Abstract
Background
Most reports regarding the obesity paradox have focused on body mass index (BMI) to classify obesity and the prognostic values of other indirect measurements of body composition remain poorly examined in heart failure (HF).
Objective
To evaluate the association between BMI and other indirect, but easily accessible, body composition measurements associated with the risk of all-cause mortality in HF.
Methods
Anthropometric parameters of body composition were assessed in 344 outpatients with a left ventricular ejection fraction (LVEF) of ≤50% from a prospective HF cohort that was followed-up for 30 ± 8.2 months. Survival was evaluated using the Kaplan-Meier method and Cox proportional hazard regression analysis.
Results
HF patients were predominantly male, of non-ischemic etiology, and had moderate to severe LV systolic dysfunction (mean LVEF = 32 ± 9%). Triceps skinfold (TSF) was the only anthropometric index that was associated with HF prognosis and had significantly lower values in patients who died (p = 0.047). A TSF ≥ 20 mm was present in 9% of patients that died and 22% of those who survived (p = 0.027). Univariate analysis showed that serum creatinine level, LVEF, and NYHA class were associated with the risk of death, while Cox proportional hazard regression analysis showed that TSF ≥ 20 was a strong independent predictor of all-cause mortality (hazard ratio = 0.36; 95% CI = 0.13-0.97, p = 0.03).
Conclusion
Although BMI is the most widely used anthropometric parameter in clinical practice, our results suggested that TSF is a better predictive marker of mortality in HF outpatients.
Keywords: Heart failure, Body mass index, Mortality, Body composition
Introdution
Obesity is defined by excessive body fat and has a long-established relationship with cardiovascular disease (CVD) and heart failure (HF)1,2. In the general CVD-free adult population, extremes in body mass index (BMI) have been associated with an increased risk of overall mortality3-5. However, there is a growing body of clinical evidence indicating that excess weight might confer a lower risk of adverse clinical events, particularly in HF patients. This phenomenon has been referred to as the "obesity paradox" or "reverse epidemiology"6-8.
Most reports regarding the obesity paradox have used BMI to classify obesity9. Although BMI is the most common method to define overweight and obese populations in epidemiological studies, it clearly does not reflect body composition10, thus depicting a relatively low sensitivity to predict fat excess11. Unfortunately, direct measurements of body mass composition, like dual energy X-ray absorptiometry (DEXA), are not practical and have not been directly related to survival in HF patients12.
Data to evaluate the prognostic value of other anthropometric and indirect measures of body composition, such as waist circumference (WC), arm muscle circumference (AMC), and triceps skinfold (TSF), have been poorly examined in HF patients. Lavie et al13 have suggested that a high body fat percentage, as estimated by TSF measurements, might be an independent predictor of cardiovascular death or heart transplantation. However, other studies have not reached a consensus regarding the role of these parameters in HF prognosis14-16.
Therefore, the aim of the present prospective study was to evaluate the association between BMI and several other indirect, but easily accessible, body composition measurements to the risk of HF mortality and hospitalization.
Methods
Study Design and Population
A prospective cohort of HF outpatients followed-up at the HF and Transplant Clinic of a university tertiary care hospital in Porto Alegre (RS, Brazil) between May 2008 and December 2009 were enrolled in the present study. This cohort included patients with an HF diagnosis, predominantly with left ventricular systolic dysfunction [left ventricular ejection fraction (LVEF) < 50%], confirmed by two-dimensional echocardiography. Pregnant women, patients with significant peripheral edema, and those with clinical conditions, in which anthropometric measurements were not feasible, were excluded. Signed and informed consent was obtained from all patients prior to enrollment and the research protocol was approved by our institutional review committee.
Anthropometric Parameters
Anthropometric measurements of weight, height, body surface area (BSA), BMI, WC, arm circumference (AC), AMC, and TSF were collected during the first medical examination. All anthropometric measurements were performed by the same trained investigator, a registered nutritionist, to avoid interobserver variability.
BMI, BSA, and Ponderal Index (PI)
BMI was calculated using the Quetelet equation as follows: BMI (Quetelet) = weight (kg)/length (m)2. Weight was measured using a balance scale (Filizola PL180; Filizola, Brazil) with capacity of 180 kg and an accuracy of 100 g. For height measurement, we used a vertical wall-mounted stadiometer. BMI was classified into three categories according to the World Health Organization classification for adults: underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2), and overweight (>25 kg/m2); and the Pan American Health Organization criteria for the elderly: underweight (<23 kg/m2), normal weight (23-28 kg/m2), and overweight (>28 kg/m2)17. In addition, we calculated BSA as weight0.5378 × height(0.3964) × 0.024265 and PI as weight/height3.
Waist circumference
WC was measured at the midpoint between the lowest rib and the iliac crest during expiration. Patients were instructed to remain in an upright position with weight evenly distributed on both sides and breathing smoothly to prevent abdominal muscle contraction.
Triceps Skinfold
The TSF thickness (in mm) was obtained at the mid-point of the non-dominant arm (between the acromial process and the olecranon) with the arm freely stretched along the body. A fold of skin was then pinched with the fingers and a scientific caliper (Cescorf Scientific, Cescorf, Brazil) was applied. The measurement was repeated three times and the mean of the measurements was used for analysis.
Triceps Skinfold
AMC (in cm) was calculated by measuring the AC and the TSF thickness, using the following formula proposed by Jelliffe: C2 = C1-3.14*S, where C2 is the muscular circumference, C1 is the arm circumference, and S is TSF thickness (in cm)18.
Outcome Evaluation
Enrolled patients were followed-up at the HF and Transplant Outpatient Clinic. At the HF clinic, patients were scheduled to have regular visits at pre-defined intervals of 1-4 months. Follow-up data were directly derived from reviewing all electronic clinical data from the institutional records (most patients had several follow-up visits). For patients who were not regularly visiting the HF clinic (or were lost to follow-up), telephone contact was attempted to obtain relevant clinical events based on a structured telephone interview performed by trained nurses. For the study participants who we were unable to contact by phone (approximately 20 patients), we checked their vital status through the State Death Certificate Database, which contains data on the main cause and date of all deaths in our state. For statistical analysis we used (1) all-cause mortality and (2) HF-related hospitalizations.
Statistical Analysis
Baseline patient clinical characteristics were expressed as mean ± SD or number and percentage. Continuous variables were compared using the Student's t-test or Mann-Whitney U test as appropriate, whereas categorical variables were compared using the chi-square test or Fisher's exact test. TSF and BMI were also analyzed according to quintiles of the distribution. Survival curves were constructed using the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazard regression analysis was performed to determine independent predictors of survival and included at least one anthropometric parameter (either BMI or TSF) and clinical predictors of risk [gender, age, New York Heart Association (NYHA) class, LVEF, and serum creatinine level]. A two-tailed p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS statistical software ver. 18 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
From May 2008 to September 2009, a total of 378 HF outpatients, who were followed-up at the HF and Transplant clinic, agreed to participate in the study and had their anthropometric parameters evaluated. We excluded 34 patients from the protocol because LV function assessment indicated a LVEF > 50%.
Baseline clinical characteristics of the remaining study population (n = 344) are listed in Table 1 and stratified by survival. Overall, HF patients were predominantly male, self-reportedly white, of non-ischemic etiology, in NYHA functional class I-II, and had moderate to severe LV systolic dysfunction (mean LVEF = 32 ± 9%). Most patients were hypertensive and 30% had diabetes. The mean follow-up period was 30.3 ± 8.2 months. Patients who died were older, had relatively high creatinine levels, low LVEFs, and depicted a trend towards higher NYHA functional class.
Table 1.
Baseline clinical characteristics of the study population
| Total (n = 344) | Alive (n = 288) | Dead (n = 56) | p | |
|---|---|---|---|---|
| Age (years) | 59 ± 13 | 59 ± 13 | 62 ± 11 | 0.031 |
| Gender (male) | 224(65) | 185 (64) | 39(73) | 0.54 |
| Ethnicity (Caucasian) | 281 (81) | 234(82) | 47(85) | 0.20 |
| Smoking | 43 (12) | 35 (13) | 8 (17) | 0.57 |
| Etiology | 0.10 | |||
| Ischemic | 118(34) | 94 (33) | 24(45) | |
| Hypertensive | 69(20) | 55(19) | 14 (26) | |
| Idiopathic | 57 (17) | 53 (18) | 4 (7) | |
| Alcoholic | 38 (11) | 32 (11) | 6 (11) | |
| Other | 52 (15) | 47 (17) | 5 (9) | |
| NYHA class | 0.08 | |||
| I-II | 286(83) | 244(84) | 42(75) | |
| III-IV | 58 (17) | 44 (16) | 14 (25) | |
| Systolic blood pressure (mmHg) | 124 ± 22 | 124 ± 22 | 125 ± 22 | 0.83 |
| Creatinine (mg/dL) | 1.2 ± 0.5 | 1.2 ± 0.5 | 1.4 ± 0.7 | 0.002 |
| Na (mEq/L) | 140 ± 3.4 | 140 ± 3 | 140 ± 3 | 0.31 |
| Left ventricle ejection fraction (%) | 32 ± 9 | 33 ± 9 | 29 ± 9 | 0.008 |
| Comorbidities | ||||
| Diabetes Melitus. | 104 (30) | 81 (29) | 23(42) | 0.078 |
| Hypertension | 224(65) | 178 (65) | 46 (85) | 0.004 |
| COPD | 27 (7.8) | 22 (10) | 5(12) | 0.078 |
| Angina | 52 (15) | 44 (18) | 8 (19) | 0.97 |
| Atrial fibrillation | 86(25) | 70 (27) | 16 (31) | 0.60 |
Data are expressed as the means ± standard deviation or absolute numbers (%). NYHA: New York Heart Association; COPD: chronic obstructive pulmonary disease.
Nutritional assessment parameters are listed in Table 2. Most HF patients were overweight when classified by BMI. There were no significant differences in most anthropometric parameters between patients who died and those who survived. In particular, mean BSA and BMI were remarkably similar in both groups, even when stratified by quintiles of the distribution. TSF was the only anthropometric index that was associated with HF prognosis. Surviving patients had a TSF 10% higher than patients who died. A TSF ≥ 20 mm was observed in only 9% of the HF patients that died during follow-up and in 22% of those that survived (p = 0.027; Table 2).
Table 2.
Anthropometric baseline parameters of the study population
| Total (n = 344) | Alive (n = 288) | Dead (n = 56) | p | |
|---|---|---|---|---|
| BMI (kg/m2) | 26 ± 5 | 26.7 ± 5.3 | 26.1 ± 4.8 | 0.47 |
| Underweight | 58 (17) | 46 (16) | 12 (21) | 0.40 |
| Normal | 131 (38) | 108 (37) | 23 (41) | |
| Overweight and obesity | 155(45) | 134 (46) | 21 (37) | |
| BMI ≥ 30.4 (superior quintile) | 69(20) | 57(20) | 12 (21) | 0.45 |
| Ponderal index | 16.1± 3.3 | 16.2 ± 3.4 | 15.7 ± 2.9 | 0.26 |
| Body surface area | 1.8 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.2 | 0.57 |
| Triceps skinfold (mm) | 14.3 ± 8 | 14.6 ± 8.3 | 12.8 ± 5.5 | 0.047 |
| TSF ≥ 20 (superior quintile) | 68(20) | 63(22) | 5(9) | 0.027 |
| Arm muscle circumference (cm) | 26.1 ± 3.4 | 26.2 ± 3.3 | 25.6 ± 3.5 | 0.18 |
| Waist circumference (cm) | 96 ± 13 | 95.7 ± 12.6 | 97.9 ± 13.3 | 0.24 |
Data are expressed as the means ± standard deviation or absolute number (%). BMI: Body mass index; TSF: Triceps skinfold.
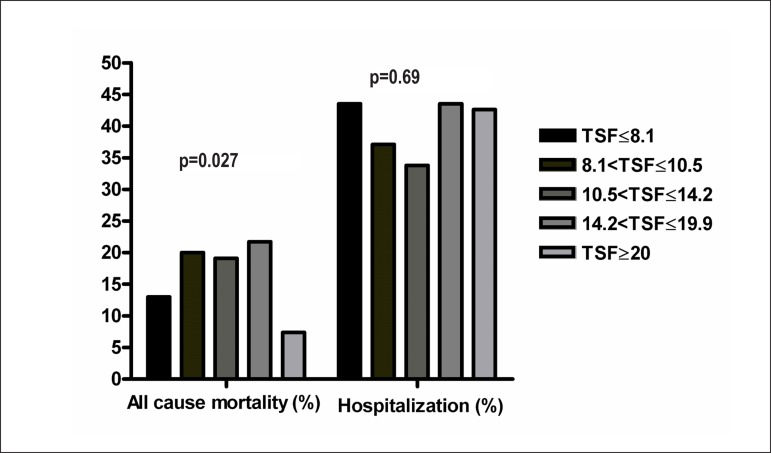
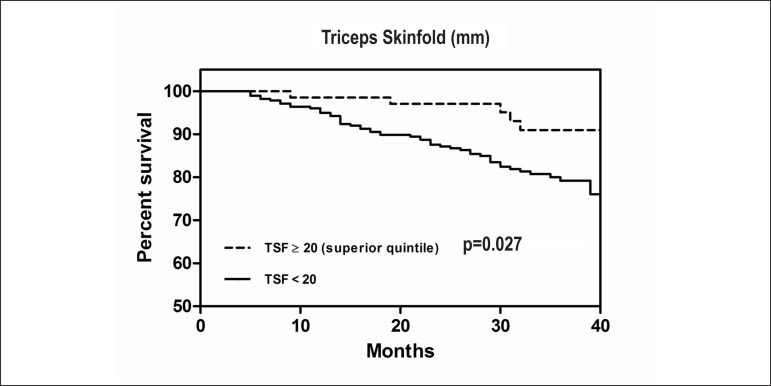
Data regarding TSF quintiles are presented in Table 3. Patients within the highest TSF quintile were younger, mostly females, with lower serum creatinine levels and higher LVEFs. As expected, patients in the highest TSF quintile had higher BMIs and WCs (p < 0.001). Figure 1 depicts HF hospitalization rates and overall mortality according to TSF quintiles. Our analysis demonstrated that HF patients within the 5th quintile had approximately a three-fold lower mortality rate than patients in the 2nd, 3rd, and 4th quintiles. No significant differences were observed in HF hospitalizations according to TSF. In addition, the Kaplan-Meier survival curves stratified by TSF progressively diverged over time (Figure 2A), but such differences were not observed in the BMI-stratified analysis.
Table 3.
Comparison of clinical and nutritional characteristics among quintiles of TSF
| Q1 (69) ≤ 8,1 | Q2 (70) 8,2-10,5 | Q3 (68) 10,6-14,2 | Q4 (69) 14,3-19,9 | Q5 (68) ≥ 20 | p | |
|---|---|---|---|---|---|---|
| Age (years) | 63 ± 15 | 62 ± 9 | 57 ± 11 | 59 ± 14 | 55 ± 13 | 0.004 |
| Gender (male) | 61 (88) | 61 (87) | 52 (76) | 35 (50) | 15 (22) | <0.001 |
| Etiology | 0.06 | |||||
| Ischemic | 22 (34) | 27 (40) | 22 (33) | 24 (35) | 23 (35) | |
| Hypertensive | 15 (23) | 13 (18) | 11 (17) | 16 (23) | 14 (21) | |
| Idiopathic | 10 (15) | 6 (8) | 15 (23) | 15 (22) | 11 (16) | |
| Alcoholic | 11 (17) | 13 (19) | 9 (14) | 3 (4) | 2 (3) | |
| Other | 7 (11) | 10 (14) | 9 (14) | 10 (15) | 16 (26) | |
| NYHA class | 0.06 | |||||
| I-II | 63 (91) | 58 (83) | 55 (81) | 59 (85) | 51 (75) | |
| III-IV | 6 (9) | 12 (17) | 13 (19) | 10 (14) | 17 (25) | |
| Systolic blood pressure (mmHg) | 120 ± 22 | 123 ± 20 | 126 ± 25 | 128 ± 21 | 127 ± 22 | 0.15 |
| Creatinine (mg/dL) | 1.4 ± 0.7 | 1.3 ± 0.4 | 1.2 ± 0.5 | 1.0 ± 0.4 | 1.0 ± 0.4 | <0.001 |
| Na (mEq/L) | 140 ± 4 | 141 ± 3 | 140 ± 3 | 141 ± 4 | 140 ± 3 | 0.42 |
| Left Ventricle Ejection fraction (%) | 30 ± 9 | 31 ± 9 | 31 ± 8 | 35 ± 9 | 35 ± 10 | 0.004 |
| Body mass index (kg/m2) | 22 ± 2 | 24 ± 3 | 25 ± 4 | 26 ± 4 | 31 ± 6 | <0.001 |
| Triceps skinfold (mm) | 5.8 ± 1.5 | 9.5 ± 0.7 | 12 ± 0.9 | 17 ± 1.7 | 27 ± 6 | <0.001 |
| Arm muscle circumference (cm) | 25 ± 3 | 26 ± 3 | 27 ± 3 | 26 ± 3 | 26 ± 4 | 0.95 |
| Waist circumference (cm) | 88 ± 9 | 94 ± 10 | 97 ± 11 | 98 ± 13 | 103 ± 14 | <0.001 |
Data are expressed as means ± standard deviations or absolute numbers (%). NYHA: New York Heart Association.
Figure 1.
HF hospitalization and overall mortality rates according to quintiles of TSF (mm). The p-value represents the difference in the 5th quintile vs. other quintiles.
Figure 2.
Kaplan–Meier results for event-free survival curves (freedom from all causes mortality) for: (A) patients in the 5th quintile of triceps skinfold (TSF ≥ 20) vs. all other quintiles (TSF < 20) and (B) patients in the 5th quintile of body mass index (BMI ≥ 30.4) vs. all other quintiles (BMI < 30.4).
Table 4 shows univariate analysisand multivariate Cox regression analysis results for all-cause mortality, including nutritional parameters and other clinical variables. In the univariate analysis, serum creatinine levels, LVEF, and NYHA class were associated with risk, but TSF was the single best predictor of mortality [hazard ratio (HR) = 0.36; 95% confidence interval (CI) = 0.14-0.91; p = 0.03]. Finally, after adjustment for these clinical characteristics, TSF remained a major independent predictor of overall mortality (HR = 0.36; 95% CI = 0.13-0.97).
Table 4.
Univariate and multivariate Cox regression analysis
| Variable | Univariate HR (95% CI) | Multivariate HR (95% CI) |
|---|---|---|
| Age | 1.01 (0.99-1.03) | |
| Gender (female) | 1.29 (0.73-2.29) | 0.88 (0.64-1.20) |
| NYHA class (I and II) | 0.54 (0.29-1.00) | 0.75 (0.55-1.03) |
| Creatinine (mg/dL) | 1.57 (1.18-2.07) | 1.40 (1.00-1.95) |
| LVEF (%) | 0.95 (0.92-0.98) | 0.96 (0.93-0.99) |
| TSF (superior quintile) | 0.36 (0.14-0.91) | 0.36 (0.13-0.97) |
| Waist circumference (cm) | 1.01 (0.99-1.03) | |
| Arm muscle circumference (cm) | 0.95 (0.88-1.02) | |
| BMI (superior quintile) | 1.06 (0.56-2.01) |
LVEF: left Ventricular ejection fraction; TSF: triceps skin fold; BMI: body mass index; NYHA: New York Heart Association.
Discussion
Despite the growing interest in the obesity paradox, there is still an ongoing debate regarding the most appropriate parameter(s) to assess the nutritional status of HF patients. Our results demonstrated that among numerous anthropometric indices (BMI, BSA, PI, TSF, WC, and AC), TSF was the only parameter that could differentiate survivors from non-survivors in a contemporary "real-world" prospective cohort of HF patients. This finding is in agreement with the concept of reverse epidemiology, as HF patients in the highest TSF quintile had lower overall mortality, even after adjustment for other important clinical predictors of risk. We did not observe a dose-response relationship between TSF and mortality, as only the superior quintile, representing a greater amount of fat mass, appeared to be an independent protective factor. In addition, unlike other studies, we did not find BMI as an adequate predictor of HF prognosis.
The correlation between BMI and HF survival remains controversial. Post-hoc analysis of large clinical trials19 demonstrated that lower BMI was associated with decreased survival. Symptomatic HF patients evaluated in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity trial with either reduced or preserved LV systolic function, underweight patients or those with a low BMI were independently associated with a substantial increased risk of death (almost 70% for BMI < 22.5 kg/m2), but primarily in patients without evidence of fluid overload7. Recently, Vara et al20 described this phenomenon in elderly, hospitalized, HF patients However, although obesity is frequently evaluated by BMI in clinical practice, several investigators have questioned the accuracy of BMI to assess different body composition components21-23. For instance, the relationship between BMI and body fat percentage was reportedly influenced by ethnicity and age24. In the elderly, BMI might represent a higher percentage of body fat, while in the relatively young this association is less evident25. Recently, similar to our findings, several other investigators have questioned the usefulness of BMI as a predictor of mortality or cardiac events10,14,16,26.
Direct indices of body composition are theoretically the best markers to evaluate the prognostic role of nutritional status in different cardiovascular scenarios. Unfortunately, until now, there were no prospective large-scale studies that evaluated the role of these parameters on HF survival. Recently, Oreopoulos et al12 evaluated the association between direct measurements of body composition by DEXA and prognostic factors in 140 chronic HF patients and demonstrated that BMI misclassified body fat status in approximately 40% of the studied patients. Also, a higher lean body mass and/or lower fat mass were independently associated with factors that are prognostically beneficial in HF, suggesting that BMI may not be a good indicator of adiposity and may, in fact, be a better surrogate for lean body mass in this population, a finding that per se might question the obesity paradox12. However, one must also consider that the association of direct body composition measurements with surrogate CVD markers may not translate into similar data regarding survival10.
Anthropometry is a simple technique that is easily applied in clinical practice or in large population surveys. Numerous anthropometric parameters have been proposed to assess nutritional status and appraise different body composition components. TSF thickness measurement allows estimation of body fat content27, while limb circumferences reflect limb muscle and, thus, protein nutritional state. It is important to point out that TSF thickness measures primarily subcutaneous fat, and therefore, is insensitive to changes or abnormalities in visceral fat. Body density and body fat can be accurately estimated from the sum of TSF measurements28. Previous studies have compared and validated different body composition techniques, such as DEXA, to assess fat mass and have demonstrated an adequate accuracy to estimate body fat mass, both for subscapular and TSF thickness29. In particular, TSF has been used more frequently than other sites, because it is easy to access, reproducible, and can measure a wide range of variation among individuals30. A recent study compared body composition assessment in 118 hemodialysis patients and reported that TSF was one of the most accurate parameters to estimate total body fat percentage using DEXA as the reference test31.
Lavie et al13 pioneered evaluation of the prognostic role of body fat percentage based on skinfold measurements in HF patients and demonstrated that for each 1% absolute reduction in percent body fat, major clinical events increased by >13%. Assessment of other anthropometric parameters, such as WC, has been proposed for HF risk stratification, but with inconsistent results14,15. Our results reinforced the concept of the obesity paradox and suggested that assessment of a simple anthropometric parameter to measure subcutaneous fat (the TSF) might be adequate to indirectly assess overall body fat mass.
Regarding hospitalization risk, most reports on the assessment of the obesity paradox opted for analysis of a combined endpoint (death and hospitalization), thereby limiting separate evaluations of these events. Furthermore, some studies have found similar results, in which obesity is a predictor of only overall/cardiovascular mortality, but not hospitalization8. One possible explanation for these findings is the fact that patients with greater adiposity are diagnosed earlier with HF, which justifies why this group is younger, has less degree of cardiac dysfunction, and consequent better survival. These patients, however, may have similar vulnerability to episodes of HF decompensation than those with normal amounts of fat mass.
The results of the present study should be evaluated by taking into account some methodological limitations. First, we used indirect measurements of body composition to evaluate body fat mass. Several studies, however, suggested that TSF measurement was apparently an adequate estimation of body fat27. Second, we acknowledged that anthropometry, particularly skinfold measurement, requires a considerable amount of technical skill and meticulousness. In our protocol, all parameters were evaluated by a single trained professional to avoid interobserver variability. Third, we opted to use only TSF measurements to predict body fat composition30,31 instead of more complex equations based on multiple skinfolds. Although this strategy might slightly reduce the accuracy of body composition assessment, we believe that if simplifies the clinical applicability of our findings.
Conclusion
Our results demonstrated that TSF might be a better predictor of mortality in HF outpatients and reinforced the concept of the obesity paradox. TSF measurement has the advantages of a simple, practical, and low cost method to assess risk and can be easily implemented in clinical practice, if performed by a trained professional.
Footnotes
Author contributions
Conception and design of the research: Souza GC; Acquisition of data: Alves FD, Zuchinali P, Souza GC, Almeida KSM; Analysis and interpretation of the data: Goldraich LA, Rohde LEP, Zuchinali P; Statistical analysis: Goldraich LA, Zuchinali P; Critical revision of the manuscript for intellectual content: Clausell NO, Souza GC, Rohde LEP, Zuchinali P.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any post-graduation program.
References
- 1.Contaldo F, Pasanisi F, Finelli C, de Simone G. Obesity, heart failure and sudden death. Nutr Metab Cardiovasc Dis. 2002;12(4):190–197. [PubMed] [Google Scholar]
- 2.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 5.Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364(8):719–729. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arena R, Lavie CJ. The obesity paradox and outcome in heart failure: is excess bodyweight truly protective? Future Cardiol. 2010;6(1):1–6. doi: 10.2217/fca.09.158. [DOI] [PubMed] [Google Scholar]
- 7.Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, Skali H, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Circulation. 2007;116(6):627–636. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, et al. The obesity paradox body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165(1):55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 9.Shirley S, Davis LL, Carlson BW. The relationship between body mass index/body composition and survival in patients with heart failure. J Am Acad Nurse Pract. 2008;20(6):326–332. doi: 10.1111/j.1745-7599.2008.00328.x. [DOI] [PubMed] [Google Scholar]
- 10.Lavie CJ, Milani RV, Ventura HO, Romero-Corral A. Body composition and heart failure prevalence and prognosis: getting to the fat of the matter in the "obesity paradox". Mayo Clin Proc. 2010;85(7):605–608. doi: 10.4065/mcp.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Bailey KR, Collazo-Clavell ML, et al. Accuracy of body mass index to diagnose obesity in the US adult population. Int J Obes. 2008;32(6):959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oreopoulos A, Ezekowitz JA, McAlister FA, Kalantar-Zadeh K, Fonarow GC, Norris CM, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85(7):609–617. doi: 10.4065/mcp.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91(7):891–894. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 14.Testa G, Cacciatore F, Galizia G, Della-Morte D, Mazzella F, Langellotto A, et al. Waist circumference but not body mass index predicts long-term mortality in elderly subjects with chronic heart failure. J Am Geriatr Soc. 2010;58(8):1433–1440. doi: 10.1111/j.1532-5415.2010.02979.x. [DOI] [PubMed] [Google Scholar]
- 15.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17(5):374–380. doi: 10.1016/j.cardfail.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Gastelurrutia P, Lupón J, Domingo M, Ribas N, Noguero M, Martinez C, et al. Usefulness of body mass index to characterize nutritional status in patients with heart failure. Am J Cardiol. 2011;108(8):1166–1170. doi: 10.1016/j.amjcard.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Wold Health Organization (WHO) Anales da 36ª Reunión del Comité Asesor de Investigaciones en salud. Encuesta multicentrica: salud, bien estar y envejecimiento (SABE) en América Latina y el Caribe. Washington (DC): Mayo. 2001. 2001. [Google Scholar]
- 18.Jelliffe DB. The Assessment of the nutritional status of the community - (with special reference to field surveys in developing regions of the world) Monogr Ser World Health Organ. 1996;53:3–271. [PubMed] [Google Scholar]
- 19.Kapoor JR, Heidenreich PA. Obesity and survival in patients with heart failure and preserved systolic function: a U-shaped relationship. Am Heart J. 2010;159(1):75–80. doi: 10.1016/j.ahj.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Casas Vara A, Santolaria F, Fernandez-Bereciartúa A, Gonzalez-Reimers E, Garcia-Ochoa A, Martinez-Riera A. The obesity paradox in elderly patients with heart failure: analysis of nutritional status. Nutrition. 2012;28(6):616–622. doi: 10.1016/j.nut.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Romero-Corral A, Somers VK, Sierra-Johnson J, Jensen MD, Thomas RJ, Squires RW, et al. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. 2007;28(17):2087–2093. doi: 10.1093/eurheartj/ehm243. [DOI] [PubMed] [Google Scholar]
- 22.Romero-Corral A, Lopez-Jimenez F, Sierra-Johnson J, Somers VK. Differentiating between body fat and lean mass: how should we measure obesity? Nat Clin Pract Endocrinol Metab. 2008;4(6):322–323. doi: 10.1038/ncpendmet0809. [DOI] [PubMed] [Google Scholar]
- 23.Salinas AM, Coca A. Ergo-anthropometric assessment [letter reply] Mayo Clin Proc. 2009;84(10):941–942. doi: 10.4065/84.10.940-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deurenberg P. Universal cut-off BMI points for obesity are not appropriate. Br J Nutr. 2001;85(2):135–136. doi: 10.1079/bjn2000273. [DOI] [PubMed] [Google Scholar]
- 25.Poirier P. Adiposity and cardiovascular disease: are we using the right definition of obesity? Eur Heart J. 2007;28(17):2047–2048. doi: 10.1093/eurheartj/ehm321. [DOI] [PubMed] [Google Scholar]
- 26.Tarastchuk JC, Guérios EE, Bueno Rda R, Andrade PM, Nercolini DC, Ferraz JG, et al. Obesity and coronary intervention: should we continue to use body mass index as a risk factor? Arq Bras Cardiol. 2008;90(5):284–289. doi: 10.1590/s0066-782x2008000500001. [DOI] [PubMed] [Google Scholar]
- 27.Woodrow G. Body composition analysis techniques in the aged adult: indications and limitations. Curr Opin Clin Nutr Metab Care. 2009;12(1):8–14. doi: 10.1097/MCO.0b013e32831b9c5b. [DOI] [PubMed] [Google Scholar]
- 28.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32(1):77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 29.Goran MI, Driscoll P, Johnson R, Nagy TR, Hunter G. Cross-calibration of body-composition techniques against dual-energy X-ray absorptiometry in young children. Am J Clin Nutr. 1996;63(3):299–305. doi: 10.1093/ajcn/63.3.299. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Thornton JC, Kolesnik NS, Pierson RN., Jr Anthropometry in body composition: an overview. Ann N Y Acad Sci. 2000;904:317–326. doi: 10.1111/j.1749-6632.2000.tb06474.x. [DOI] [PubMed] [Google Scholar]
- 31.Bross R, Chandramohan G, Kovesdy CP, Oreopoulos A, Noori N, Golden S, et al. Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis. 2010;55(5):885–896. doi: 10.1053/j.ajkd.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]