Abstract
Patients harboring brain arteriovenous malformation (bAVM) are at life-threatening risk of rupture and intracranial hemorrhage (ICH). The pathogenesis of bAVM has not been completely understood. Current treatment options are invasive and ≈ 20% of patients are not offered interventional therapy because of excessive treatment risk. There are no specific medical therapies to treat bAVMs. The lack of validated animal models has been an obstacle for testing hypotheses of bAVM pathogenesis and testing new therapies. In this review, we summarize bAVM model development; and bAVM pathogenesis and potential therapeutic targets that have been identified during model development.
Keywords: Activin-like kinase 1, Angiogenesis, Brain arteriovenous malformation, Conditional knockout, Endoglin, Hereditary hemorrhagic telangiectasia, Mouse models
Introduction
Brain arteriovenous malformations (bAVMs) are complex tangles of abnormal, dilated channels that do not have a typical artery or vein structure. They are important risk factors of intracranial hemorrhage (ICH), especially in children and young adults [1–3]. The etiopathogenesis is currently not well understood. Prevention of new or recurrent ICH is the primary rationale to treat AVMs, with some combination of resection, embolization and/or radiotherapy. All of these therapies are invasive and associated with considerable side effects [4–6]. Other than nonspecific control of symptomatology, e.g., headache and seizures, no specific medical therapy is available to directly treat AVMs or decrease spontaneous rupture risk. About 20% of patients currently are not offered treatment due to excessive risks associated with the treatment [4]. There is also considerable controversy regarding unruptured bAVMs being treated using invasive modalities, as treatment risk may outweigh the natural history risk of spontaneous rupture [6]. The lack of proper animal models has critically hampered research progress and new therapy development.
Brain AVMs are traditionally regarded as congenital lesions, which are thought to arise in the third week of gestation secondary to disordered embryogenesis. Primordial vascular channels fail to differentiate into mature intervening capillaries and veins, and instead create arteriovenous shunts without intervening capillaries [7, 8]. However, despite frequent use of prenatal ultrasound, there is remarkably little evidence to show that AVMs are congenital lesions arising during embryonic development. In fact, the mean age at presentation (detection) is roughly 40 years of age, with normal distribution. Although it is possible that the lesions uniquely arise prenatally (and a small number do), lacking sufficient data, it would be premature to infer an adequate explanation. There are multiple reports of AVM growing or regressing, and of local AVM regrowth after treatment [9]. AVMs have been shown to occasionally arise de novo after a normal angiogram and regrow after resection, either de novo from a retained fragment [10–12] or from a lesion treated with radiotherapy [12, 13]. This evidence supports the hypothesis that bAVM can form postnatally.
More than 95% of bAVMs are sporadic [14]. The genesis of bAVMs has been enigmatic. About 5% of bAVM are due to hereditary hemorrhagic telangiectasia (HHT) [15], a familial disease characterized by AVMs in multiple organs and mucocutaneous telangiectasias (small AVMs) [16]. The two main subtypes of HHT (HHT 1 & 2) are caused by mutations in two genes implicated in transforming growth factor-β (TGF-β)/bone morphogenic protein (BMP) canonical signaling pathways: Endoglin (ENG), and Activin-like kinase 1 (ALK1; ACVLR1) [17]. As a class, the inherited AVMs in HHT have some distinguishing morphological features, but are generally similar to the sporadic lesions and cannot be distinguished individually on the basis of their angioarchitecture [18, 19]. The prevalence of bAVM in HHT1 (ENG-deficient) is 1000-fold higher, and HHT2 (ALK1-deficient) is 100-fold higher than the prevalence in the general population (10/100,000) [20]. Modeling HHT1 and HHT2 bAVMs has been fruitful.
This review discusses the strategies that have been used for bAVM model development, and the pathogenesis as well as potential new therapeutic targets (Fig. 1) that have been identified using available models.
Fig. 1. Novel theories of AVM initiation and progression, and new therapeutic targets.
EC: endothelial cell; BMDC: bone marrow-derived cells; sFLT: soluble FMS-related tyrosine kinase 1 (sFLT1), also called VEGF receptor-1
Modeling Brain AVM in Animals
Historically, “AVM” models have been largely based on extradural arteriovenous (A–V) fistulas to study hemodynamic changes or develop platforms for technology development [21–38]. They can be categorized into 2 subtypes. 1) Hemodynamic models, in which the normal extracranial vasculature is surgically manipulated to create a shunt from the contralateral side through the Circle of Willis and into the ipsilateral jugular vein without an intervening capillary bed. This is most commonly accomplished by anastomosing the common carotid artery to the jugular vein [21, 22, 35]. 2) Angiographic models, which utilize essentially the same anastomotic technique to achieve high-flow, low-resistance hemodynamics that take advantage of the “AVM-like” angiographic appearance of the rete mirabile normally present in artiodactyl (even-toed ungulates) species [24, 25]. With few exceptions [38], they are extradural in nature; none display the clinical syndrome of recurrent hemorrhage into the brain parenchyma or cerebrospinal fluid (CSF) spaces. No other model has a parenchymal nidus. Therefore nidus growth and hemorrhage mimicking the human disease do not occur.
Many different kinds of developmental gene defects result in antenatal hemorrhage, which may or may not be related to brain AVMs. The proteins identified in studies of brain hemorrhage may be related to AVM biology, such as integrin αVβ8 [39]. Manipulating the proteins of interest may yield vascular structures reminiscent of the human disease. For example, endothelial expression of constitutively active Notch-4 elicited reversible “AVMs” in adult mice [40], or endothelial overexpression of Notch-4 intracellular domain resulted in brain AVMs in young mice [41]. Knockout integrin αVβ8 plus focal vascular endothelial growth factor (VEGF) stimulation induced capillary dysplasia in the brain [42]. In addition, homozygous knockout of matrix Gla protein (Mgp) have also resulted in AVM formation in the brain and multiple organs [43]. However, the story becomes more interesting when such models are focused on genes that are clearly related to the human disorder, i.e., those genes described above which underlie HHT. A logical approach to animal models is to focus on genes that are clearly related to the human disease phenotype.
An important conceptual advance in modeling brain AVMs has been to consider HHT [44] as a familial form of the more common sporadic disorder, or at least posit that HHT possesses a similar enough phenotype to sporadic brain AVM so that knowledge of the inherited gene pathways can shed light on sporadic disease pathogenesis.
Inactivating a single allele of Eng or Alk1 in mice reproduces certain aspects of the human disease in animal models [45, 46], but spontaneous lesions in the brain are rare and subtle, mostly in aged mice [45, 47]. More pronounced forms of cerebral microvascular dysplasia can be induced using VEGF stimulation in Eng+/− or Alk1+/− mice [48–50], which can be enhanced by increasing tissue perfusion rates [49]. However, the dysmorphic vessels developed in Eng+/− or Alk1+/− mice are at the capillary level. No arteriovenous (A–V) shunt can be detected.
Loss of both alleles of Eng or Alk1 in mice is embryonically lethal [51, 52]. Oh and colleagues have created A–V fistulas in the neonatal brain through knockout of Alk1 from Alk1-expressing cells [53]. Brain and spinal cord AVMs have also developed in mice with SM22α-Cre-mediated Alk1 deletion during the embryonic developmental stage [54]. However, most of the mice died shortly after birth.
Our group has developed the first adult onset brain AVM model using a combination of focal Alk1 homozygous deletion and VEGF stimulation (Table 1, Figs. 2 & 3) [55]. This model mimics many aspects of the human bAVM lesion, such as A–V shunting, microhemorrhage and macrophage infiltration [55–57]. Since an adenoviral vector is used to mediate cre expression (Ad-Cre) in this model, the inflammation caused by the adenoviral vector complicates the mechanism analysis (Table 1). In addition, Ad-Cre could not mediate significant Eng deletion in Engf/f mice [58].
Table 1.
Brain AVM mouse models
| Models | Onset | Advantages | Disadvantages |
|---|---|---|---|
| Ad-Cre/AAVVEGF/ Alk1f/f |
Adult | Low mortality. No AVM in other organs. |
Inflammation caused by adenoviral vector complicates mechanistic analysis. |
|
Pdgfb-iCreER/AAVVE GF/Alk1f/f |
Adult | Brain AVM develops in a relatively shorter time. |
AVM develops in multiple organs and mice die 10–14 days after tamoxifen- |
| Rosa-CreER/AAV-VEGF/Engf/f | Adult | Low mortality. | AVM develops in multiple organs. |
| SM22α-Cre/Engf/f | Embryonic | AVM develops spontaneously. A good model for mechanistic study and for new drug testing. |
Embryonic onset. 50% mice die before 6 weeks of age. |
Fig. 2. Development of adult onset brain AVM models.
AAV1-VEGF [2×109 viral genome (vg)] is used to stimulate brain focal angiogenesis. Ad-Cre (Ad-Cre/AAV-VEGF/Alk1f/f model), Pdgfb-icreER (pdgfb-icreER/AAV-VEGF/Alk1f/f) and Rosa-CreER (Rosa-CreER/AAV-VEGF/Engf) are used to delete Alk1 or Eng in Alk1f/f or Engf/f mice.
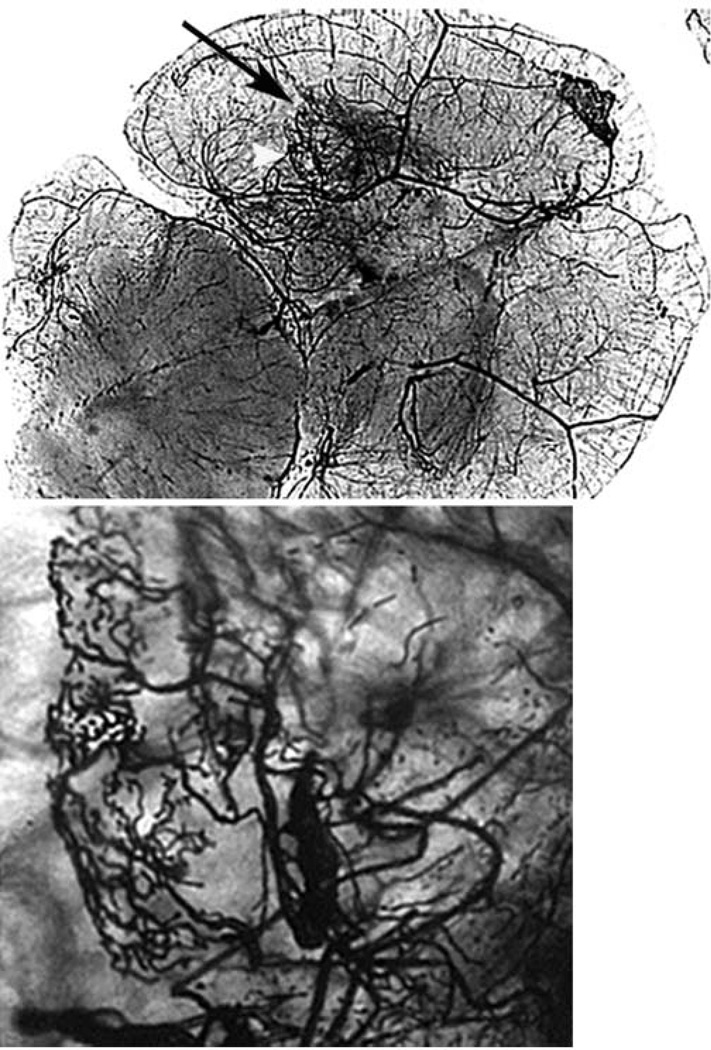
Fig. 3. Vessel casting showing AVM in the brain angiogenic region.
Large tangled vessels resembling bAVM were detected at the injection site of Ad-Cre and AAV-VEGF in the brain of Alk1-floxed mice (black arrow). Bottom images show the enlarged angiogenic foci of the images at the top. Scale bar = 100µm.
Using Cre transgenic mouse lines, we have now developed two other adult onset models (Figure 2) [59, 60] and one developmental onset bAVM model that have low mortality [60] (Table 1). Fully-developed bAVMs were detected in adult R26CreER;Engf/f mice eight weeks after induction of global Eng gene deletion and brain angiogenesis (Figs. 2 & 4), and in Pdgfb-iCre;Alk1f/f; mice four weeks after induction of brain angiogenesis and two weeks after induction of endothelial Alk1 deletion (Fig. 2). The bAVM that developed in Pdgfb-iCre;Alk1f/f mice occurred in a relatively shorter time. The mice died 10–14 days after tamoxifen-induced Alk1 deletion. The bAVM in R26CreER;Engf/f mice developed more slowly. The mice survived for an extended period, more than eight weeks after Eng deletion (Table 1, Fig. 2). Thus, this model is more suited for testing new therapies.
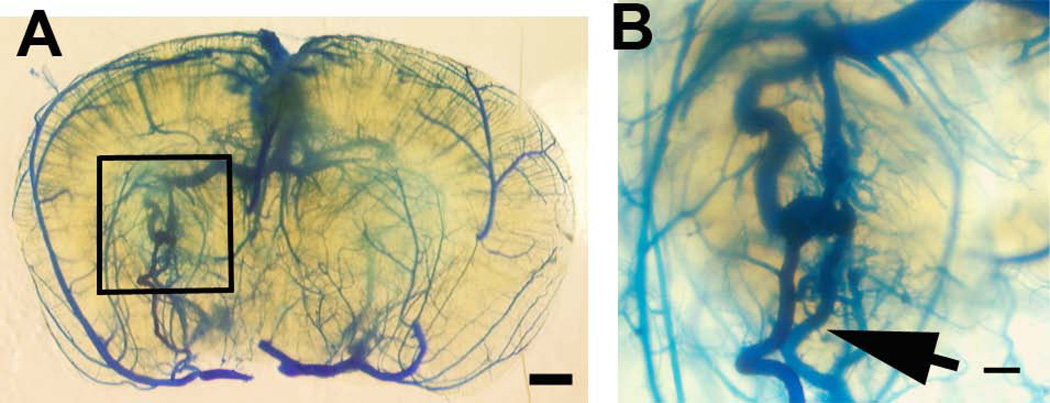
Fig. 4. Adult onset bAVM of Eng2f/2f;R26CreER mice after TM and VEGF treatment.
(A) AVM (squared region) in the brain of Eng2f/2f;R26CreER mice 8 weeks after intra-brain injection of AAV-VEGF and intraperitoneal injection of TM. (B) Enlarged image of the AVM lesion. Latex-perfused veins are clearly shown (arrows). Scale bars: 1 mm in (A) and 200 µm in (B).
The developmental onset bAVM model was developed by using SM22α-Cre transgenic line to delete Eng during the embryonic developmental stage. Unlike conventional Eng-homozygous knockout mice (Eng−/−) that are embryonically lethal [61–63], SM22α-Cre;Engf/f mice were born with, and in life developed, various degrees of AVMs in the central nervous system, with more that 95% penetrance at five weeks of age (Table 1) [60]. They showed important clinical aspects of human lesions, including A–V shunting and spontaneous hemorrhages (Fig. 5). Further, AVM phenotypes were similar to those previously observed in SM22α-Cre;Alk1f/f mice [54]. These mice, however, had less lethality. Since bAVMs in this model were developed spontaneously without local manipulation, their lesion progression more closely mimics human disease, and thus is a better model than others for bAVM mechanistic study and for new drug testing (Table 1).
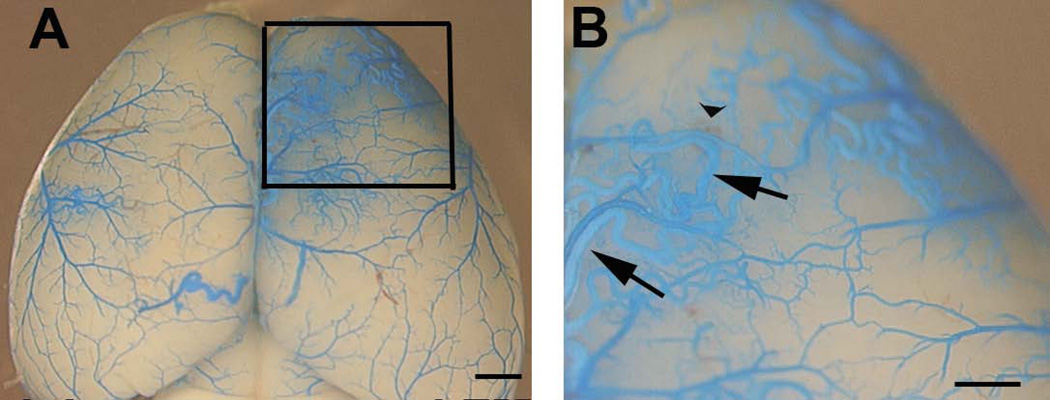
Fig. 5. Developmental onset AVMs in the postnatal brain of Eng2f/2f;SM22α-Cre mice.
(A) Representative images of latex dye casting show the AVM vessels (squared region) in the brain of 5-week-old Eng2f/2f;SM22α-Cre (B) Enlarged images of dotted boxes shown in (A). Arrows indicate latex-casted veins. Arrow head indicates hemorrhage. Due to the particle size in the latex, the dye enters the vein after intra-left cardiac perfusion only when the A–V shunts are present. Scale bars: 1 mm in (A) and 500 µm in (B).
Brain AVM Pathogenesis
Molecular and histological analysis of human bAVM specimens shows that the level of angiogenic factors and inflammatory cytokines are higher in bAVMs than in the normal brain. bAVMs are also infiltrated with inflammatory cells [16, 56, 57, 64–68]. However, the pathogenesis of bAVMs is not completely understood. By modeling HHT bAVMs in animals and analyzing the phenotype of these models, we have identified the following factors playing a role in bAVM pathogenesis (Fig. 1).
(1) Homozygous causative gene deletion in endothelial cells
The prevailing view is that HHT is caused by haploinsufficiency of one of its causative genes in somatic endothelial cells. However, inactivation of the remaining wild-type allele appears to have a powerful effect, irrespective of the mechanism by which it is inactivated, e.g., loss of heterozygosity or loss of protein during inflammation [69]. As mentioned above, the loss of a single allele of one of the causative genes for HHT is ineffective for bAVM formation in mice [45, 46]. In contrast, loss of both alleles of any HHT-causative gene is embryonically lethal [51, 52], and conditional (tissue/time-specific) homozygous deletion of Eng [69] or Alk1 [53, 54] results in striking vascular malformations resembling the AVMs found in HHT [53, 54]. We showed that homozygous knockout of Eng in just ∼1% endothelial cells in mice resulted in a more severe cerebrovascular dysplasia after VEGF stimulation than in Eng+/− mice [58]. Eng null endothelial cells were present in vessels in bAVM lesions in SM22α;Engf/f mice and RosaCreER;Engf/f mice [60]. Moreover, analysis of human brain and lung AVMs in HHT indicates that haploinsufficiency of ENG is not sufficient to cause lesion development [70]. Interestingly, bAVMs developed only in Mgp−/− mice, not in Mgp+/− mice [43]. In addition, there is compelling proof-of-principle evidence that loss of function of the wild-type allele is relevant to vascular malformations, demonstrated in two related disorders: somatic mucocutaneous venous malformations [71], and cerebral cavernous malformations [72].
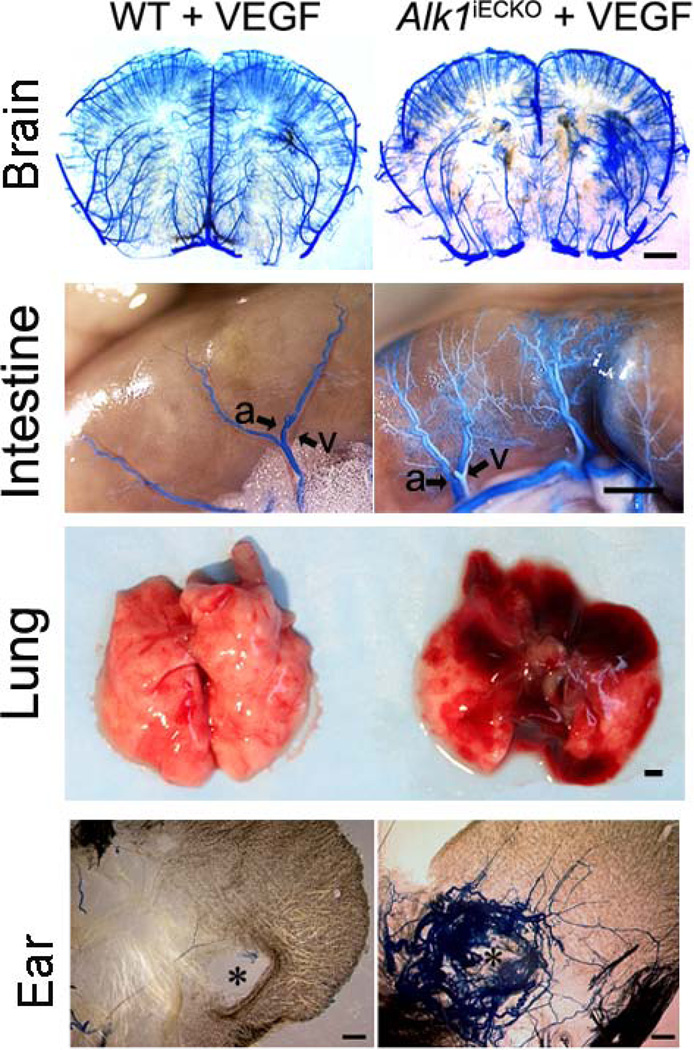
Although Alk1 has been reported to be predominantly expressed in endothelial cells [73], it has also been shown in smooth muscle cells and splenic macrophages [74]. Milton et al recently reported that Alk1 deletion driven by SM22α-Cre resulted in AVMs in the brain and spinal cord [54]. We have explored the cellular loci of endogenous Alk1 in bAVM pathogenesis, by cross breeding Alk1f/f mice with Pdgfb-iCreER (endothelial-specific promoter), NG2-iCreER (pericyte-specific promoter), or LysM-Cre (macrophage-specific promoter) mice. Neither macrophage-nor pericyte-Alk1 gene deletion caused mortality in embryonic or neonatal mice [59]. Postnatal VEGF stimulation did not lead to an AVM phenotype in any organ, including the brain. However, endothelial-Alk1 deletion led to spontaneous AVM development in the intestine and lung, and in the brain after angiogenic stimulation [59]. AVMs also developed around ear wounds (Fig. 6) [59]. Similarly, deletion of Eng in all cell-types in adult mice by Rose-CreER transgene led to AVM formation in the brain after angiogenic stimulation, and around the ear wounds[60]. No AVM developed in any organs in LysM-Cre;Engf/f mice, including the brain after angiogenic stimulation [60]. This suggests that homozygous causative gene deletion in endothelial cells is a requirement for AVM formation.
Fig. 6. Brain AVM in mice with endothelial-Alk1 deletion and focal VEGF stimulation (Alk1iECKO+VEGF).
AVMs also developed in the intestine, lung and around the ear-tag wound. a: artery: v: vein. Scale bars: 1 mm.
(2) Response-to-injury and angiogenic stimulation
The bAVM phenotype includes an active angiogenic and inflammatory component that is inconsistent with a static congenital anomaly [75]. Although HHT patients have genome-wide haploinsufficiency of one of the causative genes, AVMs did not form at random locations. In addition, only a few vascular dysplasia developed in the brain of Eng+/− [76] and Alk1+/− [45] adult mice, and was only seen in older mice [45, 47]. There were more pronounced forms of cerebral microvascular dysplasia in the VEGF-stimulated brain at the angiogenic foci in Eng+/−or Alk1+/− mice [48–50].
We hypothesize that the environmental stimulus (injury) is required for triggering bAVM formation. Induction of Alk1 gene deletion in adult mice resulted in AVM and hemorrhage in the lung and gastroinstestinal tract, but not in the skin or brain. Upon wounding, Alk1-deleted mice developed vascular dysplasia and A–V shunts around the skin wound [53]. A macroscopic level of vascular dysplasia that mimics many phenotypes of human bAVM was induced by injecting AAV-VEGF (an adeno-associated viral vector expressing VEGF) into the basal ganglia of Alk1-deleted brain [55].
Taken together, both genetic manipulation and angiogenic stimulation are required for AVM development. The angiogenic stimulus can be a minor injury, exogenous growth factor delivery, or high endogenous angiogenic factors in the brain of young and perinatal individuals.
(3) Impaired mural cell recruitment
Brain AVM can cause ICH and serious neurological disability or death. ICH is the first clinical symptom in about 50% of bAVM patients. The malformed vessels are fragile and prone to rupture, causing bleeding into the brain. We showed that 30% of unruptured and non-hemorrhagic bAVMs demonstrated microscopic evidence of hemosiderin in the vascular wall [56]. The presence of silent intralesional microhemorrhages may be a biomarker for the risk of ICH. However, the underlying mechanisms for bAVM rupture and micro-hemorrhage are not fully understood.
Analyzing our established bAVM model, we found that vascular mural cell coverage is reduced in the AVM lesion and accompanied by vascular leakage and microhemorrhage [55, 57]. Many dysplastic vessels do not have a smooth muscle cell layer. Iron-deposition (Prussian blue positive staining) is present around the dysplastic vessels, and the vascular pericytes are reduced in the bAVM lesions as well [65]. Knockdown of ALK1 attenuates the increase of PDGFB in human brain microvascular endothelial cells (HBMECs) following VEGF stimulation, and reduces the ability of HBMEC to recruit pericytes [65]. Our data suggest that PDGFB signaling could be one of the underlying mechanisms for vascular destabilization and microhemorrhage (Fig. 1).
(4) Bone marrow-derived cells
VEGF stimulation resulted in dysplasia at the capillary level in the brain of Eng+/− mice [50]. Similar degrees of cerebrovascular dysplasia developed in the brain of wild-type (WT) mice transplanted with bone marrow (BM)-derived from Eng+/− mice following VEGF stimulation. In addition, the dysplasia in Eng+/− mice could be partially rescued by transplantation of WT BM [77]. This suggests that Eng haploinsufficiency in BM-derived cells is sufficient to cause cerebrovascular dysplasia in the adult mouse after angiogenic stimulation.
So far, the cell type(s) in the BM underlying AVM formation are unknown. There is evidence for two primary—probably complementary—cell types that serve as a locus for the phenomena: (1) BM-derived endothelial cells that incorporate into the angiogenic neovasculature [77, 78]; and (2) BM-derived monocytes/macrophages that may provide critical repair functions in response to injury [79–82] and/or provide guidance involving Notch signaling during angiogenesis [83, 84]. However, deletion of Alk1 or Eng in macrophages alone did not cause AVM formation, suggesting that gene-deficiency in macrophages is not an initiating factor. The involvement of macrophages might be associated with pathological vascular remodeling and vascular destabilization.
The involvement of BM-derived endothelial cells in focal angiogenesis has been shown in several conditions, such as tumor formation. BM-derived endothelial cells seed tumor vascular beds, regulating tumor angiogenesis [85, 86], and can incorporate into vessels in the brain angiogenic foci in mouse models [77, 78, 87]. In addition, endothelial cell progenitor cells have been identified in vessels in adult human sporadic bAVMs [88].
(5) Inflammatory cells and cytokines
Supporting evidence for myeloid cells playing a critical role in AVM progression include: (1) most of the BM- derived cells that home to the brain angiogenic foci are CD68+ or CD45+ [77, 87]; and (2) intra-peritoneal administration of neutrophil neutralizing antibody reduces AAV-VEGF-mediated angiogenesis and MMP-9 activity [89]. Other experiments suggest that both neutrophils and macrophages are relevant to large vessel remodeling as well [90].
Normal human monocytes rescue the impairment of Eng+/− mice in repairing myocardial injury, whereas monocytes from HHT patients fail to improve the myocardial repair [79, 81]. HHT1 monocytes migrate to SDF1α less effectively than normal monocytes, which is associated with an increase of CD26 expression [79, 81]. These data suggest that the function of monocytes in vascular repair or remodeling is defective in HHT patients, which could result in abnormal vascular remodeling and thus promote AVM progression.
Abnormal expression patterns of inflammatory mediators and cytokines, as well as an influx of inflammatory cells into AVMs have been observed by a number of investigators [91–95]. Inflammatory markers are overexpressed in human AVMs, including myeloperoxidase (MPO) and IL-6, both of which highly correlate with matrix metalloprotease-9 (MMP-9) expression.
Remodeling of the vascular network in AVMs is facilitated by a number of proteases that can enlarge the vascular elements in the nidus. This remodeling is partially mediated through VEGF activity and modulated by pro-angiogenic signals such as MMP expression. MMPs maintain and remodel the extracellular matrix [96]. MMPs, including MMP-9, are major components of neutrophil tertiary granules and are also synthesized by monocytes and lymphocytes. MMP-9 is expressed at significantly higher levels in bAVMs than in control tissue [92, 97], but the source of this expression is unclear. MMP-9 expression and activity during inflammation are stimulated by the cytokines IL-8, IL-1 β, and IL-6. MMP-9 degrades key components of the cerebrovascular matrix including laminin, denatured collagen, and tight junction proteins such as ZO-1 leading to blood-brain barrier leakage and hemorrhage [98, 99]. MMP-9 is expressed in the endothelial cell/peri-endothelial cell layer of AVMs. Along with endothelial and smooth muscle cells, inflammatory cells seem to be a major contributor to the abnormally high levels of MMP-9 in AVM tissue [92]. The MMP-9 signal co-localizes with MPO, and expression correlates with both MPO and IL-6 levels, which suggests that the source of MMP-9 levels may be the inflammatory cells in the environment [92]. In addition, BM-derived cells that home to bAVMs might also be an MMP-9 source [78].
Soluble ENG (extracellular domain) has been shown to contribute to another vascular disease: preeclampsia [100]. Soluble ENG (sENG) is distinct from long (L) and short (S) form ENG, which have cytoplasmic tails of 47 and 14 amino acids, respectively [101]. sENG also increases in bAVMs [102]. It is not clear how sENG is formed. A related Type III TGF-β receptor, betaglycan, appears to be shed through a process that is mediated by MMP-1 [103]. Several different MMPs are also found in AVM nidal tissue [92, 104, 105], suggesting that similar mechanisms may contribute to the formation of sENG or soluble ALK1 (sALK1) [100]. TNF-α can induce the release of sENG from normal placental villous explants [106]. Thus, inflammatory proteins and cytokines in AVMs could cause shedding of sENG and promote bAVM instability. Another interesting observation is the increased levels of immunoglobulins within bAVMs when compared to the control brain [93].
Vascular inflammation is central to the pathogenesis of several vascular diseases [107, 108], including intracranial aneurysm growth [109, 110] and abdominal aortic aneurysm formation [111, 112]. In addition, associations between single nucleotide polymorphisms (SNPs) in cytokines such as TNF-α and increased bAVM intracerebral hemorrhage risk have been described [113]. SNPs in IL-6 were also associated with a hemorrhagic clinical presentation in bAVM patients [91], and the highest risk genotype IL-6 (GG) was associated with the highest IL-6 expression levels in bAVM tissue [105].
(6) Hemodynamic changes
Vessels in an AVM are subjected to higher-than-normal flow rates. High vascular flow rates in ALK1+/− mice using vasodilators (nicardipine or hydralazine) after focal VEGF stimulation increased the number of dysplastic vessels in the brain angiogenic foci [49].
Cerebral venous hypertension is a common symptom in bAVMs [114]. Cerebral venous hypertension causes at least one kind of fistula (dural arteriovenous fistula) to form [27, 30] through a mechanism involved in the induction of angiogenesis [27]. In rats, non-ischemic levels (15–23 mmHg) of cerebral venous hypertension cause expression of HIF-1α, and its downstream signal, VEGF [115]. Further, HIF-1α, VEGF, SDF-1α expression, neutrophils, macrophage and MMP-9 activity increase in the brain of the mouse cerebral venous hypertension model. Capillary density in the parasagittal cortex also increases in the mouse VH model. These findings suggest that mild nonischemic cerebral venous hypertension results in a pro-angiogenic state [116]. Thus, cerebral venous hypertension could represent a kind of injury that triggers bAVM development in subjects carrying mutant genes.
Hemodynamic stress can also trigger vascular inflammation that initiates vascular remodeling and angiogenesis. High shear stress activates endothelial cells and upregulates leukocyte adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and monocyte chemotactic protein-1 (MCP-1) [117–120]. Shear stress activates endothelial and smooth muscle cells and promotes their production and release of angiogenic factors and other cytokines critical for vascular remodeling [121, 122].
New Therapeutic Targets
Current treatment options for bAVMs are invasive and associated with excessive risk [4, 123]. There are no specific medical therapies to treat bAVMs. Through analysis of surgically resected bAVM specimens and our novel mouse models, we have identified the following targets that might be used to develop new therapies (Fig. 1).
(1) Anti-angiogenesis
Excessive VEGF expression appears to be a fundamental part of bAVM pathophysiology for both sporadic bAVM [50, 55, 124] and bAVM in HHT [125–127]. Compelling evidence supports interruption of VEGF signaling as a therapeutic strategy. Bevacizumab (Avastin) normalized cardiac output in HHT patients harboring liver AVMs [128], and was effective in the treatment of severe epistaxis caused by hemorrhage from small mucosal AVMs (telangiectasias) [129–136]. Importantly, we demonstrated that after establishment of the bAVM phenotype in a conditional Alk1 deletion mouse model [55], intra-peritoneal bevacizumab treatment reduced the number of abnormal vessels, suggesting that maintenance of the bAVM phenotype is dependent on tonic VEGF signaling [137]. However, antibody therapy has many drawbacks, including concerns regarding hemorrhage [138] and the need for prolonged periods of intermittent intravenous (i.v.) infusions.
A promising alternative is the use of AAV-mediated expression of soluble FMS-related tyrosine kinase 1 (sFLT1), also called VEGF receptor (VEGFR)-1. sFLT1 contains the extracellular domain of VEGFR-1, which binds to VEGF in the tissue, thus reducing downstream signaling through membrane-bound VEGFRs. Injection of sFLT1 in an AAV construct packaged in AAV serotype 2 capsid (AAV2) into the vitreous humor of nonhuman primates effectively inhibited laser-induced choroidal neovascularization [139]. The AAV2-sFLT1 was well tolerated and capable of mediating long-term sFLT1 expression [140]. We showed that co-injection of AAV2-sFLT with AAV1-VEGF into the brain or intravenous injection of AAV9-sFLT at the time of intra-brain injection of AAV1-VEGF completely blocked VEGF-induced brain angiogenesis [141].
(2) Vascular integrity
Unlike cancer-related chemotherapy that aims to shrink abnormal tumor tissue as cytotoxic therapy, the concept for the treatment of bAVMs would be to stabilize vascular tissue and thereby decrease the risk of spontaneous ICH [142].
Microhemorrhage is present in unruptured human bAVMs [56] and in our bAVM mouse model lesions [57]. The vessels in bAVM mouse models have less mural cell coverage, which could be due to decreased expression of platelet-derived growth factor B (PDGFB) [55, 57]. Lebrin et al [143] demonstrated that thalidomide treatment increased PDGFB expression in endothelial cells and stimulated mural cell coverage.
Thalidomide belongs to a class termed immunomodulatory drugs (IMIDs). Because of thalidomide’s well-known adverse effects that limit patient tolerance, e.g., peripheral neuropathy and drowsiness [144], the search for related molecules has yielded a second generation of IMIDs. Lenalidomide, the most widely used in the group, is effective in treating multiple myeloma and myelodysplastic syndrome [145].
IMIDs possess a number of immunomodulatory, anti-inflammatory and anti-angiogenic properties that are pertinent to bAVM therapy [144, 146, 147]. The IMIDs do not inhibit endothelial cell proliferation, but rather, migration [148, 149]. There is a remarkable overlap in the pathways targeted by IMIDs in neoplastic disorders [144] with those dysregulated in bAVM, e.g., NFκB activation [94], VEGF overexpression [66], HIF-1α [150], αvβ3 [67, 151], and cytokine elaboration [105], including TNF-α [152] and IL-1β [153]. Both thalidomide and lenalidomide improved outcomes and reduced TNF-α and IL-1β expression in an amyotrophic lateral sclerosis mouse model [154, 155], suggesting that the drug can cross the blood-brain barrier in a setting with some impairment of barrier integrity like our bAVM model [65]. In early-phase human studies, thalidomide showed promise for decreasing hemorrhage from gastrointestinal or nasal telangiectasias (epistaxis) [143, 156–163]. Only a single case of lenalidomide used to treat gastrointestinal telangiectasias has been reported [164].
The anti-angiogenic mechanism of thalidomide is poorly understood but it has shown some clinical benefit in the treatment of gastrointestinal hemorrhage and epistaxis in patients with HHT [143, 159]. We have tested thalidomide in our brain AVM mouse model. Serial injections of thalidomide over a 6-week period attenuated dysplastic vessel formation. Furthermore, it decreased hemorrhage and improved vascular smooth muscle cell coverage in the bAVM lesion [65].
(3) Anti-inflammation and BM/monocyte transfusion
As discussed above, AVMs in humans and in animal models have been associated with an increased inflammatory response [16, 56, 57, 64, 65]. Inflammatory markers, including MPO, IL-6 and MMP-9, are overexpressed in human AVMs. MMPs and proinflammatory cytokines can interact with each other to carry out both physiological and pathological vascular remodeling. We have shown that MMP-9 plays an important role in VEGF-induced brain angiogenesis [78]. Inhibition of MMP-9 might lead to a reduction of angiogenesis.
Tetracycline class drugs are emerging as clinically applicable nonspecific MMP inhibitors that have the potential to enhance vascular stability, thus reducing the risk of spontaneous hemorrhage. They also possess neuroprotective properties. Settings investigated to date include cerebral ischemia [165], ICH [166, 167], neurodegenerative disorders [168, 169], traumatic brain injury [170], and atherosclerotic disease [171]. In clinical trials, tetracyclines decreased MMP in abdominal aortic aneurysms and carotid plaques [171–173]. Doxycycline can reduce MMP levels in bAVM [174]. Further, animal studies showed that doxycycline reduces MMP-9 activity in VEGF-stimulated brain angiogenic foci and reduces parenchymal angiogenesis [175]. Our Phase I study assessing the feasibility of using minocycline and doxycycline as potential long-term vasculostatic therapy for brain vascular malformations showed that it is feasible to propose a long-term trial to assess the potential benefit of tetracycline therapy to decrease hemorrhagic risk in bAVM [142].
BM-derived cells participate in VEGF-stimulated brain angiogenesis [78, 87] and the formation of vascular dysplasia in the brain of Eng+/− mice [77]. Moreover, normal human monocytes rescue the impairment of Eng+/− mice in repairing myocardial injury [79, 81]. These data suggest that correction of gene mutations in BM or monocytes through BM transplantation or monocyte transfusion could also be therapies for bAVMs.
Summary
The pathogenesis and pathophysiology of bAVMs are complex and currently unclear. Evidence obtained from modeling HHT bAVMs suggests that the initiation and progression of AVMs require interplay among several factors (Fig. 1), including: (1) homozygous loss-of-function of causative genes in somatic endothelial cells; (2) angiogenic stimulation (response-to-injury); (3) participation of BM-derived cells; (4) inflammation; and (5) hemodynamic changes. Animal studies have also identified some potential therapeutic targets (Fig. 1).
Acknowledgments
We thank Voltaire Gungab for assistance with manuscript preparation, and members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their support. The Principal Investigator, Hua Su, is supported by grants from the National Institutes of Health (R01 NS027713 and P01 NS044155).
Abbreviations
- bAVM
Brain arteriovenous malformation
- ICH
Intracranial hemorrhage
- HHT
Hereditary hemorrhagic telangiectasia
- ENG
Endoglin
- ALK1
Activin-like kinase 1
- VEGF
Vascular endothelial growth factor
- Pdgfb
Platelet derived growth factor-b
- A-V shunt
Arteriovenous shunt
- f
Allele with 2 loxp site flanking the target sequence
Footnotes
Compliance with Ethics Requirements
This Review Article does not contain any studies with human or animal subjects. All cited studies describe ethical standards in cited manuscripts.
Conflict of Interest
Wanqiu Chen, Eun-Jung Choi, Cameron M. McDougall and Hua Su declare that they have no conflict of interest.
References
- 1.Young WL. Intracranial arteriovenous malformations: pathophysiology and hemodynamics (Chapter 6) In: Jafar JJ, Awad IA, Rosenwasser RH, editors. Vascular Malformations of the Central Nervous System. New York: Lippincott Williams & Wilkins; 1999. pp. 95–126. [Google Scholar]
- 2.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359(9309):863–873. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- 3.Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340(23):1812–1818. doi: 10.1056/NEJM199906103402307. [DOI] [PubMed] [Google Scholar]
- 4.Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98(1):3–7. doi: 10.3171/jns.2003.98.1.0003. [DOI] [PubMed] [Google Scholar]
- 5.Bambakidis NC, Cockroft K, Connolly ES, Amin-Hanjani S, Morcos J, Meyers PM, et al. Preliminary results of the ARUBA sudy. Neurosurgery. 2013;73(2):E379–E381. doi: 10.1227/NEU.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 6.Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614–621. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin D, Santillan A, Greenfield JP, Souweidane M, Riina HA. Surgical management of pediatric cerebral arteriovenous malformations. Childs Nerv Syst. 2010;26(10):1337–1344. doi: 10.1007/s00381-010-1211-1. [DOI] [PubMed] [Google Scholar]
- 8.Potter CA, Armstrong-Wells J, Fullerton HJ, Young WL, Higashida RT, Dowd CF, et al. Neonatal giant pial arteriovenous malformation: genesis or rapid enlargement in the third trimester. J Neurointerv Surg. 2009;1(2):151–153. doi: 10.1136/jnis.2009.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du R, Hashimoto T, Tihan T, Young WL, Perry VH, Lawton MT. Growth and regression of an arteriovenous malformation in a patient with hereditary hemorrhagic telangiectasia: case report. J Neurosurg. 2007;106(3):470–477. doi: 10.3171/jns.2007.106.3.470. [DOI] [PubMed] [Google Scholar]
- 10.Hino A, Fujimoto M, Iwamoto Y, Takahashi Y, Katsumori T. An adult case of recurrent arteriovenous malformation after "complete" surgical excision: a case report. Surg Neurol. 1999;52(2):156–158. doi: 10.1016/s0090-3019(99)00060-9. [DOI] [PubMed] [Google Scholar]
- 11.Kader A, Goodrich JT, Sonstein WJ, Stein BM, Carmel PW, Michelsen WJ. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J Neurosurg. 1996;85(1):14–18. doi: 10.3171/jns.1996.85.1.0014. [DOI] [PubMed] [Google Scholar]
- 12.Klimo P, Jr., Rao G, Brockmeyer D. Pediatric arteriovenous malformations: a 15-year experience with an emphasis on residual and recurrent lesions. Childs Nerv Syst. 2007;23(1):31–37. doi: 10.1007/s00381-006-0245-x. [DOI] [PubMed] [Google Scholar]
- 13.Lindqvist M, Karlsson B, Guo WY, Kihlstrom L, Lippitz B, Yamamoto M. Angiographic long-term follow-up data for arteriovenous malformations previously proven to be obliterated after gamma knife radiosurgery. Neurosurgery. 2000;46(4):803–808. doi: 10.1097/00006123-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Inoue S, Liu W, Inoue K, Mineharu Y, Takenaka K, Yamakawa H, et al. Combination of linkage and association studies for brain arteriovenous malformation. Stroke. 2007;38(4):1368–1370. doi: 10.1161/01.STR.0000260094.03782.59. [DOI] [PubMed] [Google Scholar]
- 15.Bharatha A, Faughnan ME, Kim H, Pourmohamad T, Krings T, Bayrak-Toydemir P, et al. Brain arteriovenous malformation multiplicity predicts the diagnosis of hereditary hemorrhagic telangiectasia: quantitative assessment. Stroke. 2012;43(1):72–78. doi: 10.1161/STROKEAHA.111.629865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braverman IM, Keh A, Jacobson BS. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol. 1990;95(4):422–427. doi: 10.1111/1523-1747.ep12555569. [DOI] [PubMed] [Google Scholar]
- 17.Shovlin CL. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev. 2010;24(6):203–219. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara S, Mandzia JL, ter Brugge K, Willinsky RA, Faughnan ME, Manzia JL. Angiographic and clinical characteristics of patients with cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. AJNR Am J Neuroradiol. 2000;21(6):1016–1020. [PMC free article] [PubMed] [Google Scholar]
- 19.Maher CO, Piepgras DG, Brown RD, Jr., Friedman JA, Pollock BE. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001;32(4):877–882. doi: 10.1161/01.str.32.4.877. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Marchuk DA, Pawlikowska L, Chen Y, Su H, Yang GY, et al. Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl. 2008;105:199–206. doi: 10.1007/978-3-211-09469-3_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 22.Scott BB, McGillicuddy JE, Seeger JF, Kindt GW, Giannotta SL. Vascular dynamics of an experimental cerebral arteriovenous shunt in the primate. Surg Neurol. 1978;10(1):34–38. [PubMed] [Google Scholar]
- 23.Bederson JB, Wiestler OD, Brustle O, Roth P, Frick R, Yasargil MG. Intracranial venous hypertension and the effects of venous outflow obstruction in a rat model of arteriovenous fistula. Neurosurgery. 1991;29(3):341–350. doi: 10.1097/00006123-199109000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Chaloupka JC, Vinuela F, Robert J, Duckwiler GR. An in vivo arteriovenous malformation model in swine: Preliminary feasibility and natural history study. AJNR Am J Neuroradiol. 1994;15(5):945–950. [PMC free article] [PubMed] [Google Scholar]
- 25.De Salles AAF, Solberg TD, Mischel P, Massoud TF, Plasencia A, Goetsch S, et al. Arteriovenous malformation animal model for radiosurgery: the rete mirabile. AJNR Am J Neuroradiol. 1996;17(8):1451–1458. [PMC free article] [PubMed] [Google Scholar]
- 26.Herman JM, Spetzler RF, Bederson JB, Kurbat JM, Zabramski JM. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83(3):539–545. doi: 10.3171/jns.1995.83.3.0539. [DOI] [PubMed] [Google Scholar]
- 27.Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87(2):267–274. doi: 10.3171/jns.1997.87.2.0267. [DOI] [PubMed] [Google Scholar]
- 28.Morgan MK, Anderson RE, Sundt TM., Jr. A model of the pathophysiology of cerebral arteriovenous malformations by a carotid-jugular fistula in the rat. Brain Res. 1989;496(1–2):241–250. doi: 10.1016/0006-8993(89)91071-8. [DOI] [PubMed] [Google Scholar]
- 29.Kutluk K, Schumacher M, Mironov A. The role of sinus thrombosis in occipital dural arteriovenous malformations--development and spontaneous closure. Neurochirurgia (Stuttg) 1991;34(5):144–147. doi: 10.1055/s-2008-1052075. [DOI] [PubMed] [Google Scholar]
- 30.Terada T, Higashida RT, Halbach VV, Dowd CF, Tsuura M, Komai N, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80(5):884–889. doi: 10.3171/jns.1994.80.5.0884. [DOI] [PubMed] [Google Scholar]
- 31.TerBrugge KG, Lasjaunias P, Hallacq P. Experimental models in interventional neuroradiology. AJNR Am J Neuroradiol. 1991;12(6):1029–1033. [PMC free article] [PubMed] [Google Scholar]
- 32.Kailasnath P, Chaloupka JC. Mathematical modeling of AVM physiology using compartmental network analysis: theoretical considerations and preliminary in vivo validation using a previously developed animal model. Neurol Res. 1996;18(4):361–366. doi: 10.1080/01616412.1996.11740437. [DOI] [PubMed] [Google Scholar]
- 33.Massoud TF, Ji C, Vinuela F, Turjman F, Guglielmi G, Duckwiler GR, et al. Laboratory simulations and training in endovascular embolotherapy with a swine arteriovenous malformation model. AJNR Am J Neuroradiol. 1996;17(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- 34.Massoud TF, Ji C, Vinuela F, Guglielmi G, Robert J, Duckwiler GR, et al. An experimental arteriovenous malformation model in swine: anatomic basis and construction technique. AJNR Am J Neuroradiol. 1994;15(8):1537–1545. [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan MK, Anderson RE, Sundt TM., Jr. The effects of hyperventilation on cerebral blood flow in the rat with an open and closed carotid-jugular fistula. Neurosurgery. 1989;25(4):606–611. doi: 10.1097/00006123-198910000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Murayama Y, Massoud TF, Vinuela F. Hemodynamic changes in arterial feeders and draining veins during embolotherapy of arteriovenous malformations: an experimental study in a swine model. Neurosurgery. 1998;43(1):96–104. doi: 10.1097/00006123-199807000-00064. [DOI] [PubMed] [Google Scholar]
- 37.Nagasawa S, Kawanishi M, Kondoh S, Kajimoto S, Yamaguchi K, Ohta T. Hemodynamic simulation study of cerebral arteriovenous malformations. Part 2. Effects of impaired autoregulation and induced hypotension. J Cereb Blood Flow Metab. 1996;16(1):162–169. doi: 10.1097/00004647-199601000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Pietila TA, Zabramski JM, Thellier-Janko A, Duveneck K, Bichard WD, Brock M, et al. Animal model for cerebral arteriovenous malformation. Acta Neurochir (Wien) 2000;142(11):1231–1240. doi: 10.1007/s007010070019. [DOI] [PubMed] [Google Scholar]
- 39.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, et al. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166(6):1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, et al. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci U S A. 2005;102(28):9884–9889. doi: 10.1073/pnas.0504391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy PA, Lam MT, Wu X, Kim TN, Vartanian SM, Bollen AW, et al. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci U S A. 2008;105(31):10901–10906. doi: 10.1073/pnas.0802743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su H, Kim H, Pawlikowska L, Kitamura H, Shen F, Cambier S, et al. Reduced expression of integrin alphavbeta8 is associated with brain arteriovenous malformation pathogenesis. Am J Pathol. 2010;176(2):1018–1027. doi: 10.2353/ajpath.2010.090453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao Y, Yao J, Radparvar M, Blazquez-Medela AM, Guihard PJ, Jumabay M, et al. Reducing Jagged 1 and 2 levels prevents cerebral arteriovenous malformations in matrix Gla protein deficiency. Proc Natl Acad Sci U S A. 2013;110(47):19071–10976. doi: 10.1073/pnas.1310905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchuk DA, Srinivasan S, Squire TL, Zawistowski JS. Vascular morphogenesis: tales of two syndromes. Hum Mol Genet. 2003;12(Suppl 1):R97–R112. doi: 10.1093/hmg/ddg103. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan S, Hanes MA, Dickens T, Porteous ME, Oh SP, Hale LP, et al. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet. 2003;12(5):473–482. doi: 10.1093/hmg/ddg050. [DOI] [PubMed] [Google Scholar]
- 46.Bourdeau A, Faughnan ME, Letarte M. Endoglin-deficient mice, a unique model to study hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med. 2000;10(7):279–285. doi: 10.1016/s1050-1738(01)00062-7. [DOI] [PubMed] [Google Scholar]
- 47.Satomi J, Mount RJ, Toporsian M, Paterson AD, Wallace MC, Harrison RV, et al. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke. 2003;34(3):783–789. doi: 10.1161/01.STR.0000056170.47815.37. [DOI] [PubMed] [Google Scholar]
- 48.Xu B, Wu YQ, Huey M, Arthur HM, Marchuk DA, Hashimoto T, et al. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab. 2004;24(2):237–244. doi: 10.1097/01.WCB.0000107730.66603.51. [DOI] [PubMed] [Google Scholar]
- 49.Hao Q, Su H, Marchuk DA, Rola R, Wang Y, Liu W, et al. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol. 2008;295(6):H2250–E2256. doi: 10.1152/ajpheart.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao Q, Zhu Y, Su H, Shen F, Yang GY, Kim H, et al. VEGF induces more severe cerebrovascular dysplasia in Endoglin+/− than in Alk1+/− mice. Transl Stroke Res. 2010;1(3):197–201. doi: 10.1007/s12975-010-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26(3):328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 52.Sorensen LK, Brooke BS, Li DY, Urness LD. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol. 2003;261(1):235–250. doi: 10.1016/s0012-1606(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 53.Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119(11):3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milton I, Ouyang D, Allen CJ, Yanasak NE, Gossage JR, Alleyne CH, Jr., et al. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke. 2012;43(5):1432–1435. doi: 10.1161/STROKEAHA.111.647024. [DOI] [PubMed] [Google Scholar]
- 55.Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69(6):954–962. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo Y, Saunders T, Su H, Kim H, Akkoc D, Saloner DA, et al. Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke. 2012;43(5):1240–1246. doi: 10.1161/STROKEAHA.111.647263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol. 2013;33(2):305–310. doi: 10.1161/ATVBAHA.112.300485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi EJ, Walker EJ, Shen F, Oh SP, Arthur HM, Young WL, et al. Minimal homozygous endothelial deletion of Eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc Dis. 2012;33(6):540–547. doi: 10.1159/000337762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, Sun Z, Han Z, Jun K, Camus M, Wankhede M, et al. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke. 2014;45(3):900–902. doi: 10.1161/STROKEAHA.113.003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi EJ, Chen W, Jun K, Arthur HM, Young WL, Su H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One. 2014;9(2):e88511. doi: 10.1371/journal.pone.0088511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104(10):1343–1351. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284(5419):1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 63.Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217(1):42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 64.Chen W, Guo Y, Bollen AW, Su H, Young WL. Reduced PDGFR-beta expression after regional Alk1 deletion and VEGF stimulation in the brain is associated with reduced mural cell coverage [Abstract] Stroke. 2012;43(2-Meeting Abstracts):A3169. [Google Scholar]
- 65.Chen W, Guo Y, Jun K, Wankhede M, Su H, Young WL. Alk1 deficiency impairs mural cell recruitment during brain angiogenesis [Abstract] Stroke. 2013;44(2-Meeting Abstracts) ATMP118. [Google Scholar]
- 66.Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM, Young WL. Co-expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery. 2005;56(5):1058–1065. [PubMed] [Google Scholar]
- 67.Hashimoto T, Lawton MT, Wen G, Yang GY, Chaly T, Jr., Stewart CL, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54(2):410–423. doi: 10.1227/01.neu.0000103421.35266.71. [DOI] [PubMed] [Google Scholar]
- 68.Hasan DM, Amans M, Tihan T, Hess C, Guo Y, Cha S, et al. Ferumoxytol-enhanced MRI to image inflammation within human brain arteriovenous malformations: a pilot investigation. Transl Stroke Res. 2012;3(Supplement 1):166–173. doi: 10.1007/s12975-012-0172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, et al. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res. 2010;106(8):1425–1433. doi: 10.1161/CIRCRESAHA.109.211037. [DOI] [PubMed] [Google Scholar]
- 70.Bourdeau A, Cymerman U, Paquet ME, Meschino W, McKinnon WC, Guttmacher AE, et al. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol. 2000;156(3):911–923. doi: 10.1016/S0002-9440(10)64960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet. 2009;41(1):118–124. doi: 10.1038/ng.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akers AL, Johnson E, Steinberg GK, Zabramski JM, Marchuk DA. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCM): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet. 2009;18(5):919–930. doi: 10.1093/hmg/ddn430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93(7):682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- 74.Panchenko MP, Williams MC, Brody JS, Yu Q. Type I receptor serine-threonine kinase preferentially expressed in pulmonary blood vessels. Am J Physiol. 1996;270(4 Pt 1):L547–L558. doi: 10.1152/ajplung.1996.270.4.L547. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, Zhu W, Bollen AW, Lawton MT, Barbaro NM, Dowd CF, et al. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 2008;62(6):1340–1349. doi: 10.1227/01.neu.0000333306.64683.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torsney E, Charlton R, Diamond AG, Burn J, Soames JV, Arthur HM. Mouse model for hereditary hemorrhagic telangiectasia has a generalized vascular abnormality. Circulation. 2003;107(12):1653–1657. doi: 10.1161/01.CIR.0000058170.92267.00. [DOI] [PubMed] [Google Scholar]
- 77.Choi EJ, Walker EJ, Degos V, Jun K, Kuo R, Su H, et al. Endoglin deficiency in bone marrow is sufficient to cause cerebrovascular dysplasia in the adult mouse after vascular endothelial growth factor stimulation. Stroke. 2013;44(3):795–798. doi: 10.1161/STROKEAHA.112.671974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hao Q, Su H, Palmer D, Sun B, Gao P, Yang GY, et al. Bone marrow-derived cells contribute to vascular endothelial growth factor-induced angiogenesis in the adult mouse brain by supplying matrix metalloproteinase-9. Stroke. 2011;42(2):453–458. doi: 10.1161/STROKEAHA.110.596452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Laake LW, van den Driesche S, Post S, Feijen A, Jansen MA, Driessens MH, et al. Endoglin has a crucial role in blood cell-mediated vascular repair. Circulation. 2006;114(21):2288–2297. doi: 10.1161/CIRCULATIONAHA.106.639161. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Hiroi Y, Ngoy S, Okamoto R, Noma K, Wang CY, et al. Notch1 in bone marrow-derived cells mediates cardiac repair after myocardial infarction. Circulation. 2011;123(8):866–876. doi: 10.1161/CIRCULATIONAHA.110.947531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Post S, Smits AM, van den Broek AJ, Sluijter JP, Hoefer IE, Janssen BJ, et al. Impaired recruitment of HHT-1 mononuclear cells to the ischaemic heart is due to an altered CXCR4/CD26 balance. Cardiovasc Res. 2010;85(3):494–502. doi: 10.1093/cvr/cvp313. [DOI] [PubMed] [Google Scholar]
- 82.Tang XN, Zheng Z, Yenari MA. Bone marrow chimeras in the study of experimental stroke. Transl Stroke Res. 2012;3(3):341–347. doi: 10.1007/s12975-012-0169-6. [DOI] [PubMed] [Google Scholar]
- 83.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118(12):3436–3439. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rafii S, Lyden DCancer. A few to flip the angiogenic switch. Science. 2008;319(5860):163–164. doi: 10.1126/science.1153615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 87.Hao Q, Liu J, Pappu R, Su H, Rola R, Gabriel RA, et al. Contribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leucocytes and macrophages. Arterioscler Thromb Vasc Biol. 2008;28(12):2151–2157. doi: 10.1161/ATVBAHA.108.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao P, Chen Y, Lawton MT, Barbaro NM, Yang GY, Su H, et al. Evidence of endothelial progenitor cells in the human brain and spinal cord arteriovenous malformations. Neurosurgery. 2010;67(4):1029–1035. doi: 10.1227/NEU.0b013e3181ecc49e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, et al. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27(11):1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- 90.Hashimoto T, Matsumoto M, Tsang EJ, Young WL. Critical roles of neutrophils and macrophages in flow-induced adaptive outward vascular remodeling [Abstract] J Neurosurg Anesthesiol. 2006;18(4):293. [Google Scholar]
- 91.Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35(10):2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- 92.Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, et al. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–3128. doi: 10.2741/2037. [DOI] [PubMed] [Google Scholar]
- 93.Shenkar R, Shi C, Check IJ, Lipton HL, Awad IA. Concepts and hypotheses: inflammatory hypothesis in the pathogenesis of cerebral cavernous malformations. Neurosurgery. 2007;61(4):693–702. doi: 10.1227/01.NEU.0000298897.38979.07. [DOI] [PubMed] [Google Scholar]
- 94.Aziz MM, Takagi Y, Hashimoto N, Miyamoto S. Activation of nuclear factor kappaB in cerebral arteriovenous malformations. Neurosurgery. 2010;67(6):1669–1680. doi: 10.1227/NEU.0b013e3181fa00f1. [DOI] [PubMed] [Google Scholar]
- 95.Sturiale CL, Puca A, Sebastiani P, Gatto I, Albanese A, Di Rocco C, et al. Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: where do we stand? Brain. 2013;136(Pt 2):665–681. doi: 10.1093/brain/aws180. [DOI] [PubMed] [Google Scholar]
- 96.Nagase H, Meng Q, Malinovskii V, Huang W, Chung L, Bode W, et al. Engineering of selective TIMPs. Ann N Y Acad Sci. 1999;878:1–11. doi: 10.1111/j.1749-6632.1999.tb07670.x. [DOI] [PubMed] [Google Scholar]
- 97.Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34(4):925–931. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- 98.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39(3):279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 99.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 100.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 101.Velasco S, Alvarez-Munoz P, Pericacho M, Dijke PT, Bernabeu C, Lopez-Novoa JM, et al. L- and S-endoglin differentially modulate TGFbeta1 signaling mediated by ALK1 and ALK5 in L6E9 myoblasts. J Cell Sci. 2008;121(Pt 6):913–919. doi: 10.1242/jcs.023283. [DOI] [PubMed] [Google Scholar]
- 102.Chen Y, Hao Q, Kim H, Su H, Letarte M, Karumanchi SA, et al. Soluble endoglin modulates aberrant cerebral vascular remodeling. Ann Neurol. 2009;66(1):19–27. doi: 10.1002/ana.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Velasco-Loyden G, Arribas J, Lopez-Casillas F. The shedding of betaglycan is regulated by pervanadate and mediated by membrane type matrix metalloprotease-1. J Biol Chem. 2004;279(9):7721–7733. doi: 10.1074/jbc.M306499200. [DOI] [PubMed] [Google Scholar]
- 104.Hashimoto T, Matsumoto M, Li JF, Lawton MT, Young WL. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations. BMC Neurol. 2005;5(1):1. doi: 10.1186/1471-2377-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, et al. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59(1):72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- 106.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115(13):1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 107.Hosaka K, Hoh BL. Inflammation and cerebral aneurysms. Transl Stroke Res. 2014;5(2):190–198. doi: 10.1007/s12975-013-0313-y. [DOI] [PubMed] [Google Scholar]
- 108.Chalouhi N, Jabbour P, Magnotta V, Hasan D. Molecular imaging of cerebrovascular lesions. Transl Stroke Res. 2014;5(2):260–268. doi: 10.1007/s12975-013-0291-0. [DOI] [PubMed] [Google Scholar]
- 109.Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45(5):1137–1146. doi: 10.1097/00006123-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 110.Starke RM, Raper DM, Ding D, Chalouhi N, Owens GK, Hasan DM, et al. Tumor necrosis factor-alpha modulates cerebral aneurysm formation and rupture. Transl Stroke Res. 2013;5(2):269–277. doi: 10.1007/s12975-013-0287-9. [DOI] [PubMed] [Google Scholar]
- 111.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112(2):232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 112.Thompson S, Kim L, Scott A. Screening for abdominal aortic aneurysm: screening reduces deaths related to aneurysm. BMJ. 2005;330(7491):601. doi: 10.1136/bmj.330.7491.601-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Achrol AS, Kim H, Pawlikowska L, Poon KY, Ko NU, McCulloch CE, et al. Association of tumor necrosis factor-alpha-238G>A and Apolipoprotein E2 polymorphisms with intracranial hemorrhage after brain arteriovenous malformation treatment. Neurosurgery. 2007;61(4):731–739. doi: 10.1227/01.NEU.0000298901.61849.A4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Young WL, Kader A, Pile-Spellman J, Ornstein E, Stein BM. Columbia University AVM Study Project. Arteriovenous malformation draining vein physiology and determinants of transnidal pressure gradients. Neurosurgery. 1994;35(3):389–395. doi: 10.1227/00006123-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 115.Zhu Y, Lawton MT, Du R, Shwe Y, Chen Y, Shen F, et al. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery. 2006;59(3):687–696. doi: 10.1227/01.NEU.0000228962.68204.CF. [DOI] [PubMed] [Google Scholar]
- 116.Gao P, Zhu Y, Ling F, Shen F, Lee B, Gabriel RA, et al. Nonischemic cerebral venous hypertension promotes a pro-angiogenic state through HIF-1 downstream genes and leukocyte-derived MMP-9. J Cereb Blood Flow Metab. 2009;29(8):1482–1490. doi: 10.1038/jcbfm.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, et al. Arteriogenesis proceeds via ICAM-1/Mac-1- mediated mechanisms. Circ Res. 2004;94(9):1179–1185. doi: 10.1161/01.RES.0000126922.18222.F0. [DOI] [PubMed] [Google Scholar]
- 118.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20(17):4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol. 1997;9(5):707–713. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- 120.Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci U S A. 1994;91(11):4678–4682. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31(1 Pt 2):162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 122.Malek AM, Gibbons GH, Dzau VJ, Izumo S. Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Invest. 1993;92(4):2013–2021. doi: 10.1172/JCI116796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65(4):476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 124.Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl. 2011;111:83–92. doi: 10.1007/978-3-7091-0693-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cirulli A, Liso A, D’Ovidio F, Mestice A, Pasculli G, Gallitelli M, et al. Vascular endothelial growth factor serum levels are elevated in patients with hereditary hemorrhagic telangiectasia. Acta Haematol. 2003;110(1):29–32. doi: 10.1159/000072411. [DOI] [PubMed] [Google Scholar]
- 126.Sadick H, Naim R, Sadick M, Hormann K, Riedel F. Plasma level and tissue expression of angiogenic factors in patients with hereditary hemorrhagic telangiectasia. Int J Mol Med. 2005;15(4):591–596. [PubMed] [Google Scholar]
- 127.Sadick H, Riedel F, Naim R, Goessler U, Hormann K, Hafner M, et al. Patients with hereditary hemorrhagic telangiectasia have increased plasma levels of vascular endothelial growth factor and transforming growth factor-beta1 as well as high ALK1 tissue expression. Haematologica. 2005;90(6):818–828. [PubMed] [Google Scholar]
- 128.Dupuis-Girod S, Ginon I, Saurin JC, Marion D, Guillot E, Decullier E, et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA. 2012;307(9):948–955. doi: 10.1001/jama.2012.250. [DOI] [PubMed] [Google Scholar]
- 129.Whitehead KJ, Chakinala M, Faughnan ME, Miller FJ, White RI, Jr., Vethanayagam D, et al. Use of Bevacizumab in complicated HHT: North American HHT Center experience [Abstract] Hematol Rep. 2011;3(s2):6. [Google Scholar]
- 130.Karnezis TT, Davidson TM. Efficacy of intranasal bevacizumab (Avastin) treatment in patients with hereditary hemorrhagic telangiectasia-associated epistaxis. Laryngoscope. 2011;121(3):636–638. doi: 10.1002/lary.21415. [DOI] [PubMed] [Google Scholar]
- 131.Chen S, Karnezis T, Davidson TM. Safety of intranasal Bevacizumab (Avastin) treatment in patients with hereditary hemorrhagic telangiectasia-associated epistaxis. Laryngoscope. 2011;121(3):644–646. doi: 10.1002/lary.21345. [DOI] [PubMed] [Google Scholar]
- 132.Rohrmeier C, Sachs HG, Kuehnel TS. A retrospective analysis of low dose, intranasal injected bevacizumab (Avastin) in hereditary haemorrhagic telangiectasia. Eur Arch Otorhinolaryngol. 2012;269(2):531–536. doi: 10.1007/s00405-011-1721-9. [DOI] [PubMed] [Google Scholar]
- 133.Bose P, Holter JL, Selby GB. Bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2009;360(20):2143–2144. doi: 10.1056/NEJMc0901421. [DOI] [PubMed] [Google Scholar]
- 134.Oosting S, Nagengast W, de Vries E. More on bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2009;361(9):931. doi: 10.1056/NEJMc091271. [DOI] [PubMed] [Google Scholar]
- 135.Retornaz F, Rinaldi Y, Duvoux C. More on bevacizumab in hereditary hemorrhagic telangiectasia. N Engl J Med. 2009;361(9):931. [PubMed] [Google Scholar]
- 136.Simonds J, Miller F, Mandel J, Davidson TM. The effect of bevacizumab (Avastin) treatment on epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope. 2009;119(5):988–992. doi: 10.1002/lary.20159. [DOI] [PubMed] [Google Scholar]
- 137.Walker EJ, Su H, Shen F, Degos V, Amend G, Jun K, et al. Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke. 2012;43(7):1925–1930. doi: 10.1161/STROKEAHA.111.647982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tanvetyanon T, Murtagh R, Bepler G. Rupture of a cerebral arteriovenous malformation in a patient treated with bevacizumab. J Thorac Oncol. 2009;4(2):268–269. doi: 10.1097/JTO.0b013e318195a642. [DOI] [PubMed] [Google Scholar]
- 139.Lukason M, Dufresne E, Rubin H, Pechan P, Li Q, Kim I, et al. Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol Ther. 2011;19(2):260–265. doi: 10.1038/mt.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maclachlan TK, Lukason M, Collins M, Munger R, Isenberger E, Rogers C, et al. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol Ther. 2011;19(2):326–334. doi: 10.1038/mt.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mao L, Shen F, Chen W, Akinpelu B, Lawton MT, Young WL, et al. AAV-sFLT: a potential therapeutic agent for the treatment of brain angiogenic diseases [Abstract] Stroke. 2013;44(2-Meeting Abstracts) ATMP116. [Google Scholar]
- 142.Frenzel T, Lee CZ, Kim H, Quinnine NJ, Hashimoto T, Lawton MT, et al. Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: pilot study of adverse events and tolerance. Cerebrovasc Dis. 2008;25(1–2):157–163. doi: 10.1159/000113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16(4):420–428. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- 144.Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363(9423):1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- 145.Quach H, Kalff A, Spencer A. Lenalidomide in multiple myeloma: Current status and future potential. Am J Hematol. 2012;87(12):1089–1095. doi: 10.1002/ajh.23234. [DOI] [PubMed] [Google Scholar]
- 146.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91(9):4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aragon-Ching JB, Li H, Gardner ER, Figg WD. Thalidomide analogues as anticancer drugs. Recent Pat Anticancer Drug Discov. 2007;2(2):167–174. doi: 10.2174/157489207780832478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dredge K, Marriott JB, Macdonald CD, Man HW, Chen R, Muller GW, et al. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87(10):1166–1172. doi: 10.1038/sj.bjc.6600607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69(1–2):56–63. doi: 10.1016/j.mvr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 150.Sure U, Battenberg E, Dempfle A, Tirakotai W, Bien S, Bertalanffy H. Hypoxia-inducible factor and vascular endothelial growth factor are expressed more frequently in embolized than in nonembolized cerebral arteriovenous malformations. Neurosurgery. 2004;55(3):663–669. doi: 10.1227/01.neu.0000134556.20116.30. [DOI] [PubMed] [Google Scholar]
- 151.Lim M, Haddix T, Harsh GR, Vogel H, Steinberg GK, Guccione S. Characterization of the integrin alphavbeta3 in arteriovenous malformations and cavernous malformations. Cerebrovasc Dis. 2005;20(1):23–27. doi: 10.1159/000086123. [DOI] [PubMed] [Google Scholar]
- 152.Achrol AS, Pawlikowska L, McCulloch CE, Poon KY, Ha C, Zaroff JG, et al. TNFa-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations [Abstract] Stroke. 2006;37(2):638–639. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 153.Kim H, Hysi PG, Pawlikowska L, Poon A, Burchard EG, Zaroff JG, et al. Common variants in interleukin-1-beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc Dis. 2009;27(2):176–182. doi: 10.1159/000185609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kiaei M, Petri S, Kipiani K, Gardian G, Choi DK, Chen J, et al. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci Meth. 2006;26(9):2467–2473. doi: 10.1523/JNEUROSCI.5253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Neymotin A, Petri S, Calingasan NY, Wille E, Schafer P, Stewart C, et al. Lenalidomide (Revlimid) administration at symptom onset is neuroprotective in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2009;220(1):191–197. doi: 10.1016/j.expneurol.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen CH, Hsu HH, Hu RH, Lee PH, Ho CM. Long-term therapy with thalidomide in Hereditary Hemorrhagic Telangiectasia: case report and literature review. J Clin Pharmacol. 2012;52(9):1436–1440. doi: 10.1177/0091270011417824. [DOI] [PubMed] [Google Scholar]
- 157.Amanzada A, Toppler GJ, Cameron S, Schworer H, Ramadori G. A case report of a patient with hereditary hemorrhagic telangiectasia treated successively with thalidomide and bevacizumab. Case Rep Oncol. 2010;3(3):463–470. doi: 10.1159/000323152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Penaloza A, Vekemans MC, Lambert C, Hermans C. Deep vein thrombosis induced by thalidomide to control epistaxis secondary to hereditary haemorrhagic telangiectasia. Blood Coagul Fibrinolysis. 2011;22(7):616–618. doi: 10.1097/MBC.0b013e32834a040c. [DOI] [PubMed] [Google Scholar]
- 159.Alam MA, Sami S, Babu S. Successful treatment of bleeding gastro-intestinal angiodysplasia in hereditary haemorrhagic telangiectasia with thalidomide. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.08.2011.4585. pii: bcr0820114585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Perez-Encinas M, Rabunal Martinez MJ, Bello Lopez JL. Is thalidomide effective for the treatment of gastrointestinal bleeding in hereditary hemorrhagic telangiectasia? Haematologica. 2002;87(8) ELT34. [PubMed] [Google Scholar]
- 161.Gossage JR, Chamberlain SM, Sridhar S, Kumar A. An interim report of thalidomide for treatment of recurrent angioectasia related gastrointestinal bleeding [Abstract #PC10] Hematology Meeting Reports. 2009;3(4):21. [Google Scholar]
- 162.Georgia Health Sciences University. Thalidomide reduces arteriovenous malformation related gastrointestinal bleeding (TAG) [Accessed 20 Jun 2012]; NCT00389935. 2011 http://clinicaltrials.gov/ct2/show/NCT00389935?term=thalidomide+hht&rank=2.
- 163.IRCCS Policlinico S. Matteo. Efficacy of thalidomide in the treatment of hereditary hemorrhagic telangiectasia (THALI-HHT) [Accessed 20 Jun 2012]; NCT01485224. 2011 http://clinicaltrials.gov/ct2/show/NCT01485224?term=thalidomide+hht&rank=1.
- 164.Bowcock SJ, Patrick HE. Lenalidomide to control gastrointestinal bleeding in hereditary haemorrhagic telangiectasia: potential implications for angiodysplasias? Br J Haematol. 2009;146(2):220–222. doi: 10.1111/j.1365-2141.2009.07730.x. [DOI] [PubMed] [Google Scholar]
- 165.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96(23):13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33(3):831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 167.Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53(6):731–742. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- 168.Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125(Pt 6):1297–1308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- 169.Bonelli RM, Heuberger C, Reisecker F. Minocycline for Huntington’s disease: an open label study. Neurology. 2003;60(5):883–884. doi: 10.1212/01.wnl.0000049936.85487.7a. [DOI] [PubMed] [Google Scholar]
- 170.Sanchez-Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48(6):1393–1399. doi: 10.1097/00006123-200106000-00051. [DOI] [PubMed] [Google Scholar]
- 171.Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, et al. Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg. 2000;31(2):325–342. doi: 10.1016/s0741-5214(00)90163-0. [DOI] [PubMed] [Google Scholar]
- 172.Axisa B, Loftus IM, Naylor AR, Goodall S, Jones L, Bell PR, et al. Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke. 2002;33(12):2858–2864. doi: 10.1161/01.str.0000038098.04291.f6. [DOI] [PubMed] [Google Scholar]
- 173.Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr., Kent KC, et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: Report of a prospective (Phase II) multicenter study. J Vasc Surg. 2002;36(1):1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 174.Hashimoto T, Matsumoto M, Yang GY, Lawton MT, Young WL. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations [Abstract] J Neurosurg Anesthesiol. 2004;16(4):333–334. [Google Scholar]
- 175.Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang GY, Young WL. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35(7):1715–1719. doi: 10.1161/01.STR.0000129334.05181.b6. [DOI] [PubMed] [Google Scholar]