Abstract
Deficiency of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) in humans leads to the syndrome of apparent mineralocorticoid excess (SAME), in which cortisol illicitly occupies mineralocorticoid receptors, causing sodium retention, hypokalemia, and hypertension. However, the disorder is usually incompletely corrected by suppression of cortisol, suggesting additional and irreversible changes, perhaps in the kidney. To examine this further, we produced mice with targeted disruption of the 11β-HSD2 gene. Homozygous mutant mice (11β-HSD2–/–) appear normal at birth, but ∼50% show motor weakness and die within 48 hours. Both male and female survivors are fertile but exhibit hypokalemia, hypotonic polyuria, and apparent mineralocorticoid activity of corticosterone. Young adult 11β-HSD2–/– mice are markedly hypertensive, with a mean arterial blood pressure of 146 ± 2 mmHg, compared with 121 ± 2 mmHg in wild-type controls and 114 ± 4 mmHg in heterozygotes. The epithelium of the distal tubule of the nephron shows striking hypertrophy and hyperplasia. These histological changes do not readily reverse with mineralocorticoid receptor antagonism in adulthood. Thus, 11β-HSD2–/– mice demonstrate the major features of SAME, providing a unique rodent model to study the molecular mechanisms of kidney resetting leading to hypertension.
J. Clin. Invest. 103:683–689 (1999)
Introduction
Sustained alterations of blood pressure require resetting of renal mechanisms maintaining salt–water balance (1). The latter is critically dependent on sodium resorption in the distal tubule of the nephron, which is regulated by mineralocorticoid hormones (principally aldosterone). Indeed, most human monogenic hypertensive and hypotensive syndromes reflect mutations in genes that determine electrolyte transport in the distal portion of the nephron, either directly (2, 3) or through alterations in mineralocorticoid- or glucocorticoid-signaling pathways (4, 5).
Although physiological glucocorticoids (corticosterone in rats and mice, cortisol in humans) bind to purified mineralocorticoid receptors (MR) with affinities similar to aldosterone in vitro (6), in vivo MR are selectively activated by aldosterone. This selectivity is produced by 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which catalyzes the rapid metabolism of glucocorticoids to inert 11-keto forms (11-dehydrocorticosterone, cortisone) (7). In the syndrome of apparent mineralocorticoid excess (SAME) (8), deficiency of renal 11β-HSD2 allows physiological glucocorticoids (cortisol, corticosterone) to illicitly activate MR in the distal tubule, producing sodium retention, severe hypertension, and hypokalemia (7, 9, 10). However, the disorder is usually incompletely corrected by suppression of cortisol, suggesting that irreversible changes also occur, possibly in the kidney (11). To examine the pathophysiological basis for this critical hypertensive condition further, we produced mice with targeted disruption of the 11β-HSD2 gene.
Methods
Construction of the replacement vector and gene targeting.
Two overlapping genomic fragments, encompassing 10.5 kb of the 5′ region, the complete 11β-HSD2 gene, and 9 kb of the 3′ downstream region, were cloned from a 129 Sv library in the λPS vector (12). Sequencing of all five exons showed a complete match with mouse 11β-HSD2 cDNA (13). A replacement vector was constructed in pBS-βKnpA, containing the neomycin resistance gene flanked by the human β-actin promoter and a SV40 polyadenylation signal sequence. A 5-kb EcoRI–BamHI fragment placed immediately downstream of exon 5 was used as a 3′ homology arm. The 2.7-kb SpeI–XbaI fragment from intron A (5′ arm of homology) was subcloned into the SpeI site of pBS-βKnpA, so that the 3′ XbaI site was not recreated after ligation. Replacement vector linearized with SpeI and SalI was electroporated into embryonic stem (ES) cells (line E-14), and G418-resistant clones were selected as described (14). Two hundred fifty neomycin-resistant clones were analyzed by Southern blotting, using XbaI analytical restriction digestion and hybridization with a 0.8-kb XbaI-SpeI 5′ external probe. Clones with specific recombination events were selected, and recombination at the 3′ end was confirmed by restriction digestion with SspI and NotI and subsequent hybridization with the SspI–BamHI 3′ external probe (not shown). Cells from four targeted ES cell clones were injected into C57BL/6 blastocysts and transferred into C57BL/6 × CBA foster mothers. Chimeras were bred to MF1 females and the progeny genotyped by Southern blot analysis of DNA digested with BamHI and SacI. Northern blot analysis was performed as described (15).
Assays of 11β-HSD activities.
Homogenates from placentas at embryonic day 18.5 (E18.5) were assayed for 11β-HSD2, a nicotinamide adenine dinucleotide (NAD)–dependent exclusive 11β-dehydrogenase, and for the lower-affinity 11β-HSD1 isozyme, which, in vitro, is a bidirectional reduced nicotinamide adenine dinuc 11β-HSD2 bioassays were carried out using placental tissue homogenates (0.25 mg protein/ml) incubated at 37°C with 400 μM NAD and 12 nM [3H]corticosterone (16). 11β-HSD1 was assayed in the reductase direction using 400 μM NADPH and 12 nM [3H]-11-dehydrocorticosterone. Steroids were extracted and quantified by HPLC (17).
Plasma renin activity was measured using the established assay (18). Aldosterone levels were measured by using a radioimmunoassay kit (Diagnostic Products Corp., Los Angeles, California, USA)
Mineralocorticoid actions of glucocorticoids in vivo.
Adult male wild-type and 11β-HSD2–null mice were injected with vehicle (10% ethanol/saline) subcutaneously for 3 days, followed by dexamethasone (100 μg/kg) to suppress endogenous corticosterone for 3 days, and finally dexamethasone (100 μg/kg) and replacement corticosterone (10 mg/kg) for 3 days. Urine was collected in metabolism cages for 24 h beginning 4 h after the start of each treatment, and electrolytes were measured by automatic analyzer. Basal 24-h urine collections and blood samples from chronically cannulated animals were also obtained for electrolyte estimations.
Blood pressure measurement.
Blood pressures were measured in 3-month-old chronically cannulated, conscious males and females as described previously (19). Microrenathane catheters (Braintree Scientific Inc., Braintree, Massachusetts, USA) were microsurgically implanted into the abdominal aorta. Blood pressures were recorded directly from conscious, freely moving mice for 10-min periods between 10 and 11am on 3–5 consecutive days from 48 h after the operations.
Histological preparations.
Kidneys were fixed in 10% buffered formalin and embedded in paraffin wax, and multiple adjacent 4-μm sections were cut and mounted on glass slides. After dehydration, the sections were stained with hematoxylin and eosin, periodic acid-Schiff, and Martius scarlet blue. Sections were examined by an experienced pathologist (S. Fleming), who was blinded to the genotype. Glomeruli and tubule segments were identified by positional and morphological criteria, as described previously (20), because immunocytochemistry-based identification of tubule types is unreliable in circumstances of tubule hypertrophy and hyperplasia (21, 22). Individual distal tubule diameters were measured by image analysis using a Zeiss laser scanning microscope (14). Twenty tubule diameters were measured in each of three –/– and wild-type animals at 3 months of age.
Tissue for electron microscopy was immersion-fixed as 1-mm cubes in 4% glutaraldehyde in cacodylate buffer. The tissue blocks were postfixed in osmium tetroxide, washed in cacodylate buffer, and dehydrated through graded alcohols. Blocks were embedded in araldite resin. Ultra-thin sections were cut, mounted on copper grids, and stained with uranyl acetate and lead citrate. They were examined on a Phillips CM 12 transmission electron microscope.
To determine whether changes in renal structure were readily reversible, 3-month-old 11β-HSD2–/– mice were injected subcutaneously once a day with the mineralocorticoid receptor antagonist spironolactone (10 mg/kg, in vegetable oil) for 14 days and then sacrificed. Tissue was collected for histology.
Statistics.
Data were assessed by ANOVA followed by the Newman-Keuls post hoc test or Student's t tests, as appropriate. Plasma renin activity data were not normally distributed and were analyzed by the Mann-Whitney test. Significance was set at P < 0.05. Values are means ± SEM.
Results
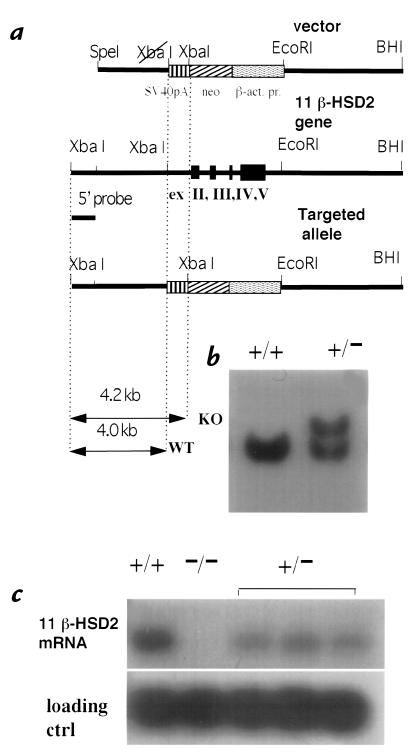
A null mutation of the 11β-HSD2 locus was generated by replacing the genomic fragment encompassing exons 2–5 with a neomycin-resistance cassette (Fig. 1a) through homologous recombination in mouse 129 ES cells. Specific recombination was detected in 12 of 250 G418-resistant colonies by Southern blot analysis with 5′ (Fig. 1b) and 3′ (not shown) external probes. Three targeted clones injected into blastocysts gave rise to chimeras capable of germline transmission. Three independent 11β-HSD2–/– transgenic lines were established by crossing chimeras to MF1 outbred females and subsequent intercross breeding of heterozygotes.
Figure 1.
Targeted inactivation of 11β-HSD2 gene. (a) Structure of the targeting vector, the 11β-HSD2 locus, and the targeted allele. (b) Southern blot hybridization analysis of XbaI-digested genomic DNA from neomycin-resistant nontargeted (+/+) and targeted (+/–) ES clones. (c) Northern blot analysis of 11β-HSD2 expression. RNA (10 μg) from kidney of wild-type (+/+), homozygous (–/–) mutants and three heterozygous (+/–) littermates was hybridized with mouse 11β-HSD2 and control (ctrl) U-1 snRNA cDNA probes. 11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; ES, embryonic stem cells.
Genotyping of 191 intercross progeny at weaning (21 days) showed clear deviation from mendelian ratios (31 –/–, 113 +/–, 47 +/+; P < 0.01), with less than half of the expected number of 11β-HSD2–/– mice found. In contrast, in fetuses at E18.5 ratios were close to mendelian, with 12 of 45 (26.7%) of the 11β-HSD2–/– genotype. 11β-HSD2–/– mice appeared normal at birth, but within 48 hours showed ∼50% mortality. Neonatal death was usually preceded by motor weakness and reduced suckling and accompanied by abdominal swelling with intestinal loop dilatation. All 11β-HSD2–/– mice surviving >48 hours after birth reached adulthood. No 11β-HSD2 mRNA was detected in 11β-HSD2–/– mouse kidney, whereas heterozygotes showed a 50% reduction of 11β-HSD2 mRNA expression (Fig. 1c). Subsequently, 16% of 11β-HSD2 males and 13% (of 75 total) females died suddenly between two and four months of age (one 11β-HSD2–/– female died suddenly four hours postpartum, and two died toward the end of lactation of their first litters). In most cases (n = 6 males and 6 females) postmortem analysis revealed no obvious cause of death (such as stroke or myocardial infarction/rupture), though one female died with a ruptured aorta and pericardial bleeding, and another had hemorrhaged around a kidney capsule. Both male and female 11β-HSD2–/– mice surviving until adulthood were fertile and gave rise to live homozygous offspring.
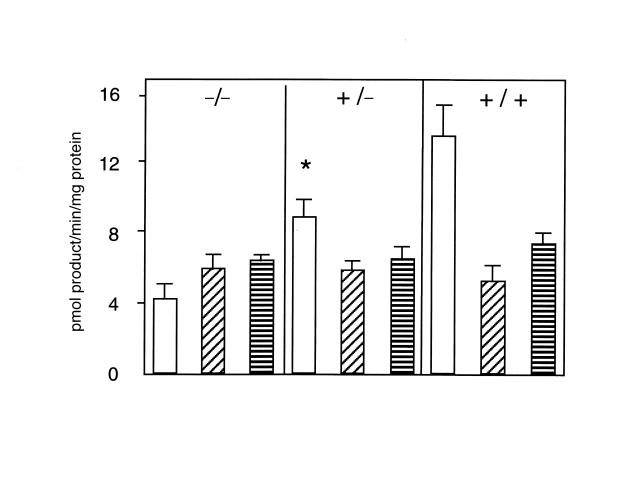
Placental NAD-dependent 11β-dehydrogenase activity at E18.5 (full gestation at E19.5) was 13.7 ± 1.9 pmol/min/mg protein (45.5 ± 6.4% conversion) of corticosterone to inert 11-dehydrocorticosterone in wild-type (+/–) 9.0 ± 0.7 pmol/ min/mg protein (30.1 ± 2.4% conversion) in heterozygous (+/–) and 4.3 ± 0.6 pmol/min/mg protein (14.4 ± 2.1%) in homozygous mutant 11β-HSD2–/– mice (Fig. 2). Mouse placentas at E18.5 also contain 11β-HSD type 1, an NADPH-dependent enzyme that acts as an oxidoreductase (regenerating active glucocorticoids) in intact cells (23). Mice homozygous for targeted disruption of the 11β-HSD1 gene do not show alterations in plasma electrolytes or blood pressure (24). 11β-HSD1 activity was equal in placentas of all three genotypes (Fig. 2). No differences in fetal or placental weights were found at E18.5 (fetus: homozygous mutant –/– 784 ± 16 mg, n = 11; heterozygous +/– 797 ± 20 mg, n = 19; wild-type +/+ 790 ± 34 mg, n = 5; placenta: –/– 142 ± 9.4 mg; +/– 147 ± 7.2 mg; +/+ 152 ± 14 mg), nor were there weight differences between genotypes (either sex) at weaning.
Figure 2.
Variation in 11β-HSD2 dehydrogenase and 11β-HSD1 oxidoreductase activities in placentas with different 11β-HSD2 genotypes. Activities (mean ± SEM) expressed as conversion of nmol [3H]-steroid substrate (either corticosterone for 11β-dehydrogenase or 11-dehydrocorticosterone for 11β-reductase) to pmol product/mg protein/min. (Open bars) NAD-dependent 11β-corticosterone dehydrogenase activity. (Diagonally striped bars) NADP-dependent 11β-corticosterone dehydrogenase activity. (Horizontally striped bars) NADPH-dependent 11-dehydrocorticosterone reductase activity. Note that activity due to 11β-HSD1 does not vary with genotype. In contrast, NAD-dependent predominantly 11β-HSD2 activity varies with genotype, resulting in overall NADP-preferring 11β-HSD activity in 11β-HSD2–/– mice. The residual NAD-dependent activity in 11β-HSD2–/– placentas presumably reflects activity of 11β-HSD1 enzyme. *Significantly greater than corresponding 11β-HSD2–/– value (P < 0.001) and significantly smaller than corresponding wild-type (+/+) values (P < 0.001). NAD, nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate.
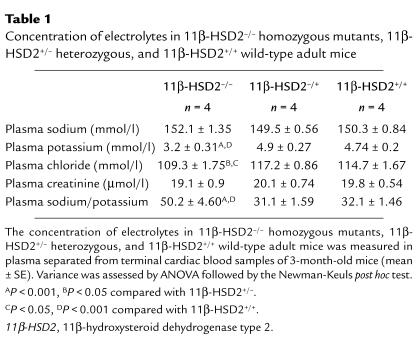
Adult male and female 11β-HSD2–/– mice showed hypotonic polyuria (24-h urine volume 3.2 ± 0.3 ml vs. 2.1 ± 0.2 ml; P < 0.005), with reduced urinary concentrations of sodium, potassium, and creatinine (61 ± 10%, 75 ± 9%, and 71 ± 5% of wild-type, respectively; P < 0.001). 11β-HSD2–/– null mice had normal plasma sodium and creatinine but showed marked hypokalemia and hypochloremia (Table 1), with plasma potassium levels as low as 2.4 ± 0.2 mmol/l in chronically catheterized males and 2.7 ± 0.2 mmol/l in females (n = 3). Plasma electrolytes in heterozygotes did not differ significantly from wild-type. Eight 11β-HSD2–/– mice had fully suppressed plasma renin activity below the level of detection of angiotensin I (AI)(<0.05 ng AI/mg protein/min), whereas levels in wild-type mouse plasma were significantly higher (0.75 ± 0.40 ng AI/mg/min; P < 0.05, n = 8). Plasma aldosterone levels were profoundly suppressed in 11β-HSD2–/– male and female mice (232 ± 93 pmol/l, n = 6) compared with wild-type controls (3085 ± 397 pmol/l, n = 6; P < 0.0001).
Table 1.
Concentration of electrolytes in 11β-HSD2–/– homozygous mutants, 11β-HSD2+/– heterozygous, and 11β-HSD2+/+ wild-type adult mice
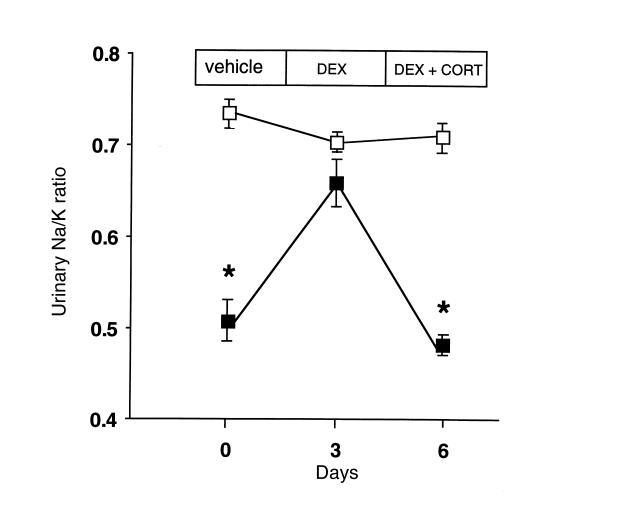
To determine whether endogenous glucocorticoids were responsible for the apparent mineralocorticoid excess, we examined the effects on urinary Na/K ratios of suppression of corticosterone by dexamethasone and subsequent corticosterone replacement. Dexamethasone reversed the low Na/K ratio in 11β-HSD2–/– mice but had no effect in wild-type controls (Fig. 3). Replacing corticosterone in dexamethasone-treated 11β-HSD2–/– mice recreated low urinary Na/K ratios, whereas controls were again unaffected.
Figure 3.
Corticosterone acts as a mineralocorticoid in 11β-HSD2–/– mice. Wild-type (open squares; n = 4) and 11β-HSD2–/– (closed squares; n = 4) mice were injected with vehicle (days –3 to 0), followed by dexamethasone (DEX; 100 μg/kg, days 0–3) to suppress endogenous corticosterone, and finally dexamethasone with replacement corticosterone (DEX + CORT; 10 mg/kg, days 3–6). Urine was collected for the last 24 h of each treatment and electrolytes measured by automated analyzer. The abnormally low urinary Na/K ratio in 11β-HSD2–/– mice is reversed by dexamethasone and recurs with corticosterone replacement, whereas the treatments have no effect in wild-type mice. *Significantly different from corresponding wild-type value (P < 0.0005).
To examine whether 11β-HSD2 gene disruption and the consequent apparent mineralocorticoid excess caused hypertension, blood pressures were measured directly in three-month-old conscious male and female mice using chronic abdominal aortic cannulae as described previously (19). 11β-HSD2–/– mice were hypertensive, with mean blood pressures of 146 ± 2 mmHg in males (n = 6) and 155 ± 4 mmHg in females (n = 3), compared with 121 ± 2 mmHg in wild-type males (n = 4, P < 0.001) and 122 ± 3 mmHg in wild-type females (n = 5, P < 0.05). Male heterozygotes had similar blood pressures (114 ± 4 mmHg, n = 5) to wild-type controls. Although the three-month-old 11β-HSD2–/– mice tended to exhibit an increased heart weight/body weight ratio compared with age-, sex-, and weight-matched wild-type controls (–/– 6.8 ± 0.7 mg/g, n = 6; +/+ 5.6 ± 0.4 mg/g, n = 9; P < 0.15), the difference was not statistically significant. Histological examination of the hearts of 11β-HSD2–/– mice (n = 6; 3 males and 3 females) showed no evidence of interstitial fibrosis, but there were clear morphological features of cardiac myocyte hypertrophy (not shown).
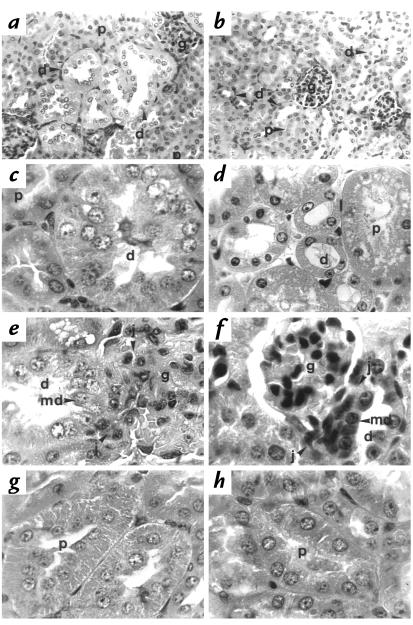
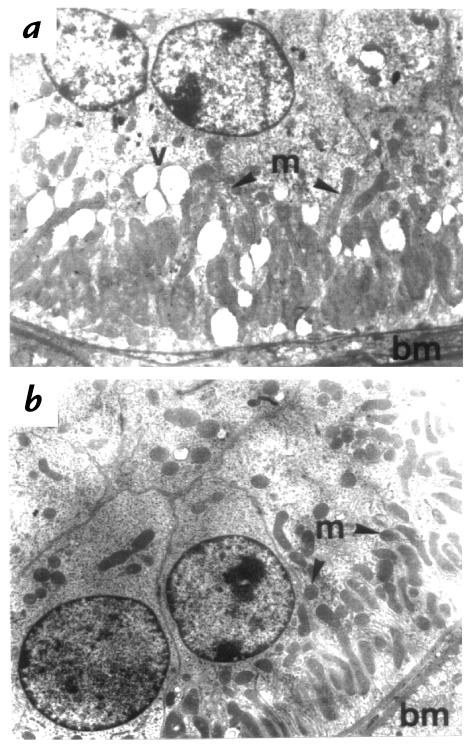
Three-month-old 11β-HSD2–/– mice exhibited marked renal enlargement (353 ± 13mg, n = 6; vs. 277 ± 14 mg, n =9; P = 0.003 in the wild-type. Histology revealed that this increase in renal mass was the consequence of distal tubular enlargement. The glomeruli, proximal tubules, medullary rays, medulla, and collecting ducts were all normal using the light (Fig. 4a) and electron microscopes (not shown). However, the distal tubules showed a two- to fourfold increase in diameter (11β-HSD2–/– mean distal tubular diameter 61.3 μm, range 42.6–96.8 μm; 11β-HSD2+/+ mean distal tubular diameter 25.7 μm, range 21.2–28.3 μm) as a result of both hyperplasia and hypertrophy of the epithelium (Fig. 4a and c). These changes were seen at the origin of the distal tubule at the juxtaglomerular apparatus and in the convoluted portion (Fig. 4e). The hypertrophy resulted in increased cell size, with apical displacement of nuclei and increased granularity. Electron microscopy revealed clear differences between the 11β-HSD2–/– animals and the wild-type controls (Fig. 5, a and b). The distal tubular epithelial cells from 11β-HSD2–/– animals showed an increase in size, with the subnuclear, (basal) appearing expanded with apical displacement of the nucleus. The 11β-HSD2–/– animals showed an increase in the number of mitochondria in each distal tubular cell, although these remained located at the expanded basal pole. Occasional membrane-bound enclosed vacuoles were seen in the cytoplasm of the distal tubular epithelial cells from the 11β-HSD2–/– animals. All described morphological tubular changes were identical in male and female mice.
Figure 4.
Hyperplasia and hypertrophy of the distal renal tubular epithelium in 11β-HSD2–/– mice. (a) In adult 11β-HSD2–/– mice, the distal tubules (d) show considerable enlargement: the tubular diameter is increased threefold compared with the distal tubule in the wild-type mice (b). Note that the proximal tubules (p) and glomeruli (g) are unaffected in the 11β-HSD2–/– mice. (c) Hyperplasia is apparent in the 11β-HSD2–/– mouse distal tubule (d), which shows over 20 cells in the cross-sectional profile compared with (d) the four to six cells observed in controls. (e) The distal tubular enlargement (d) is evident in 11β-HSD2–/– mice at the anatomical origin of the distal tubule at the juxtaglomerular apparatus (j) adjacent to the glomerulus (g), compared with (f) the nonenlarged wild-type distal tubule. md, macula densa. (g) Proximal tubule (p) is normal in 11β-HSD2–/– kidneys. 11β-HSD2–/– proximal tubules are indistinguishable from (h) wild-type proximal tubules. The tubules are of similar diameter and cell number in cross section. They are lined by cuboidal cells with granular cytoplasm and a brush border in both animals. The same magnification was used in 11β-HSD2–/– and corresponding wild-type control photographs. In a and b, H&E ×60; in c and d:, H&E ×120; in e–h, H&E ×180. H&E, hematoxylin and eosin.
Figure 5.
Electron microscopy of the distal tubular epithelial cells of 11β-HSD2–/– (a) and wild-type controls (b). Distal tubular epithelial cells from 11β-HSD2–/– animals show an increase in size. The subnuclear, or basal, pole is expanded with apical displacement of the nucleus. The number of mitochondria (m) in each distal tubular cell is increased, although the mitochondria remain located at the expanded basal pole (bm, basal membrane). Occasional membrane-enclosed vacuoles (v) are seen in the cytoplasm of the distal tubular epithelial cells from the 11β-HSD2–/– animals. ×3,800.
The kidneys of gestational day 18.5 and newborn animals were histologically normal. At one week old, two out of three of the 11β-HSD2–/– animals showed a minor degree of tubular dilatation, without hyperplasia or hypertrophy, affecting <1% of tubular profiles. By three weeks old, hypertrophy and hyperplasia of the distal tubular epithelium was evident in all of the 11β HSD2–/– kidneys examined.
Treatment of adult 11β-HSD2–/– mice with spironolactone for 14 days improved the hypokalemia (5.5 ± 0.6 mmol/l) but had no effect upon the histological hyperplasia and hypertrophy of the distal tubule.
Discussion
Targeted disruption of the 11β-HSD2 gene was successful, with no 11β-HSD2 mRNA detected in the kidneys of homozygous mutants and 50% reduction in heterozygotes. Measurement of 11β-dehydrogenase and 11β-reductase activities in the presence of different cofactors and substrates in placentas also supports the absence of 11β-HSD2 activity (a high-affinity NAD-dependent 11β-dehydrogenase) in homozygous mutants, with approximately 50% of wild-type activity in heterozygotes. Although the residual 11β-dehydrogenase activity with NAD in placentas of 11β-HSD2–/– fetuses raises the possibility of a novel 11β-HSD isoform, this seems more likely merely to reflect 11β-HSD1, which may continue moderate activity without added NADP, probably due to use of endogenous cosubstrate NADP, itself increased by exogenous NAD (17). 11β-HSD1 activity was unaffected by mutation of 11β-HSD2 in the placenta. The mutant phenotype appeared to be recessive, with detectable effects confined to homozygotes, and these findings were in accord with the lack of apparent disorders in parents or heterozygous sibs of children with SAME (25). However, heterozygous animals have only been examined up to four months of age, and effects later in life cannot be excluded.
Up to 50% of homozygous mice died within the first 48 hours after birth. Homozygous newborn pups in the intercross litters appeared weaker and less successful in competition for suckling. The abdominal swelling and intestinal dilatation observed in some 11β-HSD2–/– neonates before death suggests intestinal ileus. Other 11β-HSD2–/– pups died suddenly, possibly of cardiac arrest. Both processes are compatible with the effects of severe hypokalemia, clearly found in surviving adult 11β-HSD2–/– animals. The neonatal mortality mimics the documented perinatal and infant mortality of children with SAME (25, 26). Later deaths appeared either to reflect the hemorrhagic complications of severe hypertension or were unexplained and referred to possible cardiac events secondary to the electrolyte disturbances.
The most obvious phenotype of 11β-HSD2–/– mice was hypotonic polyuria, with 24-hour urine volumes increased by 50%. Hypokalemia causes polyuria due to nephrogenic diabetes insipidus and polydipsia in humans (27) and presumably underlies the polyuria in 11β-HSD2–/– mice. Hypokalemia appears secondary to urinary losses, driven by excessive mineralocorticoid action (low urinary Na/K ratio). In 11β-HSD2–/– mice, endogenous and exogenous corticosterone shows illicit mineralocorticoid action, effects absent from wild-type littermates. The main result of activation of mineralocorticoid receptors in the distal tubule is enhanced sodium resorption by the epithelial cells through the epithelial Na+ channel (28), which ultimately results in elevation of blood pressure (1). Indeed, male and female 11β-HSD2–/– animals have severe hypertension with mean blood pressures above 145 mmHg.
The most striking structural changes in 11β-HSD2–/– mice are in the hyperplasia and hypertrophy confined to the distal tubule, with the proximal tubules, loops of Henle, and collecting ducts appearing normal. This type of distal tubular pathology has not been reported in other polyuric or hypokalemic syndromes that result in marked cytoplasmic vacuolation affecting the proximal tubules and has minor changes only in the distal tubules with no evidence of hyperplasia or hypertrophy (29). The changes are, however, similar to the effects of chronic furosemide administration (20), in which increased distal tubular sodium transport occurs as a consequence of increased sodium delivery to the distal tubule after inhibition of inward salt transport by the loop diuretic. In 11β-HSD2–null mice, the pathology is presumably a consequence of increased mineralocorticoid activity. Hyperplasia of the distal tubular epithelium, with increased total mitochondria and basal-membrane surface area, would be predicted to be the consequence of the energy requirement of overstimulation of active ion transport by mineralocorticoid receptor activity. 11β-HSD2 and mineralocorticoid receptor mRNA are confined to the distal nephron (30). We are not aware of any studies of renal biopsies from patients with SAME to allow comparison with the mice.
Kidneys from E18.5 11β-HSD2–/– fetuses showed no morphological or histological abnormalities. High expression of mineralocorticoid receptor mRNA in the distal nephron develops between E18.5 and just after birth (at E19.5) (30). Thus, only around birth do distal tubule epithelia of 11β-HSD2–/– animals first become exposed to glucocorticoid overactivation of mineralocorticoid receptors, findings consistent with glucocorticoid mediation of the distal tubular pathology. Intriguingly, in SAME patients, hypertension is usually not fully reversed by cortisol suppression. The marked structural abnormalities found in the adult 11β-HSD2–/– mouse distal tubule and the lack of reversal of these changes with mineralocorticoid receptor antagonism in adult life may explain this and focuses attention on renal structure in patients with chronic steroid hypertension.
In contrast to the striking renal pathology, the observed cardiac myocyte hypertrophy would be compatible with a secondary effect of hypertension alone. The lack of cardiac fibrosis, which has been associated with conditions of mineralocorticoid excess (not apparent mineralocorticoid excess), conforms with suggestions that this effect is mediated by MR unprotected by 11β-HSD2 and therefore requires elevated plasma levels of corticosteroids.
The mammalian placenta (and the fetus until midgestation) highly expresses 11β-HSD2, which may exclude high levels of maternal glucocorticoids from fetal tissues during sensitive periods of prenatal growth and development. Indeed, in rats and humans, placental 11β-HSD activity near term correlates with birth weight (31, 32) and pharmacological inhibition of fetoplacental 11β-HSD reduces birth weight (33). The lack of birth-weight changes in 11β-HSD2–/– mice suggests either that there are compensatory developmental mechanisms or, more likely, that this species, which shows early midgestational loss of 11β-HSD2 gene expression in the placenta and fetal tissues (30), is less dependent on 11β-HSD2–mediated exclusion of glucocorticoids for maintenance of birth weight, which predominantly reflects growth in late gestation.
The 11β-HSD2–/– mice not only model SAME and advance our current understanding of its etiology, but will also allow more general insights into disorders of real or apparent mineralocorticoid excess, such as Conn's and Liddle's syndromes and glucocorticoid-remediable hyperaldosteronism (34, 2, 5), in which there is poorly understood variation in the degree of hypokalemia, hypertension, and the response to treatment. The mice will be useful in screening for new pharmaceutical compounds alleviating volume-dependent hypertension and also in studies of basic molecular mechanisms of mineralocorticoid responses in normal and pathological states.
Acknowledgments
We thank Andrew Smith for λPS library; William Skarnes and Meng Li for pBS-βKnpA vector; Jan Ure, Gillian Brooker, Morag Meikle, and David Fettes for skillful technical assistance; and Matthew Sharp for discussion. The work was supported by a Programme grant from the Wellcome Trust (to J.R. Seckl, C.R.W. Edwards, and J.J. Mullins), and by funding from the The Biotechnology and Biological Sciences Research Council, The Commission of the European Communities concerted action Transgeneur, and the High Blood Pressure Foundation. J.R. Seckl and J.J. Mullins are the recipients of a Wellcome Senior Research Fellowship and a Wellcome Principal Fellowship respectively. R.W. Brown is a Medical Research Council Clinician-Scientist Fellow.
References
- 1.Guyton AC. Blood pressure control—special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 2.Shimkets RA, et al. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 3.Simon DB, et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 4.White PC, Vitec A, Dupont B, New MI. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 1988;85:4436–4440. doi: 10.1073/pnas.85.12.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lifton RP, et al. A chimaeric 11β-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 6.Arriza JL, et al. Cloning of human mineralocorticoid receptor complementary DNA — structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 7.Stewart PM, et al. Mineralocorticoid activity of liquorice: 11β-hydroxysteroid dehydrogenase deficiency comes of age. Lancet. 1987;2:821–823. doi: 10.1016/s0140-6736(87)91014-2. [DOI] [PubMed] [Google Scholar]
- 8.Ulick S, et al. A syndrome of apparent mineralocorticoid excess associated with defects in the peripheral metabolism of cortisol. J Clin Endocrinol Metab. 1979;49:757–764. doi: 10.1210/jcem-49-5-757. [DOI] [PubMed] [Google Scholar]
- 9.Edwards CRW, et al. Localisation of 11β-hydroxysteroid dehydrogenase — tissue specific protector of mineralocorticoid receptor. Lancet. 1988;2:986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- 10.Mune T, Rogerson FM, Nikkila H, Agarwal AK, White PC. Human hypertension caused by mutations in the kidney isozyme of 11 beta-hydroxysteroid dehydrogenase. N at Genet. 1995;10:394–399. doi: 10.1038/ng0895-394. [DOI] [PubMed] [Google Scholar]
- 11.Stewart PM, Corrie JET, Shackleton CHL, Edwards CRW. Syndrome of apparent mineralocorticoid excess: a defect in the cortisol-cortisone shuttle. J Clin Invest. 1988;82:340–349. doi: 10.1172/JCI113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nehls M, Messerle M, Sirulnik A, Smith AJH, Boehm T. Two large insert vectors, λPS and λKO, facilitate rapid mapping and targeted disruption of mammalian genes. Biotechniques. 1994;17:770–775. [PubMed] [Google Scholar]
- 13.Cole T J. Cloning of the mouse 11-beta-hydroxysteroid dehydrogenase type-2 gene — tissue-specific expression and localization in distal convoluted tubules and collecting ducts of the kidney. Endocrinology. 1995;136:4693–4696. doi: 10.1210/endo.136.10.7664690. [DOI] [PubMed] [Google Scholar]
- 14.Clark AF, et al. Renin-1 is essential for normal renal juxtaglomerular cell granulation and macula densa morphology. J Biol Chem. 1997;272:18185–18190. doi: 10.1074/jbc.272.29.18185. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Low SC, et al. Regulation of 11β-hydroxysteroid dehydrogenase by sex steroids in vivo: further evidence for the existence of a second dehydrogenase in rat kidney. J Endocrinol. 1993;139:27–35. doi: 10.1677/joe.0.1390027. [DOI] [PubMed] [Google Scholar]
- 17.Brown RW, Chapman KE, Edwards CRW, Seckl JR. Human placental 11 beta-hydroxysteroid dehydrogenase: evidence for and partial purification of a distinct NAD-dependent isoform. Endocrinology. 1993;132:2614–2621. doi: 10.1210/endo.132.6.8504762. [DOI] [PubMed] [Google Scholar]
- 18.Millar JA, Leckie BJ, Morton JJ. A micro-assay for active and total renin concentration in human plasma cased on antibody trapping. Clin Chim Acta. 1980;101:5–15. doi: 10.1016/0009-8981(80)90050-9. [DOI] [PubMed] [Google Scholar]
- 19.Sharp MGF, et al. Targeted inactivation of the Ren-2 gene in mice. Hypertension. 1996;28:1126–1131. doi: 10.1161/01.hyp.28.6.1126. [DOI] [PubMed] [Google Scholar]
- 20.Kaissling B, Bachmann S, Kriz W. Structural adaptation of the distal convoluted to prolonged furosemide treatment. Am J Physiol. 1985;248:F374–F381. doi: 10.1152/ajprenal.1985.248.3.F374. [DOI] [PubMed] [Google Scholar]
- 21.Fleming S, Matthews TJ. Renal tubular antigens in regenerative epithelium and renal carcinoma. Br J Urol. 1987;60:103–109. doi: 10.1111/j.1464-410x.1987.tb04942.x. [DOI] [PubMed] [Google Scholar]
- 22.Grone HJ, Weber K, Grone E, Helmchen U, Osborn M. Co-expression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol. 1987;129:1–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Jamieson PM, Chapman KE, Edwards CRW, Seckl JR. 11 β-Hydroxysteroid dehydrogenase is an exclusive 11 β-reductase in primary cultures of rat hepatocytes: effect of physicochemical and hormonal manipulations. Endocrinology. 1995;136:4754–4761. doi: 10.1210/endo.136.11.7588203. [DOI] [PubMed] [Google Scholar]
- 24.Kotelevtsev Y, et al. 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid inducible responses and resist hyperglycaemia on obesity or stress. Proc Natl Acad Sci USA. 1997;94:14924–14929. doi: 10.1073/pnas.94.26.14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White PC, Mune T, Agarwal AK. 11β-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev. 1997;18:135–156. doi: 10.1210/edrv.18.1.0288. [DOI] [PubMed] [Google Scholar]
- 26.Krozowski ZS, Stewart PM, Obeyesekere VR, Li K, Ferrari P. Mutations in the 11 β-hydroxysteroid dehydrogenase type II enzyme associated with hypertension and possibly stillbirth. Clin Exp Hypertens. 1997;19:519–529. doi: 10.3109/10641969709083166. [DOI] [PubMed] [Google Scholar]
- 27.Berl T, Linas SL, Aisenbrey GA, Anderson RJ. On the mechanism of polyuria in potassium depletion. J Clin Invest. 1977;60:620–625. doi: 10.1172/JCI108813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grunder S, Rossier BC. A reappraisal of aldosterone effects on the kidney: new insights provided by epithelial sodium channel cloning. Curr Opin Nephrol Hypertens. 1997;6:35–39. doi: 10.1097/00041552-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Cherg, J., Cotran, R.S., Sinniah, R., Sakaguchi, H., and Sobin, C.H. 1985. Renal disease: classification of tubulo-interstitial diseases. WHO. Igaku-Shoin Medical Publishers. Tokyo, Japan.
- 30.Brown RW, et al. The ontogeny of 11β-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- 31.Benediktsson R, Lindsay R, Noble J, Seckl JR, Edwards CRW. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 32.Stewart PM, Rogerson FM, Mason JI. Type 2 11β-hydroxysteroid dehydrogenase messenger RNA and activity in human placenta and fetal membranes: its relationship to birth weight and putative role in fetal steroidogenesis. J Clin Endocrinol Metab. 1995;80:885–890. doi: 10.1210/jcem.80.3.7883847. [DOI] [PubMed] [Google Scholar]
- 33.Lindsay RS, Lindsay RM, Edwards CRW, Seckl JR. Inhibition of 11β- hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- 34.Gordon RD. Primary aldosteronism: a new understanding. Clin Exp Hypertens. 1997;19:857–870. doi: 10.3109/10641969709083191. [DOI] [PubMed] [Google Scholar]