Abstract
Background
The adipose tissue is considered not only a storable energy source, but mainly an endocrine organ that secretes several cytokines. Adiponectin, a novel protein similar to collagen, has been found to be an adipocyte-specific cytokine and a promising cardiovascular risk marker.
Objectives
To evaluate the association between serum adiponectin levels and the risk for cardiovascular events in patients with acute coronary syndromes (ACS), as well as the correlations between adiponectin and metabolic, inflammatory, and myocardial biomarkers.
Methods
We recruited 114 patients with ACS and a mean 1.13-year follow-up to measure clinical outcomes. Clinical characteristics and biomarkers were compared according to adiponectin quartiles. Cox proportional hazard regression models with Firth's penalization were applied to assess the independent association between adiponectin and the subsequent risk for both primary (composite of cardiovascular death/non-fatal acute myocardial infarction (AMI)/non-fatal stroke) and co-primary outcomes (composite of cardiovascular death/non-fatal AMI/non-fatal stroke/ rehospitalization requiring revascularization).
Results
There were significant direct correlations between adiponectin and age, HDL-cholesterol, and B-type natriuretic peptide (BNP), and significant inverse correlations between adiponectin and waist circumference, body weight, body mass index, Homeostasis Model Assessment (HOMA) index, triglycerides, and insulin. Adiponectin was associated with higher risk for primary and co-primary outcomes (adjusted HR 1.08 and 1.07/increment of 1000; p = 0.01 and p = 0.02, respectively).
Conclusion
In ACS patients, serum adiponectin was an independent predictor of cardiovascular events. In addition to the anthropometric and metabolic correlations, there was a significant direct correlation between adiponectin and BNP.
Keywords: Adiponectin, Metabolic Syndrome X, Insulin Resistance, Acute Coronary Syndrome, Risk Factors
Introduction
Metabolic Syndrome and Adiponectin
In pre-clinical and clinical conditions, the metabolic components of cardiovascular risk, such as obesity, insulin resistance and dyslipidemia, interact in a complex way, being associated with high cardiovascular morbidity and mortality1-11. Visceral obesity plays an increasingly relevant role as a cardiovascular risk factor. In fact, the adipose tissue is a storable energy source and an endocrine organ that secretes cytokines, which can contribute to the development of obesity-related diseases, such as diabetes mellitus (DM) and atherosclerosis. Matsuzawa et al12 have assessed the endocrine role of adipocytes and found an abundant expression of genes related to the synthesis of several bioactive substances, such as adiponectin (Arcp30, AdipoQ, apM1 or GBP28), a protein similar to collagen and identified as an adipocyte-specific cytokine.
Adiponectin is abundantly expressed in healthy individuals, has antithrombotic, antiatherogenic and anti inflammatory properties, and is downregulated in obese individuals. Similarly, adiponectin levels are reduced in male individuals, with type 2 DM, proinflammatory conditions, lipodystrophies, insulin resistance and cardiovascular disease. Inversely, serum adiponectin concentrations are elevated in women, non-obese individuals, with type 1 DM and in those undergoing treatment with peroxisome proliferator activated receptor gamma (PPARγ) agonists13-20. From the prognostic viewpoint, in healthy individuals, adiponectin has been inversely associated with cardiovascular risk, mainly in men, or directly associated with that risk, mainly in the elderly21-25. However, studies on chronic heart failure (HF) or documented cardiovascular disease (CVD) have identified hyperadiponectinemia as an independent predictor of mortality26-28. Similarly, Cavusoglu et al29 have identified a direct and independent association between adiponectin and the risk for acute myocardial infarction (AMI) and cardiovascular death in a cohort of men undergoing coronary angiography for the diagnostic investigation of chest pain. Those data suggest that adiponectin might play a different role in acute clinical scenarios. Thus, this study was aimed at assessing the association between serum adiponectin levels and the risk for cardiovascular events in patients with acute coronary syndromes (ACSs), and at establishing the correlations between adiponectin and metabolic, inflammatory and myocardial biomarkers.
Method
This study has two components: a) a cross-sectional and analytical component to determine the clinical characteristics and measures of serum biomarkers of patients with ACS on hospital admission; b) a cohort of ACS, prospectively included between 2008 and 2010, with clinical follow-up for the systematic and prospective collection of cardiovascular events. Based on the study by Cavusoglu et al29 on a subgroup of patients with ACS comprising 52.3% of a total of 325 patients and with all-cause mortality rate of 10.3% in two years of clinical follow-up, the calculation of the sample size was estimated as 112 patients, with significance of α = 0.05 and 1 − β = 0.80. It is worth noting that, due to the lack of studies about adiponectin in patients with ACSs in the Brazilian population up to the time of the elaboration of this dissertation project, and, thus, scarcity of data on the variability of that biomarker measurements, the possibility of inadequate estimation was considered. This study included patients of both sexes, over the age of 18 years, who underwent blood collection within the first 24 hours from ischemic symptom onset and who provided written informed consent, in the emergency and coronary units of the Instituto Dante Pazzanese de Cardiologia, in the city of São Paulo. Patients with the following characteristics were excluded: infectious, inflammatory and neoplastic diseases; end-stage kidney or liver disease; significant heart valve disease or heart valve disease precipitating the clinical findings; percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) in the preceding 30 days; previous and current use of insulin (patients with type 1 DM and those with type 2 DM requiring insulin); and use of oral antidiabetic drugs of the thiazolidinedione group. The following parameters were assessed: adiponectin; leptin; fasting glucose; insulin; Homeostasis Model Assessment (HOMA) index; glycated hemoglobin (HbA1c); total cholesterol, LDL- and HDL cholesterol; triglycerides; ultrasensitive C-reactive protein (us-CRP); leukocytes; fibrinogen; platelets; cardiac troponin I (TnI); CKMB mass; B-type natriuretic peptide (BNP); and demographic (age, sex, ethnicity), anthropometric (weight, body mass index and waist circumference) and angiographic (extension/severity of coronary artery disease [CAD] in patients undergoing coronary angiography) variables. Venous blood samples (10 mL) were collected in the morning following a 12-hour nocturnal fasting. Insulin resistance was expressed as the HOMA index calculated by using the formula [product of glucose (mg/dL) by insulin (µUI / mL), divided by the constant 405]30,31. Adiponectin and leptin were measured by use of ELISA assay. The kits for measuring adiponectin were Human Adiponectin ELISA Kit 96-Well Plate (Cat. # EZHADP-61K), manufactured by Millipore, United States. The kits for measuring leptin were DiaSource KAP2281 Human Leptin ELISA IVD, manufactured by DIAsource ImmunoAssays S.A., Belgium. The primary outcome comprised cardiovascular death, nonfatal AMI or reinfarction, and nonfatal stroke. The co-primary outcome comprised primary events and re-hospitalization due to recurring ischemia or ischemia considered clinically significant requiring revascularization during clinical follow up. Cases of AMI were defined by criteria proposed for the universal definition of AMI32. Cases of stroke were defined according to World Health Organization (WHO) classical criteria33. The distributions of the continuous variables were expressed as mean (± standard deviation) or median (with interquartile interval), as appropriate, and the comparisons between the groups were calculated by using Student t test or non-parametric (Kruskall-Wallis test), as appropriate. The distributions of the categorical variables were expressed as frequencies and percentages, and the comparisons calculated by using chi-square test or Fisher exact test, as appropriate. The analysis of the primary and co-primary clinical outcomes was based on the time for the occurrence of the first event. Cox univariate regression analysis gathered all demographic, metabolic, inflammatory, anthropometric and angiographic variables, and only those univariate predictors with p < 0.10 and variables with clinical significance were included in Cox proportional hazards regression models to determine whether adiponectin would be an independent risk predictor. Backward stepwise regression was used for the models to identify the independent variables of risk for the occurrence of primary and co-primary outcomes, respectively. Then, Firth's penalized likelihood method was used to adjust the potentially overestimated variables due to elevated prevalence. The results were expressed as hazard ratio and 95% confidence interval (CI), and the discriminatory capacity of the models was expressed by c-statistic (or c index). Two-tailed tests were used with significance level of α = 0.0534,35.
Results
Characteristics of the Patients
Table 1 lists the major characteristics of the 114 patients with ACS according to adiponectin quartiles. Their mean (± SD) age was 62 (± 10.5) years, 41.2% were of the female sex and 82.6%, Caucasians. The prevalence of cardiovascular risk factors was significant as follows: arterial hypertension, 90%; DM, 30%; dyslipidemia, 78%; and current tobacco use, 16.4%. The medians (interquartile interval) of the anthropometric parameters were as follows: body mass index (BMI) = 27.4 (24.6-30.4) kg / m2 and waist circumference = 98 (91-108) cm. Regarding electrocardiographic (ECG) findings, 97.3% of the patients had alterations, the most frequent (30.9%) being ST-segment depression between 0.5 and 1.0 mm. The final diagnosis defined 90.3% of the patients with ACS with no persistent ST-segment elevation.
Table 1.
Clinical characteristics according to adiponectin quartiles
| Characteristics | Q1 | Q2 | Q3 | Q4 | P |
|---|---|---|---|---|---|
| Age | 60.6 ± 9.4 | 60.7 ± 9.6 | 64.3 ± 11.2 | 64.9 ± 11.8 | 0.29 |
| Female sex | 25% | 31% | 51.7% | 57.1% | 0.037 |
| Caucasian ethnicity | 71.4% | 86.2% | 89.7% | 85.7% | 0.84 |
| Δt for admission, min | 353 ± 45 | 412 ± 62 | 482 ± 101 | 330 ± 65 | 0.47 |
| Weight, kg | 81.8 ± 14.5 | 77.2 ± 12.8 | 70.5 ± 13.2 | 66 ± 13.5 | 0.0001 |
| BMI, kg/m2 | 29.6 ± 5.2 | 28.3 ± 5.0 | 27.5 ± 4.4 | 26.4 ± 5.3 | 0.10 |
| Waist circumference, cm | 105.2 ± 11.8 | 99.2 ± 12.8 | 97.1 ± 11.4 | 93.4 ± 12.0 | 0.004 |
| SBP, mmHg | 136 ± 22.6 | 137 ± 33 | 147 ± 22.6 | 142 ± 30.4 | 0.38 |
| DBP, mmHg | 81 ± 18.2 | 81 ± 17.3 | 84 ± 13.1 | 85 ± 16.9 | 0.72 |
| HR, bpm | 73 ± 12 | 76 ± 16 | 76 ± 15 | 83 ± 23 | 0.61 |
| Killip I | 96.3% | 89.3% | 85.2% | 89.3% | 0.52 |
| Killip II/III | 3.7% | 3.6% | 11.1% | 10.7% | |
| Killip IV | 0% | 7.1% | 3.7% | 0% | |
| Diabetes mellitus | 35.7% | 24.1% | 31% | 28.6% | 0.81 |
| Dyslipidemia | 85.7% | 72.4% | 82.8% | 71.4% | 0.48 |
| Previous AMI | 60.7% | 44.8% | 44.8% | 39.3% | 0.41 |
| Previous PCI | 46.4% | 37.9% | 48.3% | 42.9% | 0.88 |
| Previous CABG | 28.6% | 25% | 27.6% | 17.9% | 0.82 |
| Previous stroke | 10.7% | 3.4% | 10.7% | 3.7% | 0.59 |
| Current smoking | 10.7% | 13.8% | 23.1% | 18.5% | 0.62 |
| Previous angina | 57.1% | 58.6% | 58.6% | 46.4% | 0.77 |
| Previous CKF | 7.1% | 0% | 6.9% | 14.3% | 0.24 |
| SAH | 92.9% | 82.8% | 96.6% | 85.7% | 0.30 |
| LVEF | 0.53 | 0.49 | 0.53 | 0.46 | 0.66 |
| Moderate/severe LV dysfunction | 33.3% | 42.3% | 19.2% | 30.4% | 0.35 |
∆t: time interval; HR: heart rate; LVEF: left ventricular ejection fraction; SAH: systemic arterial hypertension; BMI: body mass index; CKF: chronic kidney failure; DBP: diastolic blood pressure; SBP: systolic blood pressure; CABG: coronary artery bypass graft surgery; LV: left ventricle.
Biomarkers in the global sample
Table 2 shows the values of biomarkers according to adiponectin quartiles. The median adiponectin level was 9,807 (6,113-13,914) ng/mL.
Table 2.
Biomarkers according to adiponectin quartiles
| Biomarkers | Q1 | Q2 | Q3 | Q4 | p |
|---|---|---|---|---|---|
| Leptin, ng/mL | 4941 ± 4686 | 5471 ± 5142 | 7124 ± 7977 | 4037 ± 4592 | 0.41 |
| Insulin, µUI/mL | 11.36 ± 8.6 | 10.28 ± 10.1 | 9.83 ± 5.1 | 7.80 ± 6.5 | 0.06 |
| Glucose, mg/dL | 117 ± 42 | 118 ± 59 | 116 ± 91 | 110 ± 42 | 0.67 |
| HbA1c, % | 7.1 ± 1.7 | 6.6 ± 1.5 | 6.4 ± 1.12 | 6.4 ± 1.43 | 0.25 |
| HOMA index | 3.35 ± 3.13 | 2.92 ± 3.15 | 3.04 ± 3.31 | 2.23 ± 2.17 | 0.20 |
| Total cholesterol, mg/dL | 182 ± 43 | 170 ± 37 | 190 ± 42 | 191 ± 53 | 0.32 |
| LDL-cholesterol, mg/dL | 107 ± 31 | 104 ± 33 | 122 ± 40 | 116 ± 40 | 0.31 |
| HDL-cholesterol, mg/dL | 34 ± 6 | 34 ± 6 | 41 ± 8 | 43 ± 11 | 0.0001 |
| Triglycerides, mg/dL | 221 ± 130 | 174 ± 127 | 137 ± 57 | 149 ± 88 | 0.03 |
| CKMB mass, ng/mL | 12.9 ± 27.4 | 107.9 ± 394 | 20.8 ± 45.3 | 27.1 ± 62.3 | 0.62 |
| TnI, ng/mL | 6.44 ± 12.8 | 36 ± 99 | 9.44 ± 17 | 13.7 ± 26.6 | 0.65 |
| us-CRP, mg/dL | 6.13 ± 15.9 | 6.2 ± 9.96 | 3.14 ± 3.73 | 10.5 ± 21.3 | 0.89 |
| Leukocytes, /mm3 | 8689 ± 3189 | 8978 ± 4480 | 8446 ± 2921 | 7620 ± 2118 | 0.61 |
| Platelets, x103/mm3 | 239 ± 101 | 218 ± 511 | 236 ± 606 | 226 ± 596 | 0.61 |
| BNP, pg/mL | 120 ± 191 | 163 ± 222 | 169 ± 252 | 437 ± 573 | 0.21 |
| Fibrinogen, mg/dL | 392 ± 88 | 343 ± 86 | 354 ± 86 | 344 ± 83 | 0.12 |
| Creatinin, mg/dL | 1.07 ± 0.33 | 1.16 ± 0.18 | 1.06 ± 0.28 | 1.24 ± 0.68 | 0.044 |
BNP: type-B natriuretic peptide; CKMB: creatine kinase, MB fraction; HbA1c: glycated hemoglobin; HOMA: Homeostasis Model Assessment; HDL: high-density lipoprotein; LDL: low-density lipoprotein; us-CRP: ultrasensitive C-reactive protein; TnI: cardiac troponin I.
Pharmacological Treatment
Medications used before and during hospitalization
Previous treatments included the following drugs: acetylsalicylic acid (ASA), 69%; angiotensin-converting-enzyme inhibitors (ACEI), 62.2%; beta-blockers, 60.4%; statins, 59.8%; oral antidiabetic drugs, 13.2%; and insulin, 1.8%. No patient included was on previous use of thiazolidinediones. Regarding the drugs used during hospitalization to manage ACS, the following stand out: ASA, 99.1%; clopidogrel, 96.5%; beta-blockers, 89.5%; ACEI, 88.5%; statins, 98.2%; and low-molecular weight heparin (enoxaparin) for anticoagulation, 93.8%.
Procedures Performed during Hospitalization
Cardiac catheterization for coronary angiography was performed in 87.7% of the patients. Luminal stenosis ≥ 50% characterized significant CAD, which was documented in at least one vessel in 83% of the patients, and in multiple arteries in 38%. Percutaneous coronary intervention was performed in 47 patients undergoing diagnostic angiography (49%), 76.6% of whom underwent implantation of one coronary stent, and 21.3%, more than one stent. Of the stents implanted, 91% were conventional (non-pharmacological). Coronary artery bypass graft surgery was performed in 15.6% of the patients, the left internal thoracic artery being used in 100% of the patients, and grafts being applied to at least three coronary arteries in two thirds of the patients.
Clinical Outcomes
Primary outcome was observed in 18.4% of the patients, and co-primary outcome, in 21.1%, with a mean 1.13-year clinical follow-up in all 114 patients recruited. Thus, follow-up was performed in 100% of the patients, with no loss to follow-up.
Adiponectin as a Predictor of Cardiometabolic Risk
The Cox models included the following variables that showed significance for the association with primary and co-primary outcomes: adiponectin; other biomarkers; clinical characteristics; angiographic variables; and treatments and procedures performed before and during hospitalization. The models were calculated for four prespecified groups of interest: overall population (group A); patients with no DM (group B); patients with no ST-segment elevation AMI (group C); and patients with neither DM nor ST-segment elevation AMI (group D) (Tables 3-6). Some of the variables included in the model for primary outcome were as follows: previous angina; arterial hypertension; Killip classification; adiponectin; leptin; fasting glucose; creatinine; CKMB activity; CKMB mass; TnI; BNP; and urea. After adjusting for those factors, adiponectin was associated with high risk for the primary outcome in the models for groups B and D. For co-primary outcome, the following variables were included: previous angina; arterial hypertension; Killip classification; adiponectin; leptin; fasting glucose; creatinine; CKMB activity; heart rate (HR) on admission, HDL-cholesterol; HOMA index; obesity and in-hospital use of ASA. Adiponectin was consistently an independent predictor of high risk for cardiovascular events in the models of groups B and D, with borderline significance for group C.
Table 3.
Cox models for the global population (group A)
| Primary and co-primary outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | Hazard ratio | 95%CI | p | ||||||
| Troponin I (per 1) | 0.009 | 1.009 | 1.002 | 1.014 | 0.016 | ||||
| Fasting glucose (per 10) | 0.071 | 1.074 | 1.021 | 1.114 | 0.01 | ||||
| C indices per time intervals | |||||||||
| 30 days | 180 days | 365 days | |||||||
| 0.7208 | 0.6641 | 0.6641 | |||||||
| Coefficient | Hazard ratio | 95%CI | p | ||||||
| Previous angina | 0.987 | 2.684 | 0.955 | 9.038 | 0.0616 | ||||
| Adiponectin (per 1,000) | 0.047 | 1.048 | 0.996 | 1.093 | 0.0687 | ||||
| Fasting glucose (per 10) | 0.054 | 1.055 | 0.998 | 1.099 | 0.0562 | ||||
| C indices per time intervals | |||||||||
| 30 days | 180 days | 365 days | |||||||
| 0.6346 | 0.7719 | 0.7719 | |||||||
Table 6.
Cox models after excluding patients with diabetes and ST-segment elevation AMI (group D)
| Primary and co-primary outcomes | ||||||
|---|---|---|---|---|---|---|
| Coefficient | Hazard ratio | 95%CI | p | |||
| Previous angina | 1.213 | 3.364 | 0.89 | 18.16 | 0.07 | |
| Adiponectin (per 1,000) | 0.077 | 1.080 | 1.02 | 1.13 | 0.012 | |
| Troponin I (per 1) | 0.038 | 1.039 | 1.001 | 1.075 | 0.04 | |
| C indices per time intervals | ||||||
| 30 days | 180 days | 365 days | ||||
| 0.7994 | 0.8032 | 0.8032 | ||||
| Coefficient | Hazard ratio | 95%CI | p | |||
| Previous angina | 1.940 | 6.96 | 1.54 | 65.8 | 0.009 | |
| Adiponectin (per 1,000) | 0.069 | 1.072 | 1.01 | 1.12 | 0.02 | |
| C indices per time intervals | ||||||
| 30 days | 180 days | 365 days | ||||
| 0.7390 | 0.8199 | 0.8199 | ||||
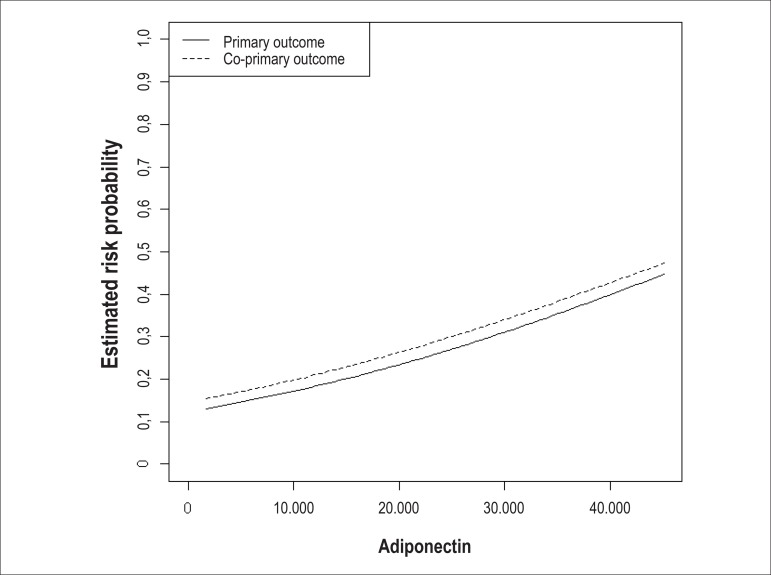
The logistic regression analysis showed an almost linear relationship between adiponectin, as a quantitative continuous variable, and the estimate of the probability of risk for primary and co-primary outcomes (Figure 1).
Figure 1.
Adiponectin values and risk estimation for the outcomes.
Correlations Between Adiponectin and Biomarkers
As prespecified, the secondary objective was to study the correlations between adiponectin and the different biomarkers, and demographic and anthropometric variables. Table 7 emphasizes the direct and significant correlation between adiponectin and BNP.
Table 7.
Correlations between adiponectin and quantitative variables
| Variables | Correlation coefficient | p |
|---|---|---|
| BNP | 0.221 | 0.02 |
| BMI | - 0.239 | 0.01 |
| Weight | - 0.412 | < 0.001 |
| Height | - 0.346 | < 0.001 |
| Waist circumference | - 0.309 | 0.001 |
| Age | 0.236 | 0.01 |
BNP: type-B natriuretic peptide; BMI: body mass index.
Discussion
This study of a cohort of patients with ACS on mid/long-term clinical follow-up detected a direct, significant and independent association between adiponectin and the risk for relevant cardiovascular clinical outcomes. There was consistent signaling of more evident risk when patients with DM or ST-segment elevation AMI were excluded. Cavusoglu et al29 have assessed 325 male patients with stable angina, unstable angina and non-ST-segment elevation AMI, who underwent coronary angiography to determine the prognostic value of serum adiponectin levels. The patients were followed up for 24 months for the occurrence of all-cause death, cardiovascular mortality and AMI. In that study, the authors have identified a direct and independent predictive association between a single baseline adiponectin measurement and the subsequent risk of death and AMI. In the subgroup of non-ST-segment elevation ACS (n = 170), adiponectin remained as an independent risk predictor. Other recent studies have also identified a direct association between adiponectin and cardiovascular risk, especially in patients with HF, mainly the elderly26-28. Wannamethee et al28have prospectively studied the relationship between adiponectin levels and mortality in 4,046 male elderly (60 79 years of age), with and without documented CVD and HF. After adjusting for important baseline characteristics, adiponectin remained directly and significantly associated with total and cardiovascular mortalities in men without CVD or HF (adjusted RR: 1.55, 95%CI: 1.19-2.02, p = 0.002; and RR: 1.53, 95%CI: 1.03 2.27, p = 0.02), for a trend in comparing the highest and the lowest tertiles, as well as in men with diagnosed HF (adjusted RR: 2.37, 95%CI: 0.64 8.79, p = 0.04; and RR: 3.43 95%CI: 0.54 21.7, p = 0.008). No association was demonstrated in those with diagnosed CVD, but no HF.
Our study has some important differences as compared with those previously discussed. Ours included patients of a wider age group, no analysis restricted only to the oldest. Similarly, this study was not restricted to the inclusion of male individuals21-23,26-29, emphasizing that there was adequate balance in the sex proportions, with significant female representation (41.2%). In fact, the mean adiponectin level is approximately 50% greater in women, mainly elderly ones, as compared with men in the same age group13,36. In terms of independent risk prediction, this study findings are in accordance with those of the most recent literature regarding the prognostic value of adiponectin in different clinical scenarios, either under stable conditions and in populations considered healthy or without documented CVD, or under acute clinical conditions or manifest CVD, as in the present study. Some studies have identified an independent association between adiponectin and higher risk for CAD (nonfatal AMI and fatal CAD) in individuals with no previous documentation, but only in the elderly, with adjusted OR of 1.69 (95%CI: 1.23-2.32) for the 5th quintile versus the 1st quintile25. However, the Rancho Bernardo study24 has reported that adiponectin levels in the 5th quintile stratified by sex were significantly associated with a 44% reduction in OR for the occurrence of CAD, which was eliminated after adjusting for HDL-cholesterol and/or triglycerides. In the 20-year prospective analysis, higher concentrations of adiponectin were predictive of reduced risk for nonfatal AMI only in men. Corroborating that information, a recent study on adiponectin and risk of CAD-related events in the context of a meta-analysis of seven prospective studies has reported an OR for CAD of 0.89 (95%CI: 0.67-1.18), comparing men in the 3rd tertile with the 1st tertile, very similar to the findings of that meta-analysis22. Wolk et al37 have assessed 499 patients undergoing coronary angiography, 168 of whom with ACS, and identified that high adiponectin levels are independently associated with a reduced risk of ACS.
Of the studied correlations between adiponectin and other biomarkers, we detected a direct and significant one with BNP. Thus, possible interactions like that cannot be ruled out, among others identified and non-measurable factors or variables, which can explain the attenuation of the strength of the association with the risk in the global model. That hypothesis is supported by the study by Schnabel et al38, in which the association of risk and adiponectin remained robust after adjusting for classical risk factors in the global population studied. However, after adjusting for BNP, adiponectin lost its independent predictive value. Because of those findings, in patients with manifest CAD, adiponectin seems to have a different role. In clinically asymptomatic individuals, high adiponectin levels seem to protect against atherosclerotic disease; however, when elevated in patients with symptomatic CAD, a direct association with risk for cardiovascular events is observed. That result of direct or paradoxical association implies that the beneficial influence of adiponectin in atherosclerotic disease would be translated into elevations in adiponectin concentrations as a contra regulatory mechanism in response to the excessive and unstable atherosclerotic process of the ACS, with a negative net result, despite the high concentrations of that biomarker. That could indicate unbalance of the entire metabolic homeostatic system, interfering with cellular bioenergetic processes.
It is worth noting that the presence of a strong association between risk factor and the outcome assessed does not necessarily mean that the risk factor provides a base for an effective predictive rule. Wang et al39have shown that, in individual risk assessment, the use of ten biomarkers added only a discrete discriminatory capacity to the classical risk factors, with practically overlapping ROC curves and similar c indices (0.76 versus 0.77).
Finally, there is the question about the potential mechanisms of the increased risk for relevant cardiovascular events. A recent review has assessed the different pathophysiological mechanisms of adiponectin and has concluded that those associations provide relevant information to understand the inflammatory and atherogenic processes associated with CAD progression and instability of atheromatous plaques40. Those mechanisms include the following: 1) involvement of adiponectin in the regulation of the necrotic core development; 2) double role in the neovascularization process due to pro/antiatherogenic properties; the ability to promote angiogenesis has been proven to be beneficial to prevent ischemia; 3) the inverse relationship between adiponectin and the ratio between matrix metalloproteinases and the tissue inhibitor of metalloproteinase-1 suggests that adiponectin modulates the stability of atheromatous plaques by balancing that relationship; 4) local adiponectin in the intima and adventitia suppresses the expression of VCAM-1 and ICAM-1 adhesion molecules in vascular walls, suggesting that adiponectin improves atherosclerosis partially by inhibiting the expression of those inflammatory molecules in vivo. Adiponectin has a documented role in the following: endothelial activation; propagation of inflammatory factors through expression of adhesion molecules; monocyte adhesion to vascular endothelium and migration to the intima; macrophage activation; transformation of macrophages into foam cells; lipid accumulation in macrophages; proliferation and migration of smooth muscle cells to the intima; and platelet aggregation. Other mechanisms involved also include the phenomena of oxidation and vascular tone regulation.
Of the metabolic variables studied, in addition to adiponectin itself, only fasting glucose showed a significant direct and independent correlation with cardiovascular risk. Our results are comparable to those of several studies on the prognostic role of hyperglycemia on ACS, especially AMI. That might either reflect an unbalance of the systems regulated by adiponectin, as previously discussed, or be influenced by the contra-regulatory mechanisms responsible for stress hyperglycemia. However, because of the associations observed between the biomarkers, specifically with adiponectin, and little or no correlation with us-CRP, leukocytes, platelets and fibrinogen, in addition to maintenance of the association with glycemia even with the strong effect of association of CKMB mass, reflecting the impact of the myocardial necrosis grade, we think that the fasting glucose findings might actually reflect interactions with the effects of adiponectin. Similarly, the results demonstrated by adiponectin quartiles suggest and strengthen that glycemia levels are directly associated with adiponectin levels, exerting an interaction or collinearity effect.
In addition, this study performed serial measurements of neither adiponectin nor the different biomarkers of metabolism, such as glycemia, insulin, HOMA index and HbA1c. Such serial measurements could provide additional relevant information for better understanding metabolic homeostasis interrelations.
This study found a significant and direct association between adiponectin and the female sex, presence of comorbidities, chronic kidney failure, current smoking and HDL-cholesterol and creatinine levels. In addition, this study found a significant and inverse association between adiponectin and DM, body weight, waist circumference and insulin and triglyceride levels. In fact, the results between the different clinical variables and biomarkers emphasize internal consistency with those previously reported, and with similar patterns, reinforce the aspect of consistency, and indicate similar trends, suggesting that the results should be considered satisfactory and interpretable. However, as in other observational cross sectional studies, there are some limitations, such as selection biases, in addition to confounding factors not completely elucidated, and, thus, without ideal adjustment. However, we emphasize that systematic efforts were planned to minimize those aspects, as follows: consecutive recruitment of eligible patients; prospective and standardized data collection, mainly of clinical events since ACS onset and representing 100% of the patients; adjustment of Cox models for important co-variables, such as age, sex, CAD extension, other metabolic, inflammatory and myocardial necrosis biomarkers, left ventricular function, coronary revascularization procedures and pharmacological therapy with proven cardiovascular benefit. Considering the unpredictability of the variability of the different measures in the population studied, the occurrence of non-measured confounding factors, and the exploratory nature of the hypotheses, the findings and conclusions of the study should be considered as suggestive or indicative, supporting their applicability to the Brazilian population and contributing to the formulation of new hypotheses.
Conclusions
The results of this study add information about the potential prognostic role of serum adiponectin levels in patients with ACS. In addition, they support the concept of metabolic homeostasis as an essential biological process that determines cardiovascular risk. Elevated total serum adiponectin levels were independent and direct predictors of the occurrence of relevant cardiovascular outcomes, such as re-hospitalization requiring revascularization. The data discussed emphasize the correlations between adiponectin and anthropometric, metabolic and myocardial biomarkers, and evoke additional investigations to determine the mechanisms involved in the following: the direct association of adiponectin with greater cardiovascular risk; the significant correlation with BNP; and the potential modulation of that biomarker as a therapeutic target.
Table 4.
Cox models after excluding the diabetic patients (group B)
| Primary and co-primary outcomes | ||||||
|---|---|---|---|---|---|---|
| Coefficient | Hazard ratio | 95%CI | p | |||
| Troponin I (per 1) | 0.012 | 1.012 | 1.004 | 1.018 | 0.004 | |
| Adiponectin (per 1,000) | 0.076 | 1.079 | 1.018 | 1.13 | 0.014 | |
| C indices per time intervals | ||||||
| 30 days | 180 days | 365 days | ||||
| 0.8314 | 0.7906 | 0.7906 | ||||
| Coefficient | Hazard ratio | 95%CI | p | |||
| Previous angina | 1.461 | 4.310 | 1.17 | 22.98 | 0.026 | |
| Adiponectin (per 1,000) | 0.067 | 1.070 | 1.01 | 1.12 | 0.023 | |
| C indices per time intervals | ||||||
| 30 days | 180 days | 365 days | ||||
| 0.6346 | 0.7719 | 0.7719 | ||||
Table 5.
Cox models after excluding ST-segment elevation AMI (group C)
| Primary and co-primary outcomes | ||||||
|---|---|---|---|---|---|---|
| Coefficient | Hazard ratio | 95%CI | p | |||
| Fasting glucose (per 10) | 0.078 | 1.081 | 1.03 | 1.12 | 0.005 | |
| Adiponectin (per 1,000) | 0.05 | 1.051 | 0.997 | 1.098 | 0.06 | |
| C indices per time intervals | ||||||
| 30 days | 180 days | 365 days | ||||
| 0.7039 | 0.7025 | 0.7025 | ||||
| Coefficient | Hazard ratio | 95%CI | P | |||
| Previous angina | 1.262 | 3.53 | 1.17 | 13.88 | 0.023 | |
| Adiponectin (per 1,000) | 0.050 | 1.051 | 0.998 | 1.096 | 0.05 | |
| C indices per time intervals | ||||||
| 30 days | 180 days | 365 days | ||||
| 0.7368 | 0.7330 | 0.7330 | ||||
Acknowledgements
This study received logistic support from the Division of Translational Epidemiology and from the Clinical Analyses Laboratory of the Instituto Dante Pazzanese de Cardiologia (IDPC). We are also grateful to the Statistics and Epidemiology Laboratory of the IDPC and the Instituto Gênese de Análises Científicas.
Footnotes
Author contributions
Conception and design of the research, Analysis and interpretation of the data, Obtaining funding and Critical revision of the manuscript for intellectual content: Oliveira GBF, Piegas LS; Acquisition of data and Writing of the manuscript: Oliveira GBF; Statistical analysis: França JID.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of Doctoral submitted by Gustavo Bernardes de Figueiredo Oliveira, from Universidade de São Paulo.
References
- 1.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk. Arch Intern Med. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Cupples LA, Ramaswami R, Stokes J, 3rd, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease; the Framingham study. J Clin Epidemiol. 1991;44(2):183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 3. (World Health Org Tech Rep Ser).Obesity: preventing and managing the global epidemic. Report of a World Health Organization. 2000;894:i-xii–i-253. [PubMed] [Google Scholar]
- 4.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart Association. National Heart, Lung, and Blood Institute Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 5.Luciano C, Hulford J, Abdullah A, Zarich S. Metabolic syndrome: a major risk factor for acute myocardial infarction in patients < 45 years of age. J Am Coll Cardiol. 2004;43(5) Suppl 1:A249. [Google Scholar]
- 6.Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141(12):1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzo C, Williams K, Hunt KJ, Hoffner SM. The National Cholesterol Education Program-Adult Treatment Panel III, International Diabetes Federation, and World Health Organization Definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30(1):8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 10.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in united states adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 11.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007;49(21):2112–2119. doi: 10.1016/j.jacc.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(2):131–141. doi: 10.2183/pjab.86.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trujillo ME, Scherer PE. Adiponectin - journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257(2):167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 14.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. Circulation. 2009;119(17):2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. Erratum in Circulation. 2009;119(25):e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 16.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K, et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221(2):286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100(25):2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103(8):1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 19.Kato H, Kashiwagi H, Shiraga M, Tadokoro S, Kamae T, Ujiie H, et al. Adiponectin acts as an endogenous antithrombotic factor. Arterioscler Throm Vasc Biol. 2006;26(1):224–230. doi: 10.1161/01.ATV.0000194076.84568.81. [DOI] [PubMed] [Google Scholar]
- 20.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose- specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Throm Vasc Biol. 2000;20(6):1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 21.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291(14):1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 22.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114(7):623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 23.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Osaka CAD Study Group Coronary artery disease. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23(1):85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectina with coronary heart disease and mortality. The Rancho Bernardo study. Am J Epidemiol. 2007;165(2):164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kizer JR, Barzilay JI, Kuller LH, Gottdiener JS. Adiponectin and risk of coronary heart disease in older men and women. J Clin Endocrinol Metab. 2008;93(9):3357–3364. doi: 10.1210/jc.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112(12):1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 27.George J, Patal S, Wexler D, Sharabi Y, Peleg E, Kamari Y, et al. Circulating adiponectin concentrations in patients with congestive heart failure. Heart. 2006;92(10):1420–1424. doi: 10.1136/hrt.2005.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167(14):1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 29.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27(19):2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira EP, Souza ML, Lima MD. Índice HOMA (homeostasis model assessment) na prática clínica: uma revisão. J Bras Patol Med Lab. 2005;41(4):237–243. [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54(5):541–553. [PMC free article] [PubMed] [Google Scholar]
- 34.Haddad N. Metodologia de estudos em ciências da saúde. São Paulo: Roca; 2004. [Google Scholar]
- 35.Hennekens CH, Buring JE. Epidemiology in medicine. Philadelphia: Lippincott Williams & Wilkins; 1987. [Google Scholar]
- 36.Lughlin GA, Barrett-Connor E, May S. Sex-specific determinants of serum adiponectina in older adults: the role of endogenous sex hormones. Intl J Obes (Lond) 2007;31(3):457–465. doi: 10.1038/sj.ijo.0803427. [DOI] [PubMed] [Google Scholar]
- 37.Wolk R, Berger P, Lennon RJ, Brilakis ES, Davison DE, Somers VK. Association between plasma adiponectin levels and unstable coronary syndromes. Eur Heart J. 2007;28(3):292–298. doi: 10.1093/eurheartj/ehl361. [DOI] [PubMed] [Google Scholar]
- 38.Schnabel R, Messow CM, Lubos E, Espinola-Klein C, Rupprecht HJ, Bickel C, et al. Association of adiponectin with adverse outcome in coronary artery disease patients: results from the AtheroGene study. Eur Heart J. 2008;29(5):649–657. doi: 10.1093/eurheartj/ehn009. [DOI] [PubMed] [Google Scholar]
- 39.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 40.Barseghian A, Gawande D, Bajaj M. Adiponectin and vulnerable atherosclerotic plaques. J Am Coll Cardiol. 2011;57(7):761–770. doi: 10.1016/j.jacc.2010.11.011. [DOI] [PubMed] [Google Scholar]