Abstract
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation intervention that modifies cortical excitability according to the stimulation parameters. Preclinical and clinical studies in healthy volunteers suggest that tDCS induces neuroplastic alterations of cortical excitability, which might explain its clinical effects in major depressive disorder (MDD). We therefore examined whether tDCS, as compared to the antidepressant sertraline, increases plasma brain-derived neurotrophic factor (BDNF) levels, a neurotrophin associated with neuroplasticity. Patients (n=73) with major depressive disorder were randomized to active/sham tDCS and sertraline/placebo (four groups) in this 6-week, double-blind, placebo-controlled trial. We measured BDNF plasma levels at baseline and endpoint, observing no significant changes of BDNF levels after treatment. In addition, no significant changes were observed in responders and non-responders as well as no relationships between BDNF levels and clinical and psychopathological variables related to depression. Thus, in one of the few placebo-controlled trials evaluating BDNF changes over an antidepressant treatment course, we did not observe BDNF increase regardless of clinical improvement in depressed patients. Regarding tDCS, BDNF plasma levels might not be a good candidate biomarker to evaluate depression improvement or be a predictor of response in patients treated with tDCS, as our results showed that BDNF increase was not necessary to induce clinical response. Finally, our findings do not support a relationship between BDNF and improvement of depression.
Keywords: Brain-derived neurotrophic factor, Transcranial direct current stimulation, Major depressive disorder, Sertraline, Non-invasive brain stimulation, Neuroplasticity
1. Introduction
Transcranial direct current stimulation (tDCS) is a non-invasive neuromodulatory technique that induces polarity-dependent changes of cortical excitability (Nitsche and Paulus, 2000). When performed for several minutes, a single tDCS session can induce cortical excitability changes outlasting the period of stimulation for more than 1 h (Batsikadze et al., 2013; Brunoni et al., 2012b; Monte-Silva et al., 2010, 2013; Nitsche et al., 2003, 2008); pointing out that changes in synaptic plasticity are involved in tDCS mechanisms. In fact, neurophysiological studies showed that tDCS-induced plasticity is calcium-dependent and involves glutamatergic synapses (for a review see Stagg and Nitsche, 2011). When applied daily for several days, tDCS seems to have therapeutic properties in the treatment of psychiatric disorders (Kuo et al., 2013), and, among those, tDCS has been particularly investigated for major depressive disorder (MDD) (Brunoni et al., 2012a), with recent and large trials showing positive outcomes (Brunoni et al., 2013b; Loo et al., 2012). Nonetheless, the mechanisms of action underlying tDCS antidepressant response are still unknown; it has been proposed that daily, anodal tDCS over the left dorsolateral prefrontal cortex (DLPFC) reverses the hypoactivity in this area, which is observed in MDD (Mayberg et al., 2000); subsequently leading to depression improvement.
In recent years, the neurotrophin hypothesis of depression has been implicated in MDD pathophysiology. In short, this hypothesis advocates that the depressive state is associated with lower expression of the brain-derived neurotrophic factor (BDNF), a neurotrophin essential to synaptic strengthening and neuronal survival (Duman and Monteggia, 2006), and that antidepressant effects would involve up-regulation of BDNF levels as a key neurobiological pathway for depression improvement. In accordance, Karege et al. (2002) and Shimizu et al. (2003) showed that BDNF blood levels are lower in depressed vs. healthy subjects and that BDNF levels increase during pharmacological treatment. Moreover, some depression symptoms, such as verbal memory impairment, are associated with low BDNF levels (Grassi-Oliveira et al., 2008). Indeed, recent meta-analyses (Brunoni et al., 2008; Molendijk et al., 2013; Sen et al., 2008) found that BDNF is lower in depressed vs. healthy participants and that it increases during treatment; although Molendijk et al. (2013) stated that such claims might be “slimmer as was initially thought and amidst a lot of noise” such as evidence of publication bias and presence of confounding factors, highlighting the need of further BDNF studies evaluating its role as a treatment biomarker. Another issue is that virtually all studies evaluating BDNF changes after antidepressant treatment were not placebo-controlled trials; therefore not disentangling treatment effects from the natural changes over the course of illness. Besides, one cannot rule out whether the BDNF changes observed in pharmacological interventions occur due to a neuroplastic effect or simply due to direct effects in BDNF peripheral levels - for instance, antidepressants release BDNF stored in platelets (Watanabe et al., 2010). In this context, evaluating BDNF changes after therapies having no pharmacokinetic properties, such as somatic treatments, might be useful.
Nevertheless, as compared to pharmacological interventions, for somatic stimulation therapies (such as electroconvulsive therapy [ECT], repetitive transcranial magnetic stimulation [rTMS] and tDCS), the role of BDNF in inducing antidepressant response has been scarcely investigated, with mixed or negative findings of BDNF increase, although most studies were not sham-controlled and therefore methodologically limited (e.g., Gedge et al., 2012; Lang et al., 2006). For tDCS, only the sham-controlled study of Palm et al. (2013) investigated whether BDNF serum levels increased after tDCS treatment of patients suffering from MDD, and report unchanged levels after treatment. However, the authors acknowledged some study limitations such as small sample size, short time period between the first and the second BDNF collections and overall absence of active vs. sham tDCS effects on clinical improvement. Further, they did not compare the antidepressant effects of tDCS to a pharmacological therapy, in which the evidence of BDNF increase might be more robust. Considering the putative advantages of tDCS in daily practice (low cost, portability, ease of use) and its clinical efficacy demonstrated in our recent study (Brunoni et al., 2013b), tDCS alone and combined with pharmacological therapy could be interesting antidepressant strategies. In this regard, investigating whether BDNF levels increase after these interventions is important to better understand the pathophysiological mechanisms involved in antidepressant response.
Therefore, considering that (1) animal studies (e.g., Fritsch et al., 2010) showed that direct current stimulation promotes BDNF-dependent synaptic plasticity; (2) tDCS effects are associated with neuroplasticity and; (3) tDCS has antidepressant effects; we hypothesized in our placebo-controlled study that BDNF levels would increase after tDCS and pharmacological antidepressant treatment and such improvement would be associated with clinical response. These hypotheses were assessed using data from our previous trial, in which we compared the effects of sertraline vs. tDCS in patients with unipolar depression, as described below.
2. Experimental procedures
2.1. Study design
The Sertraline vs. Electric Current Therapy for Treating Depression Clinical Study (SELECT-TDCS) took place from March 2010 to September 2011 at University Hospital, University of São Paulo, Brazil. This study was registered in clinicaltrials.gov (NCT01033084), and approved by the Local and National Ethics Committee with all participants providing written, informed consent. The main methodological aspects and results are described elsewhere (Brunoni et al., 2011b, 2013b).
In short, SELECT-TDCS was a factorial, sham-controlled trial in which 120 participants with depression were randomized using a 1:1:1:1 permuted block randomization method into four treatment groups: (1) sham-tDCS/placebo-pill (further referred as placebo); (2) sham-tDCS/sertraline-pill (sertraline-only); (3) active-tDCS/placebo-pill (tDCS-only); (4) active-tDCS/sertraline-pill (combined treatment). The trial duration was 6 weeks, encompassing an acute treatment phase when 10 consecutive daily neuromodulation (tDCS or sham) sessions were delivered (from Monday to Friday), followed by two tDCS sessions delivered every other week. Sertraline (50 mg/day), a selective serotonin reuptake inhibitor (SSRI), treatment duration also lasted 6 weeks. Sertraline was chosen because it is an effective antidepressant drug with few adverse effects (Cipriani et al., 2009); moreover, previous neurophysiological studies showed that tDCS effects are enhanced when combined with the SSRI citalopram (Nitsche et al., 2009).
2.2. Subjects
We enrolled subjects with non-psychotic, major depressive disorder in an acute major depressive episode. The diagnosis was established by board-certified psychiatrists (ARB and LV) using the Portuguese-validated version of the Mini International Neuropsychiatric Interview (Amorim, 2000). Only participants with at least a moderate depressive episode severity (defined as a Hamilton Depression Rating Score, 17-items [HDRS] ≥ 17) were included. Comorbid anxiety disorders were permitted.
Subjects were excluded if they were not in good physical condition or had any medical disorder as determined by physical and neurological examination, review of systems and laboratory tests. Other exclusion criteria included pregnancy or breastfeeding, history of substance abuse within the past two years, any history of psychotic disorder, bipolar disorder, current suicidal ideation, previous non-response to sertraline, or sertraline treatment in the current major depressive episode. There were no patients with diabetes melito and anorexia nervosa, conditions associated with BDNF changes.
Subjects who were not drug-naïve in the current depressive episode were gradually tapered of any psychotropic medication except for those previously taking a benzodiazepine; in such individuals, the dose was tapered to a maximum of 20 mg/day of diazepam-equivalents, which remained constant throughout the entire study. Therefore, participants remained free of psychotropic medications for at least five half-lives of the medication(s); which corresponded to a median washout time period of 18 days ((Brunoni et al., 2011b, 2013b) for further details).
In SELECT-TDCS, the primary outcome measure was the score changes of the Portuguese-validated version of the Montgomery-Åsberg Depression rating scale (MADRS) (Gorenstein et al., 2000). As a secondary outcome measure, clinical response (≥ 50% of MADRS improvement from baseline to endpoint) was also evaluated.
2.3. Procedures
We employed standard, commercial tDCS devices (Chattanooga Ionto™ Dual Channel Devices, Chattanooga Group, Hixson, TN 37343, USA). The anode was placed over the left DLPFC and the cathode over the right DLPFC (F3 and F4 areas, respectively, according to the 10/20 EEG system). The brain areas were localized 5 cm laterally and 5 cm ventrally from the central of the scalp (where the sagittal and coronal planes cross). The bifrontal setup was used in accordance to previous studies (Brunoni et al., 2011a, 2012a; Dell'Osso et al., 2009; Ferrucci et al., 2009) as this montage might be advantageous (compared to cathode placement over the right supraorbital area) considering the prefrontal activation asymmetry observed in depression, i.e. hypoactivity of the left and relative hyperactivity of the right prefrontal cortex (Mayberg et al., 2000).
A current density of 0.8 A/m2 (2 mA/25 cm2) for 30 min/day was employed. For sham condition, we used the method of Gandiga et al. (2006), in which the device is turned on for only a brief period of time and then remains turned off for the lasting 29 min. This method mimics skin side effects (tingling, itching, local discomfort) although the period of active tDCS is too short to induce any neuromodulatory effects, which outlast the stimulation (Nitsche and Paulus, 2000).
Blood samples were collected by venipuncture immediately before (2-4 pm) the first and the last tDCS session - i.e. at study baseline and endpoint. Within 30 min of sample collection, they were then spun at 3000 rpm for 15 min at 5 °C and thereafter plasma aliquots were gently collected and stored at −80 °C until analysis. Plasma levels of BDNF were measured by enzyme-linked immunosorbent assay (ELISA) according to the procedures supplied by the manufacturer (DuoSet, R&D Systems, Minneapolis, MN, USA). All samples were assayed in duplicate. Lower detection limits were 5 pg/mL. Concentrations are expressed as pg/mL. Analyses of blood samples were performed blind to group assignment and outcome.
2.4. Statistical analysis
We used Stata 12 (Statacorp, College Station, TX, USA) for all analyses, with 2-sided significance tests at the 5% significance level. For descriptive data, clinical and demographic variables were compared across groups using one-way analysis of variance (ANOVA), χ2 tests or Fisher's exact tests, when necessary. Analyses of blood samples were performed blind to group assignment.
A repeated-measures analysis of variance was performed for the primary outcome. The within-subjects factor was time (two levels: first collection and second collection) and the between-subjects factors were treatment group (four levels: placebo, sertraline-only, tDCS-only and combined therapy) and clinical response. We performed different analyses to investigate whether BDNF levels would change (1) over time and according to the interactions, (2) between time and group, (3) between time and clinical response and (4) between time, group and clinical response.
For the model using all factors (time, group and clinical response), we also performed additional analyses to examine whether other characteristics such as sociodemographic characteristics (age, gender, obesity - defined as a body mass index ≥ 30 kg/m2, smoking status, physical activity - evaluated with the IPAQ questionnaire and further explored in low, moderate and high physical activity for the present analysis); depression characteristics at baseline (melancholic depression, atypical depression, severity - with a cut-off point of baseline MADRS ≥ 30, and refractoriness - defined as the therapeutic failure to two or more antidepressants in the current depressive episode) and benzodiazepine use influenced the outcome. Each variable was explored in a separate model.
In addition, to further explore whether baseline BDNF levels were predictors of response, we used unpaired t-tests to compare responders vs. non-responders. Pearson's correlations were performed to explore the association of changes in BDNF levels with depression scores.
3. Results
3.1. Overview
Of the 120 participants enrolled, 103 completed the original study. From the study completers, 73 (71%) had their baseline and endpoint BDNF plasma levels analyzed. The remaining 30 patients were not collected due to patient refusal and technical reasons. Their clinical and demographic characteristics did not differ from the completers of the original study and the main results from the original study were replicated in this subsample regarding efficacy of clinical interventions, that is, all groups presented similar depression scores at baseline, tDCS-only was statistically superior to placebo and had similar efficacy as sertraline, and combined treatment was superior to all other groups, being also associated with a faster antidepressant response (Brunoni et al., 2013b) (Table 1).
Table 1.
Clinical and demographic characteristics of the sample of the present study at baseline.
| Placebo | Sertraline-only | tDCS-only | Combined treatment | p | Total | Total from the original study | p | |
|---|---|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||||
| Sample size | 19 | 18 | 15 | 21 | 0.79 | 73 | 120 | - |
| Age, years (SD) | 50 (12) | 41 (1) | 41 (12) | 41 (13) | 0.1 | 41 (12) | 42 (12) | 0.58 |
| Women, n (%) | 12 (63) | 11 (61) | 10 (66) | 18 (85) | 0.3 | 51 (70) | 82 (68) | 0.77 |
| Using benzodiazepines (%) | 2 (10) | 2 (11) | 1 (7) | 5 (23) | 0.53 | 10 (14) | 23 (20) | 0.3 |
| BMI, kg/m2 (SD) | 26 (6) | 25 (3) | 25 (6) | 27 (5) | 0.54 | 26 (5) | 26 (5) | 0.92 |
| Depression characteristics at baseline, n (%) or mean (SD) | ||||||||
| Refractory depression | 7 (37) | 9 (50) | 6 (40) | 7 (33) | 0.75 | 29 (40) | 50 (42) | 0.78 |
| Severe depression | 12 (63) | 11 (61) | 11 (73) | 12 (57) | 0.8 | 46 (63) | 70 (58) | 0.49 |
| MADRS | 31.5 (6) | 31 (7) | 32 (6) | 31 (6) | 0.92 | 31 (6) | 31 (6) | 0.5 |
| HDRS17 | 22 (4) | 22 (4) | 22(4) | 22 (4) | 0.99 | 22(4) | 22(4) | 0.75 |
| Depression endpoint scores, mean (SD) and response, n (%) | ||||||||
| MADRS | 24 (9) | 19 (13) | 19 (12) | 10 (6) | <0.01 | 18 (11) | 19 (11) | 0.38 |
| HDRS17 | 17 (7) | 14 (8) | 13 (7) | 9 (5) | 0.01 | 14 (8) | 15 (7) | 0.34 |
| Response | 4 (21) | 7 (39) | 7 (46) | 16 (76) | <0.01 | 34 (46) | 47 (39) | 0.52 |
tDCS, Transcranial direct current stimulation; MADRS, Montgomery–Asberg depression rating scale; HDRS17, Hamilton Depression Rating Scale, 17-items; BMI, body mass index; SD, standard deviation. Refractory depression: patients who had failed to respond to two or more antidepressants in the current major depressive episode. Severe Depression: MADRS ≥ 30. Significant p values (<0.05) are highlighted in bold.
3.2. Changes in BDNF plasma levels
According to the different models described, we did not find any significant main effects of time (F1,153=0.32, p=0.58), and also no interaction effects of time with group (F3,153=0.47, p=0.7), clinical response (F1,153=0.35, p=0.55) and time-× group × clinical response (F3,153=0.2, p=0.89). In other words, BDNF plasma levels showed no variation over time, regardless of treatment group and/or clinical response (Figure 1) (Table 2).
Figure 1.
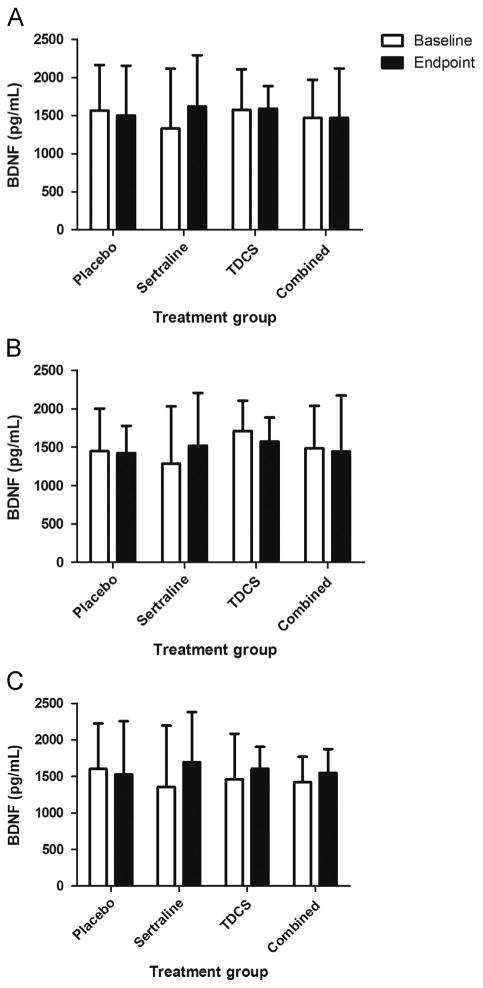
BDNF plasma levels before and after treatment. (A) Total sample (n=73), (B) results for patients presenting clinical response to treatment (n=34) and (C) for treatment non-responders (n=39). BDNF, brain-derived neurotrophic factor. Bars represent 1 standard deviation.
Table 2.
BDNF plasma levels at baseline and endpoint according to the treatment group and clinical response.
| BDNF | Placebo | Sertraline-only | tDCS-only | Combined treatment |
|---|---|---|---|---|
| Baseline | 1565 (597) | 1331 (786) | 1577 (529) | 1468 (501) |
| Endpoint | 1502 (653) | 1620 (672) | 1588 (297) | 1470 (647) |
| Responders | ||||
| Baseline | 1447 (553) | 1287 (743) | 1709 (393) | 1484 (552) |
| Endpoint | 1421 (357) | 1518 (687) | 1570 (314) | 1446 (726) |
| Non-responders | ||||
| Baseline | 1603 (623) | 1356 (841) | 1459 (626) | 1420 (349) |
| Endpoint | 1527 (729) | 1695 (685) | 1608 (299) | 1546 (329) |
BDNF, brain-derived neurotrophic factor; tDCS, transcranial direct current stimulation. Values represent mean (standard deviation) of BDNF plasma levels (pg/mL).
Finally, when analyzing tDCS and sertraline in the same model as two separate variables (i.e., tDCS vs. no-tDCS and sertraline vs. no-sertraline), neither tDCS (40 and 37 patients in the active and sham arm, respectively, F1,153=0.33, p=0.58) nor sertraline (39 and 38 participants in the real and placebo arm, respectively, F1,153=0.78, p=0.36) changed BDNF plasma levels over time and according to clinical improvement.
3.3. Influence of other variables in BDNF plasma levels
In the model exploring the effects of group and clinical response of BDNF changes over time, we also performed several analyses to assess the influence of other variables on outcome (only one variable was assessed at a time). We also found no significant effects of gender (F1,153<0.01, p=0.95), benzodiazepine use (F1,153=0.01, p=0.91), age (F37,153=0.82, p=0.71), obesity (F=0.01, p=0.97), smoking status (F1,153=0.04, p=0.85), physical activity, indexed by the IPAQ questionnaire (F2,151=0.41, p=0.63), atypical depression (F1,153=3.21, p=0.07), melancholic depression (F1,153=3.22, p=0.07), severe depression at baseline (F1,153=2.59, p=0.11) and refractoriness (F1,153=0.67, p=0.77) on the outcome.
3.4. BDNF plasma levels and depression scores
There were no significant correlations between MADRS or HDRS scores changes with BDNF plasma changes (p=0.55 and 0.56, respectively). Also, no association was significant when considering each group separately (ps>0.1).
3.5. BDNF baseline levels as predictors of antidepressant response
To assess whether BDNF baseline plasma levels predict depression improvement, we performed an analysis of covariance using MADRS depression improvement as dependent variable, group as independent variable and BDNF as covariate. No effects for BDNF were found (F1,72=0.15, p=0.69), meaning that BDNF levels at baseline were not associated with depression improvement.
4. Discussion
In this factorial, randomized, placebo-controlled trial we examined BDNF plasma levels in 73 patients before and after a 6-week treatment course of active/sham tDCS and real/placebo sertraline. Our main findings were that, contrary to our initial hypotheses, (1) BDNF plasma levels did not significantly increase after tDCS and sertraline interventions, regardless of clinical improvement; (2) BDNF baseline levels were not predictors of the antidepressant response and (3) an increase in BDNF was not necessary to induce acute antidepressant effects. Only Palm et al. (2013) examined BDNF plasma levels after tDCS treatment, finding no increase in BDNF plasma levels in 19 participants who received active or sham tDCS. However, in contrast to Palm et al. who showed no clinical response after tDCS treatment, tDCS was an effective antidepressant treatment in the present study and, in addition, we collected BDNF in subjects who were not taking antidepressants at baseline and who were not on concomitant medications (except for placebo/sertraline and low-dose benzodiazepines that did not influence the outcome) throughout the trial, factors that may influence BDNF concentrations (Brunoni et al., 2008). In addition, Palm et al. attributed their negative results partly to the fact that their sample was mainly composed of patients with treatment-resistant depression. However, in our analysis only 40% of the sample was refractory, and this variable also did not affect the outcome. Finally, Palm et al. discussed that the timeframe between BDNF collections was possibly too short (2 weeks, before the crossover phase) to show a BDNF increase, however, we found similar results with a longer timeframe of 6 weeks. Therefore, we confirm and expand the results of Palm et al. that BDNF levels do not change after tDCS treatment by examining a large sample with different clinical characteristics and in a different study design.
As no other tDCS study evaluated BDNF levels besides Palm et al. and ours, it is important to evaluate BDNF blood changes in the context of other somatic therapies. Regarding rTMS, Zanardini et al. (2006) observed enhanced BDNF levels after treatment in 16 patients, whereas Lang et al. (2006) (n=14) and Gedge et al. (2012) (n=18) showed no changes after rTMS. Finally, Yukimasa et al. (2006) observed that BDNF levels increased in rTMS responders (n=9), but not in rTMS non-responders (n=16). For ECT, Bocchio-Chiavetto et al. (2006), in 16 treatment-resistant depressed patients and, later on, Marano et al. (2007) (n=15), Okamoto et al. (2008) (n=18), Piccinni et al. (2009) (n=18) and Haghighi et al. (2013) (n=20) showed an enhancement of BDNF levels; whereas Gedge et al. (2012) (n=11) and Fernandes et al. (2009) (n=15) did not show enhancement of BDNF levels after ECT. Therefore, most rTMS and ECT studies showed mixed or negative findings regarding BDNF increase after treatment. Nonetheless, these studies were methodologically limited as most did not employ a sham-controlled design and enrolled patients with concomitant antidepressant treatments. In spite of the methodological limitations of previous reports, our results are mostly in line with previous findings. It should be noted that our study enrolled a large (n=73) sample size to assess BDNF changes after a non-invasive brain stimulation intervention, and was the first using an active control (sertraline) and a placebo control. This particular design corroborates our findings, as it allowed us to look at the stability of measures over time, ruling out natural fluctuation in the course of illness.
Interestingly, BDNF levels did not increase after sertraline treatment, apparently in contrast with meta-analyses showing that BDNF levels increase after diverse pharmacological interventions (Brunoni et al., 2008; Molendijk et al., 2013; Sen et al., 2008). One possible reason is the low sertraline dose in the present study. However, despite using 50 mg/day of sertraline, patients on sertraline (vs. placebo) showed clinical improvement and this was without correlative changes in BDNF plasma levels, supporting that changes in BDNF levels are not necessary to acute changes in depressive symptoms. In addition, the combined (tDCS/sertraline) group showed greater antidepressant effects, although BDNF levels did not change in this group as well. In this context, the recent meta-analysis of Molendijk et al. (2013) highlighted evidence for a publication bias in the BDNF literature, i.e., studies that showed no BDNF increase after antidepressant treatment might not have been published. Another critical point is whether BDNF blood levels does reflect BDNF expression in the brain or is rather influenced by peripheral sources such as platelets (Karege et al., 2002). Although technically challenging, assessment of BDNF levels in the cerebrospinal fluid could be an alternative to assess directly the effects of antidepressant therapies on BDNF expression in the CNS.
One important implication of our results is that we showed that an increase in BDNF was not necessary to induce an antidepressant response regardless of the type of the intervention, as in all groups clinical response was not associated with BDNF increasing. In this context, BDNF baseline levels were not predictors of antidepressant response either, as observed in some pharmacological studies (Kurita et al., 2012; Tadic et al., 2011; Wolkowitz et al., 2011) but not in all (Umene-Nakano et al., 2010). Nonetheless, although BDNF meta-analyses supported the notion that depression improvement is associated with BDNF increasing, virtually all BDNF trials were not controlled; therefore it was not possible to disentangle antidepressant treatment effects from time effects and also from drug effects in platelets that store BDNF in the blood. Particularly for tDCS, our results are in line with our previous finding that the BDNF Val66Met polymorphism has no major impact on tDCS antidepressant response (Brunoni et al., 2013a). In fact, the role of this polymorphism on antidepressant drug response is also unclear (Domschke et al., 2010).
The main limitation of our study is that, although we enrolled a relatively large sample size considering non-invasive brain stimulation trials, the study might have still been underpowered for detecting a subtle impact of treatment on BDNF changes; although we controlled for biases associated with lower BDNF increase, notably the enrollment of an antidepressant-free sample. Therefore, considering the paucity of studies assessing BNDF changes in depressed patients after tDCS, further tDCS trials with large sample sizes might be necessary to better define the role of BDNF in tDCS antidepressant response.
Acknowledgments
This study was partially funded by FAPESP (São Paulo Research Foundation, Grant number: 2009/05728-7), FAPEMIG and CNPq. The sponsors played no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, and preparation, review or approval of the manuscript. ARB receives a research grant from FAPESP (2012/20911-5) and is recipient of a 2013 NARSAD Young Investigator Award. CAZ is listed as a coinventor on a patent application for the use of ketamine and its metabolites in major depression. CAZ has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government. The Laboratory of Neuroscience receives financial support from the Associação Beneficente Alzira Denise da Silva (ABADHS).
Role of funding source: This study was partially funded by FAPESP (São Paulo Research Foundation, Grant number: 2009/05728-7), FAPEMIG and CNPq. The sponsors played no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, and preparation, review or approval of the manuscript.
Footnotes
Contributors: ARB: collected data, analyzed data, drafted and reviewed the manuscript.
Conflict of interest: CAZ is listed as a coinventor on a patent application for the use of ketamine and its metabolites in major depression. CAZ has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government.
References
- Amorim P. Mini International Neuropsychiatric Interview (MINI): validation of a short structured diagnostic psychiatric interview. Rev Bras Psiquiatr. 2000;22:106–115. [PubMed] [Google Scholar]
- Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591:1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Zanardini R, Bortolomasi M, Abate M, Segala M, Giacopuzzi M, Riva MA, Marchina E, Pasqualetti P, Perez J, Gennarelli M. Electroconvulsive Therapy (ECT) increases serum Brain Derived Neurotrophic Factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol. 2006;16:620–624. doi: 10.1016/j.euroneuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Ferrucci R, Bortolomasi M, Scelzo E, Boggio PS, Fregni F, Dell'Osso B, Giacopuzzi M, Altamura AC, Priori A. Interactions between Transcranial Direct Current Stimulation (tDCS) and pharmacological interventions in the Major Depressive Episode: findings from a naturalistic study. Eur Psychiatry. 2012a doi: 10.1016/j.eurpsy.2012.09.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Boggio PS, Giacopuzzi M, Barbieri S, Priori A. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011a;35:96–101. doi: 10.1016/j.pnpbp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Kemp AH, Shiozawa P, Cordeiro Q, Valiengo LC, Goulart AC, Coprerski B, Lotufo PA, Brunoni D, Perez AB, Fregni F, Bensenor IM. Impact of 5-HTTLPR and BDNF polymorphisms on response to sertraline versus transcranial direct current stimulation: implications for the serotonergic system. Eur Neuropsychopharmacol. 2013a doi: 10.1016/j.euroneuro.2013.03.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012b;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Baccaro A, Zanao TA, de Oliveira JF, Vieira GP, Bueno VF, Goulart AC, Boggio PS, Lotufo PA, Bensenor IM, Fregni F. Sertraline vs. electrical current therapy for treating depression clinical trial - SELECT TDCS: design, rationale and objectives. Contemp Clin Trials. 2011b;32:90–98. doi: 10.1016/j.cct.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Baccaro A, Zanao TA, Oliveira AC, Goulart AC, Boggio PS, Lotufo PA, Bensenor IJ, Fregni F. The sertraline versus Electrical Current Therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013b;70:383–391. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Dell'Osso B, Mundo E, D'Urso N, Pozzoli S, Buoli M, Ciabatti M, Rosanova M, Massimini M, Bellina V, Mariotti M, Altamura AC. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disorders. 2009;11:76–81. doi: 10.1111/j.1399-5618.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- Domschke K, Lawford B, Laje G, Berger K, Young R, Morris P, Deckert J, Arolt V, McMahon FJ, Baune BT. Brain-derived neurotrophic factor (BDNF) gene: no major impact on antidepressant treatment response. Int J Neuropsychopharmacol. 2010;13:93–101. doi: 10.1017/S1461145709000030. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Fernandes B, Gama CS, Massuda R, Torres M, Camargo D, Kunz M, Belmonte-de-Abreu PS, Kapczinski F, de Almeida Fleck MP, Ines Lobato M. Serum brain-derived neurotrophic factor (BDNF) is not associated with response to electroconvulsive therapy (ECT): a pilot study in drug resistant depressed patients. Neurosci Lett. 2009;453:195–198. doi: 10.1016/j.neulet.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Bortolomasi M, Brunoni AR, Vergares M, Tadini L, Giacopuzzi M, Priori A. Comparative benefits of Transcranial Direct Current Stimulation (tDCS) treatment in patients with mild/moderate vs. severe depression Clin Neuropsychiatry. 2009;6:246–251. [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Gedge L, Beaudoin A, Lazowski L, du Toit R, Jokic R, Milev R. Effects of electroconvulsive therapy and repetitive transcranial magnetic stimulation on serum brain-derived neurotrophic factor levels in patients with depression. Front Psychiatry. 2012;3:12. doi: 10.3389/fpsyt.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C, Andrade LHSG, Zuardi AW. Escalas de Avaliação Clínica em Psiquiatria e Psicofarmacologia, Sao Paulo 2000 [Google Scholar]
- Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME. Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression - a preliminary report. Biol Psychiatry. 2008;64:281–285. doi: 10.1016/j.biopsych.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Haghighi M, Salehi I, Erfani P, Jahangard L, Bajoghli H, Holsboer-Trachsler E, Brand S. Additional ECT increases BDNF-levels in patients suffering from major depressive disorders compared to patients treated with citalopram only. J Psychiatr Res. 2013;47:908–915. doi: 10.1016/j.jpsychires.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- Kurita M, Nishino S, Kato M, Numata Y, Sato T. Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study. PLoS One. 2012;7:e39212. doi: 10.1371/journal.pone.0039212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang UE, Bajbouj M, Gallinat J, Hellweg R. Brain-derived neurotrophic factor serum concentrations in depressive patients during vagus nerve stimulation and repetitive transcranial magnetic stimulation. Psychopharmacology (Berlin) 2006 doi: 10.1007/s00213-006-0399-y. [DOI] [PubMed] [Google Scholar]
- Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br J Psychiatry. 2012;200:52–59. doi: 10.1192/bjp.bp.111.097634. [DOI] [PubMed] [Google Scholar]
- Marano CM, Phatak P, Vemulapalli UR, Sasan A, Nalbandyan MR, Ramanujam S, Soekadar S, Demosthenous M, Regenold WT. Increased plasma concentration of brain-derived neurotrophic factor with electroconvulsive therapy: a pilot study in patients with major depression. J Clin Psychiatry. 2007;68:512–517. doi: 10.4088/jcp.v68n0404. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484) Mol Psychiatry. 2013 doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6:424–432. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Monte-Silva KK, Kuo MF, Liebetanz D, Paulus W, Nitsche MA. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS) J Neurophysiol. 2010;103:1735–1740. doi: 10.1152/jn.00924.2009. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Kuo MF, Karrasch R, Wachter B, Liebetanz D, Paulus W. Serotonin affects transcranial direct current-induced neuroplasticity in humans. Biol Psychiatry. 2009;66:503–508. doi: 10.1016/j.biopsych.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt. 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Yoshimura R, Ikenouchi-Sugita A, Hori H, Umene-Nakano W, Inoue Y, Ueda N, Nakamura J. Efficacy of electroconvulsive therapy is associated with changing blood levels of homovanillic acid and brain-derived neurotrophic factor (BDNF) in refractory depressed patients: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1185–1190. doi: 10.1016/j.pnpbp.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Palm U, Fintescu Z, Obermeier M, Schiller C, Reisinger E, Keeser D, Pogarell O, Bondy B, Zill P, Padberg F. Serum levels of brain-derived neurotrophic factor are unchanged after transcranial direct current stimulation in treatment-resistant depression. J Affect Disord. 2013 doi: 10.1016/j.jad.2013.03.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Piccinni A, Del Debbio A, Medda P, Bianchi C, Roncaglia I, Veltri A, Zanello S, Massimetti E, Origlia N, Domenici L, Marazziti D, Dell'Osso L. Plasma Brain-Derived Neurotrophic Factor in treatment-resistant depressed patients receiving electroconvulsive therapy. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol. 2009;19:349–355. doi: 10.1016/j.euroneuro.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Tadic A, Wagner S, Schlicht KF, Peetz D, Borysenko L, Dreimuller N, Hiemke C, Lieb K. The early non-increase of serum BDNF predicts failure of antidepressant treatment in patients with major depression: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:415–420. doi: 10.1016/j.pnpbp.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Umene-Nakano W, Yoshimura R, Ueda N, Suzuki A, Ikenouchi-Sugita A, Hori H, Otani K, Nakamura J. Predictive factors for responding to sertraline treatment: views from plasma catecholamine metabolites and serotonin transporter polymorphism. J Psychopharmacol. 2010;24:1764–1771. doi: 10.1177/0269881109106899. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Hashimoto E, Ukai W, Ishii T, Yoshinaga T, Ono T, Tateno M, Watanabe I, Shirasaka T, Saito S, Saito T. Effect of antidepressants on brain-derived neurotrophic factor (BDNF) release from platelets in the rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1450–1454. doi: 10.1016/j.pnpbp.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Wolf J, Shelly W, Rosser R, Burke HM, Lerner GK, Reus VI, Nelson JC, Epel ES, Mellon SH. Serum BDNF levels before treatment predict SSRI response in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1623–1630. doi: 10.1016/j.pnpbp.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukimasa T, Yoshimura R, Tamagawa A, Uozumi T, Shinkai K, Ueda N, Tsuji S, Nakamura J. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry. 2006;39:52–59. doi: 10.1055/s-2006-931542. [DOI] [PubMed] [Google Scholar]
- Zanardini R, Gazzoli A, Ventriglia M, Perez J, Bignotti S, Rossini PM, Gennarelli M, Bocchio-Chiavetto L. Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients. J Affect Disord. 2006;91:83–86. doi: 10.1016/j.jad.2005.12.029. [DOI] [PubMed] [Google Scholar]


