Abstract
Primary fibroblasts are not efficiently transduced by subgroup C adenovirus (Ad) vectors because they express low levels of the high-affinity Coxsackie virus and adenovirus receptor (CAR). In the present study, we have used primary human dermal fibroblasts as a model to explore strategies by which Ad vectors can be designed to enter cells deficient in CAR. Using an Ad vector expressing the human CAR cDNA (AdCAR) at high multiplicity of infection, primary fibroblasts were converted from being CAR deficient to CAR sufficient. Efficiency of subsequent gene transfer by standard Ad5-based vectors and Ad5-based vectors with alterations in penton and fiber was evaluated. Marked enhancement of binding and transgene expression by standard Ad5 vectors was achieved in CAR-sufficient fibroblasts. Expression by AdΔRGDβgal, an Ad5-based vector lacking the arginine-glycine-aspartate (RGD) αV integrin recognition site from its penton base, was achieved in CAR-sufficient, but not CAR-deficient, cells. Fiber-altered Ad5-based vectors, including (a) AdF(pK7)βgal (bearing seven lysines on the end of fiber) (b) AdF(RGD)βgal (bearing a high-affinity RGD sequence on the end of fiber), and (c) AdF9sK βgal (bearing a short fiber and Ad9 knob), demonstrated enhanced gene transfer in CAR-deficient fibroblasts, with no further enhancement in CAR-sufficient fibroblasts. Together, these observations demonstrate that CAR deficiency on Ad targets can be circumvented either by supplying CAR or by modifying the Ad fiber to bind to other cell-surface receptors.
Introduction
Subgroup C, replication-deficient recombinant adenoviruses (Ad) are used as vectors for gene transfer based on their ability to transduce many cell types efficiently (1–3). There is increasing evidence, however, that the efficiency of Ad vectors can be limited by a deficiency of the appropriate binding/entry mechanisms on the target cell (4–7). The binding/entry of subgroup C Ad is mediated through two specific cell-surface receptors. The knob of the Ad fiber first binds to the “Coxsackie virus and adenovirus receptor” (CAR), a 46-kDa transmembrane protein that functions as the high-affinity receptor for both subgroup C Ad and the Coxsackie B viruses (8–10). HLA class I molecules may also participate in Ad fiber binding to the cell surface (11). Ad internalization requires an interaction of an arginine-glycine-aspartate (RGD) sequence on the Ad penton base with αVβ3 or αVβ5 integrins on the cell surface (4, 6, 12–19).
Mesenchymal cells, such as primary human fibroblasts, are one example of cells that are not efficiently infected by subgroup C Ad. Although fibroblasts express αV integrins (20–22), they are deficient in CAR. In the present study, we have used primary human dermal fibroblasts as a model to explore strategies by which Ad vectors can be designed to enter primary human cells deficient in the high-affinity receptor but sufficient in the integrin/internalization receptor. Using an Ad vector expressing the human CAR cDNA (AdCAR) at high multiplicity of infection (moi), we have converted primary fibroblasts from being CAR deficient to CAR sufficient, enabling the study of CAR-dependent and CAR-independent mechanisms of gene transfer by subgroup C Ad vectors in primary human cells.
Methods
Cell culture.
Primary human dermal fibroblasts were cultured from a normal volunteer and maintained in RPMI-1640 medium. The A549 cell line (CCL185; American Type Culture Collection, Rockville, Maryland, USA) was maintained in Dulbecco's modified essential medium. Both media were supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, and 0.1% Fungizone (all components from GIBCO BRL, Gaithersburg, Maryland, USA).
Adenovirus vectors.
The recombinant Ad vectors used in this study are E1a–, partial E1b–, and partial E3–, vectors based on the Ad5 genome. The expression cassette in the E1 position includes the cytomegalovirus (CMV) immediate/early enhancer/promoter (except AdΔRGDβgal, which uses the Rous sarcoma virus [RSV] long terminal repeat), an artificial splice signal, the transgene, and an SV40 poly(A)/stop signal (23, 24). The vectors include (a) AdCAR, containing the human CAR cDNA (8); (b) Adβgal, with the Escherichia coli β-galactosidase (βgal) gene (24); (c) AdGFP, with a humanized version of the jellyfish Aequorea victoria green fluorescent protein (GFP) gene (25, 26); (d) AdNull with no transgene (24); (e) Ad5f7CAT, a chimeric Ad5-based vector bearing the Ad7a fiber, and the chloramphenicol acetyl transferase (CAT) gene (27); (f) AdΔRGDβgal, with a deletion of the RGD sequence in the penton base; (g) AdF(pK7)βgal, with seven lysine residues at the COOH-terminal end of each fiber protein (28, 29); (h) AdF(RGD)βgal, with a high-affinity RGD sequence GCDCRGDCFCA at the COOH-terminal end of each fiber protein (29); and (i) AdF9sKβgal, with a shortened fiber, including the tail and first eight shaft repeats of Ad5 fiber attached to the last Ad9 fiber shaft repeat and Ad9 knob (30, 31). For binding studies, AdGFP was labeled with carbocyanine dye, Cy3 (Amersham Life Sciences Inc., Arlington Heights, Illinois, USA) as described previously (32). The vectors were used on the basis of physical particle concentration and were propagated, purified, and stored at –70°C as described previously (33–35).
Assessment of transgene expression.
All reporter gene assays were performed 24 h after infection with βgal, CAT, or GFP encoding vectors. The βgal reporter gene was assessed in cell lysates in a luminometer using the Galacto-Light kit (Tropix Inc., Bedford, Massachusetts, USA) and adjusted for total protein using the BCA assay (Bio-Rad Laboratories Inc., Hercules, California, USA). The limit of detection was 103 relative light units/mg protein. CAT activity of cell lysates was quantified in a liquid scintillation counter as described by Neumann et al. (36). The limit of detection was 102 dpm/mg protein. Below the limit of detection, 10-fold differences in amount of enzyme added to a standard amount of naive cell lysate could not be differentiated by the assay. Data points labeled below this limit indicate that no activity could be detected by the assay. GFP expression was measured by counting the number of green fluorescent cells per field by fluorescence microscopy as described by Leopold et al. (32), with a minimum of 200 cells assessed for each sample.
Characterization of CAR and αV integrins on the surface of primary human fibroblasts.
Surface expression of CAR and αV integrins was assessed in fibroblasts or A549 cells (as a control) using indirect immunofluorescence techniques. The anti-CAR IgG1 monoclonal antibody (RmcB; refs. 8, 37) and the isotype-matched control antibody (H3; ref. 38), were used at 1:1,000 dilution. Primary antibodies were visualized using Texas red or FITC goat anti–mouse Ig secondary antibodies at 1:200 dilution (both from Boehringer Mannheim GmbH, Mannheim, Germany). The FITC-conjugated monoclonal mouse anti–human αV integrin, or control antibody (both from Coulter Corp., Miami, Florida, USA), was diluted according to the manufacturer's recommendations and applied after labeling with RmcB and Texas red secondary antibody. Images were collected as described by Leopold et al. (32). Exposure conditions and image manipulations were identical for comparable experimental and control panels.
Modification of Ad infection of fibroblasts by prior infection with AdCAR.
To evaluate Ad binding, fibroblasts were infected with AdCAR, AdNull (both 104 particle units [pu]/cell), or no virus, and 24 h later incubated with 1011 pu/ml of Cy3-labeled AdGFP for 10 min at 37°C. A FITC secondary antibody was used to visualize CAR in the green channel, whereas Cy3-AdGFP was visualized in the red channel. Images were collected in fields that included both CAR-positive and CAR-negative cells and triplicate gray-scale measurements made in arbitrary regions within the background (no cells) and within each cell in both green and red channels. Green and red fluorescence intensities were expressed as a ratio of cell to background fluorescence, and the correlation was calculated. Thirty-eight cells were assessed in this manner.
To quantify the efficiency of gene transfer by first generation Ad vectors in a population of cells that were either CAR-deficient or CAR-sufficient, human fibroblasts were first infected with AdCAR, AdNull (both 0–5 × 104 pu/cell), or no virus (mock-infected), then infected a second time 24 h later with 300 pu/cell of Adβgal or 200 pu/cell of AdGFP. The AdGFP-infected fibroblasts were also labeled with RcmB and Texas red secondary antibody.
To demonstrate that the improved expression of transgene in CAR-sufficient fibroblasts resulted from a fiber–CAR interaction, fibroblasts infected with AdCAR, AdNull (both 104 pu/cell), or no virus were incubated with increasing concentrations of purified Ad5 fiber (4) (0–1 μg/ml at 37°C) for 1 h before, and also during, infection with 300 pu/cell Adβgal.
For all experiments using fiber- or penton-altered Ad vectors, fibroblasts were first infected with high moi AdCAR or AdNull (both 104 pu/cell) or no virus followed 24 h later by a second infection with low moi (300 pu/cell) of altered vector or its control. Altered vectors included Ad5f7CAT, for which standard Ad5CAT served as control, and AdΔRGDβgal, AdF(pK7)βgal, AdF(RGD)βgal, or AdF9sKβgal, for which standard Adβgal served as control.
Statistical analysis.
All studies were carried out in triplicate. All data are presented as mean ± SEM. All comparisons were made using the two-tailed Student's t test. For fluorescent binding studies, correlation of Ad binding and CAR expression were calculated using Spearman's rank correlation.
Results
Gene transfer and expression of first generation Ad vectors in primary human dermal fibroblasts.
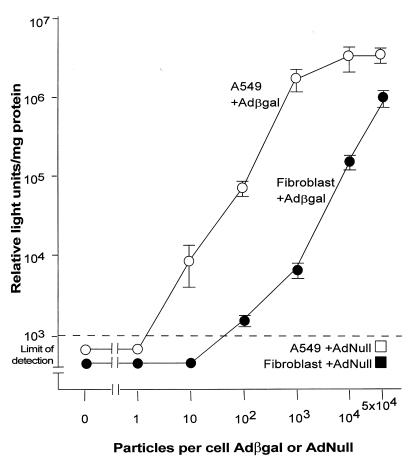
A dose–response study comparing βgal expression in human fibroblasts to A549 demonstrated that a much higher moi of Adβgal was required to generate comparable βgal expression in the fibroblasts (Fig. 1). In fibroblasts infected with 100 pu/cell, the βgal activity in these cells was approximately 100-fold less than in that of A549 cells infected with the same low moi (all P < 0.01, 10 – 5 × 104 pu/cell; Fig. 1). Interestingly, the amount of βgal expression in the A549 cells was maximal at >103 pu/cell, whereas βgal expression by the fibroblasts increased as a function of moi, so that with 5 × 104 pu/cell, the expression of βgal in the fibroblasts was only fourfold less than that of the A549 cells at the same moi. The control AdNull vector induced βgal activity below the limit of detection in either A549 cells or fibroblasts.
Figure 1.
Evaluation of the ability of subgroup C, serotype 5 Ad vectors to transfer and express the βgal transgene in primary human fibroblasts compared with the A549 lung epithelial cell line. βgal activity was assessed 24 h after infection with 0–5 × 104 pu/cell of Adβgal or 5 × 104 pu/cell AdNull. The dashed line represents the limit of detection of the assay (103 relative light units/mg protein). Each data point represents the mean ± SE of triplicate measurements. Ad, adenovirus.
Characterization of Ad cell-surface receptors.
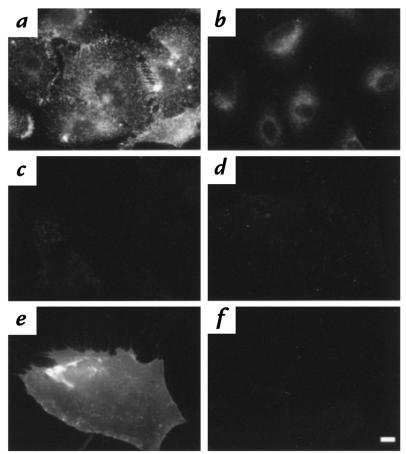
Primary human fibroblasts lack immunodetectable CAR (Fig. 2). A549 cells, which are easily infected with low moi of Ad (Fig. 1), demonstrated punctate cell-surface labeling when stained for CAR (Fig. 2a). In contrast, naive fibroblasts stained for human CAR demonstrated no fluorescence over background (Fig. 2c). Although the fibroblasts did not exhibit detectable CAR, they did express αV integrins on the cell surface (Fig. 3a).
Figure 2.
CAR expression on primary human fibroblasts. Twenty-four hours after infection with AdCAR, AdNull (both 104 pu/cell), or no virus (naive), fibroblasts were incubated with anti-CAR antibody (RmcB) or isotype-matched control antibody followed by Texas red–labeled secondary antibody and examined by fluorescent microscopy. Naive A549 cells served as positive control. (a) A549, RmcB. (b) A549, control antibody. (c) Naive fibroblasts, RmcB. (d) AdNull-infected fibroblasts, RmcB. (e) AdCAR-infected fibroblasts, RmcB. (f) AdCAR-infected fibroblasts, control antibody. Scale bar, 10 μm. CAR, Coxsackie virus and adenovirus receptor.
Figure 3.
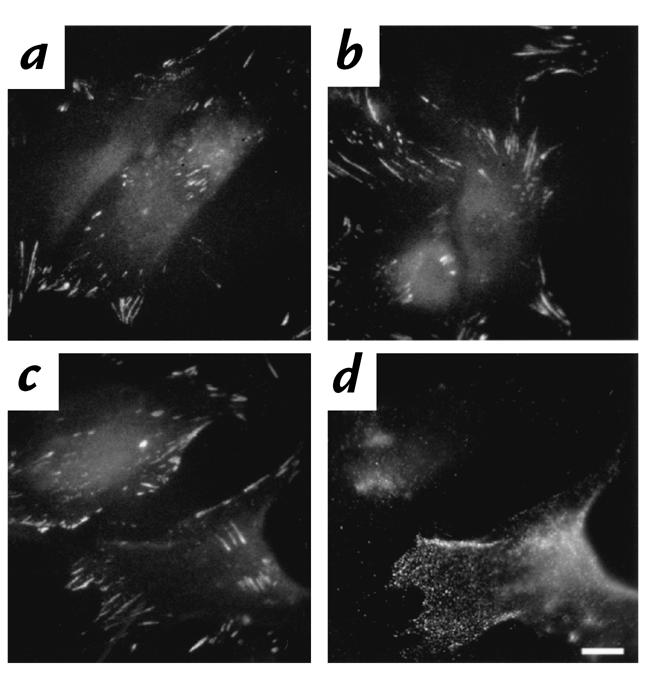
αV integrin in AdCAR- and AdNull-infected fibroblasts. Twenty-four hours after infection with AdCAR or AdNull (both 104 pu/cell) or no vector, fibroblasts were double-labeled with RmcB and anti–αV integrin antibody. (a) Naive fibroblasts, αV integrin antibody. (b) AdNull-infected fibroblasts, αV integrin antibody. (c) AdCAR-infected fibroblasts, anti–αV integrin antibody. (d) AdCAR-infected fibroblasts, RmcB. Scale bar, 10 μm.
AdCAR-induced upregulation of CAR expression in fibroblasts.
Fibroblasts infected with a high moi of AdCAR were made CAR-sufficient, demonstrating intense cell-surface staining of CAR as detected with an anti-CAR antibody (Fig. 2e) relative to a control antibody (Fig. 2f). In contrast, AdNull-infected fibroblasts demonstrated no cell-surface CAR (Fig. 2d). The immunofluorescent labeling in the AdCAR-infected fibroblasts was qualitatively more intense than that seen in the A549 cells. Neither infection with AdCAR nor AdNull altered the expression of αV integrin (Fig. 3, b–d).
Ad binding to CAR-deficient and CAR-sufficient fibroblasts.
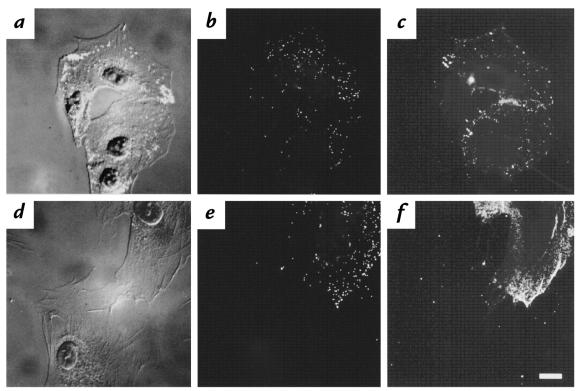
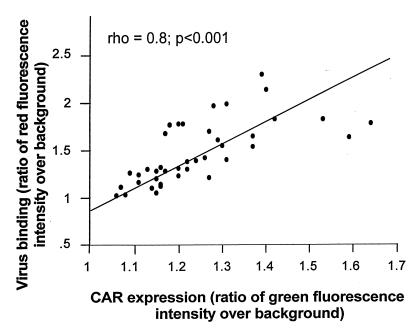
CAR-sufficient fibroblasts demonstrated greater binding of Cy3-AdGFP than did CAR-deficient controls (Fig. 4). Cy3-AdGFP binding to mock- and AdNull-infected cells was minimal (not shown). CAR expression among AdCAR-infected fibroblasts was not uniform. Quantitation of fluorescence intensity in AdCAR-infected cultures demonstrated that there was a positive correlation between green fluorescence (i.e., CAR expression) and red fluorescence (i.e., virus binding), with Spearman correlation analysis demonstrating a rho of 0.8 with P < 0.001 (Fig. 5). Thus, with increasing CAR expression, the fibroblasts were able to bind more virus.
Figure 4.
Binding of fluorescent Ad vectors by CAR-sufficient primary fibroblasts. AdCAR- infected fibroblasts were incubated with Cy3-labeled fluorescent AdGFP (1011 pu/cell) for 10 min at 37°C. The cells were then fixed and stained for CAR as described for Fig. 2, but with FITC-labeled secondary antibody. Naive A549 cells served as positive control. (a) A549 cells, DIC. (b) A549 cells, Cy3-AdGFP binding. (c) A549 cells, RmcB. (d) AdCAR-infected fibroblasts, DIC. (e) AdCAR-infected fibroblasts, Cy3-Ad5GFP binding. (f) AdCAR-infected fibroblasts, RmcB. Scale bar, 10 μm. DIC, differential interference contrast.
Figure 5.
Correlation of binding of fluorescent Ad vectors with CAR expression. Fibroblasts were infected and labeled with RmcB and Cy3-AdGFP as described in Fig. 4. Fields were selected in AdCAR-infected cultures that included intensely and minimally CAR-positive (green fluorescent) cells. Images were collected, and triplicate gray-scale measurements were made in arbitrary regions within the background (no cells) and within each cell in both green and red channels. CAR expression (green fluorescence) and virus binding (red fluorescence) in a total of 38 cells were expressed as a ratio of cell to background fluorescence. Spearman's correlation analysis revealed a significant correlation, with rho = 0.8 and P < 0.001.
Improvement of Ad transgene expression in CAR-sufficient fibroblasts.
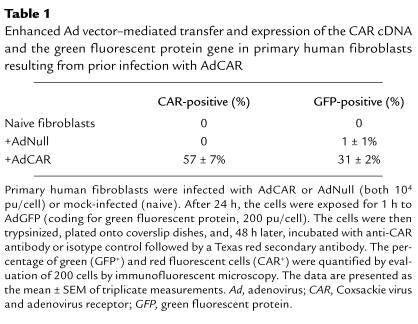
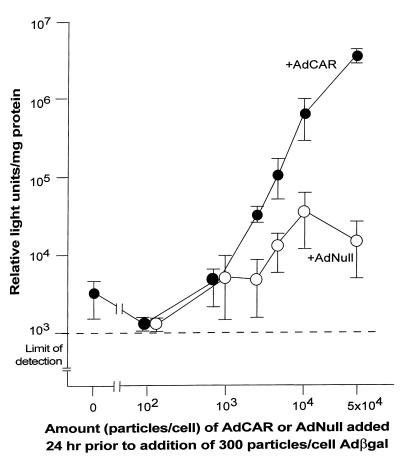
Two strategies were used to demonstrate that AdCAR infection allows fibroblasts to be subsequently infected with an Ad vector at a low moi. First, mock-, AdNull-, or AdCAR-infected fibroblasts were infected with a low moi (200 pu/cell) of AdGFP and labeled with anti-CAR antibody. The number of cells expressing CAR was compared with the number of cells expressing GFP. After infection with AdCAR and then AdGFP, 57% of fibroblasts were CAR-positive, and 31% expressed GFP (Table 1). All GFP-positive cells in this group were also CAR positive. In contrast, fibroblasts infected with AdNull and then AdGFP expressed no detectable CAR (P < 0.001), and only 1% expressed GFP (P < 0.01). As a second approach, mock-, AdNull-, or AdCAR-infected fibroblasts were infected with a low moi (300 pu/cell) of Adβgal. Compared with CAR-deficient controls, CAR-sufficient fibroblasts demonstrated higher transgene expression (Fig. 6). βgal expression derived from a fixed, low moi infection with Adβgal, depended on the dose of AdCAR used as the first vector over a 3 log range. In AdNull-infected cells, there was a minor increase in the βgal expression (a maximum of 10-fold above that of no first vector). In contrast, the βgal expression observed in AdCAR-infected fibroblasts was markedly increased (1000-fold above that of no first vector at 5 × 104 pu/cell, P < 0.01; 200-fold above that of AdNull first vector, P < 0.02).
Table 1.
Enhanced Ad vector–mediated transfer and expression of the CAR cDNA and the green fluorescent protein gene in primary human fibroblasts resulting from prior infection with AdCAR
Figure 6.
Enhanced transfer and expression in primary human fibroblasts of the βgal transgene by Adβgal after transfer and expression of CAR mediated by AdCAR. Fibroblasts were infected with increasing moi (0–5 × 104 pu/cell) of AdCAR or AdNull. Twenty-four hours later, the cells were infected with 300 pu/cell of Adβgal. βgal activity in cell lysates was assayed 24 h after the second infection. The dashed line represents the limit of detection of the assay (103 relative light units/mg protein). moi, multiplicity of infection.
Fiber–CAR interaction in the second infection of CAR-sufficient fibroblasts.
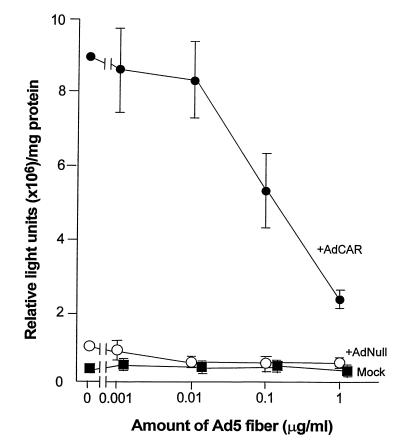
Transgene expression in CAR-sufficient, but not CAR-deficient, cells was suppressed by the presence of Ad5 fiber (Fig. 7). In CAR-sufficient fibroblasts, the competitive effect of recombinant Ad5 fiber was significant, decreasing βgal activity by up to 80% in a dose-dependent manner (Fig. 7). In contrast, the presence of recombinant Ad5 fiber only minimally affected βgal expression in the CAR-deficient controls.
Figure 7.
Ability of purified Ad5 fiber to block transfer and expression of the βgal gene by Adβgal in CAR-sufficient, but not in CAR-deficient, primary human fibroblasts. Mock-, AdNull-, or AdCAR-infected fibroblasts were incubated with increasing concentrations (0–1 μg/ml) of purified Ad5 fiber at 37°C for 1 h before and during the second infection with 300 pu/cell of Adβgal. βgal activity of cell lysates was assayed 24 h after the second infection. The data represents mean ± SE of triplicate measurements.
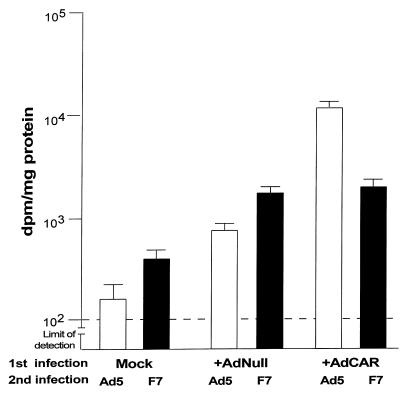
No enhancement of transgene expression in CAR-sufficient vs. CAR-deficient cells was observed when a chimeric virus that bears an Ad7a fiber rather than Ad5 fiber was used for the second infection (Fig. 8). As expected, CAT activity in CAR-sufficient cells infected with the standard Ad5-based Ad5CAT vector was 80-fold greater when compared with naive cells and 20-fold greater than AdNull-infected controls (P < 0.01, both comparisons). In contrast, CAT activity in CAR-sufficient cells did not differ significantly from that of AdNull-infected control (P > 0.6) when the second infection was with Ad5f7CAT.
Figure 8.
Fiber serotype-specific enhancement of Ad vector–mediated transgene expression in CAR-sufficient versus CAR-deficient cells. Mock-, AdNull- or AdCAR-infected fibroblasts were infected with 300 pu/cell of Ad5CAT (Ad5, a standard first-generation Ad5 vector encoding for chloramphenicol acetyl transferase) or Ad5f7CAT (F7, a chimeric Ad5-based vector with the Ad7a fiber encoding for CAT). CAT activity of cell lysates was assayed 24 h after the second infection. The data represent mean ± SE of triplicate measurements.
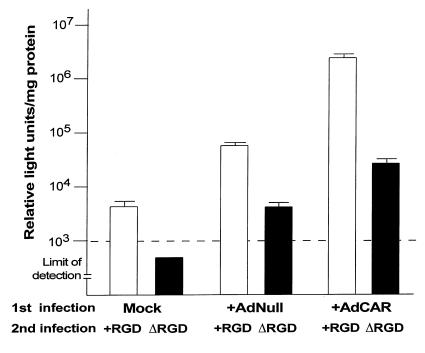
The relative importance of the fiber–CAR interaction versus the integrin–penton interaction was evaluated by comparing βgal expression in CAR-sufficient and CAR-deficient fibroblasts infected with either standard Adβgal or AdΔRGDβgal. Although βgal expression by AdΔRGDβgal in mock-infected fibroblasts was below the limit of detection, expression with the AdΔRGDβgal vector in CAR-sufficient fibroblasts was significant, approximately 10-fold above that of the background and fivefold greater than that of the AdNull control (P < 0.02; Fig. 9). This suggests that upregulation of CAR can improve Ad infection even in the absence of the penton–integrin interaction.
Figure 9.
βgal gene transfer and expression mediated by an Ad vector that lacks the penton base RGD domain in CAR-sufficient versus CAR-deficient fibroblasts. Mock-, AdNull-, or AdCAR- infected fibroblasts were infected with 300 pu/cell of Adβgal (+RGD, an Ad vector containing a normal penton base RGD sequence) or AdΔRGDβgal (ΔRGD, an Ad vector lacking the penton base RGD sequence). βgal activity of cell lysates was assayed 24 h after the second infection. The dashed line indicates limit of detection of βgal assay (103 relative light units/mg protein). The data are presented as mean ± SE of triplicate measurements.
Gene transfer and expression mediated by fiber-altered vectors.
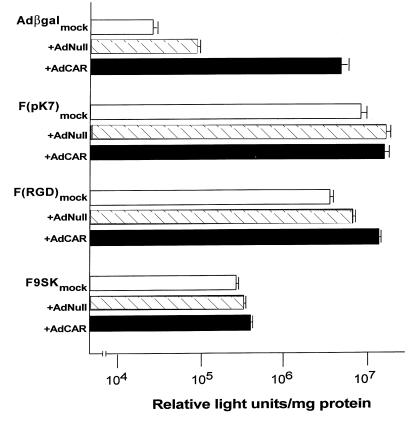
Ad vectors bearing a variety of modifications in their fiber proteins bypass the usual fiber–CAR interaction and can be used to infect CAR-deficient cells (Fig. 10). Compared with the standard Ad5-based Adβgal vector, fiber-altered vectors demonstrated improved transgene expression in CAR-deficient fibroblasts. AdF(pK7)βgal was the most effective, inducing an approximately 200-fold increase in βgal expression in naive fibroblasts versus standard Adβgal (P < 0.01). An approximately 100-fold increase was observed with AdF(RGD)βgal (P < 0.01 compared to standard Adβgal), and an approximately eightfold increase was observed with AdF9sKβgal (P < 0.01 compared with standard Adβgal). Whereas CAR-sufficient fibroblasts demonstrate a 70-fold increase in βgal expression over that of AdNull-infected controls (P < 0.001) when infected with standard Adβgal, no significant difference was found between CAR-sufficient versus CAR-deficient cells infected with AdF(pK7)βgal (P > 0.8) or AdF9sKβgal (P > 0.1). These data suggest that the mechanism for binding and/or entry by these vectors is CAR-independent. With AdF(RGD)βgal, a small (about 1.5-fold) increase in βgal expression was noted in the AdCAR- versus the AdNull-infected cells (P < 0.03).
Figure 10.
Expression of the transgene in Ad vectors with altered fibers in CAR-sufficient vs. CAR-deficient primary human fibroblasts. Mock-, AdNull-, or AdCAR-infected fibroblasts were infected with 300 pu/cell of Adβgal (first-generation Ad vector expressing the βgal gene) or AdF(pK7) βgal [F(pK7), similar to Adβgal, but seven lysine residues at the end of each fiber protein], AdβgalF(RGD) [F(RGD), similar to Adβgal, but bearing a high-affinity RGD sequence on the end of each fiber protein], or AdF9sK (F9sK, similar to Adβgal, but with a short fiber including the tail and first eight shaft repeats of Ad5 fiber attached to the last shaft repeat and knob of Ad9). βgal activity of cell lysates was assayed 24 h after the second infection. The data are presented as mean ± SE of triplicate measurements.
Discussion
Gene therapy by Ad-mediated gene transfer requires that the target cells bind and take up the Ad vector efficiently (15, 20–22). In this study, we have demonstrated that CAR is rate limiting for infection by standard Ad5 vectors in primary αV integrin–positive cells and that the upregulation of CAR in such cells leads to increased binding of Ad, as well as more efficient expression of Ad transgene, both of which depend on a subgroup-specific fiber–CAR interaction. Using a penton base RGD-deficient Ad vector, the data also demonstrate that the fiber–CAR interaction is sufficient for Ad entry and expression, although far less efficient than if the RGD–integrin interaction were available. Finally, by altering the Ad fiber to enable attachment to receptors other than CAR, it is possible to use Ad vectors effectively to transfer genes to CAR-deficient, αV integrin–positive cells.
Enhancement of Ad-mediated gene transfer in CAR-sufficient fibroblasts.
Previous studies have demonstrated that CAR functions as the high-affinity cell-surface receptor for subgroup C Ad (8, 9). Chinese hamster ovary cells (CHO) permanently transfected with CAR are able to bind subgroup C Ad but not subgroup B Ad3 (9) and are able to express genes transferred with an Ad5 vector at least 100-fold more efficiently than nontransfected CHO cells (8). Roelvink et al. (31) have demonstrated that Ad subgroups A, C, D, E, and F, but not B, compete for CAR binding. The present study demonstrates that CAR is rate limiting for Ad infection of fibroblasts, and importantly, it demonstrates that CAR can improve Ad5 binding and gene transfer to normal, primary human cells (39).
Because Ad infection of primary human dermal fibroblasts is not efficient, the extent of CAR expression after infection with AdCAR is not uniform. This variation allowed us to demonstrate that the extent of Ad binding to a given cell correlates positively with the extent of CAR expression on that individual cell. Upregulation of CAR leads not only to increased Ad binding but enhanced Ad transgene expression. Importantly, this enhancement can be eliminated by the presence of excess recombinant Ad5 fiber. Consistent with the concept that CAR expression enables subgroup-specific Ad entry, a chimeric virus that is Ad5-based but bears Ad7a fiber does not infect CAR-sufficient cells any more efficiently than CAR-deficient cells. This is in agreement with previous findings that subgroup C Ad (serotypes 2 and 5) do not compete for the same receptor as subgroup B Ad (serotypes 3 and 7) (27, 31, 40–42), and with Tomko et al. (9), who observed that Ad5 bound five times more efficiently than Ad3 to CAR-transfected NIH 3T3 cells.
CAR-independent mechanisms of Ad entry.
Whereas upregulation of CAR reflects one strategy for the improvement of Ad-mediated gene transfer to cells, an alternate strategy could be to bypass CAR by altering the fiber of the Ad vector to permit attachment to other receptors. The present study confirms that infection by such fiber-altered vectors do not rely on CAR. On a per particle basis, AdF(pK7)βgal and AdF(RGD)βgal were the most efficient at infecting CAR-deficient human fibroblasts, increasing transgene expression by about 100-fold over that of standard Adβgal. Expression in cells infected by AdF(pK7)βgal, which is designed to bind to cells via cell-surface heparan sulfate (28, 29), is not affected by the presence or absence of CAR on the cell surface. Although AdF(RGD)βgal is designed to use the RGD–αV integrin interaction for both attachment and internalization (29), the fiber modifications have left the CAR-binding domain available for interaction with CAR. Thus, a modest increase (1.5-fold) in transgene expression in CAR-sufficient vs. CAR-deficient cells is found. The main mechanism of entry, however, is most likely related to the RGD–αV integrin interaction, as the magnitude of the increased transgene expression of this vector over the standard Ad5 vector (70-fold) was much greater than that of AdF(RGD) in CAR-sufficient vs. CAR-deficient cells (1.5-fold).
The lack of significant improvement of infection by AdF9sKβgal, an Ad5-based vector with a short fiber and Ad9 knob, in CAR-sufficient versus CAR-deficient fibroblasts was somewhat surprising given that Ad9 has been demonstrated to compete for binding with CAR (30, 31). This lack of improvement suggests that the Ad9 knob–CAR interaction is bypassed. The fiber of AdF9sKβgal only contains eight of the 22 shaft repeats usually present, and this shortening is designed to enhance entry via the RGD–αv integrin pathway. Expression from this vector in CAR-deficient fibroblasts is only about eightfold greater than that of standard Ad5 vector, whereas AdF(RGD)βgal could achieve an approximately 70-fold greater expression. This observation suggests that, although binding and entry of AdF(RGD)βgal and AdF9SKβgal may be through the same RGD-mediated mechanism, trafficking of these vectors may not be the same. Alternatively, replacing a subgroup C fiber with a subgroup D fiber could adversely effect particle assembly, resulting in fewer active particles in the vector preparation.
The chimeric vector, Ad5f7CAT, represents another alteration of fiber that enhanced transgene expression relative to standard Ad5 vector in CAR-deficient fibroblasts, albeit to a modest extent (threefold). As the receptor for subgroup B Ad has not been characterized, we cannot determine whether this increase is due to Ad7a fiber binding to its receptor. As with AdF9sKβgal, the Ad7 fiber is somewhat shorter than Ad5 fiber (30, 43), and the RGD–αV integrin mechanism may dominate here as well. This may be a more likely explanation, given that Ad7 has not been reported to have a tropism for skin (44).
The relative importance of cell-surface αV integrins versus CAR for the entry of Ad into target cells is not clear. The data of Wickham et al. (4, 14) demonstrate that Ad-mediated gene transfer is facilitated by an interaction between RGD on the Ad penton base and αV integrin on the cell surface. The present study suggests that even in the absence of RGD–αV integrin interaction, significant gene transfer and expression can be achieved in primary human fibroblasts if adequate fiber–CAR interaction is available, albeit much less (approximately 20-fold) efficiently than in the presence of RGD–integrin interaction. This concept is supported by data demonstrating that RGD-deleted Ad2 can infect fiber receptor–positive cells, but not fiber receptor–negative cells (7, 12). The lack of expression by AdΔRGDβgal in the naive, CAR-deficient cells supports the reciprocal concept that RGD–integrin interaction may be the primary mechanism of CAR-independent gene transfer.
The improved ability of the short fiber vectors over standard Ad5 controls to infect naive fibroblasts also point to the importance of the penton–integrin interaction in CAR-deficient fibroblasts. However, the exclusive use of the penton–integrin entry pathway by the short fiber vectors, including Ad5f7CAT and AdF9sKβgal, has not rigorously been proved. All the vectors we have used to demonstrate CAR-independent pathways of entry have normal, RGD-bearing penton bases that probably bind to αV integrin for cell entry. Although the AdF(pK7)βgal vector is able to infect resting T cells that are αV integrin–negative (4, 5), future studies using fiber-altered vectors that are RGD negative will be necessary to discover whether the penton–integrin interaction is necessary in the absence of fiber–CAR interaction.
The data from our studies suggest that either fiber–CAR interaction or penton–integrin interaction is sufficient to permit Ad entry and gene transfer but that neither is as effective as the two-receptor system used together. Although the fiber-dependent, penton-independent pathway (AdΔRGDβgal in CAR-sufficient cells) and the fiber-independent, penton-dependent pathways (short fiber vectors in CAR-deficient cells) led to increases in transgene expression compared with their respective controls, these increases were modest. In contrast, when both mechanisms were available (e.g., standard Ad5 in CAR-sufficient, integrin-positive cells), the increase over that of the control was about 100-fold. Supporting this hypothesis is the observation that AdF(RGD)βgal demonstrates a much higher level of transgene expression than the short fiber viruses, although they all target the αV integrin pathway of entry. Unlike the short fiber vectors, AdF(RGD)βgal has both fiber and penton available for binding to cell-surface integrin.
An interesting finding in the control groups was the increase in transgene expression in cells that had been infected with AdNull, an Ad vector with no transgene. The indirect immunofluorescence studies demonstrate that neither αV integrin nor CAR is upregulated by AdNull infection of fibroblasts. That the presence of excess recombinant Ad5 fiber does not affect transgene expression in AdNull-infected cells also suggests that fiber–CAR interaction is not responsible for the increase in transgene expression. The observation of this Null vector effect with every vector tested, including the penton- and fiber-altered Ad vectors, suggests that the Null vector enhancement occurs at a step other than those related to binding or internalization and trafficking. It may be that Ad infection per se activates other biologic processes that, in turn, enhance Ad transgene expression. A recent study by R. Peila et al. (45) suggests that the CMV promoter and, to a lesser extent, the RSV promoter, can be activated by the internalization of Ad. Such activation may be occurring in the present study, because all of the vectors we used included the CMV or RSV promoters. Li et al. (46) have recently described the requirement of PI3-OH kinase activation for Ad internalization. Activation of downstream effectors and other related kinases, such as FAK and MAPK (including ERK1/ERK2), observed early during Ad internalization, may be able to activate the CMV promoter (46). Alternatively, the CMV promoter may be activated by Ad E4 products. Infection with E4-positive vectors has recently been demonstrated to be able to reactivate expression of transgene under either the CMV or RSV promoter that had declined after initial infection via an E4-negative Ad vector in immunodeficient mice (47). In the present study, only E4-positive vectors were used; thus, there could have been enhancement of the CMV-driven transgene by E4-products.
Acknowledgments
We thank B.G. Harvey of our laboratory for the dermal fibroblasts, M. Harper (Rockefeller University, New York, New York, USA) for helpful discussions regarding statistical analyses, and N. Mohamed for help in preparing the manuscript. These studies were supported in part by the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute (P01 5-P01-HL51746, 1-P01-HL59312, and HL51746-03), the Rockefeller University (GCRC NIH M01RR00102), the Cystic Fibrosis Foundation, Will Rogers Memorial Fund, and GenVec Inc. C. Hidaka is supported in part by the Institute for Sports Medicine Research. J. Bergelson is supported by NIH IR02 AI35667 and an Established Investigator Award from the American Heart Association. P. Leopold is supported in part by NIH R29 AI42250.
References
- 1.Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 2.Shenk, T. 1996. Adenoviridae: the viruses and their replication. In Fields virology. B.N. Fields, D.M. Knipe, and P.M. Howley, editors. Lippincott-Raven Publishers. Philadelphia, PA. 2111–2148.
- 3.Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 4.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins αVβ3 and αVβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 5.Wickham TJ, Carrion ME, Kovesdi I. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 1995;2:750–756. [PubMed] [Google Scholar]
- 6.Freimuth P. A human cell line selected for resistance to adenovirus infection has reduced levels of the virus receptor. J Virol. 1996;70:4081–4085. doi: 10.1128/jvi.70.6.4081-4085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh MJ. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Invest. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergelson JM, et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 9.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergelson JM, et al. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SS, Karayan L, Tournier J, Curiel DT, Boulanger PA. Adenovirus type 5 fiber knob binds to MHC class I α2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai M, Harfe B, Freimuth P. Mutations that alter an ARG-GLY-ASP (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αV integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. Integrin αVβ5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto Y, et al. Efficient transfer of genes into senescent cells by adenovirus vectors via highly expressed αVβ5 integrin. Biochem Biophys Res Commun. 1997;240:88–92. doi: 10.1006/bbrc.1997.7534. [DOI] [PubMed] [Google Scholar]
- 16.Takayama K, et al. The levels of integrin αVβ5 may predict the susceptibility to adenovirus-mediated gene transfer in human lung cancer cells. Gene Ther. 1998;5:361–368. doi: 10.1038/sj.gt.3300608. [DOI] [PubMed] [Google Scholar]
- 17.Seth P, Willingham MC, Pastan I. Adenovirus-dependent release of 51Cr from KB cells at an acidic pH. J Biol Chem. 1984;259:14350–14353. [PubMed] [Google Scholar]
- 18.Seth P. Mechanism of adenovirus-mediated endosome lysis: role of the intact adenovirus capsid structure. Biochem Biophys Res Commun. 1994;205:1318–1324. doi: 10.1006/bbrc.1994.2809. [DOI] [PubMed] [Google Scholar]
- 19.Seth P. Adenovirus-dependent release of choline from plasma membrane vesicles at an acidic pH is mediated by the penton base protein. J Virol. 1994;68:1204–1206. doi: 10.1128/jvi.68.2.1204-1206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panetti TS, Wilcox SA, Horzempa C, McKeown-Longo PJ. αVβ5 integrin receptor-mediated endocytosis of vitronectin is protein kinase C-dependent. J Biol Chem. 1995;270:18593–18597. doi: 10.1074/jbc.270.31.18593. [DOI] [PubMed] [Google Scholar]
- 21.Gailit J, Clark RA. Studies in vitro on the role of αV and β1 integrins in the adhesion of human dermal fibroblasts to provisional matrix proteins fibronectin, vitronectin, and fibrinogen. J Invest Dermatol. 1996;106:102–108. doi: 10.1111/1523-1747.ep12328177. [DOI] [PubMed] [Google Scholar]
- 22.Gailit J, et al. Human fibroblasts bind directly to fibrinogen at RGD sites through integrin αVβ3. Exp Cell Res. 1997;232:118–126. doi: 10.1006/excr.1997.3512. [DOI] [PubMed] [Google Scholar]
- 23.Mastrangeli A, et al. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J Clin Invest. 1993;91:225–234. doi: 10.1172/JCI116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hersh J, Crystal RG, Bewig B. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 1995;2:124–131. [PubMed] [Google Scholar]
- 25.Zolotukhin S, Potter M, Hauswirth WW, Guy J, Muzyczka N. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey BM, et al. High-efficiency gene transfer into ex vivo expanded human hematopoietic progenitors and precursor cells by adenovirus vectors. Blood. 1998;91:2781–2792. [PubMed] [Google Scholar]
- 27.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickham TJ, Roelvink PW, Brough DE, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotech. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 29.Wickham TJ, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roelvink PW, Kovesdi I, Wickham TJ. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roelvink, P.W., Kovesdi, I., Bergelson, J.M., Finberg, R.W., and Wickham, T.J. 1998. Adenovirus retargeting through fiber modification and bifunctional fiber receptor protein (CAR) chimeras. American Society of Gene Therapy, 1st Annual Meeting, Seattle, Washington, May 28–31, 1998; Program and Abstracts.
- 32.Leopold PL, et al. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeld MA, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 34.Rosenfeld MA, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 35.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann JR, Morency CA, Russian KO. A novel rapid assay for chloramphenicol acetyltransferase gene expression. Biotechniques. 1987;5:444–447. [Google Scholar]
- 37.Hsu KH, Lonberg-Holm K, Alstein B, Crowell RL. A monoclonal antibody specific for the cellular receptor for the group B Δviruses. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfister KK, Wagner MC, Stenoien DL, Brady ST, Bloom GS. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989;108:1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leon RP, et al. Adenoviral-mediated gene transfer to lymphocytes. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abrahamsen K, et al. Construction of an adenovirus type 7a E1a-vector. J Virol. 1997;71:8946–8951. doi: 10.1128/jvi.71.11.8946-8951.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Defer C, Belin MT, Caillet-Boudin ML, Boulanger P. Human adenovirus-host cell interactions: Comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson SC, Rollence M, White B, Weaver L, McClelland A. Human adenovirus serotypes 3 and 5 bind to two different cellular receptors via the fiber head domain. J Virol. 1995;69:2850–2857. doi: 10.1128/jvi.69.5.2850-2857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong JS, Mullis KG, Engler JA. Characterization of the early region 3 and fiber genes of Ad7. Virology. 1988;167:545–553. [PubMed] [Google Scholar]
- 44.Horwitz, M.S. 1996. Adenoviruses. In Fields virology. B.N. Fields, D.M. Knipe, and P.M. Howley, editors. Lippincott-Raven Publishers. Philadelphia, PA. 2149–2197.
- 45.Peila, R., et al. 1998. Adenovirus particle internalization upregulates transgene expression from the CMV promoter. American Society of Gene Therapy, 1st Annual Meeting, Seattle, Washington, May28–31, 1998; Program and Abstracts. 49a. (Abstr.)
- 46.Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brough DE, et al. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression from adenovirus vectors in vivo. J Virol. 1997;71:9206–9213. doi: 10.1128/jvi.71.12.9206-9213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]