Abstract
Background
The fatality attributed to pandemic influenza A H1N1 was not clear in the literature. We described the predictors for fatality related to pandemic influenza A H1N1 infection among hospitalized adult patients.
Methods
This is a multicenter study performed during the pandemic influenza A H1N1 [A(H1N1)pdm09] outbreak which occurred in 2009 and 2010. Analysis was performed among laboratory confirmed patients. Multivariate analysis was performed for the predictors of fatality.
Results
In the second wave of the pandemic, 848 adult patients were hospitalized because of suspected influenza, 45 out of 848 (5.3%) died, with 75% of fatalities occurring within the first 2 weeks of hospitalization. Among the 241 laboratory confirmed A(H1N1)pdm09 patients, the case fatality rate was 9%. In a multivariate logistic regression model that was performed for the fatalities within 14 days after admission, early use of neuraminidase inhibitors was found to be protective (Odds ratio: 0.17, confidence interval: 0.03-0.77, p = 0.022), nosocomial infections (OR: 5.7, CI: 1.84-18, p = 0.013), presence of malignant disease (OR: 3.8, CI: 0.66-22.01, p = 0.133) significantly increased the likelihood of fatality.
Conclusions
Early detection of the infection, allowing opportunity for the early use of neuraminidase inhibitors, was found to be important for prevention of fatality. Nosocomial bacterial infections and underlying malignant diseases increased the rate of fatality.
Background
In April 2009 a novel strain of human influenza A of swine origin, identified as A(H1N1)pdm09 virus, rapidly spread worldwide, and in early June 2009 the World Health Organization (WHO) raised the pandemic alert level to phase 6 [1]. Many northern countries experienced the first wave of outbreak during late spring and summer months, followed by an early 2009 fall influenza season [2]. The first laboratory confirmed case in Istanbul was reported in May 2009 [3]. According to the Ministry of Health of Turkey, approximately 6.5 million people were infected, 13,000 patients were hospitalized, and 656 persons died due to the 2009 pandemic H1N1 infection.
It was important to describe the clinical picture and define the risk factors of A(H1N1)pdm09 infection, in order to support public health policy makers in developing vaccination strategies, antiviral use, and other control measures [4]. The clinical and epidemiologic characteristics of the patients hospitalized because of A (H1N1) pdm09 infection were described in the beginning of the outbreak [2,4-7]. However, detailed studies to understand the course of the disease and the predictors of fatality are necessary for a description of such a historical outbreak. Herein, we describe the predictors of fatality among adult hospitalized patients due to A (H1N1) pdm09 infection in Istanbul, Turkey. Description of the clinical features of hospitalized patients in Istanbul, a city with the population around 13 million, will shed light on the obscure areas in fatality and therapy.
Methods
Study population
The study was performed by the İstanbul Pandemic influenza study group of The Turkish Society of Clinical Microbiology and Infectious Diseases (KLIMIK). During and after the 2009 Pandemic, all available data of the hospitalized patients in Istanbul were included in the study. The largest 11 hospitals of Istanbul participated in the study. Three of these hospitals were University Hospitals, and eight were training and research hospitals of The Ministry of Health of Turkey. All patients hospitalized with suspected A (H1N1) pdm09 infection who were ≥ 14 years of age were included in the study. In the beginning of the outbreak, all suspected imported cases were hospitalized for the purpose of disease containment regardless of their need for medical support. Accordingly, these imported cases of the first wave of the outbreak were excluded from this study. The patients from the second wave of the outbreak that started in the beginning of September 2009 were hospitalized because of clinical signs and symptoms of the A (H1N1) pdm09 infections.
The laboratory confirmation was performed by the rRT-PCR method provided by the CDC, Atlanta in one of the two National Influenza Reference Laboratories located in the Istanbul Faculty of Medicine, and at the laboratories of one university and one military hospital. Among the hospitalized patients laboratory diagnosis confirmed patients were included in the study. Infectious diseases and clinical microbiology specialists collected data electronically in individual hospitals, and the pooled data were analyzed. The hospital, official administrative and laboratory data were also reviewed for the consistency of the data related to Istanbul. The study was approved by the Medical Ethics Committee of Marmara University Medical Faculty as a non-interventional clinical research with the number of 09.2010.0097.
Statistical analysis
In univariate analysis, for comparing fatal and survived cases, categorical data were tested by chi square test and t test was used for comparison of the means of two groups (Tables 1 and 2). Parameters found to be statistically significant in univariate analyse, were tested by logistic regression to predict the risk of fatality (Table 3). The independent variables included in the model were early use of neuraminidase inhibitors, nosocomial infection, and having a malignant disease. In analysis STATA (USA, Texas, version 11) was used, with statistical significance set as <0.05.
Table 1.
Demographic characteristics of the laboratory confirmed A (H1N1) pdm09 infected cases and their risk factors for fatality
| Fatal n = 22 (%) | Survived n = 219 (%) | p | |
|---|---|---|---|
| Female gender |
10 (45) |
143 (65) |
0.065 |
| Mean age |
37 (sd 17) |
35 (sd 16) |
0.543 |
| Age ≥ 65 |
2 (9) |
17 (8) |
0.826 |
| Morbid Obesity |
1 (5) |
14 (6) |
0.732 |
| Pregnant women |
1 (10) |
50 (35) |
0.105 |
| Comorbid chronic diseases |
11 (50) |
89 (41) |
0.396 |
| Chronic heart disease |
3 (14) |
24 (11) |
0.704 |
| Diabetes mellitus |
1 (5) |
18 (8) |
0.542 |
| Chronic renal disease |
2 (9) |
11 (5) |
0.421 |
| Chronic neurologic disease |
2 (9) |
11 (5) |
0.421 |
| Chronic obstructive lung disease |
3 (14) |
21 (10) |
0.546 |
| Malignancy |
3 (14) |
5 (2) |
0.005 |
| Vaccinated against H1N1 |
0 |
3 (0.37) |
0.675 |
| Laboratory findings |
|
|
|
| Leukocyte count, median |
13, 875 |
8,700 |
0.046 |
| Thrombocyte count, median |
132,000 |
180,500 |
0.074 |
| AST, median |
59 |
25 |
<0.001 |
| ALT, median |
65 |
21 |
0.004 |
| CPK, median |
285 |
92 |
0.005 |
| LDH, median |
675 |
248 |
0.001 |
| C reactive protein, median | 31 | 25 | 0.135 |
Table 2.
Pulmonary findings of the patients
| Fatal n = 22 (%) | Survived n = 219 (%) | p | |
|---|---|---|---|
| Abnormal auscultation of the lung |
13 (59) |
79 (36) |
0.034 |
| Bilateral involvement in chest x-ray |
18/20 (90) |
59/147 (40) |
<0.001 |
| Type of involvement in chest x-ray |
|
|
|
| Lobar |
1/14 (7) |
15/137 (11) |
0.608 |
| Interstitial |
11/17 (65) |
52/137 (38) |
0.034 |
| Diffuse consolidation |
5/16 (31) |
20/136 (15) |
0.091 |
| Effusion |
3/17 (18) |
2/134 (1) |
<0.001 |
| Need for mechanic ventilation | 15 (68) | 13 (6) | <0.001 |
Table 3.
Univariate and multivariate analyses for the predictors of the fatality
| |
|
Univariate analysis |
|
|
Multivariate analysis |
|
|---|---|---|---|---|---|---|
| Odds ratio | Confidence interval | p | Odds ratio | Confidence interval | P | |
| Using neuraminidase inhibitors within two days after onset of symptoms |
0.33 |
0.14-0.79 |
0.13 |
0.17 |
0.03-0.77 |
0.022 |
| Nosocomial infection |
10 |
4.9-23.2 |
<0.001 |
5.7 |
1.84-18 |
0.013 |
| Presence of malignancy | 4.5 | 1.6-12.4 | 0.003 | 3.8 | 0.66-22.01 | 0.133 |
Results
The first cases of Pandemic Influenza A (H1N1) were recorded in May 2009 and the last cases were seen in February 2010. The patients hospitalized during the second wave of the outbreak, which started in September were included in the study (Figure 1). In the second wave of the infection, 848 patients suspected of A (H1N1) pdm09 were hospitalized. Among the hospitalized patients, 9% were admitted to the intensive care unit and 45 (5.3%) died. We limited our analysis with the 241 A(H1N1)pdm09 laboratory confirmed patients (Figure 1). Among 241 laboratory confirmed influenza A(H1N1)pdm09 patients, 22 (9%) died. Nineteen out of 22 fatal cases (86%) were in ICU, whereas 13 out of 219 (6%) survived cases were in ICU (p < 0.001).
Figure 1.
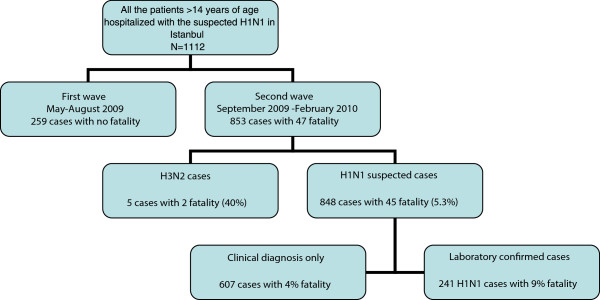
Hospitalized patients with suspected A(H1N1)pdm09 infection in Istanbul between May 2009 and February 2010.
All the co-morbid chronic diseases were more common among the fatal cases, but particularly fatality was more commonly observed among patients with malignancies (Table 1). The proportion of pregnant women among female patients was 33%. Out of 51 pregnant women 1 (2%) died, and the fatality rate among pregnant women was less than the non-pregnant women (9%). However, this difference was not statistically significant (p = 0.105). The proportion of the patients with a body mass index > 30, or clinically judged as obese, among fatal and survived cases were found to be similar (p = 0.732). The most common symptoms among H1N1 confirmed patients were fever (89%), cough (87%), shortness of breath (59%), myalgia (56%), headache (54%), sore throat (52%), and diarrhea (10%).
Leukocyte and thrombocyte counts were lower in the fatal group compared to the surviving patients. The median alanine transferase, aspartate transferase, lactate dehydrogenase, creatinin phosphokinase, and C reactive protein levels were higher among fatal cases (Table 1). Pulmonary findings were common among fatal cases (Table 2).
Among laboratory confirmed influenza A(H1N1)pdm09 cases, the median length of stay was 5 days (interquartile range 2 and 9). Secondary nosocomial bacterial infection was detected in 18 out of 241 (7%) patients. The most commonly isolated nosocomial bacterial pathogens were Acinetobacter spp. (n = 4), Methicillin resistant Staphylococcus spp. (n = 4), E.coli (n = 3), Pseudomonas spp. (n = 1), and Klebsiella spp. (n = 1).
Out of 241 laboratory confirmed patients, 222 (93%) received oseltamivir, and 4 (2%) received zanamivir. Among the patients who used neurominidase inhibitors within 48 hours after onset of symptoms the case fatality rate was 2%, whereas among the patients who did not use the CFR it was 13%. Among the hospitalized patients with confirmed diagnosis of H1N1 infection, 69% received at least one antibiotic. In decreasing order, 67 patients (28%) received respiratory quinolones, 64 patients (26%) received macrolides, 35 (15%) third generation cephalosporins, 40 (16%) ampicillin with sulbactam or amoxicillin with clavulanic acid, 25 (10%) carbapenems, 14 (6%) piperacillin-tazobactam. The rate of vaccination against H1N1 was 0.4, whereas against seasonal flu was 7%.
In multivariate logistic regression, early use of neuraminidase inhibitors was found to be protective (Odds ratio: 0.17, confidence interval: 0.03-0.77, p = 0.022), nosocomial infections (OR: 5.7, CI: 1.84-18, p = 0.013), presence of malignant disease (OR: 3.8, CI: 0.66-22.01, p = 0.133) (Table 3).
Discussion
Istanbul has a population of 13 million according to the 2011 census. Analysis of the pandemic influenza data of Istanbul is valuable because Istanbul has high population and has a potential of being an entrance gate for pandemic infections. Turkey experienced the first wave of the outbreak from May to August, followed by an early 2009 fall influenza season. Usually the influenza season in Turkey starts in December. The majority of the patients in the first wave were imported cases. Since these patients were hospitalized in order to contain the pandemic, their data was not included in this study. The first cases of the second wave were reported between September 2009 and February 2010. Since almost all cases were detected as H1N1 infection in this period, the Ministry of Health of Turkey declared that it was not necessary to confirm the laboratory diagnosis for hospitalized cases. On the other hand, the strongest feature of this study was inclusion of almost all the patients who were hospitalized because of H1N1 in Istanbul. Since the largest state hospitals participated in the study, the goal of presenting a realistic picture of the first pandemic of the 21st century was achieved.
In our study, the mean ages of fatal and survived cases were not statistically different. The fatality rate among the patients over 65 years of age were not found to be higher, although many authors reported that being older than 65 years of age was associated with fatality [8]. According to a systematic analysis which included 44 articles on A (H1N1) pdm09, early in the pandemic the disease occurred overwhelmingly in children and younger adults, and the case fatality rate was 2.9% among confirmed patients [9]. In a study from the USA, which included hospitalized patients, which included also children [10], among 255 hospitalized patients 8% died. In our study, the case fatality rate was 9.1% among the confirmed cases. The majority of the fatalities (75%) occurred within the first 2 weeks of hospitalization. Thirteen percent of confirmed patients were admitted to the intensive care unit. In another multicenter study performed in Turkey, a total of 821 children with 2009 pandemic H1N1 were hospitalized. The majority of admitted children (56.9%) were younger than 5 y of age, and 35 children (4.3%) died. The death rate was significantly higher in patients with malignancy, chronic neurological disease, immunosuppressive therapy, at least 1 pre-existing condition, or respiratory complications [11].
Cough and fever were the most common clinical symptoms as it was reported for the confirmed cases in previous reports [9]. Nosocomial bacterial infection was detected in 7% of the patients. In one study it was reported that bacterial co-infection was common and was associated with age > 50 years and co-morbidities [12]. As one of the different features from seasonal influenza, A (H1N1) pdm09 was reported to pose an increased risk of severe illness in pregnant women [10,13-15]. On the other hand, some studies reported no increased risk of fatality because of A (H1N1) pdm09 infection among the pregnant women [16,17]. In our study group, the proportion of the pregnant women among female patients was 33%, and the case fatality proportion was lower in pregnant women than the non-pregnant women. Because of the awareness about the higher fatality among pregnant women, hospitalization was high. Severely obese individuals with and without chronic conditions were reported to be at increased risk for respiratory hospitalizations during influenza seasons [10,18-20]. However, in our study the proportion of obese patients with body mass index of >30 or clinically judged, among fatal and survived cases were found to be similar (p = 0.912, Table 1). Lack of association of obesity and pregnancy with fatality could be related to low power of the study.
Early use of oseltamivir was reported to be beneficial in treatment [8,10,21,22]. Furthermore, it was reported that among the patients with A(H1N1)pdm09 infection, early use of antiviral therapy prevented development of pneumonia [23,24]. We found that the patients who received neuraminidase inhibitors within 2 days after the disease onset were found to be less likely to die in comparison with the patients who received neuraminidase inhibitors later than two days (Odds ratio: 0.16, confidence interval: 0.03-0.74, p = 0.019, Table 3). According to a recent study from USA, chest radiographs obtained at hospital admission revealed pneumonia in 103 (46%) of 255 patients. Among 255 hospitalized patients, 208 (82%) received neuraminidase inhibitors, but only 47% had treatment ≤ 2 days after illness onset [10]. The rate of vaccination against A (H1N1) pdm09 was 0.4 and was much lower than the rate of vaccination against seasonal flu. Unnecessary use of antibiotics was common among patients who were hospitalized for A (H1N1) pdm09. One of the reasons for such a high rate of unnecessary antibiotic use was obtaining the A (H1N1) pdm09 test results requires an average of 2–3 days. Early diagnosis of influenza virus infection will decrease unnecessary use of antibiotics.
Conclusion
In Influenza A (H1N1) pdm09 infection, using neuraminidase inhibitors within 2 days after onset of symptoms decreased, whereas nosocomial bacterial infections and underlying malignant diseases increased CFR.
Competing interest
The authors declare that they have no competing interests. This study was performed by the Pandemic Influenza Study Group of Turkish Society of Clinical Microbiology and Infectious Diseases.
Authors’ contributions
Substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. EÖ, AS, AÖ, SF, KA, GA, ED, ÖO, Bİİ, CB. involved in drafting the manuscript or revising it critically for important intellectual content. Ergönül ÖBN, YS ŞF, UN, İA, EG, CMA, MK, OM. Final approval of the version to be published. BS, GS, NaÖ, ÖS, ÖN, YT, AT, GP, SN, FM, DAİ, EH. All authors read and approve the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Önder Ergönül, Email: oergonul@ku.edu.tr.
Servet Alan, Email: servetalan@yahoo.com.
Öznur Ak, Email: oznurak@gmail.com.
Fatman Sargın, Email: fatmasargin2002@yahoo.com.
Arzu Kantürk, Email: arzukanturk@gmail.com.
Alper Gündüz, Email: gunduzalper@gmail.com.
Derya Engin, Email: deryaengin@gmail.com.
Oral Öncül, Email: oraloncul@yahoo.com.
Ilker Inanc Balkan, Email: iibalkan@gmail.com.
Bahadir Ceylan, Email: bahadirceylan@yahoo.com.
Nur Benzonana, Email: nbenzonana@gmail.com.
Saadet Yazıcı, Email: saadetyazici@yahoo.com.
Funda Şimşek, Email: fundasimsek67@gmail.com.
Nuray Uzun, Email: nurayuzun@yahoo.com.
Asuman İnan, Email: asumaninan@hotmail.com.
Eren Gulhan, Email: gulhaneren@yahoo.com.
Meral Ciblak, Email: mciblak@gmail.com.
Kenan Midilli, Email: kmidilli@gmail.com.
Mustafa Ozyurt, Email: mozyurt@gata.edu.tr.
Selim Badur, Email: selimbadur@gmail.com.
Serap Gencer, Email: gencerse@gmail.com.
Ozcan Nazlıcan, Email: onazlican@gmail.com.
Serdar Özer, Email: sozer@gmail.com.
Nail Özgüneş, Email: nail_ozgunes@yahoo.com.
Taner Yıldırmak, Email: mtanery@gmail.com.
Turan Aslan, Email: turanas@yahoo.com.
Pasa Göktaş, Email: pasagoktas@yahoo.com.
Nese Saltoğlu, Email: saltoglu.nese@gmail.com.
Muzaffer Fincancı, Email: mfincanci@gmail.com.
Ali Ihsan Dokucu, Email: dokucu@hotmail.com.
Haluk Eraksoy, Email: heraksoy@gmail.com.
References
- Swine Influenza, 25 April 2009. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
- Donaldson LJ, Rutter PD, Ellis BM, Greaves FE, Mytton OT, Pebody RG, Yardley IE. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;14:b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciblak MA, Albayrak N, Odabas Y, Basak Altas A, Kanturvardar M, Hasoksuz M, Sucakli B, Korukluoglu G, Bal E, Ertek M, Badur S. Cases of influenza A(H1N1)v reported in Turkey, May-July 2009. Euro Surveill. 2009;14(32):19304. [PubMed] [Google Scholar]
- Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, Carlino LO, Owen R, Paterson B, Pelletier L, Vachon J, Gonzalez C, Hongjie Y, Zijian F, Chuang SK, Au A, Buda S, Krause G, Haas W, Bonmarin I, Taniguichi K, Nakajima K, Shobayashi T, Takayama Y, Sunagawa T, Heraud JM, Orelle A, Palacios E, van der Sande MA, Wielders CC. et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;14(7):e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchemez S, Donnelly CA, Reed C, Ghani AC, Fraser C, Kent CK, Finelli L, Ferguson NM. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;14(27):2619–2627. doi: 10.1056/NEJMoa0905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Li XW, Mao Y, Wang J, Lu HZ, Chen YS, Liang ZA, Liang L, Zhang SJ, Zhang B, Gu L, Lu LH, Wang DY, Wang C. National Influenza A Pandemic (H1N1) 2009 Clinical Investigation Group of China. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;14(26):2507–2517. doi: 10.1056/NEJMoa0906612. [DOI] [PubMed] [Google Scholar]
- Rello J, Rodriguez A, Ibanez P, Socias L, Cebrian J, Marques A, Guerrero J, Ruiz-Santana S, Marquez E, Del Nogal-Saez F, Alvarez-Lerma F, Martínez S, Ferrer M, Avellanas M, Granada R, Maraví-Poma E, Albert P, Sierra R, Vidaur L, Ortiz P, Prieto del Portillo I, Galván B, León-Gil C. H1N1 SEMICYUC Working Group. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;14(5):R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Deng Y, Pang X, Shi W, Li X, Tian L, Zhang Y, Wang X, Huang F, Raina MC, Wang Q. Severe, critical and fatal cases of 2009 H1N1 influenza in China. J Infect. 2010;14(4):277–283. doi: 10.1016/j.jinf.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Khandaker G, Dierig A, Rashid H, King C, Heron L, Booy R. Systematic review of clinical and epidemiological features of the pandemic influenza A (H1N1) 2009. Influenza Other Respi Viruses. 2011;14(3):148–156. doi: 10.1111/j.1750-2659.2011.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan P, Bullion L, Neises D, Williams RM, Petruccelli BP, Vandermeer M, Lofy KH, Gindler J, Finelli L. 2009 Pandemic Influenza A H1N1 Virus Fall Hospitalizations Investigation Team. Hospitalized patients with 2009 pandemic influenza A (H1N1) virus infection in the United States–September-October 2009. Clin Infect Dis. 2011;14(Suppl 1):S50–59. doi: 10.1093/cid/ciq021. [DOI] [PubMed] [Google Scholar]
- Ciftci E, Tuygun N, Ozdemir H, Tezer H, Sensoy G, Devrim I, Dalgic N, Kara A, Turgut M, Tapisiz A, Keser M, Celebi S, Bayram N, Kocabaş E, Dinleyici EC, Ozen M, Soysal A, Kuyucu N, Tanir G, Celikel E, Belet N, Evren G, Aytaç DB, Cengiz AB, Canoz PY, Derinoz O, Ince E, Hacimustafaoglu M, Anil M, Ozgur O. et al. Clinical and epidemiological features of Turkish children with 2009 pandemic influenza A (H1N1) infection: experience from multiple tertiary paediatric centres in Turkey. Scand J Infect Dis. 2011;14(11–12):923–929. doi: 10.3109/00365548.2011.598872. [DOI] [PubMed] [Google Scholar]
- Dhanoa A, Fang NC, Hassan SS, Kaniappan P, Rajasekaram G. Epidemiology and clinical characteristics of hospitalized patients with pandemic influenza A (H1N1) 2009 infections: the effects of bacterial coinfection. Virol J. 2011;14:501. doi: 10.1186/1743-422X-8-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie JK, Wadford DA, Norman A, Jamieson DJ. 2009 pandemic influenza A (H1N1) and vaccine failure in pregnancy. Obstet Gynecol. 2011;14(2 Pt 2):470–472. doi: 10.1097/AOG.0b013e3181fd2e38. [DOI] [PubMed] [Google Scholar]
- Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z, Wiedeman C, Anand M, Nielsen CF, Waller K, Page S, Thompson JM, Avery C, Springs CB, Jones T, Williams JL, Newsome K, Finelli L, Jamieson DJ; P. H1N1 Influenza in Pregnancy Working Group. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;14(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ. Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;14(9688):451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- Anderson BL, Rouse DJ, Fitzsimmons C. Clinical characteristics of pregnant women with influenza-like illness during the 2009 H1N1 pandemic and use of a standardized management algorithm. Am J Obstet Gynecol. 2011;14(6 Suppl 1):S31–37. doi: 10.1016/j.ajog.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl W, Hell M, Herkner H, Stoiser B, Fritsche G, Schurz-Bamieh N, Poeppl G, Gattringer R, Jones N, Maass M, Egle A, Burgmann H. Clinical aspects of 2009 pandemic influenza A (H1N1) virus infection in Austria. Infection. 2011;14(4):341–352. doi: 10.1007/s15010-011-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis. 2011;14(5):413–421. doi: 10.1093/cid/cir442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, Belay B, Jain S, Cox C, Kamimoto L, Fiore A, Finelli L, Olsen SJ, Fry AM. Morbid obesity as a ssuenza A(H1N1) disease. PLoS One. 2010;14(3):e9694. doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes AL, Arguin P, Biggerstaff MS, Gindler J, Blau D, Jain S, Dhara R, McLaughlin J, Turnipseed E, Meyer JJ, Louie JK, Siniscalchi A, Hamilton JJ, Reeves A, Park SY, Richter D, Ritchey MD, Cocoros NM, Blythe D, Peters S, Lynfield R, Peterson L, Anderson J, Moore Z, Williams R, McHugh L, Cruz C, Waters CL, Page SL, McDonald CK. et al. Epidemiology of 2009 pandemic influenza A (H1N1) deaths in the United States, April-July 2009. Clin Infect Dis. 2011;14(Suppl 1):S60–68. doi: 10.1093/cid/ciq022. [DOI] [PubMed] [Google Scholar]
- McLean E, Pebody RG, Campbell C, Chamberland M, Hawkins C, Nguyen-Van-Tam JS, Oliver I, Smith GE, Ihekweazu C, Bracebridge S, Maguire H, Harris R, Kafatos G, White PJ, Wynne-Evans E, Green J, Myers R, Underwood A, Dallman T, Wreghitt T, Zambon M, Ellis J, Phin N, Smyth B, McMenamin J, Watson JM. Pandemic (H1N1) 2009 influenza in the UK: clinical and epidemiological findings from the first few hundred (FF100) cases. Epidemiol Infect. 2010;14(11):1531–1541. doi: 10.1017/S0950268810001366. [DOI] [PubMed] [Google Scholar]
- Yu H, Feng Z, Uyeki TM, Liao Q, Zhou L, Feng L, Ye M, Xiang N, Huai Y, Yuan Y, Jiang H, Zheng Y, Gargiullo P, Peng Z, Feng Y, Zheng J, Xu C, Zhang Y, Shu Y, Gao Z, Yang W, Wang Y. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis. 2011;14(4):457–465. doi: 10.1093/cid/ciq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S, Kim MN, Kim WY, Kim W, Hong SB, Lim CM, Koh Y, Kwon JW, Hong SJ, Lee SO, Choi SH, Kim YS, Woo JH, Kim SH. Prevalence and clinical features of pneumonia in patients with laboratory-confirmed pandemic influenza A H1N1 2009 infection in South Korea. Scand J Infect Dis. 2011;14(1):19–26. doi: 10.3109/00365548.2010.524656. [DOI] [PubMed] [Google Scholar]
- Jeon MH, Chung JW, Choi SH, Kim TH, Lee EJ, Choo EJ. Pneumonia risk factors and clinical features of hospitalized patients older than 15 years with pandemic influenza A (H1N1) in South Korea: a multicenter study. Diagn Microbiol Infect Dis. 2011;14(2):230–235. doi: 10.1016/j.diagmicrobio.2011.01.005. [DOI] [PubMed] [Google Scholar]


