Abstract
Total joint replacement (TJR) is a very cost-effective surgery for end-stage arthritis. One important goal is to decrease the revision rate especially because TJR has been extended to younger patients. Continuous production of ultra-high molecular weight polyethylene (UHMWPE) wear particles induces macrophage infiltration and chronic inflammation, which can lead to peri-prosthetic osteolysis. Targeting individual pro-inflammatory cytokines directly has not reversed the osteolytic process in clinical trials, due to compensatory upregulation of other pro-inflammatory factors. We hypothesized that targeting the important transcription factor NF-κB could mitigate the inflammatory response to wear particles, potentially diminishing osteolysis. In the current study, we suppressed NF-κB activity in mouse RAW264.7 and human THP1 macrophage cell lines, as well as primary mouse and human macrophages, via competitive binding with double strand decoy oligodeoxynucleotide (ODN) containing an NF-κB binding element. We found that macrophage exposure to UHMWPE particles induced multiple pro-inflammatory cytokine and chemokine expression including TNF-α, MCP1, MIP1α and others. Importantly, the decoy ODN significantly suppressed the induced cytokine and chemokine expression in both murine and human macrophages, and resulted in suppression of macrophage recruitment. The strategic use of decoy NF-κB ODN, delivered locally, could potentially diminish particle-induced peri-prosthetic osteolysis.
Keywords: wear particles, macrophage, NF-κB, decoy oligodeoxynucleotide, periprosthetic osteolysis
1. Introduction
Total joint replacement (TJR) is a cost-effective surgical procedure for end-stage arthritis. As TJR has been extended to younger more active patients, one important goal is to decrease the surgical revision rate due to wear of the bearing surfaces. The generation of wear particles from the bearing surfaces of implanted biomaterials induces a chronic inflammatory response, which may eventually lead to periprosthetic osteolysis. [1, 2]
Ultra-high molecular weight polyethylene (UHMWPE) still remains the most commonly used biomaterial in the bearing surface of TJR. UHMWPE has wear rate as high as 0.4 mm/year [3]; UHMWPE particles activate macrophages and secrete pro-inflammatory cytokines including TNF-α, IL-1β, MCP1 and others [4]. Although blocking individual cytokines showed promising effects in alleviating wear particle-induced osteolysis in vitro and in animal studies, a clinical study in humans indicated that blocking TNF-α by neutralizing antibody did not mitigate osteolysis [5]. This could be explained by the compensatory upregulation of other pro-inflammatory factors. Since wear particles may induce the expression of multiple pro-inflammatory factors, targeting their upstream signaling mechanisms could be an effective therapeutic strategy [6, 7].
Wear particles can be recognized by toll-like receptors on macrophages, which can activate NF-κB signaling and upregulate the downstream target gene expression for many chemokines and cytokines [8, 9]. The cytokines are then recognized by cell receptors which further activate the NF-κB signal intensity as positive feedback regulation. The complexity of the NF-κB-cytokine network makes it difficult to modulate NF-κB activities far upstream. Because of its central role in chronic inflammation, and regulation of the function of macrophage-osteoclast lineage cells, direct modulation of NF-κB activity is a logical therapeutic strategy to reduce tissue damage caused by wear particles [10].
Decoy oligodeoxynucleotide (ODN) is a synthesized duplex DNA, which can suppress transcription factor activity efficiently through competitive binding with endogenous target sequence in the genome [11]. Suppression of NF-κB activity via decoy ODN could be very specific with a low occurrence of adverse effects, and has been applied to many in vivo or in vitro immune mediated disease models [12-15]. However, the potential therapeutic effects of NF-κB decoy ODN in wear particle induced peri-prosthetic osteolysis have not been evaluated.
In the current study, we examine the effects of NF-κB decoy ODN in mouse and human macrophages exposed to clinically relevant UHMWPE particles with/without endotoxin in terms of cytokine expression profiles, and macrophage recruitment. Our results indicate that NF-κB targeting therapy can mitigate the inflammatory response to wear particles, potentially diminishing osteolysis.
2. Materials and Methods
2.1 Reagents
Lipopolysaccharide (LPS) was purchased from Sigma (Sigma-Aldrich St. Louis, MO). The Cationic polymer (C32-122) was generated and used for transfection as previously described [16]. Lipofectamine 2000 was purchased from Invitrogen (Life Technologies, Pleasanton, CA). Lipidoid was generated as previously described [17]. The transfection protocols for Lipofectamine 2000 and Lipoid were followed according to the instructional manual.
2.2 RAW 264.7 and THP1 Cell culture
The mouse macrophage cell line RAW 264.7 cells (Cat. TIB-71, ATCC, Manassas, VA) were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and an antibiotic/antimycotic solution (100 units of penicillin, 100μg of streptomycin, and 0.25 μg of Amphotericin B per ml; Hyclone, Thermo Scientific). Cells from the human macrophage cell line THP1 (ATCC, Manassas, VA) were grown in RPMI-1640 medium with 10% heat inactivated FBS, 50nM 2-mercaptoethanol, and the antibiotic/antimycotic solution. THP1 cells were differentiated by plating at a density of 1.5 × 105/cm2, and treated with 50 nM Phorbol-12-myristate-13-acetate (PMA, Sigma Aldrich, St. Louis, MO) for 3 days.
2.3 Generation of NF-κB luciferase reporter clone in RAW264.7
The reporter plasmid pGL4.32[luc2/NF-κB-RE/Hygro] Vector was purchased from Promega (Promega, Madison, WI). The plasmid was transfected into RAW264.7 cells with jetPEI®-Macrophage (Polyplus Transfection, France), and grown in culture medium containing 150 ng/ml hygromycin (Life Technologies, Pleasanton, CA). The luciferase activity was measured by mixing the samples with D-luciferin and read by a Luminometer (Turner Biosystem, Sunnyvale, CA) or IVIS-200 (Perkin Elmer, Santa Clara, CA).
2.4 Isolation of mouse bone marrow derived macrophage
Bone marrow was collected from the femora of C57BL6/J male mice 10 to 12 weeks of age (Jackson Laboratory). The animal protocol was approved by our institutional ethics committee. Institutional guidelines for the care and use of laboratory animals were observed in all aspects of this project. The mice were euthanized with carbon dioxide (CO2) gas, and sterilized by 70% ethanol before harvesting. The femora were surgically removed while maintaining sterile technique. Using a syringe and 25-gauge needle, the bone marrow was flushed by injecting 4 mL of culture medium (RPMI1640 medium supplemented with 10% heat inactivated FBS, and the antibiotic/antimycotic solution) through the marrow cavity into a 150 cm2 dish containing culture medium. The cells were carefully suspended and passed through a 70μm strainer, spun down, washed 3 times with culture medium, re-suspended in the culture medium containing 30% of L929 cells conditioned medium and 10 ng/ml mouse macrophage colony stimulation factor (M-CSF), and re-plated in T-175 culture flasks at a concentration of 4×107 cells per flask. Cells were allowed to expand for 5-7 days, with a medium change at the second day to remove non-adherent cells. The cells were analyzed using surface marker expression (F4/80 & CD11 b) after day 7.
2.5 Decoy oligodeoxynucleotide
The NF-κB decoy sequences used are 5′-CCTTGAAGGGATTTCCCTCC- 3′ and 3′-GGAACTTCCCTAA-AGGGAGG-5′. Scrambled decoy ODN has the following sequences 5′-TTGCCGTA-CCTGACTTAGCC-3′ and 3′-AACGGCATGGACTGAATCGG-3′ [15]. The synthetic ODNs were a kind gift from Dr. Egashira of Kyushu University, Japan. The ODNs were washed with 70% ethanol, dried, and dissolved in sterile Tris-EDTA (10 mM Tris, 1 mM EDTA), and the supernatant was purified over a NAP 10 column. For the treatment, macrophages were treated with 0.5μM ODN for 16 h, and then placed into plates coated with or without UHMWPE. The decoy ODN or scrambled ODN treatment were continually administered during the experiments.
2.6 Uhmwpe
Conventional UHMWPE particles were a gift from Dr Timothy Wright (Hospital for Special Surgery, New York) and obtained from knee joint simulator tests and isolated according to an established protocol [18]. Frozen aliquots of the particles containing serum were lyophilized for 4-7 days. The dried material was digested in 5 M sodium hydroxide at 60 °C for 1h, and ultrasonicated for 10 min. The digested particle suspension was centrifuged through a 5% sucrose gradient at 40 K rpm at 10 °C for 3 h. The collected particles at the surface of the sucrose solution were incubated at 80 °C for 1 h and centrifuged again through an isopropanol gradient (0.96 and 0.90 g/cm3) at 40K rpm at 10 °C for 1 h. The purified particles at the interface between the two layers of isopropanol were harvested and the isopropanol was evaporated from the particle mixture then lyophilized until dry. Particles were then re-suspended in 95% ethanol which was evaporated completely. The particles tested negative for endotoxin using a Limulus Amebocyte Lysate Kit (BioWhittaker, Walkersville, MD). The mean diameter of the particles was 1.0 ± 0.1 μm (mean ± SE, averaged from 125 scanned particles) measured by electron microscopy. The measurement was done by Dr. Lydia-Marie Joubert in the Cell Science Image Facility at Stanford University.
2.7 RNA extraction and quantitative PCR
Cellular RNAs were extracted by using RNeasy RNA purification kit (Qiagen, Valencia, CA). RNAs were reverse transcribed into complementary DNA (cDNA) using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Probes for 18s rRNA, TNF-α, IL-1β, IL-6, and MCP1 were purchased from Applied Biosystems. Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed in an ABI 7900HT Sequencing Detection System (Applied Biosystems), using the 18s rRNA as the internal control. The -ΔΔCt relative quantitation method was used to evaluate gene expression level.
2.8 Multiplex panel cytokine array
The Luminex assay was performed by using mouse or human multi-plex panel cytokine arrays (Life Technologies, Pleasanton, CA). The instructional manual was followed carefully. Plates were read by Luminex 100, and the results were analyzed by xPONENT® software.
2.9 Migration assay
Chemotaxis assays were modified slightly from our previous method [19] by using a ChemoTx® Disposable Chemotaxis System (NeuroProbe, Gaithersburg, MD) containing 5 μm pore. Twenty-nine μl of conditioned media from THP1 cell culture was placed in the bottom chamber and 6×104 THP-1 cells were placed in the upper chamber of the chemotaxis system. Following a 6 h incubation period, cells in the bottom chamber were transferred to a 96 well plate. The bottom chamber was washed with 30 μl of H2O and the also transferred to a 96 well plate (total volume 59μl). Cellular DNA was released by three freeze-thaw cycles. To quantify the number of migrated cells, 100μl of Picogreen dye (Life Technologies, Grand Island, NY) was added to each well and fluorescence was read at 480/520nm using a plate reader (Spectramax M2e, Molecular Devices, Sunnyvale, CA). A linear dilution of THP-1 cells served as a reference to quantify cell number.
2.10 Statistical analysis
One way ANOVA with Tukey's post-hoc test was conducted using Prism 5 (GraphPad Software, San Diego, CA). Data were reported as mean ± standard error of the mean. P<0.05 was chosen as the threshold of significance.
3. Results
3.1 Naked NF-κB decoy ODN inhibited LPS induced TNF-α production and UHMWPE induced NF-κB activation using the mouse RAW264.7 macrophage cell line
RAW 264.7 cells were used to evaluate the delivery efficiency of decoy ODN. Compared to scrambled ODN, direct treatment of 0.5 μM naked NF-κB decoy ODN was sufficient to inhibit 50% of TNF-α production induced by 1μg/ml LPS. Combining the ODN with a transfection reagent such as cationic polymer [16] or Lipofectamine 2000 was unable to inhibit TNF-α production, while ODN with Lipidoid showed similar inhibition as naked ODN (Fig.1a). In addition, cell viability testing of RAW 264.7 showed that Lipidoid decreased the percentage of viable cells by 69.9% (Fig.1b). The presence of decoy ODN decreased viable cells either with or without Lipidoid, which suggested that the ODN may suppress the survival and proliferation signals in RAW 264.7 cells. These results suggested that naked ODN is the most efficient delivery strategy for RAW 264.7 cells and has the lowest toxic effect.
Fig.1. Naked NF-κB decoy ODN suppressed LPS induced TNF-α expression in mouse macrophages.
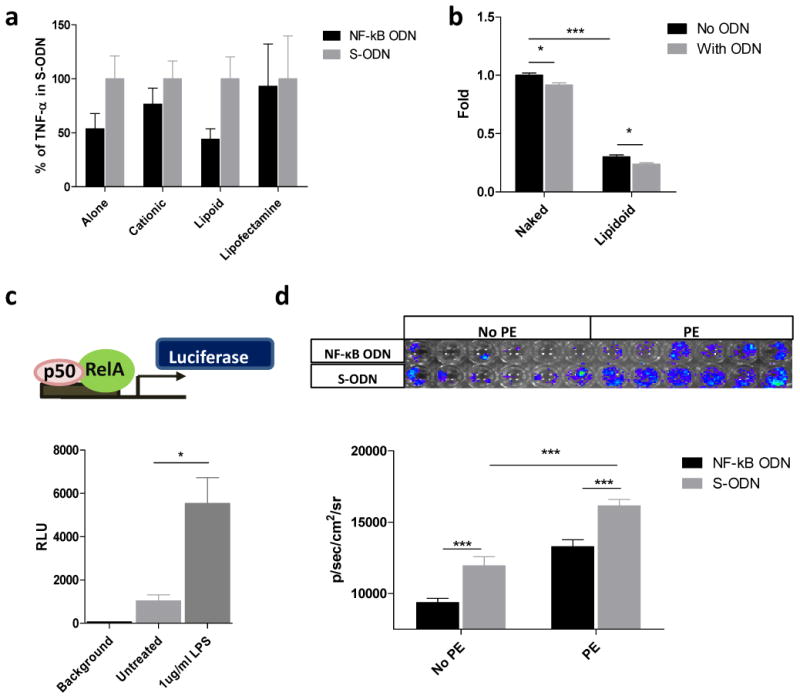
a) TNF-α production by RAW 264.7 cells exposed to 1μg/ml LPS followed by 0.5 μM ODN delivery using 3 different transducing agents: a cationic polymer (C32-122), Lipidoid, or Lipofectamine 2000 (Invitrogen). The expression of TNF-α was detected by ELISA. “Alone= no transfection agent was used. TNF-α has no difference; b) Total lactate dehydrogenase activity (an indicator of viable cell number) when cultures of RAW 264.7 cells are exposed to transducing agents with or without ODN. Total lactate dehydrogenase activity and therefore live cell viability is decreased when the ODN is delivered using a transduction agent; c) upper panel, illustration of reporter luciferase gene driven by NF-κB response elements; lower panel, effect of LPS (1 μg/ml) on the RAW264.7 NF-κB luciferase reporter cell clone; d) upper panel, RAW264.7 NF-κB luciferase reporter cells in response to PE particles with decoy ODN (NF-κB ODN) or scrambled ODN (S-ODN) for 24h, the result was read by IVIS imaging system; lower panel, quantification result of luciferase activity. *p< .05, ** p< .01, *** p<.005
The Luciferase reporter plasmid driven by the NF-κB response element was transfected into RAW264.7 cells to generate a stable cell line (Fig.1c, upper panel). The luciferase expression level increased 6-fold in response to NF-κB activation by 1 μg/ml LPS treatment (Fig.1c, lower panel). The NF-κB activity was increased by 50% in response to UHMWPE, whereas the decoy ODN decreased the particle induced activation when compared with scrambled ODN (Fig.1d) Together, the results confirmed that the particles could induce NF-κB activation in macrophages, which can be mitigated by decoy ODN.
3.2 Decoy ODN inhibited UHMWPE and LPS induced cytokine production in murine bone marrow derived primary macrophages
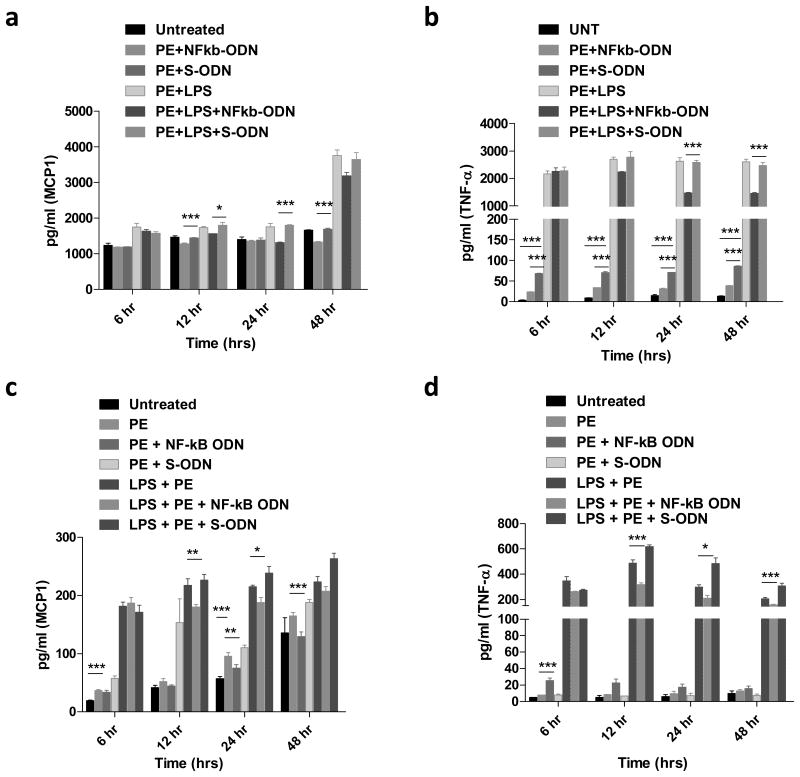
Murine bone marrow derived macrophages were isolated and characterized for their cell surface marker expression (Fig.2a). Cytokine expression profiles in macrophages including MCP1, TNF-α, IL-1β, and IL-6 were first analyzed by quantitative PCR for their significant roles in wear particle-induced periprosthetic osteolysis [20-22] Macrophages exposed to UHmWPE particles induced TNF-α (3.8 fold, peak at 24h), IL-1β (67.9 fold, peak at 48h), but not IL-6 or MCP1 expression. Combined exposure of macrophage with UHMWPE plus LPS induced TNF-α (7.3 fold, peak at 12h), MCP1 (3.5 fold, peak at 48h), IL-1β (6489 fold, peak at 48h), and IL-6 (607 fold, peak at 24h) expression, respectively (Fig.2b-e). Alternatively, decoy ODN significantly inhibited cytokine expression when compared with scrambled ODN or no ODN control group.
Fig.2. NF-κB decoy ODN suppressed pro-inflammatory cytokines mRNA expression in primary mouse macrophages exposed to UHMWPE and/or LPS.
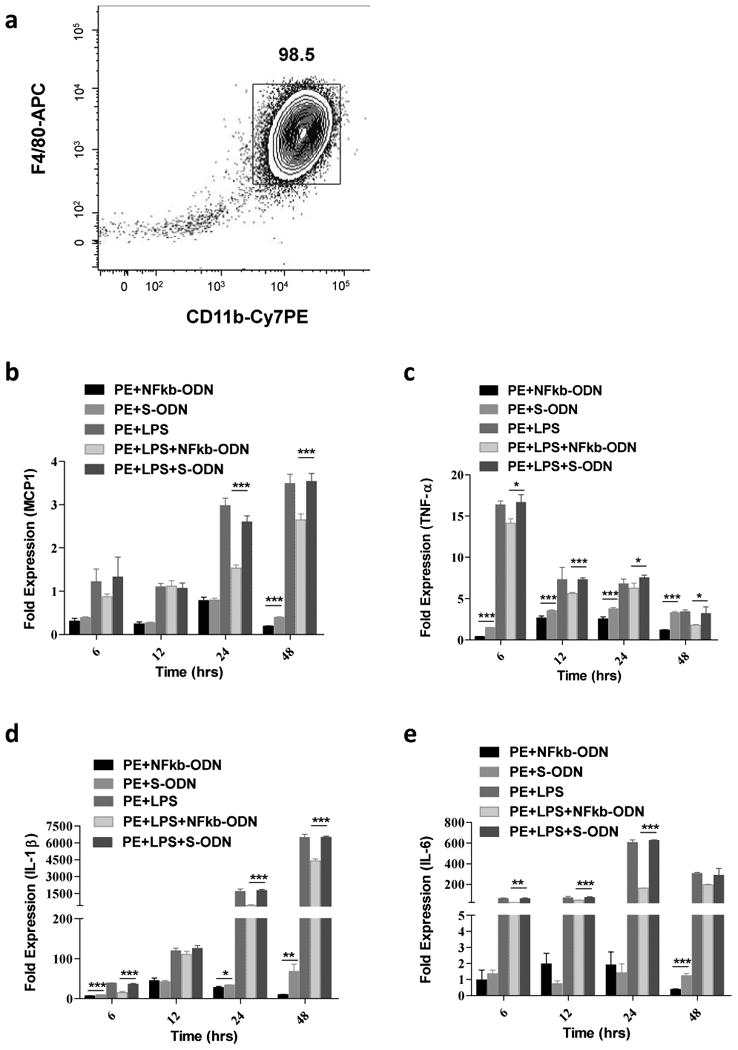
Characterization of mouse bone marrow derived macrophage (a). Primary mouse macrophages cultured for one week were stained for macrophage surface marker including F4/80 (APC) and CD11b (Cy7PE) and analyzed by flow cytometry. Effect of NF-κB decoy ODN (0.5μM) on TNF-α, MCP1, IL-1β, and IL-6 expression by mouse bone marrow-derived macrophages stimulated with UHMWPE and LPS (1 μg/ml) for 6, 12, 24 and 48 h (b-e). The RNA samples were collected at indicated time points and converted into complementary DNA. Quantitative PCR analysis was performed by using the Taq-Man system as described in the Materials and Methods. *p< .05, ** p< .01, *** p<.005.
Cytokine production in the supernatants was analyzed by using the mouse 20-plex panel array. Consistent with the quantitative PCR results, production of TNF-α and IL-1β but not MCP1 and IL-6 was increased by exposure of macrophages to particles (Fig.4a, b). In addition, chemokine production including MIP1α and CXCL1 was also increased by particle exposure (see summary in Table 1 and Supplementary Table 1). The chemokines (MCP1, MIP1α, CXCL1) and pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) in the array panels were all inhibited by the decoy ODN (see summary in Table 2 and Supplementary Table 2).
Fig.4. NF-κB decoy ODN suppressed TNF-α and MCP1 cytokine expression in primary mouse macrophages and THP1 cells exposed to UHMWPE and/or LPS.
Effect of NF-κB decoy ODN (0.5μM) on TNF-α and MCP1 production by mouse bone marrow-derived macrophages (a, b) and THP1 cells (c, d) stimulated with UHMWPE and LPS (1 μg/ml) for 6, 12, 24 and 48 h. The supernatant samples were collected at indicated time points and analyzed by magnetic beads cytokine assay system. *p< .05, ** p< .01.
Table 1. Macrophage cytokine expression in response to UHMWPE particles.
| Classification | Name | Mouse | THP1 |
|---|---|---|---|
| Chemokine -Macrophage | MCP1 | N.S. | *↑ 3.73 fold |
| MIP1α | *↑ 11.78 fold | *↑ 2.19 fold | |
| MIP1β | N.A. | N.S. | |
| RANTES | N.A. | ↓ 47 % | |
| -Neutrophil | IL-8 | N.A. | *↑ 6.18 fold |
| CXCL1 | *↑ 1.52 fold | N.A. | |
| -T cell | IP-10 | N.S. | N.S. |
|
| |||
| Pro-inflammatory cytokines | TNF-α | *↑ 22.33 fold | N.D. |
| IL-1α | N.D. | N.A. | |
| IL-1β | *↑ 1.42 fold | N.S. | |
| IL-1Ra | N.S. | *↑ 2.03 fold | |
| IL-6 | N.S. | N.S. | |
| IL-12 | N.S. | N.S. | |
|
| |||
| Cellular immunity | IL-2R | N.A. | N.S. |
| IL-7 | N.A. | N.D. | |
| IL-10 | N.S. | N.D. | |
| IL-13 | N.S. | N.D. | |
| IL-15 | N.A. | N.S. | |
| IFN-α | N.A. | N.D. | |
N.A. Not available; N.D. Non-detectable; N.S. Not significant.
Statistically significant
Table 2. Macrophage cytokine expression in response to NF-κB decoy ODN.
| Classification | Name | Mouse | THP1 |
|---|---|---|---|
| Chemokine -Macrophage | MCP1 | *↓ 27% | *↓ 20% |
| MIP1α | *↓ 83% | *↓ 65% | |
| MIP1β | N.A. | *↓ 66% | |
| RANTES | N.A. | *↓ 63% | |
| -Neutrophil | IL-8 | N.A. | *↓ 54% |
| CXCL1 | *↓ 36% | N.A. | |
| -T cell | IP-10 | N.S. | N.S. |
|
| |||
| Pro-inflammatory cytokines | TNF-α | *↓ 43% | *↓ 49% |
| IL-1α | N.S. | N.A. | |
| IL-1β | *↓ 38% | N.S. | |
| IL-1Ra | N.S. | *↓ 27% | |
| IL-6 | *↓ 84% | *↓ 68% | |
| IL-12 | *↓ 19% | *↓ 37% | |
|
| |||
| Cellular immunity | IL-2R | N.A. | *↓ 19% |
| IL-7 | N.A. | N.D. | |
| IL-10 | N.S. | N.D. | |
| IL-13 | N.S. | N.D. | |
| IL-15 | N.A. | N.S. | |
| IFN-α | N.A. | N.D. | |
N.A. Not available; N.D. Non-detectable; N.S. Not significant.
Statistically significant
3.3 Decoy ODN inhibited UHMWPE and LPS induced cytokine production in human THP1 macrophages
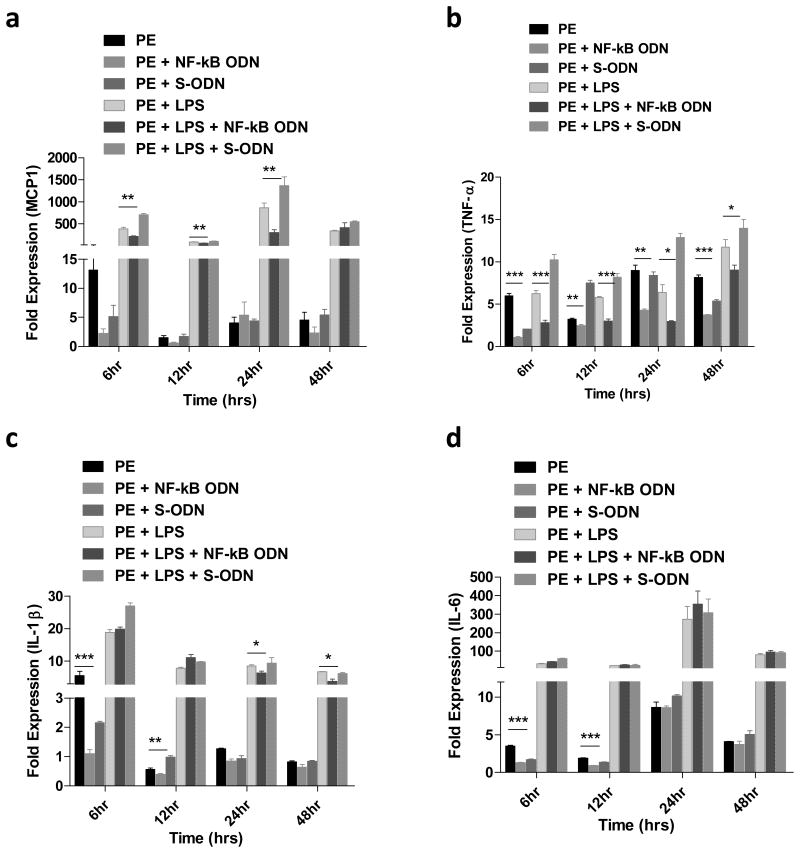
Human THP1 macrophage exposed to UHMWPE particles induced TNF-α (8.4 fold, peak at 24h), MCP1 (5.4 fold, peak at 48h), IL-1β (2 fold, peak at 6h), and IL-6 (10.2 fold, peak at 24h) expression at the mRNA level. Macrophages exposed to the combination of UHMWPE particles plus LPS induced TNF-α (11.7 fold, peak at 48h), MCP1 (863 fold, peak at 24h), IL-1β (18.9 fold, peak at 6h), and IL-6 (271 fold, peak at 24h), respectively (Fig.3). Decoy ODN significantly inhibited cytokine expression when compared with scrambled ODN or the no ODN control group. Cytokine production in the supernatant was also analyzed using the human 25-plex panel array. Compared to the quantitative PCR results, production of MCP1 and IL-6 but not TNF-α and IL-1β were increased by macrophage exposure to particles (Fig.4c,d and Table 1). In addition, chemokine production including MIP1α, MIP1β, and IL-8 were also increased by particle exposure. Notably, production of RANTES was also significantly suppressed by particle exposure (Table 1). The chemokines MCP1, MIP1α, MIP1β, RANTES, and IL-8 and the pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-12) were all inhibited by the decoy ODN (Table 2).
Fig.3. NF-κB decoy ODN suppressed pro-inflammatory cytokines mRNA expression in human THP1 macrophage cells exposed to UHMWPE and/or LPS.
Effect of NF-κB decoy ODN (0.5μM) on TNF-α, MCP1, IL-1β, and IL-6 expression by human THP1 macrophages stimulated with UHMWPE and LPS (1 μg/ml) for 6, 12, 24 and 48 h (a-d). The RNA samples were collected at indicated time points and converted into complementary DNA. Quantitative PCR analysis was performed by using the Taq-Man system as described in the Materials and Methods. *p< .05, ** p< .01,*** p<.005..
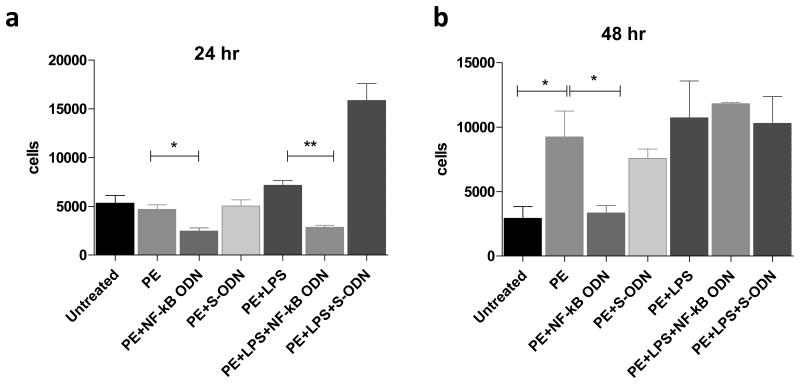
3.4 Decoy ODN suppressed THP1 cell migration to the conditioned media from THP1 macrophage exposure to UHMWPE and LPS
Using the THP1 cell migration assay, conditioned media from THP1 cells treated with decoy ODN induced less THP1 cell migration at 24h and 48h exposure (Fig.5a & b, respectively); however, cell migration studies showed no significant differences at 6h and 12h exposure times (data not shown). Consistent with MCP1 production levels (Fig.4c), the migrated cell numbers were not affected by decoy ODN treatment in the group of THP1 cells exposed to UHMWPE and LPS for 48hr. The migrated cell number in the particle treated group increased to 2.6 fold when compared to the untreated cell samples (Fig.5b), suggesting that cells exposed to UHMWPE induced more macrophage infiltration.
Fig.5. NF-κB decoy ODN suppressed THP1 cell migration attracted by the conditioned media from cells exposed to UHMWPE and/or LPS.
The supernatant were collected from THP1 cells stimulated with ODNs, UHMWPE, and LPS (0.1 μg/ml) for 24 (a) and 48 h (b). The migration assay was performed using a ChemoTx® Disposable Chemotaxis System. The migrated cell number was quantified by detecting the double strand DNA concentration using pico-green detection dye. *p< .05, ** p< .01.
4. Discussion
Our current findings show that targeting the NF-κB pathway could suppress pro-inflammatory cytokine expression induced by UHMWPE wear particles and endotoxin in macrophages. Suppression of the inflammatory response to wear debris in TJR patients using this novel strategy could potentially mitigate particle-induced inflammation in the early high wear “bedding in” stage during the first several months after joint replacement, thus producing a more robust interface per primum. Furthermore, if this strategy is implemented at a later time in the early stages of periprosthetic osteolysis, bone loss could be reduced while the wear process continues. It is recognized that the current study reports the results of in vitro experiments, in contrast to the long term process of particle-induced periprosthetic osteolysis in humans. We plan to extend these encouraging in vitro studies to evaluate the potential of decoy ODN treatment in longer term experiments using our in vivo murine continuous femoral particle infusion model [23].
Decoy ODN mediated suppression of NF-κB activity inhibited the production of multiple cytokine and chemokines in primary mouse macrophages and human THP1 cells. This strategy could potentially block wear particle-mediated osteolysis by several mechanisms. First, decoy NF-κB ODN inhibited the production of pro-inflammatory cytokines including TNF-α and IL-1β, which could mitigate particle-induced tissue damage. Second, inhibition of macrophage attracting chemokines could further reduce the infiltration of circulating macrophages to the inflammatory site. Third, RANK/RANKL mediated osteoclastogenesis is also regulated by the NF-κB pathway and is critical for the osteolytic process. However, the overall biological effects of decoy ODN on the regulation of RANK/RANKL remains to be clarified in vivo since their expression is more dominant in other cell types [24].
The direct effects of NF-κB signaling on bone is complicated due to the complexity of cell types involved in this specialized tissue micro-environment. NF-κB activation can induce Type I collagen expression in osteoprogenitor cells, [25], which can induce bone formation [26]. NF-κB signaling can also modulate inflammation via the secretion of anti-inflammatory cytokines such as IL-10 by mesenchymal stem cells [27, 28]. However, NF-κB can suppress osteogenic differentiation via suppression of β-catenin signaling [29-31], or can enhance bone formation via up-regulation of the transcriptional co-activator, TAZ [32]. Therefore, the overall effects of NF-κB signaling on osteogenesis remain to be clarified in vivo.
Importantly, the neutrophil attracting chemokines, including IL-8 and CXCL1, were both induced by the exposure to UHMWPE particles. Previous studies have reported that titanium particles trigger neutrophil recruitment in an IL-1α/β dependent manner [33]. In addition, when neutrophils were exposed to UHMWPE particles, there was a significant correlation between the bactericidal activity of neutrophils with the number and size of the particles [34]. It is likely that the generation of UHMWPE wear particles could recruit neutrophils with impaired bactericidal function, which may further enhance the pro-inflammatory response and inhibit the normal clearance of bacteria.
Previous studies using titanium wear particles and differentiated THP-1 cells have shown variable results [35, 36], which may be due to the type and dose of particles and cell differentiation strategy. Our current studies using PMA stimulated THP-1 cells which were exposed to UHMWPE particles moderately increased TNF-α expression, and is consistent with a previous study with a similar dose (∼1 × 109 particles/ml) of polyethylene particles [36].
Despite the specificity and biological safety, the clinical application of decoy ODN may be limited due to its short half-life. Nevertheless, chemical modifications such as using a phosphothioate bond to replace the phosphodiester bond with sulfur have been shown to be more resistant to DNAase and more stable in serum [11]. In addition, the decoy ODN could be delivered locally by coating it on the implanted biomaterials. Recent studies have reported that the surface of implanted devices can be coated with proteins, small molecules, or other potential therapeutic compounds [37, 38]. This novel strategy could affect long term release in an anatomically localized region, and reduce concerns of potential systemic toxicity. Previous reports showed that NF-κB decoy ODN can be coated on nanoparticles for local delivery [39], or directly coated onto a catheter for implantation in cardiovascular diseases [40]. In this manner, the application of decoy ODN technology coated on the biomaterials used in TJR may have great potential to mitigate wear particle induced osteolysis.
Interestingly, our data also showed that direct treatment of NF-κB decoy ODN was sufficient to inhibit NF-κB activity and target gene expression in macrophage cells. This could be explained by the fact that CD11b, which is a macrophage surface marker, has been reported to be a cellular surface receptor for oligonucleotides [41]. The ODN used in current study may be taken up by macrophages through a receptor-mediated endocytosis pathway. Recent studies have reported that direct treatment of decoy ODN cannot become translocated into the cells without an appropriate carrier [42]. The contradictory results reported herein might be due to ODN sequence differences, because cell receptor-mediated ODN uptake is known to be sensitive to the sequence specificity [43].
5. Conclusion
Local administration of NF-κB decoy ODN may potentially mitigate wear particle and endotoxin induced pro-inflammatory cytokine expression in macrophages. Thus, the strategic use of NF-κB ODN, delivered locally, could potentially diminish wear particle-induced peri-prosthetic osteolysis.
Supplementary Material
Acknowledgments
This work was supported by NIH grants 2R01AR055650, 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsao A, Jones L, Lewallen D Implant Wear Symposium Clinical Work G. What patient and surgical factors contribute to implant wear and osteolysis in total joint arthroplasty? The Journal of the American Academy of Orthopaedic Surgeons. 2008;16(Suppl 1):13. doi: 10.5435/00124635-200800001-00004. [DOI] [PubMed] [Google Scholar]
- 2.Purdue P, Koulouvaris P, Nestor B, Sculco T. The central role of wear debris in periprosthetic osteolysis. HSS journal: the musculoskeletal journal of Hospital for Special Surgery. 2006;2:102–13. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sochart D. Relationship of acetabular wear to osteolysis and loosening in total hip arthroplasty. Clinical orthopaedics and related research. 1999:135–50. [PubMed] [Google Scholar]
- 4.Johnston R. Current concepts: immunology. Monocytes and macrophages. The New England journal of medicine. 1988;318:747–52. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz E, Campbell D, Totterman S, Boyd A, O'Keefe R, Looney R. Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: a 1-year clinical pilot of etanercept vs. placebo. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2003;21:1049–55. doi: 10.1016/S0736-0266(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 6.Yang SY, Wu B, Mayton L, Mukherjee P, Robbins P, Evans C, et al. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene therapy. 2004;11:483–91. doi: 10.1038/sj.gt.3302192. [DOI] [PubMed] [Google Scholar]
- 7.Wei S, Kitaura H, Zhou P, Ross F, Teitelbaum S. IL-1 mediates TNF-induced osteoclastogenesis. The Journal of clinical investigation. 2005;115:282–90. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashima Y, Sun D, Trindade M, Maloney W, Goodman S, Schurman D, et al. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. The Journal of bone and joint surgery American volume. 1999;81:603–15. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Rao AJ, Gibon E, Ma T, Yao Z, Smith RL, Goodman SB. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8:2815–23. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin TH, Tamaki Y, Pajarinen J, Waters HA, Woo DK, Yao Z, et al. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater. 2014;10:1–10. doi: 10.1016/j.actbio.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osako M, Nakagami H, Morishita R. Modification of decoy oligodeoxynucleotides to achieve the stability and therapeutic efficacy. Current topics in medicinal chemistry. 2012;12:1603–7. doi: 10.2174/156802612803531397. [DOI] [PubMed] [Google Scholar]
- 12.Dinh TD, Higuchi Y, Kawakami S, Yamashita F, Hashida M. Evaluation of osteoclastogenesis via NF-kappaB decoy/mannosylated cationic liposome-mediated inhibition of pro-inflammatory cytokine production from primary cultured macrophages. Pharmaceutical research. 2011;28:742–51. doi: 10.1007/s11095-011-0366-0. [DOI] [PubMed] [Google Scholar]
- 13.Desmet C, Gosset P, Pajak B, Cataldo D, Bentires-Alj M, Lekeux P, et al. Selective blockade of NF-kappaB activity in airway immune cells inhibits the effector phase of experimental asthma. Journal of immunology (Baltimore, Md: 1950) 2004;173:5766–75. doi: 10.4049/jimmunol.173.9.5766. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura H, Aoki M, Tamai K, Oishi M, Ogihara T, Kaneda Y, et al. Prevention and regression of atopic dermatitis by ointment containing NF-kB decoy oligodeoxynucleotides in NC/Nga atopic mouse model. Gene therapy. 2002;9:1221–9. doi: 10.1038/sj.gt.3301724. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu H, Nakagami H, Morita S, Tsukamoto I, Osako M, Nakagami F, et al. New treatment of periodontal diseases by using NF-kappaB decoy oligodeoxynucleotides via prevention of bone resorption and promotion of wound healing. Antioxidants & redox signaling. 2009;11:2065–75. doi: 10.1089/ars.2008.2355. [DOI] [PubMed] [Google Scholar]
- 16.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. 2010;107:3317–22. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SW, Goldberg M, Son SM, Xu Q, Yang F, Mei Y, et al. Lipid-like Nanoparticles for Small Interfering RNA Delivery to Endothelial Cells. Advanced functional materials. 2009;19:3112–8. doi: 10.1002/adfm.200900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell P, Ma S, Yeom B, McKellop H, Schmalzried T, Amstutz H. Isolation of predominantly submicron-sized UHMWPE wear particles from periprosthetic tissues. Journal of biomedical materials research. 1995;29:127–31. doi: 10.1002/jbm.820290118. [DOI] [PubMed] [Google Scholar]
- 19.Yao Z, Keeney M, Lin TH, Pajarinen J, Barcay K, Waters H, et al. Mutant monocyte chemoattractant protein 1 protein attenuates migration of and inflammatory cytokine release by macrophages exposed to orthopedic implant wear particles. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman S, Huie P, Song Y, Schurman D, Maloney W, Woolson S, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. The Journal of bone and joint surgery British volume. 1998;80:531–9. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 21.Goodman S, Chin R, Chiou S, Schurman D, Woolson S, Masada M. A clinical-pathologic-biochemical study of the membrane surrounding loosened and nonloosened total hip arthroplasties. Clinical orthopaedics and related research. 1989:182–7. [PubMed] [Google Scholar]
- 22.Goodman S, Huie P, Song Y, Lee K, Doshi A, Rushdieh B, et al. Loosening and osteolysis of cemented joint arthroplasties. A biologic spectrum. Clinical orthopaedics and related research. 1997:149–63. doi: 10.1097/00003086-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Ma T, Huang Z, Ren PG, McCally R, Lindsey D, Smith RL, et al. An in vivo murine model of continuous intramedullary infusion of polyethylene particles. Biomaterials. 2008;29:3738–42. doi: 10.1016/j.biomaterials.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 25.Novitskiy G, Potter J, Rennie-Tankersley L, Mezey E. Identification of a novel NF-kappaB-binding site with regulation of the murine alpha2(I) collagen promoter. The Journal of biological chemistry. 2004;279:15639–44. doi: 10.1074/jbc.M311499200. [DOI] [PubMed] [Google Scholar]
- 26.Ollivere B, Wimhurst JA, Clark IM, Donell ST. Current concepts in osteolysis. J Bone Joint Surg Br. 2012;94:10–5. doi: 10.1302/0301-620X.94B1.28047. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–8. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 28.Cao S, Zhang X, Edwards J, Mosser D. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. The Journal of biological chemistry. 2006;281:26041–50. doi: 10.1074/jbc.M602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang J, Liu F, Lee M, Wu B, Ting K, Zara JN, et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci U S A. 2013;110:9469–74. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–9. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Hu C, Wang G, Li L, Kong X, Ding Y, et al. Nuclear factor-kappaB modulates osteogenesis of periodontal ligament stem cells through competition with beta-catenin signaling in inflammatory microenvironments. Cell Death Dis. 2013;4:e510. doi: 10.1038/cddis.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, et al. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010;223:168–77. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- 33.St Pierre CA, Chan M, Iwakura Y, Ayers DC, Kurt-Jones EA, Finberg RW. Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles. J Orthop Res. 2010;28:1418–24. doi: 10.1002/jor.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernard L, Vaudaux P, Merle C, Stern R, Huggler E, Lew D, et al. The inhibition of neutrophil antibacterial activity by ultra-high molecular weight polyethylene particles. Biomaterials. 2005;26:5552–7. doi: 10.1016/j.biomaterials.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 35.Akisue T, Bauer T, Farver C, Mochida Y. The effect of particle wear debris on NFkappaB activation and pro-inflammatory cytokine release in differentiated THP-1 cells. Journal of biomedical materials research. 2002;59:507–15. doi: 10.1002/jbm.1264. [DOI] [PubMed] [Google Scholar]
- 36.Baumann B, Seufert J, Jakob F, Noth U, Rolf O, Eulert J, et al. Activation of NF-kappaB signalling and TNFalpha-expression in THP-1 macrophages by TiAlV- and polyethylene-wear particles. J Orthop Res. 2005;23:1241–8. doi: 10.1016/j.orthres.2005.02.017.1100230602. [DOI] [PubMed] [Google Scholar]
- 37.Keeney M, Waters H, Barcay K, Jiang X, Yao Z, Pajarinen J, et al. Mutant MCP-1 protein delivery from layer-by-layer coatings on orthopedic implants to modulate inflammatory response. Biomaterials. 2013;34:10287–95. doi: 10.1016/j.biomaterials.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman SB, Yao Z, Keeney M, Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013;34:3174–83. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y, Wang Hf, Sun Wq, Xie Cs, Wei Wn, Zheng Je, et al. [Regulation of tissue factor expression in brain microvascular endothelial cells by PLA nanoparticles coating NF-kappaB decoy oligonucleotides] Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi. 2005;26:534–8. [PubMed] [Google Scholar]
- 40.Kalinowski M, Viehofer K, Hamann C, Barry J, Kleb B, Klose K, et al. Local administration of NF-kappa B decoy oligonucleotides to prevent restenosis after balloon angioplasty: an experimental study in New Zealand white rabbits. Cardiovascular and interventional radiology. 2005;28:331–7. doi: 10.1007/s00270-003-0239-y. [DOI] [PubMed] [Google Scholar]
- 41.Benimetskaya L, Loike J, Khaled Z, Loike G, Silverstein S, Cao L, et al. Mac-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nature medicine. 1997;3:414–20. doi: 10.1038/nm0497-414. [DOI] [PubMed] [Google Scholar]
- 42.Dinh TD, Higuchi Y, Kawakami S, Yamashita F, Hashida M. Evaluation of osteoclastogenesis via NFkappaB decoy/mannosylated cationic liposome-mediated inhibition of pro-inflammatory cytokine production from primary cultured macrophages. Pharm Res. 2011;28:742–51. doi: 10.1007/s11095-011-0366-0. [DOI] [PubMed] [Google Scholar]
- 43.Juliano RL, Ming X, Nakagawa O. Cellular uptake and intracellular trafficking of antisense and siRNA oligonucleotides. Bioconjug Chem. 2012;23:147–57. doi: 10.1021/bc200377d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.