Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) gene-targeted mice (GM–/–) cleared group B streptococcus (GBS) from the lungs more slowly than wild-type mice. Expression of GM-CSF in the respiratory epithelium of GM–/– mice improved bacterial clearance to levels greater than that in wild-type GM+/+ mice. Acute aerosolization of GM-CSF to GM+/+ mice significantly enhanced clearance of GBS at 24 hours. GBS infection was associated with increased neutrophilic infiltration in lungs of GM–/– mice, while macrophage infiltrates predominated in wild-type mice, suggesting an abnormality in macrophage clearance of bacteria in the absence of GM-CSF. While phagocytosis of GBS was unaltered, production of superoxide radicals and hydrogen peroxide was markedly deficient in macrophages from GM–/– mice. Lipid peroxidation, assessed by measuring the isoprostane 8-iso-PGF2α, was decreased in the lungs of GM–/– mice. GM-CSF plays an important role in GBS clearance in vivo, mediated in part by its role in enhancing superoxide and hydrogen peroxide production and bacterial killing by alveolar macrophages.
Introduction
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a 23-kDa glycoprotein produced by multiple cell types including monocytes/macrophages, B lymphocytes, neutrophils, eosinophils, pulmonary type II cells, and other respiratory epithelial cells (1, 2). The functions of GM-CSF include regulation of hematopoietic cell proliferation and differentiation (1), and modulation of the function of mature hematopoietic cells. These effects include enhanced antigen presentation (3), increased complement- and antibody-mediated phagocytosis (4), augmented microbiocidal capacity (5, 6), and heightened leukocyte chemotaxis and adhesion (7, 8).
Alveolar macrophages are thought to play a critical role in host defense of the lung. Alveolar macrophages bind, phagocytose, and kill bacteria in association with cellular activation, release of intracellular proteases and reactive oxygen species. Reactive oxygen species are released by activated alveolar macrophages directly killing bacteria. Phagocytic cells exposed to GM-CSF demonstrate increased phagocytosis (6, 9), oxidative metabolism (9, 10), activation of Fc receptors (7), release of chemotactic factors (4, 11), and stimulation of mechanisms for killing intracellular viruses, fungi, bacteria, and protozoa (12, 13).
GM-CSF administration to mice before burn injury and infection with Escherichia coli significantly reduced systemic infection and enhanced survival (14). GM-CSF improved survival in neonatal rats treated before Staphylococcus aureus infection (15). However, survival did not improve in rats treated with GM-CSF after the onset of infection from cecal ligation and puncture (16). Although there is compelling evidence that GM-CSF enhances host defense, its role in the clearance of respiratory pathogens in vivo, has not been demonstrated. To assess the function of GM-CSF in vivo, mice carrying a null allele of the GM-CSF gene were generated through the use of gene targeting techniques in embryonic stem cells (17, 18). Mice homozygous for the GM-CSF null allele (GM–/–) have normal hematopoietic function but develop lung pathology characteristic of pulmonary alveolar proteinosis (17, 18). Lung lavage from GM–/– mice revealed markedly increased tubular myelin figures, surfactant proteins A, B, and C, phospholipid and enlarged, foamy alveolar macrophages. Alveolar proteinosis and alveolar macrophage morphology were corrected in the GM–/– mice by direct expression of mouse GM-CSF in lung epithelial cells using the human surfactant protein C (SP-C) gene promoter (SP-C-GM transgenic mice) (19).
Group B streptococcus (GBS) is the most common bacterial infection causing pneumonia and sepsis in newborn infants. Host responses to GBS include activation of both alveolar macrophages and polymorphonuclear leukocytes (PMNs). Phagocytosis and killing of GBS in the lungs is enhanced by surfactant protein A, which increases phagocytosis and reactive oxygen species–mediated killing (20–22). Because macrophage function is strongly influenced by GM-CSF, the present study tested whether GBS clearance from the lungs was influenced by GM-CSF in vivo. Chronic and acute exposure to GM-CSF protected mice from GBS pneumonia mediated in part, by oxygen radical production.
Methods
Animal husbandry.
GM-CSF–deficient mice (GM–/–) were bred from the C57BL/6/129Sv F2 homozygous GM-CSF–deficient mice described previously by Dranoff et al. (17) and were maintained in the C57BL/6 background for several years. GM–/– mice have alveolar proteinosis with elevated phospholipid and surfactant proteins in the lung. The human surfactant protein C (SP-C) gene promoter was used to direct expression of mouse GM-CSF in lung epithelial cells in GM-CSF null mutant (GM–/–) mice, resulting in correction of the alveolar proteinosis (SP-C-GM mice) (19). SP-C-GM mice have increased bronchoalveolar lavage (BAL) GM-CSF, normal concentrations of surfactant proteins A and B and phospholipid in BAL fluid and increased numbers of alveolar macrophages under vivarium conditions. The C57BL/6 strain of mice (Taconic Farms, Germantown, New York, USA) used for comparison (GM+/+), GM–/– and SP-C-GM mice were housed and studied under Institutional Animal Care and Use Committee–approved protocols in the animal facility of the Children's Hospital Research Foundation. Male and female mice weighing ∼20–25 grams (35–42 days old) were used.
Preparation of bacteria.
A stock culture GBS was obtained from a clinical isolate from a newborn infant with systemic infection. Bacteria were suspended in sterile PBS containing 20% glycerol and frozen in aliquots at –80°C. Bacteria from the same passage were used to minimize variations in virulence related to culture conditions. Before each experiment, an aliquot was thawed and plated on tryptic soy/5% defibrinated sheep blood agar and then inoculated into 4 ml of Todd-Hewitt broth (Difco Laboratories, Detroit, Michigan, USA) and grown for 14–16 h at 37°C with continuous shaking. The broth was centrifuged, and the bacteria were washed in PBS at pH 7.2 and resuspended in 4 ml of the buffer. To facilitate studies, a growth curve was generated so the bacterial concentration could be determined spectrophotometrically which was confirmed by quantitative culture of the intratracheal inoculum.
Labeling of bacteria with FITC.
Bacteria were harvested from agar plates 24 h after streaking, suspended in 5 ml PBS (pH 7.2), and centrifuged 1 min at 228 g to remove any large aggregates or agar. The OD at 660 nm of the resulting supernatant was measured to determine bacterial concentration. The suspension was then pelleted at maximum speed in a microfuge and the pellet resuspended in 0.9 ml PBS (pH 7.2) and heated to 95°C for 10 min to kill the bacteria. The heat-killed bacteria were then pelleted and resuspended in 1 ml 0.1 M sodium carbonate (pH 9.0). FITC (Molecular Probes, Eugene, Oregon, USA) was added as a 10 mg/ml stock in DMSO to a final concentration of 0.01 mg/ml, and the suspension was incubated for 1 h in the dark at room temperature with gentle agitation. Labeled bacteria were washed four times for 5 min each time with PBS (pH 7.2) to remove unconjugated fluorophore and finally diluted in PBS and stored in aliquots of 100 μl at –80°C.
Intratracheal inoculation.
Administration of GBS into the respiratory tract was performed by intratracheal inoculation of 104 CFU/ml diluted in sterile PBS. Mice were anesthetized with isofluorane, and an anterior midline incision was used to expose the trachea. A 30-gauge needle attached to a tuberculin syringe was inserted into the trachea and a 100 μl inoculum was dispersed into the lungs. The incision was closed with one drop of Nexaband. Nonpyogenic PBS was injected intratracheally as control.
Bacterial clearance.
Quantitative cultures of lung and spleen homogenates were performed 6, 24, and 48 h after inoculation of the animals with bacteria. Mice were exsanguinated after a lethal intraperitoneal injection of sodium pentobarbital. The abdomen was opened by a midline incision and the animal exsanguinated by transection of the inferior vena cava to reduce pulmonary hemorrhage. The lung and spleen were removed, weighed, and each homogenized in 2 ml of sterile PBS. Homogenate (100 μl) and further dilutions were plated on blood agar plates to quantitate bacteria.
Pathology.
Lungs were inflated via a tracheal cannula at 20 cm of pressure with 4% paraformaldehyde and removed en bloc from the thorax. Lungs were dehydrated and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin.
BAL.
Lung cells were recovered by BAL. Animals were sacrificed as described for bacterial clearance and lungs were lavaged three times with 1 ml of sterile PBS. The fluid was centrifuged at 2,000 rpm for 10 min and resuspended in 1 ml of PBS. Differential cell counts were performed on cytospin preparations stained with Diff-Quick (Scientific Products, McGaw Park, Indiana, USA).
Macrophage phagocytosis.
GBS phagocytosed by alveolar macrophages in vivo were quantitated by counting FITC-labeled GBS under confocal microscopy (Molecular Dynamics, Sunnyvale, California, USA). Lung lavage fluid was centrifuged at 1,200 rpm for 10 min, rat anti–mouse CD16/CD32 monoclonal antibody (Fc block) and phycoerythrin (PE)-conjugated anti–mouse Mac-3 antibody (PharMingen, San Diego, California, USA) were added to the cell pellet and incubated in the dark for 30 min on ice. The Mac-3 antibody binding to the surface of the macrophage was used to delineate the outline of the cell. The pellet was washed with 2 ml of PBS to remove unbound antibody, and a cytospin preparation was examined by confocal microscopy by a blinded observer to assess the presence of intracellular bacteria. Serial sections through 100 random macrophages were performed to determine the percentage of macrophages with phagocytosed bacteria.
Cytokine production.
Lung homogenates were centrifuged at 2,000 rpm and the supernatants stored at –20°C. Interleukin-6 (IL-6), interferon-γ (IFN-γ), and macrophage inflammatory protein-2 (MIP-2) were quantitated using quantitative murine sandwich ELISA kits (R&D Systems, Minneapolis, Minnesota, USA) according to the manufacturer's directions. Tumor necrosis factor-α (TNF-α) levels were measured by ELISA with goat anti-murine antibody (R&D Systems) directed against TNF-α. All plates were read on a microplate reader (Molecular Devices, Sunnyvale, California, USA) and analyzed with the use of a computer-assisted analysis program (Softmax; Molecular Devices). Only assays having standard curves with a calculated regression line value >0.95 were accepted for analysis.
Nitric oxide production.
Nitric oxide (NO) was quantified by measuring its oxidation products nitrite and nitrate after enzymatic conversion to nitrite. Nitrite was measured as previously described (23) by adding 100 μl of Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamide in 5% phosphoric acid) to 100 μl of BAL fluid. The OD at 550 nm (OD550) was measured using a Spectramax 250 microplate reader (Molecular Devices). Nitrite concentrations were calculated by comparison with the OD550 of standard solutions of sodium nitrite. Nitrate concentrations were also determined. Nitrate in the BAL fluid was reduced to nitrite by incubation with nitrate reductase (670 mU/ml) and nicotinamide dinucleotide phosphate (NADPH) (160 μM) at room temperature. After 2 h, nitrite concentration in the samples was measured by the Griess reaction.
Oxygen radical and superoxide anion generation by macrophages.
Superoxide anion production by alveolar macrophages was determined as described (22). Eighteen hours after intratracheal inoculation of GBS (104 CFU), alveolar macrophages were collected by BAL with 1 ml of dye-free RPMI media (GIBCO BRL, Grand Island, New York, USA) three times. Red blood cells in the lavage fluid were lysed with red cell lysis buffer (Sigma Chemical Co., St. Louis, MO), the lavage was centrifuged at 1,200 rpm for 10 min and the pellet resuspended in 200 μl of PBS. Differential analysis of the cells revealed >90% macrophages. One hundred thousand cells were placed in wells of a 96-well plate with 1.2 mg/ml (∼100 μmol/liter) cytochrome C, with or without 20 μg/ml superoxide dismutase, in a final volume of 200 μl of HBSS. Superoxide anion production was determined after activation with 100 ng/ml PMA. OD at 550 nm was determined using a THERMOmax microplate reader (Molecular Devices) linked to a laboratory computer. Measurements were made initially at 5, 10, and 15 min, and then every 15 min until 2 h, at 37°C. OD was converted to nanomoles of cytochrome C reduced using a molar extinction coefficient of 21.1 mM/cm. Each measurement was the mean of at least two replicates with eight determinations at each time. Data were expressed as nanomoles cytochrome C reduced per 105 cells for total oxygen radical production. Superoxide production was assessed by subtracting activity in the presence of superoxide dismutase from total oxygen radical production. Hydrogen peroxide production was assessed by subtracting activity in the presence of catalase (200 U/105 cells) from total oxygen radical production.
Superoxide anion generation by neutrophils.
Alveolar neutrophils were collected by BAL. Mice infected with 109 CFU of GBS had >95% neutrophils in BAL. Because previous studies have demonstrated that surfactant can decrease superoxide generation by neutrophils (24), BAL neutrophils were studied directly after lavage and, in separate experiments, after removal of the surfactant. To separate neutrophils from surfactant, BAL fluid was placed on neutrophil isolation medium (Cardinal Associates, Santa Fe, New Mexico, USA), centrifuged at 1,200 rpm for 30 min, and the band of neutrophils collected. Superoxide generation by neutrophils was analyzed as described for macrophages. Superoxide levels were slightly lower after separation of the surfactant, most likely resulting from partial activation with the additional time and handling before PMA stimulation.
Measurements of 8-iso-PGF2α.
Lung homogenates and BAL fluid were centrifuged at 2,000 rpm and the supernatants stored at –70°C. An enzyme immunoassay (EIA) kit for 8-iso-prostaglandin F2α (8-iso-PGF2α) was purchased from Oxford Biomedical Research Inc. (Oxford, Michigan, USA) and then used for analysis of lung homogenate and BAL fluid 8-iso-PGF2α levels.
Aerosolized GM-CSF.
Recombinant murine GM-CSF, provided by Immunex Inc. (Seattle, Washington, USA), was prepared with mouse albumin fraction as a protein carrier and nebulized in sterile 0.9% NaCl. Studies with technetium-sulfur colloid dissolved in 0.9% NaCl administered by aerosol revealed a volume of 1.5 μl deposited in the lung. Based on these findings, 32.8 μg of GM-CSF in a volume of 12 ml of 0.9% NaCl was aerosolized to provide a dose of 4 ng/mouse. GM+/+ mice sacrificed at 6 h received two doses of GM-CSF (20 h before and at the time of bacterial infection). Mice sacrificed at 24 h received an additional dose of GM-CSF 6 h after infection (three doses total).
Statistical methods.
ANOVA was performed to assess differences between the groups. Individual determinations for each time point were compared using the median scores nonparametric test. Findings were considered statistically significant at P < 0.05.
Results
Pulmonary pathology after GBS administration.
To determine an appropriate bacterial dose for study, wild-type mice were inoculated intratracheally with GBS at concentrations of 104–108 colony-forming units (CFU) (four mice per group). The 106 CFU dose resulted in 50% mortality with the deaths occurring after 24 hours at this dose. A sub-lethal dose of GBS was chosen for the study. Intratracheal administration of 104 CFU GBS was well-tolerated, and all animals survived the 48-hour study period. No alterations in activity or physical appearance of the animals were detected throughout the 48 hours of study.
In GM–/– mice, GBS caused pulmonary infiltrates consisting of both macrophages and polymorphonuclear leukocytes. In contrast, GBS infection caused predominately macrophage infiltrates in GM+/+ and SP-C-GM mice 24 and 48 hours after infection. GBS administration to GM–/– mice caused mild-to-moderate pulmonary inflammation in four of five mice studied at 48 hours. A similar infiltrate was detected in only one of five GM+/+ mice 48 hours after infection (data not shown). GM–/– mice have baseline histologic changes in the lung consisting of accumulation of eosinophilic material in the alveolar spaces and mononuclear cell infiltrates that progress with age. Uninfected GM–/– mice used in the current study (five to six weeks of age) had minimal pulmonary infiltrates. Pulmonary infiltrates were not observed in GM+/+ mice inoculated with sterile PBS (n = 6).
Decreased bacterial clearance in GM–/– mice.
GBS proliferated in the lungs of GM–/– mice and bacteria were more numerous in lung homogenates compared with GM+/+ controls (Fig. 1). GBS clearance was markedly improved in the bitransgenic SP-C-GM mice, to levels better than that seen in GM+/+ mice at 6 hours (Fig. 1). Systemic dissemination of the bacteria was assessed by quantitative culture of the spleen. At 6 hours, no GBS was isolated from the spleens of GM+/+, SP-C-GM, or GM–/– mice. GBS was detected in spleen homogenates in both GM–/– and GM+/+ mice at 24 hours; however, colony counts were not different (data not shown).
Figure 1.
Increased GBS in lung homogenates from GM–/– mice. Concentration of GBS was determined by quantitative cultures of lung homogenates. Colony counts were significantly greater 6, 24, and 48 h after administration of 104 CFU GBS in GM–/– (solid bars) compared with GM+/+ (hatched bars) mice. Six hours after GBS infection, clearance of GBS from the lung was enhanced in SP-C-GM (open bars) mice compared with GM+/+ mice. Data were not obtained for SP-C-GM mice at 48 h. Data are mean ± SEM with n = 10 mice per group. *P < 0.05 compared with GM+/+ mice. GBS, group B streptococcus; GM–/–, GM-CSF gene-targeted mice; SP-C-GM, human surfactant protein C (SP-C) gene promoter expressing GM-CSF.
Phagocytosis of GBS by alveolar macrophages.
GBS infection (104 CFU) increased the number of macrophages and PMNs in BAL fluid from GM+/+ and GM–/– mice. However, BAL fluid from GM–/– mice infected with GBS contained a greater percentage of neutrophils at 6 (5.4 ± 1.2 vs. 1.8 ± 0.8), 24 (27.8 ± 0.8 vs. 0), and 48 hours (34.0 ± 2.4 vs. 0) compared with BAL fluid from GM+/+ mice, respectively; mean ± SEM, n = 5 mice per group, P < 0.05 compared with GM+/+ mice. Control experiments in which sterile 0.9% NaCl was injected intratracheally showed that the inoculation procedure did not alter the cell counts in BAL fluid. BAL fluid from uninfected GM–/– mice contained a slightly lower percentage of macrophages than BAL fluid from uninfected GM+/+ mice (93.4 ± 0.4 vs. 98.6 ± 0.6, respectively; mean ± SEM, n = 5 mice per group, P < 0.05). FITC-labeled bacteria and stained macrophages from the infected animals were visualized under confocal microscopy with three-dimensional imaging. Serial sections through the cell confirmed the presence of intracellular bacteria. Phagocytosis of GBS by alveolar macrophages was similar in GM–/– and GM+/+ mice (15 ± 1.2% and 17.5 ± 1.8%, respectively; mean ± SEM, n = 6).
Decreased superoxide production by alveolar macrophages from GM–/– mice after GBS infection.
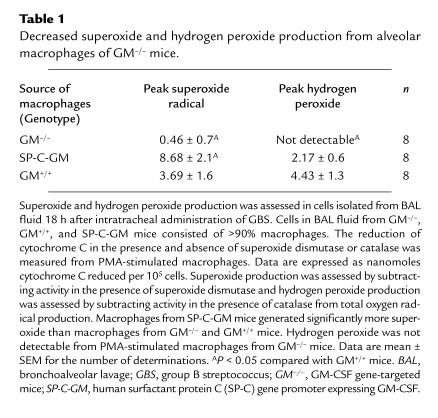
Superoxide production was assessed in cells isolated from BAL fluid 18 hours after intratracheal administration of GBS. BAL fluid from both GM–/–, GM+/+, and SP-C-GM mice contained >90% macrophages after GBS infection (104 CFU). After stimulation with PMA, superoxide radical, and hydrogen peroxide production by alveolar macrophages was significantly decreased in GM–/– compared with GM+/+ mice. Macrophages from SP-C-GM mice generated greater superoxide and similar amounts of hydrogen peroxide compared with macrophages from GM+/+ mice (Table 1).
Table 1.
Decreased superoxide and hydrogen peroxide production from alveolar macrophages of GM–/– mice.
Superoxide production by neutrophils is unaltered in GM–/– mice.
Superoxide production was assessed in cells isolated from BAL fluid 18 hours after intratracheal administration of GBS (109 CFU). At this concentration of GBS, BAL fluid from both GM–/– and GM+/+ mice contained > 90% neutrophils. Stimulation of BAL neutrophils with PMA produced a maximal level of superoxide at 60–75 minutes. Superoxide radical production by neutrophils was similar in cells from GM–/– and GM+/+ mice (4.6 ± 1.8 and 8.5 ± 3.1 nm cytochrome C reduced per 105 cells, respectively; mean ± SEM, n = 8). Similar results were obtained when neutrophils were separated from surfactant with neutrophil isolation medium; superoxide radical production being 2.5 ± 0.9 for GM–/– and 2.8 ± 1.1 nm cytochrome C reduced per 105 cells (mean ± SEM, n = 8) for GM+/+ mice. Thus, abnormalities of superoxide production by GM–/– neutrophils were not a determinant for the impaired clearance of GBS.
Decreased 8-iso-PGF2α in lung homogenates and BAL fluid from GM–/– mice.
Eighteen hours after GBS infection, 8-iso-PGF2α levels were greater in lung homogenates and BAL fluid from GM+/+ compared with GM–/– mice (Fig. 2). F2-isoprostanes are prostanoids produced independently of cyclooxygenase by free radical–catalyzed peroxidation of arachidonic acid–containing lipids. These findings are consistent with a role of GM-CSF in oxygen radical generation in the lung.
Figure 2.
Decreased 8-iso-PGF2α in lung homogenates and BAL fluid from GM–/– mice. Concentrations of 8-iso-prostaglandin F2α (8-iso-PGF2α) were assessed in lung homogenates (a) and BAL fluid (b) from GM–/– (solid bars), and GM+/+ (hatched bars) mice. Prostaglandin F2–like compounds are formed by free radical–catalyzed peroxidation of arachidonic acid, independent of cyclooxygenase enzyme. Decreased concentrations of 8-iso-PGF2α were found in lung homogenates and BAL fluid from the GM –/– mice 18 h after GBS infection. Data are expressed as ng/ml for the lung homogenates and pg/ml for BAL fluid and represent mean ± SEM with n = 8 mice per group. *P < 0.05 compared with GM+/+ mice. BAL, bronchoalveolar lavage.
Cytokine levels in lung homogenates.
Twenty-four hours after GBS infection, proinflammatory cytokines TNF-α, IL-6, and MIP-2 were significantly increased in lung homogenates from GM–/– compared with GM+/+ mice (see Fig. 3). TNF-α concentrations in lung homogenates were increased in SP-C-GM mice, while MIP-2 and IL-6 were similar to those in GM+/+ mice 24 hours after infection (Fig. 3). In contrast, IFN-γ levels were increased in GM–/– (27.2 ± 1.3 pg/ml) compared with GM+/+ (6.7 ± 0.8 pg/ml; mean ± SEM, P < 0.05) mice 24 hours after infection. Increased concentrations of TNF-α in lung homogenates from SP-C-GM mice were associated with enhanced bacterial clearance in these animals. However, increased TNF-α was associated with decreased bacterial clearance in the GM–/– mice. TNF-α was undetectable in lung homogenates from uninfected GM+/+, GM–/–, and SP-C-GM mice (data not shown).
Figure 3.
Increased TNF-α, IL-6, and MIP-2 in lung homogenates from GM–/– mice after GBS infection. Concentrations of TNF-α, IL-6, and MIP-2 were assessed in lung homogenates from GM–/– (solid bars), GM+/+ (hatched bar), and SP-C-GM (open bars) mice. Increased concentrations of the proinflammatory cytokines TNF-α, IL-6, and MIP-2 were found in lung homogenates from the GM–/– mice at 24 h. TNF-α levels were increased in lung homogenates from SP-C-GM compared with GM+/+ mice at 24 h. Data are expressed as pg/ml and represent mean ± SEM with n = 10 mice per group. *P < 0.05 compared with GM+/+ mice. IL-6, interleukin-6; MIP-2, macrophage inflammatory protein-2; TNF-α, tumor necrosis factor-α.
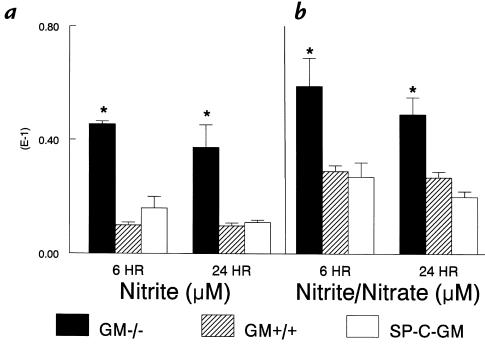
Increased nitrite in BAL fluid from GM–/– mice.
Nitric oxide production after GBS infection was measured as nitrite in BAL fluid. Six and 24 hours after infection, increased nitrite levels were found in BAL fluid from the GM–/–, compared with GM+/+ and SP-C-GM mice (Fig. 4). To estimate nitrate production, nitrate reductase was used to convert nitrate to nitrite and total nitrite/nitrate measured in BAL fluid (20). Six and 24 hours after infection, increased nitrite/nitrate levels were found in BAL fluid from the GM–/– compared with GM+/+ and SP-C-GM mice (Fig. 4). Nitrite was undetectable in BAL fluid from uninfected GM–/–. The observed impairment of bacterial clearance in GM–/– mice, despite increased nitrite concentrations in BAL fluid, suggests that the nitrite production was not a critical determinant of clearance of GBS.
Figure 4.
Increased nitrite concentrations in BAL fluid from GM–/– mice after GBS infection. Nitrite in the BAL fluid was measured by the Griess reaction as described in Methods. Six and 24 h after intratracheal inoculation of 104 CFU GBS, nitrite (a) and nitrite/nitrate (b) levels in BAL fluid from GM–/– mice (solid bars) were increased compared with GM+/+ (hatched bars) and SP-C-GM (open bars) mice. Data represent mean ± SEM with n = 5 mice per group. *P < 0.05 compared with GM+/+ mice.
Aerosolized GM-CSF enhances GBS clearance.
Wild-type mice were treated with exogenous GM-CSF to determine whether acute administration of GM-CSF to the lung enhanced bacterial clearance. Two doses of aerosolized GM-CSF (4 ng/mouse/dose, 20 hours before and at time of infection) did not increase clearance of GBS from the lungs of GM+/+ mice at 6 hours. Treatment of GM+/+ mice with three doses of aerosolized GM-CSF (additional dose 6 hours after infection) significantly enhanced clearance of GBS at 24 hours (Fig. 5).
Figure 5.
Exogenous GM-CSF enhances GBS clearance from the lung. Concentration of GBS was determined by quantitative cultures of lung homogenates. Colony counts were significantly decreased 24 h after administration of 104 CFU GBS to wild-type mice (solid) treated with three doses of aerosolized recombinant mouse GM-CSF (4 ng/mouse) compared with untreated controls (hatched bars). Data are mean ± SEM with n = 15 mice per group. *P < 0.05 compared with untreated wild-type mice.
Discussion
Pulmonary clearance of intratracheally administered GBS was reduced in GM–/– mice when compared with GM+/+ mice. Expression of mouse GM-CSF in the respiratory epithelium of GM–/– mice under control of the SP-C promoter and acute administration of GM-CSF to GM+/+ mice improved bacterial clearance to levels greater than wild-type mice. While phagocytosis of bacteria by macrophages was similar in GM–/– and GM+/+ mice, superoxide radical and hydrogen peroxide production were markedly decreased in alveolar macrophages from GM–/– mice. These findings support the concept that GM-CSF plays an important role in pulmonary host defense, mediated in part by increased alveolar macrophage oxygen radical production and bacterial killing.
Surprisingly, impaired pulmonary clearance of GBS in GM–/– mice was not associated with abnormalities in macrophage phagocytosis. Previous in vitro studies demonstrated that GM-CSF enhanced phagocytosis of serum opsonized Staphylococcus aureus by neutrophils (6) and Cryptococcus neoformans by macrophages (4, 25). The present study did not demonstrate abnormalities in phagocytosis, suggesting that other mechanisms operative in vivo may compensate for the lack of GM-CSF. For example, surfactant proteins A and D (SP-A, SP-D) are markedly increased in BAL from GM–/– mice (17). These surfactant proteins play an important role in host defense against bacterial pathogens. Surfactant proteins A and D stimulate macrophage chemotaxis (26, 27) and SP-A enhances binding of bacteria to macrophages (20). SP-A gene–deficient mice are highly susceptible to infection with GBS (28). Thus, the increased concentrations of SP-A and SP-D in BAL fluid from GM–/– mice may have contributed to the maintenance of phagocytic activity by alveolar macrophages.
Phagocytic cells generate reactive oxygen species that are involved in bacterial killing. Phagocytosis of invading microorganisms leads to increased oxygen consumption, reduction of oxygen to superoxide, which is then secreted into the phagosome where it dismutates to hydrogen peroxide. Production of superoxide and hydrogen peroxide contributes to microbial killing. In the present study, stimulated macrophages from GM–/– mice generated less superoxide and hydrogen peroxide than GM+/+ macrophages, demonstrating a critical role of GM-CSF in oxidant production. In previous studies, GM-CSF enhanced superoxide anion generation from monocytes in vitro (29), and inhibited replication of Trypanosoma cruzi with enhanced oxidative metabolism (30). Elevated superoxide production (both basal state and after stimulation) was also observed in peritoneal and pleural macrophages isolated from transgenic mice expressing GM-CSF with a retroviral promoter (31), consistent with the present observations that superoxide production was decreased in GM–/– mice. Likewise, local expression of GM-CSF in the respiratory epithelium of the transgenic SP-C-GM mice was associated with enhanced superoxide generation by alveolar macrophages. Concentrations of 8-iso-PGF2α in the lung, a measure of free radical–induced lipid peroxidation, were decreased in GM–/– mice, consistent with the role of GM-CSF in oxygen radical production. F2-isoprostanes are biologically active prostaglandin F2 (PGF2)-like compounds formed nonenzymatically by free radical–catalyzed peroxidation of arachidonic acid (32). Macrophages from GM–/– mice had impaired production of oxygen radicals (superoxide, hydrogen peroxide), consistent with the finding of decreased lipid peroxidation. The defects in superoxide production and lipid peroxidation by alveolar macrophages could be primary defects resulting from the lack of GM-CSF signaling or alternatively could be secondary defects resulting from the abnormal alveolar macrophages with characteristic enlarged, foamy cytoplasm with lipid inclusions.
Oxidative killing of bacteria can be mediated by the production of nitric oxide, which in the presence of reactive oxygen species is converted to peroxynitrite, a potent bactericidal free radical (33). However, decreased bacterial clearance was associated with increased nitrite concentrations in BAL fluid from GM–/– mice. Therefore, nitrite production was not a determinant in the clearance of GBS from the lungs. In vitro, NO production by lipopolysaccharide (LPS)-stimulated peritoneal macrophages from GM–/– mice was decreased; however, LPS and interferon-γ increased NO production similarly in wild-type and GM–/– macrophages (34). Nitric oxide has antimicrobial effects against a variety of intracellular pathogens (35); however, it remains unclear whether NO contributes to the ability of murine macrophages to eliminate pyogenic microorganisms in vivo.
Clearance of GBS from the lungs of GM–/– mice was deficient in spite of increased neutrophil recruitment. Furthermore, differences in superoxide radical generation by neutrophils in GM–/– and GM+/+ mice were not a determinant in the impaired pulmonary clearance of GBS. Increased infiltration of neutrophils after GBS administration was associated with increased cytokine production in the lungs of GM–/– mice. MIP-2, a neutrophil chemoattractant,was elevated, consistent with increased neutrophils in GM–/– BAL fluid. In vitro studies have suggested that GM-CSF may regulate cytokine release, in particular, TNF-α and IL-6 (36, 37). GM-CSF deficiency had no apparent effect on GBS-induced peak TNF-α response, because both GM–/– and SP-C-GM mice had greater TNF-α levels in BAL fluid compared with GM+/+ mice. Previous studies found decreased serum IL-6 and IFN-γ in GM–/– mice treated with LPS (34), in contrast to the current study demonstrating elevated IL-6 and IFN-γ in BAL fluid from GM–/– mice after GBS infection. It is unclear from the current study whether these differences are directly related to the absence of GM-CSF or to the severity of infection and failure of early bacterial clearance.
Pulmonary clearance of GBS was improved acutely by administration of aerosolized GM-CSF and by genetic replacement of GM-CSF in the respiratory epithelium of transgenic mice. This finding is consistent with previous studies demonstrating that intraperitoneal GM-CSF increased survival and decreased bacterial load in mice infected with Salmonella typhimurium (38). Additionally, GM-CSF decreased the severity of Pneumocystis carinii pneumonia in vivo (39). Although GM–/– mice had greater pulmonary bacterial burden, systemic dissemination was similar in GM+/+ and GM–/– mice. Enhanced infection was observed in GM–/– mice infected intraperitoneally with Listeria monocytogenes (a facultative intracellular bacterium) (40), suggesting that GM-CSF may have a role in host defense outside of the lung. SP-A and SP-D enhance phagocytosis (20), chemotaxis (26, 27), macrophages activation (21, 41), and enhance GBS clearance from the lung (28). The increased concentrations of SP-A and SP-D (17) failed to correct pulmonary infection or to limit systemic spread of GBS in GM–/– mice.
In summary, the present study demonstrates the important role of GM-CSF in pulmonary clearance of GBS in vivo. Effects of GM-CSF on GBS clearance were associated with increased superoxide radical and hydrogen peroxide generation by alveolar macrophages, demonstrating the critical role of GM-CSF in oxidant generation by these cells. Exogenous administration of GM-CSF enhanced clearance of GBS in wild-type mice and therefore, may represent a strategy to prevent or treat pulmonary infections.
Acknowledgments
We thank Jo Rae Wright for the FITC-labeled group B streptococcus, G. Dranoff (Massachusetts Institute of Technology, Cambridge, Massachusetts, USA) for the GM–/– mice, Ann Maher for assistance with manuscript preparation, and Peter Gartside for assistance with statistical methods. Supported by National Institutes of Health grants HL-56387 (to J.A. Whitsett and J.A. Reed) and T32HL-07752 (to J.A. Reed), and Cystic Fibrosis Research and Development Program. A.M. LeVine is a Procter Fellow at the Children's Hospital Medical Center.
References
- 1.Tarr PE. Granulocyte-macrophage colony-stimulating factor and the immune system. Med Oncol. 1996;13:133–140. doi: 10.1007/BF02990841. [DOI] [PubMed] [Google Scholar]
- 2.Smith SM, Lee DKP, Lacy J, Coleman DL. Rat tracheal epithelial cells produce granulocyte/macrophage colony-stimulating factor. Am J Respir Cell Mol Biol. 1990;2:59–68. doi: 10.1165/ajrcmb/2.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Morrissey PJ, Bressler L, Park LS, Alpert A, Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J Immunol. 1987;139:1113–1119. [PubMed] [Google Scholar]
- 4.Collins HL, Bancroft GJ. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-α and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992;22:1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- 5.Weiser WY, van Niel A, Clark SC, David JR, Remold HG. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987;166:1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann J, Golde DW, Weisbart RH, Gasson JC. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood. 1986;68:708–711. [PubMed] [Google Scholar]
- 7.Weisbart RH, Kacena A, Schuh A, Golde DW. GM-CSF induces human neutrophil IgA-mediated phagocytosis by an IgA Fc receptor activation mechanism. Nature. 1988;332:647–648. doi: 10.1038/332647a0. [DOI] [PubMed] [Google Scholar]
- 8.Arnaout MA, Wang EA, Clark SC, Sieff CA. Human recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins on mature granulocytes. J Clin Invest. 1986;78:597–601. doi: 10.1172/JCI112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman DL, Chodakewitz JA, Bartiss AH, Mellors JW. Granulocyte-macrophage colony-stimulating factor enhances selective effector functions of tissue derived macrophages. Blood. 1988;72:573–578. [PubMed] [Google Scholar]
- 10.Wing EJ, Ampel NM, Waheed A, Shadduck RK. Macrophage colony-stimulating factor (M-CSF) enhances the capacity of murine macrophages to secrete oxygen reduction products. J Immunol. 1985;135:2052–2056. [PubMed] [Google Scholar]
- 11.Wang JM, Colella S, Allavena P, Mantovani A. Chemotactic activity of human recombinant granulocyte-macrophage colony-stimulating factor. Immunology. 1987;60:439–444. [PMC free article] [PubMed] [Google Scholar]
- 12.Ruef C, Coleman DL. Granulocyte-macrophage colony stimulating factor: pleiotropic cytokine with potential clinical usefulness. Rev Infect Dis. 1990;12:41–62. doi: 10.1093/clinids/12.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Jones TC. The effect of granulocyte-macrophage colony stimulating factor (rGM-CSF) on macrophage function in microbial disease. Med Oncol. 1996;13:141–147. doi: 10.1007/BF02990842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gennari R, Alexander JW, Gianotti L, Eaves-Pyles T, Hartmann S. Granulocyte macrophage colony-stimulating factor improves survival in two models of gut-derived sepsis by improving gut barrier function and modulating bacterial clearance. Ann Surg. 1994;220:68–76. doi: 10.1097/00000658-199407000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenck RW, Sarman G, Harper TE, Buescher ES. The ability of recombinant murine granulocyte-macrophage colony-stimulating factor to protect neonatal rats from septic death due to Staphylococcus aureus. J Infect Dis. 1990;162:109–114. doi: 10.1093/infdis/162.1.109. [DOI] [PubMed] [Google Scholar]
- 16.Toda H, et al. Effect of granulocyte-macrophage colony-stimulating factor on sepsis-induced organ injury in rats. Blood. 1994;83:2893–2898. [PubMed] [Google Scholar]
- 17.Dranoff G, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 18.Stanley E, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huffman JA, Hull WM, Dranoff G, Mulligan RC, Whitsett JA. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest. 1996;97:649–655. doi: 10.1172/JCI118461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Iwaarden F, Welmers B, Verhoef J, Haagsman HP, van Golde LMG. Pulmonary surfactant protein A enhances the host-defense mechanisms of rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990;2:91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]
- 21.Weissbach S, Neuendank A, Pettersson M, Schaberg T, Pison U. Surfactant protein A modulates release of reactive oxygen species from alveolar macrophages. Am J Physiol. 1994;267:L660–L666. doi: 10.1152/ajplung.1994.267.6.L660. [DOI] [PubMed] [Google Scholar]
- 22.LeVine, A.M., et al. Surfactant protein-A (SP-A) binds group B streptococcus enhancing phagocytosis and clearance from lungs of surfactant protein-A deficient mice. Am. J. Respir. Cell Mol. Biol. In press. [DOI] [PubMed]
- 23.Green LC, et al. Analysis of nitrate, nitrite, and 15N nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 24.Ahuja A, Oh N, Chao W, Spragg RG, Smith RM. Inhibition of the human neutrophil respiratory burst by native and synthetic surfactant. Am J Respir Cell Mol Biol. 1996;14:496–503. doi: 10.1165/ajrcmb.14.5.8624255. [DOI] [PubMed] [Google Scholar]
- 25.Chen GH, et al. Effect of granulocyte-macrophage colony-stimulating factor on rat alveolar macrophage anticryptococcal activity in vitro. J Immunol. 1994;152:724–734. [PubMed] [Google Scholar]
- 26.Wright JR, Youmans DC. Pulmonary surfactant protein A stimulates chemotaxis of alveolar macrophages. Am J Physiol. 1993;264:L338–L344. doi: 10.1152/ajplung.1993.264.4.L338. [DOI] [PubMed] [Google Scholar]
- 27.Crouch EC, Persson A, Griffin GL, Chang D, Senior RM. Interactions of pulmonary surfactant protein D (SP-D) with human blood leukocytes. Am J Respir Cell Mol Biol. 1995;12:410–415. doi: 10.1165/ajrcmb.12.4.7695920. [DOI] [PubMed] [Google Scholar]
- 28.LeVine AM, et al. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- 29.Smith PD, et al. Granulocyte-macrophage colony-stimulating factor augments human monocyte fungicidal activity for Candida albicans. J Infect Dis. 1990;161:999–1005. doi: 10.1093/infdis/161.5.999. [DOI] [PubMed] [Google Scholar]
- 30.Reed SG, et al. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. J Exp Med. 1987;166:1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott MJ, Strasser A, Metcalf D. Selective up-regulation of macrophage function in granulocyte-macrophage colony-stimulating factor transgenic mice. J Immunol. 1991;147:2957–2963. [PubMed] [Google Scholar]
- 32.Morrow JD, Roberts LJ., II The isoprostanes: current knowledge and directions for future research. Biochem Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- 33.Fang FC. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu S, et al. Increased tolerance to endotoxin by granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1997;159:1412–1417. [PubMed] [Google Scholar]
- 35.Miller RA, Britigan BE. The formation and biologic significance of phagocyte-derived oxidants. J Invest Med. 1995;43:39–49. [PubMed] [Google Scholar]
- 36.Sisson SD, Dinarello CA. Production of interleukin-1α, interleukin-1β and tumor necrosis factor by human mononuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood. 1988;72:1368–1374. [PubMed] [Google Scholar]
- 37.Cicco NA, et al. Inducible production of interleukin-6 by human polymorphonuclear neutrophils: role of granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-alpha. Blood. 1990;75:2049–2052. [PubMed] [Google Scholar]
- 38.Morrissey PJ, Charrier K. GM-CSF administration augments the survival of ITY-resistant A/J mice, but not ITY-susceptible C57BL/6 mice, to a lethal challenge with Salmonella typhimurium. J Immunol. 1990;144:557–561. [PubMed] [Google Scholar]
- 39.Mandujana JF, et al. Granulocyte-macrophage colony stimulating factor and Pneumocystis carinii pneumonia in mice. Am J Respir Crit Care Med. 1995;151:1233–1238. doi: 10.1164/ajrccm/151.4.1233. [DOI] [PubMed] [Google Scholar]
- 40.Zhan Y, Lieschke GJ, Grail D, Dunn AR, Cheers C. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood. 1998;91:863–869. [PubMed] [Google Scholar]
- 41.van Iwaarden JF, Shimizu H, van Golde PHM, Voelker DR, van Golde LMG. Rat surfactant protein D enhances the production of oxygen radicals by rat alveolar macrophages. Biochemistry. 1992;286:5–8. doi: 10.1042/bj2860005. [DOI] [PMC free article] [PubMed] [Google Scholar]