Abstract
The adenosine triphosphate (ATP)–sensitive K+ (KATP) channel is the most abundant K+ channel active in the skeletal muscle fibers of humans and animals. In the present work, we demonstrate the involvement of the muscular KATP channel in a skeletal muscle disorder known as hypokalemic periodic paralysis (HOPP), which is caused by mutations of the dihydropyridine receptor of the Ca2+ channel. Muscle biopsies excised from three patients with HOPP carrying the R528H mutation of the dihydropyridine receptor showed a reduced sarcolemma KATP current that was not stimulated by magnesium adenosine diphosphate (MgADP; 50–100 μM) and was partially restored by cromakalim. In contrast, large KATP currents stimulated by MgADP were recorded in the healthy subjects. At channel level, an abnormal KATP channel showing several subconductance states was detected in the patients with HOPP. None of these were surveyed in the healthy subjects. Transitions of the KATP channel between subconductance states were also observed after in vitro incubation of the rat muscle with low-K+ solution. The lack of the sarcolemma KATP current observed in these patients explains the symptoms of the disease, i.e., hypokalemia, depolarization of the fibers, and possibly the paralysis following insulin administration.
Introduction
The adenosine triphosphate (ATP)–sensitive K+ channel (KATP) is a metabolically regulated K+ channel present in high density in different tissues, including skeletal muscle (1–4). Although in the last few years the role of this type of ion channel in cardiac and pancreatic β cells has been extensively investigated, not much is known about the role of the KATP channel in the skeletal muscle. A recent report (5) suggested a possible role of this ion channel in muscle disorders related to hypokalemia. For example, we have shown that in the K+-depleted rat (6), in which a chronic hypokalemia was produced by feeding the rats with a K+-free diet, the activity of the sarcolemma KATP channels is abnormally reduced. In these animals, the in vitro administration of insulin to the muscles abolishes the residual KATP current and depolarizes the fibers (5). In contrast, in normokalemic rats, the hormone induces stimulation of the sarcolemma KATP channels and hyperpolarizes the fibers (7, 8). Similarly, in humans affected by an inherited muscle disorder known as hypokalemic periodic paralysis (HOPP; ref. 9), insulin produces fiber depolarization and muscle paralysis associated with a fall of the serum K+ concentration (from 3.5 to <2 mEq; ref. 9) but produces fiber hyperpolarization in healthy subjects (7, 9).
Linkage studies have shown that the human HOPP gene maps to chromosome 1q31–32 and is colocalized with the gene encoding the α1 subunit of the skeletal muscle L-type Ca2+ channel (10–14). Three point mutations were found within the coding sequence of the α1 subunit containing the dihydropyridine (DHP) receptor, resulting in arginine→histidine (R528H, R1239H) and arginine→glycine (R1239G) substitutions (14). However, functional studies have shown that these mutations do not significantly affect the macroscopic L-type Ca2+ current of myotubes cultured from muscle of patients with HOPP, or the Ca2+ current carried by channels expressed in the cell line (15–17). It has recently been proposed (17) that factor(s) directly or indirectly related to the DHP mutation play a role in the pathogenesis of HOPP. This poses the question of how the genotype is related to the phenotype of the muscle pathology (16–18). Indeed, although the mutations of the α1 subunit of the Ca2+ channel have been found in two of three of the patients with HOPP, the link between the mutations of the DHP receptor, the depolarization of the fibers, the characteristic muscle paralysis induced by insulin, and the hypokalemia occurring during the attacks is not yet understood (17, 18). These clinical and laboratory findings suggest the possible involvement of the KATP channel also in the pathogenesis of human HOPP. This is also supported by the observations that the K+ channel openers, cromakalim and pinacidil, are, respectively, capable of repolarizing the skeletal muscle fibers from humans affected by HOPP and restoring the muscle strength in the same patients (18–22).
We therefore performed patch clamp experiments (3–5) on muscle biopsies excised from three patients with HOPP, two members of a HOPP family and one belonging to another family, but all carrying the common R528H mutation of the DHP receptor. We also successfully screened human muscle biopsies excised for orthopedic surgery from healthy subjects.
To investigate the link between the DHP receptor mutation of the patients with HOPP and the impairment of the KATP channels, experiments were performed to evaluate the effects of the Ca2+ ion on KATP channels of rat skeletal muscle fibers preincubated in vitro with low-K+ solution.
Methods
Human biopsies.
For diagnostic purposes, biopsies were excised from vastus lateralis and deltoid muscles of patients with HOPP; biopsies were excised from healthy subjects for orthopedic surgery. After dissection, the muscles were pinned in a Sylgard-coated dish and immersed in Krebs solution (118.1 mM NaCl, 3.4 mM KCl, 0.8 mM MgSO4·7H2O, 1.2 mM KH2PO4, 25 mM NaHCO3, 2.5 mM CaCl2, 11.1 mM glucose; pH 7.4) for removal of the connective tissue and fascia. This solution was then replaced with Krebs solution enriched with 3 mg/ml collagenase (3.3 U/mg) type XI-S (Sigma Chemical Co., Munich, Germany). The biopsies were incubated for 30–60 min at 30°C under a 95% O2/5% CO2 atmosphere, in a shaking incubator. The isolated fibers were washed several time with Krebs solution before recording. Only fibers with clearly visible sarcomere-cross striations were patched.
Molecular biology.
Mutations were detected by K. Jurkat-Rott in the laboratories of R. Rüdel and F. Lehmann-Horn (Universität Ulm). In brief, total RNA was isolated from muscle specimens obtained from three patients with HOPP belonging to two different families and from healthy subjects (12). RNA was reverse transcribed, and regions of the CACNL1A3 cDNA were amplified by use of PCR primers derived from human cDNA sequence. Amplified DNA fragments obtained by asymmetrical PCR were purified and directly sequenced. Of the full-length cDNA (∼5,600 bp), 4,100 bp encoding the four (highly conserved) domains have been sequenced. We identified the G→A transition of nucleotide 1,583 for the index patients of the two HOPP families, which results in an arginine→histidine substitution at position 528. All procedures were in accordance with the Helsinki Convention and were approved by the Ethical Committee of the Technical University of Munich (Munich, Germany).
Patch clamp experiments.
Experiments were performed in inside-out configurations using standard patch clamp techniques (3–5). KATP currents, sampled at 5 kHz and filtered at 0.5 kHz, were recorded during voltage steps of 53-s duration going from 0 mV of holding potential to different voltages (from –60 mV to +40 mV), at 22°C. Single-channel currents, sampled at 20 kHz and filtered at 2 kHz, were recorded at –60 mV voltage membrane (Vm) or at various potentials, at 22°C, in the presence of 150 mM KCl on both sides of the membrane. Acquisition of the currents and analysis were performed using Axon hardware (Axon Instruments Inc., Foster City, California, USA) or List 7 amplifier (List Electronic, Darmstadt, Germany) and the pClamp 6 software package (Axon Instruments Inc.), respectively, as described previously (5). The following procedure was performed to measure the KATP current in the excised patches: currents were recorded after ∼20 s from excision in the presence of 150 mM KCl in the bath (control condition), after perfusion of the macropatches with 50–100 μM concentrations of magnesium adenosine diphosphate (MgADP) that ensured the maximal stimulation of the currents (23), and after exposure of the macropatches with 500 μM concentration of magnesium adenosine triphosphate (MgATP) to measure the residual currents (leak currents + other currents). The residual currents were then subtracted from the control currents and MgADP-stimulated currents to measure the effective KATP currents.
The effects of cromakalim on the current were evaluated at –60 mV (Vm), at 22°C, in the presence of 150 mM KCl on both sides of the membrane. The single-channel conductance was calculated as the slope of the voltage–current relationship of the channel in the range of potentials from –70 mV to –10 mV, and in the range of potentials from +10 mV to +70 mV. The open probability of each conductance level was measured as the ratio between the time spent by the channel in the open states — O1, O1.5, O2, O2.5, O3, and O4 — over the total time of recording. This analysis was performed in the first 260 s from patch excision on maximally activated channels. The single-channel data was collected in the presence of the only single active unit in the patches.
The curves of the normalized KATP currents vs. ATP or Ca2+ concentrations were fitted by the following equation: KATP current blocker/KATP current control = 1/1 + ([chemical])n /IC50.
The equation was based on a least squares fitting routine. The fitted parameters were: n, the slope ofcurve; IC50, the concentration of the blocker required to produce a 50% decrease of the KATP current. The plot of the number of levels (x) vs. the corresponding amplitudes (y) were fitted with the following linear equation: y = ax+b. The equation was based on a least squares fitting routine. The fitted parameters were: a, slope of the linear regression; b, the intercept on the x axis. The data are reported as mean ± SE unless otherwise specified. The macropatch currents are expressed as absolute values or values normalized on the pipette area.
The solutions used had the following compositions: pipette, 150 mM KCl, 2 mM CaCl2, 10 mM MOPS (pH 7.2); bath, 150 mM KCl, 0.5 mM EGTA, 10 mM MOPS (pH 7.2). Stock solutions (5 mM) of the nucleotide tested, Na2ATP, MgATP, and MgADP were prepared by dissolving the chemicals in the bath solution. Cromakalim (Sigma Chemical Co.) was first dissolved in DMSO at concentrations of 0.28 M. In the range of the cromakalim concentrations tested, the corresponding DMSO concentrations did not mimic the effects of the drug on KATP channels (solvent control). Microliter amounts of the stock solutions of the nucleotides and drug tested were then diluted in the bath solutions as needed. The Ca2+-containing solutions were prepared as described previously (24). In brief, different amounts of CaCl2 were added to the symmetrical K+ solution to give free Ca2+ concentrations between 1 μM and 50 μM. The calculation of the free Ca2+ ion has been performed by EQCAL software (Biosoft, Cambridge, United Kingdom). The activity of the free Ca2+ ion was then measured by Ca2+ selective electrodes (World Precision Instruments, Sarasota, Florida, USA). The exact concentration of the Ca–EGTA complex, which is also related to the purity of the EGTA used, was established by a pH–metric method.
Pipettes were pulled from Corning 7052 glass (Garner Glass Co., Claremont, California, USA) in two stages on a patch electrode puller (DMZ universal puller; Zeitz Instruments, Augsburg, Germany). The electrodes were then coated with Sylgard and fire polished. The tip opening area of the pipettes was measured by scanning electron microscopy (Cambridge Instruments, London, United Kingdom). Measurements of the patch conductances and tip opening area were performed on the same pipettes according to the method of Sakmann and Neher (25). A linear correlation between the pipette conductance and the tip opening area has been found in the range of conductance from 50 × 10–9 S to 1,600 × 10–9 S. The slope of the straight line was 0.00698, the intercept was 0.302, and the coefficient of correlation was 0.778. Macropipettes having an average tip opening area of 5.2 ± 1 μm2 (n = 41) were used to measure the current sustained by multiple channels and the pharmacologic properties of the KATP channels. Single-channel conductance and open probability were measured using micropipettes having a tip opening area of 0.9 ± 0.1 μm2 (n = 15). Using this type of pipette, no more than two to three open channels were observed in each patch. Few micropatches of healthy subjects (three of nine) and patients with HOPP (four of six) contained single units. This was tested by observing the single-channel transitions for long periods (123–260 s), and in healthy subjects, also in the presence of 50 μM MgADP in the bath, a condition that ensured the maximum stimulation of the channel open probability.
Rat experiments.
The evaluation of the in vitro effects of different concentrations of free Ca2+ ion on the KATP channel was performed after incubation of the flexor digitorum brevis muscles of male adult Wistar rats with Krebs solution containing 4.6 mEq/l of K+ ion (normokalemic solution) or with Krebs solution containing 0.5 mEq/l of K+ ion (low-K+ solution). Single fibers were obtained by enzymatic treatment of the muscles in the presence of low-Ka+ or normokalemic solution. The total time of incubation with low-K+ or normokalemic solution including the time for the enzymatic dissociation was 80 min. After this time, both type of fibers were exposed to the solution containing 150 mM KCl for patch clamp recordings as detailed in the previous section of this article.
Results
Macropatch currents of skeletal muscle KATP channels of healthy subjects and patients with HOPP.
A 39-year-old woman with HOPP had a long history of episodic weakness that began in the first decade of life. Also, her son, an 18-year-old boy, had developed severe episodes of paralysis that began at 13 years of age. A 40-year-old woman from another family with HOPP had a less severe history of episodic weakness. In the fibers of vastus lateralis and deltoid muscles of healthy subjects and patients with HOPP, cell-attached recordings, made by macropatches at potential similar to the resting potential with 150 mM KCl in the bath and in the pipette solutions, revealed inward currents. As expected for currents sustained by KATP channels, the excision of the macropatches from the fibers into ATP-free solution increased the currents. This effect was routinely observed in the healthy subjects, whereas in the patients with HOPP, the increase of the current upon excision was abnormally low and in some macropatches it was not observed. The cytosolic MgATP (100–500 μM) or ATP (10–500 μM) reduced the macropatch currents of patients with HOPP and healthy subjects, confirming that these currents were sustained by KATP channels. In the healthy subjects, the IC50 of the ATP calculated by fitting routine was 16 ± 4 μM. Unfortunately, the extremely low residual current present in the fibers of the patients with HOPP did not allow us to calculate this parameter in these fibers.
The analysis of the macropatch current revealed that the mean KATP current of the fibers excised from the deltoid muscle of the 18-year-old HOPP boy was abnormally low at –0.36 ± 0.02 pA/μm2 (eight macropatches; pipette area 5.1 ± 0.9 μm2) at –60 mV of membrane potential (Fig. 1a). In the biopsy of the HOPP mother, no KATP current was recorded (four macropatches; pipette area 4.9 ± 1 μm2). Similarly, in the biopsy of the 40-year-old HOPP woman, the mean KATP current was low at –0.716 ± 0.36 pA/μm2 (14 macropatches; pipette area 4.59 ± 0.35 μm2) (Fig. 1b). In contrast, the magnitude of the KATP currents recorded in the biopsies of the healthy 17-year-old boy and 40-year-old woman was significantly higher in the patients with HOPP: –9.03 ± 0.8 pA/μm2 (eight macropatches; pipette area 5.2 ± 0.4 μm2) and –9.1 ± 0.9 pA/μm2 (seven macropatches; pipette area 4.9 ± 0.9 μm2), respectively (Fig. 1, c and d).
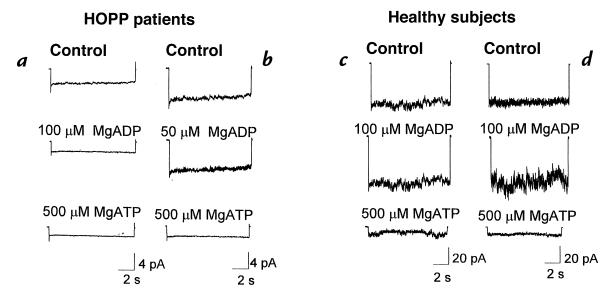
Figure 1.
Digital average of the sarcolemma KATP currents of patients with HOPP and healthy subjects. KATP currents of the 18-year-old boy with HOPP (seven macropatches) (a) and of the 40-year-old woman with HOPP (14 macropatches) are shown (b). MgADP at 50 μM concentration failed to stimulate the current, whereas at 100 μM, current was reduced. In both patients with HOPP, MgATP (500 μM) completely reduced the currents. KATP currents of the 17-year-old healthy boy (eight macropatches) (c) and 40-year-old healthy woman (seven macropatches) are shown (d). In contrast to the patients with HOPP, in the healthy subjects large KATP currents were recorded that were stimulated by MgADP (100 μM) and completely reduced by MgATP (500 μM). HOPP, hypokalemic periodic paralysis; KATP, adenosine triphosphate–sensitive K+ channel; MgADP, magnesium adenosine diphosphate; MgATP, magnesium adenosine triphosphate.
To ensure a maximal stimulation of the KATP currents, the macropatches of the patients with HOPP were exposed to 50–100 μM concentrations of MgADP. It is known that the nucleotide diphosphate at low concentrations (50–100 μM) behaves as a physiological agonist of the KATP channels in various tissues including skeletal muscle, whereas at higher concentrations, it causes a dose-dependent reduction of the KATP currents (23). However, in the fibers of the 18-year-old boy with HOPP, MgADP at 50 μM concentration failed to stimulate the current, whereas at 100 μM concentration, it reduced the current to –1.15 ± 0.9 pA in the control condition and to –0.3 ± 0.01 pA after 100 μM concentration of MgADP (eight macropatches; pipette area 5.1 ± 0.9 μm2) (Fig. 1a). Similarly, in the 40-year-old woman with HOPP, MgADP at 50 μM failed to stimulate the currents: –2.39 ± 1.07 pA in the control condition and –2.39 ± 1.1 pA after MgADP (nine macropatches; pipette area 4.63 ± 0.4 μm2) (Fig. 1b). At 100 μM concentration, MgADP reduced the currents close to the baseline level. In contrast, in the 17-year-old healthy boy and in the 40-year-old healthy woman, MgADP enhanced the sarcolemma KATP currents: –60.4 ± 3 pA and –58 ± 5 pA in the control condition, and –77.5 ± 4 pA (eight macropatches; pipette area 5.2 ± 0.4 μm2) and –74.1 ± 4 pA (seven macropatches; pipette area 4.9 ± 0.9 μm2) in the presence of 100 μM concentration of MgADP in the boy and woman, respectively (Fig. 1, c and d). A certain stimulatory effect of the MgADP was also observed at 50 μM concentration.
To recruit as much KATP channels as we could, we perfused the macropatches of the patients with HOPP with cromakalim (10–100 μM). However, we found that the drug only partially restored the current: –1.14 ± 0.9 pA in the control condition and –3.85 ± 0.2 pA (three macropatches; pipette area 5.2 ± 0.36 μm2) after application of 10 μM concentration of cromakalim. Also, higher concentrations of the drug (100 μM) did not restore the current. The incubation of the macropatches with solution containing cromakalim (10–100 μM) and ATP Mg2+ salt or K+ salt (100 μM) exerted effects similar to those observed in the absence of nucleotide triphosphate.
Single-channel currents of skeletal muscle KATP channels of healthy subjects and patients with HOPP.
At the channel level, several open conductance states that were all blocked by ATP were routinely recorded by micropatches in the fibers of the patients with HOPP (Fig. 2, a and b). In both patients with HOPP, at least five levels were counted and labeled (O1, O1.5, O2, O3, and O4), with amplitudes of –0.86 ± 0.03 pA, –1.5 ± 0.01 pA, –2.1 ± 0.09 pA, –2.7 ± 0.08 pA, and –4.5 ± 0.3 pA, respectively, at –60 mV of membrane potentials (Fig. 3a). The depolarization of the patches led to the disappearance of the subconductance levels; at +40 mV, only one level was counted, with an amplitude of +3.2 ± 0.7 pA, which was not different from that measured in the healthy subjects. This phenomenon should predict the loss of the characteristic inward rectification properties of the KATP channel. This is clearly shown in the current–voltage relationships constructed for the O1 and O2 levels of both patients with HOPP (Fig. 2, d and e), whereas the O4 level (Fig. 2e) had a slope conductance similar to that calculated for the healthy subjects (Fig. 2f). In contrast, only one conductance state, O4 (Fig. 3d), blocked by ATP was routinely detected in the healthy subjects (Fig. 2c). This had an amplitude of –4.4 ± 0.3 pA and +3.3 ± 0.1 pA at –60 mV and +40 mV of membrane potentials, respectively. The current–voltage relationship showed the characteristic inward rectification of the KATP channel (Fig. 2f).
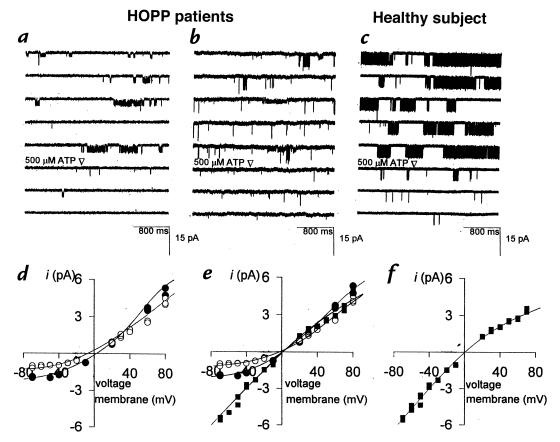
Figure 2.
Sample traces of single sarcolemma KATP channel from two patients with HOPP and from a healthy subject. The KATP channel of the 40-year-old woman with HOPP (a) and of the 18-year-old boy with HOPP (b) transits through subconductance states of low open probability, all blocked by ATP. In the 17-year-old healthy boy, only one open level blocked by ATP is clearly visible (c). In the 40-year-old woman with HOPP, the O1 (open circles) and O2 (closed circles) levels had slope conductances of 25 pS and 32 pS at negative potentials, and 40 pS and 47 pS at positive potentials, respectively (d). In the 18-year-old boy with HOPP, the slope conductances of O1 (open circles), O2 (closed circles), and O4 (closed squares) levels were 27 pS, 35 pS, and 72 pS at negative potentials, and 40 pS, 47 pS, and 46 pS at positive potentials, respectively (e). In the 18-year-old healthy boy, the O4 level (closed squares) had a slope conductances of 72 pS and 45 pS at the negative and positive potentials, respectively (f).
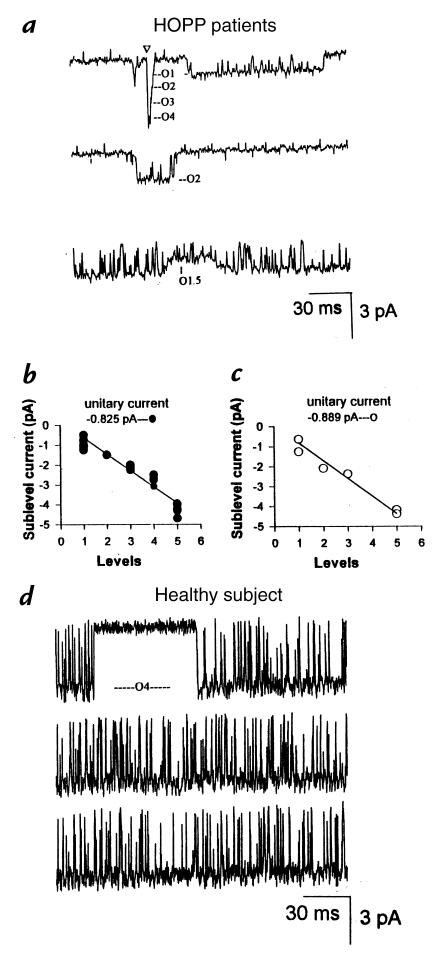
Figure 3.
Conductance levels of the sarcolemma KATP channels in the patients with HOPP and in a healthy subject. Five subconductance levels are clearly visible in these sample traces (a). They are labeled on the basis of their specific amplitudes. The arrow marks the simultaneous opening from the closed state to the O4 level. Linear relationships between the number of levels vs. the amplitude counted in the fibers of the 40-year-old woman with HOPP (b) and of the 18-year-old boy with HOPP (c). In the 40-year-old healthy woman (d), only one open conductance state, O4, was clearly visible.
In both patients with HOPP, the subconductance levels appeared to be equally spaced. The plots of the number of levels against the corresponding amplitude were well fitted with straight lines with similar slopes that represent the theoretical values of the unitary current of each fluctuation (Fig. 3, b and c). Although in most of our recordings these conductance levels appeared to act independently, we also observed direct openings from the closed state to the O4 level (Fig. 3a). This would predict that the conductance levels counted in the fibers of the patients with HOPP came from intermediate fluctuations of a common pore.
Open probability analysis performed at –60 mV of membrane potential revealed that the O1 and O2 levels contributed significantly to the channel activity in the excised patches of the patients with HOPP, whereas the contribution of the O1.5, O3, and O4 levels (Fig. 4a) was negligible. In contrast, in the healthy subjects, the KATP channel spent 12.5% of the time in the O4 state without evident fluctuations between subconductance states (Fig. 4b).
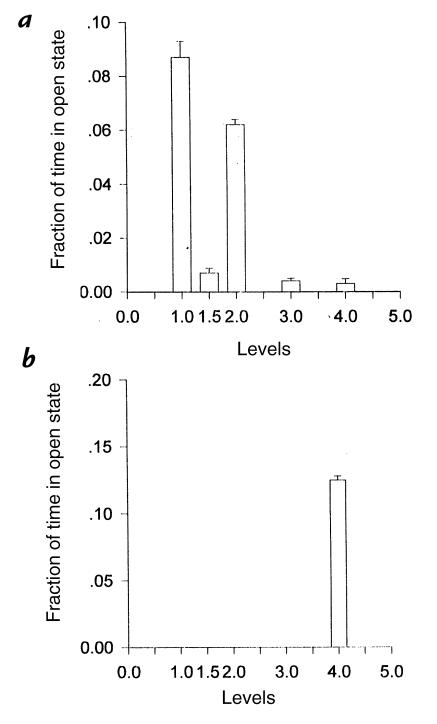
Figure 4.
Fraction of time spent in open state by each subconductance level counted in the fibers of the 40-year-old woman with HOPP (a) and in the 40-year-old healthy woman (b). In the patient with HOPP, the KATP channel resides mainly in the subconductance levels, whereas in the healthy subject, the KATP channel resides only in the O4 level.
In vitro effects of low-K+ solution and different concentrations of free Ca2+ ion on rat KATP channel.
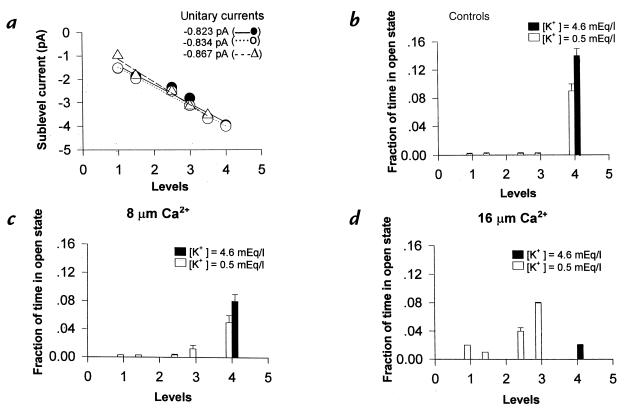
Basically, we have tried to answer the question of whether the DHP receptor mutation can directly or indirectly modify the availability of the Ca2+ ion, which in turn might block the channel, forcing it into subconductance states. Surprisingly, we found that the preincubation of the muscle with low-K+ solution led to the appearance of at least six subconductance states, all blocked by ATP (500 μM) of unitary amplitude similar to that measured in the patients with HOPP but with a different open probability (Fig. 5a). In particular, in this experimental condition, the KATP channel spent ∼9% of the time in the O4 level and <1% of the time in the sublevels (Fig. 5b). The internal application to the patches of increasing concentrations of free Ca2+ ion (8–16 μM) led to a Ca2+-dependent reduction of the open probability of the O4 level and to the parallel increase of the open probability of the sublevels (Fig. 5, c and d). In this condition, the rat KATP channels showed open probability of the sublevels similar to that measured for the KATP channels of the patients with HOPP. The free Ca2+ ion did not significantly changes the unitary current of the sublevels (Fig. 5a). As expected, the KATP channel from muscles preincubated with normokalemic solution transits exclusively through the O4 level without showing fluctuations between subconductance states (Fig. 5b). In these fibers, in contrast to what was observed in the fibers pretreated with low-K+ solution, the Ca2+ ion did promote transitions of the KATP channels between intermediate levels. The Ca2+ ion reduced only the open probability of the main level, O4 (Fig. 5, c and d), with an IC50 of 12 ± 2 μM. The effects described here of the Ca2+ ion on KATP channels were irreversible upon replacement of the bath solution with a solution enriched with EGTA and free of the Ca2+ ion.
Figure 5.
In vitro effects of low-K+ solution and different concentrations of free Ca2+ ion on KATP channel. The muscle fibers were preincubated for 80 min with low-K+ solution (open bars) or with normokalemic solution (closed bars) before recordings. A linear relationship between the number of levels of the KATP channels vs. the corresponding amplitude in the absence (closed circles), or in the presence, of 8 μM (open circles) and 16 μM (open triangle) concentrations of free Ca2+ ion (a) was found. Fraction of time spent in open state by each subconductance level in the absence (b) or in the presence of 8 μM (c) and 16 μM (d) concentrations of free Ca2+ ion (a). Only after incubation of the muscle with the low-K+ solution, the Ca2+ ion forced the channel into the subconductance states, reducing the open probability of the O4 level and increasing the open probability of the sublevels.
Discussion
To our knowledge, this is the first report that shows the presence of an abnormal muscular KATP channel in patients with HOPP carrying the common R528H mutation of the α subunit of the L-type Ca2+ channel. This KATP channel is characterized by having a reduced channel open probability and several subconductance states. None of these open states were detected on fibers from muscles of healthy subjects. Low-conductance channel states may have originated either from substates of a single population of channel or from separate channel populations. The frequent fluctuations observed between the fully open (O4 level) and the lower conductance states, and the occurrence of direct transitions from fully open to closed state crossing the intermediate levels (Fig. 3a, arrows), would strongly suggest that these levels arose from a common pore. Interestingly, we have also found that the KATP channel of rat muscle fibers preincubated in vitro with low-K+ solution transit through subconductance levels with unitary current amplitude similar to those calculated in the patients with HOPP but with different open probability. This comes as an unexpected finding because it was not observed in the fibers exposed to normokalemic solution. Similarly, we have recently shown (5) that in rats made hypokalemic by a K+-free diet, the KATP channels behaves abnormally; indeed, they transit through subconductance states of low open probability, and the corresponding macropatch current is abnormally reduced. The presence of the subconductance states in the muscle KATP channel of the patients with HOPP is relevant, considering that this particular behavior has never been observed in other physiological and pathological conditions, such as aging (3) and hyperinsulinemic hypoglycemia of infancy (26), situations characterized by abnormal closure of the KATP channels of skeletal muscle and of pancreatic β cell.
The mechanism(s) responsible for the appearance of the subconductance states in the KATP channel is still obscure. It has been reported (27–29) that the lowering of the internal pH or the exposure of the patches to adenine leads to the appearance of several subconductance states in cardiac and skeletal muscle KATP channels. These two maneuvers cause reversible modifications of the channel pore, such as changes in the surface charge distribution or internal block of the pore. In the patients with HOPP, the modifications of the KATP channel were irreversible (our experiments were performed in excised patches in which the influence of the metabolic factors on channel properties is negligible); this excludes the involvement of the low internal pH or adenine as factors responsible for the appearance of the subconductance states in our experiments. One possible factor can be the Ca2+ ion. This is supported by several findings. First, the Ca2+ ion forced the KATP channels into subconductance states, irreversibly modifying the channel gating. This is clearly shown in our experiments, in which the KATP channel of rat muscle preincubated in vitro with low-K+ solution, when exposed to Ca2+ ion, transits through subconductance states having open probability and unitary current amplitude similar to that measured for the channels of the patients with HOPP. Second, the Ca2+ ion is a positively charged factor that can modify the conduction pathway, reducing the unitary current amplitude; indeed, in our experiments, the subconductance states disappeared when the patches were depolarized. Finally, Jurkat-Rott et al. (17) recently pointed out that other factors directly or indirectly related to the DHP mutation are involved in the etiopathogenesis of HOPP. They showed that a third type of Ca2+ current is twofold overexpressed in the myotubes of human patients with HOPP and in the cell lines expressing the R528H mutation of DHP receptor (17). It is likely that this abnormality contributes to alter the Ca2+ ion homeostasis in the fibers of patients withHOPP.
It seems from our experiments, that in patients with HOPP, not only the pore of the muscle KATP channel complex is altered but also the sulfonylurea receptor (30). This is supported by the fact that the abnormally low KATP current recorded in the fibers of the patients with HOPP is not stimulated by MgADP and is only partially restored by cromakalim, a molecule that binds the sulfonylurea receptor, activating the channels (30–31).
We should stress that the lack of the KATP channel activity in the skeletal muscle fibers may result in a cascade of events. In various tissues, including skeletal muscle (7), the KATP channels are functionally coupled to the Na+/K+ ATPase, so that in healthy subjects, the insulin stimulation of the pump leads to influx of K+ ion into the muscle, transient hypokalemia, and finally activation of the KATP current that sustains the hyperpolarization of the fibers (7). In patients with HOPP, the pump appears to work properly (9), but we propose here that no efflux of K+ ion occurs because of the low basal activity of the KATP channels. This mechanism can explain the hypokalemia, the membrane depolarization, and the insulin-induced paralysis affecting patients with HOPP. This is supported by the finding that in the K+-depleted rats, insulin reduces the residual currents, aggravating the fiber depolarization and provoking paralysis (5, 32), rather than inducing activation of the KATP channel as it does in the normokalemic rats (7).
Acknowledgments
We are grateful to Fedele Natuzzi, Mariagrazia Barbieri, Antonella Spinazzola, Giulia De Rosa, Erhard Schoch, and Werner Klingler for help with human biopsies. This work was supported by Telethon-Italy project no. 579.
References
- 1.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 2.Sakura H, Ämmälä C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- 3.Tricarico D, Conte Camerino D. ATP-sensitive K+ channels of skeletal muscle fibers from young adult and aged rats: possible involvement of thiol-dependent redox mechanisms in the age-dependent modifications of their biophysical and pharmacological properties. Mol Pharmacol. 1994;46:754–761. [PubMed] [Google Scholar]
- 4.Tricarico D, Petruzzi R, Conte Camerino D. Different sulfonylurea and ATP sensitivity characterizes the juvenile and the adult form of KATP channel complex of rat skeletal muscle. Eur J Pharmacol. 1997;321:369–378. doi: 10.1016/s0014-2999(96)00965-x. [DOI] [PubMed] [Google Scholar]
- 5.Tricarico D, et al. The biophysical and pharmacological characteristics of skeletal muscle KATP channels are modified in K+ depleted rat, an animal model of hypokalemic periodic paralysis. Mol Pharmacol. 1998;54:197–206. doi: 10.1124/mol.54.1.197. [DOI] [PubMed] [Google Scholar]
- 6.Dengler R, Hofmann WW, Rüdel R. Effects of potassium depletion and insulin on resting and stimulated skeletal rat muscle. J Neurol Neurosurg Psychiatry. 1979;42:818–826. doi: 10.1136/jnnp.42.9.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tricarico D, Mallamaci R, Barbieri M, Conte Camerino D. Modulation of ATP-sensitive K+ channel by insulin in rat skeletal muscle fibers. Biochem Biophys Res Commun. 1997;232:536–539. doi: 10.1006/bbrc.1997.6320. [DOI] [PubMed] [Google Scholar]
- 8.Iannaccone ST, Li KX, Speralakis N, Lathrop DA. Insulin-induced hyperpolarization in mammalian skeletal muscle. Am J Physiol. 1989;256:C368–C374. doi: 10.1152/ajpcell.1989.256.2.C368. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann-Horn, F., Engel, A.G., Ricker, K., and Rüdel, R. 1994. The periodic paralyses and paramyotonia congenita. In Myology. 2nd ed. A.G Engel and C. Franzini-Armstrong, editors. McGraw-Hill. New York, NY. 1303–1327.
- 10.Lapie P, Lory P, Fontaine B. Hypokalemic periodic paralysis: an autosomal dominant muscle disorder caused by mutations in a voltage-gated calcium channel. Neuromusc Disord. 1997;7:234–240. doi: 10.1016/s0960-8966(97)00435-5. [DOI] [PubMed] [Google Scholar]
- 11.Fontaine B, et al. Mapping of the hypokalemic periodic paralysis to chromosome 1q31–q32 (Hyopp) locus in three European families. Nat Genet. 1994;6:267–272. doi: 10.1038/ng0394-267. [DOI] [PubMed] [Google Scholar]
- 12.Jurkat-Rott K, et al. A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet. 1994;3:1415–1419. doi: 10.1093/hmg/3.8.1415. [DOI] [PubMed] [Google Scholar]
- 13.Ptacek LJ, et al. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell. 1994;77:863–868. doi: 10.1016/0092-8674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann-Horn F, Rüdel R. Molecular pathophysiology of voltage-gated ion channels. Rev Physiol Biochem Pharmacol. 1996;128:195–268. doi: 10.1007/3-540-61343-9_9. [DOI] [PubMed] [Google Scholar]
- 15.Lapie P, Goudet C, Nargeot J, Fontaine B, Lory P. Electrophysiological properties of the hypokalaemic periodic paralysis mutation (R528) of the skeletal muscle a1s subunit as expressed in mouse L cells. FEBS Lett. 1996;382:244–248. doi: 10.1016/0014-5793(96)00173-1. [DOI] [PubMed] [Google Scholar]
- 16.Cannon SC. Ion-channel defects and aberrant excitability in myotonia and periodic paralysis. Trends Neurosci. 1996;19:3–10. doi: 10.1016/0166-2236(96)81859-5. [DOI] [PubMed] [Google Scholar]
- 17.Jurkat-Rott K, et al. Calcium currents and transients of native and heterologous expressed mutant skeletal muscle DHP receptor alpha1 subunits. FEBS Lett. 1998;423/2:198–204. doi: 10.1016/s0014-5793(98)00090-8. [DOI] [PubMed] [Google Scholar]
- 18.Fouad G, et al. Genotype-phenotype correlations of DHP receptor α1-subunit gene mutations causing hypokalemic periodic paralysis. Neuromusc Disord. 1997;7:33–38. doi: 10.1016/s0960-8966(96)00401-4. [DOI] [PubMed] [Google Scholar]
- 19.Grafe P, Quasthoff S, Strupp M, Lehmann-Horn F. Enhancement of K+ conductance improves in vitro the contraction force of skeletal muscle in hypokalemic periodic paralysis. Muscle Nerve. 1990;13:451–457. doi: 10.1002/mus.880130513. [DOI] [PubMed] [Google Scholar]
- 20.Spuler A, Lehmann-Horn F, Grafe P. Cromakalim (BRL 34915) restores in vitro the membrane potential of depolarized human skeletal muscle fibres. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:327–331. doi: 10.1007/BF00173587. [DOI] [PubMed] [Google Scholar]
- 21.Links TP, Smit AJ, Oosterhuis HJGH, Reitsma WD. Potassium channels in hypokalaemic periodic paralysis: a key to the pathogenesis? Clin Sci. 1993;85:319–325. doi: 10.1042/cs0850319. [DOI] [PubMed] [Google Scholar]
- 22.Ligtenberg JJ, et al. Normal insulin release during sustained hyperglycaemia in hypokalaemic periodic paralysis: role of the potassium channel opener pinacidil in impaired muscle strength. J Clin Sci. 1996;91:583–589. doi: 10.1042/cs0910583. [DOI] [PubMed] [Google Scholar]
- 23.Allard B, Lazdunski M. Nucleotide diphosphates activate the ATP-sensitive potassium channel in mouse skeletal muscle. Pflugers Arch. 1992;422:185–192. doi: 10.1007/BF00370419. [DOI] [PubMed] [Google Scholar]
- 24.Tricarico D, Petruzzi R, Conte Camerino D. Changes of the biophysical properties of calcium-activated potassium channels of rat skeletal muscle fibres during aging. Pflugers Arch. 1997;434:822–829. doi: 10.1007/s004240050471. [DOI] [PubMed] [Google Scholar]
- 25.Sakmann, B., and Neher, E. 1983. Geometric parameters of pipettes and membrane patches. In Single-channel recording. B. Sakmann and E. Neher, editors. Plenum Press. New York, NY. 37–51.
- 26.Aguilar-Bryan L, Bryan J. ATP-sensitive potassium channels, sulfonylurea receptors and persistent hyperinsulinemic hypoglycemia of infancy. Diabetes Rev. 1996;4:336–346. [Google Scholar]
- 27.Weik R, Lönnendonker U, Neumcke B. Low-conductance states of K+ channels in adult mouse skeletal muscle. Biochem Biophys Acta. 1989;983:127–134. doi: 10.1016/0005-2736(89)90225-3. [DOI] [PubMed] [Google Scholar]
- 28.Fan Z, Furukawa T, Sawanobori T, Makielski JC, Hiraoka M. Cytoplasmic acidosis induces multiple conductance states in ATP-sensitive potassium channels of cardiac myocytes. J Membr Biol. 1993;136:169–179. doi: 10.1007/BF02505761. [DOI] [PubMed] [Google Scholar]
- 29.Vivaudou M, Forestier C. Modification by protons of frog skeletal muscle KATP channel: effects on ion conduction and nucleotide inhibition. J Physiol (Lond) 1995;486:629–645. doi: 10.1113/jphysiol.1995.sp020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguilar-Bryan L, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 31.Löffler C, Quast U. Pharmacological characterization of the sulphonylurea receptor in rat isolated aorta. Br J Pharmacol. 1997;120:476–480. doi: 10.1038/sj.bjp.0700919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tricarico D, Capriulo R, Conte Camerino D. Insulin modulation of ATP-sensitive K+ channel of rat skeletal muscle is impaired in the hypokalemic state. Pflugers Arch. 1998;437:235–240. doi: 10.1007/s004240050774. [DOI] [PubMed] [Google Scholar]