Abstract
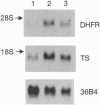
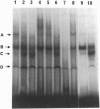
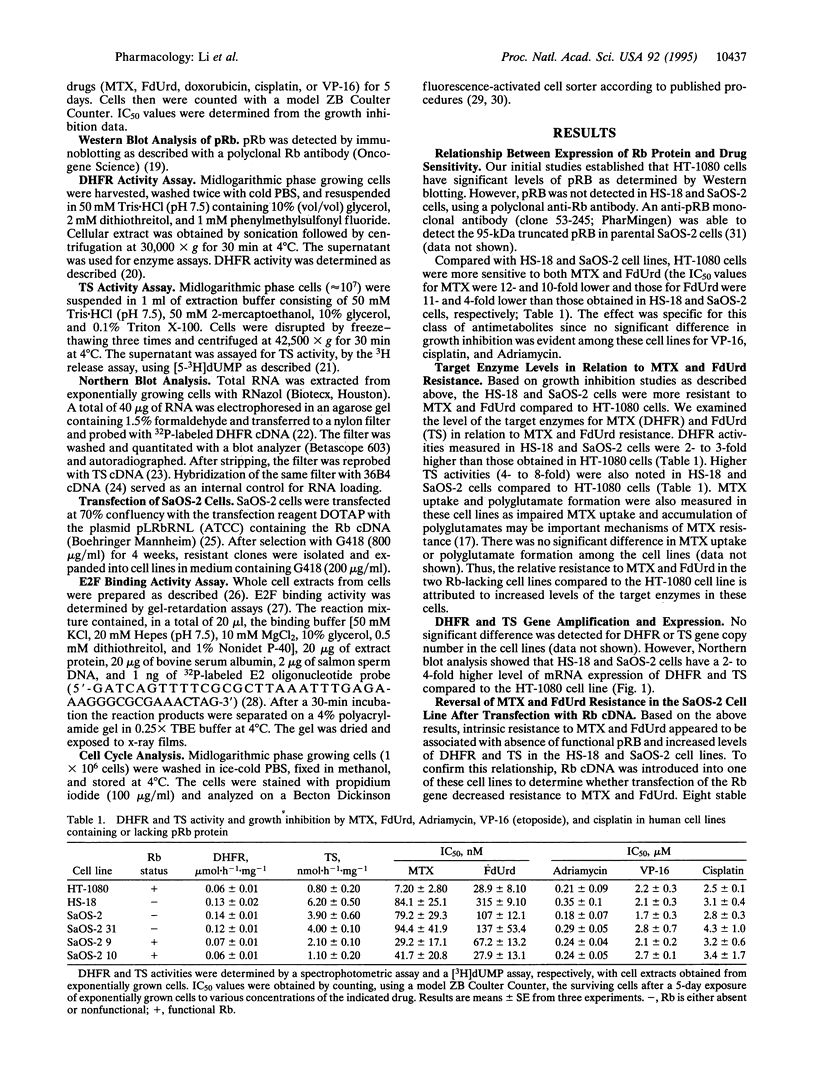
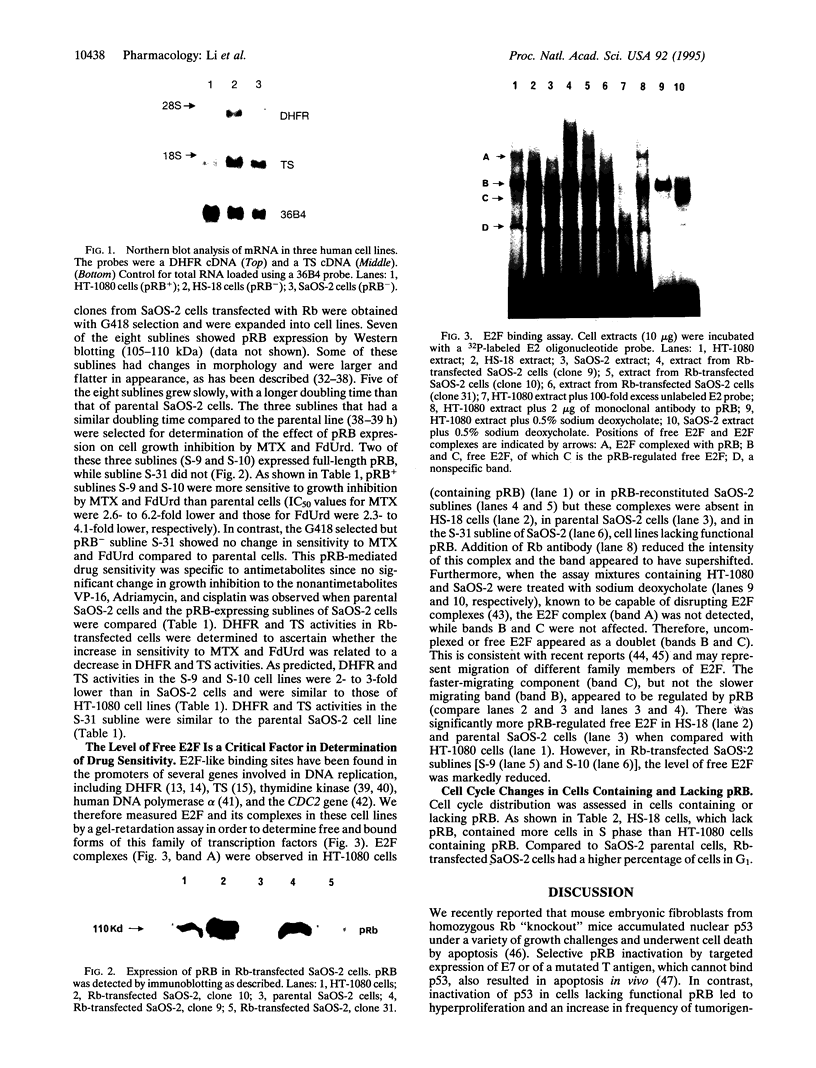
Growth inhibition assays indicated that the IC50 values for methotrexate (MTX) and 5-fluorodeoxyuridine (FdUrd) in HS-18, a liposarcoma cell line lacking retinoblastoma protein (pRB), and SaOS-2, an osteosarcoma cell line with a truncated and nonfunctional pRB, were 10- to 12-fold and 4- to 11-fold higher, respectively, than for the HT-1080 (fibrosarcoma) cell line, which has wild-type pRB. These Rb-/- cell lines exhibited a 2- to 4-fold increase in both dihydrofolate reductase (DHFR) and thymidylate synthase (TS) enzyme activities as well as a 3- to 4-fold increase in mRNA levels for these enzymes compared to the HT-1080 (Rb+/+) cells. This increase in expression was not due to amplification of the DHFR and TS genes. Growth inhibition by MTX and FdUrd was increased and DHFR and TS activities and expression were correspondingly decreased in Rb transfectants of SaOS-2 cells. In contrast, there was no significant difference in growth inhibition among these cell lines for the nonantimetabolites VP-16, cisplatin, and doxorubicin. A gel mobility-shift assay showed that parental SaOS-2 cells had increased levels of free E2F compared to the Rb-reconstituted SaOS-2 cells. These results indicate that pRB defective cells may have decreased sensitivity to growth inhibition by target enzymes encoded by genes whose transcription is enhanced by E2F proteins and suggest mechanisms of interaction between cytotoxic agents and genes involved in cell cycle progression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almasan A., Yin Y., Kelly R. E., Lee E. Y., Bradley A., Li W., Bertino J. R., Wahl G. M. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5436–5440. doi: 10.1073/pnas.92.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTINO J. R., PERKINS J. P., JOHNS D. G. PURIFICATION AND PROPERTIES OF DIHYDROFOLATE REDUCTASE FROM EHRLICH ASCITES CARCINOMA CELLS. Biochemistry. 1965 May;4:839–846. doi: 10.1021/bi00881a007. [DOI] [PubMed] [Google Scholar]
- Björklund S., Skogman E., Thelander L. An S-phase specific release from a transcriptional block regulates the expression of mouse ribonucleotide reductase R2 subunit. EMBO J. 1992 Dec;11(13):4953–4959. doi: 10.1002/j.1460-2075.1992.tb05602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. C., Azizkhan J. C. Transcription factor E2F is required for efficient expression of the hamster dihydrofolate reductase gene in vitro and in vivo. Mol Cell Biol. 1989 Nov;9(11):4994–5002. doi: 10.1128/mcb.9.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein R., Shew J. Y., Chen P. L., Scully P., Lee W. H. Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science. 1990 Feb 9;247(4943):712–715. doi: 10.1126/science.2300823. [DOI] [PubMed] [Google Scholar]
- Cao L., Faha B., Dembski M., Tsai L. H., Harlow E., Dyson N. Independent binding of the retinoblastoma protein and p107 to the transcription factor E2F. Nature. 1992 Jan 9;355(6356):176–179. doi: 10.1038/355176a0. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Canton M. D., Gallie B. L. Chemosensitivity and multidrug resistance to antineoplastic drugs in retinoblastoma cell lines. Anticancer Res. 1989 Mar-Apr;9(2):469–474. [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Livingston D. M., DeCaprio J. A. Cell cycle analysis of E2F in primary human T cells reveals novel E2F complexes and biochemically distinct forms of free E2F. Mol Cell Biol. 1993 Jul;13(7):3975–3983. doi: 10.1128/mcb.13.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D., Whyte P., Peeper D. S., Jacks T., Weinberg R. A. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993 Dec;7(12A):2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO J. 1992 May;11(5):1797–1804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Furukawa Y., Ajchenbaum F., Griffin J. D., Livingston D. M. The retinoblastoma-susceptibility gene product becomes phosphorylated in multiple stages during cell cycle entry and progression. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1795–1798. doi: 10.1073/pnas.89.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T. L., Li D. W., Jenh C. H., Johnson L. F. Structure of the gene for mouse thymidylate synthase. Locations of introns and multiple transcriptional start sites. J Biol Chem. 1986 Dec 5;261(34):16000–16005. [PubMed] [Google Scholar]
- Di Leonardo A., Linke S. P., Clarkin K., Wahl G. M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994 Nov 1;8(21):2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- Dou Q. P., Zhao S., Levin A. H., Wang J., Helin K., Pardee A. B. G1/S-regulated E2F-containing protein complexes bind to the mouse thymidine kinase gene promoter. J Biol Chem. 1994 Jan 14;269(2):1306–1313. [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Slansky J. E., Kollmar R. The role of E2F in the mammalian cell cycle. Biochim Biophys Acta. 1993 Aug 23;1155(2):125–131. doi: 10.1016/0304-419x(93)90001-s. [DOI] [PubMed] [Google Scholar]
- Goldman I. D., Matherly L. H. The cellular pharmacology of methotrexate. Pharmacol Ther. 1985;28(1):77–102. doi: 10.1016/0163-7258(85)90083-x. [DOI] [PubMed] [Google Scholar]
- Goodrich D. W., Wang N. P., Qian Y. W., Lee E. Y., Lee W. H. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991 Oct 18;67(2):293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- Goodrich D. W., Wang N. P., Qian Y. W., Lee E. Y., Lee W. H. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991 Oct 18;67(2):293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Haas-Kogan D. A., Kogan S. C., Levi D., Dazin P., T'Ang A., Fung Y. K., Israel M. A. Inhibition of apoptosis by the retinoblastoma gene product. EMBO J. 1995 Feb 1;14(3):461–472. doi: 10.1002/j.1460-2075.1995.tb07022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hande K. R., Oldham R. K., Fer M. F., Richardson R. L., Greco F. A. Randomized study of high-dose versus low-dose methotrexate in the treatment of extensive small cell lung cancer. Am J Med. 1982 Sep;73(3):413–419. doi: 10.1016/0002-9343(82)90745-8. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Brill J. A., Fink G. R., Weinberg R. A. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes Dev. 1994 Aug 1;8(15):1759–1771. doi: 10.1101/gad.8.15.1759. [DOI] [PubMed] [Google Scholar]
- Hensel C. H., Hsieh C. L., Gazdar A. F., Johnson B. E., Sakaguchi A. Y., Naylor S. L., Lee W. H., Lee E. Y. Altered structure and expression of the human retinoblastoma susceptibility gene in small cell lung cancer. Cancer Res. 1990 May 15;50(10):3067–3072. [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Howes K. A., Ransom N., Papermaster D. S., Lasudry J. G., Albert D. M., Windle J. J. Apoptosis or retinoblastoma: alternative fates of photoreceptors expressing the HPV-16 E7 gene in the presence or absence of p53. Genes Dev. 1994 Jun 1;8(11):1300–1310. doi: 10.1101/gad.8.11.1300. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Johnson D. G., Cress W. D., Jakoi L., Nevins J. R. Oncogenic capacity of the E2F1 gene. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12823–12827. doi: 10.1073/pnas.91.26.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. G., Schwarz J. K., Cress W. D., Nevins J. R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993 Sep 23;365(6444):349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- Johnson L. F. Posttranscriptional regulation of thymidylate synthase gene expression. J Cell Biochem. 1994 Apr;54(4):387–392. doi: 10.1002/jcb.240540405. [DOI] [PubMed] [Google Scholar]
- Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991 Jul 25;19(14):3998–3998. doi: 10.1093/nar/19.14.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J. A., Buchkovich K. J., Marshak D. R., Anderson C. W., Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991 Dec;10(13):4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. W., Lin J. T., Schweitzer B. I., Tong W. P., Niedzwiecki D., Bertino J. R. Intrinsic resistance to methotrexate in human soft tissue sarcoma cell lines. Cancer Res. 1992 Jul 15;52(14):3908–3913. [PubMed] [Google Scholar]
- Li W. W., Tong W. P., Bertino J. R. Antitumor activity of antifolate inhibitors of thymidylate and purine synthesis in human soft tissue sarcoma cell lines with intrinsic resistance to methotrexate. Clin Cancer Res. 1995 Jun;1(6):631–636. [PubMed] [Google Scholar]
- Lin B. T., Gruenwald S., Morla A. O., Lee W. H., Wang J. Y. Retinoblastoma cancer suppressor gene product is a substrate of the cell cycle regulator cdc2 kinase. EMBO J. 1991 Apr;10(4):857–864. doi: 10.1002/j.1460-2075.1991.tb08018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Morgenbesser S. D., Williams B. O., Jacks T., DePinho R. A. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994 Sep 1;371(6492):72–74. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Pan H., Griep A. E. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994 Jun 1;8(11):1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- Pearson B. E., Nasheuer H. P., Wang T. S. Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells. Mol Cell Biol. 1991 Apr;11(4):2081–2095. doi: 10.1128/mcb.11.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X. Q., Chittenden T., Livingston D. M., Kaelin W. G., Jr Identification of a growth suppression domain within the retinoblastoma gene product. Genes Dev. 1992 Jun;6(6):953–964. doi: 10.1101/gad.6.6.953. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Rooney R., Nevins J. R. Identification of an E1A-inducible cellular factor that interacts with regulatory sequences within the adenovirus E4 promoter. EMBO J. 1987 Dec 20;6(13):4073–4081. doi: 10.1002/j.1460-2075.1987.tb02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. An isotopic assay for thymidylate synthetase. Biochemistry. 1966 Nov;5(11):3546–3548. doi: 10.1021/bi00875a022. [DOI] [PubMed] [Google Scholar]
- Rosowsky A., Wright J. E., Cucchi C. A., Lippke J. A., Tantravahi R., Ervin T. J., Frei E., 3rd Phenotypic heterogeneity in cultured human head and neck squamous cell carcinoma lines with low-level methotrexate resistance. Cancer Res. 1985 Dec;45(12 Pt 1):6205–6212. [PubMed] [Google Scholar]
- Sardet C., Vidal M., Cobrinik D., Geng Y., Onufryk C., Chen A., Weinberg R. A. E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2403–2407. doi: 10.1073/pnas.92.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert E. L., Hansen M. F., Strong L. C. The retinoblastoma gene and its significance. Ann Med. 1994 Jun;26(3):177–184. doi: 10.3109/07853899409147887. [DOI] [PubMed] [Google Scholar]
- Shew J. Y., Lin B. T., Chen P. L., Tseng B. Y., Yang-Feng T. L., Lee W. H. C-terminal truncation of the retinoblastoma gene product leads to functional inactivation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):6–10. doi: 10.1073/pnas.87.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodkar S., Ewen M., DeCaprio J. A., Morgan J., Livingston D. M., Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992 Jan 10;68(1):157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Slansky J. E., Li Y., Kaelin W. G., Farnham P. J. A protein synthesis-dependent increase in E2F1 mRNA correlates with growth regulation of the dihydrofolate reductase promoter. Mol Cell Biol. 1993 Mar;13(3):1610–1618. doi: 10.1128/mcb.13.3.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srimatkandada S., Medina W. D., Cashmore A. R., Whyte W., Engel D., Moroson B. A., Franco C. T., Dube S. K., Bertino J. R. Amplification and organization of dihydrofolate reductase genes in a human leukemic cell line, K-562, resistant to methotrexate. Biochemistry. 1983 Dec 6;22(25):5774–5781. doi: 10.1021/bi00294a015. [DOI] [PubMed] [Google Scholar]
- Stamatatos L., Leventis R., Zuckermann M. J., Silvius J. R. Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry. 1988 May 31;27(11):3917–3925. doi: 10.1021/bi00411a005. [DOI] [PubMed] [Google Scholar]
- Takahashi R., Hashimoto T., Xu H. J., Hu S. X., Matsui T., Miki T., Bigo-Marshall H., Aaronson S. A., Benedict W. F. The retinoblastoma gene functions as a growth and tumor suppressor in human bladder carcinoma cells. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5257–5261. doi: 10.1073/pnas.88.12.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton D. J., Park S. H., Lanier L., Weinberg R. A. Nonfunctional mutants of the retinoblastoma protein are characterized by defects in phosphorylation, viral oncoprotein association, and nuclear tethering. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3033–3037. doi: 10.1073/pnas.88.8.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. J., Kokontis J. M., Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 1994 Dec 1;8(23):2817–2830. doi: 10.1101/gad.8.23.2817. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Knudsen E. S., Welch P. J. The retinoblastoma tumor suppressor protein. Adv Cancer Res. 1994;64:25–85. doi: 10.1016/s0065-230x(08)60834-9. [DOI] [PubMed] [Google Scholar]
- Wang N. P., To H., Lee W. H., Lee E. Y. Tumor suppressor activity of RB and p53 genes in human breast carcinoma cells. Oncogene. 1993 Feb;8(2):279–288. [PubMed] [Google Scholar]
- Wu X., Levine A. J. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A. S., Reichel R., Kovesdi I., Nevins J. R. Promoter interaction of the E1A-inducible factor E2F and its potential role in the formation of a multi-component complex. EMBO J. 1987 Jul;6(7):2061–2068. doi: 10.1002/j.1460-2075.1987.tb02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]