Abstract
Patients with pemphigus foliaceus (PF) have blisters on skin, but not mucous membranes, whereas patients with pemphigus vulgaris (PV) develop blisters on mucous membranes and/or skin. PF and PV blisters are due to loss of keratinocyte cell–cell adhesion in the superficial and deep epidermis, respectively. PF autoantibodies are directed against desmoglein (Dsg) 1; PV autoantibodies bind Dsg3 or both Dsg3 and Dsg1. In this study, we test the hypothesis that coexpression of Dsg1 and Dsg3 in keratinocytes protects against pathology due to antibody-induced dysfunction of either one alone. Using passive transfer of pemphigus IgG to normal and DSG3null neonatal mice, we show that in the areas of epidermis and mucous membrane that coexpress Dsg1 and Dsg3, antibodies against either desmoglein alone do not cause spontaneous blisters, but antibodies against both do. In areas (such as superficial epidermis of normal mice) where Dsg1 without Dsg3 is expressed, anti-Dsg1 antibodies alone can cause blisters. Thus, the anti-desmoglein antibody profiles in pemphigus sera and the normal tissue distributions of Dsg1 and Dsg3 determine the sites of blister formation. These studies suggest that pemphigus autoantibodies inhibit the adhesive function of desmoglein proteins, and demonstrate that either Dsg1 or Dsg3 alone is sufficient to maintain keratinocyte adhesion.
Introduction
Pemphigus is a life-threatening blistering skin disease in which patients’ autoantibodies are directed against desmosomal glycoproteins, resulting in the loss of keratinocyte cell–cell adhesion (1). The two classic forms of pemphigus are pemphigus foliaceus (PF) and pemphigus vulgaris (PV) (2). In PF, patients develop skin erosions that result from blisters within the granular layers of the superficial epidermis. Patients with PF, however, do not develop blisters or erosions of the mucous membranes. Two-thirds of patients with PV develop mucous membrane blisters early in the course of their disease, and as the disease progresses, they develop skin blisters as well (3). Histologically, PV blisters occur deep in the epidermis between the basal and most immediate suprabasal keratinocytes, as well as between the basal cells themselves.
Pemphigus autoantibodies recognize cell-surface antigens of keratinocytes (4). These antigens have been identified as desmogleins (Dsg's), transmembrane desmosomal glycoproteins belonging to the cadherin supergene family of calcium-dependent adhesion molecules (5–7). Dsg1 is the autoantigen recognized by PF antibodies, whereas Dsg3 is specifically recognized by PV autoantibodies (8–13). However, about one-half to two-thirds of PV sera also contain antibodies against Dsg1 (10, 14–16). Most patients with early PV and only mucous membrane lesions have only anti-Dsg3 antibodies, whereas most patients with later disease, involving the skin, have both anti-Dsg3 and anti-Dsg1 antibodies (13, 16).
There is compelling evidence for the pathogenicity of these autoantibodies in pemphigus. One example is the development of skin blisters in neonatal mice when injected with pemphigus IgG (17, 18). Similar to the pathology of human pemphigus, PF IgG induces blisters in the superficial epidermis, and PV IgG induces deep suprabasilar blisters in neonatal mice. Immunoadsorption and affinity chromatography of pemphigus sera have confirmed that the anti-desmoglein antibodies are pathogenic in pemphigus. PF sera that are immunoadsorbed with the extracellular domain of Dsg1 are no longer pathogenic in neonatal mice, whereas IgG that has been affinity purified from PF sera on Dsg1 causes superficial skin blisters (19). Similarly, PV sera immunoadsorbed with the extracellular domain of Dsg3 lose pathogenic activity (20, 21).
Although these anti-desmoglein antibodies have been shown to cause the blister in pemphigus, the pathophysiological mechanism by which they do so has been controversial. Some studies have suggested that pemphigus antibody binding mediates protease release that, in turn, causes loss of cell adhesion (22–25). However, we have postulated a more direct effect in which antibodies block the function of the Dsg's in stabilizing cell adhesion in desmosomes (7). Lending credence to this theory has been the characterization of DSG3null mice that are essentially equivalent to mice whose Dsg3 function has been completely blocked (26). These mice have a similar phenotype to patients with PV, with oral mucous membrane lesions in neonates due to suckling and skin erosions at sites of trauma. Both types of lesions show histology typical of PV.
It has been suggested (27–30) that the distribution and expression levels of Dsg1 and Dsg3 might account for the characteristic distribution of lesions. For example, Dsg3 is expressed throughout the oral mucosa, whereas it is only expressed in the basal and immediate suprabasal layer of the epidermis (29, 30). Conversely, Dsg1 is expressed throughout the epidermis and oral mucosa, but more intensely superficially, and very weakly in the deep epidermis. These type of observations have led to the hypothesis, first advanced by Shirakata et al. (30), that where Dsg3 and Dsg1 are coexpressed, antibodies against either one alone are not efficient at causing spontaneous blistering (e.g., anti-Dsg1 in the oral mucous membrane where Dsg3 is highly expressed throughout). On the other hand, in areas where Dsg1 is expressed without concomitant Dsg3, anti-Dsg1 alone is efficient at causing spontaneous blister formation (e.g., in the superficial epidermis of patients with PF).
In this report, we use passive transfer of pemphigus IgG to normal and DSG3null neonatal mice to test this hypothesis. We will show that if both Dsg1 and Dsg3 are present concomitantly at a tissue site, antibodies against either alone are inefficient at causing a spontaneous blister. In contrast, antibodies against both Dsg's are highly efficient at blister formation if both are concomitantly expressed in the tissue, or antibodies against one alone are efficient if the other is not coexpressed. These findings are consistent with an explanation for blister formation in which autoantibodies against either Dsg1 or Dsg3 specifically block only its function, in contrast to causing release of proteases that nonspecifically cause loss of cell adhesion. Finally, by knowing the anti-desmoglein specificity of pemphigus sera and the Dsg distributions in stratified squamous epithelia, the localization of blister formation in PV and PF can be explained.
Methods
Serum IgG purification and characterization.
Ten human sera were used: one normal human serum (NHS3019), sera from two patients with PF (PF982 and PF1239), and sera from seven patients with PV (JPV21, NIH2054, PV1236, PV1581, PV3014, PV3015, and PV3024). The IgG fractions of the sera were isolated by chromatography using DEAE Affi-Gel Blue according to the manufacturer's protocol (Bio-Rad Laboratories Inc., Richmond, California, USA). For each purification, serum (20 ml) was dialyzed overnight at 4°C against 20 mM KH2PO4 (pH 8.0) and 0.02% NaN3 and loaded on a 100-ml column bed. The eluted IgG was concentrated using Centriprep 10 (Amicon, Lexington, Massachusetts, USA) and dialyzed against PBS (pH 7.4; GIBCO BRL, Grand Island, New York, USA) overnight at 4°C. The protein concentration was then determined using Bio-Rad Protein Assay (Bio-Rad Laboratories Inc.). The final IgG concentrates were sterile-filtered through 0.22-μm membranes (Millipore Corp., Bedford, Massachusetts, USA) and stored at –20°C.
Before affinity purification, all sera were tested by indirect immunofluorescence for cell-surface staining on monkey esophagus cryosections (Scimedx Corp., Denville, New Jersey, USA). The titers were greater than 320 for all PV and PF sera.
Dsg1 and Dsg3 ELISA.
Procedures for ELISA using baculovirus-expressed recombinant Dsg1 and Dsg3 have been described previously (13). The methods were slightly modified, and index values were used for ELISA scores instead of reaction units. Briefly, all sera were diluted 200-fold and incubated for 1 h at room temperature on the recombinant Dsg-coated 96-well ELISA plates. After washing, the plate was incubated with peroxidase-conjugated mouse monoclonal anti–human IgG antibody (MBL, Nagoya, Japan) for 1 h at room temperature. After washing again, the color was developed with a tetramethylbenzidine solution for 30 min, and the reaction was stopped with 2N H2SO4. The absorbance was measured at 450 nm by ELISA reader (Bio-Rad Laboratories Inc.). Index values were obtained from the following formula: Index = [(OD of tested serum – OD of negative control) / (OD of positive control serum – OD of negative control)] × 100. Positive controls for Dsg1 and Dsg3 ELISAs were diluted standard PF and PV sera, respectively. Negative control was a diluted serum obtained from a normal individual.
IgG injection into neonatal mice.
C57Bl/6J mice were obtained from Jackson Laboratories (Bar Harbor, Maine, USA). The DSG3null mice were obtained by DSG3+/– heterozygote intercrosses (26). Newborn mice (1–2 days old) weighing between 1.3 and 1.8 g were injected subcutaneously between the shoulder blades using a 1-cc insulin syringe (Becton Dickinson, Franklin Lakes, New Jersey, USA). Each animal received between 1 and 26 mg of the purified IgGs and was sacrificed 16 h later for evaluation. The mice were injected with 100 units of heparin (Lyphomed, Deerfield, Illinois, USA) and decapitated 10 min later. Skin and head sections were fixed in 10% PBS-buffered formalin (Sigma Chemical Co., St. Louis, Missouri, USA) for histology. Tails were collected for DNA extraction and genotyping.
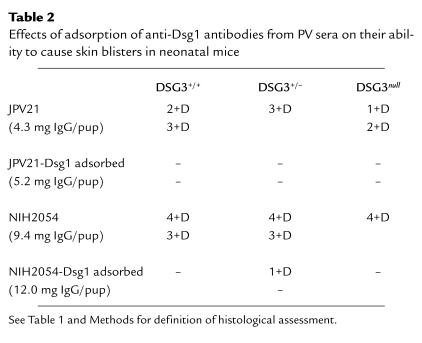
After immunoadsorption with Dsg1, the ELISA readings for anti-Dsg3 antibodies decreased by 17% (from 0.561 to 0.468) with JPV21 serum and by 21% (from 0.894 to 0.704) with NIH2054 serum. To compensate for the decrease of anti-Dsg3 antibodies, we increased the amount of IgG injected per pup: from 4.3 mg/pup of unadsorbed JPV21 to 5.2 mg/pup of Dsg1-adsorbed JPV21 and from 9.4 mg/pup of unadsorbed NIH2054 to 12 mg/pup of Dsg1-adsorbed NIH2054.
To score the animals or histological tissues based on the degree of blistering, we have devised the following protocols: We scored gross blisters on animals before sacrifice on a scale from 1+ to 4+, where 1+ denotes blisters only when handled; 2+, localized blisters <30% (of tissue); 3+, extensive blisters >30%; and 4+, very extensive blisters >80%. Animals without any blisters received a (–) mark. Histological blisters were scored from 0.5+ to 4+, where 0.5+ denotes very minor blisters; 1+, blisters at the edge; 2+, localized blisters <50%; 3+, extensive blisters >50%; and 4+, very extensive blisters >75%. Tissues that did not show any blisters received a (–) mark.
PCR and DSG3 genotyping.
The DSG3 exon 1 (200 bps) was amplified with primers 5′- GGAGGAACAGACTAACAGGC and 5′-ACCATCAGGAGGGCCAGAGA, and the neomycin DNA fragment (300 bps) was amplified with primers 5′-CTTGGGTGGAGAGGCTATTC and 5′-AGGTGAGATTACAGGAGATC under the following conditions: 94°C for 2 min, 58°C for 2 min, 72°C for 2 min, then 29 cycles of 94°C for 40 sec, 58°C for 1 min, and 72°C for 1 min; the final step was a 10-min incubation at 72°C. The products were electrophoresed on a 2% agarose gel, and the DNA was stained with ethidium bromide.
Indirect immunofluorescence.
Mouse tissues were either frozen in OCT (Electron Microscopy Sciences, Fort Washington, Pennsylvania, USA) or fixed in 10% formalin (Sigma Chemical Co.). With frozen OCT tissue, sections (4 μm) were fixed either by air drying or 5 min in methanol/acetone (–20°C) and then washed in PBS (GIBCO BRL) for 15 min. The tissue sections were then blocked for 1 h in blocking buffer (1% BSA, fraction V; Sigma Chemical Co.) in PBS and incubated with PF982 or PF1239 sera in blocking buffer overnight at 4°C. After a 15-min wash in PBS, the tissue sections were incubated with FITC-conjugated goat anti–human IgG (1:80 in blocking buffer, Cappel Laboratories, Cochranville, Pennsylvania, USA) for 1 h at room temperature. The sections were again washed for 15 min in PBS, dipped briefly in 95% ethanol (Sigma Chemical Co.), and mounted in Elvanol (DuPont,Wilmington, Delaware, USA) for viewing and photography with a BX60 photomicroscope (Olympus Corp., Lake Success, New York, USA) (26).
Titers of the sera and IgG fractions were determined by indirect immunofluorescence on monkey esophagus cryosections (Scimedx Corp.). Briefly, monkey esophagus sections were washed for 15 min in PBS, blocked for 15 min in blocking buffer, and incubated with the sera or IgG fractions suspended in blocking buffer for 1 hr at room temperature. The remaining steps were as described earlier here.
To stain for Dsg3, AP904, an affinity purified rabbit anti–mouse Dsg3 antibody, was used. This antibody was shown by immunoblot to recognize a 130-kDa protein in DSG3+/+ but not DSG3null mouse skin extracts (31). Formalin-fixed paraffin-embedded tissues (4 μm) were deparaffinized for 5 min each in xylene (2 times), 100% ethanol (2 times), 95% ethanol, 75% ethanol, and 50% ethanol. After a brief rinse in PBS, the tissues were microwaved at 900 W for 4.5 min in an antigen-retrieving medium (TUF; Signet, Dedham, Massachusetts, USA). The tissues were then treated at 41°C for 7 min each with 0.1% trypsin and then 3% hydrogen peroxide. After a 7-min incubation in 20% ethanol in PBS, the tissues were incubated in blocking buffer for 1 h at room temperature and then with AP904 (1:100) in blocking buffer overnight at 4°C. For immunofluorescence microscopy, slides were then washed in PBS for 15 min and incubated with Texas red–conjugated goat anti–rabbit IgG (1:200) (Molecular Probes Inc., Eugene, Oregon, USA). The tissues were then washed for 15 min in PBS, rinsed briefly in 95% ethanol, and mounted for viewing. For immunoperoxidase staining, after the overnight incubation with AP904 antibody, the tissues were washed in PBS for 15 min and then incubated with biotinylated goat anti–rabbit IgG (1:200) (Vector Laboratories, Burlingame, California, USA). The tissues were washed in PBS for 15 min and incubated with “Vectastain” (Vector Laboratories) for 30 min at room temperature. The antibody binding was then detected with Stable DAB (Research Genetics, Huntsville, Alabama, USA). The DAB reactions were stopped with a 2-min rinse in H2O, and the tissues were counterstained with hematoxylin (Gill's Formulation #3; Fisher Scientific, Pittsburgh, Pennsylvania, USA), dehydrated in ethanol, and mounted with Elvanol.
Results
Distribution of Dsg1 and Dsg3 in normal neonatal mouse skin and oral mucous membranes.
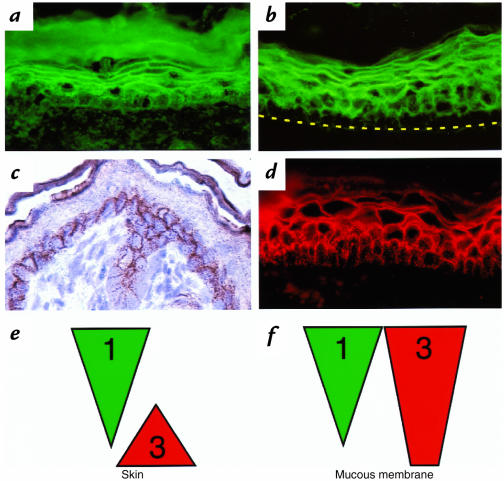
To test the hypothesis that the distribution of Dsg1 and Dsg3 and the anti-desmoglein specificity of autoantibodies determine the blister localization in pemphigus, we first defined the expression profiles of Dsg1 and Dsg3 in mouse skin and mucous membranes by immunohistochemistry. In the skin, Dsg1, as detected with PF serum, was on the cell surface throughout the epidermis, more intense in the superficial layers, and less intense in the deep basal layer (Fig. 1a). Cell-surface staining for Dsg3, with rabbit anti–mouse Dsg3 antibodies, was limited to the basal and most immediate suprabasal cells of the skin (Fig. 1c). Similar staining was seen with anti-Dsg3 specific PV serum (not shown). The distribution of Dsg1 and Dsg3 in mouse skin is similar to that previously observed in human skin (29, 30). In the palate, the staining for Dsg1 was throughout the epithelium, however much more intense in the superficial layers (Fig. 1b). In some areas of the mucosa, the borders between the cells of the basal and the suprabasal layers were stained for Dsg1, but no staining was detected on the lateral cell membranes of the basal cells. The staining for Dsg3 was present throughout the mouse mucosal epithelium (Fig. 1d). Thus, as determined by immunohistochemistry, the distributions of Dsg1 and Dsg3 in mouse and human skin and mucous membrane are similar, as shown schematically in Fig. 1, e and f. Unlike previous reports in the human (30), however, Dsg1 is expressed in mouse mucosa and skin at about the same intensity (Fig. 1, a and b).
Figure 1.
Distribution of Dsg1 and Dsg3 in neonatal mouse skin and mucous membrane. Immunofluorescence staining of neonatal mouse skin (a) and mucous membrane (b) with human PF1239 serum. Immunoperoxidase (c) and immunofluorescence (d) staining of neonatal mouse skin and mucous membrane with rabbit anti-Dsg3 antibodies. Schematic diagram of the Dsg1 and Dsg3 expression in the neonatal mouse skin (e) and oral mucous membrane (f). Note that Dsg1 is expressed throughout all cell layers, more in the superficial layers and less in the deep layers of both epidermis and mucous membrane. In the epidermis, Dsg3 is expressed only in the deep basal and most immediate suprabasal layers, whereas in the oral mucosa Dsg3 is expressed throughout all cell layers. Dsg, desmoglein.
PF autoantibodies induce blisters where Dsg1 is expressed without coexpressed Dsg3.
In the skin of normal mice, PF autoantibodies are known to cause blisters in the superficial epidermis, where Dsg1 is expressed without concomitant Dsg3. If, as we hypothesized, Dsg3 protects against blisters due to anti-Dsg1 antibodies, then DSG3null mice, which do not express Dsg3, should exhibit more severe blistering than wild-type mice when injected with PF antibodies. To show that the presence of Dsg3 limits PF blister formation, low doses of IgG from sera of two patients with PF were transferred into two sets of neonatal mice from DSG3+/– parents. In the first experiment, nine pups from one litter were each injected with 3 mg of PF IgG. The next day, we recorded the extent of blistering without yet knowing the genotype of the pups. We observed that one pup had no gross blisters (Fig. 2a). Six pups had either localized or extensive blisters (example shown in Fig. 2b). Two pups had very extensive blisters (example shown in Fig. 2c). Subsequent genotyping of the animals revealed that the one pup that did not develop gross blisters was a wild-type animal (DSG3+/+). The other six pups that showed localized to extensive blisters were heterozygous for the DSG3 mutation (DSG3+/–). The two neonates that had very extensive blisters were DSG3null. In a second experiment, seven pups from another litter were injected with 1 mg of PF IgG. The next day, five animals developed localized blisters covering less than 30% of their bodies, and two animals had very extensive blisters covering greater than 80% of their bodies. Subsequent genotyping showed that of the five animals with localized blisters, two were wild-type (DSG3+/+) and three were heterozygous (DSG3+/–) for the DSG3 mutation. The two animals that demonstrated very extensive blisters were DSG3null. Even with higher doses of PF IgG, we repeatedly observed more extensive blister formation in the DSG3null animals. These findings demonstrate that animals devoid of Dsg3 were more susceptible to gross blister formation by anti-Dsg1 antibodies in PF sera.
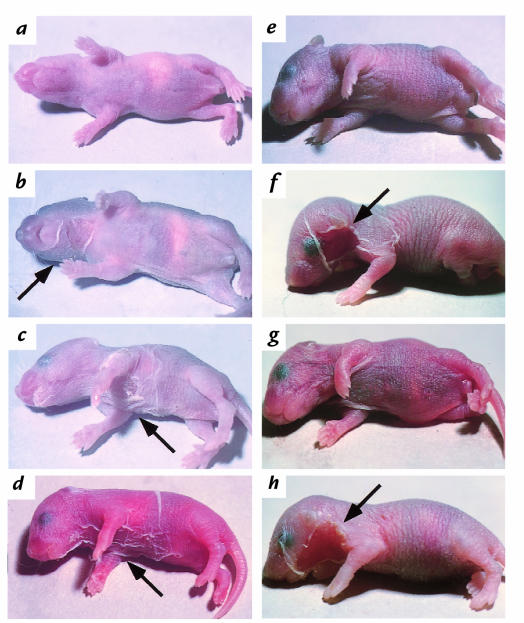
Figure 2.
Neonatal mice injected with PF and PV IgG. (a–c) Injection of low-dose (3 mg) PF982 IgG. DSG3+/+ has no detectable blisters (a); DSG3+/– has localized blisters (b); DSG3null shows extensive blisters (c). These results show that the presence of Dsg3 limits blisters due to anti-Dsg1 in PF sera. (d) DSG3null injected with 10 mg of IgG from PV3014 containing both anti-Dsg3 and anti-Dsg1 antibodies shows extensive blisters, demonstrating that anti-Dsg1 antibodies in PV serum are pathogenic. (e) Wild-type mouse injected with high-dose (26 mg) IgG from PV3024 containing only anti-Dsg3 antibodies (without anti-Dsg1 antibodies) developed no skin blisters. (f) Wild-type mouse injected with 9.4 mg from NIH2054, containing anti-Dsg3 and anti-Dsg1 antibodies, shows extensive blisters. (g) Wild-type mouse injected with 12.0 mg of IgG from NIH2054 adsorbed with Dsg1 no longer demonstrates blister formation. (h) Wild-type mouse injected with 10 mg IgG from PV3024 (anti-Dsg3 antibodies) plus 1 mg IgG from PF982 (anti-Dsg1 antibodies) shows extensive blisters. (e–h) These results show the importance of anti-Dsg1 for efficient blister formation by the anti-Dsg3 in PV sera. Arrows mark the sites of blister formation. PF, pemphigus foliaceus; PV, pemphigus vulgaris.
Similar to humans, neonatal mice express Dsg3 throughout the oral epithelium. Therefore, we hypothesized that this expression should protect against blister formation from the anti-Dsg1 antibodies in PF sera. PF (PF982 and PF1239) IgG (10 mg/pup) was injected into C57Bl/6J (n = 12), DSG3+/+ (n = 6), and DSG3+/– (n = 12) neonatal mice within one to two days after birth. Within 16 hours after the IgG transfer, all 30 animals had gross blisters over 80% of their skin. Histologically, the blisters were observed in the superficial epidermis between cells within the granular layers (Fig. 3a). In spite of these extensive skin blisters, none of the animals developed blisters in the oral mucosa (Fig. 3b). These results show that in both the deep epidermis and throughout the epithelia of oral mucous membrane, expression of Dsg3 protects against blister formation by anti-Dsg1 antibodies.
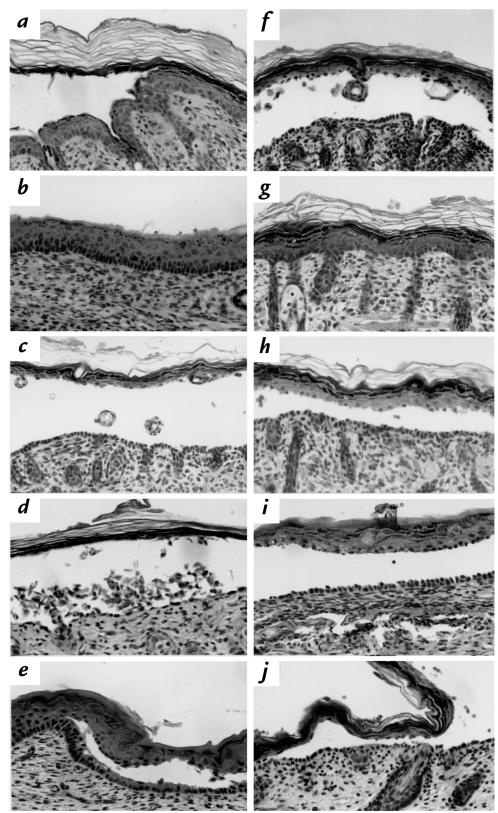
Figure 3.
Histology of skin and oral mucosa of neonatal mice injected with PF and PV IgG. (a) Skin from a wild-type mouse injected with (10 mg) PF982 (anti-Dsg1) IgG shows superficial blisters. (b) Mucous membrane from a wild-type mouse injected with (10 mg) PF982 IgG shows no blisters. Skin from a DSG3null mouse injected with (10 mg) PF982 IgG reveals deep suprabasilar blisters (c) and extensive acantholysis (d). (e) Mucous membrane from a DSG3null neonatal mouse injected with (10 mg) PF982 IgG shows deep suprabasilar blisters. (c–e) These results demonstrate that in DSG3null mice, in which there is no Dsg3 to compensate, PF IgG causes deep suprabasilar blisters in both the skin and mucous membranes. (f) Skin from a DSG3null mouse injected with (10 mg) PV3014 (anti-Dsg1 and anti-Dsg3) IgG shows suprabasilar blisters. (g) Skin from a wild-type mouse injected with (26 mg) PV3024 (anti-Dsg3) IgG shows no blisters. (h) Skin from a wild-type mouse injected with (10 mg) PV3014 (anti-Dsg1 and anti-Dsg3) IgG show suprabasilar blisters. (i) Mucous membrane from a wild-type mouse injected with (10 mg) PV3014 IgG shows suprabasilar blisters. (j) Skin from a wild-type mouse injected with (10 mg) PV3024 IgG and (1 mg) PF982 IgG shows suprabasilar blisters. (f–j) These results demonstrate that anti-Dsg1 antibodies in PV are pathogenic and that both anti-Dsg1 and anti-Dsg3 antibodies are necessary for efficient blister formation in PV.
If, indeed, Dsg3 protects against blistering from anti-Dsg1 antibodies, then in DSG3null mice injected with PF antibodies, we would expect to see loss of cell adhesion extending to the deep epidermis and throughout the mucous membranes, sites protected by Dsg3 expression in normal mice. To test this prediction, six DSG3null neonatal mice were injected with 10 mg IgG from two different PF sera (PF982 and PF1239). All six animals developed extensive gross blisters. The histology of the skin mostly showed acantholytic blisters deep in the epidermis (Fig. 3c); however, in some areas, extensive acantholysis was observed throughout the entire epidermis, from the suprabasal to the granular layers (Fig. 3d). We assayed these mice for mucous membrane lesions by histological analysis of the anterior palate, a region that never shows blistering in uninjected DSG3null neonates. All PF IgG–injected DSG3null mice developed extensive suprabasilar blisters on the anterior palate (Fig. 3e). Thus, in DSG3null mice, PF IgG causes blistering in areas normally protected from pathology by coexpressed Dsg3 in wild-type mice.
Anti-Dsg1 antibodies in PV sera are pathogenic.
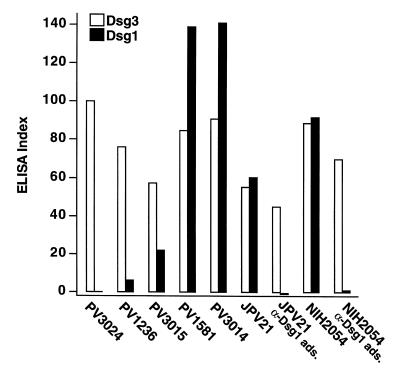
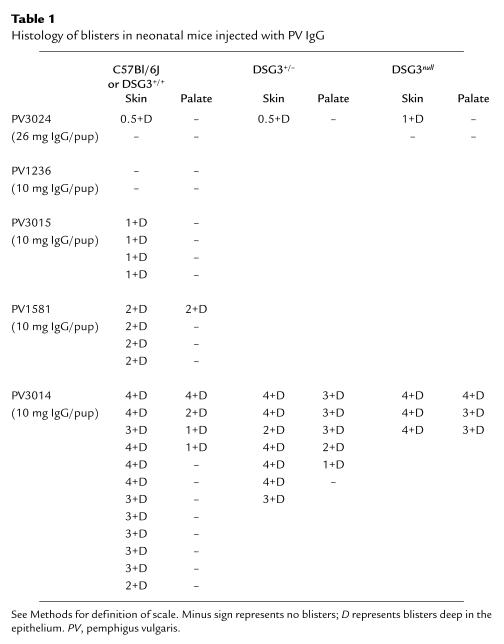
All PV sera contain antibodies against Dsg3, whereas one-half to two-thirds also contain antibodies against Dsg1 (10, 14–16). To determine whether the anti-Dsg1 antibodies in PV sera are pathogenic, we injected six DSG3null animals from three different litters with IgG from three different PV sera (PV3014, JPV21, and NIH2054) that contained high levels of anti-Dsg1 antibodies, as determined by enzyme-linked immunosorbent assay (ELISA) (Fig. 4). All six animals developed extensive gross skin blisters (example shown in Fig. 2d). By histology, the blisters were localized to the deep suprabasal layer of the epidermis (Tables 1 and 2; Fig. 3f). To demonstrate that it is the anti-Dsg1 antibodies that are pathogenic in these mice, we adsorbed two PV sera (JPV21 and NIH2054) with the extracellular domain of Dsg1 produced in baculovirus. ELISA assay confirmed that these sera no longer contained anti-Dsg1 antibodies (Fig. 4). IgG from these adsorbed sera no longer caused blisters in DSG3null mice (Table 2). These data show that anti-Dsg1 antibodies from these PV sera result in the same pathology in DSG3null mice as those from PF sera (Fig. 3c).
Figure 4.
Levels of anti-Dsg1 and anti-Dsg3 antibodies in PV sera measured by ELISA. Dsg1 ads. indicates that the serum was adsorbed with the extracellular domain of Dsg1 produced in baculovirus. The ELISA index is defined in Methods.
Table 1.
Histology of blisters in neonatal mice injected with PV IgG
Table 2.
Effects of adsorption of anti-Dsg1 antibodies from PV sera on their ability to cause skin blisters in neonatal mice
Both anti-Dsg3 and anti-Dsg1 antibodies are necessary for efficient blister formation by PV sera.
To determine whether anti-Dsg1 antibodies increase the pathogenicity of PV sera, we injected neonatal wild-type mice with different PV sera, all containing anti-Dsg3 antibodies, but with varying amounts of anti-Dsg1 antibodies, as determined by ELISA (Fig. 4 and Table 1). Mice injected with IgG from PV 1236 (10 mg/pup) or high-dose IgG from PV3024 (26 mg/pup), both containing insignificant anti-Dsg1 antibodies, did not develop gross or significant histological blisters (Figs. 2e and 3g). In contrast, PV sera with increasing amounts of anti-Dsg1 antibodies caused increasingly severe blistering, even when injected at lower doses (10 mg/pup). In pups injected with PV3015, which by ELISA contained a low level of anti-Dsg1 antibodies, no gross blisters were observed, but suprabasilar blisters were detected by histology in most animals. The two PV sera with the highest level of anti-Dsg1 antibodies, PV1581 and PV3014, caused gross skin blisters in all pups and oral blisters in 30%, all suprabasilar by histology (Fig. 3, h and i). These observations demonstrate that PV sera containing anti-Dsg3 alone are inefficient at causing blister formation and that PV sera that contain both anti-Dsg3 and anti-Dsg1 are much more pathogenic.
To demonstrate further the importance of anti-Dsg1 antibodies in the pathogenesis of PV, we adsorbed these antibodies from pathogenic PV sera (JPV21 and NIH2054), leaving only the anti-Dsg3 antibodies, which were then were tested for their ability to cause skin blisters in neonatal mice (Table 2). ELISA assay demonstrated that before adsorption both sera contained anti-Dsg3 and anti-Dsg1 antibodies, but after adsorption with the extracellular domain of Dsg1, essentially only anti-Dsg3 antibodies were detectable (Fig. 4). The unadsorbed sera induced extensive gross blisters (Fig. 2f) that were histologically in the deep epidermis. In contrast, after adsorption, even though higher amounts of IgG were injected to compensate for the slight decrease in anti-Dsg3 antibody titers, mice did not develop gross (Fig. 2g) or histological blisters. These results confirm that the anti-Dsg1 antibodies in PV sera are critical for efficient pathogenicity.
Final proof that anti-Dsg1 antibodies are critical to PV sera pathogenicity was provided by taking nonpathogenic PV sera that contained only anti-Dsg3 antibodies and adding small amounts of anti-Dsg1 antibodies from PF sera. Even when injected at a dose of 26 mg per neonatal mouse, PV3024, which contains only anti-Dsg3 antibodies (Fig. 4), was extremely inefficient at causing blisters (Table 1). However, 10 mg of IgG from this PV serum plus 1 mg of PF IgG produced extensive suprabasilar blisters in wild-type mice (Figs. 2h and 3j)
These data demonstrate the importance of both the anti-Dsg3 and anti-Dsg1 antibodies in the pathophysiology of PV blister formation and support the hypothesis that it is necessary to interfere with the function of both Dsg's in areas of skin in which they overlap to produce blisters efficiently. According to this hypothesis, the reason that anti-Dsg3 antibodies alone are inefficient at causing loss of cell adhesion is because Dsg1 is expressed throughout the epidermis and can compensate for loss of function of Dsg3.
Discussion
In this study, we correlate pemphigus antibody profiles with Dsg tissue distribution to explain blister localization in PF and PV. We explain blister formation in PF as follows. In PF sera, the anti-Dsg1 antibodies cause blisters only in the superficial epidermis of neonatal mice because that is the only area in which Dsg1 is present without coexpressed Dsg3 (Fig. 1, e and f). Although the anti-Dsg1 antibodies bind to mucosa of these mice (data not shown), no blisters are formed because of the coexpression of Dsg3. In DSG3null mice, in which there is no Dsg3 to compensate, PF IgG causes blisters in both the skin and mucous membranes. These blisters are usually deep in the epithelium. We speculate that the reason the blisters are deep is twofold. First, the injected antibodies penetrate from the dermis to the epidermis and first interfere with the function of the Dsg1 in the lower epidermis; thus, the blister occurs there before pathogenic levels of antibodies penetrate to the higher levels. By direct immunofluorescence, we have confirmed that at 4 hours after injection, IgG is found only in the lower epidermis (our unpublished observation). Second, the cell–cell adhesion deep in the epidermis might be weaker than that found more superficially, because there are fewer desmosomes and less Dsg1 in the former area.
We explain blister formation in PV as follows. In early PV, there may only be antibodies against Dsg3 (13, 16). These antibodies are very inefficient at causing blisters because in the skin and mucosa of neonatal mice, Dsg1 is expressed from the superficial epidermis at least down to the apical membrane of the basal cells (Fig. 1, e and f) and can compensate for lost Dsg3 function. However, in adult humans, as opposed to mice, there is much less Dsg1 in oral mucosa than in skin; therefore, in the deep mucosa, where the intensity of Dsg1 expression falls off dramatically, there may be little, if any, Dsg1 (30). Therefore, in humans, anti-Dsg3 alone may be efficient at causing oral mucous membrane blisters in early disease. In later disease, patients with PV develop anti-Dsg1 antibodies in addition to the anti-Dsg3 antibodies. We propose that in later disease, mice and humans develop deep epidermal blisters because the PV anti-Dsg3 and anti-Dsg1 antibodies interfere with the function of both Dsg's and penetrate from the dermis into the deep epidermis, where cell–cell adhesion may be weakest, as already discussed.
The results discussed here do not support the theory that antibodies in pemphigus indirectly cause loss of cell adhesion through mediation of protease release from keratinocytes. If antibody binding caused protease release, which in turn caused blisters, then in areas where Dsg3 and Dsg1 are coexpressed, antibody binding to one of the two antigens would be expected to produce blisters. However, we show here that these areas are actually protected from blister formation.
Our results strongly suggest that antibodies in pemphigus interfere with the function of Dsg's and that if either Dsg1 or Dsg3 is still functional, there is limited spontaneous blistering, but that if neither is functional, then extensive blistering results. These observations also underscore the importance of Dsg's in maintaining cell adhesion in stratified squamous epithelia.
A logical extension of our findings is that it would be reasonable to screen for pharmacologic agents that increase the expression of Dsg's that are not bound by antibodies in particular types of pemphigus, thus protecting against blister formation. It is our hope that an understanding of the pathophysiology of pemphigus at this level may lead to innovative therapy for these life-threatening diseases.
Acknowledgments
We thank Dorothy Campbell and Valerie Johnson of the Department of Dermatology at the University of Pennsylvania for the tissue histology processing. This work was supported by grants from the National Institutes of Health. M.G. Mahoney was a recipient of a Dermatology Foundation Research Fellowship.
References
- 1.Stanley, J.R. 1993. Pemphigus. In Dermatology in general medicine. T.B. Fitzpatrick, A.Z. Eisen, K. Wolff, I. M. Freedberg, and K.F. Austen, editors. McGraw-Hill. New York, NY. 606–615.
- 2.Lever, W.F. 1965. Pemphigus and pemphigoid. Charles C. Thomas. Springfield, IL. 15–66.
- 3.Meurer M, Millns JL, Rogers RS, III, Jordon RE. Oral pemphigus vulgaris. A report of ten cases. Arch Dermatol. 1977;113:1520–1524. [PubMed] [Google Scholar]
- 4.Beutner EH, Jordon RE. Demonstration of skin antibodies in sera of pemphigus vulgaris patients by indirect immunofluorescent staining. Proc Soc Exp Biol Med. 1964;117:505–510. doi: 10.3181/00379727-117-29622. [DOI] [PubMed] [Google Scholar]
- 5.Rappersberger K, Roos N, Stanley JR. Immunomorphological and biochemical identification of the pemphigus foliaceus autoantigen within desmosomes. J Invest Dermatol. 1992;99:323–330. doi: 10.1111/1523-1747.ep12616659. [DOI] [PubMed] [Google Scholar]
- 6.Karpati S, Amagai M, Prussick R, Cehrs K, Stanley JR. Pemphigus vulgaris antigen, a desmoglein type of cadherin, is localized within keratinocyte desmosomes. J Cell Biol. 1993;122:409–415. doi: 10.1083/jcb.122.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanley JR. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv Immunol. 1993;53:291–325. doi: 10.1016/s0065-2776(08)60503-9. [DOI] [PubMed] [Google Scholar]
- 8.Koulu L, Kusumi A, Steinberg MS, Klaus Kovtun V, Stanley JR. Human autoantibodies against a desmosomal core protein in pemphigus foliaceus. J Exp Med. 1984;160:1509–1518. doi: 10.1084/jem.160.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanley JR, Koulu L, Klaus Kovtun V, Steinberg MS. A monoclonal antibody to the desmosomal glycoprotein desmoglein I binds the same polypeptide as human autoantibodies in pemphigus foliaceus. J Immunol. 1986;136:1227–1230. [PubMed] [Google Scholar]
- 10.Eyre RW, Stanley JR. Identification of pemphigus vulgaris antigen extracted from normal human epidermis and comparison with pemphigus foliaceus antigen. J Clin Invest. 1988;81:807–812. doi: 10.1172/JCI113387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto T, Ogawa MM, Konohana A, Nishikawa T. Detection of pemphigus vulgaris and pemphigus foliaceus antigens by immunoblot analysis using different antigen sources. J Invest Dermatol. 1990;94:327–331. doi: 10.1111/1523-1747.ep12874456. [DOI] [PubMed] [Google Scholar]
- 12.Amagai M, Klaus Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 13.Ishii K, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–2017. [PubMed] [Google Scholar]
- 14.Emery DJ, et al. Pemphigus foliaceus and pemphigus vulgaris autoantibodies react with the extracellular domain of desmoglein-1. J Invest Dermatol. 1995;104:323–328. doi: 10.1111/1523-1747.ep12665364. [DOI] [PubMed] [Google Scholar]
- 15.Kowalczyk AP, et al. Pemphigus sera recognize conformationally sensitive epitopes in the amino-terminal region of desmoglein-1 (Dsg1) J Invest Dermatol. 1995;105:147–152. doi: 10.1111/1523-1747.ep12316680. [DOI] [PubMed] [Google Scholar]
- 16.Ding X, et al. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J Invest Dermatol. 1997;109:592–596. doi: 10.1111/1523-1747.ep12337524. [DOI] [PubMed] [Google Scholar]
- 17.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 18.Roscoe JT, et al. Brazilian pemphigus foliaceus autoantibodies are pathogenic to BALB/c mice by passive transfer. J Invest Dermatol. 1985;85:538–541. doi: 10.1111/1523-1747.ep12277362. [DOI] [PubMed] [Google Scholar]
- 19.Amagai M, Hashimoto T, Green KJ, Shimizu N, Nishikawa T. Antigen-specific immuoabsorption of pathogenic autoantibodies in pemphigus foliaceus. J Invest Dermatol. 1995;104:895–901. doi: 10.1111/1523-1747.ep12606168. [DOI] [PubMed] [Google Scholar]
- 20.Amagai M, Hashimoto T, Shimizu N, Nishikawa T. Absorption of pathogenic autoantibodies by the extracellular domain of pemphigus vulgaris antigen (Dsg3) produced by baculovirus. J Clin Invest. 1994;94:59–67. doi: 10.1172/JCI117349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memar OM, et al. Recombinant desmoglein 3 has the necessary epitopes to adsorb and induce blister-causing antibodies. J Invest Dermatol. 1996;106:261–268. doi: 10.1111/1523-1747.ep12340663. [DOI] [PubMed] [Google Scholar]
- 22.Schiltz JR, Michel B, Papay R. Appearance of “pemphigus acantholysis factor” in human skin cultured with pemphigus antibody. J Invest Dermatol. 1979;73:575–581. doi: 10.1111/1523-1747.ep12541618. [DOI] [PubMed] [Google Scholar]
- 23.Morioka S, Lazarus GS, Jensen PJ. Involvement of urokinase-type plasminogen activator in acantholysis induced by pemphigus IgG. J Invest Dermatol. 1987;89:474–477. doi: 10.1111/1523-1747.ep12460937. [DOI] [PubMed] [Google Scholar]
- 24.Esaki C, Seishima M, Yamada T, Osada K, Kitajima Y. Pharmacologic evidence for involvement of phospholipase C in pemphigus IgG-induced inositol 1,4,5-trisphosphate generation, intracellular calcium increase, and plasminogen activator secretion in DJM-1 cells, a squamous cell carcinoma line. J Invest Dermatol. 1995;105:329–333. doi: 10.1111/1523-1747.ep12319948. [DOI] [PubMed] [Google Scholar]
- 25.Stanley JR. Pemphigus and pemphigoid as paradigms of organ-specific, autoantibody-mediated diseases. J Clin Invest. 1989;83:1443–1448. doi: 10.1172/JCI114036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch PJ, et al. Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J Cell Biol. 1997;137:1091–1102. doi: 10.1083/jcb.137.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioannides D, Hytiroglou P, Phelps RG, Bystryn JC. Regional variation in the expression of pemphigus foliaceus, pemphigus erythematosus, and pemphigus vulgaris antigens in human skin. J Invest Dermatol. 1991;96:159–161. doi: 10.1111/1523-1747.ep12460927. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu H, et al. Pemphigus vulgaris and pemphigus foliaceus sera show an inversely graded binding pattern to extracellular regions of desmosomes in different layers of human epidermis. J Invest Dermatol. 1995;105:153–159. doi: 10.1111/1523-1747.ep12316695. [DOI] [PubMed] [Google Scholar]
- 29.Amagai M, Koch PJ, Nishikawa T, Stanley JR. Pemphigus vulgaris antigen (desmoglein 3) is localized in the lower epidermis, the site of blister formation in patients. J Invest Dermatol. 1996;106:351–355. doi: 10.1111/1523-1747.ep12343081. [DOI] [PubMed] [Google Scholar]
- 30.Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, Hashimoto K. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol. 1998;110:76–78. doi: 10.1046/j.1523-1747.1998.00085.x. [DOI] [PubMed] [Google Scholar]
- 31.Koch PJ, et al. Desmoglein 3 anchors telogen hair in the follicle. J Cell Sci. 1998;111:2529–2537. doi: 10.1242/jcs.111.17.2529. [DOI] [PubMed] [Google Scholar]