0.001 versus control (shUCP2) or HG + BBR.
Berberine inhibited mitochondrial ROS production in INS-1E cells
The UCP2 expression was elevated by berberine (Figure 2B), and UCP2 overexpression attenuates mitochondrial ROS production in arteries from diet-induced obese mice (Tian et al., 2012). We next used the fluorescence ROS indicator MitoSOX™ to measure mitochondrial ROS generation triggered by Ang II in INS-1E cells. Ang II (100 nmol·L−1) acutely elevated mitochondrial ROS in cells following 8 h exposure to HG (30 mmol·L−1), and this elevation was reversed by co-treatment with berberine (5 μmol·L−1). Both compound C (10 μmol·L−1) and genipin (1 μmol·L−1) reversed the inhibitory effect of berberine on mitochondrial ROS production (Figure 3C and Supporting Information Figure S2A). Adenovirus ucp2 short hairpin RNA augmented the Ang II-stimulated mitochondrial ROS production and berberine failed to normalize the HG-elevated mitochondrial ROS production when ucp2 was knockdown (Figure 3D and Supporting Information S2B).
Berberine increased insulin secretion in INS-1E cells
Over-production of ROS impairs islet function (Koulajian et al., 2013; Lee et al., 2013) and decreases insulin secretion (Li et al., 2012; Barlow and Affourtit, 2013; Zhang et al., 2013). Thus, we measured insulin secretion in INS-1E cells. Eight hour incubation with HG (30 mmol·L−1) reduced GSIS, and this reduction was reversed by co-treatment with berberine (5 μmol·L−1) (Figure 4A). In addition, adenovirus ucp2 short hairpin RNA significantly inhibited insulin secretion in transfected cells (Figure 4B). Furthermore, compound C (10 μmol·L−1), genipin (1 μmol·L−1) (Figure 4A) and adenovirus ucp2 short hairpin RNA (Figure 4B) all inhibited the stimulatory effect of berberine on insulin secretion.
Figure 4.
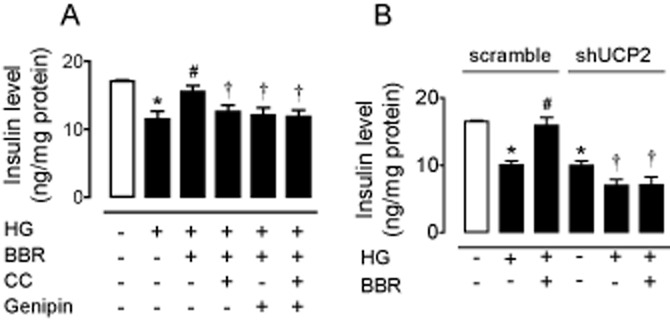
(A) HG (30 mmol·L−1, 8 h)-induced reduction of glucose-stimulated insulin secretion in INS-1E cells was reversed by berberine (BBR; 5 μmol·L−1). Compound C (CC; 10 μmol·L−1), genipin (1 μmol·L−1) (A) and adenovirus ucp2 short hairpin RNA (B) inhibited the effect of BBR on insulin secretion. Results are means ± SEM of six to eight experiments. *P < 0.001 versus control or control (scramble); #P < 0.05 versus HG or HG (scramble); †P < 0.05 versus HG + BBR or control (shUCP2).
Berberine inhibited oxidative stress and restored insulin secretion in HG-treated rat islets
Although berberine exerts protective effects against oxidative stress in HG-treated rat insulinoma INS-1E cells, we next explored the effects of HG and berberine in isolated rat islets. Western blot analysis showed that 8 h exposure to HG reduced AMPK phosphorylation (Figure 5A) and UCP2 expression (Figure 5B), elevated nitrotyrosine level (Figure 5C) and decreased SOD1 content (Figure 5D) in rat islets. In addition, HG impaired GSIS in rat islets (Figure 5E). The effects of HG were reversed by berberine (5 μmol·L−1) (Figure 5A–E). Likewise, UCP2 inhibitor genipin (1 μmol·L−1) also inhibited the effect of berberine on nitrotyrosine content (Figure 5C) and insulin secretion (Figure 5E) without affecting phosphorylation of AMPK (Figure 5A) or expressions of UCP2 (Figure 5B) and SOD1 (Figure 5D).
Figure 5.
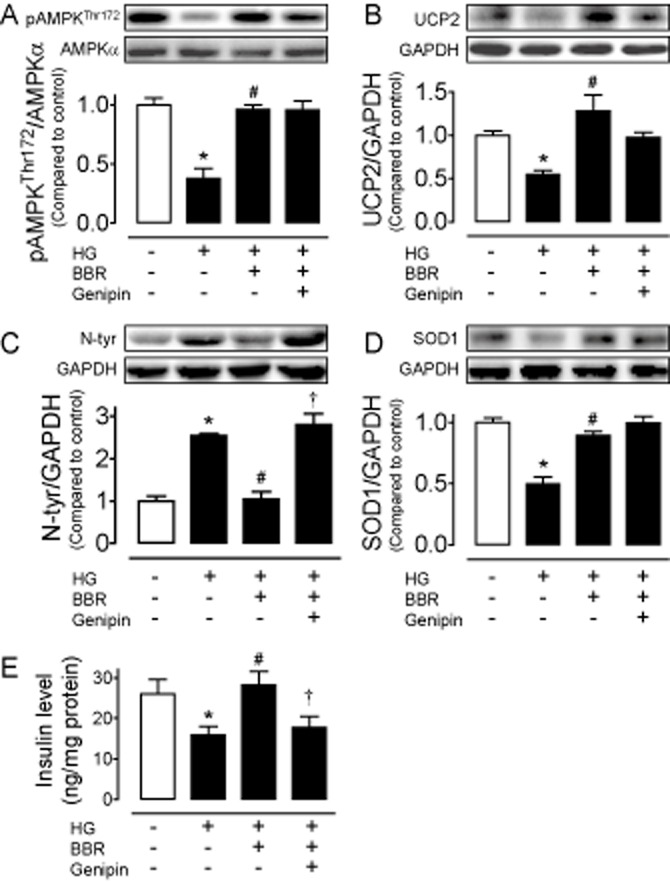
Eight hour treatment with HG (30 mmol·L−1) reduced AMPK phosphorylation (A) and UCP2 expression (B), elevated the nitrotyrosine (N-tyr) content (C), decreased SOD1 expression (D), and impaired glucose-stimulated insulin secretion (E) in isolated rat islets. Berberine (BBR) reversed the effects of HG (A–E), whereas co-incubation with genipin (1 μmol·L−1) inhibited the beneficial effects of BBR (C and E). Results are means ± SEM of four experiments. *P < 0.05 versus control; #P < 0.05 versus HG; †P < 0.05 versus HG + BBR.
Berberine inhibited oxidative stress and restored insulin secretion in islets from diabetic db/db mice
We finally examined whether berberine can also exert a beneficial effect in isolated islets from diabetic db/db mice. Diabetic mouse islets exhibited a reduced AMPK phophorylation (Figure 6A) and UCP2 expression (Figure 6B), an elevated nitrotyrosine content (Figure 6C), a decreased SOD1 level (Figure 6D) and an impaired GSIS (Figure 6E) compared with islets of non-diabetic db/m+ mice. Eight hour treatment with berberine (5 μmol·L−1) inhibited oxidative stress and restored insulin secretion in db/db mouse islets (Figure 6A–E). Co-treatment with genipin (1 μmol·L−1) reversed the effect of berberine on nitrotyrosine content (Figure 6C) and insulin secretion (Figure 6E). By contrast, genipin did not alter phosphorylation of AMPK (Figure 6A) or expressions of UCP2 (Figure 6B) and SOD1 (Figure 6D).
Figure 6.
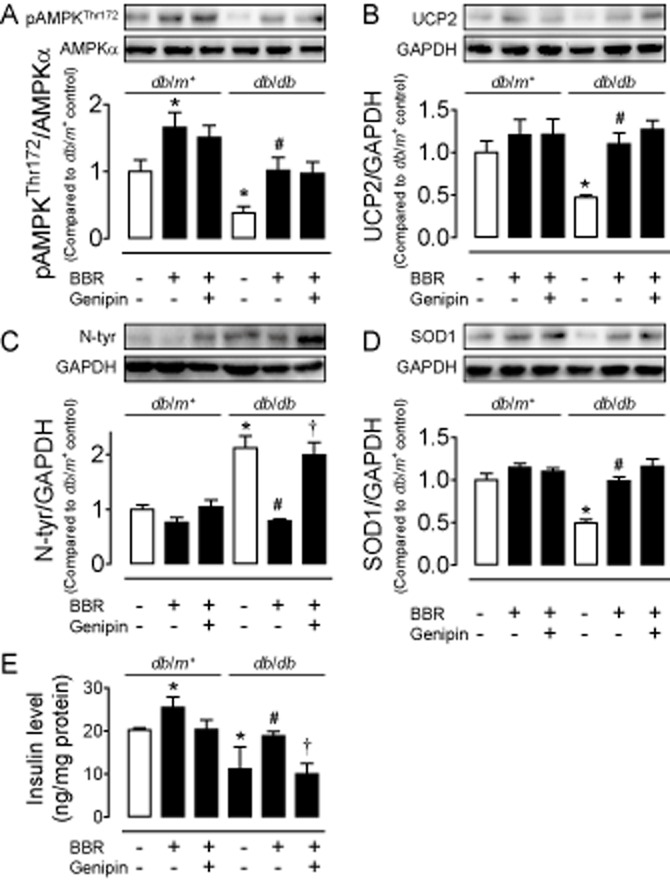
The reduced AMPK phosphorylation (A) and UCP2 expression (B) elevated nitrotyrosine (N-tyr) content (C), decreased SOD1 expression (D) and impaired glucose-stimulated insulin secretion (E) in islets from diabetic db/db mice as compared with those from db/m+ mice. These changes in islets from diabetic db/db mice were normalized by treatment with berberine (BBR; A–E) while co-incubation with genipin inhibited the effects of BBR (C and E). Results are means ± SEM of four experiments. *P < 0.05 versus control (db/m+); #P < 0.05 versus control (db/db); †P < 0.05 versus BBR (db/db).
Discussion
The present study reveals a functional importance of UCP2 in mediating the beneficial effect of berberine against oxidative stress and related impairment of insulin secretion as demonstrated in HG-treated rat insulinoma cell line (INS-1E) and rat islets as well as in the islets from diabetic db/db mice. The major novel findings include (1) HG exposure reduces AMPK phosphorylation and UCP2 expression in INS-1E cells and rat islets; (2) berberine treatment normalizes the increased level of nitrotyrosine (a cellular oxidative stress index) and restores the diminished expression of SOD1 in HG-treated INS-1E cells and rat islets; (3) berberine inhibits Ang II-stimulated mitochondrial ROS production in HG-treated INS-1E cells; (4) berberine treatment restores the impaired insulin secretion through activation of AMPK and UCP2 signalling in INS-1E cells and rat islets; and (5) berberine inhibits oxidative stress and restores insulin secretion through AMPK/UCP2 activation in the islets of diabetic mice.
Increased oxidative stress is actively involved in the development and progression of diabetes and its complications. Hyperglycaemia-related oxidative stress impairs vascular function in association with activation of the PKC, polyol pathway and formation of advanced glycation end products (Lau et al., 2013; Renaud et al., 2014). Hyperglycaemia causes excessive ROS formation, particularly superoxide anions (Jay et al., 2006), and ROS overproduction is involved in the development of beta cell dysfunction (Malin et al., 2014). Both clinical and experimental studies show that berberine is effective in treating the metabolic syndrome, correcting hyperinsulinaemia, increasing insulin sensitivity and stimulating insulin secretion through mechanisms including triglyceride reduction, augmented secretion of glucagon-like peptide (GLP)-2 and increased release of GLP-1 (Lu et al., 2009; Yu et al., 2010; Perez-Rubio et al., 2013; Shan et al., 2013). The berberine analogue, 8,8-dimethyldihydroberberine, improved glucose tolerance and alleviates insulin resistance in db/db mice (Cheng et al., 2010). Most recently, berberine exerted nephroprotective and neuroprotective effects through inhibiting oxidative stress (Domitrovic et al., 2013; Moghaddam et al., 2014). Consistent with these earlier reports, the present study showed that in vitro berberine treatment restored insulin secretion through inhibiting HG-stimulated oxidative stress as berberine normalized HG-induced elevation in nitrotyrosine level and HG-induced reduction in SOD1 expression in INS-1E cells and in isolated rat islets. More importantly, berberine treatment also normalized the elevated nitrotyrosine content, increased the reduced SOD1 expression and restored the impaired insulin secretion in islets from diabetic mice.
UCP2 can limit mitochondrial ROS production and thus protects against oxidative stress (Arsenijevic et al., 2000; Robson-Doucette et al., 2011). The increased UCP2 activity contributes to beta cell pathogenesis and development of type 2 diabetes (Affourtit and Brand, 2008a). Up to now, whether UCP2 stimulates or inhibits GSIS in beta cells remains debated (Affourtit and Brand, 2008b; Affourtit et al., 2011; Calegari et al., 2011; Barlow et al., 2013). The present study showed that 8 h exposure to HG increased Ang II-stimulated acute production of mitochondrial ROS in INS-1E cells, down-regulated the expression of UCP2 and attenuated insulin secretion in both INS-1E cells and rat islets. In addition, the UCP2 level was lower in islets from db/db mice compared with those from db/m+ mice. In vitro treatment with berberine reversed the harmful effects of HG on the UCP2 expression, mitochondrial ROS production and insulin release. The present results suggest a significant role of UCP2 in controlling basal levels of mitochondrial ROS and insulin release, as silencing UCP2 with adenovirus ucp2 short hairpin RNA increased the un-stimulated content of nitrotyrosine and lowered insulin concentration in the culture medium of INS-1E cells. The present study provides novel evidence to support a key role of UCP2 in mediating the beneficial effects of berberine in INS-1E cells. Both the UCP2 inhibitor genipin and UCP2 knockdown reversed berberine-induced inhibition of oxidative stress and increase of insulin secretion in INS-1E cells. Furthermore, the UCP2 inhibitor genipin inhibited the protective effects of berberine in both HG-treated rat islets and db/db mouse islets, thus supporting a critical role of UCP2 in berberine-treated INS-1E cells.
AMPK involves UCP2 activation not only in endothelial cells (Wang et al., 2011; Xu et al., 2011) but also in beta cells (Calegari et al., 2011; Beall et al., 2013). The present study shows that berberine stimulated the AMPK activity which in turn up-regulated expression of UCP2, leading to reduced oxidative stress and improved insulin secretion in INS-1E cells, based on the following observations. First, the AMPK inhibitor compound C reversed berberine-stimulated AMPK phosphorylation and UCP2 up-regulation in INS-1E cells. Second, the UCP2 inhibitor genipin did not affect berberine-stimulated AMPK phosphorylation in both INS-1E cells and islets from mice or rats. Third, compound C reversed not only the inhibitory effect of berberine on oxidative stress but also its stimulatory effect on insulin secretion in INS-1E cells. By contrast, neither compound C nor genipin affected berberine-stimulated expression of SOD1, an anti-oxidant enzyme, implying that other mechanisms rather than AMPK-UCP2 signalling contribute to the SOD1 up-regulation, which may also play a role in curtailing oxidative stress. In addition, drug treatments and viral transduction did not alter cell viability (Supporting Information S3). Taken together, the present study adds new mechanistic insights into the anti-diabetic properties of berberine. Berberine activated AMPK and subsequently elevated UCP2 expression and activity to reduce mitochondrial ROS production, which helped to restore the HG-impaired insulin secretion in INS-1E cells and rat islets. More importantly, berberine in vitro inhibited oxidative stress and restored insulin secretion in islets from db/db mice also through genipin-sensitive mechanisms. The novel findings of this investigation highlight UCP2 as a useful target for drug intervention to protect beta cell function in diabetes.
Acknowledgments
This study is supported by the National Nature Science Foundation of China (No. 81200183), National Basic Research Program of China (2012CB517805) and CUHK Focused Investment Scheme B.
Glossary
- AMPK
AMP-activated protein kinase
- Ang II
angiotensin II
- GLP
glucagon-like peptide
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- UCP2
uncoupling protein-2
Author contributions
L. L. and J. L. designed and performed the experiments, acquired most of data and drafted the manuscript. Y. H. handled funding and supervision, and made critical revision of the manuscript. Y. G. and G. X. were involved in data discussion and made critical revision of the manuscript. X. Y. was involved in insulin measurement.
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12666
Figure S1 High glucose (30 mmol·L−1, 8 h) had no effect on SOD2 level in INS-1E cells. Results are means ± SEM of four experiments. HG, high glucose.
Figure S2 (A) Images of mitochondrial ROS production triggered acutely by Ang II (100 nmol·L−1) in INS-1E cells following 8 h incubation with high glucose (30 mmol·L−1) or co-incubation with berberine (5 μmol·L−1) in the presence or absence of compound C (10 μmol·L−1) or genipin (1 μmol·L−1). (B) Images of Ang II-stimulated mitochondrial ROS production when silencing UCP2 with adenovirus ucp2 short hairpin RNA or scramble control. Ang II, angiotensin II; BBR, berberine; CC, compound C; HG, high glucose.
Figure S3 Cell viability was unaffected by 8 h incubation with high glucose (30 mmol·L−1), berberine (5 μmol·L−1), CC (10 μmol·L−1) or genipin (1 μmol·L−1) (A) and scramble or shUCP2 (B). Results are means ± SEM of six to eight experiments. BBR, berberine; CC, compound C; HG, high glucose.
References
- Affourtit C, Brand MD. On the role of uncoupling protein-2 in pancreatic beta cells. Biochim Biophys Acta. 2008a;1777:973–979. doi: 10.1016/j.bbabio.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Affourtit C, Brand MD. Uncoupling protein-2 contributes significantly to high mitochondrial proton leak in INS-1E insulinoma cells and attenuates glucose-stimulated insulin secretion. Biochem J. 2008b;409:199–204. doi: 10.1042/BJ20070954. [DOI] [PubMed] [Google Scholar]
- Affourtit C, Jastroch M, Brand MD. Uncoupling protein-2 attenuates glucose-stimulated insulin secretion in INS-1E insulinoma cells by lowering mitochondrial reactive oxygen species. Free Radic Biol Med. 2011;50:609–616. doi: 10.1016/j.freeradbiomed.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Barlow J, Affourtit C. Novel insights in pancreatic beta cell glucolipotoxicity from real-time functional analysis of mitochondrial energy metabolism in INS-1E insulinoma cells. Biochem J. 2013;456:417–426. doi: 10.1042/BJ20131002. [DOI] [PubMed] [Google Scholar]
- Barlow J, Hirschberg V, Brand MD, Affourtit C. Measuring mitochondrial uncoupling protein-2 level and activity in insulinoma cells. Methods Enzymol. 2013;528:257–267. doi: 10.1016/B978-0-12-405881-1.00015-X. [DOI] [PubMed] [Google Scholar]
- Beall C, Watterson KR, McCrimmon RJ, Ashford ML. AMPK modulates glucose-sensing in insulin-secreting cells by altered phosphotransfer to KATP channels. J Bioenerg Biomembr. 2013;45:229–241. doi: 10.1007/s10863-013-9509-9. [DOI] [PubMed] [Google Scholar]
- Calegari VC, Zoppi CC, Rezende LF, Silveira LR, Carneiro EM, Boschero AC. Endurance training activates AMP-activated protein kinase, increases expression of uncoupling protein 2 and reduces insulin secretion from rat pancreatic islets. J Endocrinol. 2011;208:257–264. doi: 10.1530/JOE-10-0450. [DOI] [PubMed] [Google Scholar]
- Chan SH, Wu KL, Kung PS, Chan JY. Oral intake of rosiglitazone promotes a central antihypertensive effect via upregulation of peroxisome proliferator-activated receptor-gamma and alleviation of oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertension. 2010;55:1444–1453. doi: 10.1161/HYPERTENSIONAHA.109.149146. [DOI] [PubMed] [Google Scholar]
- Chang W, Zhang M, Li J, Meng Z, Wei S, Du H, et al. Berberine improves insulin resistance in cardiomyocytes via activation of 5′-adenosine monophosphate-activated protein kinase. Metabolism. 2013;62:1159–1167. doi: 10.1016/j.metabol.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Chen AF, Wu F, Sheng L, Zhang HK, Gu M, et al. 8,8-Dimethyldihydroberberine with improved bioavailability and oral efficacy on obese and diabetic mouse models. Bioorg Med Chem. 2010;18:5915–5924. doi: 10.1016/j.bmc.2010.06.085. [DOI] [PubMed] [Google Scholar]
- Domitrovic R, Cvijanovic O, Pernjak-Pugel E, Skoda M, Mikelic L, Crncevic-Orlic Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem Toxicol. 2013;62C:397–406. doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR, et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296:E812–E819. doi: 10.1152/ajpendo.90710.2008. [DOI] [PubMed] [Google Scholar]
- King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko BS, Choi SB, Park SK, Jang JS, Kim YE, Park S. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol Pharm Bull. 2005;28:1431–1437. doi: 10.1248/bpb.28.1431. [DOI] [PubMed] [Google Scholar]
- Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- Koulajian K, Desai T, Liu GC, Ivovic A, Patterson JN, Tang C, et al. NADPH oxidase inhibition prevents beta cell dysfunction induced by prolonged elevation of oleate in rodents. Diabetologia. 2013;56:1078–1087. doi: 10.1007/s00125-013-2858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau YS, Tian XY, Huang Y, Murugan D, Achike FI, Mustafa MR. Boldine protects endothelial function in hyperglycemia-induced oxidative stress through an antioxidant mechanism. Biochem Pharmacol. 2013;85:367–375. doi: 10.1016/j.bcp.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Lee J, Moulik M, Fang Z, Saha P, Zou F, Xu Y, et al. Bmal1 and beta-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced beta-cell failure in mice. Mol Cell Biol. 2013;33:2327–2338. doi: 10.1128/MCB.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Robson-Doucette CA, Wheeler MB. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. J Endocrinol. 2009;203:33–43. doi: 10.1677/JOE-09-0117. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- Li N, Li B, Brun T, Deffert-Delbouille C, Mahiout Z, Daali Y, et al. NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion. Diabetes. 2012;61:2842–2850. doi: 10.2337/db12-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Liu J, Tian XY, Wong WT, Lau CW, Xu A, et al. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5519. doi: 10.1089/ars.2013.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SS, Yu YL, Zhu HJ, Liu XD, Liu L, Liu YW, et al. Berberine promotes glucagon-like peptide-1 (7–36) amide secretion in streptozotocin-induced diabetic rats. J Endocrinol. 2009;200:159–165. doi: 10.1677/JOE-08-0419. [DOI] [PubMed] [Google Scholar]
- Malin SK, Kirwan JP, Ling Sia C, Gonzalez F. Glucose-stimulated oxidative stress in mononuclear cells is related to pancreatic beta-cell dysfunction in polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:322–329. doi: 10.1210/jc.2013-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam HK, Baluchnejadmojarad T, Roghani M, Khaksari M, Norouzi P, Ahooie M, et al. Berberine ameliorate oxidative stress and astrogliosis in the hippocampus of STZ-induced diabetic rats. Mol Neurobiol. 2014;49:820–826. doi: 10.1007/s12035-013-8559-7. [DOI] [PubMed] [Google Scholar]
- Perez-Rubio KG, Gonzalez-Ortiz M, Martinez-Abundis E, Robles-Cervantes JA, Espinel-Bermudez MC. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord. 2013;11:366–369. doi: 10.1089/met.2012.0183. [DOI] [PubMed] [Google Scholar]
- Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diabetes Obes Metab. 2010;12(Suppl. 2):141–148. doi: 10.1111/j.1463-1326.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- Pi J, Bai Y, Daniel KW, Liu D, Lyght O, Edelstein D, et al. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic beta-cell function. Endocrinology. 2009;150:3040–3048. doi: 10.1210/en.2008-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J, Bournival J, Zottig X, Martinoli MG. Resveratrol protects DAergic PC12 cells from high glucose-induced oxidative stress and apoptosis: effect on p53 and GRP75 localization. Neurotox Res. 2014;25:110–123. doi: 10.1007/s12640-013-9439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KM, Janes MS, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protoc. 2008;3:941–947. doi: 10.1038/nprot.2008.56. [DOI] [PubMed] [Google Scholar]
- Robson-Doucette CA, Sultan S, Allister EM, Wikstrom JD, Koshkin V, Bhattacharjee A, et al. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes. 2011;60:2710–2719. doi: 10.2337/db11-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MC, Wheeler MB, Chan CB. Endogenous islet uncoupling protein-2 expression and loss of glucose homeostasis in ob/ob mice. J Endocrinol. 2006;190:659–667. doi: 10.1677/joe.1.06715. [DOI] [PubMed] [Google Scholar]
- Shan CY, Yang JH, Kong Y, Wang XY, Zheng MY, Xu YG, et al. Alteration of the intestinal barrier and GLP2 secretion in berberine-treated type 2 diabetic rats. J Endocrinol. 2013;218:255–262. doi: 10.1530/JOE-13-0184. [DOI] [PubMed] [Google Scholar]
- Shen N, Huan Y, Shen ZF. Berberine inhibits mouse insulin gene promoter through activation of AMP activated protein kinase and may exert beneficial effect on pancreatic beta-cell. Eur J Pharmacol. 2012;694:120–126. doi: 10.1016/j.ejphar.2012.07.052. [DOI] [PubMed] [Google Scholar]
- Tian XY, Wong WT, Xu A, Lu Y, Zhang Y, Wang L, et al. Uncoupling protein-2 protects endothelial function in diet-induced obese mice. Circ Res. 2012;110:1211–1216. doi: 10.1161/CIRCRESAHA.111.262170. [DOI] [PubMed] [Google Scholar]
- Wallace M, Whelan H, Brennan L. Metabolomic analysis of pancreatic beta cells following exposure to high glucose. Biochim Biophys Acta. 2013;1830:2583–2590. doi: 10.1016/j.bbagen.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, Zou MH. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS ONE. 2011;6:e25436. doi: 10.1371/journal.pone.0025436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Gu D, Li J, Cui K, Zhang Y. Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS ONE. 2011;6:e24520. doi: 10.1371/journal.pone.0024520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56:1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- Xu MJ, Song P, Shirwany N, Liang B, Xing J, Viollet B, et al. Impaired expression of uncoupling protein 2 causes defective postischemic angiogenesis in mice deficient in AMP-activated protein kinase alpha subunits. Arterioscler Thromb Vasc Biol. 2011;31:1757–1765. doi: 10.1161/ATVBAHA.111.227991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Liu L, Wang X, Liu X, Xie L, Wang G. Modulation of glucagon-like peptide-1 release by berberine: in vivo and in vitro studies. Biochem Pharmacol. 2010;79:1000–1006. doi: 10.1016/j.bcp.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yamamoto T, Hisatome I, Li Y, Cheng W, Sun N, et al. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic beta cells. Mol Cell Endocrinol. 2013;375:89–96. doi: 10.1016/j.mce.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang X, Shao L, Yang Y, Shang W, Yuan G, et al. Berberine acutely inhibits insulin secretion from beta-cells through 3′,5′-cyclic adenosine 5′-monophosphate signaling pathway. Endocrinology. 2008;149:4510–4518. doi: 10.1210/en.2007-1752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 High glucose (30 mmol·L−1, 8 h) had no effect on SOD2 level in INS-1E cells. Results are means ± SEM of four experiments. HG, high glucose.
Figure S2 (A) Images of mitochondrial ROS production triggered acutely by Ang II (100 nmol·L−1) in INS-1E cells following 8 h incubation with high glucose (30 mmol·L−1) or co-incubation with berberine (5 μmol·L−1) in the presence or absence of compound C (10 μmol·L−1) or genipin (1 μmol·L−1). (B) Images of Ang II-stimulated mitochondrial ROS production when silencing UCP2 with adenovirus ucp2 short hairpin RNA or scramble control. Ang II, angiotensin II; BBR, berberine; CC, compound C; HG, high glucose.
Figure S3 Cell viability was unaffected by 8 h incubation with high glucose (30 mmol·L−1), berberine (5 μmol·L−1), CC (10 μmol·L−1) or genipin (1 μmol·L−1) (A) and scramble or shUCP2 (B). Results are means ± SEM of six to eight experiments. BBR, berberine; CC, compound C; HG, high glucose.


