Abstract
The cricket's auditory system is a highly directional pressure difference receiver whose function is hypothesised to depend on phase relationships between the sound waves propagating through the auditory trachea that connects the left and right hearing organs. We tested this hypothesis by measuring the effect of experimentally constructed phase shifts in acoustic stimuli on phonotactic behavior of Gryllus bimaculatus, the oscillatory response patterns of the tympanic membrane, and the activity of the auditory afferents. The same artificial calling song was played simultaneously at the left and right sides of the cricket, but one sound pattern was shifted in phase by 90 deg (carrier frequencies between 3.6 and 5.4 kHz). All three levels of auditory processing are sensitive to experimentally induced acoustic phase shifts, and the response characteristics are dependent on the carrier frequency of the sound stimulus. At lower frequencies, crickets steered away from the sound leading in phase, while tympanic membrane vibrations and auditory afferent responses were smaller when the ipsilateral sound was leading. In contrast, opposite responses were observed at higher frequencies in all three levels of auditory processing. Minimal responses occurred near the carrier frequency of the cricket's calling song, suggesting a stability at this frequency. Our results indicate that crickets may use directional cues arising from phase shifts in acoustic signals for sound localisation, and that the response properties of pressure difference receivers may be analysed with phase-shifted sound stimuli to further our understanding of how insect auditory systems are adapted for directional processing.
KEY WORDS: Auditory orientation, Phase shift, Cricket
INTRODUCTION
The ability to locate a sound's origin is important for many animals to find a mate, avoid predators and detect prey (Bradbury and Vehrencamp, 1998; Stumpner and von Helversen, 2001). While humans and other vertebrates use interaural intensity and timing differences (IID and ITD, respectively) to perform these tasks (Middlebrooks and Green, 1991), these cues decrease and become difficult to process neuronally when the distance between the ears is small compared with the wavelength of the sound being localised. Such is the case for many insects (Michelsen, 1992; Michelsen and Larsen, 2008; Hennig et al., 2004). However, in some species, such as the Mediterranean field cricket Gryllus bimaculatus deGeer, adaptations of the auditory system have evolved that improve directional hearing. Females of this species use sound localisation to walk towards a male producing an attractive calling song (Weber and Thorson, 1989). Remarkably, female crickets are able to accurately steer towards a calling song presented only 1 deg off their length axis under open-loop laboratory settings (Schöneich and Hedwig, 2010).
In G. bimaculatus, the amplitude and phase differences measured between both tympanic membranes vary between 1 and 2 dB and 0 and 70 deg, respectively, as a sound source broadcasting 4.5 kHz pulses is moved 360 deg around the animal (Michelsen et al., 1994). Such small amplitude and phase differences are not likely to be sufficient for precise directional steering, suggesting that the physical structure and functional properties of the peripheral auditory system must play a role in enhancing the directional tuning of the system (Michelsen et al., 1994). The peripheral auditory system of G. bimaculatus is a pressure difference receiver made up of four sound inputs: two tympanic membranes on the posterior side of the frontal tibiae, and two auditory spiracles on the lateral surfaces of the prothorax (Larsen et al., 1989). The inputs are connected by acoustic trachea, bilateral air-filled tubes that meet at a septum along the midline and allow propagation of, and interactions between, the sound waves entering the auditory system from both sides of the insect. The potential for interactions between sound waves within the acoustic trachea is the key advantage of the cricket's pressure difference receiver for improving directional hearing, as constructive and destructive interference may enhance the contrast between the two ears (Robert, 2005).
Two factors affect the internal interactions between the sound waves: first, which inputs the sound waves enter through, and second, the means by which the phase of the sound waves is altered. To start, previous findings (Larsen and Michelsen, 1978; Fletcher and Thwaites, 1979; Larsen, 1981) suggested that the transverse acoustic trachea has a significant impact on tympanic membrane oscillations, indicating that the sound waves entering by way of both the ipsilateral and the contralateral inputs play a role in activating the ipsilateral auditory afferents. Michelsen et al. (Michelsen et al., 1994) confirmed these findings and additionally revealed that the sound waves entering the auditory trachea via the contralateral inputs contribute more to the directionality of the system than those entering through the ipsilateral spiracle. Between the two sound properties amplitude and phase, Michelsen et al. (Michelsen et al., 1994) predicted that the phase of the internal sound waves relative to one another is the more influential parameter affecting the directionality of the hearing organs. However, the phase difference across the tympanic membrane created by the additional physical distance that the sound waves travel in the acoustic trachea is not likely to be large enough to provide effective directionality cues. Therefore, the cricket's peripheral auditory system requires a way of enhancing the phase difference. Indeed, Fletcher and Thwaites (Fletcher and Thwaites, 1979) introduced an extra phase delay into their model of the Teleogryllus commodus peripheral auditory system to achieve the cardioid-shaped directional tuning observed experimentally at the level of both the posterior tympanic membrane and the auditory afferents (Michelsen et al., 1994; Boyd and Lewis, 1983; Löhe and Kleindienst, 1994). The phase-shifting element has since been proposed to be the double-walled medial septum, which bisects the acoustic tracheal system at the midline, and through which sound waves from the contralateral side must pass (Fletcher and Thwaites, 1979; Larsen, 1981). In support of the medial septum's involvement, reduced directional tuning occurs when the septum is perforated [phonotactic steering (Wendler and Löhe, 1993; Hirtenlehner et al., 2014); auditory afferent activity (Löhe and Kleindienst, 1994); tympanic membrane oscillations (Michelsen and Löhe, 1995)].
Based on this evidence, the phase relationships between the internally propagating sound waves are important for the directional tuning of G. bimaculatus. This suggests that phase-shifted acoustic stimuli presented externally from two simultaneously active sound sources could be used to influence these phase interactions within the cricket's peripheral auditory system. If the phase of sound waves in the peripheral auditory system is indeed important for directional processing, then such manipulations should lead to reliable steering responses during phonotaxis, and should have an impact on the tympanic membrane oscillations and auditory afferent activity of the tympanal nerve. Phase-shifted binaural stimuli may therefore be used as an analytical tool for studying the cricket's peripheral auditory processing. Here we present acoustic stimuli comprising a range of carrier frequencies (CFs) and degrees of phase shift, and examine their effect on phonotactic steering, tympanic membrane oscillation amplitudes and activity of the tympanal nerve. Additionally, we measure directional tuning curves from the tympanic membrane oscillations, for comparison.
RESULTS
Phonotactic response to 90 deg phase-shifted calling song
Using an open-loop trackball system (see Materials and methods), we tested whether phase-shifted calling song stimuli had an effect on phonotactic steering (n=12). The sound waves of equal-amplitude pulses broadcast simultaneously from the left and right speakers were shifted 90 deg in phase relative to each other, and the CF of the song stimuli was systematically increased in 0.1 kHz steps across the 3.6–5.4 kHz range. At each frequency, the right speaker led for 30 s, and then the lead switched to the left.
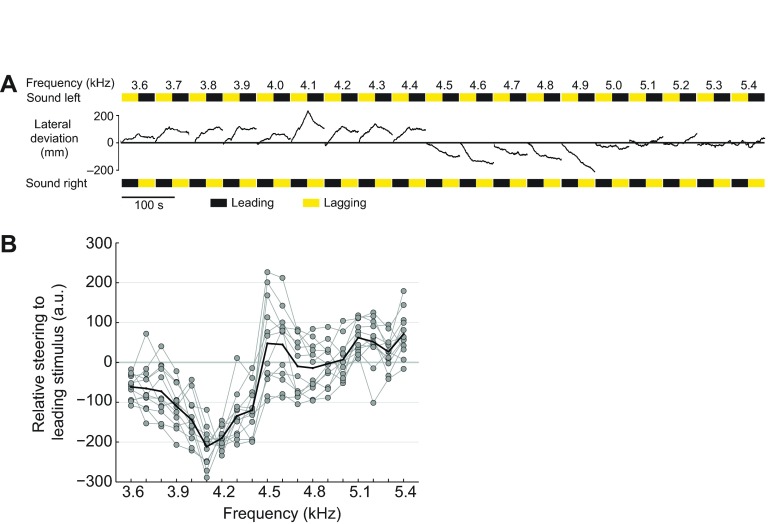
The crickets' forward walking movements and lateral deviations towards the sound pattern were recorded; a typical individual response is shown in Fig. 1A, and is detailed here. Although the overall steering was less pronounced than steering towards a single lateral speaker (Hedwig and Poulet, 2005), this female did perform phonotactic steering by altering her directional heading with the change in the leading speaker at some frequencies. The cricket's lateral deviations demonstrate that she steered away from the leading speaker between 3.6 and 4.4 kHz. Within this frequency range, the steering response was most obvious at 4.1 kHz. Between 4.5 and 4.9 kHz, the female altered her steering response to walk towards the leading speaker, with a best response at 4.6 kHz. Beyond 5.0 kHz, this animal continued walking, but did not display a clear steering preference.
Fig. 1.
Phonotactic steering behaviour recorded on a trackball in response to presentation of 90 deg phase-shifted sound stimuli. (A) Sample walking trace of an individual cricket. Steering velocity was integrated to calculate the lateral deviation in response to the acoustic stimulation. Time is depicted on the x-axis, while lateral movement is depicted on the y-axis (upwards trajectories are leftward movement, and downward trajectories are rightward movement). Black and yellow bars indicate that the designated speaker is leading or lagging in phase, respectively. (B) Normalised individual responses to the 90 deg phase-shifted sound stimuli (n=12, grey filled circles), and the average across individuals (black line). Steering responses towards the leading speaker are defined as positive. Steering data were normalised using an average measurement of steering across carrier frequencies (a.u.; see Materials and methods for detailed description of normalisation).
Data were pooled across individuals, and a Wilcoxon signed rank test for zero median was used to determine whether the trajectories in response to each CF significantly differed from zero (i.e. straight forward walking, indicating no steering preference). Individual and average data are plotted in Fig. 1B. The crickets steered away from the leading speaker between 3.6 and 4.4 kHz (P≤0.001), and towards the leading speaker between 4.5–4.6 kHz and 5.1–5.4 kHz (4.5–4.6 kHz: P≤0.05; 5.1–5.2 kHz: P≤0.001; 5.3 kHz: P≤0.05; 5.4 kHz: P≤0.001). Between 4.7 and 5.0 kHz the crickets exhibited no steering preference. The peak lateral deviation occurred at 4.1 kHz, as the crickets steered away from the leading speaker. These data demonstrate that crickets phonotactically respond to phase-shifted sound cues, and that the amplitude and direction of steering depend on the stimulus' CF.
Effect of phase-shifted sounds on tympanic membrane oscillations
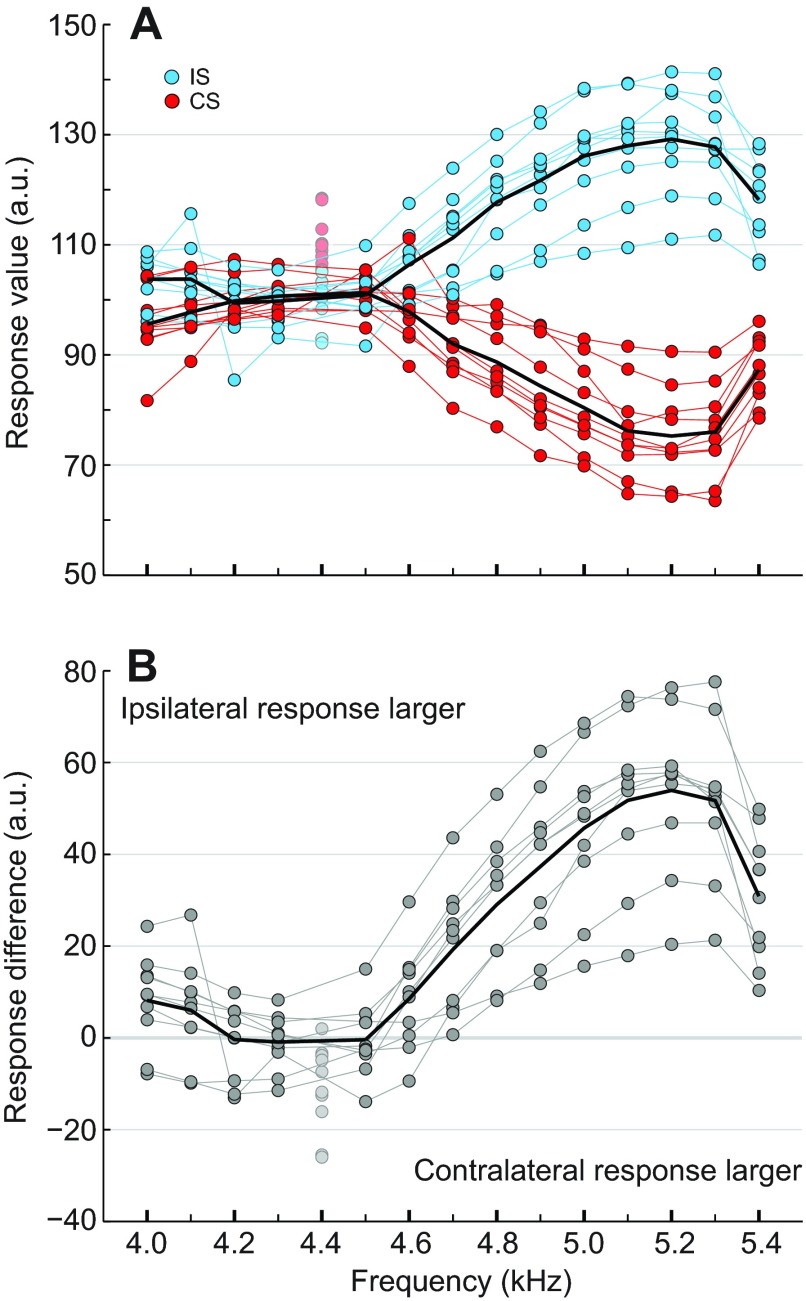
As cricket phonotaxis is sensitive to 90 deg phase-shifted sound stimuli, the stimuli must be activating the ipsilateral and contralateral hearing organs to a differing degree in a frequency-dependent manner. We therefore examined how 90 deg phase-shifted sound stimuli affected tympanic membrane oscillations across the 4.0–5.4 kHz frequency range, as measured by laser vibrometry (n=10). Normalised tympanic membrane velocities measured in response to the ipsilateral or contralateral speaker leading in phase are plotted in Fig. 2A, and the differences between the ipsilateral and contralateral tympanic membrane responses are plotted in Fig. 2B. Positive difference values indicate that the ipsilateral leading stimulus pulse caused a higher velocity of the tympanic membrane than the contralateral leading pulse.
Fig. 2.
Posterior tympanic membrane velocity measured in response to 90 deg phase-shifted sound stimuli using laser vibrometry. (A) Normalised velocity measurements for individual crickets in response to the ipsilateral (IS, blue circles) or contralateral speaker (CS, red circles) leading in phase. The black lines represent the average responses across all individuals (n=10) for IS and CS. Original velocity measurements were normalised at each respective carrier frequency using reference velocities measured in response to 75 dB SPL pulses from the ipsilateral speaker (a.u.; see Materials and methods). (B) The difference between the normalised velocity responses to the ipsilateral and contralateral speakers leading in phase of individual crickets (grey circles), and the average difference across all individuals (black line). Lighter coloured dots refer to data points that were not included in the analysis; see Materials and methods for details.
The shapes of the responses to the ipsilateral and contralateral speaker leading in phase are mirror images, with minor differences between 4.0 and 4.5 kHz (both responses near the 100 a.u. reference value) and increasingly stronger and weaker responses to higher frequencies, respectively. At 4.6 kHz the tympanic responses diverged, and the responses to the ipsilateral leading stimuli maximally increased to 125 a.u. while the responses to the contralateral leading stimuli decreased to 75 a.u. Correspondingly, significant differences were found between the ipsilateral and contralateral leading responses at 4.0 kHz and between 4.6 and 5.4 kHz, when the response to the ipsilateral speaker leading dominated (Wilcoxon signed rank test; 4.0 kHz: P≤0.05; 4.6 kHz: P≤0.05; 4.7–5.4 kHz: P≤0.01; 4.1–4.5 kHz: P=n.s.). The strongest difference occurred at 5.2 kHz. In reference to the 4.2, 4.45 and 5.15 kHz intensity tuning curves (supplementary material Fig. S1A), the response differences between the ipsilateral and contralateral speakers leading at 4.2 and 4.5 kHz correspond to less than a 1 dB SPL difference, whereas the response difference at 5.2 kHz corresponds to a 4.4 dB SPL difference.
To understand how the peripheral auditory system responds to other degrees of phase shift, we measured the velocity of the left tympanic membrane in response to sound stimuli presented in 22.5 deg phase-shifted steps from 0 to 180 deg, across the 4.0–5.4 kHz frequency range (n=10; supplementary material Fig. S2). Wilcoxon signed rank tests revealed that only the ipsilateral and contralateral leading responses to degrees of phase shift from 22.5 to 157.5 deg in the frequency range of 4.7–5.4 kHz were significantly different (P≤0.01), along with at a phase shift of 22.5 deg at 4.6 kHz (P≤0.01). In reference to the 4.5, 4.8 and 5.1 kHz intensity tuning curves (supplementary material Fig. S1C), the difference values for all degrees of phase shift at 4.5 kHz correspond to less than a 1 dB SPL difference, whereas 4.8 and 5.1 kHz have peaks of 2.5 and 4.2 dB SPL at 67.5 deg, respectively.
Effect of 90 deg phase-shifted sound stimuli on auditory afferent activity
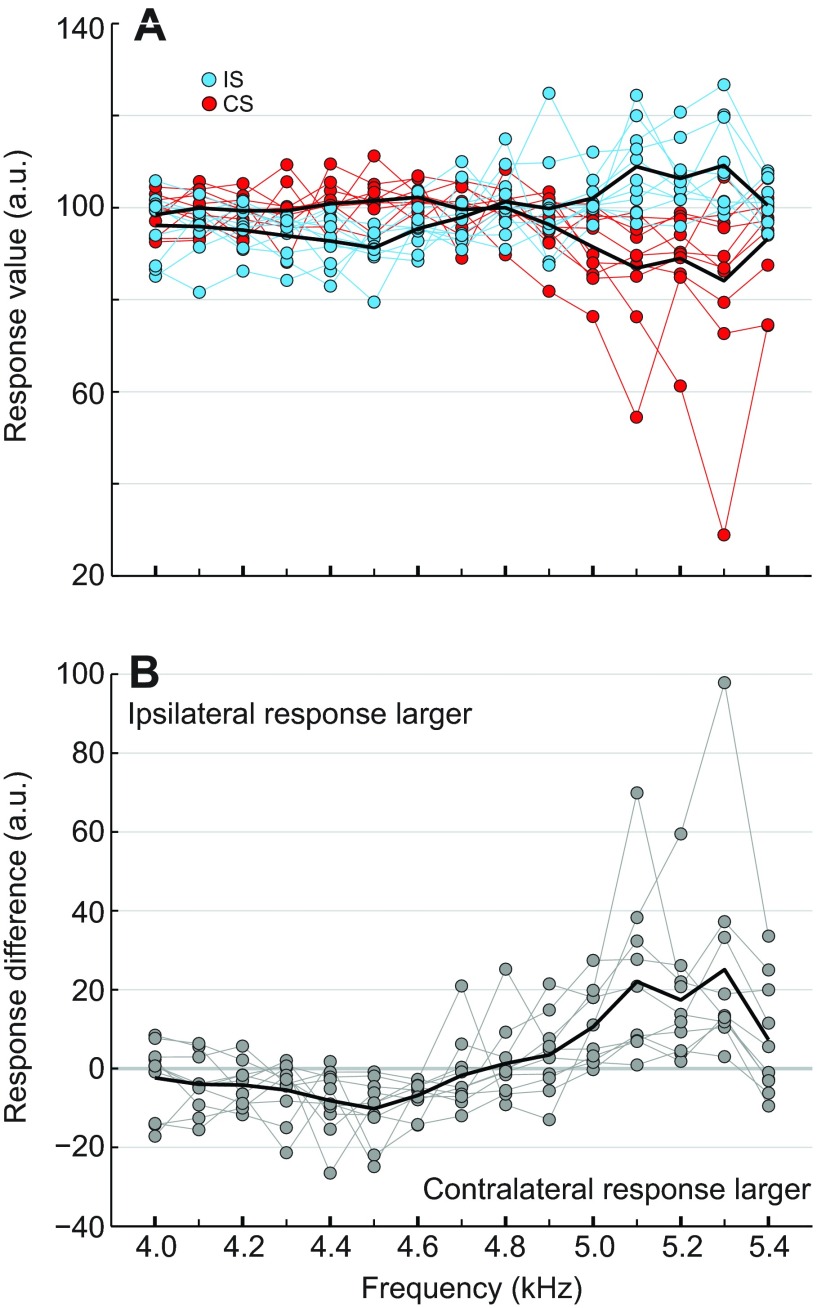
The sound waves and accompanying pressure changes within the acoustic trachea are forwarded to the hearing organ located in the tibia and activate the auditory afferents. To reveal the impact of the phase-shifted sound stimuli on the afferent activity, we measured the summed activity of the left tympanal nerve in response to 90 deg phase-shifted sound stimuli (n=10). Upon acoustic stimulation, the auditory nerve responded with a typical multiphasic potential that lasted for the duration of the stimulus (see Materials and methods).
The normalised responses to the ipsilateral and contralateral speakers leading in phase are given in Fig. 3A. When the ipsilateral speaker was leading between 4.0 and 4.7 kHz, the afferent response was reduced compared with the reference value (100 a.u.), with response values between 91 and 98 a.u. At higher frequencies (5.0–5.4 kHz), however, the afferent response increased to a range of 100–110 a.u. The responses of the auditory afferents to sound pulses when the contralateral speaker was leading were nearly a mirror image of the ipsilateral leading response pattern. When the contralateral speaker was leading between 4.0 and 4.8 kHz, the afferent responses were from 98 to 103 a.u., and from 4.9 to 5.4 kHz the responses were between 84 and 96 a.u.
Fig. 3.
Summed neuronal activity of the auditory afferents measured in response to 90 deg phase-shifted sound stimuli. (A) Normalised neuronal responses from individual crickets to the ipsilateral (IS, blue circles) or contralateral speaker (CS, red circles) leading in phase by 90 deg (n=10). Original neuronal responses were normalised at each respective carrier frequency using reference neuronal responses to 75 dB SPL pulses from the ipsilateral speaker (a.u.; see Materials and methods). (B) Difference between the normalised individual neuronal responses to the ipsilateral and contralateral speaker leading in phase by 90 deg (grey circles), and the average difference across all crickets (black line).
The difference between the ipsilateral and contralateral speakers leading in phase is shown in Fig. 3B, and indicates which signal dominated at each CF. At 4.2 kHz and between 4.5 and 4.6 kHz, the afferent response was significantly larger when the contralateral speaker was leading in phase (Wilcoxon signed rank test; 4.2 kHz: P≤0.05; 4.5–4.6 kHz: P≤0.01), and between 5.0 and 5.3 kHz the response was larger when the ipsilateral speaker was leading (Wilcoxon signed rank test; 5.0–5.3 kHz: P≤0.01). There was not a significant difference in the neuronal response between 4.0 and 4.1 kHz, at 4.3 kHz, between 4.7 and 4.9 kHz, or at 5.4 kHz. The response differences at 4.2, 4.5 and 5.2 kHz correspond to differences of 1.6, 3.9 and 4.7 dB SPL, respectively. Notably, between 4.7 and 4.8 kHz the phase shift had the smallest effect on afferent activity. This frequency represented the transition point where the afferent activity changed from less activity as the ipsilateral speaker was leading to more activity.
Frequency-dependent directional tuning of the tympanic membrane oscillations
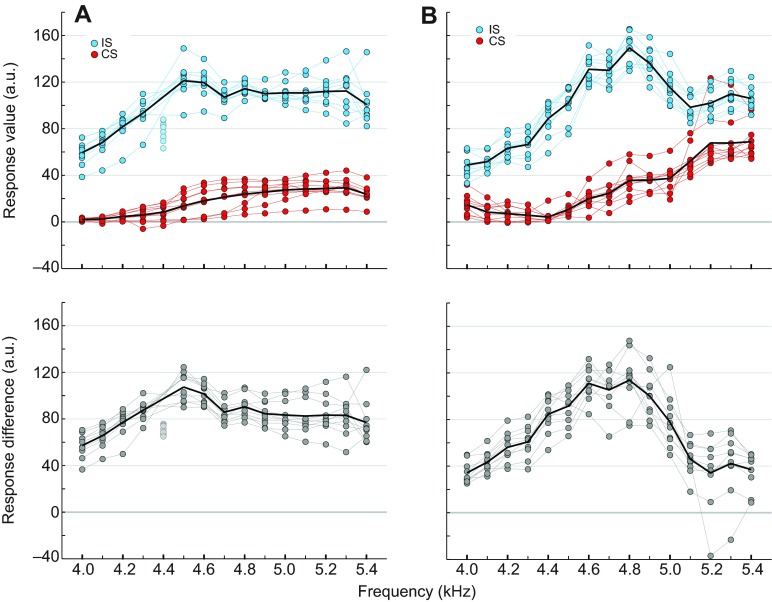
Measurements of the tympanic membrane oscillations while the ipsilateral and contralateral speakers were independently active (no phase shift) allowed us to analyse CF-dependent directional tuning across the 4.0–5.4 kHz range. The difference between the ipsilateral and contralateral responses revealed the extent of the frequency-dependent directional tuning.
Acoustic stimuli were broadcast from both conical speakers (Fig. 4A) and free-field speakers (Fig. 4B). For both speaker types, the responses to the contralateral stimuli were smaller than responses to the ipsilateral stimuli (Fig. 4, top diagrams). Although the responses to contralateral stimuli from both speakers increased with CF, neither response curve showed a clear peak. In contrast, both ipsilateral response curves had distinct maxima. There was a peak at 4.5 kHz in the conical speaker curve (120 a.u.), and a clear but broad peak around 4.8 kHz in the free-field speaker curve (140 a.u.).
Fig. 4.
Frequency tuning curves measured from tympanic membrane velocity responses to sound stimuli presented with conical and free-field speakers. (A) Conical speakers. (B) Free-field speakers. The top panels show normalised tympanic membrane responses from individual crickets to sound stimuli presented from the ipsilateral (blue circles) and contralateral (red circles) speakers (n=10). Original tympanic membrane velocity responses were normalised with reference to the mean of the ipsilateral responses across all carrier frequencies (a.u.; see Materials and methods). The bottom panels show the difference between the normalised responses to ipsilateral and contralateral stimulation of individual crickets (grey circles), and the average difference across individuals (black line). The best frequency tuning is expected to occur at the largest difference between the responses to ipsilateral and contralateral stimulation. Lighter coloured dots refer to data points that were not included in the analysis; see Materials and methods for details.
The difference between the responses to ipsilateral and contralateral stimuli from both speaker types showed a similar pattern: the difference increased between 4.0 kHz and the middle of the frequency range, then decreased again as the frequency approached 5.4 kHz (Fig. 4, bottom diagrams). However, the range of differences was larger in the free-field speaker tuning curve, with average values between 35 and 115 a.u., compared with the 55–110 a.u. difference range in the conical speaker directional tuning curve. The differences calculated from the intensity tuning curves for the conical speaker tuning curves were 25, 19.9 and 11.5 dB SPL for 4.2, 4.5 and 5.2 kHz, respectively, and 14.7, 17.5 and 3.3 dB SPL for 4.2, 4.5 and 5.2 kHz, respectively, on the free-field tuning curve (supplementary material Fig. S1D,E). For both tuning curves, all of the ipsilateral and contralateral responses were significantly different (Wilcoxon signed rank test; conical speakers: 4.0–5.4 kHz: P≤0.01; free-field speakers: 4.0–5.1 kHz: P≤0.01, 5.2 kHz: P≤0.05, 5.3–5.4 kHz: P≤0.01).
DISCUSSION
Considerable interest has been paid to understanding the biophysical mechanisms of the G. bimaculatus peripheral auditory system that provide the basis for directional tuning (Rheinlaender and Blätgen, 1982; Larsen et al., 1989; Michelsen et al., 1994; Schöneich and Hedwig, 2010). Like other insects, crickets face the fundamental problem that the distance between their ears (~1.6 cm) is small compared with the wavelength of their communication signals (~7.15 cm at 4.8 kHz) Therefore, binaural cues between the tympana (IID and ITD) are so small as to be at the functional limit for use in directional hearing and sound localisation. For example, the maximum ITD occurs when the sound source is 90 deg off the cricket's length axis and is only 47 μs.
Previous studies established that the internal interactions between the sound waves travelling within the cricket's peripheral auditory system are important for effective directional tuning (Larsen and Michelsen, 1978; Fletcher and Thwaites, 1979; Larsen, 1981; Hill and Boyan, 1977). It was further hypothesised that phase-interference effects generated within the acoustic tracheal system by differences in path lengths between the auditory inputs or by internal changes to the propagation velocity of the sound waves (Larsen, 1981; Michelsen et al., 1994) may play a key role in directional hearing [mechanism first proposed by Autrum (Autrum, 1940)]. In the present study we sought to build on these hypotheses by examining how dichotic presentation of external phase differences affects the directional responses of the cricket's auditory system as measured at the levels of phonotactic steering, tympanic membrane oscillations and activity of the tympanal nerve. To our knowledge, dichotic phase-shifted sound stimuli of this kind have only been used to study one other model hearing system, that of the barn owl Tyto alba (Moiseff and Konishi, 1981).
Phonotactic steering and auditory afferent activity responses to 90 deg phase-shifted sound stimuli are CF dependent
Measurements of phonotactic steering provide a direct method for testing how an acoustic stimulus affects sound localisation (Weber et al., 1981; Schmitz et al., 1982; Hedwig and Poulet, 2004). In our study, female crickets presented with 90 deg phase-shifted sound stimuli of identical amplitude displayed significant steering responses when the side from which the sound signal was leading in phase was switched. These data indicate that 90 deg phase shifts between calling songs broadcast simultaneously from two sound sources do generate directional cues that affect phonotactic steering. The behavioural data also demonstrate that the CF of the phase-shifted sound stimulus has a significant effect on directional steering, as shown by the variation in the steering response observed across the 3.6–5.4 kHz CF range. The crickets steered away from the speaker leading in phase when the frequency of the calling song was low (3.6–4.4 kHz), then changed their steering behaviour to move towards the leading speaker when the calling song was presented at higher frequencies (4.5–4.6 kHz, 5.1–5.4 kHz). As the phase angle between internal and external sound waves depends on the CF of the sound stimulus, these observations are in agreement with the equation set out by Larsen et al. (Larsen et al., 1989). They additionally corroborate Michelsen et al.'s (Michelsen et al., 1994) findings that the phase angle across the tympanic membrane changes dramatically as the CF is increased from 2 to 10 kHz.
Directional cues are forwarded from the periphery to the central nervous system and therefore the behavioural responses to 90 deg phase-shifted sound stimuli should be reflected in the activity of the tympanal nerve. As expected, changes in the auditory afferent activity occurred when the side of the leading stimulus was switched, and the response was frequency dependent. Like the behavioural data, at lower CFs the auditory afferents had a larger response when the contralateral speaker was leading in phase, but as the frequency increased the sign of the response difference changed and at higher frequencies the auditory afferents responded more strongly to the ipsilateral speaker leading in phase. The dB differences between the afferent responses to ipsilateral and contralateral leading stimuli fall above the 1–2 dB SPL minimum intensity difference found necessary to elicit phonotactic steering (Hedwig and Poulet, 2005; Schöneich and Hedwig, 2010). Thus, the tympanal nerve activity measured in response to phase-shifted stimuli is sufficient to explain the matching phonotactic response.
Of particular interest are the responses between 4.7 and 4.9 kHz, which fall near the 4.7 kHz average CF of the natural calling song (Kostarakos et al., 2009). In this frequency range, the phonotactic steering in response to phase-shifted stimuli was not directed and the afferent responses were not significantly different. There are two possible explanations for these results. First, the 90 deg external phase shifts may add to the internal phase interactions in such a way that the resulting activity of the left and right ears is equal, thus resulting in a null response of the animal. Second, the crickets' peripheral auditory system may be more stable in response to phase shifts in the 4.7–4.9 kHz frequency range, resulting in little effect of the phase-shifted stimuli on the cricket's directional tuning at those frequencies. If the second hypothesis is true, such stabilisation could be helpful when females are trying to locate a singing male in a complex natural environment, as reflection of the sound off objects in the surrounding would have less of an effect on the directional cues leading the female.
Tympanic membrane oscillations do not respond to phase-shifted sound stimuli as predicted by behavioural and afferent data
The tympanic membrane's oscillatory responses to sound stimuli are thought to reflect auditory afferent activity (Kleindienst et al., 1983; Michelsen et al., 1994; Michelsen and Löhe, 1995; Schöneich and Hedwig, 2010). We therefore expected that tympanic membrane oscillations driven by 90 deg phase-shifted stimuli would have a similar response pattern to that seen in both the behavioural and afferent activity data. The response pattern of the tympanic membrane was indeed dependent on the CF of the sound stimulus, as the influence of the ipsilateral leading stimulus changed with CF. However, the membrane's response differences were consistently positive, indicating that the ipsilateral leading stimulus was dominant at all CFs. Thus, although the shape of the tympanic membrane's response pattern matches that of the behavioural and auditory afferent data, it appears DC-shifted towards stronger responses to the ipsilateral leading sound stimuli.
Mathematical descriptions of the phase angle between the external and internal sound pressures across the tympanic membrane are reliant not only on the CF, but also on the angle of sound incidence (Larsen et al., 1989). This effect is demonstrated by the cardioid-shaped directional tuning measured as the sound source is moved 360 deg around a cricket [tympanic membrane (Michelsen et al., 1994); tympanal nerve (Boyd and Lewis, 1983; Löhe and Kleindienst, 1994)]. Thus, the maximum directional tuning at each CF should be dependent on the degree of phase shift. To address this hypothesis, we measured tympanic membrane oscillations while presenting phase-shifted sound stimuli with degrees of phase shift ranging from 0 to 180 deg across the 4.0–5.4 kHz frequency range, stimuli that may correspond to the changing degree of phase shift that accompanies modulation in the angle of sound incidence. The data show that the degree of phase shift does have an effect on tympanic membrane oscillations, with significant response differences to phase shifts as small as 22.5 deg at some frequencies (4.7–5.4 kHz). However, 67.5 or 90 deg phase shifts produced the largest response difference at all effective frequencies (4.7–5.4 kHz), indicating that the maximum response difference was not CF dependent. These responses to the range of degrees of phase shifts, together with the tympanic responses to the 90 deg phase-shifted stimuli, provide evidence that measurements of the tympanic membrane oscillations may not directly represent the effect of phase interactions on directional tuning. As the tympanic membranes and auditory sensilla are not directly coupled (Michel, 1974; Ball et al., 1989), it is still unknown how tympanal oscillations contribute to driving the activity of the auditory afferents. Future studies measuring afferent activity in response to a range of degrees of phase shift are required to shed further light on this discrepancy and confirm the difference between tympanic and afferent responses to phase-shifted sound stimuli.
It is worth noting that the smallest degree of phase shift that elicited significant differences between ipsilateral and contralateral tympanic responses was 22.5 deg, which falls well within the range of phase shifts arising from diffraction and time of arrival differences between the ipsilateral and contralateral auditory inputs measured by Michelsen et al. (Michelsen et al., 1994) and Kleindienst (Kleindienst, 1978). This suggests that the tympanic membranes may respond significantly to even the small degrees of phase shift arising from ITDs. Although further experiments are needed to assess how the tympanic responses to phase-shifted stimuli affect activity of the auditory afferents, the findings of Schöneich and Hedwig (Schöneich and Hedwig, 2010) lend support to the hypothesis that crickets may be able to use these small degrees of phase shift as cues for phonotactic steering. They found that crickets accurately steer towards a sound source located only 1 deg off the cricket's length axis, which is much smaller than the 16 deg incident angle that is required to produce a 22.5 deg phase shift resulting from binaural differences in time of arrival (at 4.8 kHz).
CF-dependent directional tuning is broader than previously reported
To isolate the effects of sound waves interacting within the auditory system, we presented acoustic stimuli with conical speakers that attenuate the effects of sound diffraction around the crickets' bodies and interference between the sound waves. To determine how these changes in the stimuli presentation affected the tympanic membrane's directional tuning, we compared the directional response to stimuli presented with conical speakers with equivalent directional responses to free-field stimuli, similar to those measured previously by Michelsen and Löhe (Michelsen and Löhe, 1995).
The free-field directional tuning curve had a broad peak between 4.6 and 4.8 kHz (115 a.u.), which decayed by 80 a.u. as the CF was increased or decreased to 5.2 or 4.0 kHz, respectively. In contrast, the directional tuning measured using the conical speakers had a single frequency peak at 4.5 kHz (110 a.u.), and the decay to each side was shallower (55 a.u. to 4.0 kHz, and only 30 a.u. to 5.4 kHz). In both cases, the differences in the shapes of the directional tuning curves were strongly driven by the responses to the ipsilateral stimuli, though the contralateral stimuli clearly had a greater effect in the free-field measurements. Overall, the effect of the sound presentation method on the directional tuning curves indicates that diffraction of sound waves around the cricket's body enhances the cricket's frequency-dependent directional tuning.
Our free-field measurement of the tympanic membrane's directional tuning is in partial disagreement with the sharp directional tuning published for a single cricket by Michelsen and Löhe (Michelsen and Löhe, 1995). Michelsen and Löhe's free-field directionality curve has a peak at 4.5 kHz, which is more similar to our conical response curve. Additionally, the dB SPL difference between the left and right responses that we measured at 4.5 kHz (17.5 dB SPL) is also almost twice as large as that reported by Michelsen and Löhe [10 dB, although their dB reference value was not stated; see their fig. 2B (Michelsen and Löhe, 1995)]. These differences might arise from the differing speaker placements used in the two studies (Michelsen and Löhe's 30 deg versus our 45 deg off the cricket's length axis); however, the broad tuning peak in our data is not the consequence of averaging the responses across several animals, as we did not observe a sharp tuning in any female. Based on these results, the frequency-dependent tuning of the cricket's peripheral auditory system may not be as tightly tuned as previously reported for a single specimen. Nevertheless, both of our directional tuning curves and the curve measured by Michelsen and Löhe (Michelsen and Löhe, 1995) do agree in that the peaks all fall near the average male calling song CF reported by Kostarakos et al. [4.7 kHz (Kostarakos et al., 2009)], and in each curve the directional responses decrease as the CF is shifted away from the average.
Conclusions
Pressure difference receiving ears are found in some insects (grasshoppers, crickets and katydids) and a number of vertebrates (reviewed by Christensen-Dalsgaard, 2011), e.g. frogs (Gerhardt and Huber, 2002), reptiles (Christensen-Dalsgaard and Manley, 2005) and birds (Lewis and Coles, 1980; Larsen et al., 2006). The connections between the ears differ markedly from organism to organism, but the common mechanism that defines them is that sound waves entering through at least two auditory inputs interact within the peripheral auditory system to influence and enhance directional hearing. Although we are far from understanding the detailed physics of the sound wave interactions occurring within the cricket's auditory trachea, the results of the experiments performed in this study demonstrate that the cricket's auditory system is sensitive to experimentally induced acoustic phase shifts. Remarkably, the system seems to be stable at the CF of the calling song under these experimental conditions. Future studies that examine the movements of the acoustic trachea and measurements of medial septum oscillations will be required to understand the finer details of these sound wave interactions and the mechanisms by which they activate the hearing organs. However, in the absence of methods to perform these experiments, the results of this study present evidence that dichotic sound stimuli can be used to analyse response properties of pressure difference receivers in a systematic way, thus providing greater opportunity to further our understanding of how pressure difference receivers utilise phase shifts within their auditory systems for enhanced directional sensitivity.
MATERIALS AND METHODS
Animals and acoustic setup
Last instar female crickets (Gryllus bimaculatus) from the Department of Zoology, Cambridge, were isolated and reared individually. All crickets were given protein- and fat-rich food and water. Only adult females with intact tympanic membranes and spiracles were used, and each cricket was tested during the 1–5 weeks following its final molt. The crickets used in the behavioural assay were later used for electrophysiological recordings; another group was used for laser vibrometry experiments.
Experiments were performed in a dark sound-damped chamber lined with Illsonic tiles (Sonex 65/125; Illbruck, Bodenwöhr, Germany). Sound stimuli were developed using Cool Edit 2000 audio software (Syntrillium, Phoenix, AZ, USA, now Adobe Audition) and were presented with 3 cm (diameter) Sinus Live, Neo 13s speakers (Conrad Electronics, Hirschau, Germany). Two speaker configurations were used, depending on the experiment. In the first configuration, two free-field speakers were positioned 57.3 cm away from the centre of the trackball or cricket platform at the height of the cricket stage, and forward at +45 and −45 deg azimuth from the cricket's length axis (referred to as ‘free-field speakers’). In the second configuration, the speakers had 17.5 cm copper cones attached that funnelled the sound to a 4 mm opening, which was placed 10 mm away from the lateral surface of the cricket's thorax, and was aimed at the auditory spiracles (referred to as ‘conical speakers’). For all sound stimuli and speaker configurations, the speakers were individually calibrated to 75 dB SPL at the location of the cricket using a Brüel and Kjær measuring amplifier and microphone (models 2610 and 4939, respectively; Nærum, Denmark). A downwards facing microphone was hung 2.5 cm above the cricket during experiments to check for effects of acoustic interference on the amplitude of the sound field.
Phonotactic steering on a trackball system
A trackball system was used to record phonotactic steering behaviour in response to acoustic stimuli presented from free-field speakers (Fig. 5B) (Hedwig and Poulet, 2005). The system allowed measurements of the crickets' forward and lateral steering movements with a resolution of 120 μm and 0.3 ms. Only crickets that demonstrated a phonotactic response to a standard 4.8 kHz calling song pretest (Thorson et al., 1982) were used in experiments with phase-shifted calling song (n=12 out of 14).
Fig. 5.
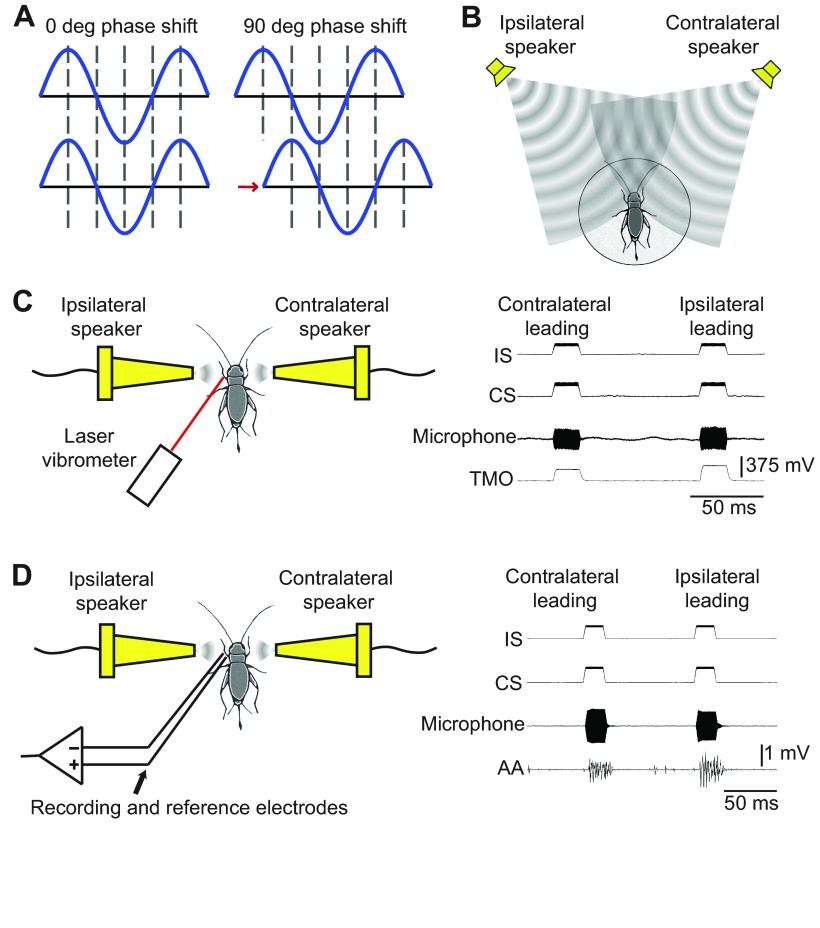
Schematics of experimental methods. (A) Diagram of a 90 deg phase shift in a sinusoidal sound wave, including a time delay (shown for clarity, but not considered experimentally). (B) Cricket steering in response to free-field sound stimuli presented from two speakers positioned at ±45 deg off the insect's length axis. (C) Laser vibrometry measurements of the left posterior tympanic membrane's velocity in response to sound stimuli presented from conical speakers (left), and example traces of the ipsilateral speaker (IS), contralateral speaker (CS), microphone and laser vibrometer measurements of tympanic membrane oscillations (TMO) during two sound pulse presentations (right). (D) Extracellular recordings of the left tympanal nerve in response to sound stimuli presented from conical speakers (left), and example traces of the IS, CS, microphone and auditory afferent activity (AA) during two sound pulse presentations (right).
The acoustic stimuli were computer-generated calling songs with CFs between 3.6 and 5.4 kHz (0.1 kHz intervals, 19 frequencies total). They were constructed such that the crickets were exposed to the even frequencies first, followed by the odd frequencies, both in ascending order.
A total of 200 chirps were presented at each CF. Chirps consisted of four 20 ms pulses (including 2 ms rise and fall times), with 20 ms of silence in between. A silence of 200 ms occurred between chirps. Both speakers were simultaneously active and presented stimuli at 75 dB SPL during all pulses. However, the sound pattern from one speaker was shifted forward in phase by 90 deg (termed ‘leading’; Fig. 5A). At each frequency, 100 chirps were presented with the right speaker leading in phase (30 s), followed by 100 chirps with the left speaker leading in phase, to control for side biases (1 s silence at left–right transitions and a 5 s silence between CFs).
The trackball movement and the envelopes of the sound stimuli were recorded and analysed as described by Hedwig and Poulet (Hedwig and Poulet, 2005). Each cricket was tested once a day for 3 days and the data from all tests were averaged. Walking biases were eliminated by defining steering responses towards the leading speaker as positive, and then averaging the responses to the right and left speaker leading. Because of differences in walking distance magnitude between individuals, the responses at each CF were normalised by dividing them by the average of the absolute values of all phonotactic responses from the respective test sequence (reported in arbitrary units, a.u.). The sign of the average indicated whether the cricket had steered towards (positive) or away (negative) from the leading speaker. A Wilcoxon signed rank test for zero median was used to determine whether the degree of steering at each CF differed from zero (i.e. forward walking and a lack of directional steering; P≤0.05) across all animals.
Tympanic membrane oscillations in response to phase shifts
Sound-induced oscillations of the left posterior tympanic membrane were measured with a PSV-400 laser vibrometer (Polytec, Waldbronn, Germany) (Fig. 5C; n=10). The crickets were cold anaesthetised and the thoracic ganglia were removed ventrally to reduce motor activity. Then the animals were secured dorsal side up, with the frontal legs set at a right angle to the body, and the femur and tibia set at a right angle to each other. The posterior tympanic membranes and auditory spiracles were kept unobstructed and the laser was centred onto the tympanic membrane of the left front leg (referred to herein as the ‘ipsilateral’ leg). A few glass spheres (0.3 μm diameter) were brushed onto the tympanic membrane to increase its reflectivity. The following laser vibrometer settings were used: velocity = 2 mm s−1 V−1, tracking filter = fast, low-pass filter = 20 kHz, high-pass filter = 100 Hz.
Acoustic stimuli with CFs from 4.0 to 5.4 kHz (0.1 kHz intervals) were presented from both the conical and the free-field speakers, in separate experiments. At each frequency, the crickets were stimulated sequentially with a pulse from the ipsilateral (left) speaker only, a pulse from the contralateral (right) speaker only, two simultaneous pulses of the same amplitude with the contralateral speaker leading in phase by 90 deg, and finally simultaneous pulses with the ipsilateral speaker leading in phase by 90 deg. Sound pulses were 20 ms in duration (including 2 ms rise and fall times), and were separated by 80 ms of silence; a 200 ms silent interval occurred when the CF changed.
For calibration purposes, stimuli for three intensity tuning curves with CFs at 4.2, 4.45 and 5.15 kHz were presented from the ipsilateral and contralateral speakers individually following the 90 deg phase-shifted stimuli. They had an initial pulse at 75 dB SPL, and then consisted of 21 pulses (20 ms pulse durations, 80 ms silent intervals in between) ascending from 60 to 80 dB SPL in 1 dB steps. There were 500 ms of silence between presentation of the left and right intensity curves.
The RMS of the laser vibrometer signal, the microphone signal, and the envelopes of the sound pulses were sampled at 40 kHz per channel with an AD board (PCI-Mio 16-E-4, National Instruments, Newbury, UK) controlled under LabView 5.01 and stored on the hard disk of a PC. Data analysis was performed offline using open source scripts (www.bio.brandeis.edu/marderlab/scripts.html) written for use with Spike2 software (v7, Cambridge Electronic Design, Cambridge, UK).
The laser vibrometer was also used to measure the amplitudes of tympanic membrane oscillations in response to sound stimuli with varying degrees of phase shift (n=10). The following stimuli were presented with CFs between 4.0 and 5.4 kHz (0.1 kHz steps; 11–33 stimuli repetitions). First, simultaneous pulses of equal amplitude from the contralateral and ipsilateral speakers (20 ms pulse duration, 80 ms silent interval) alternated leading in phase as the degree of phase shift was systematically increased by 22.5 deg from 0 to 180 deg (9 deg of phase shift for each frequency, 18 pulses total). Then, intensity tuning curves with the same CF were presented from the ipsilateral and contralateral speakers independently (constructed as described previously). A 1 s silent interval occurred between each test sequence.
The microphone recordings revealed an unexpected drop in the intensity of 4.4 kHz stimuli presented with the conical speakers (supplementary material Fig. S3), suggesting that a non-linearity of the speaker reduced the sound field amplitude at that frequency; therefore, the data in response to 4.4 kHz stimuli, while shown in lighter colour, will be ignored.
Frequency tuning of the tympanic membranes
The oscillatory responses of the tympanic membrane to pulses independently presented from the ipsilateral and contralateral speakers that were recorded during both the conical speaker and free-field speaker laser vibrometry experiments were used to construct frequency tuning curves (n=10).
Neuronal activity of the auditory afferents
The procedures used for the laser vibrometry experiments were also used during electrophysiology experiments (n=10) to measure the response of the auditory afferents to the sound stimuli. A detailed description of the dissection was previously reported by Schöneich and Hedwig (Schöneich and Hedwig, 2010); the reference electrode was positioned in the exposed leg cavity distal to the location of recording (Fig. 5D).
The neural activity was amplified using a differential AC amplifier (Microelectrode AC Amplifier Model 1800, A-M Systems, Carlsborg, WA, USA), then sampled and digitised as described for the laser vibrometry recordings above. Data analysis was performed offline using custom-written software, and neural responses were rectified before further processing (Hedwig and Knepper, 1992).
Data analysis and statistics
Similar analysis and statistical methods were used for all of the tympanic membrane and auditory afferent data. The response to each stimulus pulse was measured by taking the integral above baseline across the duration of the tympanic membrane or afferent response. Depending on the stability of the recording, tympanic membrane or afferent responses from a single cricket were averaged across a number of repetitions of the sound stimuli (90 deg phase-shifted stimuli and directional tuning, laser vibrometer: 10–43 repetitions; 90 deg phase-shifted stimuli, afferent recordings: 106–112 repetitions). The data were then normalised and converted into percentages (a.u.) by dividing the response values by a reference value (see below), and multiplying by 100. Thus, 100 a.u. is equal to the reference value. The reference values used to normalise the 90 deg phase-shifted data collected in both laser vibrometer and electrophysiological experiments were the responses to 75 dB SPL pulses from the singly active ipsilateral speaker, and the directional tuning data collected via laser vibrometry were normalised with the mean of the ipsilateral responses across all CFs. Intensity tuning curves were normalised using their initial 75 dB SPL pulses.
A Wilcoxon signed rank test was used for each combination of CF and degree of phase shift to determine whether the responses to the ipsilateral and contralateral speakers leading differed significantly (P≤0.05), and the difference between the ipsilateral and contralateral responses was determined. By definition, the difference was positive when the response to the ipsilateral speaker leading in phase was larger.
Based on the intensity tuning curves (supplementary material Fig. S1), dB SPL values were calculated for select phase-shifted and directional responses. First, the log10 was taken of the phase-shifted, directional and tuning curve response values, and then a line was fitted to the new shape of the tuning curve data. Each log-transformed phased-shifted or directional tuning value was then plugged into the equation for the line to find the corresponding dB SPL value.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Engineering and Physical Sciences Research Council (EPSRC) for the loan of the laser vibrometer to B.H., and Manfred Hartbauer for providing the glass microspheres. We thank Glenn Harrison and Steve Ellis for electronics and machining assistance, respectively, and Stefan Schöneich for technical help and fruitful discussions. Thanks also go to the Part II Neuroscience students Tim Bayley and Bryony Shelton for collecting data in pilot experiments. Finally, we thank our two reviewers for their helpful comments.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This work was supported by the Howard Hughes Medical Institute's Janelia Farm Graduate Scholars Program.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.101402/-/DC1
References
- Autrum H. (1940). Über lautäusserungen und schallwahrnehmung bei arthropoden: ii. das richtungshören von locusta und versuch einer hörtheorie für tympanalorgane vom locustidentyp. Z. Vgl. Physiol. 28, 326-352. [Google Scholar]
- Ball E. E., Oldfield B. P., Rudolph K. M. (1989). Auditory organ structure, development, and function. In Cricket Behavior and Neurobiology (ed. Huber F., Moore T. E., Loher W.), pp. 391-422. Ithaca, NY: Cornell University Press. [Google Scholar]
- Boyd P., Lewis B. (1983). Peripheral auditory directionality in the cricket (Gryllus campestris L., Teleogryllus oceanicus Le Guillou). J. Comp. Physiol. A 153, 523-532. [Google Scholar]
- Bradbury J. W., Vehrencamp S. L. (1998). Principles of Animal Communication. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- Christensen-Dalsgaard J. (2011). Vertebrate pressure-gradient receivers. Hear. Res. 273, 37-45. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J., Manley G. A. (2005). Directionality of the lizard ear. J. Exp. Biol. 208, 1209-1217. [DOI] [PubMed] [Google Scholar]
- Fletcher N. H., Thwaites S. (1979). Acoustical analysis of the auditory system of the cricket Teleogryllus commodus (Walker). J. Acoust. Soc. Am. 66, 350-357. [DOI] [PubMed] [Google Scholar]
- Gerhardt H. C., Huber F. (2002). Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. Chicago, IL: University of Chicago Press. [Google Scholar]
- Hedwig B., Knepper M. (1992). NEUROLAB, a comprehensive program for the analysis of neurophysiological and behavioural data. J. Neurosci. Methods 45, 135-146. [DOI] [PubMed] [Google Scholar]
- Hedwig B., Poulet J. F. A. (2004). Complex auditory behaviour emerges from simple reactive steering. Nature 430, 781-785. [DOI] [PubMed] [Google Scholar]
- Hedwig B., Poulet J. F. A. (2005). Mechanisms underlying phonotactic steering in the cricket Gryllus bimaculatus revealed with a fast trackball system. J. Exp. Biol. 208, 915-927. [DOI] [PubMed] [Google Scholar]
- Hennig R. M., Franz A., Stumpner A. (2004). Processing of auditory information in insects. Microsc. Res. Tech. 63, 351-374. [DOI] [PubMed] [Google Scholar]
- Hill K. G., Boyan G. S. (1977). Sensitivity to frequency and direction of sound in the auditory system of crickets (Gryllidae). J. Comp. Physiol. A 121, 79-97. [Google Scholar]
- Hirtenlehner S., Römer H., Schmidt A. K. (2014). Out of phase: relevance of the medial septum for directional hearing and phonotaxis in the natural habitat of field crickets. J. Comp. Physiol. A 200, 139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst H. U. (1978). Schallbeugung und reflexion am grillenkörper im frequenzbereich 5-20 kHz. Verh. Dtsch Zool. Gesellsch. 71, 160. [Google Scholar]
- Kleindienst H. U., Wohlers D. W., Larsen O. N. (1983). Tympanal membrane motion is necessary for hearing in crickets. J. Comp. Physiol. A 151, 397-400. [Google Scholar]
- Kostarakos K., Hennig M. R., Römer H. (2009). Two matched filters and the evolution of mating signals in four species of cricket. Front. Zool. 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen O. N. (1981). Mechanical time resolution in some insect ears. II. Impulse sound transmission in acoustic tracheal tubes. J. Comp. Physiol. A 143, 297-304. [Google Scholar]
- Larsen O. N., Michelsen A. (1978). Biophysics of the ensiferan ear. III. The cricket ear as a four-input system. J. Comp. Physiol. A 123, 217-227. [Google Scholar]
- Larsen O. N., Kleindienst H. U., Michelsen A. (1989). Biophysical aspects of sound reception. In Cricket Behavior and Neurobiology (ed. Huber F., Moore T. E., Loher W.), pp. 364-390. Ithaca, NY; London: Cornell University Press. [Google Scholar]
- Larsen O. N., Dooling R. J., Michelsen A. (2006). The role of pressure difference reception in the directional hearing of budgerigars (Melopsittacus undulatus). J. Comp. Physiol. A 192, 1063-1072. [DOI] [PubMed] [Google Scholar]
- Lewis B., Coles R. (1980). Sound localization in birds. Trends Neurosci. 3, 102-105. [Google Scholar]
- Löhe G., Kleindienst H. U. (1994). The role of the medial septum in the acoustic trachea of the cricket Gryllus bimaculatus. II. Influence on directionality of the auditory system. J. Comp. Physiol. A 174, 601-606. [Google Scholar]
- Michel K. (1974). Das tympanalorgan von Gryllus bimaculatus DeGeer (Saltatoria, Gryllidae). Z. Morph. Tiere 77, 285-315. [Google Scholar]
- Michelsen A. (1992). Hearing and sound communication in small animals: evolutionary adaptations to the laws of physics. In The Evolutionary Biology of Hearing (ed. Webster D. B., Fay R. R., Popper A. N.), pp. 61-77. New York, NY: Springer Verlag. [Google Scholar]
- Michelsen A., Larsen O. N. (2008). Pressure difference receiving ears. Bioinspir. Biomim. 3, 011001. [DOI] [PubMed] [Google Scholar]
- Michelsen A., Löhe G. (1995). Tuned directionality in cricket ears. Nature 375, 639. [Google Scholar]
- Michelsen A., Popov A. V., Lewis B. (1994). Physics of directional hearing in the cricket Gryllus bimaculatus. J. Comp. Physiol. A 175, 153-164. [Google Scholar]
- Middlebrooks J. C., Green D. M. (1991). Sound localization by human listeners. Annu. Rev. Psychol. 42, 135-159. [DOI] [PubMed] [Google Scholar]
- Moiseff A., Konishi M. (1981). Neuronal and behavioral sensitivity to binaural time differences in the owl. J. Neurosci. 1, 40-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinlaender J., Blätgen G. (1982). The precision of auditory lateralization in the cricket, Gryllus bimaculatus. Physiol. Entomol. 7, 209-218. [Google Scholar]
- Robert D. (2005). Directional hearing in insects. In Sound Source Localization (ed. Fay R. R., Popper A. N.), pp. 6-35. New York, NY: Springer. [Google Scholar]
- Schmitz B., Scharstein H., Wendler G. (1982). Phonotaxis in Gryllus campestris L. (Orthoptera, Gryllidae). I: Mechanism of acoustic orientation in intact female crickets. J. Comp. Physiol. A 148, 431-444. [Google Scholar]
- Schöneich S., Hedwig B. (2010). Hyperacute directional hearing and phonotactic steering in the cricket (Gryllus bimaculatus deGeer). PLoS ONE 5, e15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpner A., von Helversen D. (2001). Evolution and function of auditory systems in insects. Naturwissenschaften 88, 159-170. [DOI] [PubMed] [Google Scholar]
- Thorson J., Weber T., Huber F. (1982). Auditory behavior of the cricket. II. Simplicity of calling-song recognition in Gryllus, and anomalous phonotaxis at abnormal carrier frequencies. J. Comp. Physiol. A 146, 361-378. [Google Scholar]
- Weber T., Thorson J. (1989). Phonotactic behavior of walking crickets. In Cricket Behavior and Neurobiology (ed. Huber F., Moore T. E., Loher W.), pp. 310-339. Ithaca, NY: Cornell University Press. [Google Scholar]
- Weber T., Thorson J., Huber F. (1981). Auditory behavior of the cricket. I: Dynamics of compensated walking and discrimination paradigms on the Kramer treadmill. J. Comp. Physiol. A 141, 215-232. [Google Scholar]
- Wendler G., Löhe G. (1993). The role of the medial septum in the acoustic trachea of the cricket Gryllus bimaculatus. I. Importance for efficient phonotaxis. J. Comp. Physiol. A 173, 557-564. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.