Abstract
In the evolution of caste-based societies in Hymenoptera, the classical insect hormones juvenile hormone (JH) and ecdysteroids were co-opted into new functions. Social wasps, which show all levels of sociality and lifestyles, are an ideal group in which to study such functional changes. Virtually all studies on the physiological mechanisms underlying reproductive division of labor and caste functions in wasps have been done on independent-founding paper wasps, and the majority of these studies have focused on species specially adapted for overwintering. The relatively little-studied tropical swarm-founding wasps of the Epiponini (Vespidae) are a diverse group of permanently social wasps, with some species maintaining caste flexibility well into the adult phase. We investigated the behavior, reproductive status, JH and ecdysteroid titers in hemolymph, ecdysteroid content of the ovary and cuticular hydrocarbon (CHC) profiles in the caste-monomorphic, epiponine wasp Polybia micans Ducke. We found that the JH titer was not elevated in competing queens from established multiple-queen nests, but increased in lone queens that lack direct competition. In queenless colonies, JH titer rose transiently in young potential reproductives upon challenge by nestmates, suggesting that JH may prime the ovaries for further development. Ovarian ecdysteroids were very low in workers but higher and correlated with the number of vitellogenic oocytes in the queens. Hemolymph ecdysteroid levels were low and variable in both workers and queens. Profiles of P. micans CHCs reflected caste, age and reproductive status, but were not tightly linked to either hormone. These findings show a significant divergence in hormone function in swarm-founding wasps compared with independently founding ones.
KEY WORDS: Challenge hypothesis, Cuticular hydrocarbons, Ecdysteroids, Epiponini, Juvenile hormone, Polistes
INTRODUCTION
Eusocial insects within the order Hymenoptera (bees, wasps and ants) are characterized by the production of distinct phenotypes (caste polyphenism) in the female sex: the queen, the worker and often additional physiologically or morphologically specialized types within the worker caste (Wilson, 1971; Hunt, 2006; Hunt et al., 2007; Robinson, 2009; Tibbetts and Izzo, 2009; Shukla et al., 2013). In most caste-based societies the development of such specialized phenotypes is induced or at least biased in a pre-imaginal stage, where qualitative or quantitative differences in diet, pheromonal signals, mechanical stimuli or other environmental cues trigger endogenous signaling cascades toward alternative developmental trajectories (Wilson, 1971; Chavarría-Pizarro and West-Eberhard, 2010; Suryanarayanan et al., 2011; Hartfelder and Emlen, 2012; Penick and Liebig, 2012). As with most polyphenisms in insects, juvenile hormone (JH) and ecdysteroids were co-opted to orchestrate distinct ontogenetic pathways on top of their well-conserved role in regulating the episodic, molt-based growth events in immature animals (Hartfelder and Emlen, 2012). This is typically accomplished during particular hormone-sensitive periods that serve as bifurcation points in development (Nijhout, 1994; Hartfelder and Emlen, 2012).
In many insects, JH has a gonadotropic role in females, stimulating vitellogenin synthesis in the fat body and/or its uptake into the oocyte (Nijhout, 1994; Wyatt and Davey, 1996). In some insects, such as flies, JH only primes the ovaries and fat body to respond to other hormonal signals, and the ecdysteroids have taken over the regulatory functions (Raikhel et al., 2005). A gonadotropic function for JH is conserved in many primitively eusocial hymenopteran species, such as bumble bees (Bombus) and paper wasps (Polistes and Ropalidia) (Röseler, 1991; Bloch et al., 2002; Agrahari and Gadagkar, 2003), and ecdysteroids are likewise at high levels in reproductive individuals. Yet both hormones are at low levels in the hemolymph of adults of more advanced eusocial bees, and JH has lost reproductive functions in most ant species studied (Ramamurty and Engels, 1977; Sommer et al., 1993; Cuvillier-Hot et al., 2004; Brent et al., 2006; Hartfelder et al., 2006; Penick et al., 2011). In Polistes, nests are often founded by groups of competing foundresses, and JH and the ecdysteroids both augment behavioral dominance (Röseler et al., 1984; Röseler, 1985; Tibbetts et al., 2011b) and (at least for JH) strongly correlate with fertility signals encoded by cuticular hydrocarbons (CHCs) (Izzo et al., 2010). The dominant foundress typically becomes the lone reproductive female (i.e. queen), but if she disappears, former workers fighting to inherit the nest show increased JH levels (Tibbetts and Huang, 2010). Finally, as in other hymenopteran societies (O'Donnell and Jeanne, 1993; Robinson and Vargo, 1997; Lengyel et al., 2007; Penick et al., 2011; Dolezal et al., 2012), JH appears to modulate age-related changes in worker activity, a function that is condition and context dependent in adults of Polistes (West-Eberhard, 1996; Giray et al., 2005; Shorter and Tibbetts, 2009).
In the Neotropical swarm-founding wasps (Epiponini: Vespidae), queens and workers migrate to new sites as a coordinated group, and the colony cycle lacks a solitary phase (cf. individual dispersal and
List of symbols and abbreviations
- 3MeC25
methylpentocosane (a hydrocarbon)
- Acc.
accepted (former attackee) females
- AQR
after queen removal
- Atke
attackees
- Atkr
attackers
- BQR
before queen removal
- CHC
cuticular hydrocarbon
- JH
juvenile hormone
- LME
length of a mature egg
- NQ
new queen
- PR
prospective reproductives
- PW
presumptive workers
- Q
queen(s)
- QL
queenless
- QR
queenright
- W
workers
- Ω
final queen removed from a multiple-queen nest
association of foundresses in Polistes). During colony foundation and subsequent nest expansion events, workers build the nest and cells into which queens oviposit. In many species, such as Metapolybia aztecoides, Synoeca surinama and Parachartergus colobopterus, the caste fate of the developing brood is entirely subject to the social environment; namely, the presence or absence of queens (West-Eberhard, 1977; West-Eberhard, 1978; West-Eberhard, 1981; Strassmann et al., 2002). As a result, castes may only be distinguished by their behavior (West-Eberhard, 1978). Queenright (i.e. worker-fated) females pass through a period of idleness, during which they may be actively suppressed by nestmates (West-Eberhard, 1977; West-Eberhard, 1978; West-Eberhard, 1981). After a number of days, they take up tasks in and on the nest before transitioning into foraging roles (West-Eberhard, 1981; Jeanne, 1991). Incipient (i.e. young queenless females) and actual queens signal their dominance through ritualized acts of aggression – by abdomen-bending gestures – in response to other queens and the spasmodic approaches of workers, a ritualized behavior called the ‘queen-dance’ (West-Eberhard, 1978). Queens that begin to respond submissively to the queen-dance are typically attacked and may eventually become workers themselves (Forsyth, 1978; West-Eberhard, 1978). In smaller nests, a single queen eventually emerges as the winner and will henceforth monopolize egg laying, and, as a consequence, within-colony relatedness will then increase (West-Eberhard, 1978; Hughes et al., 1993).
In Polistes, as mentioned, the hormones involved in reproductive and social dominance are JH-III and ecdysteroids. As the ritualized threats seen in Epiponini are likely derived from acts of outright combat (West-Eberhard, 1981), we hypothesized a conserved, Polistes-like function for JH and/or ecdysteroids in augmenting aggression, maintaining reproductive growth and effecting CHC signaling in caste-flexible, swarm-founding wasps. At the same time, JH may have condition- and context-dependent effects on female behavior [cf. honeybees and Polistes (see Hartfelder and Emlen, 2012)]. This effect is strongly suggested by O'Donnell and Jeanne (O'Donnell and Jeanne, 1993) who showed that JH mimic (JHM) treatments to young queenright females induced the early onset of several activities (e.g. foraging tasks) in the well-studied epiponine Polybia occidentalis, but physiological measurements have not been done to validate these results (Zera, 2007).
Polybia micans Ducke belongs to a derived genus within the Epiponini (Pickett and Carpenter, 2010), can form very small colonies (30 individuals) and they lack morphologically defined castes (Richards, 1978), strongly suggesting (and confirmed herein) that caste determination occurs in the adult stage (although a physiological bias cannot be excluded). Here, we report the first endogenous hormone titers of any swarm-founding wasp to assess the endocrine physiology under an array of natural and manipulated social conditions, thereby gaining insight into the evolution of hormone functions in a permanently social wasp. We also present the first report of intraspecific differences in cuticular pheromone signaling in any epiponine species.
RESULTS
Colony states and demographics
Nests were observed in a variety of states (Table 1). Each non-founding colony (colonies 1–8) was in a period of nest expansion. Colony 7 swarmed three times over the course of a month. Colonies 9 and 10 were studied as they were founding a nest.
Table 1.
Basic information for colonies used in this study
All females who received the queen-dance had large ovaries and were inseminated, and thus were considered queens. Queen number estimates ranged from 1 to over 27. Ovary size (oocytes >70% length of mature egg, LME) was inversely related to queen number in established colonies where the exact (colonies 3–5 and 8) or an approximate (colonies 2, 6 and 7) number of queens was known (Spearman's ρ=0.0162). In the established first nest of colony 7, one queen was found to have only one oocyte >70% LME, and the proximal oviducts of her ovarioles were devoid of oocytes, yet full of yellow bodies, indicative of a long history of ovipositions (Tyndale-Biscoe, 1984).
Behavior
Queenright conditions
When a queen emerged from the inside of the nest to the outermost (and newest) comb, virtually all workers performed the queen-dance when they encountered a queen. In response, queens would usually not show an obvious response but would sometimes elicit trophallactic exchanges or, much more rarely, perform the abdomen-bending display. Unexpectedly, single queens (colonies 1 and 4) bent more frequently and at sharper angles (N=2) than ones from multiple-queen nests. In colony 7, the queen with regressing ovaries (see above) was found crouched on the very periphery of the outermost comb, a rare resting place for a reproductively active queen. Passing workers would perform the queen-dance toward her and occasionally bite, although the confrontations did not escalate beyond this in the 2 h prior to her collection.
Queenless conditions
On the day after all queens were removed, particular individuals were targeted for attack and were temporarily banished from the colony (colonies 1, 4, 5 and 8, with observations based on paint-marked females restricted to the last three colonies). These attackees, which consisted of unmarked females (i.e. they had not been observed to work), builders and/or pulp foragers, found refuge off the nest (e.g. resting in isolation on the surrounding foliage) and intermittently attempted to return to the colony, where they were met by an assembly of aroused workers, most of which had been observed to build, or forage for pulp, water and/or meat under queenright conditions. Upon landing, the attackees were bitten and pinned down to the comb (Fig. 1A) while receiving mock stings from their attackers. In three instances, a female that flew to the nest charged through attempted attacks and disappeared inside the nest. No fatal or serious injury was observed as a result of the attacks. The outbreak of worker aggression persisted over a period of 1 (colony 5) to 3 days (colony 4) after queen removal. In colony 5, some workers engaged in indiscriminant aggression (e.g. attackers became attackees and vice versa) within hours of queen removal, a situation resembling a ‘mêlée’, i.e. a disorganized close combat involving multiple fighters. Whether this queenless mêlée preceded the inception of targeted attacks in other nests is not known.
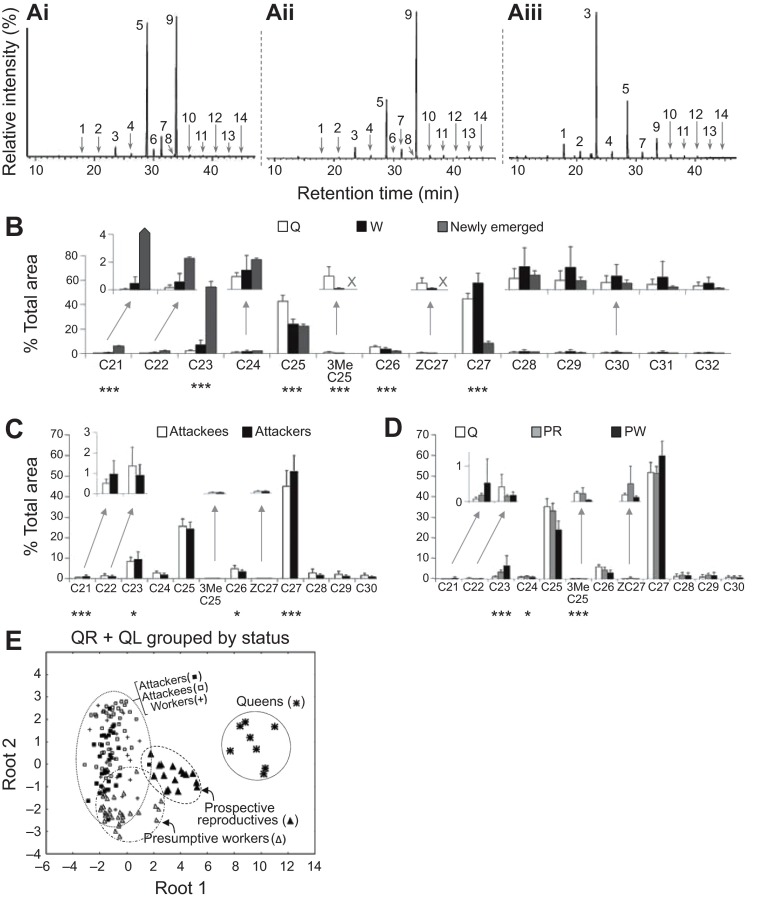
Fig. 1.
Description of attackers and attackees from queenless nests of Polybia micans. (A) One day after queen removal, attackers surround, bite and pin down an attackee (yellow arrow) to the comb. (B) Relative age of attackees and attackers on queenless nests based on the analysis of apodeme darkening on the 5th sternite. Gray, black and white circles indicate females with partially developed, filamentous and unknown ovaries, respectively. (C) Ovarian status (designated A–H) of females from four colonies sampled 1, 1–3 or 7 days after queen removal (AQR). Attackees (Atke) have larger ovaries and are younger than their attackers (Atkr). Some ‘accepted’ (Acc.) females, those who were attacked 1–3 days AQR but later were accepted back into the nest, had larger ovaries than those attacked in colony 4, but not in colony 5. Inside wasps (In) are a sampling of females found in the nest who were not observed to be attacked.
Ovarian status
Queen ovaries contained mature oocytes (see above) whereas worker ovaries were all judged to be pre-vitellogenic (ovary scores A–D; see Materials and methods). To assess the effect of queen removal, all queens were removed from four colonies (colonies 1, 4, 5 and 8) and attacks were observed. The attackees were relatively young according to cuticular scores (Fig. 1B) and had non-filamentous ovaries (91%, N=58) (Fig. 1C), whereas the attackers were relatively old (Fig. 1B) and had filamentous ovaries (98%, N=36) (Fig. 1C). All eight mêlée aggressors sampled had filamentous ovaries. In colonies 4 and 5, which were collected 1 week after queen removal, some marked attackees were found inside and will be referred to as ‘accepted’. In monogynous colony 4, all accepted females had comparable or significantly larger ovaries (ovary sizes C–H) than the attackees sampled in the days following queen removal (N=6) (Fig. 1C). Also, 11 of 44 unmarked females collected from inside the nest (referred to as ‘inside’) had developing eggs. In contrast, the ovaries of accepted females from colony 5 that had many queens were not larger than those of the attackees (N=6) (Fig. 1C), and three marked attackees (A–C type ovaries) were observed to work (e.g. adding pulp to the nest envelope) only 3 days after queen removal. In this case, most inside females had small ovaries, although four relatively young females (<3%, N=174) possessed developing eggs (Fig. 1C). Thus, removal of all queens from a colony initiates a process whereby the older workers with filamentous ovaries attack relatively young females, some of which had worked when the nest was queenright. In the monogynous colony, some of the attackees were subsequently accepted as prospective queens and began to mature eggs.
JH titer
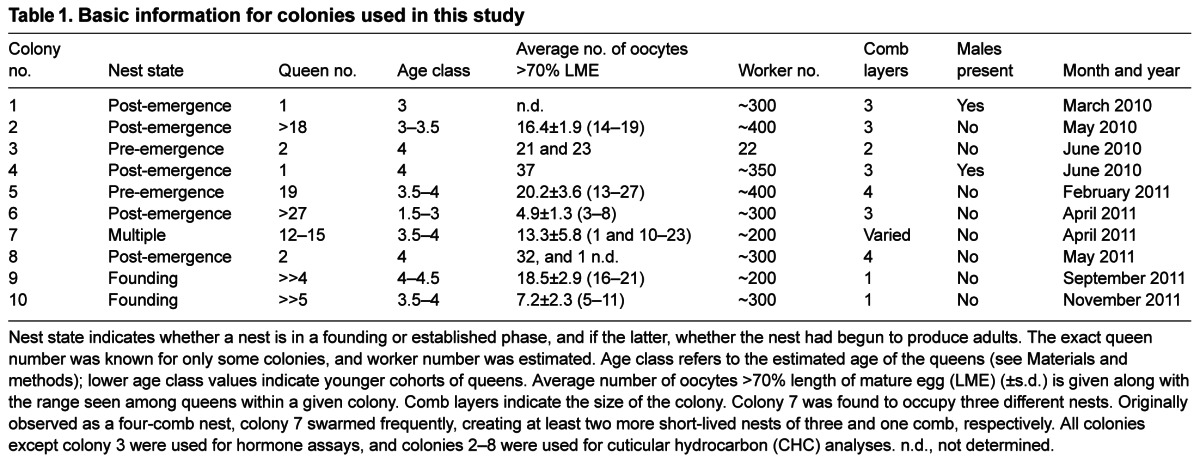
The JH titer of queens and workers under various social conditions was assessed. Fig. 2A shows that in the four established, multiple-queen nests studied, there was no significant difference in JH titer between queens and workers. The isolated queen with regressed ovaries from the non-swarming nest of colony 7 (see above) had a low JH titer (1.3 pg μl−1), comparable to that of a normal queen taken a few days later (2.2 pg μl−1), and within the range of six workers sampled alongside these queens (data not shown). In contrast, in the two monogynous colonies, the lone queen had at least a 10-fold higher level of JH (Fig. 2B). Moreover, in multiple-queen colonies where all queens were eventually removed, the last queen from each colony (who reigned alone for 3–4 days) had a relatively high JH titer compared with that of the other queens (Fig. 2A, see Ω values). In nest-founding phase colonies (combining colonies 7, 9 and 10), queens also had higher titers than the workers constructing new cells (Fig. 2C), although the titers were very low.
Fig. 2.
JH titer of queens and workers from colonies in different phases. (A) No significant differences in JH titer were found between queens (Q) and workers (W) from established, multiple-queen nests. Ω indicates the last queen removed from colonies 5 and 8 (not all queens could be removed from colonies 2 and 6). Box plots show the median and the inner two quartiles; the whiskers indicate the 1.5 interquartile range; the dots represent the respective individual data points. (B) In colonies 1 and 4, ruled by single queens, the JH titer in the queen was higher than that in queenright workers. (C) In nest-founding colonies, queens had higher JH titers than workers (t=4.95, P<0.0001) (mixed model analysis including colonies 7, 9 and 10: F1=24.51, P<0.0001). Colony had no effect on the differences in JH titer. *P<0.05, ***P<0.001.
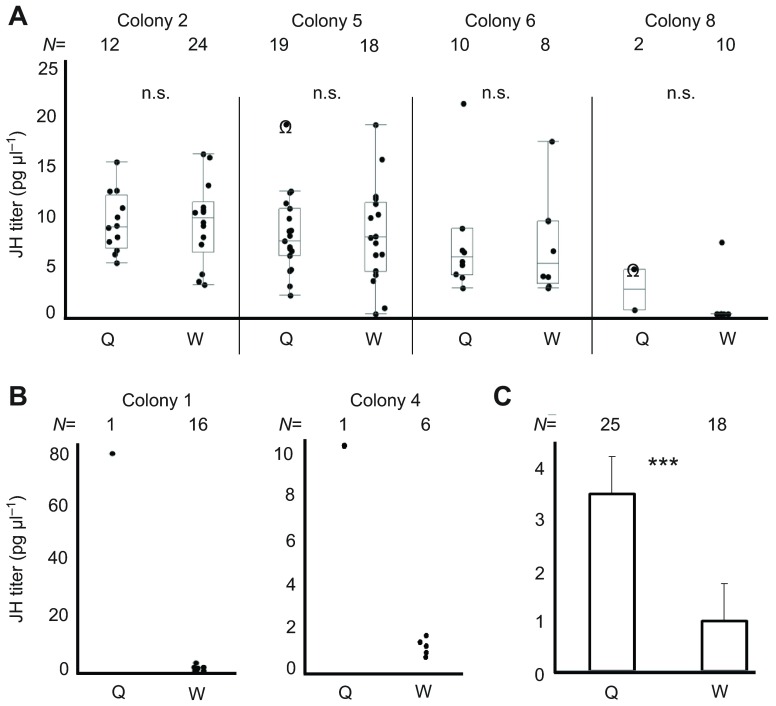
When all the queens were removed from two of the multiple-queen colonies (colonies 5 and 8) and from the two single-queen colonies (colonies 1 and 4), the JH titers of the attacked young females were elevated compared with those of older attackers (Fig. 3), although the difference was only significant for colonies 4 and 8. The JH titers of the attackees were also significantly higher than those of workers in queenright colonies sampled at the same time as the remaining queens (Fig. 3). When queenright workers, attackers and attackees were combined from colonies 1, 4, 5 and 8, the JH titers differed significantly between these groups (F2=23.41, P<0.0001). Attackees (22.94±4.59 pg μl−1) had the highest JH titer compared with queenright workers (1.62±4.47 pg μl−1, t=6.54, P<0.0001) and attackers (6.65±4.88 pg μl−1, LSD P<0.0001); the last two groups did not differ significantly from each other. Colony had no effect on the differences in JH titer of females in the three status categories. Within the attackees there was no relationship between JH titer and oocyte length (N=39, Spearman's ρ=−0.149, P=0.365, Zr=0.15). Thus, JH titer appeared to increase in attacked females, most of whom were relatively young and possessed partially developed ovaries before the final queen was removed.
Fig. 3.
JH titer and aggressive behavior. Day of sampling is indicated with respect to queen removal (BQR, before queen removal; AQR, after queen removal). (A) In colony 4, there was a significant difference in JH titer between workers, attackers and attackees (Kruskal–Wallis test: H2=10.946, P=0.004). JH titer was elevated in attackees compared with attackers sampled on day 1 AQR (Steel–Dwass all pairs test: Z=2.65, P=0.022) and queenright workers sampled on the day of queen removal (day 0) (Z=2.65, P=0.022) NQ, new queen. (B) In colony 5, there was a significant difference in JH titer between workers, attackers, attackees and the mêlée group (Kruskal–Wallis test: colony 5: H3=20.585, P<0.0001). The attackees tended to have higher JH titers than the other groups (Steel–Dwass all pairs test, versus attackers: Z=−2.51 P=0.06; versus mêlée: Z=2.12, P=0.14; versus workers: Z=3.32, P=0.005). Mêlée attackers had higher JH titers than queenright workers (Steel–Dwass all pairs test: Z=3.42, P=0.004). The majority of females sampled thereafter had low JH titers. (C) In colony 1, there was a significant difference in JH titer between workers, attackers and attackees (Kruskal–Wallis test: colony 1: H2=8.041, P=0.018). The attackees had higher JH titers than queenright workers (Steel–Dwass all pairs test: Z=−2.68, P=0.02) but not their attackers (Steel–Dwass all pairs test: Z=1.74, P=0.19), probably due to low sample size. (D) In colony 8, there was a significant difference in JH titer between workers, attackers and attackees. The attackees had significantly higher JH titers than both queenright workers (Steel–Dwass all pairs test: Z=−2.97, P=0.009) and their attackers (Z=3.19, P=0.004). Box plots show the median and the inner two quartiles; the whiskers indicate the 1.5 interquartile range; the dots represent the respective individual data points. *P<0.05, **P<0.01.
In both the multiple-queen colony 5 and the monogynous colony 4, attackees showed an initial rise in JH on day 1 and then a subsequent decline, irrespective of continued aggression in colony 4 (Fig. 3A) or the cessation of attacks (colony 5) (Fig. 3B). Accepted attackees that were captured 7 days after queen removal had low JH titers in colony 4 (accepted females in Fig. 3A) but a great range of titers in colony 5 (Fig. 3B). An unmarked female with a swollen gaster from colony 4 had well-developed queen-like ovaries and a very high JH titer (‘new queen’ of Fig. 3A). Yet in colony 5, no female with comparable ovaries was bled for JH measurement (N=28), although such females were present (Fig. 1C). A range of ovary sizes was observed among both accepted and inside wasps from colony 5 (Fig. 1C), but there was no relationship between JH titer and oocyte length (N=33, Spearman's ρ=0.14, P=0.44, Zr=0.12).
Ecdysteroids
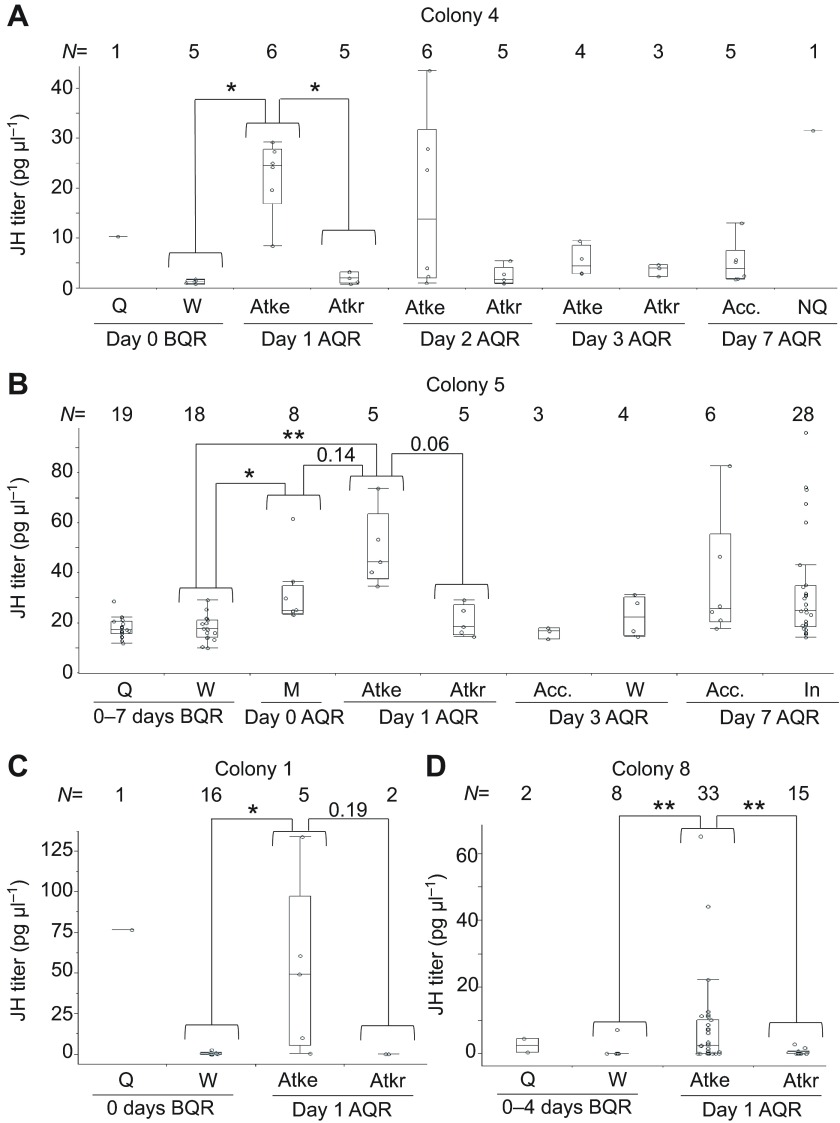
The ovaries of queens contained considerably more ecdysteroids than those of workers in colony 2 (Fig. 4A). The maturing ovaries of accepted females from colony 4 also had significantly more ecdysteroids than attackers and attackees; the last two groups did not significantly differ from one another (Fig. 4A, inset) despite differences in ovary size (Fig. 1C). A separate radioimmunoassay (RIA) analysis of slightly developed ovaries (sizes B–C) of queenless females from colony 5 showed no detectable ecdysteroids (N=8), whereas vitellogenic ovaries (sizes E–G) contained a mean of 45 pg (N=4). This data set indicates that ovarian ecdysteroids increase at the onset of vitellogenin uptake by the oocyte.
Fig. 4.
Ovarian ecdysteroids and hemolymph ecdysteroid titer in P. micans. (A) Ecdysteroid content of the ovaries of queens, workers and transitional types. Queens, whose ovaries are shown [black areas represent yellow bodies indicating a history of ovipositions (Tyndale-Biscoe, 1984)], have higher levels of ecdysteroids than their worker nestmates (Mann–Whitney U-test: Z=4.19, P<0.001). MQ, multiple queens; SQ, single queen. The inset in A represents an expanded scale for attackees, attackers and accepted females, showing that accepted females with well-developed ovaries had higher levels of ovarian ecdysteroids than the attackers (Steel–Dwass all pairs test: Z=2.34, P=0.045) and attackees (Z=2.52, P=0.03). Ovary size ranges are indicated in parentheses. (B) A pooled analysis of ovarian ecdysteroid content for 45 queens from colonies 5–10 revealed a significant correlation with ovary size (number of oocytes >70% length of mature egg, LME) (Spearman's ρ=0.84, P<0.001); Ω indicates last queens removed from a nest. (C) Mixed model analysis of hemolymph ecdysteroid titer from four colonies (2, 6, 9 and 10) showing that queens have higher titers than workers (F1=7.39, P=0.008; t=2.72, P=0.008). Colony had no effect on the difference in JH titer. *P<0.05, **P<0.01.
Ovarian ecdysteroids were significantly correlated with ovary size in queens (Fig. 4B). Yet the ovaries of the last queen remaining in colonies 5 and 8 contained more ecdysteroids than would be expected based on the size of her ovaries alone (Fig. 4B, see Ω values). Similarly, the smaller ovaries of a presumed new queen from colony 4 contained substantially more ecdysteroids than the larger ovaries of established queens from colony 2 (Fig. 4A).
In contrast to their high ovarian ecdysteroid content, queens had higher hemolymph ecdysteroid titers than workers in only one out of two colonies with sample sizes over 10 (Mann–Whitney U-test: colony 2: N=22, Z=2.38, P=0.017; colony 6: N=27, Z=0.37, P=0.71, Zr=0.14). When all colonies (2, 6, 9 and 10) were combined, queens had higher ecdysteroid titers than workers. Yet there was no significant correlation between ovarian and hemolymph ecdysteroid titers in these queens (N=24, Spearman's ρ=0.333, P=0.112, Zr=0.37). Finally, no hemolymph ecdysteroids were detected in attackees (N=3), attackers (N=4) or an accepted female with vitellogenic ovaries from colony 4.
CHCs
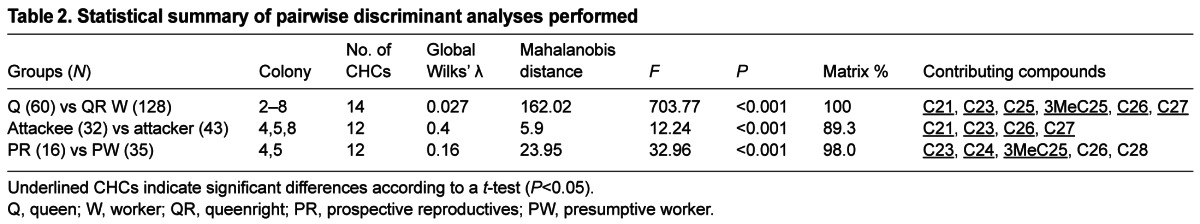
Analysis of the worker CHCs identified 14 shared unambiguous hydrocarbons (supplementary material Table S1), although others were inconsistently represented at very low levels (0.0–0.5%). Twelve of the 14 compounds were linear alkenes. The other two hydrocarbons were 3-methylpentocosane (3MeC25) and a C27 alkene (ZC27). Three pairwise comparisons were made, and the main results of the discriminant analyses are presented in Table 2.
Table 2.
Statistical summary of pairwise discriminant analyses performed
Queens, workers and newly emerged females
Queens and workers were separately pooled from all colonies from which cuticular compounds were extracted (colonies 2–8). Selected chromatograms for each phenotype are shown in Fig. 5Ai,ii. The CHC profiles of queens and workers are clearly distinct, with the C25 pentacosane and 3MeC25 significantly higher in queens (shaded in supplementary material Table S1; Fig. 5B). The defeated queen from colony 7 had an unmistakable queen-like profile, and the lone queen from colony 4 had similar levels of C25 and 3MeC25 as did the nest-sharing queens. Hence, these two CHCs are associated with caste and not queen numbers or reproductive dominance.
Fig. 5.
Cuticular hydrocarbon (CHC) profiles of P. micans. (Ai–iii) Chromatogram of P. micans CHCs extracted from a queen (Ai), a worker (Aii) and a newly emerged female (Aiii). For compound numbering and identification, see supplementary material Table S1. (B–D) CHC profile comparison between co-existing types of females under queenright conditions (B) and queenless conditions (C,D) (means ± s.d.). Asterisks indicate the main contributors of separation (discriminant analysis) between types of females (*P<0.05; ***P<0.0001) in log-transformed compound relative proportions. Inset graphs in B–D show compounds present at lower percentages. (B) Queens versus workers from colonies 2–8. The profile of four newly emerged females is shown for comparison but was not included in the statistical analysis because of the small sample size. Compounds that were not detected in the respective female type are marked with a cross. (C) CHC profile comparison between attackees and attackers from colonies 4, 5 and 8. (D) Prospective reproductives (PR) and presumptive workers (PW) from queenless colonies 4 and 5. Queen data from the same colonies are shown for comparison but were not included in the statistical analysis. (E) Discriminant analysis for CHC composition of queenright (QR) females (Queens, Workers), queenless (QL) females 1–3 days after queen removal (Attackees, Attackers) and QL females 7 days after queen removal (Prospective reproductive, Presumptive workers) from colonies 4, 5 and 8. Dotted ellipses encompass all females from a particular group.
Four newly emerged females from colony 1, collected within 3 h of eclosion, showed a dramatically different CHC profile (Fig. 5Aiii,B), but the sample size was too small for a discriminant analysis. Nonetheless, C23 was clearly predominant at this time while the level of C27 (high in both workers and queens) was very much reduced, and only linear alkanes were detected (Fig. 5B; supplementary material Table S1).
Attackees versus attackers
In a pooled analysis of attackees and attackers from colony 4 (N=29), colony 5 (N=10) and colony 8 (N=36) 1–3 days after the final queen was removed, the discriminant analysis significantly separated individuals according to their role in the attacks (Table 2). Although there was overlap in the relative proportions of CHCs among individuals (resulting in a lower Mahalanobis distance), there was a robust matched classification (89.3%) in their respective predicted groups (Table 2; Fig. 5D).
To evaluate whether age contributed to the CHC profile differences between the groups, we added young (cuticular scores 1–3) and old (cuticular scores 4–5) workers from colony 2 (all of which had filamentous ovaries) to the discriminant analysis. The attackees separated from the attackers in the same direction as young females separated from old females (supplementary material Fig. S1), suggesting that age, at least in part, is an important factor contributing to the differences seen between attackees and attackers.
Attacks persisted for 3 days in colony 4, providing an opportunity to see how the CHC profile changed in potential reproductives (i.e. attackees) within the first 3 days following queen removal (supplementary material Fig. S2). Both C25 and 3MeC25, the queen-associated alkanes, showed a slight rise in the attackees.
Prospective reproductives versus presumptive workers in a queenless nest
Females found inside the nests on day 7 after final queen removal (colonies 4 and 5) were grouped based on ovarian state. Those females with developing or non-degenerating oocytes, from stages D–H (N=16), were considered ‘prospective reproductives’ and were separated from the ‘presumptive workers’ with A–C type ovaries (N=35). When data from colonies 4 (N=19) and 5 (N=32) were pooled, the discriminant analysis showed a significant separation based on ovarian state (Table 2). Fig. 5E shows that 3MeC25 was relatively high in both queens and the potential reproductives, but not in presumptive workers. Supplementary material Fig. S3 shows that the level of 3MeC25 was always higher in queens than in workers (five out of five colonies) and, on average, also higher in potential replacement reproductives than in queenless workers of the same nest (two out of two colonies). Finally, a discriminant analysis based on pooled data from queens, queenright workers, attackees, attackers, prospective reproductives and queenless presumptive workers from colonies 4, 5 and 8 revealed that prospective reproductives show a clear shift towards a queen-like CHC profile (Fig. 5E), confirming the pattern seen in Fig. 5D. Also, females without a queen for an extended period (7 days), irrespective of status, separated from the cluster of queenright workers, queenless attackees and queenless attackers (Fig. 5E).
DISCUSSION
This is the first study providing a comprehensive picture of the reproductive status, endocrine physiology and chemical signaling of individuals in colonies of a swarm-founding wasp. In contrast to the independent-founding paper wasps (Röseler et al., 1985), in P. micans ecdysteroids were found primarily in vitellogenic ovaries of queens and were not consistently detected in the hemolymph, and JH titer was not correlated with reproductive status. Rather, JH titer was relatively low in queens, even during periods of intense competition, and showed a rise only once all direct competing queens were eliminated (i.e. monogynic colonies), whether by experimental removal (Fig. 2A), or likely as an outcome of queen–queen competition (Fig. 2B). Nonetheless, JH titer increased in potential reproductives in queenless nests of both P. micans (Fig. 3) and Polistes dominula (Tibbetts and Huang, 2010), suggesting a conserved, context-dependent role for JH in caste determination. Yet the two species show distinct differences in terms of queen selection among potential reproductives (see below). Also, the decline in hemolymph JH titer among prospective queens indicates that JH titer does not correlate with CHC signals linked to fertility as shown in P. dominula (Izzo et al., 2010). Thus, despite sharing a common caste-based ancestor (Pickett and Carpenter, 2010), the Polistini and the Epiponini wasps have, apparently, diverged into widely different endocrine regulatory patterns.
JH titer differences between castes are contingent on nesting phase and queen number
As JH is known to directly influence relocation behaviors in a variety of insects (Dingle and Winchell, 1997), the best data set for assessing a role of JH in social competition among queens comes from established colonies. In founding colonies of P. micans, queens had higher JH titers than workers (Fig. 2B), while virtually no difference existed between the castes in established multiple-queen nests with new combs (Fig. 2A). Both types of nest contained many empty cells, meaning reproductive competition was high (West-Eberhard, 1977). Thus, in contrast to the situation in Polistes (Röseler, 1991), JH does not appear to be important for reproductive dominance per se in P. micans, although the effect of removal of the corpora allata, the source of JH, is necessary to confirm that it has no role.
Still more surprising, single queens of monogynous P. micans colonies had much higher JH titers than workers (Fig. 2B). Unexpectedly, these single queens were observed to exhibit more ritualized aggression than queens of multiple-queen societies, suggesting that JH may, paradoxically, fuel aggressive behavior in queens that lack direct competition. Possibly, a lone queen may be pushed to the limit of her ability to restrict the ascension of other reproductives, and so the intensification of her abdomen bending may serve to supplement inhibitory or warning pheromones to suppress the development of potential rivals. Alternatively, high JH levels may augment ovarian growth, perhaps in response to a diminution of signals from other queens. Along with established single queens, a new queen from colony 4 also had a high JH titer (Fig. 3A) and her ovaries, like those of the last remaining queen from colony 5 (Fig. 4B), contained more ecdysteroids than expected based on their size (Fig. 4A). Clearly, more hormone measurements, coupled with JH application experiments, will be necessary to elucidate the possibly special function of JH in lone queens.
The enigmatic rise and fall of JH titer in potential reproductives
A prospective role for JH in social competition and/or reproductive competence was observed when young potential reproductives experienced a surge in JH following queen removal (Fig. 3). In P. dominula, prospective queen replacements had high JH titers (Tibbetts and Huang, 2010), whereas in P. micans, JH titer was only transiently elevated, declined by the third day, and thereafter remained low (Fig. 3A). The transient rise of JH in P. micans was concomitant with an opportunity for reproduction, suggesting a gonadotropic function, but this window also coincided with the reception of attacks from testing nestmates, a mechanism of queen succession different from that of other studied caste-flexible epiponine wasps where queenless young adults are accepted as new queens without a fight (West-Eberhard, 1977; West-Eberhard, 1978; West-Eberhard, 1981; Strassmann et al., 2002; Platt et al., 2004).
Whether JH rises or falls in response to a stressor is species dependent, often leading to a physiologically appropriate response. For example, in the tobacco hornworm Manduca sexta, the JH titer increases in response to starvation (Cymborowski et al., 1982) and other stressors, including microbial infection, cutaneous injury, episodic movement and temperature elevation (Tauchman et al., 2007). Stress by way of crowding in caste-totipotent workers of the termite Coptotermes formosanus causes an increase in JH levels that induces the development of soldiers whose presence lessens the density-dependent rise of JH in workers (Mao and Henderson, 2010). As for hymenopterans, honeybee workers showed a JH response to being caged and cold anesthetized (Lin et al., 2004). Although caged nurse bees experienced a dramatic upswing in JH, overall the response to the stressors was inconsistent (and sometimes reversed), leading the authors to conclude that JH is not a ‘stress’ hormone in honeybees (Lin et al., 2004).
Although increased JH titer was correlated with the onset of attacks in P. micans, levels decreased during continued attacks (Fig. 3A). If JH were a stress-response hormone in this species, it would be expected to remain high during such transitory stress. In social vertebrates, for example, stress hormones rise in challenging, unstable situations and remain high for the duration of the event (e.g. mating opportunities) before returning to normal levels (Sapolsky, 1992). We thus hypothesize that JH has retained a reproductive function in P. micans by priming the ovaries of potential reproductives for further development, a known physiological function of JH in numerous other insects (Wyatt and Davey, 1996).
While a spike of JH alone may not guarantee reproductive maturation of young queenless females, the contest for reproduction appears to select for the most resilient (i.e. dominant) potential reproductives, and the attacks may counter JH action in females of inferior quality. In periods of instability (e.g. queenlessness), elevated JH titers in workers of P. dominula are thought to function analogously to testosterone in vertebrates: they increase an individual's ability to compete (Tibbetts and Huang, 2010) but likely come at a cost (Tibbetts and Banan, 2010). In P. micans, JH may fuel dominance indirectly: only the most competitive females can assert themselves as future queens (e.g. by charging into the nest). At the same time, the discovery of many unmarked (i.e. probably non-attacked) females in colonies 4 and 5 with well-developed ovaries (Fig. 1C) suggests that some females may be allowed to ascend to queenhood without a fight (by exhibiting some show of dominance), as observed in other caste-flexible epiponines (West-Eberhard, 1978).
The rise of JH titer in attackees also preceded a subtle shift in their CHC profile toward that of a queen's (supplementary material Fig. S2), but the conversion was probably too slow to account for the increased attention received by the attackers. Although the production of other pheromones may occur immediately after queen removal, it may be that pre-existing cues of age and/or ovarian condition between attackees and attackers are sufficient for attackers to detect potential reproductives. The occurrence of indiscriminant attacks by workers within hours of final queen removal in colony 5 (the ‘mêlée’ group) supports the notion of an immediate behavioral transformation, followed by accurate targeting of young nestmates with partially developed ovaries. The proximate factor(s) leading to the attacks remains unresolved, but our results suggest that JH does not maintain the production of a fertility signal, as has been implicated in P. dominula (Izzo et al., 2010), marking yet another difference between P. micans and Polistes in how reproductive dominance becomes established.
Ecdysteroids and reproduction
As JH titer modulation can explain some, but not all, aspects of the physiology of incipient and established competing queens of P. micans, we also measured circulating ecdysteroids. In Polistes, hemolymph ecdysteroids have been shown to be important for establishing dominance, a function partially redundant with JH (Röseler et al., 1985; Röseler and Röseler, 1989; Strambi, 1990; Röseler, 1991). Yet in P. micans queens, ecdysteroid titer was generally low (Fig. 4C), only sometimes higher than that of workers, and failed to correlate with ovarian ecdysteroid content in queens. Moreover, hemolymph ecdysteroids were not detected in potential reproductives from queenless nests, and it is therefore unlikely that they affect cuticular pheromones of emerging reproductives [cf. houseflies (Blomquist, 2003)]. The high ecdysteroid content in ovaries of highly reproductive P. micans queens furthermore shows that it is not a lack of ecdysteroid production in the ovaries that caused the low and inconsistent hemolymph ecdysteroid titers, but a restriction of the release of these hormones into the hemolymph. This is different from P. dominula (Röseler et al., 1985) and the bumble bee Bombus terrestris (Geva et al., 2005), where hemolymph ecdysteroid titers are correlated with social status. Thus, as has occurred with the evolution of other swarm-founding lineages of Hymenoptera (Hartfelder et al., 2002), a hormonal function for ecdysteroids has, apparently, also been lost in swarming wasps.
A frequent finding on ovarian ecdysteroid content in social insects is a correlation with the size of developing oocytes. This holds true both in primitively eusocial species, such as bumble bees (Bloch et al., 2000; Geva et al., 2005) and paper wasps (Röseler, 1985), and also in the highly eusocial honey bees (Feldlaufer et al., 1986). But such correlations are far from simple. Our findings, illustrated in Fig. 4A, point in the same direction, leading one to infer that the size of the compartment that contains early vitellogenic oocytes is more important in determining ovarian ecdysteroid content than is the overall ovary size, and is thus indicative of an enhanced ovarian ecdysteroid production. This interpretation is in accordance with findings on the spatial distribution of transcripts of the early ecdysone-response gene E74 in the ovary of honey bee queens (Paul et al., 2005) and the expression of a battery of ecdysone synthesis-related genes in the ovaries of queens and nurse bees (Yamazaki et al., 2011).
CHC profiles associate with age, fertility and caste
The overall CHC profile of P. micans is dominated by straight-chain alkanes, accounting for over 98% of the total composition in each individual sample. In contrast, the CHC profiles of workers from fellow epiponines Polybia occidentalis and Parachartergus aztecus are dominated by methyl-alkanes and alkenes, respectively (Espelie and Hermann, 1988; Singer et al., 1998). The diversity of CHC class representation among these three species indicates that cuticular pheromone evolution is quite labile in the Epiponini, at least compared with Vespine wasps (van Zweden et al., 2013). With regards to intraspecific differences, this is the first study to compare CHC blends within any epiponine wasp. Our results suggest that the CHC profiles of P. micans convey age-, fertility - and/or status-related signals (Fig. 5), but causal experiments will be required to test functionality. For each nest, the 3MeC25 and C25 alkanes were present at greater concentrations in queens than in workers, suggesting that like most social Hymenoptera (Van Oystaeyen et al., 2014), saturated hydrocarbons are important fertility signaling and/or regulatory pheromones in P. micans as well. Interestingly, C25 has been shown to inhibit ovarian development in bumble bee workers (Van Oystaeyen et al., 2014).
As might be expected, these queen-associated hydrocarbons were upregulated in queenless prospective reproductives with borderline or advanced vitellogenic oocytes (supplementary material Table S1; Fig. 5E). In all colonies, the 3MeC25 alkane was found to be proportionally several times more abundant in queens than in workers (Table 2; supplementary material Table S1), but among queens from different colonies, there was no correlation with ovary size (supplementary material Fig. S3) or with reproductive dominance, as even an attacked, descending queen with regressed ovaries had a 3MeC25 level equivalent to that of her reproductive cohorts. Moreover, neither 3MeC25 nor C25 differed between attackees with partially developed ovaries and their effectively sterile attackers. Thus, in P. micans, these queen-associated compounds are more closely associated with caste than with fertility per se (see van Zweden et al., 2013). Curiously, the CHC profile of Ropalidia opifex, a southeast Asian wasp which has independently evolved a swarming lifestyle, showed no correlation with fertility (Dapporto et al., 2006).
Shared and divergent patterns of endocrine activity in social wasps
Against the background of previous studies on hormone titer and function in wasps (Röseler, 1991; O'Donnell and Jeanne, 1993; Giray et al., 2005; Tibbetts et al., 2011b), our results on the endocrine physiology of a swarm-founding epiponine species show that the endocrinology of social wasps has undergone extensive remodeling. The low JH titer of queens and developing queens in P. micans suggests that JH is not a key driver of reproduction or competition, in stark contrast to the situation in Polistes wasps (Strambi, 1990; Röseler, 1991; Tibbetts et al., 2011b). Still, the hormone titers presented here suggest that JH may augment ovarian growth in queens with limited or no competition, and/or serve to prime the ovaries of potential reproductives for subsequent growth (in young, good quality females). Indeed, amongst all the differences between the endocrine profiles of Polistes wasps and P. micans, the prominent rise of JH in potential reproductives during episodes of social instability, with winners becoming queens, is intriguing. Future work must test JH function through hormone manipulation experiments.
Although some aspects of JH and ecdysteroid function may be conserved across social wasps, the differences are more evident. Fortunately, there are many wasp species phylogenetically intermediate to Polistes and Polybia (Noll and Wenzel, 2008; Pickett and Carpenter, 2010), making it possible to track how such contrasting endocrine patterns may have evolved. Also, swarm founding has independently evolved in two genera of Old World wasps, making it feasible to test whether specific changes (or conservation) of hormone functions in socially advanced groups are due to lineage-specific peculiarities or are indeed correlates of more complex societies. Furthermore, the finding that morphologically distinct castes have evolved several times independently in the Epiponini, ranging from subtle to very conspicuous differences (Noll and Wenzel, 2008), makes possible comparative intra-genus studies of how endocrine-mediated caste differentiation may have shifted developmentally from the imaginal to the pre-imaginal stage, and also how the roles of hormones changed in adults once castes became pre-imaginally fixed.
With regard to caste origins, the co-option of an ancestral solitary ground plan for regulating maturational behaviors and physiologies among members of caste-based insect societies is well supported (West-Eberhard, 1996; Amdam et al., 2006; Tibbetts et al., 2011a; Smith et al., 2013; Tibbetts et al., 2013), although specific hypotheses will require more studies on the appropriate solitary hymenopterans (West-Eberhard, 1987; West-Eberhard, 1996). But what accounts for the remarkable divergence in the endocrine profiles of Polistes and Polybia, along with the parallel departures of hormone function between bumble bees and highly eusocial swarm-founding bees (Hartfelder et al., 2006), remains a challenging question. It is nonetheless an emergent view that a fundamental shift in the endocrine activity of caste-based societies is not necessarily contingent on the evolution of ontogenetically defined castes.
MATERIALS AND METHODS
Field observations and age determination of queens and workers
All behavioral and physiological data were collected from P. micans nests on the campus of Universidade Federal de Sergipe (UFS), São Cristóvão, Sergipe, Brazil. All nests, except colony 6, were studied in situ (found affixed to trees and man-made structures).
The nest architecture of P. micans consists of horizontal combs that are encapsulated by a paper envelope, with an opening located at the bottom. The least intrusive way to observe activity within a nest is to find an expanding nest, as this provides a natural, temporary view of the outermost comb. The expansion phase can be perpetuated by daily removal of freshly built sections of the envelope. All non-founding colonies were in a phase of growth when observations started, including colony 6, which began to expand weeks after relocation.
Empty cells on the outermost comb attracted queens from within the colony, facilitating their removal, one-by-one, without destroying the nest. The arrival of queens from the nest interior was often brief, but their presence was usually announced by the attention received from workers (e.g. the queen-dance). Queens were removed with forceps as soon as their status was clear.
Wasps were color marked with oil-based Sharpie pens according to the task they performed or their social status, and females could receive multiple marks (e.g. one female could be marked as a builder, a pulp forager and, following queen removal, an attacker).
To avoid sample variance by possible circadian endocrine changes (Zera, 2007), the vast majority of wasps were collected for processing between 13:00 h and 17:00 h. They were placed in clean glass vials on ice within 15 s of removal. Collection events for individual wasps always included multiple types of females, with the relevant pair determined by the objective of the assay (e.g. queens versus workers). Some assays required the removal of entire nests in order to access young, less active females (e.g. prospective reproductives). To facilitate the search, collected nests were agitated for 5–10 s to encourage the exit of early responders. The nest was then captured with a butterfly net, placed in a plastic bag and immediately buried in ice.
The relative age of females was estimated by scoring (blind) the degree of apodeme darkening on the 5th gastral sternite (West, 1969; Forsyth, 1978) (see Fig. 1B for the scoring system). The rate of pigmentation likely differs between queens and workers (Forsyth, 1978), and so these age classes are likely affected by overall activity levels and/or environmental factors.
Collecting hemolymph for hormone measurements, CHC wash, and ovary measurements
After 20–120 min, the cold-anesthetized wasps were fastened to a wax plate with intersecting insect pins. Hemolymph was withdrawn from between the anterior-most segments of the gaster with a 5 μl graduated microcapillary (Drummond Scientific Company, Broomall, PA, USA) pulled to a point over a flame. In total, 1–10 μl of hemolymph was taken and measured by transferring it to a non-manipulated pipet. Samples for JH measurement were transferred to 500 μl of acetonitrile in a 2 ml screw-top glass vial capped with a Teflon-lined rubber septum. Samples destined for ecdysteroid measurement were preserved in 500 μl of methanol.
Subsequent to bleeding, CHCs were extracted from females submersed in 2 ml of hexane for 2–2.2 min. Hormone and CHC samples were kept at −20°C until processing.
Ovaries were carefully removed in cold E&B (Ephrussi and Beadle, 1936) Ringer solution and photographed with a Leica EZ4D microscope camera. Oocyte length (not including the adjoining trophocyte chamber) was measured using ImageJ (NIH, Bethesda, MD, USA). Ovaries were then placed in 500 μl of methanol for ecdysteroid measurement.
The stage of ovarian development among non-queens was scored based on a pictorial index presented in supplementary material Fig. S4 (and as drawn in Fig. 1C). Queen ovaries were quantified by counting the number of oocytes that were larger than 70% LME.
Hemolymph JH titer analysis by RIA
Juvenile hormone was extracted from the hemolymph as detailed previously (Huang et al., 1994), and quantified by RIA using a JH-specific antiserum (Goodman et al., 1990), [10-3H(N)]-JH III (specific activity 19.4 Ci nmol−1, Perkin Elmer Life Sciences, Waltham, MA, USA), and JH-III (Fluka, Munich, Germany) as the non-radioactive competitor. The assay has previously been validated for JH titer analyses in Hymenoptera (Goodman et al., 1993) and is detailed elsewhere (Hartfelder et al., 2013). JH titers of the samples were calculated by non-linear four-parameter regression on standard curve values (ImmunoAssay Calculations spreadsheet, Bachem, Bubendorf, Switzerland) and are expressed as JH-III equivalents (pg μl−1 hemolymph).
Hemolymph titer and ovarian ecdysteroid content analysis by RIA
Hemolymph extracts in 500 μl of methanol were directly quantified by RIA. The extracts from ovaries kept in methanol were first passed through a SepPak-C18 cartridge (Waters, Milford, MA, USA) to remove excess lipids, as previously established for measuring ecdysteroids in bumble bee ovaries (Geva et al., 2005).
Ecdysteroids were quantified by RIA, as previously described (Feldlaufer and Hartfelder, 1997; Hartfelder et al., 2013), using an antiserum prepared against a hemisuccinate derivative of ecdysone (Bollenbacher et al., 1983; Warren and Gilbert, 1986), [23,24-3H(N)]ecdysone (Perkin Elmer; NEN, specific activity 102 Ci mmol−1). Standard curves were established using 20-hydroxyecdysone (20E; Sigma) as a non-radioactive ligand. Accordingly, results are expressed as 20E equivalents, calculated by the same four-parameter regression algorithm used for JH titer (see above). Results are expressed as pg μl−1 for the hemolymph samples, or as pg ovary−1 for the ovary samples.
CHC analysis
After the hexane solvent was evaporated under a fume-hood, the apolar extract was suspended in 50 μl of hexane; 1 μl of this was injected into a combined gas chromatography-mass spectrometer (GC/MS; model QP2010, Shimadzu, Kyoto, Japan). Separation was achieved on a 30 m DB-5MS column, with a helium gas carrier at 1.0 ml min−1. Oven temperature was initially set to 150°C, and ramped up 3°C min−1 until it reached 280°C and held for 20 min. Analyses were performed in the splitless mode. The mass spectra were obtained by 70 eV ionization. The chromatographs were analyzed with GC/MS solutions software (Shimadzu). CHCs were identified using synthetic standards (linear alkanes) and/or by their molecular diagnostics ions.
Statistical analyses
For hormone titer data, a Shapiro-Wilk W-test was applied prior to a one-way ANOVA test, followed by a Tukey HSD post hoc test. When the requirement for homogeneity of variance was not fulfilled, a corresponding non-parametric test, the Kruskal–Wallis test, was used, followed by a Dunn's post hoc test. For comparisons between more than two groups, each pair was tested using the Steel–Dwass method, a non-parametric version of the all pairs, Tukey HSD. Within-nest statistics were calculated with JMP 10 (SAS Institute, Cary, NC, USA). When comparing JH titers between female types across colonies, we used SPSS 21 (IBM, Armonk, NY, USA) to construct a linear mixed model (restricted maximum likelihood method), designating status as a fixed factor and colony as a random one. In cases where no relationship was found between two variables, a power analysis was employed and reported as Fischer's refined Z (Zr) using Statistica 12.0 (StatSoft, Tulsa, OK, USA).
To avoid errors in the compositional sample data for the CHC analysis, the area under each hydrocarbon peak was transformed according to the following formula: Z=ln[Ap/g(Ap)], where Ap is the area under the peak, g(Ap) is the geometric mean for each individual compound group and Z is the transformed peak area (Aitchison, 1986). The proportions of compounds were compared for each wasp group using the t-test. In the discriminant analyses, only compounds that were shared among colony members were entered, and the relative concentrations of these compounds were readjusted to 100%. Following the discriminant analysis, a stepwise discriminant function analysis was performed to see whether and which combinations of variables could be useful for group predictions. Wilks' λ-values were used to verify the individual contribution of each variable to the model. The statistical analyses were performed using the software Statistica 10.0.
Supplementary Material
ACKNOWLEDGEMENTS
This work was made possible by the generosity of the Laboratório de Entomologia at the Universidade Federal de Sergipe (UFS). Biologists Yana T. Reis, Leandro S. Souto, José O. Dantas, Bianca Ambrogi, Ana Paula Marques Costa and others provided lab space, equipment and continuous support with the procurement of materials required for this research. Michel Precisão and Adriano Aquino from the UFS Laboratório de Análise de Compostos Orgânicos Poluentes supplied the solvents. For assistance in the field, we thank Eduardo Nascimento, Beryl Jones and Lucas Oliveira for their tireless work. Finally, Isabel Cristina Turatti helped with the processing of CHC samples at the Universidade de São Paulo.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This project was approved by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [010157/2009-3] to H. K. and F.S.N., and funded by a Sargent Award from the Department of Biology, University of Washington to H.K., Janelia Farm, Howard Hughes Medical Institute to H.K. and L.M.R., CNPq [300311/2010-9] to K.H., and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [Proc. 2010/10027-5] to F.S.N. Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.096750/-/DC1
References
- Agrahari M., Gadagkar R. (2003). Juvenile hormone accelerates ovarian development and does not affect age polyethism in the primitively eusocial wasp, Ropalidia marginata. J. Insect Physiol. 49, 217-222. [DOI] [PubMed] [Google Scholar]
- Aitchison J. (1986). The Statistical Analysis of Compositional Data. London: Chapman and Hall. [Google Scholar]
- Amdam G. V., Csondes A., Fondrk M. K., Page R. E., Jr (2006). Complex social behaviour derived from maternal reproductive traits. Nature 439, 76-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch G., Hefetz A., Hartfelder K. (2000). Ecdysteroid titer, ovary status, and dominance in adult worker and queen bumble bees (Bombus terrestris). J. Insect Physiol. 46, 1033-1040. [DOI] [PubMed] [Google Scholar]
- Bloch G., Wheeler D., Robinson G. (2002). Endocrine influences on the organization of insect societies. In Hormones, Brains and Behavior, Vol. 3 (ed. Pfaff D., Arnold A., Etgen A., Fahrbach S., Moss R., Rubin R.), pp. 195-237. New York, NY: Academic Press. [Google Scholar]
- Blomquist G. J. (2003). Biosynthesis anmd ecdysteroid regulation of housefly sex pheromone production. In Insect Pheromone Biochemistry and Molecular Biology (ed. Blomquist G. J., Vogt R. G.), pp. 231-252. San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Bollenbacher W. E., O'Brien M. A., Katahira E. J., Gilbert L. I. (1983). A kinetic analysis of the action of the insect prothoracicotropic hormone. Mol. Cell. Endocrinol. 32, 27-46. [DOI] [PubMed] [Google Scholar]
- Brent C., Peeters C., Dietemann V., Crewe R., Vargo E. (2006). Hormonal correlates of reproductive status in the queenless ponerine ant, Streblognathus peetersi. J. Comp. Physiol. A 192, 315-320. [DOI] [PubMed] [Google Scholar]
- Chavarría-Pizarro L., West-Eberhard M. J. (2010). The behavior and natural history of Chartergellus, a little-known genus of neotropical social wasps (Vespidae Polistinae Epiponini). Ethol. Ecol. Evol. 22, 317-343. [Google Scholar]
- Cuvillier-Hot V., Lenoir A., Peeters C. (2004). Reproductive monopoly enforced by sterile police workers in a queenless ant. Behav. Ecol. 15, 970-975. [Google Scholar]
- Cymborowski B., Bogus M., Beckage N. E., Williams C. M., Riddiford L. M. (1982). Juvenile-hormone titers and metabolism during starvation-induced supernumerary larval molting of the tobacco hornworm, Manduca-sexta L. J. Insect Physiol. 28, 129-135. [Google Scholar]
- Dapporto L., Fondelli L., Turillazzi S. (2006). Nestmate recognition and identification of cuticular hydrocarbons composition in the swarm founding paper wasp Ropalidia opifex. Biochem. Syst. Ecol. 34, 617-625. [Google Scholar]
- Dingle H., Winchell R. (1997). Juvenile hormone as a mediator of plasticity in insect life histories. Arch. Insect Biochem. Physiol. 35, 359-373. [Google Scholar]
- Dolezal A. G., Brent C. S., Hölldobler B., Amdam G. V. (2012). Worker division of labor and endocrine physiology are associated in the harvester ant, Pogonomyrmex californicus. J. Exp. Biol. 215, 454-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi B., Beadle G. W. (1936). A technique of transplantation for Drosophila. Am. Nat. 70, 218-225. [Google Scholar]
- Espelie K. E., Hermann H. R. (1988). Congruent cuticular hydrocarbons: biochemical convergence of a social wasp, an ant and a host plant. Biochem. Syst. Ecol. 16, 505-508. [Google Scholar]
- Feldlaufer M. F., Hartfelder K. (1997). Relationship of the neutral aterols and ecdysteroids of the parasitic mite, Varroa jacobsoni to those of the honey bee, Apis mellifera. J. Insect Physiol. 43, 541-545. [DOI] [PubMed] [Google Scholar]
- Feldlaufer M., Svoboda J., Herbert E. W., Jr (1986). Makisterone A and 24-methylenecholesterol from the ovaries of the honey bee, Apis mellifera L. Experientia 42, 200-201. [Google Scholar]
- Forsyth A. B. (1978). Studies on the behavioral ecology of polygynous social wasps. PhD thesis, Harvard University, Cambridge, MA, USA. [Google Scholar]
- Geva S., Hartfelder K., Bloch G. (2005). Reproductive division of labor, dominance, and ecdysteroid levels in hemolymph and ovary of the bumble bee Bombus terrestris. J. Insect Physiol. 51, 811-823. [DOI] [PubMed] [Google Scholar]
- Giray T., Giovanetti M., West-Eberhard M. J. (2005). Juvenile hormone, reproduction, and worker behavior in the neotropical social wasp Polistes canadensis. Proc. Natl. Acad. Sci. USA 102, 3330-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman W. G., Coy D. C., Baker F. C., Xu L., Toong Y. C. (1990). Development and application of a radioimmunoassay for the juvenile hormones. Insect Biochem. 20, 357-364. [Google Scholar]
- Goodman W. G., Huang Z. H., Robinson G. E., Strambi C., Strambi A. (1993). Comparison of two juvenile hormone radioimmunoassays. Arch. Insect Biochem. Physiol. 23, 147-152. [DOI] [PubMed] [Google Scholar]
- Hartfelder K., Emlen D. (2012). Endocrine control of insect polyphenism. In Insect Endocrinology (ed. Gilbert L. I.), pp. 464-522. San Diego, CA: Academic Press. [Google Scholar]
- Hartfelder K., Bitondi M. M. G., Santana W. C., Simões Z. L. P. (2002). Ecdysteroid titer and reproduction in queens and workers of the honey bee and of a stingless bee: loss of ecdysteroid function at increasing levels of sociality? Insect Biochem. Mol. Biol. 32, 211-216. [DOI] [PubMed] [Google Scholar]
- Hartfelder K., Makert G. R., Judice C. C., Pereira G. A. G., Santana W. C., Dallacqua R., Bitondi M. M. G. (2006). Physiological and genetic mechanisms underlying caste development, reproduction and division of labor in stingless bees. Apidologie (Celle) 37, 144-163. [Google Scholar]
- Hartfelder K., Bitondi M., Brent C., Guidugli-Lazzarini K., Simões Z., Stabentheiner A., Tanaka E., Wang Y. (2013). Standard methods for physiology and biochemistry research in Apis mellifera. J. Apic. Res. 52, 1-47. [Google Scholar]
- Huang Z. Y., Robinson G. E., Borst D. W. (1994). Physiological correlates of division of labor among similarly aged honey bees. J. Comp. Physiol. A 174, 731-739. [DOI] [PubMed] [Google Scholar]
- Hughes C., Queller D., Strassmann J., Solis C., Negron J., Gastreich K. (1993). The maintenance of high genetic relatedness in multi-queen colonies of social wasps. In Queen Number and Sociality in Insects (ed. Keller L.), pp. 153-170. Oxford: Oxford University Press. [Google Scholar]
- Hunt J. H. (2006). Evolution of castes in Polistes. Ann. Zool. Fennici 43, 407-422. [Google Scholar]
- Hunt J. H., Kensinger B. J., Kossuth J. A., Henshaw M. T., Norberg K., Wolschin F., Amdam G. V. (2007). A diapause pathway underlies the gyne phenotype in Polistes wasps, revealing an evolutionary route to caste-containing insect societies. Proc. Natl. Acad. Sci. USA 104, 14020-14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A., Wells M., Huang Z., Tibbetts E. (2010). Cuticular hydrocarbons correlate with fertility, not dominance, in a paper wasp, Polistes dominulus. Behav. Ecol. Sociobiol. 64, 857-864. [Google Scholar]
- Jeanne R. L. (1991). Polyethism. In The Social Biology of Wasps (ed. Ross K., Matthews R.), pp. 389-425. Ithaca, NY: Comstock Publishing Associates. [Google Scholar]
- Lengyel F., Westerlund S. A., Kaib M. (2007). Juvenile hormone III influences task-specific cuticular hydrocarbon profile changes in the ant Myrmicaria eumenoides. J. Chem. Ecol. 33, 167-181. [DOI] [PubMed] [Google Scholar]
- Lin H. R., Dusset C., Huang Z. Y. (2004). Short-term changes in juvenile hormone titers in honey bee workers due to stress. Apidologie (Celle) 35, 319-327. [Google Scholar]
- Mao L., Henderson G. (2010). Group size effect on worker juvenile hormone titers and soldier differentiation in Formosan subterranean termite. J. Insect Physiol. 56, 725-730. [DOI] [PubMed] [Google Scholar]
- Nijhout H. (1994). Insect Hormones. Princeton, NJ: Princeton University Press. [Google Scholar]
- Noll F. B., Wenzel J. W. (2008). Caste in the swarming wasps: ‘queenless’ societies in highly social insects. Biol. J. Linn. Soc. Lond. 93, 509-522. [Google Scholar]
- O'Donnell S., Jeanne R. L. (1993). Methoprene accelerates age polyethism in workers of a social wasp (Polybia occidentalis). Physiol. Entomol. 18, 189-194. [Google Scholar]
- Paul R. K., Takeuchi H., Matsuo Y., Kubo T. (2005). Gene expression of ecdysteroid-regulated gene E74 of the honeybee in ovary and brain. Insect Mol. Biol. 14, 9-15. [DOI] [PubMed] [Google Scholar]
- Penick C. A., Liebig J. (2012). Regulation of queen development through worker aggression in a predatory ant. Behav. Ecol. 23, 992-998. [Google Scholar]
- Penick C. A., Liebig J., Brent C. S. (2011). Reproduction, dominance, and caste: endocrine profiles of queens and workers of the ant Harpegnathos saltator. J. Comp. Physiol. A 197, 1063-1071. [DOI] [PubMed] [Google Scholar]
- Pickett K. M., Carpenter J. M. (2010). Simultaneous analysis and the origin of eusociality in the Vespidae (Insecta: Hymenoptera). Arthropod Syst. Phylogeny 68, 3-33. [Google Scholar]
- Platt T. G., Queller D. C., Strassmann J. E. (2004). Aggression and worker control of caste fate in a multiple-queen wasp, Parachartergus colobopterus. Anim. Behav. 67, 1-10. [Google Scholar]
- Raikhel A., Brown M., Bellés X. (2005). Hormonal control of reproductive processes. In Comprehensive Molecular Insect Science, Vol. 3 (ed. Gilbert L., Iatrou K., Gill S.), pp. 433-491. Oxford: Elsevier. [Google Scholar]
- Ramamurty P. S., Engels W. (1977). Allatectomy and juvenile hormone effects on synthesis and incorporation of vitellogenin in honeybee queen (Apis mellifera). Zool. Jahrb. Physiol. 81, 165-176. [Google Scholar]
- Richards O. (1978). The Social Wasps of the Americas, Excluding the Vespinae. London: British Museum of Natural History. [Google Scholar]
- Robinson E. J. H. (2009). Physiology as a caste-defining feature. Insectes Soc. 56, 1-6. [Google Scholar]
- Robinson G. E., Vargo E. L. (1997). Juvenile hormone in adult eusocial Hymenoptera: gonadotropin and behavioral pacemaker. Arch. Insect Biochem. Physiol. 35, 559-583. [DOI] [PubMed] [Google Scholar]
- Röseler P. (1985). Endocrine basis of dominance and reproduction in polistine paper wasps. Fortschr. Zool. 31, 259-272. [Google Scholar]
- Röseler P. F. (1991). Reproductive competition during colony establishment. In The Social Biology of Wasps (ed. Ross K., Matthews R.), pp. 309-335. Ithaca, NY: Cornell University Press. [Google Scholar]
- Röseler P. F., Röseler I. (1989). Dominance of ovariectomized foundresses of the paper wasp, Polistes gallicus. Insectes Soc. 36, 219-234. [Google Scholar]
- Röseler P. F., Röseler I., Strambi A., Augier R. (1984). Influence of insect hormones on the establishment of dominance hierarchies among foundresses of the paper wasp, Polistes gallicus. Behav. Ecol. Sociobiol. 15, 133-142. [Google Scholar]
- Röseler P. F., Röseler I., Strambi A. (1985). Role of ovaries and ecdysteroids in dominance hierarchy establishment among foundresses of the primitively social wasp, Polistes gallicus. Behav. Ecol. Sociobiol. 18, 9-13. [Google Scholar]
- Sapolsky R. (1992). Endocrinology of the stress-response. In Behavioral Endocrinology (ed. Becker J. B., Breedlove S. M., Crews D.), pp. 409-450. Cambridge, MA: MIT Press. [Google Scholar]
- Shorter J. R., Tibbetts E. A. (2009). The effect of juvenile hormone on temporal polyethism in the paper wasp Polistes dominulus. Insectes Soc. 56, 7-13. [Google Scholar]
- Shukla S., Chandran S., Gadagkar R. (2013). Ovarian developmental variation in the primitively eusocial wasp Ropalidia marginata suggests a gateway to worker ontogeny and the evolution of sociality. J. Exp. Biol. 216, 181-187. [DOI] [PubMed] [Google Scholar]
- Singer T., Espelie K., Gamboa G. (1998). Nest and nestmate discrimination in independent-founding paper wasps. In Pheromone Communication in Social Insects (ed. Vander Meer R., Breed M., Winston M., Espelie K.), pp. 104-125. Boulder, CO: Westview Press. [Google Scholar]
- Smith A. R., Kapheim K. M., Pérez-Ortega B., Brent C. S., Wcislo W. T. (2013). Juvenile hormone levels reflect social opportunities in the facultatively eusocial sweat bee Megalopta genalis (Hymenoptera: Halictidae). Horm. Behav. 63, 1-4. [DOI] [PubMed] [Google Scholar]
- Sommer K., Hölldobler B., Rembold H. (1993). Behavioral and physiological-aspects of reproductive control in a Diacamma species from Malaysia (Formicidae, Ponerinae). Ethology 94, 162-170. [Google Scholar]
- Strambi A. (1990). Physiology and reproduction in social wasps. In Social Insects – An Evolutionary Approach to Castes and Reproduction (ed. Engels W.), pp. 59-75. Heidelberg: Springer. [Google Scholar]
- Strassmann J. E., Sullender B. W., Queller D. C. (2002). Caste totipotency and conflict in a large-colony social insect. Proc. Biol. Sci. 269, 263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayanan S., Hermanson J. C., Jeanne R. L. (2011). A mechanical signal biases caste development in a social wasp. Curr. Biol. 21, 231-235. [DOI] [PubMed] [Google Scholar]
- Tauchman S. J., Lorch J. M., Orth A. P., Goodman W. G. (2007). Effects of stress on the hemolymph juvenile hormone binding protein titers of Manduca sexta. Insect Biochem. Mol. Biol. 37, 847-854. [DOI] [PubMed] [Google Scholar]
- Tibbetts E. A., Banan M. (2010). Advertised quality, caste and food availability influence the survival cost of juvenile hormone in paper wasps. Proc. R. Soc. B 277, 3461-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts E. A., Huang Z. Y. (2010). The challenge hypothesis in an insect: juvenile hormone increases during reproductive conflict following queen loss in Polistes wasps. Am. Nat. 176, 123-130. [DOI] [PubMed] [Google Scholar]
- Tibbetts E. A., Izzo A. S. (2009). Endocrine mediated phenotypic plasticity: condition-dependent effects of juvenile hormone on dominance and fertility of wasp queens. Horm. Behav. 56, 527-531. [DOI] [PubMed] [Google Scholar]
- Tibbetts E. A., Levy S., Donajkowski K. (2011a). Reproductive plasticity in Polistes paper wasp workers and the evolutionary origins of sociality. J. Insect Physiol. 57, 995-999. [DOI] [PubMed] [Google Scholar]
- Tibbetts E. A., Izzo A., Huang Z. Y. (2011b). Behavioral and physiological factors associated with juvenile hormone in Polistes wasp foundresses. Behav. Ecol. Sociobiol. 65, 1123-1131. [Google Scholar]
- Tibbetts E. A., Mettler A., Donajkowski K. (2013). Nutrition-dependent fertility response to juvenile hormone in non-social Euodynerus foraminatus wasps and the evolutionary origin of sociality. J. Insect Physiol. 59, 339-344. [DOI] [PubMed] [Google Scholar]
- Tyndale-Biscoe M. (1984). Age-grading methods in adult insects – a review. Bull. Entomol. Res. 74, 341-377. [Google Scholar]
- Van Oystaeyen A., Oliveira R. C., Holman L., van Zweden J. S., Romero C., Oi C. A., d'Ettorre P., Khalesi M., Billen J., Wäckers F., et al. (2014). Conserved class of queen pheromones stops social insect workers from reproducing. Science 343, 287-290. [DOI] [PubMed] [Google Scholar]
- van Zweden J. S., Bonckaert W., Wenseleers T., d'Ettorre P. (2013). Queen signaling in social wasps. Evolution 68, 976-986. [DOI] [PubMed] [Google Scholar]
- Warren J. T., Gilbert L. I. (1986). Ecdysone metabolism and distribution during the pupal-adult development of Manduca sexta. Insect Biochem. 16, 65-82. [Google Scholar]
- West M. J. (1969). The social biology of polistine wasps. PhD thesis, University of Michigan, Ann Harbor, MI, USA. [Google Scholar]
- West-Eberhard M. J. (1977). The establishment of dominance of the queen in social wasp colonies. In Proceedings of the 8th Congress International Union Study Social Insects, pp. 223-227. Centre for Agricultural Publishing and Documentation: Wageningen, Netherlands. [Google Scholar]
- West-Eberhard M. J. (1978). Temporary queens in Metapolybia wasps: nonreproductive helpers without altruism? Science 200, 441-443. [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J. (1981). Intragroup selection and the evolution of insect societies. In Natural Selection and Social Behavior (ed. Alexander R. D., Tinkle D. W.), pp. 3-17. New York, NY: Chiron Press. [Google Scholar]
- West-Eberhard M. J. (1987). Flexible strategy and social evolution. In Animal Societies: Theories and Facts (ed. Ito Y., Brown L., Kikkawa L.), pp. 35-51. Tokyo, Japan: Japan Scientific Societies Press. [Google Scholar]
- West-Eberhard M. J. (1996). Wasp societies as microcosms for the study of development and evolution. In Natural History and Evolution of Paper Wasps (ed. Turillazzi S., West-Eberhard M. J.), pp. 290-317. London: Oxford University Press. [Google Scholar]
- Wilson E. (1971). The Insect Societies. Cambridge, MA: Harvard University Press. [Google Scholar]
- Wyatt G. R., Davey K. G. (1996). Cellular and molecular actions of juvenile hormone. 2. Roles of juvenile hormone in adult insects. Adv. In Insect Phys. 26, 1-155. [Google Scholar]
- Yamazaki Y., Kiuchi M., Takeuchi H., Kubo T. (2011). Ecdysteroid biosynthesis in workers of the European honeybee Apis mellifera L. Insect Biochem. Mol. Biol. 41, 283-293. [DOI] [PubMed] [Google Scholar]
- Zera A. J. (2007). Endocrine analysis in evolutionary-developmental studies of insect polymorphism: hormone manipulation versus direct measurement of hormonal regulators. Evol. Dev. 9, 499-513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.