Abstract
We have shown previously that a group IIA phospholipase A2 (PLA2) is responsible for the potent bactericidal activity of inflammatory fluids against many Gram-positive bacteria. To exert its antibacterial activity, this PLA2 must first bind and traverse the bacterial cell wall to produce the extensive degradation of membrane phospholipids (PL) required for bacterial killing. In this study, we have examined the properties of the cell-wall that may determine the potency of group IIA PLA2 action. Inhibition of bacterial growth by nutrient deprivation or a bacteriostatic antibiotic reversibly increased bacterial resistance to PLA2-triggered PL degradation and killing. Conversely, pretreatment of Staphylococcus aureus or Enterococcus faecium with subinhibitory doses of β-lactam antibiotics increased the rate and extent of PL degradation and/or bacterial killing after addition of PLA2. Isogenic wild-type (lyt+) and autolysis-deficient (lyt–) strains of S. aureus were equally sensitive to the phospholipolytic action of PLA2, but killing and lysis was much greater in the lyt+ strain. Thus, changes in cell-wall cross-linking and/or autolytic activity can modulate PLA2 action either by affecting enzyme access to membrane PL or by the coupling of massive PL degradation to autolysin-dependent killing and bacterial lysis or both. Taken together, these findings suggest that the bacterial envelope sites engaged in cell growth may represent preferential sites for the action and cytotoxic consequences of group IIA PLA2 attack against Gram-positive bacteria.
J. Clin. Invest. 103:715–721 (1999)
Introduction
Multicellular organisms developed multiple defense mechanisms to deal with invasion and infection by microorganisms. The inflammatory reaction elicited by the invasion of bacteria mobilizes both cellular (1–4) and extracellular (5) antimicrobial factors. Using a rabbit model of sterile inflammation, we have shown that proteins secreted by the cells in the exudate or entering from the circulation accumulate in the ascitic fluid and exert potent bactericidal activity against both Gram-positive (6) and Gram-negative bacteria (5), as well as fungi (Weinrauch, Y., and Foreman-Wykert, A.K., unpublished observations). A 14-kDa, secretory group IIA PLA2 present in concentrations ranging from 100 to 1,000 ng/ml is largely responsible for the potent bactericidal activity of these inflammatory fluids against many Gram-positive bacteria (ref. 6; and Liang, N.S., et al., manuscript in preparation).
For this PLA2 to exert its bactericidal effects, the enzyme must first bind to the bacteria and then traverse the multiple peptidoglycan layers of the cell wall to reach and hydrolyze the phospholipids in the cell membrane. The thick peptidoglycan layer of Gram-positive bacteria can be highly cross-linked (7) and may constitute a barrier to PLA2 access of the cell membrane. In line with this supposition, protoplasts of Staphylococcus aureus and Bacillus subtilis are more susceptible to PLA2 than intact bacteria (Liang, N.S., et al., manuscript in preparation). However, the potency of mammalian group IIA PLA2 (LD90 1–10 nM) (6) against S. aureus that contain highly cross-linked cell-wall peptidoglycans (7) implies that, even in these organisms, regions may exist that permit enzyme penetration to the cell membrane. In this study, we have examined the effect of cell growth, bacterial autolysins, and cell-wall active antibiotics on the phospholipolytic and bactericidal activity of PLA2. Our findings suggest that bacterial envelope sites engaged in the normal cell-wall turnover associated with cell growth, division, and separation are the sites at which PLA2 preferentially acts against Gram-positive bacteria.
Methods
Bacteria.
Staphylococcus aureus RN450 (8325-4) (8) is a laboratory strain provided by B. Kreiswirth (Public Health Research Institute, New York, New York, USA). S. aureus Lyt– is an autolysis-deficient, isogenic mutant of strain RN450 (9) and was provided by R.K. Jayaswal (Illinois State University, Normal, Illinois, USA). S. aureus strain 18 and Enterococcus faecium strains 4 and 6 are multi-drug–resistant clinical isolates obtained from P. Tierno and K. Inglima (Department of Clinical Microbiology, Tisch Hospital, New York, New York, USA). E. faecium strain 4 is highly susceptible to PLA2 (LD50 ∼50 ng/ml), whereas E. faecium strain 6 is less susceptible to PLA2 (LD50 ∼500 ng/ml).
The S. aureus strains were grown overnight at 37°C, washed once, and then subcultured for either 2.5–3 h (mid-logarithmic phase) or ∼18 h (stationary phase) in fresh Trypticase Soy Broth (TSB; Difco Laboratories, Detroit, Michigan, USA) at a starting concentration of 1.5 × 107 bacteria/ml. The E. faecium strains were grown overnight (>16 h) in Brain Heart Infusion Broth (Difco Laboratories) supplemented with 0.5% (vol/vol) heat-inactivated horse serum (GIBCO BRL, Gaithersburg, Maryland, USA). After harvesting, the bacteria were sedimented by centrifugation at 3,000 g for 5 min and resuspended in sterile physiological saline (Baxter Healthcare Corp., Deerfield, Illinois, USA) to a concentration of 109 bacteria/ml. The bacteria were used within 30 min of harvesting.
Collection of ascitic fluid, preparation of ascitic fluid filtrate, and purification of PLA2.
Sterile inflammatory exudates were elicited in New Zealand white rabbits by intraperitoneal injection of 300 ml of sterile physiological saline (Baxter Healthcare Corp.) supplemented with oyster glycogen (2.5 mg/ml; United States Biochemical Corp., Cleveland, Ohio, USA). After 16 h, the inflammatory exudate was collected, and the ascitic fluid (AF) was prepared as described previously (5, 6). Protein-poor (<1% of AF protein concentration) AF filtrate was prepared by filtration of the cell-free inflammatory AF in a centrifugal concentrator (Centricon-10; Amicon, Danvers, Massachusetts, USA) (1600 g for 1 h) at 4°C (6). The AF filtrate was sterile filtered (0.45 μm; Nalge Nunc International, Rochester, New York, USA) and stored at –20°C until used. Group IIA PLA2 from rabbit AF was purified as described previously (6, 10, 11).
Assay of PLA2 catalytic activity.
The catalytic activity of PLA2 in various assay media was measured against autoclaved [1-14C]oleate–labeled Escherichia coli as described previously (10–12). Arbitrary units of PLA2 activity were measured as described previously (6, 12).
Recombinant human PLA2.
Recombinant human group IIA PLA2 was expressed in E. coli and purified as described previously (ref. 13; and Liang, N.S., et al., manuscript in preparation).
Radiolabeling of S. aureus lipids during growth.
To radiolabel the lipids of S. aureus, the bacteria were subcultured to mid-logarithmic phase in TSB supplemented with 1 μCi/ml of [1-14C]oleic acid (40–60 mCi/mmol) (Du Pont NEN Research Products, Boston, Massachusetts, USA) and 0.1% BSA. Stationary phase cultures were grown overnight in TSB supplemented with 2 μCi/ml of [1-14C]oleic acid and 0.1% BSA. Bacteria harvested at mid-logarithmic and stationary phases were incubated in medium without [1-14C]oleic acid at 37°C for 20 min to chase incorporated, unesterified precursor fatty acid into ester positions and then were washed as described previously (6), except that stationary phase bacteria were washed in medium (TSB) derived from stationary phase cultures (used TSB). The composition of lipids in bacteria harvested at mid-logarithmic or stationary phase was determined by extraction in CHCl3/CH3OH (14) and analysis by TLC of radiolabeled material recovered in the CHCl3 phase (>95% of total) (6). In both mid-logarithmic and stationary phase bacteria, ∼70% of radiolabeled material was phospholipid, nearly all of which was phosphatidyl glycerol.
Assay of bacterial phospholipid degradation.
Because phospholipid breakdown products formed during PLA2 treatment are quantitatively recovered in the extracellular medium complexed to albumin, whereas undigested phospholipid remains within the bacterial envelope, phospholipid degradation was routinely measured as accumulation of radioactive material in the supernatant recovered after sedimentation of the bacteria (11,000 g for 4 min). To confirm that release corresponded quantitatively to phospholipid degradation, the lipids of S. aureus, with and without PLA2 treatment, were extracted using the method of Bligh and Dyer (14) and resolved as described previously (6). Phospholipid degradation of the control samples (no PLA2) was ≤5%.
Assay of bacterial viability.
To determine the effect of purified PLA2 on bacterial viability, the colony-forming ability of the bacteria was measured before and after incubation with various doses of PLA2. Typical incubation mixtures contained 1–4 × 107 bacteria/ml in the desired medium (AF filtrate, TSB, used TSB, 70% pooled human serum [PHS] or RPMI-1640 [BioWhittaker Inc., Walkersville, Maryland, USA]) supplemented with 10 mM HEPES (pH 7.4; 20 mM for filtrate) and 1% (wt/vol) BSA (not added to serum samples). Calcium chloride (2 mM) was added to TSB, used TSB, and RPMI incubation mixtures. Incubations were carried out at 37°C for ≤ 3 h. Used TSB was prepared by centrifugation (11,000 g for 5 min) and sterile filtration (0.45 μm; Nalge Nunc International) of overnight bacterial cultures (grown in TSB) to remove bacteria and particulate matter. PHS was collected from blood of healthy human volunteers and prepared as described previously (15). After incubation, aliquots of bacterial suspensions were serially diluted in sterile physiological saline and plated in 5 ml of molten (50°C) trypticase soy agar (Difco Laboratories). Bacterial viability was measured after 18–24 h of incubation at 37°C. When antibiotics were used, the bacteria were incubated with the desired antibiotic for 15 or 30 min at 37°C before the addition of PLA2. All antibiotics were obtained from the Tisch Hospital Pharmacy. The sensitivity of RN450 to PLA2 was comparable in all media used.
OD measurements.
To monitor the growth or lysis of bacteria during the course of a viability assay, 500-μl aliquots of assay samples (4 × 107 bacteria/ml) were removed, placed in a microcuvette (Fisher Scientific Co., Pittsburgh, Pennsylvania, USA), and the OD measured at 550 nm on a Beckman DU-30 spectrophotometer.
Gram staining.
Gram stains of bacterial samples with and without PLA2 treatment were performed using the Bacto-3-Step Gram stain kit (Difco Laboratories) according to the manufacturer's directions.
Results
Stationary phase and starved S. aureus are less susceptible to PLA2.
To determine whether bacterial populations with different growth rates differed in susceptibility to PLA2, S. aureus RN450 was harvested from mid-logarithmic and stationary phases; after resuspension at 107/ml in TSB, it was tested for PLA2 sensitivity. Under these experimental conditions, bacteria harvested from mid-logarithmic or stationary phase displayed different growth rates for an extended time (Fig. 1a), providing an experimental setting in which the effects of growth differences on bacterial sensitivity to PLA2 could be tested. Figure 1 (a and b) shows that purified rabbit group IIA PLA2 produced more rapid and extensive degradation of the phospholipids of bacteria harvested from mid-logarithmic than from stationary phase. More dramatic differences in PLA2 sensitivity were observed when bacteria harvested from mid-logarithmic phase and exposed to PLA2 were compared in either fresh TSB or used TSB (Fig. 1, c and d). In the latter medium, the bacteria did not grow and were neither killed by PLA2 nor suffered appreciable phospholipid degradation. PLA2 activity toward autoclaved bacteria was the same in fresh and used TSB (data not shown), indicating that the inhibition of PLA2 antibacterial action in used medium reflected an effect on the bacteria and not on the PLA2 itself. Thus, these findings suggest that the growth state of the bacteria is an important determinant of bacterial sensitivity to PLA2.
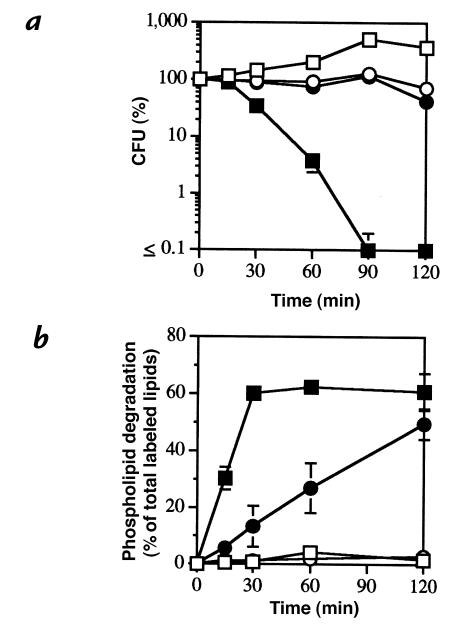
Figure 1.
Stationary phase and starved S. aureus are less susceptible to PLA2-mediated killing and phospholipiddegradation. [1-14C]oleate–labeled S. aureus RN450 (107/ml), harvested from mid-logarithmic and stationary phase, were incubated in TSB supplemented with 1% BSA, 2 mM CaCl2, and 10 mM HEPES (pH 7.4) with or without 250 ng/ml of purified rabbit AF group IIA PLA2 at 37°C. At the indicated times, samples were removed to measure viability (a) and phospholipid degradation (b) as described in Methods. Alternatively, [1-14C]oleate–labeled S. aureus RN450 (107/ml) harvested from mid-logarithmic phase were incubated in fresh or used (nutrient-depleted) TSB supplemented with 1% BSA, 2 mM CaCl2 and 10 mM HEPES (pH 7.4) with and without 150 ng/ml of purified rabbit AF PLA2 at 37°C (c and d). At the indicated times, samples were removed to measure viability (c) and phospholipid degradation (d). The results shown represent the mean ± SEM of three or more experiments. Some error bars are masked by the symbols. (open squares) Mid-logarithmic bacteria in TSB; (closed squares) mid-logarithmic bacteria + PLA2 in TSB; (open circles) stationary bacteria in TSB; (closed circles) stationary bacteria + PLA2 in TSB; (open triangles) mid-logarithmic bacteria in used TSB; (closed triangles) mid-logarithmic bacteria + PLA2 in used TSB. AF, ascitic fluid; PLA2, phospholipase A2; TSB, Trypticase Soy Broth.
A bacteriostatic antibiotic also reduces the susceptibility of S. aureus to PLA2.
To further test this hypothesis, we examined the effect of a bacteriostatic antibiotic that promptly inhibits bacterial growth under conditions less likely to produce the broad metabolic effects that accompany bacterial starvation or the transition to stationary phase. For this purpose, we used a multi-drug–resistant clinical isolate of S. aureus (strain 18) that is growth inhibited, but not killed, by treatment with erythromycin. In the absence of erythromycin, this strain of S. aureus was highly susceptible to PLA2; 50 ng/ml of this enzyme was sufficient to cause virtually quantitative degradation of membrane phospholipids within 30 minutes and >3 logs killing within 90 minutes (Fig. 2, a and b). In contrast, pretreatment of strain 18 with erythromycin for 15 minutes before PLA2 exposure greatly retarded the degradation of bacterial membrane phospholipids by PLA2 and rendered the bacteria largely resistant to the bactericidal action of PLA2 (Fig. 2, a and b).
Figure 2.
Erythromycin pretreatment reduces the susceptibility of S. aureus to PLA2 [1-14C]oleate–labeled S. aureus strain 18 (107/ml) was preincubated with and without erythromycin (5 μg/ml) in AF filtrate supplemented with 1% BSA and 20 mM HEPES (pH 7.4) for 15 min before treatment with 50 ng/ml of purified rabbit AF group IIA PLA2 at 37°C. At the indicated times, samples were removed to measure viability (a) and phospholipid degradation (b) as described in Methods. The results shown represent the mean ± SEM of three experiments. Some error bars are masked by the symbols. (open squares) Bacteria alone; (closed squares) bacteria + PLA2; (open circles) erythromycin + bacteria; (closed circles) erythromycin + bacteria + PLA2.
Erythromycin had no adverse effect on PLA2 activity toward autoclaved bacteria (data not shown), indicating that erythromycin was not directly inhibiting the catalytic activity of PLA2. In addition, erythromycin did not inhibit the initial binding of PLA2 to the bacteria (data not shown), an essential first step in the antibacterial action of mammalian group IIA PLA2 (Liang, N.S., et al., manuscript in preparation). Thus, these results support the hypothesis that bacterial growth and cell division promote the susceptibility of S. aureus to PLA2-mediated killing, apparently by facilitating the access of PLA2 to the phospholipids in the bacterial membrane. To further explore the possibility that the integrity of the cell wall is a major variable in the sensitivity of S. aureus to host PLA2, the effect on PLA2 sensitivity of antibiotics that interfere with peptidoglycan synthesis was examined.
Ampicillin promotes PLA2 action against S. aureus.
The preceding findings suggested that cell-wall turnover associated with cell growth and division may promote the antibacterial action of PLA2. If this is correct, weakening of the cell-wall structure by antibiotics should also render the bacteria more susceptible to PLA2. To explore this possibility, S. aureus strain 18 was treated with ampicillin, erythromycin, or no antibiotic for 15 minutes before treatment with increasing concentrations of PLA2. As shown above, in a different medium, bacteria pretreated with erythromycin were more resistant to the antibacterial and phospholipolytic effects of PLA2 at all concentrations tested (Fig. 3, a and b). In contrast, bacteria pretreated with subinhibitory doses of ampicillin were more susceptible than the untreated bacteria to PLA2 (Fig. 3, a and b). Thus, ampicillin pretreatment of S. aureus significantly increased bacterial susceptibility to PLA2-mediated phospholipid degradation at sublethal concentrations of PLA2 (Fig. 3b).
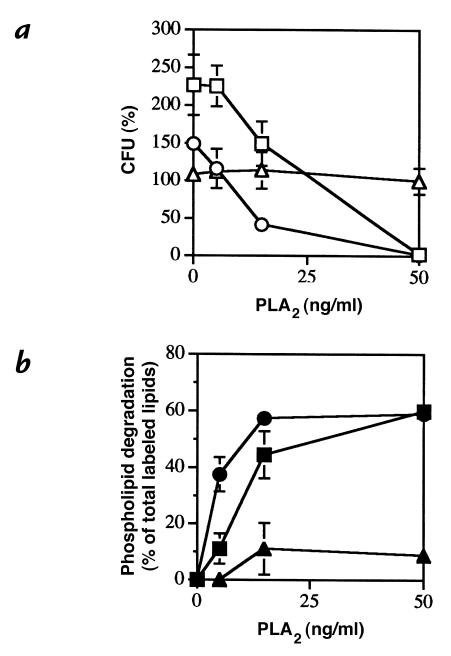
Figure 3.
Ampicillin promotes and erythromycin inhibits PLA2-mediated killing and phospholipid degradation of S. aureus. [1-14C]oleate–labeled S. aureus strain 18 (107/ml) was treated with ampicillin (1 μg/ml) (open circles), erythromycin (5 μg/ml) (open triangles), or no antibiotic (open squares) for 15 min in PHS buffered with 10 mM HEPES (pH 7.4) before incubation with increasing doses of purified rabbit AF group IIA PLA2 (5, 15, and 50 ng/ml) at 37°C. After 90 min, aliquots of the samples were removed to measure (a) viability (open symbols) and (b) phospholipid degradation (closed symbols) as described in Methods. The results represent the mean ± SEM of three experiments. Some error bars are masked by the symbols.
Antibacterial synergy between cell-wall active antibiotics and PLA2 against E. faecium.
Although pretreatment of S. aureus with subinhibitory doses of ampicillin appreciably increased the PLA2 degradation of bacterial membrane phospholipids, effects on PLA2-triggered killing were only modest. Reasoning that bactericidal synergy might be more pronounced toward Gram-positive bacteria that are inherently more resistant to PLA2, we examined the effects of cell-wall active antibiotics on PLA2-triggered killing of two multi-drug–resistant clinical isolates of E. faecium.
As expected, treatment of these two strains with either β-lactam antibiotics or vancomycin alone had little or no effect on bacterial viability (Fig. 4, a–f). However, with both strains, pretreatment with either ampicillin, cephalothin, or vancomycin increased bacterial killing produced when LD50 doses of PLA2 were added (Fig. 4, a–f). The effects of PLA2 were synergistic with both β-lactams (Fig. 4, a, b, d, and e) and additive with vancomycin (Fig. 4, c and f). These results indicate that PLA2 and β-lactam antibiotics act synergistically toward both multi-drug–resistant S. aureus and E. faecium.
Figure 4.
Cell-wall active antibiotics can act synergistically or additively with PLA2 to kill E. faecium. E. faecium strains 4 (a–c) and 6 (d–f) (105/ml) were treated with ampicillin (16 μg/ml; a and d), cephalothin (32 μg/ml; b and e),vancomycin (30 μg/ml; c and f), or no antibiotic for 30 mi n in PHS buffered with 10 mM HEPES (pH 7.4) before treatment with 50 ng/ml (strain 4) or 500 ng/ml (strain 6) of purified rabbit AF group IIA PLA2 at 37°C. At the indicated times, samples were removed to measure viability as described in Methods. The results represent the mean ± SEM of four experiments. Some error bars are masked by the symbols. (open squares) Bacteria alone; (closed squares) antibiotic + bacteria; (open circles) bacteria + PLA2; (closed circles) antibiotic + bacteria + PLA2.
Susceptibility of autolysis-deficient S. aureus to PLA2.
Bacterial cell-wall–degrading enzymes (autolysins) play an important role in cell-wall morphogenesis that accompanies cell growth, division/separation, and peptidoglycan turnover (16, 17). Thus, these enzymes could contribute to the greater sensitivity of growing bacteria to PLA2 action by producing localized sites of weakened and thinned peptidoglycan (18, 19). To test this hypothesis, we compared the sensitivity of S. aureus RN450 (lyt+) and an isogenic, autolysis-deficient mutant (Lyt–) to PLA2.
In contrast to the effect of bacterial growth inhibition (Fig. 1, b and d; Fig. 2b; and Fig. 3b), the reduced autolysin content of the lyt– RN450 mutant did not render these bacteria more resistant to the phospholipolytic action of PLA2. Thus, PLA2-triggered phospholipid degradation in RN450 (lyt+) and Lyt– is essentially the same (Fig. 5a). However, the lyt– strain is much less susceptible to killing by PLA2 (Fig. 5b), and, in contrast to the wild-type strain, is not lysed during PLA2 treatment (Fig. 5c). Gram stains confirmed the much greater lysis of the lyt+ than of the lyt– strain (data not shown). We conclude that bacterial autolysins play a major role in PLA2-triggered killing and post-killing lysis of S. aureus.
Figure 5.
Autolysis-deficient S. aureus are poorly killed and not lysed by PLA2. [1-14C]oleate–labeled S. aureus RN450 (Lyt+) and Lyt– (4 × 107/ml) were incubated in RPMI-1640 supplemented with 1% BSA, 10 mM HEPES (pH 7.4), and 1 mM CaCl2 with or without 300 ng/ml of purified rabbit AF group IIA PLA2 at 37°C. At the indicated times, aliquots were removed to measure the phospholipid degradation (a), viability (b), and OD (c) of the samples, as described in Methods. The results represent the mean ± SEM of three experiments. Some error bars are masked by the symbol. (open squares) RN450 control; (closed squares) RN450 + PLA2; (open circles) Lyt– control; (closed circles) Lyt– + PLA2.
Discussion
In this study, we have shown that the susceptibility of Gram-positive bacteria to the bactericidal action of mammalian group IIA PLA2 is profoundly affected by the bacterial growth rate. Thus, conditions that affect cell-wall structure — cell growth, division, and separation, treatment with cell-wall active antibiotics, and the action of autolysins — all correlate with bacterial sensitivity to PLA2. S. aureus was less susceptible to the antibacterial actions of PLA2 when bacterial growth was slowed by reaching stationary phase (Fig. 1, a and b), by incubation in a nutrient-depleted medium (Fig. 1, c and d), or by antibiotic-induced bacteriostasis (Fig. 2). Conversely, susceptibility to PLA2 was increased when cell-wall active antibiotics (Figs. 3 and 4) or endogenous autolysins weakened envelope structure (Fig. 5).
Bacteria undergo multiple structural and physiological changes during the transition from a growing to a nongrowing state. Alterations include increased cross-linking and thickening of the peptidoglycan (16, 20), decreased cell-wall turnover (21), transmembrane potential (22), autolysis (9), and changes in membrane phospholipid composition (23) and protein synthesis (24). Many of these changes alter the susceptibility of the bacteria to antibacterial agents (20, 25, 26). Therefore, under our experimental conditions the factor(s) responsible for the decreased sensitivity of the nongrowing bacteria cannot yet be defined precisely. However, the results of this study lead us to propose that the structure of the envelope peptidoglycan is a major determinant of the actions of PLA2 against Gram-positive bacteria.
First, the transition from active bacterial growth to a nongrowing state is accompanied by increased O-acetylation and thickening of the peptidoglycan layer (16, 20) and a dramatic increasein resistance of S. aureus to the antibacterial actions of the enzyme, most likely because of reduced access to membrane phospholipids. In contrast, the autolytic activity associated with cell-wall biogenesis may account for the high sensitivity of growing bacteria to PLA2. Second, β-lactam antibiotics, which reduce the cross-linking of peptidoglycan, also promote the antibacterial action of PLA2.
However, the ability of the PLA2 to reach and hydrolyze the bacterial phospholipids is not sufficient to kill the bacteria. The demonstration that PLA2-treated, autolysis-deficient S. aureus undergo as much phospholipolysis as their wild-type counterparts but largely escape killing and lysis (Fig. 5) indicates that the phospholipolytic and bactericidal actions of the PLA2 need not be linked; and it implies that activation of autolytic events is also required for killing of S. aureus by PLA2. Because the lyt+ and lyt– strains of S. aureus are equally susceptible to phospholipid degradation by PLA2, differences in their autolytic activity (9) apparently do not affect the access of PLA2 to the bacterial membrane. The survival of the lyt– strain despite the nearly quantitative degradation of its membrane phospholipids further points to remarkable repair capabilities of the bacteria in the absence of autolytic activity.
The activities of autolysins are tightly regulated (27), both temporally and spatially, to permit the discrete action needed for cell-wall biogenesis (16, 18, 19), without causing diffuse cell-wall alterations that would be harmful to the bacteria. Because the growth rates of the lyt+ and lyt– strains are comparable, the actual level of autolytic activity coupled to cell growth processes may be similar in both strains, despite the large difference in overall autolytic activity.
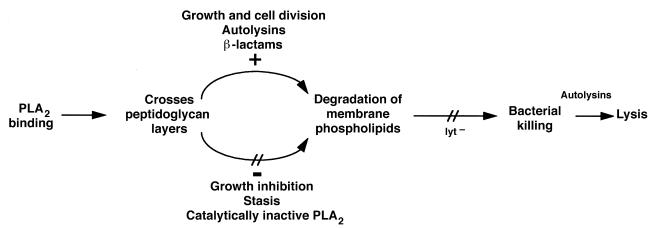
Based on these observations, we propose a model for mammalian group IIA PLA2 action against Gram-positive bacteria (outlined in Fig. 6). First, the PLA2 must bind and penetrate the cell-wall, a feature that distinguishes this PLA2 from other closely related, but nonbactericidal, PLA2's (ref. 6; and Liang, N.S., et al., manuscript in preparation). These initial interactions are dependent on electrostatic attraction between the very cationic group IIA PLA2 and anionic bacterial envelope constituents. One bacterial envelope component that could be particularly important in this context is lipoteichoic acid (LTA), an abundant anionic membrane glycolipid that extends through the cell-wall to the outer surface. We speculate that the initial binding of PLA2 to LTA on the outer surface of the bacteria is followed by penetration of the PLA2 down the repeating glycerol phosphate chain of LTA permitting PLA2 to reach the cell membrane. Binding of PLA2 to LTA might also displace the less cationic autolysins, relieving this negative constraint on autolysin activity (28) and producing discrete cell-wall changes at sites of PLA2 binding that further promote PLA2 penetration. Penetration of PLA2 may be favored at envelope sites with decreased cross-linking and/or increased active autolysins, e.g., sites of new cell-wall growth.
Figure 6.
Model for PLA2 action against Gram-positive bacteria.
When the PLA2 causes massive phospholipid degradation, thereby destabilizing the association of LTA with the cell membrane, a more diffuse activation of autolysins (in lyt+ strains) may follow and, consequently, result in irreversible bacterial injury. The activities of cytosolic autolysins, which are controlled by close association with membrane phospholipids (29, 30), may also be activated because of the membrane disruption caused by PLA2. The decreased autolysin content of the autolysis-deficient strain of S. aureus may explain why, in this situation, the bacteria are not lysed, but instead remain largely intact and able to repair the membrane damage caused by PLA2.
Intrinsic differences in cell-wall peptidoglycan cross-linking and/or autolytic activity among Gram-positive bacterial species may account for different sensitivities to PLA2 (6). For example, B. subtilis, which is exquisitely sensitive to PLA2, has high autolytic activity (31), whereas less susceptible species display lower autolytic activity or possess fewer autolysins (32). These differences could be linked to different growth patterns of the bacterial species. It has been speculated that B. subtilis may undergo random or diffuse cell-surface extension by the addition of new cell wall at many sites (33), thereby creating more sites at which PLA2 can cross the cell wall. Conversely, enterococcal species, which are much less sensitive to PLA2 (6), exhibit patterns of zonal growth (34), where new cell-wall material is inserted at a single region or zone, resulting in fewer sites for PLA2 access to the membrane phospholipids.
The demonstration of synergism between sublethal doses of β-lactam antibiotics and low concentrations of PLA2 against two drug-resistant enterococcal pathogens (Fig. 4) that differ in their intrinsic susceptibilities to PLA2 not only further supports the contention that peptidoglycan structure is an important variable in the antibacterial actions of PLA2, but may also have broader biological implications. These findings suggest that even in antibiotic-resistant bacteria, discrete changes in the cell wall caused by subinhibitory doses of β-lactam antibiotics (35, 36) are sufficient to promote access of PLA2 to the membrane phospholipids, setting in motion both their degradation and autolytic events resulting in killing.
In contrast to the synergistic effects of β-lactam antibiotics and PLA2, the combined effects of PLA2 and vancomycin are additive. This difference is observed over a wide range of vancomycin concentrations, including doses at which β-lactam antibiotics and vancomycin, by themselves, have similar effects on bacterial growth and viability (data not shown). The finding that β-lactam antibiotics, which decrease peptidoglycan cross-linking, acted synergistically with PLA2, whereas combinations with vancomycin (an agent that inhibits cell-wall synthesis) were only additive, may again reflect an important role of the peptidoglycan structure in PLA2 action.
In conclusion, this study provides an expansion of our understanding of the antibacterial action of a group IIA PLA2. Recently it has become apparent that this enzyme is an important component of host defense against many Gram-positive pathogens (6, 37–40). Much effort has been directed in the past at developing inhibitors of this PLA2 based upon the belief that the products of its action might exacerbate inflammation. More work is needed to determine the relative prominence of the role of this PLA2 in antibacterial host defense and the extent of its potential contribution to harmful or beneficial inflammatory responses by acting against host lipids. The demonstration in this study of the synergistic and additive actions of this PLA2 against common pathogens, including multi-drug–resistant organisms, raises the prospect of new therapeutic approaches.
Acknowledgments
We gratefully acknowledge the generous help of Barry Kreiswirth, Radheshyam Jayaswal, and Philip Tierno, and of Ken Inglima in making bacterial strains and clinical isolates available for study. We also thank Ning-Sheng Liang for making recombinant human D48S PLA2 available for use, and Jake Kaufman for technical help. This work was supported by United States Public Health Service grant AI-18571 and a Ford Foundation Predoctoral Fellowship (to A.K. Foreman-Wykert).
References
- 1.Elsbach, P., and Weiss, J. 1992. Oxygen-independent antimicrobial systems of phagocytes. In Inflammation: basic principles and clinical correlates. J.I. Gallin, I.M. Goldstein, and R. Snyderman, editors. Raven Press. New York, NY. 603–636.
- 2.Klebanoff, S.J. 1992. Oxygen metabolites from phagocytes. In Inflammation: basic principles and clinical correlates. J.I. Gallin, I.M. Goldstein, and R. Snyderman, editors. Raven Press. New York, NY. 541–588.
- 3.Ganz T, Lehrer RI. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Levy O. Antibiotic proteins of polymorphonuclear leukocytes. Eur J Haematol. 1996;56:263–277. doi: 10.1111/j.1600-0609.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 5.Weinrauch Y, et al. Extracellular accumulation of potently microbicidal bactericidal/permeability-increasing protein and p15s in an evolving sterile rabbit peritoneal inflammatory exudate. J Clin Invest. 1995;95:1916–1924. doi: 10.1172/JCI117873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinrauch Y, Elsbach P, Madsen LM, Foreman A, Weiss J. The potent anti–Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson, B.J. 1997. Biology. In The staphylococci in human disease. K.B. Crossley and G.L. Archer, editors. Churchill Livingstone. New York, NY. 1–38.
- 8.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 9.Mani N, Tobin P, Jayaswal RK. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J Bacteriol. 1993;175:1493–1499. doi: 10.1128/jb.175.5.1493-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forst S, et al. Structural and functional properties of a phospholipase A2 purified from an inflammatory exudate. Biochemistry. 1986;25:8381–8385. doi: 10.1021/bi00374a008. [DOI] [PubMed] [Google Scholar]
- 11.Wright GW, Ooi CE, Weiss J, Elsbach P. Purification of a cellular (granulocyte) and an extracellular (serum) phospholipase A2 that participate in the destruction of Escherichia coli in a rabbit inflammatory exudate. J Biol Chem. 1990;265:6675–6681. [PubMed] [Google Scholar]
- 12.Elsbach P, Weiss J. Utilization of labeled Escherichia coli as phospholipase substrate. Methods Enzymol. 1991;197:24–31. doi: 10.1016/0076-6879(91)97130-q. [DOI] [PubMed] [Google Scholar]
- 13.Weiss J, Inada M, Elsbach P, Crowl RM. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J Biol Chem. 1994;269:26331–26337. [PubMed] [Google Scholar]
- 14.Bligh ES, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Madsen LM, Inada M, Weiss J. Determinants of activation by complement of group II phospholipase A2 acting against Escherichia coli. Infect Immun. 1996;64:2425–2430. doi: 10.1128/iai.64.7.2425-2430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong W, Chatterjee AN, Young FE. Regulation of bacterial cell walls: correlation between autolytic activity and cell wall turnover in Staphylococcus aureus. J Bacteriol. 1978;134:555–561. doi: 10.1128/jb.134.2.555-561.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugai M, et al. Identification of endo-beta-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J Bacteriol. 1995;177:1491–1496. doi: 10.1128/jb.177.6.1491-1496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugai M, et al. Localized perforation of the cell wall by a major autolysin: atl gene products and the onset of penicillin-induced lysis of Staphylococcus aureus. J Bacteriol. 1997;179:2958–2962. doi: 10.1128/jb.179.9.2958-2962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada S, et al. An autolysin ring associated with cell separation of Staphylococcus aureus. J Bacteriol. 1996;178:1565–1571. doi: 10.1128/jb.178.6.1565-1571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johannsen L, Labischinski H, Reinicke B, Giesbrecht P. Changes in the chemical structure of walls of Staphylococcus aureus grown in the presence of chloramphenicol. FEMS Microbiol Lett. 1983;16:313–316. [Google Scholar]
- 21.Boothby D, et al. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973;248:2161–2169. [PubMed] [Google Scholar]
- 22.Koo SP, Bayer AS, Sahl HG, Proctor RA, Yeaman MR. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect Immun. 1996;64:1070–1074. doi: 10.1128/iai.64.3.1070-1074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Short SA, White DC. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J Bacteriol. 1971;108:219–226. doi: 10.1128/jb.108.1.219-226.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson SP, Clements MO, Foster SJ. Characterization of the starvation-survival response of Staphylococcus aureus. J Bacteriol. 1998;180:1750–1758. doi: 10.1128/jb.180.7.1750-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986;132:1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- 26.Koo SP, Yeaman MR, Bayer AS. Staphylocidal action of thrombin-induced platelet microbicidal protein is influenced by microenvironment and target cell growth phase. Infect Immun. 1996;64:3758–3764. doi: 10.1128/iai.64.9.3758-3764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snowden MA, Perkins HR. Cross-linking and O-acetylation of peptidoglycan in Staphylococcus aureus (strains H and MR-1) grown in the presence of sub-growth-inhibitory concentrations of beta-lactam antibiotics. J Gen Microbiol. 1991;137:1661–1666. doi: 10.1099/00221287-137-7-1661. [DOI] [PubMed] [Google Scholar]
- 28.Qoronfleh MW, Wilkinson BJ. Effects of growth of methicillin-resistant and -susceptible Staphylococcus aureus in the presence of beta-lactams on peptidoglycan structure and susceptibility to lytic enzymes. Antimicrob Agents Chemother. 1986;29:250–257. doi: 10.1128/aac.29.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunskill EW, Bayles KW. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J Bacteriol. 1996;178:611–618. doi: 10.1128/jb.178.3.611-618.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer W. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med Microbiol Immunol. 1994;183:61–76. doi: 10.1007/BF00277157. [DOI] [PubMed] [Google Scholar]
- 31.Cleveland RF, Daneo-Moore L, Wicken JA, Shockman GD. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976;127:1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cleveland RF, Wicken AJ, Daneo-Moore L, Shockman GD. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976;126:192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster SJ. Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J Bacteriol. 1992;174:464–470. doi: 10.1128/jb.174.2.464-470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers, H.J., Perkins, H.R., and Ward, J.B. 1980. Microbial cell walls and membranes. In The bacterial autolysins. Chapman and Hall. London, United Kingdom. 437–459.
- 35.Doyle RJ, Koch AL. The functions of autolysins in the growth and division of Bacillus subtilis. Crit Rev Microbiol. 1987;15:169–222. doi: 10.3109/10408418709104457. [DOI] [PubMed] [Google Scholar]
- 36.Higgins ML, Shockman GD. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970;101:643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harwig S, et al. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95:603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu XD, Lloyd KC, Walsh JH, Lehrer RI. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal Paneth cells. Infect Immun. 1996;64:5161–5165. doi: 10.1128/iai.64.12.5161-5165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu XD, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinrauch Y, Abad C, Liang N-S, Lowry SF, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge: role of group IIA phospholipiase A2. J Clin Invest. 1998;102:633–638. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]