Abstract
Frontline chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR) is associated with superior overall survival (OS) for patients with chronic lymphocytic leukemia (CLL). Alemtuzumab (A) was added to FCR (CFAR) in a phase 2 trial for high-risk untreated patients < 70 years with serum β-2 microglobulin (β2M) ≥ 4 mg/L. Sixty patients were enrolled; median age was 59 years (range, 42-69); 75% were male; median β2M was 5.1 mg/L (range, 4-11.6); and 51% were Rai III-IV. Complete remission (CR) was achieved in 70%, partial remission (PR) in 18%, nodular PR in 3%, for an overall response of 92%. Of 14 patients with 17p deletion, CR was achieved by 8 (57%). Of 57 BM samples evaluated by 3-color flow cytometry at the end of treatment, 41 (72%) were negative for residual disease. Grade 3-4 neutropenia and thrombocytopenia occurred with 33% and 13% courses, respectively. The median progression-free survival was 38 months and median OS was not reached. In conclusion, CFAR is an active frontline regimen for high-risk CLL. Response rates and survival are comparable with historic high-risk FCR-treated patients. CFAR may be a useful frontline regimen to achieve CR in patients with 17p deletion before allogeneic stem cell transplantation.
Introduction
Chemoimmunotherapy combines chemotherapy with mAbs and represents a significant advance in treatment for patients with chronic lymphocytic leukemia (CLL). A complete remission (CR) rate of 72% and an overall response rate (ORR) of 96% was reported for the combination of fludarabine, cyclophosphamide, and rituximab (FCR) in 300 previously untreated patients at the M. D. Anderson Cancer Center (MDACC).1 After a median follow-up of 72 months, the actuarial 6-year overall survival (OS) and failure-free survival was 77% and 51%, respectively.2 Among responders, the median time to progression was 80 months. The superiority of the FCR regimen over fludarabine and cyclophosphamide (FC) was confirmed in the randomized phase 3 CLL8 study of the German CLL Study Group (GCLLSG). Superior CR rate (44% vs 22%, P < .001) and ORR (95 vs 88%, P < .001) were reported and after a median follow-up of 38 months, superior median progression-free survival (PFS; 52 months vs 33 months, P < .001) and OS (84% vs 79%, P = .01) were reported for patients treated with FCR versus FC.3
In patients treated with MDACC FCR-based frontline chemoimmunotherapy trials, the subset of patients with serum β-2 microglobulin (β2M) ≥ 4 mg/L had a lower CR rate (60% vs 72%) and shorter median PFS (55 months vs 72 months), thereby characterizing this group as high risk. Alemtuzumab, the CD52 mAb, was shown to have activity as a monotherapy and in combination with fludarabine in patients with relapsed/refractory CLL.4–7 Therefore, we added alemtuzumab to FCR (CFAR regimen), in a single institution, single-arm, phase 2 clinical trial as frontline therapy for treatment-naive, high-risk patients with CLL who were younger than 70 years of age.
Methods
Study design and patients
The MDACC Institutional Review Board approved the trial. All patients provided written informed consent according to MDACC institutional guidelines; this trial was conducted in accordance with the Declaration of Helsinki. Patients 70 years of age or older are more susceptible to myelosuppression from chemoimmunotherapy combinations, which may be further increased with the addition of alemtuzumab. Therefore, we restricted eligibility to patients younger than 70 years. Eligible patients had the following characteristics: (1) previously untreated CLL with a National Cancer Institute-Working Group (NCI-WG)8 indication for treatment; (2) 70 years of age or younger; (3) β2M ≥ 4 mg/L; (4) Eastern Cooperative Oncology Group (ECOG) performance status 0-2; (5) normal organ function (ie, total bilirubin ≤ 2 mg/dL, aspartate aminotransferase [AST] and alanine aminotransferase [ALT] < 2.5× upper limit of normal and serum creatinine < 2 mg/dL); and (6) no active or uncontrolled infection. Historically, there were 96 high-risk patients of 300 (32%) treated on the FCR frontline trial. For this subgroup, the CR rate was 60%, nodular partial remission (nPR) 14%, partial remission (PR) 20%, and 6% were nonresponders.2 Of this high-risk FCR subgroup, 53% had < 5% CD 5/19+ B cells in BM after 3 courses of chemotherapy. The primary objective of the CFAR regimen was to improve this rate to 66% after 3 courses of CFAR.
Treatment
Treatment was as follows: fludarabine 20 mg/m2/d IV and cyclophosphamide 200 mg/m2/d IV on days 3 through 5; rituximab 375 mg/m2 IV (500 mg/m2 IV for courses 2-6) on day 2; and alemtuzumab 30 mg IV on days 1, 3, and 5. All patients received 6 mg of pegfilgrastim subcutaneously on day 6 of each course of treatment as primary prophylaxis. Courses of CFAR were repeated every 4 weeks as permitted by recovery of neutrophil and platelet counts for a planned total of 6 courses. To decrease the incidence and severity of infusion-related reactions, acetaminophen 500 mg per oral (PO), diphenhydramine 25-50 mg PO or IV, and hydrocortisone 100 mg IV were administered before each infusion of rituximab and alemtuzumab. All patients received allopurinol 300 mg PO for at least the first week of course 1 for tumor lysis prophylaxis. Pneumocystis jirovecii prophylaxis was trimethoprim/sulfamethoxazole double strength (160-800 mg), and herpes virus prophylaxis was valacyclovir 500 mg daily throughout treatment and for at least 3-6 months after last course. Some patients received valganciclovir 450 mg PO twice daily for prophylaxis against herpes virus and CMV, instead of valacyclovir. All patients received their first course at MDACC and then were carefully monitored on an outpatient basis by their treating physician at MDACC or by a community physician in collaboration with their treating physician at MDACC.
Dose reduction to the next lower level (fludarabine −1 and −2 dose levels were 17.5 mg/m2 and 15 mg/m2 for 3 days, respectively; and cyclophosphamide levels were 175 mg/m2 and 150 mg/m2 for 3 days, respectively) was made if pneumonia, sepsis, or other life-threatening infections occurred during the treatment phase of the protocol. If recovery of the platelet count to > 100 000/μL or within 20% of pretreatment level, or absolute neutrophil count to > 1000/μL or within 20% of pretreatment level exceeded 35 days, the dose was decreased to level −1. If grade 3 or 4 toxicities to other organ systems developed, the dose level was lowered to level −1 or −2, respectively. Dose reduction of rituximab and alemtuzumab were not recommended, but were left to the discretion of the treating physician.
Baseline, follow-up, and response assessment
Pretreatment evaluation consisted of complete history and physical examination, complete blood count (CBC) with differential, serum chemistries, liver function test, lactate dehydrogenase (LDH), β2M, quantitative Igs, and blood CMV Ag assay. A pretreatment BM aspiration and biopsy were performed on all patients for immunophenotyping to demonstrate light chain–restricted monoclonal population of CD5+/19+/23+ B cells, and determine metaphase karyotype; Ig heavy chain (IGHV) gene mutation status; CD38 expression; and ζ-chain–associated protein kinase 70 (ZAP70) expression by IHC. Interphase FISH was performed on BM samples after culturing cells for 24 hours without stimulation, using the Vysis CLL probe panel, according to the manufacturer's recommendations. The panel included probes specific to TP53 (17p13.1), ATM (11q22.3), D13S319 (13q14.3), LAMP1 (13q34), and the centromeric region of chromosome 12 (12p11.1-q11). For analysis, nuclei for 200 cells were counted, ≤ 5% positive cells were considered negative. CBC with differential, serum chemistries, serum bilirubin, and ALT were monitored weekly for the first course and at least once before each subsequent course of therapy. CMV antigenemia was evaluated before each course.
All formal response assessment evaluations were performed at MDACC. Formal response assessment was according to 1996 NCI-WG criteria before course 4 and at least 2 months after the last course.8 Patients who were responding with at least PR after course 3 continued treatment. Patients with progressive disease or no response before course 4 were removed from treatment. All patients underwent end-of-treatment BM aspiration and biopsy for response assessment to assess minimal residual disease (MRD) by 3-color flow cytometry (flow MRD) and by a PCR-based ligase assay for patient-specific clonal IGHV gene (PCR MRD).2 Flow MRD-negative status was defined as < 5% cells in the marrow lymphocyte gate coexpressing CD5 and CD19 and which have a κ:λ ratio of < 3:1 or > 1:3. PCR MRD results were normalized to the RAS oncogene, with ratios < 0.001 considered negative, between 0.001 and 0.10 considered low positive and higher ratios considered positive.
Statistical analyses
The primary objective of this phase 2 clinical trial was to determine whether treatment with CFAR rendered 66% of treated high-risk patients negative for disease by flow cytometry after course 3 and before course 4, while maintaining an infection rate < 25%. Secondary objectives were to assess responses and remission duration. The method of Thall, Simon, and Estey was used to perform interim efficacy and safety monitoring.9 Patient characteristics were summarized using median and range for continuous variables and frequency (percentage) for categorical variables. The association between patient characteristics and CR was assessed using logistic regression analysis. OS was defined as time from first treatment to death because of any cause or last follow-up time, whichever occurred first. PFS was defined as the time from first treatment to disease progression or death because of any reason. Patients who were alive and had no disease progression were censored at last follow-up. The Kaplan-Meier method was used to estimate the probability of PFS and OS; Cox proportional hazards models were used to evaluate associations between PFS or OS and patient prognostic factors. All statistical analyses were conducted using SAS Version 9.1.
Results
Response to treatment
Sixty patients were enrolled from July 2005 through August 2008 (Table 1). The median time from CLL diagnosis to CFAR was 30 months (range, 1-191 months). One patient (2%) was not evaluable for overall response. In an intent-to-treat analysis, overall, 70% achieved CR (95% confidence interval [CI] 57,80), 3% nPR, and 18% PR, for an ORR of 92% (95% CI 82,97). Of patients who achieved PR, 5% had persistent cytopenia with no residual disease and 13% had persistent disease at the end of treatment. Four patients (7%) did not respond to therapy; they received a median of 3 courses of CFAR (range 2-4). Three of these 4 patients had 17p deletion and 1 patient had 11q deletion by FISH.
Table 1.
NCI-WG response and progression-free survival by pretreatment characteristics
| Patient characteristic | n | % CR | % ORR | Median PFS, mo |
|---|---|---|---|---|
| Overall | 60 | 70 | 92 | 38 |
| Age, y | ||||
| < 60 | 33 | 70 | 88 | 32 |
| 61-70 | 27 | 70 | 96 | 38 |
| Sex | ||||
| Male | 45 | 64 | 89 | 32 |
| Female | 15 | 87 | 100 | 38+ |
| Rai stage | ||||
| I-II | 30 | 70 | 90 | 32 |
| III-IV | 30 | 70 | 93 | 42+ |
| ECOG PS | ||||
| 0 | 13 | 69 | 85 | 32 |
| 1-2 | 47 | 70 | 95 | 38+ |
| WBC, ×109/L | ||||
| ≤ 50 | 17 | 70 | 88 | 42+ |
| 51-150 | 25 | 72 | 92 | 32 |
| > 150 | 18 | 68 | 94 | 28 |
| β2-microglobulin, mg/L | ||||
| 4-5.0 | 26 | 69 | 96 | 38 |
| > 5.0 | 34 | 70 | 88 | 42+ |
| LDH | ||||
| < 1.5× ULN | 40 | 67 | 92 | 42+ |
| > 1.5× ULN | 20 | 75 | 90 | 32 |
| IGHV gene status | ||||
| Mutated (> 2%) | 20 | 70 | 100 | 42+ |
| Unmutated (< 2%) | 37 | 73 | 92 | 32 |
| Unavailable | 3 | 33 | 33 | 32 |
| Cytogenetics by FISH | ||||
| 17p deletion | 14 | 57* | 78* | 15* |
| 11q deletion | 10 | 80 | 90 | 27 |
| Trisomy 12 | 15 | 93 | 100 | 42+ |
| None | 10 | 50 | 90 | 42+ |
| 13q (sole) deletion | 11 | 64 | 100 | 42+ |
| CD38 expression | ||||
| ≤ 30% | 39 | 62 | 94 | 38 |
| > 30% | 21 | 86 | 90 | 28* |
| ZAP 70 (by IHC) | ||||
| Positive | 33 | 57* | 82 | 32 |
| Negative | 16 | 94 | 100 | 42+ |
| Unavailable | 11 | 73 | 91 | 38 |
P < .05.
NCI-WG indicates National Cancer Institute–Working Group; CR, complete remission; ORR, overall response rate; PFS, progression-free survival; ECOG, Eastern Cooperative Oncology Group; PS, performance status; LDH, lactate dehydrogenase; IHC, immunohistochemistry; ULN, upper limit of normal; and +, median not reached.
Response to CFAR by baseline characteristics is shown in Table 1. Notably, patients who had ZAP70 expression by IHC had a lower CR rate compared with those who did not (57% vs 94%). Similarly, patients with 17p deletion (in > 5% of CLL cells) by FISH had a lower CR rate compared with those in other FISH categories (57% vs 76%). There were 3 patients with 17p deletion in 5%-20% of CLL cells, all achieved CR; among the 11 patients with 17p deletion in > 20% of CLL cells, 45% achieved CR and the ORR was 73%. A multivariable logistic regression model identified 17p deletion (in > 5% of CLL cells) by FISH and expression of ZAP70 by IHC as independently associated with a lower probability of achieving CR (data not shown).
A total of 260 courses of CFAR were administered in this study. The median number of courses given was 4 (range 2-6). One hundred six (40%) courses were administered by community physicians and the remaining 154 by physicians at MDACC. Thirty-six patients (60%) could not complete all 6 courses of therapy (Table 2). Significantly higher CR rate (P = .002) and ORR (P < .001) were observed in patients who received > 3 courses of CFAR compared with those who received fewer.
Table 2.
Reasons for not completing 6 courses of therapy according to number of courses received
| Reason off treatment | C2 | C3 | C4 | C5 | Total |
|---|---|---|---|---|---|
| Decreased ANC/platelet count | 2 | 9 | 3 | 4 | 18 |
| Patient preference (in CR) | 1 | 1 | 2 | ||
| AIHA | 2 | 2 | 4 | ||
| Infection | 2 | 3 | 3 | 8 | |
| Failed therapy | 1 | 2 | 1 | 4 | |
| Total | 3 | 16 | 10 | 7 | 36 |
C indicates course number; ANC, absolute neutrophil count; CR, complete remission; and AIHA, autoimmune hemolytic anemia.
BM aspirate samples from 55 (92%) patients were available for 3-color flow cytometry evaluation for residual disease after 3 courses (before course 4). Of the 55 samples evaluated after course 3, 33 (60%; 95% CI 47,72) were negative for residual disease by 3-color flow cytometry. The primary objective was to determine whether 66% of high-risk patients could be rendered negative for residual disease by 3-color flow cytometry after course 3. Of the 57 BM samples evaluated by 3-color flow cytometry at final response assessment, 41 (72%; 95% CI 59,82) were negative for residual disease as previously defined. Of these 41 patients, 33 (80%) had achieved CR, 6 (15%) PR, and 2 (5%) patients had achieved nPR. Of the 40 patients in CR for whom 3-color flow cytometry data were available at the end of treatment, 34 (85%) patients were negative. BM aspirate samples from 42 patients were evaluated for residual disease by PCR-based ligase assay at final response assessment. Twenty-five (60%) patients had negative and low-positive ratios (0.001-0.10) by PCR-based assay; 17 (40%) were positive (ratio > 0.1).
Progression-free survival and overall survival
The median follow-up time for all 60 patients was 25 months (range, 3-51+). Of the 56 responders, 19 (34%) have progressed. Of these 19, 12 (63%) had achieved CR, 6 (32%) PR, and 1 (5%) patient had achieved nPR. Four patients (7%) developed Richter transformation after a median of 8.3 months (range, 1.7-12.2 months) and 2 patients (4%) developed acute myeloid leukemia (AML) after a median of 19.1 months (range, 17.9-20.3 months). There were no baseline characteristics (including cytogenetics by FISH) nor was the number of CFAR courses received associated with increased risk for developing Richter transformation or AML, although the patient number is small. Fifteen (27%) patients received subsequent salvage treatment, and 4 (7%) underwent allogeneic stem cell transplantation. The median PFS for all patients was 38 months; median PFS by pretreatment characteristics are shown in Table 1. In a multivariable Cox proportional hazards model for PFS, elevated serum LDH (hazard ratio [HR], 4.04; P = .004) and CD38 expression ≥ 30% (HR, 1.49; P = .01) were associated with higher risk for progression. The presence of 17p deletion by FISH was significant in univariable analysis for shorter PFS; however, when added into the multivariable model, it was not significant.
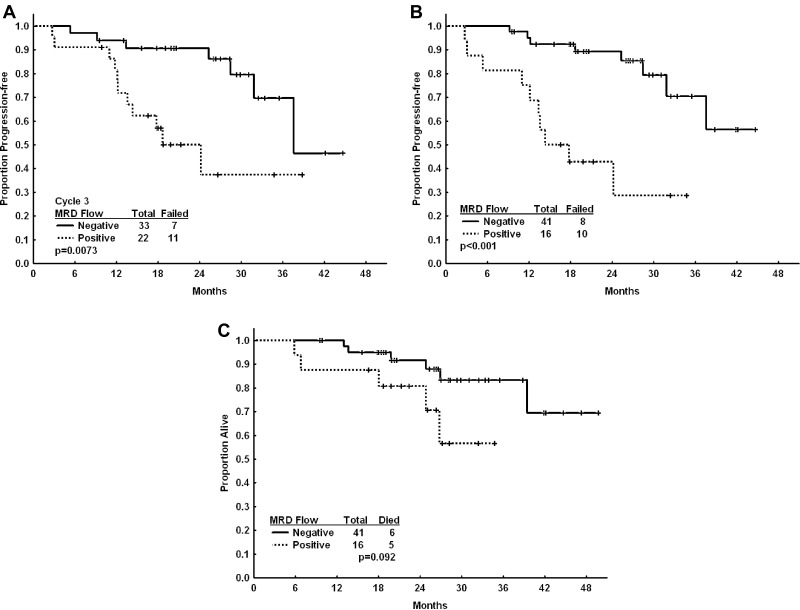
Flow cytometry residual disease status in BM after course 3 correlated with PFS (Figure 1). Patients who were flow MRD-negative after course 3 had longer PFS compared with patients who were positive (38 vs 19 months; P = .007; Figure 1A). Furthermore, flow cytometry residual disease status in BM at final response assessment correlated with PFS; flow MRD-negative patients had longer PFS compared with positive patients (not reached vs 15 months; P ≤ .001; Figure 1B). There was a trend for longer overall survival for BM flow MRD-negative patients; however, this was not statistically significant, perhaps because of a relatively short follow-up time for overall survival (Figure 1C). Patients who achieved PCR MRD-negative or low positive status did not have a significantly longer PFS compared with patients who did not (data not shown).
Figure 1.
Progress-free and overall survival based on flow MRD status with frontline CFAR. Kaplan-Meier analyses showing progression-free survival from start of treatment by 3-color flow cytometry BM residual disease (MRD flow) status (negative, solid line; positive, dashed line) after course 3 (A) and at end of treatment (B). Overall survival is shown from start of treatment by 3-color flow cytometry BM residual disease (negative, solid line; positive, dashed line) status at end of treatment (C).
Of the 60 patients, 11 (18%) have died, 7 (12%) because of disease progression after achieving CR; 2 (3%) did not respond to therapy; 1 (2%) died of metastatic lung cancer; and 1 (2%) died of CMV pneumonia. No early (< 3 months of enrollment) deaths were observed. The estimated median OS for the 60 patients has not been reached. Cox proportional hazards model identified a higher serum LDH (HR, 12.90; P = .0001) as the only pretreatment characteristic associated with shorter survival.
Toxicity
Grade 3 and 4 neutropenia occurred in 12% and 21% courses, and grade 3 and 4 thrombocytopenia occurred in 9% and 4% courses, respectively. Infectious complications occurred in 18% of courses. Major infections (pneumonia, sepsis, septic shock, or fever of unknown origin requiring hospitalization) were noted in 11% of courses. Organisms identified in documented major infections were: Histoplasma capsulatum (n = 2); Staphylococcus aureus (n = 2); Pseudomonas aeruginosa (n = 1); Clostridium difficile (n = 1); and Parainfluenza (n = 1). Minor infections (cellulitis, urinary tract infection, sinusitis or bronchitis or upper respiratory tract infection) occurred in 7% of courses. Urinary tract infection because of BK virus was diagnosed in 2 patients, which were successfully treated with outpatient cidofovir. Reactivation of herpes simplex occurred in 1 (2%) patient and reactivation of herpes zoster occurred in 2 (4%) patients; all were on valacyclovir prophylaxis. The risk of infection per course of therapy according to response and number of courses received is shown in Table 3. Late infections, defined as major infections diagnosed 3 or more months after completing therapy occurred in 6 patients, 5 of whom had achieved a CR; 1 patient had achieved a nPR. Organisms identified in these late infections were H capsulatum (n = 1); endemic fungal infection (n = 1), Moraxella catarrhalis (n = 1), CMV (n = 1); P jirovecii (n = 1), and Cryptococcus neoformans (n = 1). All late infections occurred within the first year after completing therapy.
Table 3.
Incidence of infections (all grades) with CFAR therapy by NCI-WG response and course number
| Infection | No. of courses | Total no. of infections | No. of major infections | Risk per course |
|---|---|---|---|---|
| Response | ||||
| CR | 191 | 36 | 19 | 0.18 |
| nPR | 9 | 1 | 2 | 0.11 |
| PR | 48 | 7 | 6 | 0.14 |
| NR | 12 | 2 | 0.17 | |
| Course no. | ||||
| 1-3 | 175 | 24 | 8 | 0.14 |
| 4-6 | 85 | 22 | 18 | 0.23 |
Risk per course refers to the total number of infections/number of courses. Major infections refers to pneumonia, sepsis, fever of unknown origin requiring hospitalization.
CFAR indicates alemtuzumab added to FCR; NCI-WG, National Cancer Institute–Working Group; CR, complete remission; nPR, nodular partial remission; PR, partial remission; and NR, no response.
Alemtuzumab-related infusion reactions occurred in 42 (70%) patients; all were grade 1-2 and were easily attenuated with the use of additional diphenhydramine and hydrocortisone. No grade 3-4 infusion reactions were noted. Of the 60 patients enrolled in this study, 11 (18%) received valacyclovir and 49 (82%) received valganciclovir as CMV reactivation prophylaxis. CMV monitoring consisted of monthly blood CMV Ag assay and repeat testing for any patient presenting with fever. Six (10%) patients developed CMV antigenemia while on study; 3 (5%) were receiving valacyclovir, 2 (4%) were previously on valganciclovir but were switched to valacyclovir before developing reactivation, and 1 (2%) was on valganciclovir. Of these 6 events, 4 (7%) were asymptomatic reactivation, treated with valganciclovir (n = 2) or observed (n = 2). One (2%) patient on valacyclovir prophylaxis who had symptomatic reactivation of CMV was treated with valganciclovir with complete resolution of antigenemia. One patient (2%) who stopped his prophylactic valganciclovir 3 months before developing the infection died of CMV pneumonia 8 months after achieving CR.
Comparison with historic high-risk patients treated with FCR-based regimens at MDACC
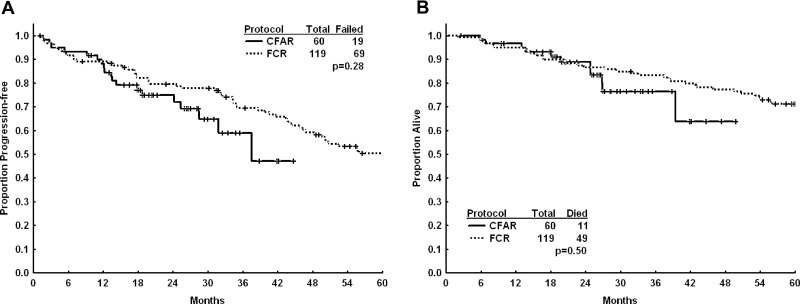
We identified 119 previously untreated patients younger than 70 years with β2M ≥ 4 mg/L who had been treated with an FCR-based regimen (fludarabine 25 mg/m2/d IV and cyclophosphamide 250 mg/m2/d IV on days 2-4 and rituximab 375 mg/m2 on day 1; every 4 weeks for a total of 6 courses) at MDACC as the historic high-risk comparison group. There was a trend toward higher CR rate in patients who received the CFAR regimen (70% vs 60%); however, as illustrated in the Kaplan-Meir analysis in Figure 2A and B, there was no statistically significant difference in median PFS or median OS between the 2 groups. There was no significant difference in the proportion of courses associated with grade 3-4 neutropenia, thrombocytopenia, and anemia (although all patients in the CFAR study received pegfilgrastim with each course of treatment), or major and minor infectious complications during treatment between the 2 groups (Table 4).
Figure 2.
Progression-free and overall survival for patients treated with frontline CFAR versus historic FCR. Kaplan-Meier analyses showing progression-free survival (A) and overall survival (B) from start of treatment for patients treated with frontline CFAR (solid line) versus an historic group of patients treated with frontline FCR (dashed line).
Table 4.
Comparison of hematologic toxicity and infectious complications by proportion of courses in patients who received CFAR (260 courses in 60 patients) versus historic high-risk patients (543 courses in 119 patients) who received FCR
| % of Courses |
||||
|---|---|---|---|---|
| CFAR (n = 260) |
High-risk FCR (n = 543) |
|||
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Hematologic toxicity | ||||
| Neutropenia | 12 | 21 | 15 | 31 |
| Thrombocytopenia | 9 | 4 | 6 | 3 |
| Anemia | 3 | < 1 | 5 | < 1 |
| Infectious complications | ||||
| Major infection | 11 | 12 | ||
| Minor infection | 7 | 5 | ||
| Herpes simplex | < 1 | < 1 | ||
| Herpes zoster | < 1 | < 1 | ||
All patients in the CFAR study received pegfilgrastim on day 6 of each course of therapy whereas growth-factor support was not routine with FCR; major infection includes pneumonia, sepsis, septic shock, and fever of unknown origin requiring hospitalization; minor infection includes cellulitis, urinary tract infection, sinusitis, bronchitis, or upper respiratory tract infection.
FCR indicates fludarabine, cyclophosphamide, and rituximab; and CFAR, alemtuzumab added to FCR.
There were 18 (15%) patients in the comparison high-risk FCR group who had 17p deletion (in > 5% CLL cells) by FISH; 3 (17%) achieved CR and the ORR was 78%. Among the 18 patients in this FCR comparison group with 17p deletion, there were 2 patients with 17p deletion in 5% to 20% of CLL cells, 1 achieved CR; and among the 16 patients with 17p deletion in > 20% of CLL cells, 13% achieved CR and their ORR was 75%. Although there was a higher CR rate (P = .03) for patients with 17p deletion treated with CFAR, the median PFS and OS were not significantly different from the high-risk patients with 17p deletion who had received standard FCR-based treatment (Table 5).
Table 5.
A comparison of key features of patients with 17p deletion treated with CFAR versus historic high-risk group treated with FCR
| Parameter | CFAR (n = 14) | FCR (n = 18) |
|---|---|---|
| CR | 57%* | 17%* |
| ORR | 79% | 67% |
| Median PFS | 15 mo | 12 mo |
| Median OS | 25+ mo | 55 mo |
FCR indicates fludarabine, cyclophosphamide, and rituximab; CFAR, alemtuzumab added to FCR; CR, complete remission; ORR, overall response rate; PFS, progression-free survival; and OS, overall survival.
Fisher exact, 2-tailed test P = .03.
Discussion
The FCR regimen is the cornerstone of frontline therapy and treatment for patients with CLL in first relapse who meet NCI-WG criteria for treatment. The phase 3 randomized CLL8 trial of the GCLLSG showed superior PFS and OS for patients who received frontline FCR versus FC.3 In this phase 2 trial, we evaluated the effectiveness of FCR combined with alemtuzumab, the CD52 mAb, for improving response rates and remission duration for high-risk patients (defined as those with a β2M ≥ 4 mg/L) younger than 70 years. The results of this phase 2, single-arm study showed that CFAR is an active frontline regimen in treatment-naive, high-risk patients with CLL. There was a trend for a higher CR rate with CFAR; however, with current follow-up the time-to-event endpoints are comparable with a historic high-risk patients treated with FCR-based regimens at MDACC. In subgroup analysis, there was a higher CR rate with CFAR for patients with 17p deletion compared with the historic CR rate with frontline FCR in this patient population.
Attempts to improve on the results obtained with the standard FCR regimen have included additional agents with alternative mechanisms of action and nonoverlapping toxicities, especially for high-risk patients with untreated CLL. In a study from Mayo Clinic, rituximab was combined with subcutaneous alemtuzumab to improve response rates for untreated, high-risk patients (17p deletion or 11q deletion or a combination of unmutated IGHV and CD38+/ZAP70+) who did not have an NCI-WG indication for treatment.10 For the 30 treated patients, the CR rate was 37% and ORR was 90%; the duration of response was 14 months. The addition of mitoxantrone to FCR (FCM-R) has been explored in 2 phase 2 trials. Patients were not selected by risk status. ORR ranged from 93% to 96% and median PFS was not reached after a median follow-up of 38.5 months in the study reported by Faderl et al.11,12 Both studies suggest minimal additional benefit by adding another agent to the standard FCR regimen for standard-risk patients. An ongoing phase 3 study comparing FCR to FCM-R will clarify the use of adding mitoxantrone to FCR in the frontline setting for standard-risk CLL.
Patients with 17p deletion and/or TP53 gene mutations represent a high-risk category because of lower CR rates and shorter PFS with standard chemotherapy-, mAb-, and chemoimmunotherapy-based treatments. In contemporary chemoimmunotherapy trials, CR rates for these patients have ranged from 0% to 21%, and median PFS of < 6 to 15 months have been reported.3,13–16 Interestingly, nearly a quarter of patients in the current study had 17p deletion, much higher than expected for an unselected, treatment-naive population going on frontline therapy. To our knowledge, the CR rate of 57% is the highest reported in a frontline CLL trial in this subset of patients. However, the median PFS of 15 months with CFAR in the 17p deletion subgroup was not superior to historic patients with 17p deletion treated by FCR in our experience. Nevertheless, a high CR rate achieved by the CFAR regimen may represent an important finding if the intent is to achieve response, and then immediately move patients to allogeneic stem cell transplantation in first remission, as has been recently suggested.17,18
The use of alemtuzumab has been associated with immune suppression and prolonged increased risk for infection. Indeed, myelosuppression was routinely observed in patients treated in this study and prolonged cytopenias prevented almost a third of patients from completing the planned 6 courses of therapy, despite consistent use of pegfilgrastim with each course of treatment. However, the rates of major and minor infection were not significantly increased compared with historic high-risk patients treated with FCR. This may be attributed to the use of prophylactic antibiotics directed against opportunistic pathogens. Although the rates of late infections (> 3 months) were not significantly increased compared with previously treated patients at our institution; the occurrence of opportunistic infections up to 1 year after completion of therapy underscores the importance to remain vigilant and monitor patients carefully. In addition, supportive measures such as prolonged use of prophylactic antiviral therapy and IV immune globulin for hypogammaglobulinemia should be considered. In the current study, routine anti-infective prophylaxis was recommended for 3-6 months after completion of treatment courses for all patients. In the future, objective parameters such as Ig levels to assess B-cell recovery and CD4 count and CD4/CD8 ratios to assess T-cell function may be considered before discontinuing anti-infective prophylactic medications.
MRD-negative response has been proposed to be an important endpoint to improve both PFS and OS in previously untreated patients with CLL.19,20 Our results add to the growing body of literature demonstrating with MRD-free status associated with longer PFS. This is observed even using less sensitive methods of 3-color flow cytometry and PCR-ligase based assays to evaluate for MRD in our study. In a long-term follow-up analysis of patients who received frontline FCR for CLL at our institution,2 the median PFS among patients who achieved flow MRD-negative status was 85 months versus 49 months for patients who were flow MRD-positive. Although the median PFS for patients in this study is shorter (48+ months for flow MRD-negative patients vs 15 months for flow MRD-positive), we speculate that these differences illustrate a higher intrinsic leukemia cell proliferation for the high-risk patients with β2M ≥ 4 mg/L enrolled in this study, especially for those who were MRD-positive after treatment.
We used β2M ≥ 4 mg/L (twice the upper limit of normal) to identify high-risk patients. This group of patients had a lower CR rate and shorter median PFS with FCR-based treatments in our historic experience. Other pretreatment characteristics including IGVH gene mutation status, 17p deletion and 11q deletion, and expression of ZAP70 and CD38 have also been associated with inferior outcomes.21 When this study was designed in 2005, analysis of these newer prognostic factors was not routinely done. Therefore, we used β2M, which remains a very important prognostic factor, to identify high-risk patients with CLL. Serum β2M can be elevated in patients with renal insufficiency. Serum creatinine is a blunt measure of renal function, nevertheless, we used this to evaluate baseline renal function in these patients younger than 70 years.
In summary, the CFAR regimen represents an active frontline treatment for patients with high-risk CLL; however, with current follow-up, the PFS and OS are not superior to historic high-risk patients treated with FCR at our institution. This may be a useful regimen to achieve CR in patients with 17p deletion who are planned to proceed to allogeneic stem cell transplantation.
Acknowledgments
W.G.W. is a Leukemia & Lymphoma Society Clinical Scholar.
Footnotes
Presented in abstract form at the American Society of Hematology annual meetings of December 8-11, 2007, Atlanta, GA; December 6-9, 2008, San Francisco, CA; and December 5-8, 2009, New Orleans, LA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.A.P. collected data, analyzed and interpreted data, wrote the manuscript, and gave final approval of the manuscript; M.J.K. designed research, performed research, collected data, and gave final approval of the manuscript; S.O., A.F., S.F., J.B., C.K., and Z.E. performed research and gave final approval of the manuscript; X.W. designed research, performed statistical analysis, and gave final approval of the manuscript; X.B. performed research, collected data, analyzed and interpreted data, and gave final approval of the manuscript; S.L. collected data and gave final approval of the manuscript; and W.G.W. designed research, performed research, collected data, analyzed and interpreted data, wrote the manuscript, and gave final approval of the manuscript
Conflict-of-interest disclosure: W.G.W. is a consultant and speaker for, and served on the advisory boards of, Genentech and DSMB, and was also a speaker for Roche. The remaining authors declare no competing financial interests.
Correspondence: William G. Wierda, MD, PhD, M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX, 77030; e-mail: wwierda@mdanderson.org.
References
- 1.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 2.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112(4):975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 4.Elter T, Borchmann P, Schulz H, et al. Fludarabine in combination with alemtuzumab is effective and feasible in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: results of a phase II trial. J Clin Oncol. 2005;23(28):7024–7031. doi: 10.1200/JCO.2005.01.9950. [DOI] [PubMed] [Google Scholar]
- 5.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy B, Rawstron A, Carter C, et al. Campath-1H and fludarabine in combination are highly active in refractory chronic lymphocytic leukemia. Blood. 2002;99(6):2245–2247. doi: 10.1182/blood.v99.6.2245. [DOI] [PubMed] [Google Scholar]
- 7.Rai KR, Freter CE, Mercier RJ, et al. Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol. 2002;20(18):3891–3897. doi: 10.1200/JCO.2002.06.119. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–4997. [PubMed] [Google Scholar]
- 9.Thall PF, Simon RM, Estey EH. New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. J Clin Oncol. 1996;14(1):296–303. doi: 10.1200/JCO.1996.14.1.296. [DOI] [PubMed] [Google Scholar]
- 10.Zent CS, Call TG, Shanafelt TD, et al. Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer. 2008;113(8):2110–2118. doi: 10.1002/cncr.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faderl S, Wierda W, O'Brien S, Ferrajoli A, Lerner S, Keating MJ. Fludarabine, cyclophosphamide, mitoxantrone plus rituximab (FCM-R) in frontline CLL < 70 years. Leuk Res. 2010;34(3):284–8. doi: 10.1016/j.leukres.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch F, Abrisqueta P, Villamor N, et al. Rituximab, fludarabine, cyclophosphamide, and mitoxantrone: a new, highly active chemoimmunotherapy regimen for chronic lymphocytic leukemia. J Clin Oncol. 2009;27(27):4578–4584. doi: 10.1200/JCO.2009.22.0442. [DOI] [PubMed] [Google Scholar]
- 13.Fischer K, Cramer P, Stilgenbauer S, et al. Bendamustine combined with rituximab (BR) in first- line therapy of advanced CLL: a multicenter phase II trial of the German CLL Study Group (GCLLSG) [abstract]. Blood (ASH Annual Meeting Abstracts) 2009;114(22) Abstract 205. [Google Scholar]
- 14.Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27(4):498–503. doi: 10.1200/JCO.2008.17.2619. [DOI] [PubMed] [Google Scholar]
- 15.Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007;109(2):405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zenz T, Hoth P, Busch R, et al. TP53 mutations and outcome after fludarabine and cyclophosphamide (FC) or FC plus rituximab (FCR) in the CLL8 trial of the GCLLSG [abstract]. Blood (ASH Annual Meeting Abstracts) 2009;114(22) Abstract 1267. [Google Scholar]
- 17.Delgado J, Milligan DW, Dreger P. Allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia: ready for prime time? Blood. 2009;114(13):2581–2588. doi: 10.1182/blood-2009-05-206821. [DOI] [PubMed] [Google Scholar]
- 18.Hallam S, Gribben JG. Transplantation in chronic lymphocytic leukemia: timing and expectations. Clin Lymphoma Myeloma. 2009;9(suppl 3):S186–S193. doi: 10.3816/CLM.2009.s.010. [DOI] [PubMed] [Google Scholar]
- 19.Kwok M, Rawstron AC, Varghese A, Hillmen P. Minimal residual disease is a predictor for progression-free and overall survival in chronic lymphocytic leukemia (CLL) that is independent of the type or line of therapy [abstract]. Blood (ASH Annual Meeting Abstracts) 2009;114(22) Abstract 540. [Google Scholar]
- 20.Sayala HA, Rawstron AC, Hillmen P. Minimal residual disease assessment in chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2007;20(3):499–512. doi: 10.1016/j.beha.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Wierda WG, O'Brien S, Wang X, et al. Characteristics associated with important clinical end points in patients with chronic lymphocytic leukemia at initial treatment. J Clin Oncol. 2009;27(10):1637–1643. doi: 10.1200/JCO.2008.18.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]