Abstract
Background:
Morbidity due to cardiovascular disease is high among First Nations people. The extent to which this may be related to the likelihood of coronary angiography is unclear. We examined the likelihood of coronary angiography after acute myocardial infarction (MI) among First Nations and non–First Nations patients.
Methods:
Our study included adults with incident acute MI between 1997 and 2008 in Alberta. We determined the likelihood of angiography among First Nations and non–First Nations patients, adjusted for important confounders, using the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) database.
Results:
Of the 46 764 people with acute MI, 1043 (2.2%) were First Nations. First Nations patients were less likely to receive angiography within 1 day after acute MI (adjusted odds ratio [OR] 0.73, 95% confidence interval [CI] 0.62–0.87). Among First Nations and non–First Nations patients who underwent angiography (64.9%), there was no difference in the likelihood of percutaneous coronary intervention (PCI) (adjusted hazard ratio [HR] 0.92, 95% CI 0.83–1.02) or coronary artery bypass grafting (CABG) (adjusted HR 1.03, 95% CI 0.85–1.25). First Nations people had worse survival if they received medical management alone (adjusted HR 1.38, 95% CI 1.07–1.77) or if they underwent PCI (adjusted HR 1.38, 95% CI 1.06–1.80), whereas survival was similar among First Nations and non–First Nations patients who received CABG.
Interpretation:
First Nations people were less likely to undergo angiography after acute MI and experienced worse long-term survival compared with non–First Nations people. Efforts to improve access to angiography for First Nations people may improve outcomes.
Although cardiovascular disease has been decreasing in Canada,1 First Nations people have a disproportionate burden of the disease. First Nations people in Canada have a 2.5-fold higher prevalence of cardiovascular disease than non–First Nations people,2 with hospital admissions for cardiovascular-related events also increasing.3
The prevalence of cardiovascular disease in First Nations populations is presumed to be reflective of the prevalence of cardiovascular risk factors.4–7 However, the disproportionate increase in rates of hospital admission suggests that suboptimal management of cardiovascular disease or its risk factors may also influence patient outcomes.2,3 Racial disparities in the quality of cardiovascular care resulting in adverse outcomes have been documented, although most studies have focused on African-American, Hispanic and Asian populations.8,9 As a result, it is unclear whether suboptimal delivery of guideline-recommended treatment contributes to increased cardiovascular morbidity and mortality among First Nations people.10–12
We undertook a population-based study involving adults with incident acute myocardial infarction (MI) to examine the receipt of guideline-recommended coronary angiography among First Nations and non–First Nations patients.10–12 Among patients who underwent angiography, we sought to determine whether there were differences between First Nations and non–First Nations patients in the likelihood of revascularization and long-term survival.
Methods
Study population
We did a population-based cohort study involving people 18 years of age and older using databases from Alberta Health and from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH).13–15 The APPROACH database is a prospective data collection initiative that collects detailed clinical information on all patients undergoing coronary angiography in Alberta since 1995, including information on coronary anatomy, left ventricular ejection fraction and smoking status.15 Alberta has a population of about 3.6 million, and there are 3 cardiac catheterization laboratories in Alberta: 2 located in Edmonton and 1 located in Calgary.
Patients admitted to hospital with incident acute MI (International Classification of Diseases, 9th revision, code 410; International Statistical Classification of Diseases and Related Health Problems, 10th revision, code I21) from Apr. 1, 1997, until Mar. 31, 2008, were identified from the Alberta Health database of hospital admissions. To identify patients, we used the most responsible diagnosis or complication that occurred after admission.16 This algorithm has a sensitivity of 88.8%, specificity of 92.8% and positive predictive value of 88.5% for identifying acute MI.16 We excluded patients who had an acute MI during the 3-year washout period (Apr. 1, 1994, to Mar. 31, 1997) to ensure that only incident cases of acute MI were included. The date of hospital admission for incident acute MI served as the index date for all analyses. For patients with multiple acute MIs during the study, we evaluated care for only the first acute MI. Patients were linked between the databases using their personal health number.13–15
First Nations status and covariates
We used the Alberta Health Population Registry to identify patients with First Nations status, defined as any individual registered under the Indian Act. Health care for First Nations people is administered through the First Nations and Inuit Health Branch of Health Canada.17 Based on the 2006 Canadian census, about 81% of the self-identified First Nations population is registered under the Indian Act.18 However, people with First Nations status represent about 53% of the Aboriginal population.17 We searched the registry for patients from Apr. 1, 1993, to Mar. 31, 2008, who had an indicator of First Nations status at any time. We classified this group as “First Nations” and the remainder as “non–First Nations” (reference cohort). Aboriginal people in Alberta not registered under the Indian Act were included in the non–First Nations comparison group.18
We used the 2006 Canadian census and the postal code of each participant to determine median household income.19 We used ArcGIS software (version 9.1, Esri) to determine the shortest distance by road (in kilometres) between each patient’s residence and the closest cardiac catheterization laboratory.20 This distance was categorized, as in prior work, into the following categories: 0–50 km, 50.1–150 km, 150.1–300 km and greater than 300 km.21 We used validated algorithms to define diabetes and hypertension.22,23 Comorbidities, excluding diabetes and acute MI, were based on the Deyo classification of the Charlson comorbidity index validated by Quan and colleagues.24 We included the year of acute MI diagnosis to account for changing treatment recommendations over time.
Outcomes
Primary outcomes were receipt of coronary angiography within 1 day, 30 days and 1 year after admission for acute MI. Secondary outcomes included receipt of coronary revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass grafting [CABG]) up to 1 year after angiography, and long-term survival.
Given that coronary angiography and revascularization are recommended for those with ST elevation MI (including those with failed thrombolytic therapy),10–12 and for those with non–ST elevation MI in certain circumstances,10–12,25–27 we conducted a sensitivity analysis among the subgroup with ST elevation MI who underwent coronary angiography.
We identified all-cause mortality from the Statistics Canada Vital Statistics database of Alberta Health.28
Statistical analysis
We compared baseline characteristics by First Nations status. We used logistic regression models to compare the likelihood of coronary angiography within 1 day, and Cox proportional hazards models to compare the likelihood of angiography within 30 days and 1 year of acute MI among First Nations and non–First Nations patients, adjusting for all covariates. We tested for potential interaction between First Nations status and both quintiles of median household income and distance to the nearest cardiac catheterization laboratory in each model.
Among the subset of patients who underwent coronary angiography, we evaluated likelihood of revascularization within 1 year and long-term survival (follow-up to Mar. 31, 2009) using Cox proportional hazards models, adjusting for covariates, as well as smoking status, coronary anatomy and ejection fraction (as a measure of severity of coronary disease). In these models, we calculated time to outcome or censoring from the date of angiography to the date on which the patients were censored (i.e., Mar. 31, 2009, or emigration from the province) or the date when the outcome occurred, ensuring there were no losses to follow-up. A Cox proportional hazards model was fitted to determine if survival after angiography varied by treatment received, followed by analyses stratified by treatment strategy. Finally, we assessed survival for all patients after acute MI by plotting the risk-adjusted survival curves from the proportional hazards model using the corrected group prognosis method.29 A survival curve for each of the risk factors was calculated, after which an average survival curve was calculated as a weighted average of the individual survival curves. We then used the resulting risk-adjusted survival curves to compare the survival of First Nations and non–First Nations patients while adjusting for the differences in risk factors, irrespective of the size of the differences.
The proportional hazards assumption was satisfied for all multivariable models. We also modelled the cumulative incidence of coronary angiography and revascularization within 1 year of acute MI, treating death as a competing risk, and using the competing proportional subdistribution hazard approach.30 Also, because sociodemographic factors, including income and distance of a person’s residence to the closest cardiac catheterization laboratory, may be considered a component of ethnic background and result in overadjustment, we did not adjust for these variables initially, but repeated all analyses, adjusting for income and distance to the closest cardiac catheterization laboratory from each patient’s residence in the full models.31 Patients with missing income data were included in the analysis as a “missing” category, whereas those with missing distance to the closet cardiac catheterization laboratory (< 0.2%) were excluded from the analysis. We did the analyses using SAS version 9.3 (SAS Institute Inc.) and Stata 11.1 MP (StataCorp LP).
Ethics approval
The Conjoint Health Research Ethics Board at the University of Calgary gave ethics approval.
Results
Of the 46 764 patients who experienced an incident acute MI during the study period, 1043 (2.2%) were First Nations. Compared with the non–First Nations group, First Nations patients were younger (mean age 59.2 v. 67.9 yr), more likely to have diabetes (54.2% v. 33.9%) and less likely to live within 50 km of the closest cardiac catheterization laboratory (34.3% v. 58.6%) (Table 1). The median follow-up was 3.7 (interquartile range [IQR] 1.4–6.8) years.
Table 1:
Characteristics of patients with acute myocardial infarction, by First Nations status
| Characteristic | No. (%) of patients* | p value | |
|---|---|---|---|
| First Nations, n = 1043 | Non–First Nations, n = 45 721 | ||
| Age, yr, mean ± SD | 59.2 ± 12.7 | 67.9 ± 14.1 | < 0.001 |
| Sex, female | 351 (33.7) | 15 558 (34.0) | 0.8 |
| Distance to closest cardiac catheterization laboratory, km | < 0.001 | ||
| Missing | 2 (0.2) | 98 (0.2) | |
| ≤ 50 | 358 (34.3) | 26 802 (58.6) | |
| 50.1–150 | 248 (23.8) | 7 177 (15.7) | |
| 150.1–300 | 303 (29.1) | 9 192 (20.1) | |
| > 300 | 132 (12.7) | 2 452 (5.4) | |
| Income quintile | < 0.001 | ||
| Missing | 142 (13.6) | 2 130 (4.7) | |
| 1st (lowest) | 456 (43.7) | 9 353 (20.5) | |
| 2nd | 143 (13.7) | 9 404 (20.6) | |
| 3rd | 112 (10.7) | 8 942 (19.6) | |
| 4th | 90 (8.6) | 8 235 (18.0) | |
| 5th (highest) | 100 (9.6) | 7 657 (16.7) | |
| Diabetes | 565 (54.2) | 15 487 (33.9) | < 0.001 |
| Hypertension | 844 (80.9) | 38 084 (83.3) | 0.047 |
| Year of acute myocardial infarction diagnosis | 0.1 | ||
| 1997 | 61 (5.8) | 2 741 (6.0) | |
| 1998 | 83 (8.0) | 3 673 (8.0) | |
| 1999 | 80 (7.7) | 3 793 (8.3) | |
| 2000 | 72 (6.9) | 3 785 (8.3) | |
| 2001 | 80 (7.7) | 4 040 (8.8) | |
| 2002 | 93 (8.9) | 4 402 (9.6) | |
| 2003 | 95 (9.1) | 4 506 (9.9) | |
| 2004 | 97 (9.3) | 4 378 (9.6) | |
| 2005 | 127 (12.2) | 4 431 (9.7) | |
| 2006 | 116 (11.1) | 4 360 (9.5) | |
| 2007 | 112 (10.7) | 4 437 (9.7) | |
| 2008† | 27 (2.6) | 1 175 (2.6) | |
| Comorbidities | |||
| Cancer | 58 (5.6) | 5 075 (11.1) | < 0.001 |
| Cerebrovascular disease | 93 (8.9) | 6 002 (13.1) | < 0.001 |
| Heart failure | 299 (28.7) | 13 887 (30.4) | 0.2 |
| COPD | 423 (40.6) | 12 925 (28.3) | < 0.001 |
| Dementia | 21 (2.0) | 2 853 (6.2) | < 0.001 |
| HIV/AIDS | 2 (0.2) | 33 (0.1) | 0.2 |
| Metastatic cancer | 7 (0.7) | 978 (2.1) | 0.002 |
| Mild liver disease | 42 (4.0) | 728 (1.6) | < 0.001 |
| Moderate/severe liver disease | 14 (1.3) | 223 (0.5) | < 0.001 |
| Paraplegia/hemiplegia | 21 (2.0) | 914 (2.0) | 0.9 |
| Peptic ulcer disease | 122 (11.7) | 2 782 (6.1) | < 0.001 |
| Peripheral vascular disease | 106 (10.2) | 5 825 (12.7) | 0.02 |
| Renal disease | 122 (11.7) | 4 752 (10.4) | 0.2 |
| Rheumatologic disease | 58 (5.6) | 1 621 (3.5) | 0.001 |
COPD = chronic obstructive pulmonary disease, SD = standard deviation.
Unless stated otherwise.
Data ended Mar. 31, 2008.
Coronary angiography after acute MI
Overall, 30 358 (64.9%) patients underwent coronary angiography after acute MI. Of these, 186 (26.3%) First Nations patients and 10 279 (34.7%) non–First Nations patients underwent coronary angiography within 1 day after their acute MI (unadjusted odds ratio [OR] 0.75, 95% CI 0.64–0.88) (Table 2). After adjustment for all covariates, First Nations patients were still less likely to receive coronary angiography within 1 day compared with non–First Nations patients (OR 0.73, 95% CI 0.62–0.87). First Nations patients were also less likely to receive angiography within 30 days (HR 0.82, 95% CI 0.76–0.90) and 1 year (HR 0.83, 95% CI 0.77–0.90), after adjustment for all covariates (Table 3). We did not find a significant interaction between First Nations status and income or distance to the closest cardiac catheterization laboratory.
Table 2:
Full model with unadjusted and adjusted estimates for odds of angiography within 1 day after acute myocardial infarction
| Covariate | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| First Nations status | ||
| Non–First Nations | 1.00 (ref) | 1.00 (ref) |
| First Nations | 0.75 (0.64–0.88) | 0.73 (0.62–0.87) |
| Age, yr | ||
| 18–45 | 1.00 (ref) | 1.00 (ref) |
| 46–65 | 0.79 (0.73–0.86) | 0.81 (0.74–0.88) |
| 66–75 | 0.47 (0.43–0.51) | 0.59 (0.54–0.66) |
| 76–85 | 0.26 (0.24–0.28) | 0.36 (0.32–0.40) |
| ≥ 86 | 0.07 (0.06–0.09) | 0.10 (0.09–0.12) |
| Sex | ||
| Male | 1.00 (ref) | 1.00 (ref) |
| Female | 0.61 (0.58–0.64) | 0.84 (0.80–0.89) |
| Distance from residence to the nearest cardiac catheterization laboratory, km | ||
| ≤ 50 | 1.00 (ref) | 1.00 (ref) |
| 50.1–150 | 0.49 (0.46–0.52) | 0.48 (0.45–0.51) |
| 150.1–300 | 0.32 (0.30–0.34) | 0.30 (0.28–0.33) |
| > 300 | 0.33 (0.29–0.37) | 0.27 (0.24–0.31) |
| Income quintile | ||
| 1st (lowest) | 1.00 (ref) | 1.00 (ref) |
| 2nd | 1.07 (1.00–1.15) | 1.07 (0.99–1.15) |
| 3rd | 1.05 (0.98–1.12) | 1.04 (0.96–1.12) |
| 4th | 1.03 (0.96–1.11) | 0.99 (0.92–1.07) |
| 5th (highest) | 1.13 (1.05–1.21) | 1.10 (1.02–1.19) |
| Missing | 0.77 (0.68–0.86) | 1.07 (0.93–1.22) |
| Diabetes | 0.74 (0.71–0.78) | 0.86 (0.81–0.90) |
| Hypertension | 0.73 (0.69–0.77) | 0.94 (0.88–1.00) |
| Year of acute MI diagnosis | ||
| 1997 | 1.00 (ref) | 1.00 (ref) |
| 1998 | 1.48 (1.26–1.75) | 1.50 (1.26–1.78) |
| 1999 | 1.88 (1.60–2.21) | 1.99 (1.68–2.35) |
| 2000 | 2.13 (1.81–2.49) | 2.25 (1.91–2.65) |
| 2001 | 2.63 (2.26–3.07) | 2.88 (2.45–3.38) |
| 2002 | 3.53 (3.04–4.11) | 4.00 (3.42–4.68) |
| 2003 | 3.47 (2.99–4.03) | 4.00 (3.42–4.68) |
| 2004 | 3.80 (3.27–4.41) | 4.44 (3.80–5.20) |
| 2005 | 4.40 (3.79–5.10) | 5.43 (4.65–6.34) |
| 2006 | 4.19 (3.61–4.86) | 5.09 (4.36–5.94) |
| 2007 | 5.42 (4.68–6.28) | 6.41 (5.49–7.48) |
| 2008 | 5.49 (4.59–6.58) | 6.67 (5.51–8.09) |
| Cancer | 0.56 (0.52–0.61) | 0.85 (0.77–0.93) |
| Cerebrovascular disease | 0.40 (0.37–0.43) | 0.74 (0.67–0.81) |
| Heart failure | 0.44 (0.41–0.46) | 0.93 (0.87–0.99) |
| COPD | 0.54 (0.51–0.57) | 0.76 (0.72–0.81) |
| Dementia | 0.18 (0.15–0.21) | 0.39 (0.33–0.46) |
| HIV/AIDS | 1.39 (0.67–2.89) | 0.78 (0.34–1.76) |
| Metastatic cancer | 0.26 (0.21–0.34) | 0.38 (0.29–0.50) |
| Mild liver disease | 0.68 (0.56–0.83) | 0.77 (0.63–0.95) |
| Moderate/severe liver disease | 0.32 (0.20–0.50) | 0.36 (0.22–0.58) |
| Paraplegia/hemiplegia | 0.36 (0.29–0.45) | 0.64 (0.50–0.82) |
| Peptic ulcer disease | 0.61 (0.55–0.67) | 0.87 (0.78–0.97) |
| Peripheral vascular disease | 0.46 (0.43–0.50) | 0.79 (0.72–0.86) |
| Renal disease | 0.33 (0.30–0.36) | 0.49 (0.43–0.54) |
| Rheumatologic disease | 0.68 (0.59–0.77) | 0.96 (0.84–1.11) |
Note: CI = confidence interval, COPD = chronic obstructive pulmonary disease, MI = myocardial infarction, OR = odds ratio, ref = reference group.
Adjusted based on all variables in the table.
Table 3:
Unadjusted and adjusted likelihood of coronary angiography (n = 46 764)*
| Time since acute MI | Unadjusted OR/HR (95% CI) | Adjusted OR/HR (95% CI) | |
|---|---|---|---|
| Partial adjustment† | Full adjustment‡ | ||
| 1 d | OR: 0.75 (0.64–0.88) | OR: 0.57 (0.48–0.67) | OR: 0.73 (0.62–0.87) |
| 30 d | HR: 0.96 (0.88–1.04) | HR: 0.72 (0.66–0.78) | HR: 0.82 (0.76–0.90) |
| 1 yr | HR: 0.99 (0.92–1.07) | HR: 0.74 (0.68–0.80) | HR: 0.83 (0.77–0.90) |
Note: CI = confidence interval, HR = hazard ratio, OR = odds ratio, MI = myocardial infarction.
Reference category is non–First Nations status.
Adjusted for age, sex, diabetes, hypertension, year of acute MI diagnosis and comorbidities listed in Table 1.
Adjusted for age, sex, diabetes, hypertension, year of acute MI diagnosis, comorbidities listed in Table 1, distance to closest cardiac catheterization laboratory and income quintiles.
Among the subset of patients with ST elevation MI who underwent coronary angiography, we found no difference in timing of coronary angiography after adjustment for differences in all covariates (1 d: OR 0.88, 95% CI 0.62–1.18; 30 d: HR 0.98, 95% CI 0.86–1.11; 1 yr: HR 0.97, 95% CI 0.85–1.11).
Treatment and survival after coronary angiography
First Nations patients were more likely to be current smokers across all treatment modalities (Table 4). Among those who underwent CABG, coronary anatomy and ejection fraction were similar for First Nations and non–First Nations patients. However, First Nations patients who received PCI or medical management had low-risk to normal coronary anatomy compared with non–First Nations patients.
Table 4:
Coronary anatomy, ejection fraction and smoking status, by treatment after angiography and First Nations status
| Variable | CABG | PCI | Medical management | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Non–First Nations, % (n = 4 179) | First Nations, % (n = 108) | p value | Non–First Nations, % (n = 17 503) | First Nations, % (n = 381) | p value | Non–First Nations, % (n = 7 970) | First Nations, % (n = 217) | p value | |
| Coronary anatomy | 0.7 | 0.02 | 0.002 | ||||||
|
|
|
|
|||||||
| Normal | 0.2 | 0.0 | 0.3 | 1.3 | 7.8 | 14.7 | |||
|
|
|
|
|||||||
| Minimal disease* | 0.3 | 0.0 | 0.8 | 0.5 | 13.1 | 14.3 | |||
|
|
|
|
|||||||
| Low risk | 10.7 | 14.8 | 64.0 | 64.3 | 40.5 | 42.9 | |||
|
|
|
|
|||||||
| High risk | 58.8 | 60.2 | 31.9 | 29.4 | 29.6 | 19.8 | |||
|
|
|
|
|||||||
| Left main | 29.4 | 25.0 | 2.2 | 3.1 | 7.9 | 7.4 | |||
|
|
|
|
|||||||
| Missing | 0.6 | 0.0 | 0.6 | 1.3 | 1.2 | 0.9 | |||
|
| |||||||||
| Ejection fraction, % | 0.4 | 0.001 | 0.07 | ||||||
|
|
|
|
|||||||
| > 50 | 45.7 | 45.4 | 53.1 | 46.7 | 52.9 | 47.0 | |||
|
|
|
|
|||||||
| 35–50 | 32.6 | 35.2 | 27.5 | 26.5 | 24.8 | 22.6 | |||
|
|
|
|
|||||||
| < 35 | 9.5 | 12.0 | 4.8 | 8.7 | 9.2 | 12.0 | |||
|
|
|
|
|||||||
| Ventriculogram not done | 8.2 | 3.7 | 9.3 | 12.1 | 7.9 | 10.1 | |||
|
|
|
|
|||||||
| Missing | 4.0 | 3.7 | 5.4 | 6.0 | 5.2 | 8.3 | |||
|
| |||||||||
| Smoking status | < 0.001 | < 0.001 | < 0.001 | ||||||
|
|
|
|
|||||||
| No smoking | 34.5 | 17.6 | 34.9 | 17.8 | 37.9 | 22.1 | |||
|
|
|
|
|||||||
| Prior smoking | 34.0 | 32.4 | 27.0 | 19.2 | 31.2 | 22.1 | |||
|
|
|
|
|||||||
| Present smoking | 31.5 | 50.0 | 38.1 | 63.0 | 31.0 | 55.8 | |||
Note: CABG = coronary artery bypass grafting, PCI = percutaneous coronary intervention.
Defined as a lesion with less than 50% stenosis.
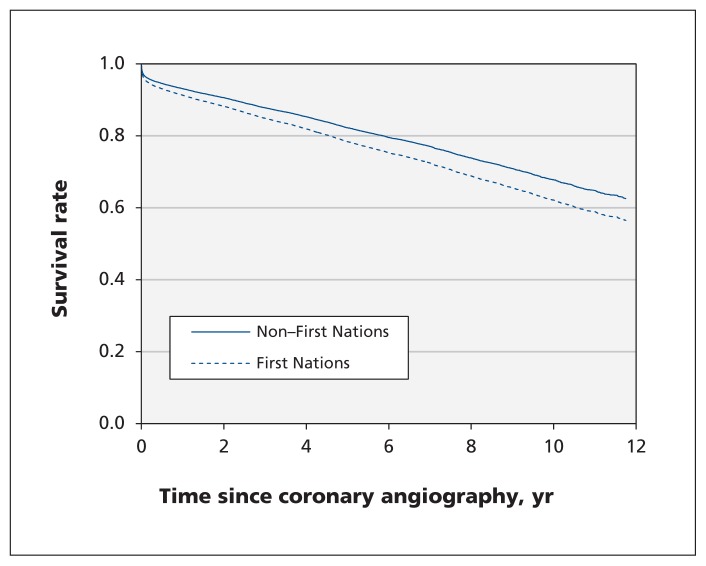
We found no statistically significant difference for time to PCI (median 0.5 d for both groups, p = 0.5) or CABG (median 13 d among First Nations patients v. 11 d among non–First Nations patients, p = 0.7). After adjustment, First Nations patients were as likely to receive PCI (HR 0.92, 95% CI 0.83–1.02) and CABG (HR 1.03, 95% CI 0.85–1.25) as non–First Nations patients (Table 5). However, First Nations patients had increased long-term mortality (HR 1.30, 95% CI 1.09–1.55) (Figure 1). Mortality was higher among First Nations than non–First Nations patients who received medical management (HR 1.38, 95% CI 1.07–1.77) or PCI (HR 1.38, 95% CI 1.06–1.80), with similar survival for those who underwent CABG (HR 0.91, 95% CI 0.56–1.47) (Table 6).
Table 5:
Hazard ratios for likelihood of percutaneous coronary intervention and coronary artery bypass grafting after coronary angiography (n = 30 358)*
| Treatment | No. (%) of patients | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||
|---|---|---|---|---|---|
| Non–First Nations (n = 29 652) | First Nations (n = 706) | Partial adjustment† | Full adjustment‡ | ||
| PCI | 17 503 (59.0) | 381 (54.0) | 0.89 (0.80–0.98) | 0.89 (0.80–0.99) | 0.92 (0.83–1.02) |
| CABG | 4 179 (14.1) | 108 (15.3) | 0.96 (0.79–1.16) | 1.04 (0.86–1.25) | 1.03 (0.85–1.25) |
Note: CABG = coronary artery bypass grafting, CI = confidence interval, HR = hazard ratio, MI = myocardial infarction, PCI = percutaneous coronary intervention.
Reference category is non–First Nations status.
Adjusted for age, sex, diabetes, hypertension, year of acute MI diagnosis, comorbidities listed in Table 1, ejection fraction, smoking status and coronary anatomy.
Adjusted for age, sex, diabetes, hypertension, year of acute MI diagnosis, comorbidities listed in Table 1, ejection fraction, smoking status, coronary anatomy, income quintiles and distance to closest cardiac catheterization laboratory.
Figure 1:
Survival rate after coronary angiography among First Nations and non–First Nations patients, adjusted for treatment type (percutaneous coronary intervention, coronary artery bypass grafting, no revascularization), age, sex, diabetes, hypertension, comorbidities, year of acute myocardial infarction diagnosis, quintile of median household income, driving distance to the closest cardiac catheterization laboratory, ejection fraction, smoking status and coronary anatomy.
Table 6:
Hazard ratios for all-cause mortality after coronary angiography (n = 30 358), by treatment subgroup*
| Treatment | No. (%) of patients | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||
|---|---|---|---|---|---|
| Non–First Nations (n = 29 652) | First Nations (n = 706) | Partial adjustment† | Full adjustment‡ | ||
| Medical management | 2 365 (8.0) | 61 (8.6) | 0.99 (0.76–1.28) | 1.40 (1.09–1.80) | 1.38 (1.07–1.77) |
| PCI | 2 672 (9.0) | 69 (9.8) | 1.24 (0.98–1.57) | 1.38 (1.06–1.80) | 1.38 (1.06–1.80) |
| CABG | 997 (3.4) | 17 (2.4) | 0.67 (0.42–1.07) | 0.89 (0.55–1.44) | 0.91 (0.56–1.47) |
Note: CABG = coronary artery bypass grafting, CI = confidence interval, HR = hazard ratio, MI = myocardial infarction, PCI = percutaneous coronary intervention.
Reference category is non–First Nations.
Adjusted for age, sex, diabetes, hypertension, year of acute MI diagnosis, comorbidities listed in Table 1, ejection fraction, smoking status and coronary anatomy.
Adjusted for age, sex, diabetes, hypertension, year of acute MI diagnosis, comorbidities listed in Table 1, ejection fraction, smoking status, coronary anatomy, income quintiles and distance to closest cardiac catheterization laboratory.
In analyses that accounted for the competing risk of death, similar patterns in the cumulative incidence of coronary angiography and revascularization at 1 year were evident (data available on request).
Interpretation
In this large population-based study, we evaluated receipt of guideline-recommended angiography for acute MI among First Nations people. We found that First Nations patients were less likely to undergo coronary angiography after acute MI than non–First Nations patients, even after adjusting for differences in important sociodemographic variables. Further, First Nations patients had an increased risk of mortality after acute MI compared with non–First Nations patients. Given that early invasive interventions improve outcomes in ST elevation MI, and in some cases in non–ST elevation MI, efforts to improve access to angiography for First Nations patients may improve their long-term outcomes.10–12,25–27 Our results provide insight into the treatment and outcomes of coronary disease in this high-risk population.
Prior studies have documented that First Nations people have less access to specialists than non–First Nations people for conditions treated medically and those requiring an invasive procedure.31–34 Shah and colleagues33 reported that relative utilization rates for referral care–sensitive procedures (i.e., procedures for which a referral from primary care is necessary to access the specialists or centres that provide the procedure) were significantly lower in the Aboriginal than non-Aboriginal population in Ontario. Similarly, we found that First Nations patients were less likely than non–First Nations patients to undergo angiography, but were as likely to receive revascularization procedures once they had undergone angiography. This suggests that decisions for further management after angiography are applied similarly for First Nations and non–First Nations patients but that differences exist in decisions about angiography. This may be related to factors pertaining to the patient, provider or health care system. We also found that, despite similar revascularization rates, First Nations patients experienced worse survival after undergoing PCI or receiving medical management. The extent to which treatment gaps may be contributing to health inequities for various ethnic groups has been described.35 Potential explanations for the treatment gaps are varied, ranging from cultural barriers for medication use to cost of therapies, urban versus rural location of residence and health care delivery that is not culturally sensitive.36 Although we were able to adjust for distance to the closest cardiac catheterization laboratory in our analysis, differences in processes of care and follow-up for First Nations people, such as access to cardiac rehabilitation programs, may be contributing to their increased likelihood of mortality after acute MI.
Strengths and limitations
The strengths of our study include its population-based design spanning more than a decade, and detailed clinical information, which permitted us to adjust for important prognostic factors.
Our study also has important limitations. It is likely that some First Nations patients were identified as non–First Nations if they were not registered under the Indian Act. Therefore, our results may not be generalizable to the overall Aboriginal population, or to First Nations people who are not registered under the Indian Act.17 However, according to the 2006 Canadian census, 81% of the self-identified First Nations population are registered under the Indian Act.18
Our data set does not contain complete information on receipt of medications for acute MI, including long-term therapies such as angiotensin receptor blockers or angiotensin-converting enzyme inhibitors, β-blockers, antiplatelet agents and statins, or acute therapies such as thrombolytics.37 As a result, we were unable to assess whether there was a difference in receipt of medications between First Nations and non–First Nations patients.
We were unable to differentiate between ST elevation MI and non–ST elevation MI in our full study cohort (i.e., those identified as having acute MI based on International Classification of Diseases codes). Recognizing that treatment can differ for these 2 events,10–12,25–27 particularly timing of cardiac catheterization, we evaluated likelihood of receipt of angiography at different time points (1 d, 30 d and 1 yr) in both our full cohort and in our subgroup of patients identified with ST elevation MI who underwent angiography (i.e., those identified as having ST elevation MI using APPROACH data).
We were unable to account for patient or provider preferences, or other factors that may explain differences in receipt of angiography. We do not believe this negates our study results, given that the primary aim of our study was to evaluate likelihood of angiography following acute MI.
Conclusion
We found that First Nations patients were less likely than non–First Nations patients to receive coronary angiography after hospital admission for acute MI, but were equally likely to receive revascularization after angiography. Survival, however, was worse among First Nations patients after angiography. Given the benefit of invasive intervention in people with acute MI, efforts to improve access to angiography after acute MI among First Nations patients along with the use of medical therapy that has been proven effective may improve outcomes.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the design of the study. Jianguo Zhang completed the study analysis. Lauren Bresee and Brenda Hemmelgarn drafted the manuscript, which all of the other authors revised. All of the authors approved the final version submitted for publication.
Brenda Hemmelgarn had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by a Canadian Institutes of Health Research (CIHR) operating grant and by an interdisciplinary team grant from Alberta Innovates Health Solutions (AIHS). The Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) initiative has received contributions from Alberta Health, Merck Frosst Canada Ltd., Monsanto Canada Inc. — Searle division, Eli Lilly Canada Inc., Guidant Corporation, Boston Scientific, Hoffman–LaRoche Ltd., and Johnson & Johnson InCordis. These unrestricted grants provide the project with “general use” funds that support the basic infrastructure of this cardiac registry.
The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. This study is based in part on data provided by Alberta Health. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta.
At the time of this work, Lauren Bresee was supported by the 4th ICPC/HSFC/CCS (4th International Conference On Preventive Cardiology/Heart and Stroke Foundation of Canada/Canadian Cardiovascular Society) Fellowship in Preventive Cardiology and a fellowship award from AIHS. Brenda Hemmelgarn, Sofia Ahmed, William Ghali, Merril Knudtson, Braden Manns and Marcello Tonelli are supported by a joint initiative between Alberta Health and the Universities of Alberta and Calgary. Brenda Hemmelgarn is supported by the Roy and Vi Baay Chair in Kidney Research. Sofia Ahmed is supported by AIHS and CIHR. Hude Quan is supported by AIHS.
References
- 1.Lee DS, Chiu M, Manuel DG, et al. ; Canadian Cardiovascular Outcomes Research Team. Trends in risk factors for cardiovascular disease in Canada: temporal, socio-demographic and geographic factors. CMAJ 2009;181:E55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand SS, Yusuf S, Jacobs R, et al. Risk factors, atherosclerosis, and cardiovascular disease among Aboriginal people in Canada: the Study of Health Assessment and Risk Evaluation in Aboriginal Peoples (SHARE-AP). Lancet 2001;358:1147–53 [DOI] [PubMed] [Google Scholar]
- 3.Shah BR, Hux JE, Zinman B. Increasing rates of ischemic heart disease in the native population of Ontario, Canada. Arch Intern Med 2000;160:1862–6 [DOI] [PubMed] [Google Scholar]
- 4.Sarkar J, Lix LM, Bruce S, et al. Ethnic and regional differences in prevalence and correlates of chronic diseases and risk factors in northern Canada. Prev Chronic Dis 2010;7:A13. [PMC free article] [PubMed] [Google Scholar]
- 5.Welty TK, Rhoades DA, Yeh F, et al. Changes in cardiovascular disease risk factors among American Indians. The Strong Heart Study. Ann Epidemiol 2002;12:97–106 [DOI] [PubMed] [Google Scholar]
- 6.Healthy children, healthy families, healthy communities: the road to wellness. BC First Nations Regional Longitudinal Health Survey 2002/2003. Ottawa (ON): First Nations Centre; 2005. Available: www.fnhc.ca/pdf/RHS_2002_2003_Regional_Report.pdf (accessed 2013 Oct. 16). [Google Scholar]
- 7.Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases: Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 2001;104:2855–64 [DOI] [PubMed] [Google Scholar]
- 8.Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med 2001;135:352–66 [DOI] [PubMed] [Google Scholar]
- 9.Khan NA, Grubisic M, Hemmelgarn B, et al. Outcomes after acute myocardial infarction in South Asian, Chinese, and white patients. Circulation 2010;122:1570–7 [DOI] [PubMed] [Google Scholar]
- 10.Tu JV, Khalid L, Donovan LR, et al. Indicators of quality of care for patients with acute myocardial infarction. CMAJ 2008;179: 909–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krumholz HM, Anderson JL, Bachelder BL, et al. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to develop performance measures for ST-elevation and non-ST-elevation myocardial infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation 2008;118:2596–648 [DOI] [PubMed] [Google Scholar]
- 12.Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2009;53:530–53 [DOI] [PubMed] [Google Scholar]
- 13.Interdisciplinary Chronic Disease Collaboration. The research to health policy cycle: a tool for better management of chronic noncommunicable diseases. J Nephrol 2008;21:621–31 [PubMed] [Google Scholar]
- 14.Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol 2009;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghali WA, Knudtson ML. Overview of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease. On behalf of the APPROACH investigators. Can J Cardiol 2000;16:1225–30 [PubMed] [Google Scholar]
- 16.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J 2002;144:290–6 [DOI] [PubMed] [Google Scholar]
- 17.Health Canada. First Nations and Inuit Health Branch. Ottawa (ON): Health Canada; 2013. Available: www.hc-sc.gc.ca/ahc-asc/branch-dirgen/fnihb-dgspni/index-eng.php (accessed 2013 Oct. 16). [Google Scholar]
- 18.Aboriginal peoples and communities. Ottawa (ON): Aboriginal Affairs and Northern Development Canada; 2013. Available: www.aadnc-aandc.gc.ca/eng/1100100013785/1304467449155 (accessed 2013 Oct. 16). [Google Scholar]
- 19.Population and dwelling counts, for Canada, provinces and territories, 2006 and 2001 censuses — 100% data. Ottawa (ON): Statistics Canada; 2010. Available: www12.statcan.ca/census-recensement/2006/dp-pd/hlt/97-550/Index.cfm?TPL=P1C&Page=RETR&LANG=Eng&T=101 (accessed 2013 Oct. 16). [Google Scholar]
- 20.Brabyn L, Gower P. Comparing three GIS techniques for modeling geographical access to general practitioners. Cartographica 2004; 39:41–9 [Google Scholar]
- 21.Tonelli M, Manns B, Culleton B, et al. ; Alberta Kidney Disease Network. Association between proximity to the attending nephrologist an mortality among patients receiving hemodialysis. CMAJ 2007;177:1039–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–6 [DOI] [PubMed] [Google Scholar]
- 23.Tu K, Campbell NR, Chen ZL, et al. Accuracy of administrative databases in identifying patients with hypertension. Open Med 2007;1:e18–26 [PMC free article] [PubMed] [Google Scholar]
- 24.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9 [DOI] [PubMed] [Google Scholar]
- 25.Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011;57:e215–367 [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Weintraub WS, Demopoulos LA, et al. TACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)–Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001;344:1879–87 [DOI] [PubMed] [Google Scholar]
- 27.Damman P, Hirsch A, Windhausen F, et al. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes Trial). J Am Coll Cardiol 2010;55:858–64 [DOI] [PubMed] [Google Scholar]
- 28.Statistics Canada. Data accuracy. Vital statistics — death database. Available: www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3233#a3 (accessed 2013 Oct. 16).
- 29.Ghali WA, Quan H, Norris CM, et al. Plotting adjusted survival curves from proportional hazards models: a comparison of two methods [abstract]. Med Decis Making 1998;18:490 [Google Scholar]
- 30.Zhang X, Zhang MJ, Fine J. A proportional hazards regression model for the subdistribution with right-censored and left-truncated competing risks data. Stat Med 2011;30:1933–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao S, Manns BJ, Culleton BF, et al. ; Alberta Kidney Disease Network. Access to health care among status Aboriginal people with chronic kidney disease. CMAJ 2008;179:1007–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jetté N, Quan H, Faris P, et al. Health resource use in epilepsy: significant disparities by age, gender, and aboriginal status. Epilepsia 2008;49:586–93 [DOI] [PubMed] [Google Scholar]
- 33.Shah BR, Gunraj N, Hux JE. Markers of access to and quality of primary care for aboriginal people in Ontario, Canada. Am J Public Health 2003;93:798–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens PJ, Sanderson D, Jebamani L. Health services use of Manitoba First Nations people: Is it related to underlying need? Can J Public Health 2005;96:S39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreatsoulas C, Anand SS. The impact of social determinants on cardiovascular disease. Can J Cardiol 2010;26:8C–13C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi R, Jan S, Wu Y, et al. Global inequalities in access to cardiovascular health care: our greatest challenge. J Am Coll Cardiol 2008;52:1817–25 [DOI] [PubMed] [Google Scholar]
- 37.Armstrong PW, Gershlick AH, Goldstein P, et al. ; STREAM Investigative Team. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med 2013;368:1379–87 [DOI] [PubMed] [Google Scholar]