Abstract
Chronic hypoxia induces polycythemia, pulmonary hypertension, right ventricular hypertrophy, and weight loss. Hypoxia-inducible factor 1 (HIF-1) activates transcription of genes encoding proteins that mediate adaptive responses to hypoxia, including erythropoietin, vascular endothelial growth factor, and glycolytic enzymes. Expression of the HIF-1α subunit increases exponentially as O2 concentration is decreased. Hif1a–/– mouse embryos with complete deficiency of HIF-1α due to homozygosity for a null allele at the Hif1a locus die at midgestation, with multiple cardiovascular malformations and mesenchymal cell death. Hif1a+/– heterozygotes develop normally and are indistinguishable from Hif1a+/+ wild-type littermates when maintained under normoxic conditions. In this study, the physiological responses of Hif1a+/– and Hif1a+/+ mice exposed to 10% O2 for one to six weeks were analyzed. Hif1a+/– mice demonstrated significantly delayed development of polycythemia, right ventricular hypertrophy, pulmonary hypertension, and pulmonary vascular remodeling and significantly greater weight loss compared with wild-type littermates. These results indicate that partial HIF-1α deficiency has significant effects on multiple systemic responses to chronic hypoxia.
J. Clin. Invest. 103:691–696 (1999)
Introduction
Many cardiopulmonary disorders, including chronic obstructive lung disease and Eisenmenger's syndrome, are associated with chronic hypoxia. The principal medical consequences of chronic hypoxia include polycythemia, pulmonary hypertension, and weight loss, all of which are associated with greatly increased mortality (1–3). Laboratory animals subjected to decreased ambient O2 concentrations manifest similar physiological responses (4–9). The use of gene-targeting techniques has provided a potential means to determine the contribution of specific genes to these responses (10, 11). Polycythemia is attributable to increased plasma levels of erythropoietin, which stimulates the survival and proliferation of erythroid progenitor cells (reviewed in ref. 12). The pathophysiology of hypoxic pulmonary hypertension is more complex and involves vasoconstriction as well as neomuscularization and thickening of the media and adventitia of pulmonary arterioles (8, 13–15). Weight loss under conditions of chronic hypoxia may reflect multiple changes in cardiovascular function, hormone production, energy metabolism, and other aspects of cellular and systemic physiology.
Physiological responses to chronic hypoxia result from altered patterns of gene expression. An essential mediator of transcriptional responses to decreased O2 availability is hypoxia-inducible factor 1 (HIF-1) (16, 17). Among the hypoxia-inducible genes that contain functionally important HIF-1 binding sites are those encoding erythropoietin (16), transferrin (18), vascular endothelial growth factor (VEGF; refs. 19, 20), VEGF receptor 1 (21), inducible nitric oxide synthase (22, 23), heme oxygenase 1 (24), and endothelin 1 (ET-1; ref. 25). The protein products of many of these genes have been implicated in the development of polycythemia or pulmonary hypertension in response to chronic hypoxia (4, 6, 26–29).
HIF-1 is a heterodimer consisting of HIF-1α and HIF-1β subunits (17, 30, 31). Whereas HIF-1β, which is also known as the aryl hydrocarbon receptor nuclear translocator (32), can dimerize with several different basic-helix-loop-helix-PAS transcription factors, HIF-1α is unique to HIF-1: its expression is tightly regulated by the cellular O2 concentration and determines the levels of HIF-1 activity (17, 33, 34). Several recent studies have demonstrated physiological regulation of HIF-1 expression and its consequences in vivo. In fetal sheep subjected to chronic anemia, cardiac hypertrophy was associated with increased myocardial vascularization and concomitantly increased myocardial expression of VEGF and HIF-1α protein (35). Expression of HIF-1α protein was also induced in isolated ferret lung preparations in a time-dependent and O2 concentra-tion–dependent manner (36). Immunohistochemical analyses of hypoxic lungs demonstrated markedly increased HIF-1α protein levels in the bronchial and alveolar epithelium and in blood vessel walls (36).
To provide definitive evidence for the role of HIF-1 in development and physiology, null alleles at the Hif1a locus encoding HIF-1α were generated by homologous recombination in mouse embryonic stem (ES) cells (37, 38). Hif1a+/– and Hif1a–/– ES cells, which were heterozygous and homozygous for the null allele, demonstrated partial and complete loss of HIF-1α expression and HIF-1 DNA-binding activity, respectively. The expression of 13 different genes encoding glucose transporters and glycolytic enzymes decreased in parallel, thus representing one of the most striking examples of coordinate genetic control of a metabolic pathway in mammalian cells (37). HIF-1α–deficient ES cells also manifested markedly decreased Vegf expression. Hif1a–/– mouse embryos that were homozygous for the mutant allele died at midgestation, with major defects in cardiovascular development and massive cell death within mesenchymal cell populations (37, 38). These results demonstrated that HIF-1α was essential for normal embryonic development, but the involvement of HIF-1α in postnatal physiology could not be determined. In contra st to Hif1a–/– embryos, Hif1a+/– mice developed normally and were indistinguishable from their wild-type Hif1a+/+ littermates under normoxic conditions. We therefore investigated whether partial deficiency of HIF-1α in adult Hif1a+/– mice would affect physiological responses to chronic hypoxia.
Methods
Animal care and use.
All procedures were approved by the Animal Care and Use Committee of The Johns Hopkins University School of Medicine. The generation of Hif1a+/– mice on a C57B6/129 genetic background was described previously (37). Offspring of Hif1a+/+ x Hif1a+/– matings were genotyped by PCR (37). All experiments involved male Hif1a+/+ and Hif1a+/– littermates that were 8 weeks old at the start of the study. Animals that were not subjected to hypoxia were studied at the same age as the hypoxic mice at the end of the study (i.e., 11 or 14 weeks old) depending on whether the study was 3 or 6 weeks in duration. To subject mice to chronic hypoxia, mice were placed in a plexiglass chamber after measurement of weight and hematocrit. Blood samples were obtained by retro-orbital sinus puncture. The chamber was maintained at 21% or 10% O2 by controlling the inflow rates of air and nitrogen. The O2 concentration was monitored continuously (OM-11 analyzer; Sensormedics, Anaheim, California, USA). CO2 levels were monitored (LB-2 gas analyzer; Sensormedics) and maintained at <0.3%. At the end of the study period, mice were anesthetized with sodium pentobarbital (60 mg/kg). Body weights and hematocrits were measured. For measurement of right ventricular pressure, mice were lightly anesthetized with sodium pentobarbital, the trachea was cannulated, and the lungs were ventilated with 10% O2 or room air at a tidal volume of 0.2 ml and a rate of 90 breaths per minute. An incision was made in the abdomen, and the diaphragm was visualized. A 23-gauge needle connected to a pressure transducer was inserted through the diaphragm into the right ventricle (RV), and pressures were recorded on a polygraph (Model 7; Grass Instruments, Quincy, Massachusetts, USA). Right ventricular puncture was verified by postmortem examination. For histological studies, the trachea was cannulated and the lungs were fixed by tracheal instillation of 10% buffered formalin while maintaining a constant tracheal pressure of 20 cm H2O. The thorax was opened, and the heart and lungs were removed en bloc. The lungs were immersed and stored in 10% buffered formalin for sectioning. The RV was dissected from the left ventricle (LV) and septum after removal of the atria. The ventricles were blotted dry and weighed.
Pulmonary vascular morphometry.
Formalin-fixed lungs were transferred to 70% ethanol, embedded in paraffin, sectioned into 5-μm slices parallel to the hilum, and stained with hematoxylin and eosin. For each lung section, vessels with an external diameter ≤100 μm were classified as nonmuscularized, partially muscularized, or completely muscularized. Approximately 500 vessels from the lungs of at least three different Hif1a+/– and Hif1a+/+ mice were scored. For all muscular vessels, video images were captured, and measurements of arterial diameter and area were obtained using a computerized image analysis program (Image 1.55; National Institutes of Health, Bethesda, Maryland, USA). The area bounded by the internal elastic lamina was subtracted from the area bounded by the external elastic lamina to obtain the area of the medial layer, which was then expressed as a percentage of the total vessel area. Percent wall thickness was also calculated as the diameter of the external elastic lamina minus the diameter of the internal elastic lamina divided by the diameter of the external elastic lamina (5).
Statistical analyses.
Differences in the development of polycythemia and RV hypertrophy in Hif1a+/– and Hif1a+/+ mice exposed to 10% O2 for 1–6 weeks were analyzed using ANOVA with a post hoc Dunnet's test. Differences in RV pressure, weight loss, and pulmonary artery wall thickness were analyzed using Student's t test. Differences in the muscularization of pulmonary arterioles were analyzed using the χ2 test. P ≤ 0.05 was considered significant.
Results
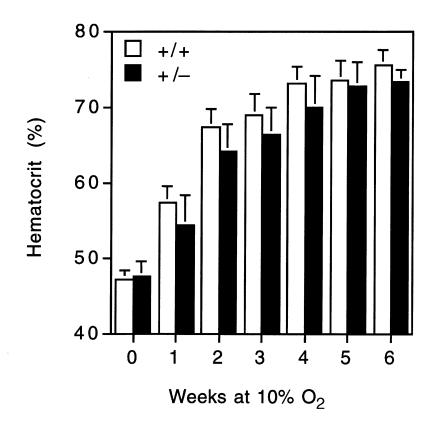
To determine the effect of partial HIF-1α deficiency on physiological responses to chronic hypoxia, 8-week-old male Hif1a+/– and Hif1a+/+ mice were exposed to 10% O2 for 0, 1, 2, 3, 4, 5, or 6 weeks. The mice were weighed, and blood was obtained for hematocrit before and after the hypoxic exposure. The mice were sacrificed, the hearts were excised, and the masses of the RV and LV plus interventricular septum (LV+S) were determined. There was no difference in the hematocrits of Hif1a+/– (47.5 ± 2.0%; mean ± SE) and Hif1a+/+ (47.1 ± 1.3%) mice maintained under normoxic conditions (Fig. 1). In contrast, there was a significant difference with respect to the development of polycythemia in Hif1a+/– and Hif1a+/+ mice (P = 0.025 by ANOVA). Compared with their wild-type littermates, Hif1a+/– mice showed a significantly delayed erythropoietic response. The differences between genotypes were most significant at 1 and 2 weeks. Thereafter, the differences gradually decreased, such that at 5 and 6 weeks, there was no difference between the two groups.
Figure 1.
Development of polycythemia in mice subjected to chronic hypoxia. Hematocrits of Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice exposed to room air or 10% O2 for 1–6 weeks were determined. Results are expressed as mean ± SE (n = 8–10 mice for 0–5 weeks; n = 5–7 mice for 6 weeks). ANOVA with a post hoc Dunnet's test revealed a significant difference between genotypes (P = 0.025).
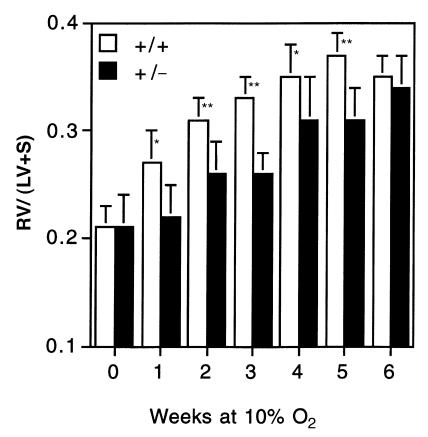
As shown in Figure 2, there was no difference between Hif1a+/– and Hif1a+/+ mice, maintained under normoxic conditions, with respect to the mass ratio of the RV to LV and septum [RV/(LV+S) ratio] (Fig. 2). However, compared with the effect of genotype on hematocrit, the Hif1a+/– mice showed an even more dramatic impairment in the development of hypoxia-induced RV hypertrophy (P = 0.0001 by ANOVA). Despite the small numbers in each group (n ≤ 10), the differences between genotypes at 1–5 weeks were highly significant (P < 0.001 at 2, 3, and 5 weeks; and P ≤ 0.01 at 1 and 4 weeks, by Student's t test). However, as in the case of the erythropoietic response, there was no significant difference between genotypes at 6 weeks. Thus, the development of both polycythemia and RV hypertrophy, two well-documented responses to chronic hypoxia, were significantly delayed, but not eliminated, in Hif1a+/– mice.
Figure 2.
Development of right ventricular hypertrophy in response to chronic hypoxia. The mass ratio of the right ventricle (RV) to left ventricle and septum (LV+S) was determined for the same Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice analyzed in Fig. 1. Results are expressed as mean ± SE. ANOVA with a post hoc Dunnet's test revealed a significant difference between genotypes (P = 0.0001). *P < 0.01 ; **P < 0.001 (Student's t test).
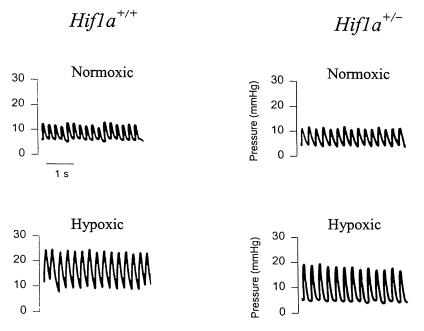
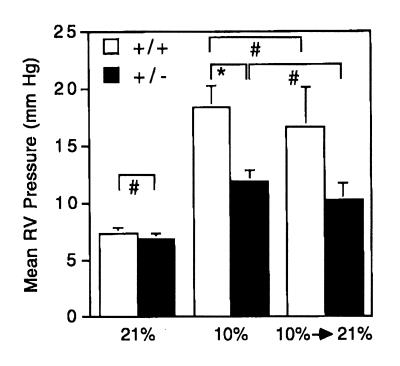
To determine whether the RV hypertrophy observed in hypoxic mice was associated with pulmonary hypertension, RV pressures were measured directly (Fig. 3). There was no significant difference in the mean RV pressures of Hif1a+/– (6.83 ± 0.48 mmHg) and Hif1a+/+ (7.33 ± 0.49 mmHg) mice under normoxic conditions (Fig. 4a). Hif1a+/– and Hif1a+/+ mice were exposed to 10% O2 for 3 weeks, and RV pressures were measured during ventilation with 10% O2. Mean RV pressure was increased in hypoxic Hif1a+/– and Hif1a+/+ mice, but the degree of pulmonary hypertension was significantly greater in Hif1a+/+ mice (18.36 ± 1.88 vs. 11.87 ± 0.95 mmHg; P = 0.003).
Figure 3.
Measurement of right ventricular pressures. Shown are representative polygraph tracings obtained from Hif1a+/+ and Hif1a+/– mice exposed to room air (Normoxic) or 10% O2 (Hypoxic) for 3 weeks.
Figure 4.
Right ventricular pressures of normoxic, hypoxic, and reoxygenated mice. Mean right ventricular (RV) pressure (± SE) was determined for Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice exposed to 21% (n = 6) or 10% (n = 11–15) O for 3 weeks, or exposed to 10% O for 3 weeks followed by 21% O for 3 h (n = 5). *P = 0.003; #P = NS (Student's t test). NS, not significant.
The difference in mean RV pressure between Hif1a+/– and Hif1a+/+ mice could reflect altered vasomotor and/or vasoproliferative responses to chronic hypoxia. Because these represent dynamic and fixed changes, respectively, we sought to distinguish between them by exposing mice to 10% O2 for 3 weeks and then returning the mice to room air for 3 hours before measuring RV pressures while the animals were ventilated with room air. The mean RV pressures of the reoxygenated mice were not significantly different from those of the chronically hypoxic mice (Fig. 4). These data suggested that partial deficiency of HIF-1α resulted in impaired hypoxia-induced vascular remodeling in Hif1a+/– mice.
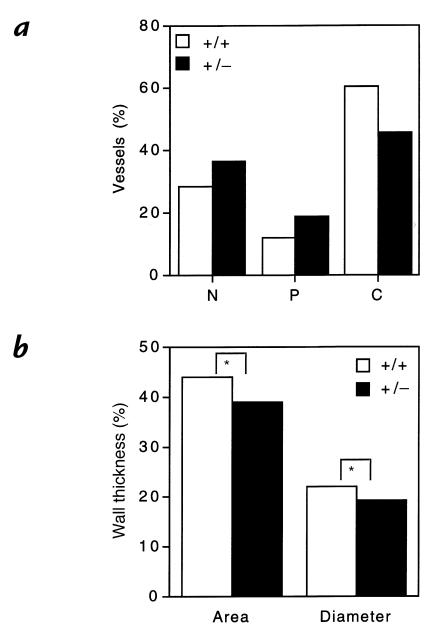
To investigate the effects of partial HIF-1α deficiency on remodeling of pulmonary arterioles directly, histological sections of lungs from Hif1a+/– and Hif1a+/+ mice exposed to 10% O2 for 3 weeks were prepared for morphometric analysis (Fig. 5). The proportion of nonmuscularized, partially muscularized, and completely muscularized pulmonary arterioles with an external diameter of ≤100 μm in Hif1a+/– and Hif1a+/+ mice (Fig. 6a) was significantly different by χ2 analysis (P = 0.00001). The decreased proportion of completely muscularized and increased proportion of nonmuscularized pulmonary arterioles in hypoxic Hif1a+/– mice was restricted to vessels with a diameter of ≤50 μm (data not shown).
Figure 5.
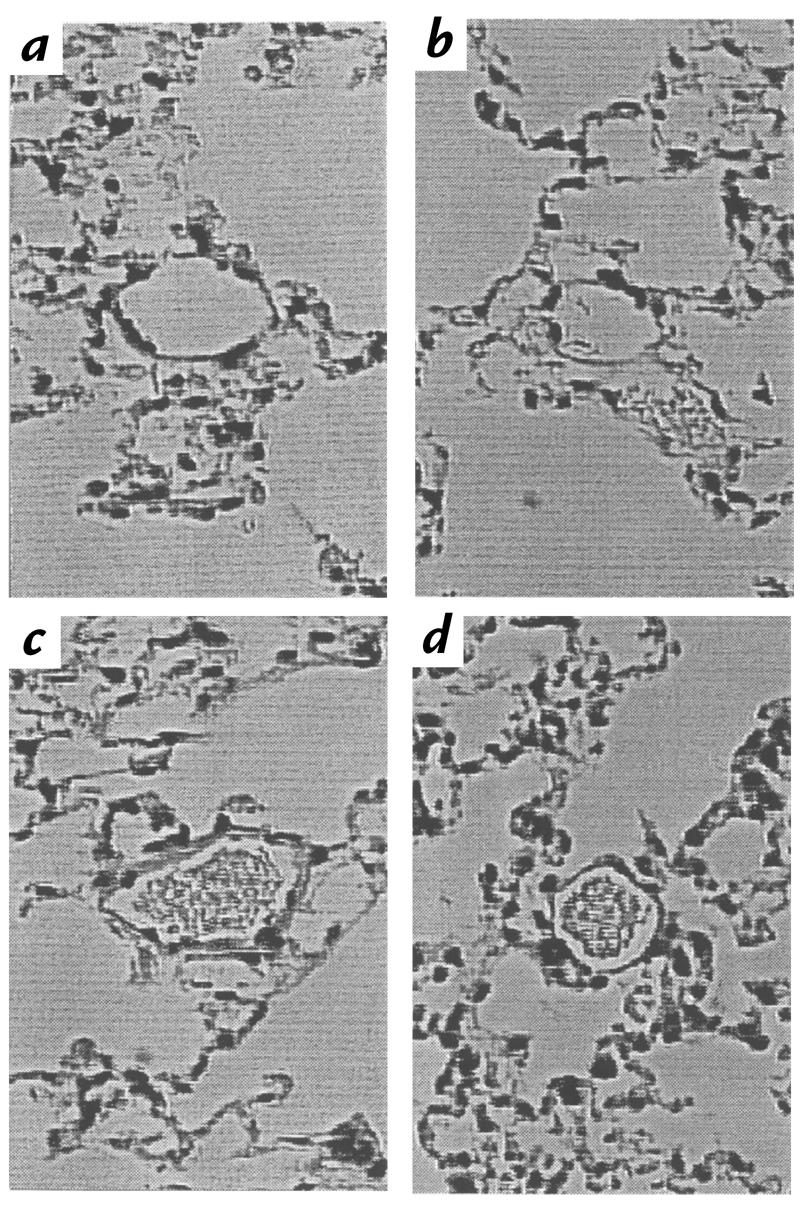
Pulmonary histology. Lungs from Hif1a+/+ (a and c) and Hif1a+/+ (b and d) mice exposed to 21% (a and b) or 10% (c and d) O for 3 weeks were formalin-fixed, paraffin-embedded, sectioned, and stained with hematoxylin and eosin for videomicroscopy. Each field shows a representative pulmonary arteriole. ×400.
Figure 6.
Morphometric analysis of pulmonary vasculature in chronically hypoxic mice. (a) Neomuscularization of pulmonary arterioles. Lung sections from Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice exposed to 10% O for 3 weeks were scored for nonmuscularized (N), partially muscularized (P), or completely muscularized (C) arterioles with an external diameter of ≤100 μm. For each genotype, >400 vessels were analyzed in lung sections from three to four mice to generate the mean data shown. χ2 analysis revealed a significant difference between genotypes (P = 0.00001). (b) Quantitative analysis of medial thickening. Percent wall thickness (% WT) was calculated for completely muscularized arterioles, based on analysis of area or diameter, according to the following formulae: % WT = ([areaext – areaint] / areaext) × 100; and % WT = ([diameterext – diameterint] / diameterext) × 100. Dimensions were demarcated by the external (ext) and internal (int) elastic laminae. For each genotype, >100 vessels were analyzed in multiple lung sections from three to four mice. Mean values are shown (SD ≤ 0.6% for each). *P < 0.001 (Student's t test).
The wall thickness of completely muscularized pulmonary arterioles with a diameter of ≤100 μm was also determined, using two different methods. In both cases, Hif1a+/– mice demonstrated a significant (P < 0.001) reduction in wall thickness when compared with Hif1a+/+ mice (Fig. 6b). These results indicate that not only did chronically hypoxic Hif1a+/– mice have fewer completely muscularized pulmonary arterioles, but the degree of muscularization in such vessels was reduced.
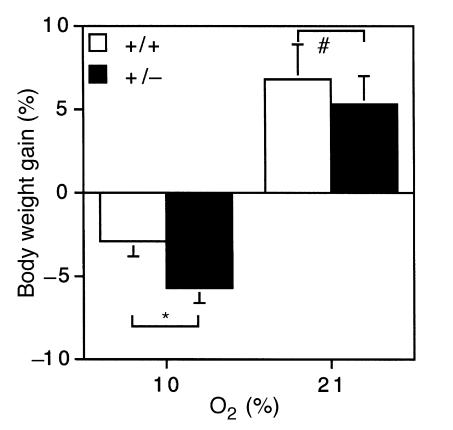
The effect of O2 concentration and Hif1a genotype on body weight was also analyzed. There was no significant difference in weight gain by Hif1a+/– and Hif1a+/+ mice maintained under normoxic conditions for 6 weeks (5.25 ± 1.74% vs. 6.73 ± 2.16%; P = 0.60), and there was no significant difference between normoxic Hif1a+/– and Hif1a+/+ mice with respect to mean body weight at the beginning or end of the study period (Fig. 7, and data not shown). Both Hif1a+/– and Hif1a+/+ mice lost weight when maintained at 10% O2 and, because weight loss was maximal after 1 week, data from the groups of mice subjected to hypoxia for 1–6 weeks were pooled to increase statistical power (Fig. 7). Hif1a+/– mice lost a significantly greater percentage of their body weight than Hif1a+/+ mice in response to chronic hypoxia (5.79 ± 0.83% vs. 2.99 ± 0.88%; P = 0.02).
Figure 7.
Analysis of weight gain under normoxic and hypoxic conditions. Percent body weight gain (% BW gain) was determined for Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice exposed to 21% O for 6 weeks (n = 10) or 10% O for 1–6 weeks (n = 54–57), using the following formula: % BW gain = ([BWfinal – BWinitial] / BWinitial) × 100. *P = 0.02; #P = NS (Student's t test).
Discussion
The results of this study demonstrate that partial HIF-1α deficiency has a significant effect on multiple physiological responses to chronic hypoxia. Despite the presence of one normally functioning allele, Hif1a+/– mice were impaired in the development of polycythemia, RV hypertrophy, pulmonary hypertension, and pulmonary vascular remodeling. Hif1a+/– mice also lost more weight than Hif1a+/+ mice. HIF-1α expression increases exponentially as O2 concentration is decreased, both in cultured cells (33) and in vivo (36), and levels of HIF-1α correlate with the expression of downstream target genes, both in cultured cells (19, 37) and in vivo (35). These results suggest that the more severe the hypoxic stimulus, the greater the magnitude of HIF-1α expression, HIF-1 DNA binding, transcription of downstream genes, and ultimate physiological responses. The data presented in this article provide a definitive connection between HIF-1α expression and physiological responses to hypoxia in adult animals. The role of HIF-1α in each of these physiological responses is considered below.
Polycythemia.
HIF-1 was initially identified as a nuclear factor that bound to the hypoxia response element of the human erythropoietin (EPO) gene and was shown to be an essential mediator of its function (16). The effect of HIF-1α deficiency on hypoxia-induced erythropoiesis may therefore be due to decreased EPO gene transcription in response to hypoxia. However, there is a wide normal range of plasma EPO levels, and small chronic changes in EPO concentration are sufficient to have major effects on hematocrit (39). Thus, demonstration of a significant difference in plasma EPO levels in chronically hypoxic Hif1a+/– and Hif1a+/ +mice may require very large sample sizes.
Pulmonary hypertension.
Analysis of Hif1a+/– and Hif1a+/+ mice suggested that the impaired development of pulmonary hypertension associated with partial HIF-1α deficiency was due to decreased muscularization of pulmonary arterioles. The significant difference in RV pressures was related to both a decrease in the number of fully muscularized resistance vessels and a decrease in the extent of medial thickening in those vessels that were fully muscularized. The observed morphometric differences may be sufficient to account for the differences in RV pressure and hypertrophy, although the possibility that long-acting vasoconstrictors such as ET-1 also contribute to the difference in pulmonary hypertension cannot be ruled out. Partial HIF-1α deficiency had a greater effect on pulmonary vascular remodeling than on erythropoiesis, suggesting that the latter effect was not sufficient to explain the former. Indeed, augmentation of polycythemia in hypoxic rats by EPO administration did not worsen pulmonary hypertension but instead was associated with decreased vascular remodeling (40). The pathophysiology of hypoxic pulmonary hypertension is exceedingly complex and incompletely understood. As described in the Introduction, several genes whose protein products have been implicated in this process are induced by hypoxia and contain HIF-1 binding sites. The observed physiological effects of partial HIF-1α deficiency may therefore represent the integrated effect of reduced expression of multiple genes, as has been demonstrated previously in ES cells (37, 38). Using an isolated perfused/ventilated ferret lung preparation, HIF-1α protein expression was analyzed by immunoblot assay as a function of inspired O2 concentration (36). This analysis revealed large amounts of HIF-1α protein in lungs ventilated with 0% O2, modest amounts at 4%, and no detectable HIF-1α protein at 7% or 10% O2. We have also been unable to detect HIF-1α expression in the lungs of wild-type mice exposed to 10% O2 (data not shown). Characterization of ES cells and mouse embryos demonstrated partial and complete loss of HIF-1α protein expression in Hif1a+/– and Hif1a–/– cells, respectively (37, 38). We therefore conclude that physiologically relevant levels of HIF-1α expression in the lung are below the sensitivity of our immunoblot assay and that partial HIF-1α deficiency is associated with decreased pulmonary vascular remodeling.
Weight loss.
Factors contributing to hypoxia-induced weight loss are also complex and incompletely defined. In addition to effects on energy metabolism via regulation of genes encoding glucose transporters and glycolytic enzymes (34, 37, 38), HIF-1α expression has recently been shown to be modulated by the insulin and insulin-like growth factor (IGF) pathway (41, 42), which in turn is regulated by hypoxia (43–45). Hypoxia has been shown to induce expression of the IGF-binding protein 1 (IGF-BP1) gene, which contains a hypoxia response element with an essential HIF-1 binding site, and levels of IGF-BP1 correlate with chronic intrauterine hypoxia and growth retardation (46). Hypoxia-induced intrauterine growth retardation was prevented in rats by administration of an ETA receptor antagonist (47), implicating ET-1 in the pathophysiology of growth retardation.
Conclusions.
Analysis of Hif1a–/– mouse embryos demonstrated the essential role of HIF-1α in prenatal development (37, 38). In this study, analysis of adult Hif1a+/– mice has revealed the importance of HIF-1α for postnatal physiological responses to hypoxia. Taken together, these results indicate that HIF-1α regulates O2 homeostasis by controlling both the establishment of key physiological systems during embryogenesis and their subsequent utilization throughout life. In addition to pulmonary hypertension, hypoxia also plays an important role in the pathophysiology of cancer, myocardial infarction, and stroke, the major causes of mortality in the United States. The role of HIF-1α and its potential as a therapeutic target in these clinical conditions are presently under investigation.
Acknowledgments
We thank Elizabeth Wagner for advice on pulmonary morphometry. This work was supported by grants from the National Institutes of Health (R01-DK39869 and R01-HL55338 to G.L. Semenza; R01-HL51912 to J.T. Sylvester), and grants from the American Heart Association National Center and Maryland Affiliate (to G.L. Semenza). G.L. Semenza is an Established Investigator of the American Heart Association.
References
- 1.Hultgren HN, Grover RF. Circulatory adaptation to high altitude. Annu Rev Med. 1968;19:119–152. doi: 10.1146/annurev.me.19.020168.001003. [DOI] [PubMed] [Google Scholar]
- 2.Moraes D, Loscalzo J. Pulmonary hypertension: newer concepts in diagnosis and management. Clin Cardiol. 1997;20:676–682. doi: 10.1002/clc.4960200804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naeije R. Pulmonary circulation at high altitude. Respiration. 1997;64:429–434. doi: 10.1159/000196719. [DOI] [PubMed] [Google Scholar]
- 4.DiCarlo VS, et al. ETA-receptor antagonism prevents and reverses chronic hypoxia-induced pulmonary hypertension in rat. Am J Physiol. 1995;269:L690–L697. doi: 10.1152/ajplung.1995.269.5.L690. [DOI] [PubMed] [Google Scholar]
- 5.Hales CA, Kradin RL, Brandstetter RD, Zhu Y-J. Impairment of hypoxic pulmonary artery remodeling by heparin in mice. Am Rev Respir Dis. 1983;128:747–751. doi: 10.1164/arrd.1983.128.4.747. [DOI] [PubMed] [Google Scholar]
- 6.Li H, et al. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J Appl Physiol. 1994;77:1451–1459. doi: 10.1152/jappl.1994.77.3.1451. [DOI] [PubMed] [Google Scholar]
- 7.Ostadal B, et al. The effect of beta adrenergic blockade on pulmonary hypertension, right ventricular hypertrophy and polycythaemia, induced in rats by intermittent high altitude hypoxia. Basic Res Cardiol. 1978;73:422–432. doi: 10.1007/BF01906523. [DOI] [PubMed] [Google Scholar]
- 8.Rabinovitch M, Gamble W, Nades AS, Miettinen OS, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamics and structural features. Am J Physiol. 1979;236:H818–H827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- 9.Widimsky J, et al. Effect of intermittent altitude hypoxia on the myocardium and lesser circulation in the rat. Cardiovasc Res. 1973;7:798–808. doi: 10.1093/cvr/7.6.798. [DOI] [PubMed] [Google Scholar]
- 10.Steudel W, et al. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest. 1998;101:2468–2477. doi: 10.1172/JCI2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voelkel NF, et al. Inhibition of 5-lipoxygenase-activating protein (FLAP) reduces pulmonary vascular reactivity and pulmonary hypertension in hypoxic rats. J Clin Invest. 1996;97:2491–2498. doi: 10.1172/JCI118696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang GL, Semenza GL. Molecular basis of hypoxia-induced erythropoietin expression. Curr Opin Hematol. 1996;3:156–162. doi: 10.1097/00062752-199603020-00009. [DOI] [PubMed] [Google Scholar]
- 13.Jin HK, et al. Hemodynamic effects of arginine vasopressin in rats adapted to chronic hypoxia. J Appl Physiol. 1989;66:151–160. doi: 10.1152/jappl.1989.66.1.151. [DOI] [PubMed] [Google Scholar]
- 14.Jones AT, Evans TW. NO: COPD and beyond. Thorax. 1997;52:S16–S21. doi: 10.1136/thx.52.2008.s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka M, Morris KG, McMurtry IF. NIP-121 is more effective than nifedipine in acutely reversing chronic hypoxic pulmonary hypertension. J Appl Physiol. 1993;75:1074–1080. doi: 10.1152/jappl.1993.75.3.1075. [DOI] [PubMed] [Google Scholar]
- 16.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang GL, Jiang B-H, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272:20055–20062. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- 19.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Circ Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 21.Gerber H-P, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes: Flt-1, but not Flk-1, is upregulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 22.Melillo G, et al. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995;182:1683–1693. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol. 1998;274:L212–L219. doi: 10.1152/ajplung.1998.274.2.L212. [DOI] [PubMed] [Google Scholar]
- 24.Lee PJ, et al. Hypoxia-inducible factor 1 mediates transcriptional activation of heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- 25.Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor 1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- 26.Kourembanas S, Morita T, Liu Y, Christou H. Mechanisms by which oxygen regulates gene expression and cell-cell interaction in the vasculature. Kidney Int. 1997;51:438–443. doi: 10.1038/ki.1997.58. [DOI] [PubMed] [Google Scholar]
- 27.Le Cras TD, Xue C, Rengasamy A, Johns RA. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am J Physiol. 1996;270:L164–L170. doi: 10.1152/ajplung.1996.270.1.L164. [DOI] [PubMed] [Google Scholar]
- 28.Ou LC, et al. Polycythemic responses to hypoxia: molecular and genetic mechanisms of chronic mountain sickness. J Appl Physiol. 1998;8:1242–1251. doi: 10.1152/jappl.1998.84.4.1242. [DOI] [PubMed] [Google Scholar]
- 29.Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia: modulation of gene expression by nitric oxide. J Clin Invest. 1995;95:1798–1807. doi: 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 31.Jiang B-H, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman EC, et al. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- 33.Jiang B-H, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1998;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 35.Martin C, et al. Cardiac hypertrophy in chronically anemic fetal sheep: increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am J Obstet Gynecol. 178:527–534. doi: 10.1016/s0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- 36.Yu AY, et al. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor 1 in the lung. Am J Physiol. 1998;275:L818–L826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 37.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semenza GL, Traystman MD, Gearhart JD, Antonarakis SE. Polycythemia in transgenic mice expressing the human erythropoietin gene. Proc Natl Acad Sci USA. 1989;86:2301–2305. doi: 10.1073/pnas.86.7.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petit RD, Warburton RR, Ou LC, Hill NS. Pulmonary vascular adaptations to augmented polycythemia during chronic hypoxia. J Appl Physiol. 1995;79:229–235. doi: 10.1152/jappl.1995.79.1.229. [DOI] [PubMed] [Google Scholar]
- 41.Agani F, Semenza GL. Mersalyl is a novel inducer of vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Mol Pharmacol. 1998;54:749–754. doi: 10.1124/mol.54.5.749. [DOI] [PubMed] [Google Scholar]
- 42.Zelzer E, et al. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim KW, et al. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998;58:348–351. [PubMed] [Google Scholar]
- 44.Tapanainen PJ, et al. Hypoxia-induced changes in insulin-like growth factors and their binding proteins in pregnant rats. Horm Res. 1997;48:227–234. doi: 10.1159/000185520. [DOI] [PubMed] [Google Scholar]
- 45.Tucci M, et al. Modulation of insulin-like growth factor (IGF) and IGF binding protein biosynthesis by hypoxia in cultured vascular endothelial cells. J Endocrinol. 1998;157:13–24. doi: 10.1677/joe.0.1570013. [DOI] [PubMed] [Google Scholar]
- 46.Tazuke SI, et al. Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia. Proc Natl Acad Sci USA. 1998;95:10188–10193. doi: 10.1073/pnas.95.17.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thaete LG, Neerhof MG, Caplan MS. Endothelin receptor A antagonism prevents hypoxia-induced intrauterine growth restriction in the rat. Am J Obstet Gynecol. 1997;176:73–76. doi: 10.1016/s0002-9378(97)80014-2. [DOI] [PubMed] [Google Scholar]