Parallel evolution of two functionally linked gene families has resulted in diel regulatory characteristics in the phosphorylation of PEPC that differs between Flaveria spp. with C3 and C4 photosynthetic types.
Abstract
The key enzyme for C4 photosynthesis, Phosphoenolpyruvate Carboxylase (PEPC), evolved from nonphotosynthetic PEPC found in C3 ancestors. In all plants, PEPC is phosphorylated by Phosphoenolpyruvate Carboxylase Protein Kinase (PPCK). However, differences in the phosphorylation pattern exist among plants with these photosynthetic types, and it is still not clear if they are due to interspecies differences or depend on photosynthetic type. The genus Flaveria contains closely related C3, C3-C4 intermediate, and C4 species, which are evolutionarily young and thus well suited for comparative analysis. To characterize the evolutionary differences in PPCK between plants with C3 and C4 photosynthesis, transcriptome libraries from nine Flaveria spp. were used, and a two-member PPCK family (PPCKA and PPCKB) was identified. Sequence analysis identified a number of C3- and C4-specific residues with various occurrences in the intermediates. Quantitative analysis of transcriptome data revealed that PPCKA and PPCKB exhibit inverse diel expression patterns and that C3 and C4 Flaveria spp. differ in the expression levels of these genes. PPCKA has maximal expression levels during the day, whereas PPCKB has maximal expression during the night. Phosphorylation patterns of PEPC varied among C3 and C4 Flaveria spp. too, with PEPC from the C4 species being predominantly phosphorylated throughout the day, while in the C3 species the phosphorylation level was maintained during the entire 24 h. Since C4 Flaveria spp. evolved from C3 ancestors, this work links the evolutionary changes in sequence, PPCK expression, and phosphorylation pattern to an evolutionary phase shift of kinase activity from a C3 to a C4 mode.
C4 plants evolved from C3 plants by developing a spatial separation for the process of carbon fixation in the leaves and carrying it out in two cell types, mesophyll and bundle-sheath cells. This special leaf anatomy is known as Kranz anatomy and includes enlarged, chloroplast-rich bundle-sheath cells around the closely spaced veins to ensure an intense contact between the mesophyll and bundle-sheath cells (Sage et al., 2012). During C4 photosynthesis, atmospheric CO2 is initially fixed by Phosphoenolpyruvate Carboxylase (PEPC) in the mesophyll cells. Here, PEPC catalyzes the β-carboxylation of phosphoenolpyruvate, using HCO3− as a substrate, leading to the formation of the four-carbon organic acid oxaloacetate. Oxaloacetate is reduced to malate or transaminated to Asp and transported into the bundle-sheath cells, where CO2 is released by a decarboxylating enzyme (Edwards and Walker, 1983). By this process, CO2 is effectively concentrated in the bundle-sheath cells, bringing its concentration up to 1,500 µL L−1 (Hatch, 1987; Sage et al., 2012). CO2 is refixed by Rubisco, which in C4 plants is restricted to bundle-sheath cells, and further metabolized by the Calvin-Benson cycle (Sage, 2004). This characteristic allows C4 plants to survive in more extreme environments, where heat and the lack of water cause the closing of stomata and result in low CO2 concentrations in the intercellular spaces around the mesophyll cells (Ehleringer et al., 1997).
However, the evolution of C4 photosynthesis involved more than just the development of Kranz anatomy. It included changes in the transcriptional regulation of genes that encode components of the C4 pathway and the adjustment of enzyme properties and their regulation (Sheen, 1999; Sage, 2004; Akyildiz et al., 2007; Engelmann et al., 2008; Aubry et al., 2011; Brown et al., 2011; Ludwig, 2011; Wiludda et al., 2012; Paulus et al., 2013a, 2013b). In the process of evolution toward C4 photosynthesis, PEPC was influenced by a series of single-amino acid exchanges that raised the Michaelis-Menten constant for the substrate phosphoenolpyruvate (Bläsing et al., 2000) and lowered the sensitivity to malate and Asp (Engelmann et al., 2002; Paulus et al., 2013a, 2013b). So far, there is no information if such exchanges have also occurred in the regulatory protein Phosphoenolpyruvate Carboxylase Protein Kinase (PPCK), whose phosphorylation of PEPC changes the kinetic and regulatory properties of the enzyme (Nimmo, 2003). In order to shed light on two questions (What are the differences in PEPC regulation, by phosphorylation, between plants with C3 and C4 photosynthesis? And how did PEPC phosphorylation in C4 plants evolve to the state it is in today?), we turned to species in the genus Flaveria.
Although the first C4 plants originated about 30 million years ago (Pagani et al., 2005; Tipple and Pagani, 2007), the much younger genus Flaveria is the preferred model for studying the evolution of C4 photosynthesis. Having evolved in the last 3 million years, the species contained within this genus still retain a high level of similarity and allow the study of changes driven predominantly by the evolution of C4 photosynthesis (Christin et al., 2011; Sage et al., 2012). Most importantly, the genus contains C4, C3-C4 intermediate, and C3 species, such as Flaveria trinervia, Flaveria ramosissima, and Flaveria pringlei, respectively (Sage, 2004; McKown et al., 2005). This palette of species with various photosynthetic types allows investigation of the gradual development of C4-related characteristics by comparative analysis.
Contrary to its photosynthetic function in C4 plants, in C3 plants PEPC is nonphotosynthetic and involved in diverse functions, such as replenishment of the tricarboxylic acid cycle, carbon-nitrogen interactions, carbon storage, and pH maintenance (O’Leary et al., 2011). This multifaceted role of PEPC in plant metabolism is underlined by a complex regulation system (O’Leary et al., 2011).
In order to carry out all these functions, PEPC in all investigated angiosperms is encoded by small gene families (Lepiniec et al., 1994). In Flaveria spp., the PEPC gene family is composed of four genes, ppcA to ppcD, with the latter being a bacterial PEPC and out of the scope of this article (Hermans and Westhoff, 1990, 1992). The ppcA gene of C4 Flaveria spp. encodes the C4-type photosynthetic PEPC isoform, which is highly expressed in the mesophyll cells of C4 Flaveria spp., while its evolutionary ortholog in C3 Flaveria spp. encodes a typical nonphotosynthetic C3-type PEPC (Hermans and Westhoff, 1992; Svensson et al., 2003). ppcB and ppcC of both C3 and C4 Flaveria spp. encode nonphotosynthetic PEPC isoforms with a ubiquitous expression pattern (Ernst and Westhoff, 1997; Svensson et al., 2003).
As a primary enzyme in C4 photosynthesis or primary metabolism in C3 plants, PEPC is controlled both metabolically and posttranslationally. Malate, Asp, and oxaloacetate function as negative feedback regulators, while the PEPC activity increases in the presence of triose and hexose phosphate (Rajagopalan et al., 1994; Law and Plaxton, 1997; Bläsing et al., 2002; Svensson et al., 2003; Takahashi-Terada et al., 2005; Jacobs et al., 2008). The C4 ppcA PEPC isoform has been shown to have a lower sensitivity for the allosteric inhibitors mentioned above, a characteristic that is explained by a single-amino acid mutation from Arg-884 to Gly (Paulus et al., 2013a, 2013b).
Both phosphorylation and ubiquitination have been indicated as posttranslational modifications that affect PEPC activity. Monoubiquitination of PEPC has been identified only recently; it was found to be tissue specific and to influence the feedback regulation of PEPC by various metabolites (Agetsuma et al., 2005; Uhrig et al., 2008; O’Leary et al., 2011; Shane et al., 2013).
Phosphorylation of the photosynthetic PEPC of C4 and Crassulacean acid metabolism (CAM) plants has been known for decades (Budde and Chollet, 1986; Nimmo et al., 1986; Jiao and Chollet, 1989). Phosphorylation of PEPC was shown to be induced by several factors, such as light or the availability of nitrogen and phosphorus (Leport et al., 1996). Diel regulation of phosphorylation is evident for CAM plants, Arabidopsis (Arabidopsis thaliana), maize (Zea mays), sorghum (Sorghum bicolor), Digitaria sanguinalis, and wheat (Triticum aestivum; Jiao and Chollet, 1988; Echevarría et al., 1990; McNaughton et al., 1991; Duff and Chollet, 1995; Giglioli-Guivarc’h et al., 1996; Nimmo, 2000; Fontaine et al., 2002). However, these same studies outline some crucial differences among species when grouped by photosynthesis type. In C4 plants, the phosphorylation of PEPC is induced by light and decreased in the dark (Ueno et al., 2000). In CAM plants, both the expression of the PPCK transcript and the phosphorylation level of PEPC peak at night (Nimmo, 2000), while in C3 plants, mixed results were found (Van Quy et al., 1991; Duff and Chollet, 1995; Li et al., 1996; Fukayama et al., 2006; Meimoun et al., 2009).
In the context of the changes occurring during C4 evolution, it is crucial to understand whether the above differences result from the different photosynthetic types among the studied species or are because of species individuality. The effects of malate and Glc-6-P on PEPC activity are modulated by phosphorylation, which decreases the inhibition of PEPC by malate and renders the enzyme more sensitive to the activator Glc-6-P. Thus, phosphorylation seems to broaden the conditions under which PEPC can be active and seems to decrease the Km, while the Vmax of PEPC is only modestly affected (Duff et al., 1995; Tovar-Méndez et al., 2000; Takahashi-Terada et al., 2005). Interestingly, the knockdown of PEPC phosphorylation in Flaveria bidentis (C4) by RNA interference inhibition of PPCK did not affect the CO2 assimilation rate, although the response to malate was observed as in previous studies (Furumoto et al., 2007). The latter indicates that phosphorylation is probably required to adjust PEPC activity in response to various signals or fluctuating conditions or to potentially mediate the interaction with another protein, such as 14-3-3 proteins (O’Leary et al., 2011; Grieco et al., 2012). The implicated phosphorylation site is located on the N terminus of PEPC and has been identified as Ser-6, Ser-8, Ser-11, or Ser-15 among various plants (Jiao and Chollet, 1990; Wang et al., 1992; Lepiniec et al., 1994; Chollet et al., 1996; Vidal and Chollet, 1997; Tripodi et al., 2005).
PEPC phosphorylation is carried out by a kinase named PPCK found to range in size from 30 to 39 kD in diverse species (Chollet et al., 1996). PPCK is most similar to a Ca2+-independent Ser/Thr protein kinase. It has been classified as belonging to the Ca2+-sensing calmodulin-like regulated calcium-dependent protein kinase SNF1-related kinase superfamily (Hartwell et al., 1996, 1999; Halford and Hardie, 1998; Hrabak et al., 2003). The kinase is composed of a single core domain without broader N- and C-terminal extensions. The interaction between the kinase catalytic domain and the substrate has been studied for other CDP kinases whose catalytic domain is composed of two lobes: an N-terminal lobe containing β-sheets and a C-terminal lobe composed of α-helices. It is thought that the ATP molecule is bound in a cleft between these two lobes, while the substrate binds along the cleft (Ubersax and Ferrell, 2007). On the substrate protein, the amino acids flanking the phosphorylation site, probably up to four residues on either side, are thought to contribute to substrate recognition. However, residues farther away from the phosphorylation site can interact with portions of the kinase just outside the active site (Ubersax and Ferrell, 2007). Echevarria and Vidal (2003) list the consensus domain acid-base-X-X-S-I-D-A-Q-L-R as characteristic for phosphorylation by PPCK.
The protein amount of PPCK seems to be regulated at the level of transcription and protein synthesis/turnover in C4 maize and seeds from C3 plants (Jiao et al., 1991; Hartwell et al., 1999; Osuna et al., 1999; Echevarria and Vidal, 2003). In addition, the activity and/or expression of the PPCK protein are known to be influenced by the reduction of disulfide bonds (Saze et al., 2001; Tsuchida et al., 2001; Nimmo, 2003), a proteinaceous inhibitor (Nimmo, 2000), and inhibition by malate (Borland et al., 1999; Uhrig et al., 2008).
PPCK proteins were found to be encoded by a small gene family (PPCK), which in most investigated species is composed of at least two isoforms (Fontaine et al., 2002; Nimmo, 2003; Fukayama et al., 2006; Shenton et al., 2006). The genus Flaveria is an exception, with only one characterized PPCK gene, encoding a 31.8-kD protein identified in F. trinervia and F. bidentis (Tsuchida et al., 2001; Furumoto et al., 2007). This raises the question of whether these species are a true exception in possessing only a single PPCK gene or if the nucleic acid sequences for further isoforms simply have been missed so far. Furthermore, the PPCK genes are not described in the C3 and C3-C4 intermediate Flaveria spp. This study, therefore, aimed to identify the genomic representation of PPCK genes in the genus Flaveria and to investigate the evolutionary characteristics related to C4 photosynthesis.
RESULTS
PPCK Is Encoded by a Two-Member Gene Family in Flaveria spp., and Both Genes Were Altered in the Process of C4 Evolution
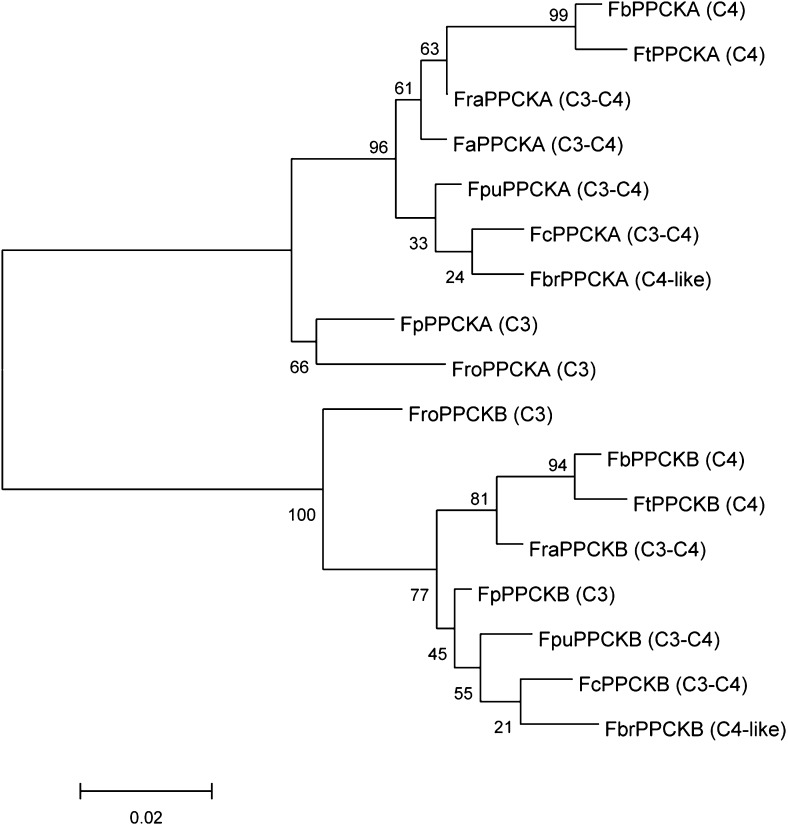
To investigate the number of PPCK isoforms in Flaveria spp., previous leaf transcriptome data for the two C3 species F. pringlei and Flaveria robusta, the C3-C4 intermediate F. ramosissima, and the two C4 species F. trinervia and F. bidentis (Gowik et al., 2011) were complemented by RNA sequencing (RNAseq) data that included four additional C3-C4 intermediates, namely Flaveria chloraefolia, Flaveria pubescens, Flaveria anomala, and Flaveria brownii (Mallmann et al., 2014). In all nine Flaveria spp., we consistently found two PPCK isoforms (Supplemental File S1). A phylogenetic analysis of the PPCK proteins showed that the two isoforms clearly differ from each other and belong to two separate branches of a maximum-likelihood phylogram (Fig. 1). We will thus refer to them as PPCKA and PPCKB. The PPCKA isoform branching reflects the photosynthetic type of the species. At the base of the branch are the sequences from the C3 plants, and the evolutionary time increases toward the sequence form of the C4 species. In the case of PPCKB, there is high similarity between the PPCKB isoforms from the species with C3 and intermediate photosynthesis types. These results are similar to previous phylogenetic analyses of PEPC isoforms in Flaveria spp. (Svensson et al., 2003; Fig. 1).
Figure 1.
Phylogenetic analysis of the PPCK family in Flaveria spp. Two PPCK isoforms, A and B, are found in each Flaveria spp. that cluster together to form individual groups. In both situations, the isoforms from the C4 species show the largest evolutionary distance from the C3 species. Species are indicated as follows: F. trinervia (Ft), F. bidentis (Fb), F. brownii (Fbr), F. ramosissima (Fra), F. anomala (Fa), F. pubescence (Fpu), F. chloraefolia (Fc), F. pringlei (Fp), and F. robusta (Fro). FaPPCKB was not used in the phylogeny due to partial sequence coverage. The photosynthesis type for each species is indicated on the phylogenetic tree. Evolutionary analyses were conducted in MEGA5 (Tamura et al., 2011; Hall, 2013). Sequences were aligned using MUSCLE (Edgar, 2004) with default settings for 1,000 iterations. The evolutionary history was inferred by using the maximum likelihood method based on the Jones-Taylor-Thornton matrix-based model (Jones et al., 1992). The tree with the highest log likelihood (−1,268.7007) is shown. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 17 amino acid sequences. All positions containing gaps and missing data were eliminated. The reliability of the evolutionary relationship was estimated using bootstrap variances (1,000 replicates), indicated in percentages at the branches.
Extending the analysis to a selection of known PPCKs from C3, CAM, and C4 species, we found that the PPCKs cluster primarily based on their systematic relationships rather than photosynthetic type or PPCK isoform (Supplemental File S2). All PPCK isoforms from the genus Flaveria cluster together, just as the PPCKs from Magnoliophyta and the rosids do. Second, within the systematics groups, further organization driven by PPCK isoform is observed with Flaveria spp., Fabales (Lotus and Soja spp.), and the order Brassicales (Arabidopsis, C3; Cleome gynandra, C4; Bräutigam et al., 2011). Similarly, in the family Poaceae, here represented by maize (C4), sorghum (C4), Setaria italica (C4), and rice (Oryza sativa; C3), all PPCKs are grouped together, but within this clade the subdivision is driven by the high similarity of PPCK1 proteins. These data indicate that the PPCK isoforms evolved independently, after speciation of the highly divergent species (Supplemental File S2).
In order to pinpoint any evolutionary changes among the Flaveria spp. that were associated with the transition from C3 to C4 photosynthesis in this genus, we compared the amino acid differences in the PPCK sequences. Amino acid exchanges of interest were defined as conserved changes found in both C3 species (F. robusta and F. pringlei) on one side and both C4 species (F. bidentis and F. trinervia) on the other, while the exchange could be absent or present in the intermediate species. Following these guidelines, three types of mutations were observed: (1) exchanges found in all intermediate and C4 species; (2) exchanges found in some intermediate and C4 species; and (3) exchanges found only in the C4 species. In the case of PPCKA, exchanges of type 1 are E80D, G160A, G165R, G185M and L257F; type 2 exchanges are E163D and S270N; and type 3 exchanges are A4T, I135L, S147G, Y211H, E217D, and E273K (Fig. 2; Table I).
Figure 2.
Amino acid exchanges between Flaveria spp. with C3 and C4 photosynthesis. A, Multiple sequence alignment of PPCK A and B isoforms from nine Flaveria spp. using MUSCLE (Edgar, 2004). B, Schematic model of PPCKA from F. pringlei, with marked positions of amino acid exchange among C3 and C4 species for both isoforms. C, Three-dimensional model of PPCKA from F. trinervia (blue) aligned onto the model from F. pringlei (red); the positions of the amino acid exchanges are marked in yellow and numbered according to the alignment shown in A. The protein kinase catalytic core domain starts at amino acid position 15 at the N terminus and ends at position 268 at the C terminus (according to BAB71853 and BAF4832). Amino acids involved in ATP binding are indicated in red, and those involved in substrate binding are marked in light blue. Amino acids in green are involved in ATP and substrate binding; dark blue boxes refer to the A-loop. Highlighted background or stars indicate amino acid residues that differ between species with C3 and C4 photosynthesis. PPCK sequences from C4 Flaveria spp. were taken from the accessions BAB71853 and BAF4832. The remaining Flaveria spp. PPCK sequences were cloned and/or obtained from RNAseq. Sequences used for the alignment and modeling are listed in Supplemental File S1. Species are indicated as follows: C4 species: F. trinervia (Ft) and F. bidentis (Fb); C4-like species: F. brownii (Fbr); C3-C4 species: F. ramosissima (Fra), F. anomala (Fa), F. pubescens (Fpu), and F. chloraefolia (Fch); and C3 species: F. pringlei (Fp) and F. robusta (Fro). The three-dimensional models are calculated using SWISS-MODEL (Schwede et al., 2003; Arnold et al., 2006; Kiefer et al., 2009) using the sequences for FtPPCKA and FpPPCKA listed in Supplemental File S1. PPCKAs from F. pringlei and F. trinervia are modeled onto a calmodulin domain protein kinase 1 from Toxoplasma gondii, with sequence similarities of 0.4 (sequence identity of 38.49%) and 0.4 (sequence identity of 39.62%), respectively. The models with highest Qualitative Model Energy Analysis values (−0.85 and −0.78 for FpPPCKA and FtPPCKA, respectively) were aligned in iPBA (de Brevern et al., 2000; Joseph et al., 2010; Benkert et al., 2011), and the resulting file was displayed in PyMol version 1.3 (http://www.pymol.org/).
Table I. Amino acid exchanges in PPCK isoforms occurring among C3 and C4 Flaveria spp. compared with C3 and C4 species from Brassicales and Poaceae.
Amino acids are listed using standard single-letter code, with positions marked according to the F. pringlei PPCKA sequence. An alignment of all sequences is presented in Supplemental File S3. Boldface letters indicate amino acid exchanges found in some intermediate species and species with C4; italic letters indicate amino acid exchanges found in all intermediate species and species with C4; underlined letters indicate amino acid exchanges found only in species with C4 photosynthesis. Species indicated with single asterisks perform C3 photosynthesis; those indicated with double asterisks perform C4 photosynthesis; all others are C3-C4 intermediates and the C4-like F. brownii (Fbr). Dashes indicate that sequence information is missing or a residue is not present. Species abbreviations are as follows: Arabidopsis (At), C. gynandra (Cg), maize (Zm), rice (Os), S. italica (Si), and sorghum (Sb). The accession numbers for the sequences are listed in Supplemental File S2, except for C. gynandra; the sequence for C. gynandra is included at the end of Supplemental File S1.
| Species | Residue Position Based on FpPPCKA Protein Sequence |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPCK | 4 | 49 | 80 | 87 | 109 | 123 | 135 | 147 | 160 | 163 | 165 | 185 | 211 | 217 | 233 | 257 | 270 | 273 | |
| Flaveria spp. | |||||||||||||||||||
| Fp* | A* | A | T | E | I | T | S | I | S | G | E | G | R | Y | E | L | L | S | E |
| Fro* | A* | A | T | E | I | A | S | I | S | G | E | G | R | Y | E | L | L | S | E |
| Fc | A | A | T | D | I | T | S | I | S | A | E | R | M | Y | E | L | F | S | E |
| Fa | A | A | T | D | I | T | S | I | S | A | D | R | M | Y | E | L | F | S | E |
| Fpu | A | A | T | D | I | T | S | I | S | A | E | R | M | Y | E | L | F | S | E |
| Fra | A | A | T | D | I | T | S | I | S | A | D | R | M | Y | E | L | F | N | E |
| Fbr | A | A | T | D | I | T | S | I | S | A | E | R | M | Y | E | L | F | S | E |
| Fb** | A** | T | T | D | I | T | S | L | G | A | D | R | M | H | D | L | F | N | K |
| Ft** | A** | T | T | D | I | T | S | L | G | A | D | R | M | H | D | L | F | N | K |
| Fp* | B* | V | T | E | I | S | S | I | S | A | E | G | R | Y | D | I | L | S | E |
| Fro* | B* | V | T | E | I | S | S | I | N | A | E | G | R | Y | D | I | L | S | E |
| Fc | B | – | T | E | I | S | S | I | S | A | E | G | R | Y | D | M | L | S | E |
| Fpu | B | – | T | E | I | S | S | I | S | A | E | G | R | Y | D | M | L | S | E |
| Fra | B | – | T | E | M | A | S | I | S | A | E | G | R | Y | D | I | L | S | E |
| Fbr | B | V | T | E | I | S | S | I | S | A | E | G | R | Y | D | M | L | S | E |
| Fb** | B** | V | I | E | M | A | G | I | S | G | E | G | R | Y | D | M | L | S | E |
| Ft** | B** | – | I | E | M | A | G | I | S | A | E | G | R | Y | D | M | L | S | E |
| Brassicales | |||||||||||||||||||
| At* | 1* | T | L | D | M | P | S | I | T | G | – | E | Y | Y | E | I | F | R | E |
| Cg** | T | L | N | M | A | S | I | V | G | – | E | R | Y | E | I | F | N | A | |
| At* | 2* | E | L | E | M | S | A | V | G | G | – | E | R | N | D | K | F | N | N |
| Poaceae | |||||||||||||||||||
| Os* | 1* | A | L | D | V | H | A | V | V | G | – | R | R | Y | E | A | F | S | G |
| Zm** | 1** | A | L | A | L | P | A | V | A | G | – | R | R | S | E | A | L | T | G |
| Sb** | 1** | A | L | A | L | P | A | V | A | G | – | R | R | S | D | A | L | T | G |
| Si** | 1** | A | L | A | L | P | A | V | A | G | – | R | R | G | E | A | F | T | G |
| Bd* | 1* | E | L | E | M | P | A | V | R | G | – | G | G | S | D | L | F | N | G |
| Bd* | 2* | E | L | E | M | P | A | V | R | G | – | G | G | S | D | L | F | N | G |
| Os* | 3* | E | L | D | M | P | A | V | R | G | – | I | G | G | D | L | F | S | G |
| Sb** | 3** | – | – | D | M | V | A | V | G | G | G | R | G | – | D | – | – | S | G |
| Zm** | 4** | Q | L | D | M | P | A | V | R | G | A | G | G | G | D | L | F | S | G |
| Si** | 3** | Q | L | D | M | P | A | V | R | G | G | G | G | G | E | L | F | S | G |
| Os* | 2* | E | L | D | M | P | A | V | R | G | – | G | G | G | E | L | F | S | G |
| Bd* | E | L | E | M | P | A | V | R | G | E | G | G | G | E | L | F | S | G | |
| Sb** | 2** | E | L | E | M | P | A | V | R | G | G | G | G | G | E | L | F | S | G |
| Zm** | 3** | E | L | E | M | P | A | V | R | G | G | G | G | G | E | L | F | S | G |
| Zm** | 2** | E | L | E | I | P | A | V | R | G | G | G | G | G | E | L | F | S | G |
| Si** | 2** | E | L | E | M | P | A | V | R | G | G | G | G | G | E | L | F | S | G |
In line with the parallel evolution of the PPCK isoforms seen in the phylogenetic analysis (Supplemental File S2), these amino acid exchanges seem characteristic for the genus Flaveria (Table I; Supplemental File S3). A comparison of sequences from the order Brassicales and the family Poaceae, both of which have C3 and C4 representatives, shows a similar trend of C3-to-C4 exchanges primarily for PPCK1 (Supplemental File S3). However, it must be noted that for Brassicales, only single PPCK1 representatives from C3 and C4 species are available, and in Poaceae, we have a single C3 PPCK1 representative from rice (Supplemental File S2). It is thus difficult to conclude if the observed differences are conserved among related species with the same type of photosynthesis or if we are seeing species-specific characteristics. The presence of the three PPCK1 sequences in Poaceae originating from C4 species that all show the same amino acid difference from the rice PPCK1 sequence supports the hypothesis that we are observing C3- to C4-related changes, as in the genus Flaveria (Table I).
In order to visualize the distribution of these exchanges in Flaveria spp., we first summarized the mutations in a schematic model (Fig. 2B) and could observe that most of the conserved differences between C3 and C4 species are in the C-terminal part of the kinase. Subsequently, the tertiary structure of PPCKA from F. pringlei (C3) and F. trinervia (C4) was modeled using SWISS-MODEL (de Brevern et al., 2000; Joseph et al., 2010; Benkert et al., 2011); the models were aligned in iPBA (Gelly et al., 2011) and displayed using PyMol version 1.3. The sequence identity of the template (Protein Data Bank identifier 3ku2.1.A) used for modeling was 38.49% and 39.62% for F. pringlei (C3) and F. trinervia (C4), respectively (Fig. 2C). The structure of the kinase was composed of N- and C-terminal lobes with an unstructured protein region between them. The cluster of amino acid exchanges on positions 135, 160, 163, 165, and 185 was found on the unstructured protein region in the three-dimensional model, while most other exchanges were located on the C-terminal lobe (Fig. 2, B and C).
For PPCKB, only five amino acid exchanges were found among C3 and C4 species according to the above criteria. I87M, S109A, and I233M are found in some intermediates and the C4 species (type 2), while T49I and S123G are found in the C4 species only (type 3; Fig. 2A; Table I). The smaller number of differences in this isoform, which are related to the photosynthetic type of the species, illustrates that higher evolutionary pressure was put on optimization of the structure of PPCKA.
PPCK Genes Have Diel Expression Patterns in Flaveria spp., and Their Levels Vary between C3 and C4 Species
The differences in the protein sequences among PPCK isoforms led to the question of whether changes in the regulation of transcript abundance were also present among species with C3 and C4 photosynthesis. Therefore, we investigated PPCK transcript levels by a 24-h harvest experiment and profiled the samples by Illumina RNAseq. Plants from F. trinervia (C4) and F. pringlei (C3) were grown in a 10/14-h (light/dark) period, and harvesting was performed every 4 h from the onset of light (Fig. 3).
Figure 3.
Transcript abundances of the PPCK genes from Flaveria spp. along a light/dark cycle. Each graph represents the circadian transcript expression of PPCKA (A), normalized PPCKA (B), PPCKB (C), and normalized PPCKB (D) genes from F. pringlei (C3) and F. trinervia (C4) plants grown under light (white background) and dark (gray background) conditions, also indicated as the white (light) and black (dark) horizontal bars. The transcript abundances of PPCK genes are presented in RPMK as averages of three biological replicates measured by RNAseq. Normalized RPMK values for each time point were expressed as percentages from the sum of RPMK values for the respective time series for each transcript (B and D). F. trinervia (C4), blue lines; F. pringlei (C3), red lines; PPCKA, black circles; PPCKB, white circles. Lights were turned on at 0 h and off at 10 h diurnal time. The se was calculated for each time course of three biological replicates. Significant differences between each time point were determined with the RPMK values using a one-way ANOVA (Tukey test) and additionally by Student’s t test (P ≤ 0.05; Supplemental File S4).
Figure 3 depicts that PPCKA and PPCKB transcripts exhibit strikingly different patterns of accumulation. Both PPCKA and PPCKB transcripts, in F. trinervia (C4) as well as in F. pringlei (C3), fluctuate with a phase shift of 12 h. Both transcripts can be classified as having diel regulation, since a significant difference in the P values (P ≤ 0.05) and at least a 3-fold change can be simultaneously observed between a reference time (4 h of light) and three consecutive time points (Facella et al., 2008). However, PPCKA and PPCKB transcripts differ in the temporal order of maxima and minima. While PPCKA transcript amounts of F. trinervia and F. pringlei reach their maximum during the day, PPCKB transcripts peak in the dark.
The abundance of PPCKA transcripts differs between species with varying photosynthesis types. At the time of expression maximum (4 h after the onset of light), the amounts of PPCKA transcripts are at least 10-fold higher in F. trinervia (C4) than in F. pringlei (C3). As can be seen in Figure 3B, the relative levels in F. pringlei seem to drop a bit faster than in the C4 species, resulting in higher differences in later time points (Fig. 3; Supplemental File S4).
This increase in PPCK expression was only found in PPCKA. In the case of the PPCKB transcript, we found that the amounts in F. pringlei (C3) are generally higher than in F. trinervia (C4) and that the differences among species are less expressed (Fig. 3C; Supplemental File S4). During the night, at the time of the expression maximum for PPCKB, this transcript is 2 to 6 times higher in the C3 species. The only time point that showed higher levels of PPCKB in the C4 species was at the end of night at 0/24 h (Fig. 3D).
Comparison of the isoform transcripts within each species shows that, at midday, the level of PPCKA in F. trinervia (C4) is more than 160 times higher than the level of PPCKB, whereas in F. pringlei (C3), the PPCKA transcript is only 5 times more abundant than PPCKB. During the night, PPCKB is twice as abundant as PPCKA in F. trinervia (C4) and 44 times more abundant in F. pringlei (C3).
In the context of the evolution of C4 photosynthesis, it is the PPCKA isoform that shows the characteristic increase in transcription seen for many transcripts involved in this process (Gowik et al., 2011; Christin et al., 2013), suggesting that the PPCKA isoform is responsible for the daytime phosphorylation of PEPC. On the other hand, in C4 species, there is a decrease in the amount of PPCKB transcripts during the night, while this isoform is still relatively highly expressed in C3 species. This supports an hypothesis for the differential importance of these isoforms in the two compared species, which might be reflected in the levels of PEPC phosphorylation.
The Phosphorylation Pattern of PEPC from Species with C3 and C4 Photosynthesis Is Consistent with the Characteristic Expression Patterns of the PPCK Isoforms
To confirm the above hypothesis, we started two approaches: (1) investigation of the protein levels of PPCKA, and (2) monitoring of the in vivo phosphorylation state of PEPC in F. trinervia (C4) and F. pringlei (C3). In the first approach, we generated antibodies against PPCKA isoforms, which showed satisfactory specificity; however, due to their very low sensitivity, they were not usable in a complex plant extract and could only be applied against higher amounts of recombinant protein (Supplemental File S5). We then attempted to monitor the kinase protein using selected reaction monitoring (SRM; Lange et al., 2008), but the kinase levels were below the detection limit as well.
Consequently, we took the transcript levels of PPCK as a proxy for the protein amount and proceeded to monitor the in vivo phosphorylation state of the substrate using the phosphorylated and nonphosphorylated versions of the peptide, which contain the Ser-11 phosphorylation sites LA(pS)IDAQLR and LASIDAQLR, respectively (Svensson et al., 1997; O’Leary et al., 2011; Supplemental File S6). Using the plants harvested over the course of 24 h, we extracted proteins and spiked known concentrations of the labeled synthetic peptides mentioned above. The signal from the standard was used to determine the amount of the native PEPC peptide, following which the ratio of phosphorylated to nonphosphorylated PEPC was calculated.
The results in Figure 4 show one crucial difference between F. trinervia (C4) and F. pringlei (C3): while the phosphorylation of the PEPC from the species with C4 photosynthesis peaks during the day, the PEPC from the C3 species does not; instead, it maintains a certain level of phosphorylation throughout the entire day, and this level drops only briefly after the end of night.
Figure 4.
Circadian variation of PEPC phosphorylation levels in comparison with PPCK transcripts. The ratio of PEPC phosphorylation was calculated as the ratio of the concentrations of the phosphorylated and nonphosphorylated versions of the peptide LASIDAQR monitored by SRM and is expressed as a percentage from the total sum of ratios for each species. The diel variation of PPCKA and PPCKB transcripts for each species is expressed as percentage RPMK as in Figure 3. Three biological replicates were averaged for each time point. A, F. trinervia. B, F. pringlei. PPCKA, gray lines and gray circles; PPCKB, gray lines and white circles; ratio of phosphorylated PEPC, black lines and black squares. The horizontal bars above the graphs and the graph backgrounds indicate the light conditions: light, white; dark, black/gray. The original results for each measurement are listed in Supplemental File S4.
Comparison of the phosphorylation trend for PEPC from F. trinervia (C4) with the transcript levels of PPCKA and PPCKB from this species shows that the peak of phosphorylation closely matches the accumulation pattern of the PPCKA transcript, while at night the phosphorylation levels are negligible (Fig. 4A; Supplemental File S4).
On the other hand, PEPC from F. pringlei (C3) maintains a certain phosphorylation level throughout the entire day, fitting the expression profiles of both PPCKA and PPCKB, and the maximum phosphorylation point is during the dark period (Fig. 4B; Supplemental File S4).
In the context of C4 evolution, these results show that the Flaveria spp. plants with C4 photosynthesis probably abolished the phosphorylation of PEPC in the dark, while this action is still important in C3 species, where PEPC does not play a photosynthesis-related role but functions in other primary metabolic pathways.
Functional Characterization of Recombinant PPCKA Proteins Shows Cross Phosphorylation of PEPC among Flaveria spp. and Indicates Changes in Activity
The first part of “Results” outlined the changes that have occurred in the amino acid sequence of PPCKA, which we believe is responsible for the phosphorylation of the photosynthetic PEPC isoform in F. trinervia. As can be seen in Figure 2B, many of these amino acid exchanges occurred near or at sites that are implicated in binding either ATP or PEPC. Thus, we investigated if these changes are sufficient to abolish substrate recognition between species with C3 and C4 photosynthesis.
To this aim, we first isolated the PPCKA complementary DNAs (cDNAs) from F. pringlei (C3) and F. trinervia (C4), based on existing studies (Tsuchida et al., 2001) and our sequencing data, and purified the recombinant PPCKA proteins to near homogeneity. The purity of the protein differed between the two kinases, as can be seen in Supplemental File S5.
In two independent experiments, either recombinant ppcA PEPC from F. pringlei (C3) or F. trinervia (C4) was used as a substrate (obtained from Paulus et al., 2013b). Regardless of the origin of PEPC, there was successful phosphorylation by both F. pringlei and F. trinervia PPCKA recombinant proteins (Fig. 5). Consequently, the interspecies cross phosphorylation is not a trait restricted only to the C4 species.
Figure 5.
In vitro phosphorylation of ppcA PEPC protein from Flaveria spp. Recombinant ppcA PEPC proteins from species with C3 and C4 photosynthesis were phosphorylated in vitro using radioactive ATP and recombinant PPCK from two Flaveria spp. PPCKs are not specific to the PEPC from the respective species and can cross phosphorylate in vitro. A, The phosphorylation of the F. pringlei ppcA PEPC (1 µg) was tested, with equal amounts of purified recombinant kinase based on Supplemental File S5: FpPPCKA (25 µg of total protein) and FtPPCKA (1 µg of total protein). B, Indications of higher activity by the PPCK from C4 species appeared only when FtPEPC (3 µg) was used as a substrate: FpPPCK (25 µg of total protein) and FtPPCK (1 µg of total protein). Each lane represents an independent PPCK expression and purification. Controls are as follows: C1, temperature-deactivated PPCK; C2, no PEPC; C3, no FpPPCK; C4, no FtPPCK.
We found that the signals from the combination of F. trinervia (C4) PPCK and PEPC were much stronger than the signals resulting from the phosphorylation of the F. trinervia (C4) PEPC with the F. pringlei (C3) PPCK (Fig. 5B). In comparison, even though both kinases could successfully phosphorylate the PEPC from F. pringlei (C3), the signals were of similar intensity (Fig. 5A).
Consequently, although the PPCKs can cross phosphorylate among species, the stronger signals from the F. trinervia (C4) PPCKA and PEPC suggest that the changes in both proteins are important for the observed difference in phosphorylation.
To identify possible changes near or on the substrate recognition site, we investigated the N terminus of PEPC in the nine sequenced Flaveria spp. and the PEPCs from Brassicales, Amaranthaceae, and Poaceae, all of which contain C3 and C4 species. The conserved motif from this interspecies comparison was identified as E-K-X-X-S-I-D-A-Q-L-R (Fig. 6A; Supplemental File S7; Bailey and Elkan, 1994; Echevarria and Vidal, 2003).
Figure 6.
Analysis of the phosphorylation motif on the N terminus of PEPC. The first 18 to 21 amino acids (based on the sequence alignment in Supplemental File S7) were analyzed for conserved motifs using the online tool MEME (http://meme.nbcr.net/meme/; Bailey and Elkan, 1994). A, The consensus motif E-K-X-X-S-I-D-A-Q-L-R was found in 50 of the 56 submitted sequences from Flaveria spp., Brassicales, Amaranthaceae, and Poaceae. Several C3-to-C4 amino acid exchanges can be observed in front of the phosphorylated Ser (Supplemental File S7). B, The consensus sequence for Flaveria shows the exchanges L6V (found in C4 ppcA PEPC) and Q15H (found in C4 ppcB PEPCs), which differ between C3 and C4 species. C, Virtual mutagenesis was applied to the crystal structure of the PEPC tetramer from F. pringlei, which starts at position Leu-6. The applied mutations L6V and Q15H lie on the surface of the molecule and are accessible to the solute. Shades of gray indicate the individual monomers as part of the PEPC tetramer; Val, cyan; Ser-11, yellow; His, green.
Focusing on the genus Flaveria, where the N-terminal region of the photosynthetic ppcA PEPC is well conserved, we found a single L6V exchange, which is different between the C3 and C4 Flaveria spp. (Fig. 6B). The species in the genus are phylogenetically subdivided into two clades, A and B, with the C4 species belonging to clade A, whereas the furthest evolved species in clade B is the C4-like F. brownii (McKown et al., 2005; Gowik et al., 2011). The exchange L6V was found in the C4 species F. trinervia (C4) and F. bidentis (C4), in clade A, as well as in F. brownii (C4 like) and the C3-C4 intermediate species F. pubescens and F. anomala, all belonging to clade B. Thus, Val has been introduced in the further evolved C3-C4 intermediates and the C4 species in two independent evolutionary paths. Furthermore, the same type of exchange was observed in the C4 species in Amaranthaceae, which represent a totally different origin of C4 photosynthesis, but was not found in the Brassicales or Poaceae (Supplemental File S7).
Surprisingly, a second amino acid exchange, Q15H, was discovered in the C4 nonphotosynthetic ppcB PEPC isoforms in Flaveria spp. (Fig. 6A; Supplemental File S7).
Using the recently deduced crystal structure of PEPC from plants (Paulus et al., 2013b), we used virtual mutagenesis to display the above-mentioned C4-related changes (L6V and Q15H) onto the F. pringlei PEPC tetramer (Fig. 6C). This in silico investigation shows that, in both cases, the amino acids lie on the surface of the protein and are exposed to the solvent. Consequently, they could be accessible to interact with a PPCK. These findings show that the C4-related changes, which have been identified to take place in PEPC, also extend to its N terminus.
DISCUSSION
This study underlines the existence of a two-member PPCK family in the genus Flaveria whose isoforms are expressed in an inverse day/night manner. PPCKA underwent changes characteristic for proteins recruited to C4 photosynthesis in F. trinervia (Gowik et al., 2011; Christin et al., 2013), while PPCKB seems to have a higher importance in the C3 Flaveria spp.
Parallel Evolution of PEPC and PPCK Proteins
Phylogenetic analyses for PPCK (Supplemental File S2) and PEPC (Bläsing et al., 2002; Svensson et al., 2003) show that, in both cases, the ancestor proteins diverged at the time of speciation and the respective protein isoforms evolved in parallel within each species. This is in agreement with the polyphyletic evolution of C4 photosynthesis, where multiple independent origins led to the full C4 photosynthesis (Sage et al., 2012).
PPCK isoforms and the ppcA PEPC from species with C4 photosynthesis are always found on branches marking the longest evolutionary distance. This illustrates (1) the ongoing evolutionary process of both kinase and substrate (Wang et al., 2009) and (2) the presence of a large number of C4-related amino acid exchanges, as we were able to demonstrate with the case of PPCKA (Fig. 2A). PPCKA and ppcA PEPC are thus indicated as the kinase-substrate pair that was under strong evolutionary pressure.
In contrast, PPCKB does not show large differences between species with C3 and C4 photosynthesis (Fig. 1), nor do the ppcB and ppcC PEPC isoforms from F. trinervia (C4), which is marked by shorter evolutionary distance in previous studies (Bläsing et al., 2002; Svensson et al., 2003).
Changes in the Quantity of PPCK Transcripts in Leaves of C3 and C4 Flaveria spp.: Potential Mechanisms for the Regulation of Transcript Stability and Transcription Rhythms
Suitable expression patterns seem to be one of the prerequisites necessary for the recruitment of genes in the C4 pathway (Christin et al., 2013). We showed that PPCKA was present in the C3 Flaveria spp. that diverged earlier in evolution, and already there it has a daytime maximum in transcript accumulation. This expression pattern is much more suitable for C4 photosynthesis than the nighttime peak in transcription found for PPCKB. Similar to the case of ppcA (Ernst and Westhoff, 1997), PPCKA also showed the characteristic increase in expression between C3 and C4 species. Taken together with the phylogenetic analysis, this change in transcription supports the conclusion that PPCKA is the isoform that was recruited in the transition from C3 to C4 photosynthesis mode.
PPCKB has opposite characteristics from PPCKA. In addition to the maximal transcript accumulation at night in both species, a decrease in transcript levels is observed from the C3 to the C4 species (Fig. 3). Thus, it appears that this isoform has decreased importance in the leaves of the newly evolved C4 Flaveria spp., which was also reflected in the decreased phosphorylation levels of PEPC during the night. On the other hand, PPCKB is fully functional in the C3 species, where nighttime phosphorylation is relatively abundant. As a side finding, the existence of daytime- and nighttime-expressed PPCK in the C3 species, especially if this expression pattern for the PPCK family is confirmed for other C3 plants, is an explanation for the varying PEPC phosphorylation results observed among various C3 plants (Fukayama et al., 2006; Meimoun et al., 2009).
PEPC has been found to be under the influence of the circadian clock in a number of plants (O’Leary et al., 2011). The closely related function of PPCKA in Flaveria spp., in addition to the similar diel expression pattern in C4 photosynthesis, allows for speculation that the same will be the case for this kinase. Indeed, extended light experiments in CAM plants show such regulation for PPCK (Taybi et al., 2000), but no evidence for circadian regulation has been found in maize (C4; Shenton et al., 2006). In soybean (Glycine max; C3), PPCK has been found under circadian regulation in leaves but not in roots (Sullivan et al., 2005), and in rice (C3), it appears that there is no circadian regulation (Fukayama et al., 2006). Additional experiments are needed to test whether the accumulation of PPCKA and PPCKB transcripts in Flaveria spp. is controlled by the circadian clock. An analysis of cis-regulatory elements in the PPCK promoter regions would also be useful to investigate any regulatory differences between Flaveria spp. with varying photosynthetic types (Mockler et al., 2007).
The main mechanisms influencing PPCKA transcript levels could be (1) increased transcription rate and/or (2) increased transcript stability. We inspected the 3′ untranslated region (UTR) from the PPCK isoforms from the available data for the presence of the 10 most significant destabilization or stabilization 6-mers (Tsuchida et al., 2001; Narsai et al., 2007). On average, 80% of all identified 6-mers in the PPCKA 3′ UTR were destabilization motifs implicated in rapid RNA turnover, while this number was a little lower (72%) for PPCKB (Supplemental File S1; disregarding the partial UTR sequences). Thus, in both cases, we found indications for rapid transcript turnover that agree with a previously postulated theory (Tsuchida et al., 2001).
The Evolutionary Changes in PPCKA Proteins and Their Potential Functional Significance
Eight of the 13 amino acid exchanges conserved among the PPCKA proteins from C3 and C4 plants are concentrated in the region of amino acids 135 to 217 (Fig. 2). Some amino acids in this region are tentatively indicated to participate in ATP and substrate binding based on the National Center for Biotechnology Information Conserved Domains Database (GenBank accession nos. BAB71853.1 and BAF48321.1; accession no. cd05123; Marchler-Bauer et al., 2013). This is an indication of the strong evolutionary pressure to optimize the kinetic properties and/or the activation mechanism of the kinase.
Some of the potentially important differences are at positions 147, 160 to 165, and 211. At position 147 there is a Ser-to-Gly exchange. In contrast to the nonpolar amino acid Gly, Ser is a polar amino acid that can be subject to phosphorylation. Moreover, intramolecular or intermolecular hydrogen bonds can be formed with the Ser side chain. At positions 160, 163, and 165, three amino acid exchanges were identified. The first two are exchanges among amino acids with similar chemical properties, but the last exchange, at position 165, replaces Gly, a small nonpolar amino acid, with Arg, a larger basic (polar) amino acid, whose side chain is fully protonated at neutral pH, consequently bringing in positive charge at this position (Taniguchi, 2010). This cluster of point mutations is followed by an Arg-to-Met exchange at position 185. In the three-dimensional structure, Arg-185 is found opposite the region 160 to 165. Together with a third amino acid exchange at position 135, these amino acid exchanges are predicted to lie in an unstructured protein region, partly annotated as the activation loop (A-loop; Fig. 2). Current structural annotations state that phosphorylation of the A-loop results in transition from a disordered to an ordered state of the kinase and is part of the kinase activation mechanism (accession no. cd05123; Marchler-Bauer et al., 2013). As the putative A-loop contains Ser (positions 155 and 168), Thr (positions 166 and 173), and Tyr (position 175), which are all susceptible to phosphorylation, the nearby exchanges might influence the interaction with another regulating protein. Toward the C terminus, at position 211, instead of a Tyr in C3 plants, which has been indicated to play a part in substrate binding, is a His (a basic amino acid) in C4 plants, which is known to function as an acid/base catalyst (Taniguchi, 2010). However, we also must bear in mind that the crystal structure onto which PPCKA was modeled has sequence identity of about 40% with the Flaveria spp. PPCKAs and that PPCK has not been crystalized from any plant species. The latter will be necessary for proper determination of the influence of these amino acid exchanges in the tertiary structure of PPCK.
If we assume that the evolutionary trajectories are accompanied by gains of fitness and that the evolution of the PPCK gene family optimized the C4 evolutionary processes (Sage et al., 2012; Heckmann et al., 2013), then amino acid exchanges observed in the PPCKA sequences from various species could indicate the order of structure optimization. Starting from a common ancestor in the genus Flaveria, species with C3 photosynthesis diverged earlier, whereas the C4 species diverged later. If an amino acid exchange is conserved among all intermediates and the C4 species (type 1), then this point mutation probably occurred before those found only in the C4 species (type 3; Table I). Thus, in the case of PPCKA, we believe that the exchanges E80D, G160A, G165R, R185M, and L257F (type 1) probably precede the amino acid exchanges A4T, I135L, S147G, Y211H, E217D, and E273K (type 3). In the two cases where the C4 residue is found in some but not all intermediate species (E163D and S270N; type 2), it is difficult to determine what happened. Creating a series of mutated PPCKA proteins will be needed to investigate the importance of each of these amino acid exchanges for the kinase enzymatic activity.
The recombinant PPCKA experiments presented here show that these amino acid differences are not enough to abolish substrate recognition among the species in the genus Flaveria (Fig. 5), which is in agreement with previous findings where PPCK proteins from several plants can phosphorylate exogenous PEPC (Li and Chollet, 1994; Tsuchida et al., 2001; Ermolova et al., 2003). Since a higher signal results from the C4 form of both kinase and substrate, it is the parallel optimization of these molecules that is responsible for this increased activity (Fig. 5B). In this context, the so-far ignored amino acid differences in the N terminus of PEPC, and in the vicinity of the phosphorylated Ser (Fig. 6; Supplemental File S7), gain in importance and will require further study (Echevarria and Vidal, 2003). Simulation of the C3-to-C4 exchanges on the N-terminal part of PEPC that take into account that PEPC is a tetramer show that, despite the macromolecular conformation, these amino acids would still be accessible for protein-protein interaction (Fig. 6C; Paulus et al., 2013b). The L6V exchange on the N terminus of ppcA PEPC, characteristic for the intermediate and C4 species, is one of two exchanges in the otherwise conserved N-terminal region in Flaveria spp. (Supplemental File S7). The side chains of these two amino acids have similar properties, but the smaller side chain from Val might allow easier uncoupling of the kinase from the substrate, by providing larger separation between the PEPC- and PPCK-interacting residues. Consequently, this might be one of the reasons for observing a stronger signal in the in vitro phosphorylation assay between the C4 recombinant PEPC and PPCK proteins. ppcB PEPC, on the other hand, showed a Q15H exchange only in the C4 species (Fig. 6). This introduces a positive charge close to Ser-11, but the true consequence of this exchange will only be known after further investigation, as it will depend on the interacting residue from PPCK. Cocrystallization of both proteins should uncover the amino acid residues involved in the protein-protein interaction and allow the investigation of the observed amino acid exchanges between isoforms from C3 and C4 plants.
Although the evolution of the photosynthetic PEPC from C4 Flaveria spp. has been studied extensively and the influence of crucial amino acid exchanges on enzymatic activity has been clarified (Bläsing et al., 2000; Engelmann et al., 2002, 2008; Paulus et al., 2013a, 2013b), no such information exists for PPCK at this time. Additionally, as the cumulative findings from previous and these studies reinforce the differences in phosphorylation patterns of PEPC from C3 and C4 species, and at a time with ongoing efforts for engineering C4 traits in C3 crops, we can only stress the need for detailed knowledge of the regulation of one of the key enzymes of the C4 pathway.
MATERIALS AND METHODS
Plant Material
Plants were initially grown in greenhouse conditions, and before harvest for the Illumina and SRM experiment, they were acclimated for the last 10 d in an open phytochamber at 120 µmol quanta m−2 s−1 irradiance with a 10-h photoperiod, at 19°C night and 22°C day. Leaf blades were harvested from wild-type 4- to 6-week-old plants from Flaveria trinervia, Flaveria ramosissima, and Flaveria pringlei. Leaf samples were collected at 4-h intervals over a 24-h period. Plant material was immediately frozen in liquid nitrogen for further storage at –80°C. Three separate biological replicates for each time point were ground in liquid nitrogen prior to the respective RNA and protein preparations.
Library Construction and Illumina Sequencing
F. trinervia and F. pringlei were used for transcriptome sequencing. Total RNA was extracted from Flaveria spp. leaves using TRIsure Reagent according to the protocol for plant tissue (Bioline) transcriptome sequencing. Digestion with DNase was performed for 15 min. The RNA was treated with phenol and chloroform and precipitated overnight with a sodium acetate/isopropyl alcohol solution. The RNA was washed with 70% (v/v) ethanol and dissolved in water. The RNA concentration was determined with the nanodrop (Peqlab Biotechnologie).
Total RNA was set to a final concentration of 1 µg in 50 µL as a starting volume. The DNA libraries were generated according to the manufacturer’s TruSeq RNA Sample Preparation Kit via the Low-Throughput Protocol (Illumina catalog no. RS-930-2001, part no. 15008136 Rev. A, November 2010). RNA aliquots and libraries were validated for qualification and quantitation with the Agilent2100 bioanalyzer (Agilent Technologies). Validated DNA libraries were normalized and pooled to a final concentration of 2 nm. Clusters were generated with the TruSeq SR Cluster Kit version 2 according to the Reagent Preparation Guide with the Illumina cBot device. Single-read sequencing was performed with the Illumina HiSeq 2000.
Transcriptome Mapping and Analysis
The PPCK sequences for various species were obtained by aligning contigs from two de novo assembly pipelines, CLC Genomics Workbench (version 6.5) combined with the CLC Genomics Server (version 5.5; http://www.clcbio.com) and Velvet/Oases (version 1.2.08/0.2.08; Schulz et al., 2012), using the automatic k-mer determination setting with CLC and a k-mer length of 25 bp with Velvet/Oases. These were then combined and aligned to the F. trinervia cDNA (PEPCK-A accession no. AB272061), allowing us to combine the contigs of the two pipelines for several species and ensure the largest possible coverage of the PPCK transcript. In case of PPCKB, we used a contig generated by de novo assembly of 454 and Illumina reads (Gowik et al., 2011; J. Mallmann, D. Heckmann, A. Bräutigam, M.J. Lercher, A.P.M. Weber, P. Westhoff, and U. Gowik, unpublished data). To obtain the abundance of reads for the kinase, clean Illumina reads were aligned against F. trinervia cDNA sequences either obtained from public databases or during this work (Supplemental File S1). Reads were mapped with Bowtie2 (version 2.0.6.) using the end-to-end mode with sensitive settings and allowing one mismatch per seed for multiseeding (Langmead and Salzberg, 2012). We counted the number of unambiguous best hits per transcript and normalized these values to reads per million mapped reads and kilobase transcript length (RPMK).
Means and se were calculated from three biological replicates. The data were evaluated with a one-way ANOVA by multiple comparison using a Tukey test (multiplicity-adjusted P ≤ 0.05). In parallel, with a two-tailed paired Student’s t test, we obtained significance for transcripts of two time points, with major differences in abundance (P ≤ 0.05). Statistical analyses were performed with the program GraphPad PRISM 6 (GraphPad Software).
Isolation, Cloning, and Sequencing of PPCK for Recombinant Protein Purification
Total RNA was extracted from F. pringlei and F. trinervia leaves using the RNeasy Plant Mini Purification Kit (Qiagen), including the RNase-Free DNase Set (Qiagen). The RNA concentration was determined with the nanodrop (Peqlab Biotechnologie). The synthesis of 5′ and 3′ RACE-ready first-strand DNAs was carried out according to the SMARTer RACE cDNA Amplification Kit (Clontech Laboratories) with 1 µg of total RNA using SMARTScriber Reverse Transcriptase (Clontech Laboratories). The 3′ and 5′ RACE-PCRs were performed using Universal Primer A Mix (Clontech Laboratories) and the Smart-II-A-5′RACE oligonucleotides, respectively (Supplemental File S8). The gene-specific oligonucleotides were selected according to the available PPCK sequence from F. trinervia (GenBank accession no. AB065100). The noncoding regions of PPCKA from F. pringlei were identified with the PPCKA 5′ end (PEPC-PK_Fp_5′RACE) and PPCKA 3′ end (PEPC-PK_Fp_3′RACE) oligonucleotides. PCR was performed with the Advantage 2 Polymerase Mix according to Clontech Laboratories. The obtained DNA fragments were cloned with the CloneJET PCR Cloning Kit (Fermentas). Several independent clones were verified via PCR with the oligonucleotides pJET_fw and pJET_rew (Supplemental File S8). Potential positive clones were verified by DNA sequencing (LGC Genomics). The cDNA full-length PPCK gene from Flaveria spp. was amplified with the oligonucleotides PEPC-PK_CDS_Fp, PEPC-PK_CDS_Fra, and PEPC-PK_CDS_Ft listed in Supplemental File S8. Additional restriction sites were amplified to PPCK with the oligonucleotides PEPC-PK-Fp-5′-XhoI and PEPC-PK-Fp-3′-BamHI (F. pringlei) and PEPC-PK-Ft-5′-NdeI and PEPC-PK-Ft-3′-BamHI (F. trinervia), as listed in Supplemental File S8. All PCRs were carried out by means of the Phusion High-Fidelity DNA Polymerase (New England Biolabs). PCR products were sequenced, digested with XhoI/BamHI or NdeI/BamHI, and cloned into the corresponding XhoI/BamHI-digested or XhoI/NdeI-digested pETEV15b (+) vector (pET system; Novagen) together with the coding sequence of an N-terminal 6×His tag and Tobacco Etch Virus cleavage site. All generated plasmids were verified by DNA sequencing.
Alignments and Phylogenetic Analysis
Calculations of sequence identity between the PPCK proteins from Flaveria spp. were performed with the Web site http://emboss.bioinformatics.nl. The phylogenetic analysis was performed using MEGA5 (Tamura et al., 2011; Hall, 2013), as listed in the figures. Predicted protein parameters for PPCK, which has been isolated with RACE-PCR, were acquired using the ExPASy program (http://web.expasy.org/protparam).
Heterologous Expression of Recombinant PPCK in Escherichia coli
The QIAexpressionist protocol was used for the heterologous expression of native protein (Qiagen). E. coli BL21 (DE3) cells (Agilent Technologies) were transformed with PPCK-pETEV15b. Culture growth was performed using 2YT medium (5 g L−1 NaCl, 10 g L−1 yeast extract, and 16 g L−1 peptone) with ampicillin at 37°C after reaching an optical density of 0.7. Expression of recombinant proteins was induced by the addition of 500 µm isopropyl β-d-thiogalactopyranoside, and cells continued to grow for 5 h at 20°C.
Affinity Purification of the Recombinant PPCK Proteins
Recombinant PPCKA protein was purified by nickel-nitrilotriacetic acid affinity chromatography according to the manufacturer’s instructions (Protino; Macherey-Nagel). In short, intact E. coli cells were incubated on ice and subsequently resuspended in lysis buffer (50 mm NaH2PO4, 300 mm NaCl, and 1 mg mL−1 lysozyme, pH 8.0). The cells were disrupted by sonification. Native recombinant proteins were purified with a preequilibrated nickel-nitrilotriacetic acid affinity column (Protino; Macherey-Nagel) on the Äktaprime Plus system (GE Healthcare). In order to protect protein integrity, a gradient from 0 to 500 mm imidazole in 50 mm steps was used. Proteins were concentrated with the Amicon Ultra-10 centrifugal filter units (10-kD cutoff; Millipore) and desalted and buffered in 50 mm Tris and 5 mm MgCl2, pH 8, using PD-10 columns (GE Healthcare).
The concentrations of purified recombinant proteins were determined with a standard procedure (Bio-Rad; Bradford, 1976) and examined by Coomassie Blue staining and immunoblotting by SDS-PAGE on a 12% polyacrylamide gel (Laemmli, 1970). Signals were quantified using the program Multi Gauge 3.0 (Fuji Photo Film). The blotted nitrocellulose membrane was incubated with the primary (anti-PPCK) antibody in a dilution of 1:400 for 12 h at 4°C. Immunoblot analysis was examined using the reagent of the chemiluminescence assay (SuperSignal West; Thermo Scientific).
After investigating the protein purity using SDS-PAGE and Coomassie Blue staining, the intensities of the kinase bands from F. pringlei were compared with those from F. trinervia, and the protein content used for the in vitro phosphorylation assays was adjusted accordingly to equalize the kinase amounts (Supplemental File S5).
Enzymatic Activity of PPCK
The in vitro phosphorylation activity of recombinant PPCKA was detected by labeling recombinant PEPC with 32P in a 15-µL reaction mixture containing 50 mm Tris-HCl, 5 mm MgCl2, 1 mm EGTA, and 1 mm dithiothreitol, pH 8. After 20 min of incubation on ice, radiolabeled ATP was added as a final step to the reaction at a concentration of 1 µCi mmol−1 [γ-32P]ATP per reaction. The reaction was run for 15 min at 30°C and terminated by the addition of 2× SDS loading buffer (Laemmli, 1970). Samples were boiled at 100°C for 1 min and centrifuged at 10,000g for 10 min before loading on a 10% SDS polyacrylamide gel. Following electrophoresis, the radioactive bands were autographically visualized on a FLA3000 Bio-Imaging Analyzer (Fuji Photo Film).
PPCK Antibody Synthesis
Peptides conserved among the PPCK from F. pringlei, F. ramosissima, and F. trinervia were considered for the antibody synthesis. Rabbit polyclonal PPCK antibodies were raised against the peptides NH2-CLQKEPKILHILG-CONH2, PPCK residues 53 to 65, and NH2-CRLGIAHRDLKPDNV-CONH2, PPCK residues 127 to 140, by Agrisera. An additional anti-PPCK antibody was synthesized by Agrisera that had been obtained from a mix of purified recombinant PPCK proteins from the same Flaveria spp. Unfortunately, although specific, the antibody sensitivity was determined to be above 0.6 µg, which was sufficient for recombinant protein work but not sensitive enough to detect PPCK in plant extracts.
SRM: Peptide Selection, Sample Preparation, Liquid Chromatography-Tandem Mass Spectrometry, and Quantitation
The amino acid sequences of PPCK and PEPC were aligned, and in silico tryptic peptides conserved among F. trinervia and F. pringlei were considered for SRM. Initial testing was performed on the recombinant proteins for F. trinervia and F. pringlei both in unmodified and phosphorylated states. For the phosphorylated PEPC test, proteins were phosphorylated as above, using 1 µg of kinase to 10 µg of PEPC in a 7.5-µL reaction and nonradioactive ATP.
The peptides that showed good properties in the mass spectrometer were checked against the Flaveria spp. transcriptomes (BLASTp) supplemented with the experimentally determined sequences of PEPC and PPCK as a control. The BLASTp was performed with increasing e-values until the control peptide was found. The peptide covering the PEPC phosphorylation site is conserved among the three isoforms in F. pringlei (C3) and among the two most abundant isoforms in F. trinervia (C4; Ernst and Westhoff, 1997; Supplemental File S6). The peptides then underwent a second round of selection using plant extract, and those peptides that could be successfully monitored in this complex sample were obtained as heavy peptides (13C and 15N) from Thermo Fisher Scientific. None of the peptides from PPCK could be monitored in complex plant extracts using our setup.
Proteins were extracted from F. trinervia and F. pringlei as already described (Willige et al., 2011) with minor modifications. In short, 300 mg of leaf material was treated with 600 µL of buffer containing 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, protease inhibitor cocktail (Sigma-Aldrich), phosphatase inhibitor cocktail (Serva), and 1% (w/v) polyvinylpolypyrrolidone. Proteins were precipitated overnight at −20°C using a 5× volume of ice-cold acetone (Sigma-Aldrich). The protein pellet was dissolved in 6 m urea and 2 m thiourea (Merck and Sigma-Aldrich, respectively), and 10 µg of protein was digested and desalted as described by Arsova et al. (2012). Prior to desalting, the synthetic peptides for PEPC (listed in Supplemental File S6) were added to the samples in species-specific amounts. Per 500 ng of total protein, the amounts of synthetic peptides were as follows: F. trinervia (nonphosphorylated, 10 fmol; phosphopeptide, 5 fmol); F. pringlei (nonphosphorylated, 2.5 fmol; phosphopeptide, 1.25 fmol).
The tryptic digests were measured using the ultra-HPLC system UltiMate 3000 RSLCnano in combination with a TSQ Vantage triple quadrupole mass spectrometer (both Thermo Fisher Scientific). A total of 500 ng of digested plant protein was loaded at a rate of 20 µL min−1 and separated on a 20-min gradient (5%–30% B, where solution A is 0.1% formic acid in water and solution B is 80% acetonitrile, 10% trifluoroethanol, and 0.1% formic acid in water), using a column of 75-µm i.d., 15-cm length, C18, and 2-µm particle size (Acclaim PepMap RSLC; Thermo Fisher Scientific). Samples were sprayed into the mass spectrometer by electrospray ionization. The resolution of Q1 and Q3 was set to 0.7 u full width at half maximum. Cycle time was 1 s (dwell time was more than 50 ms for each transition). Peptides were fragmented using argon gas for collision-induced dissociation at collision energies specific for each peptide (Supplemental File S6). Instrument scan mode was iSRM, and three primary and two secondary fragment ions per peptide were collected; allowed time was 0.1 min, threshold intensity was 300 counts, and dynamic exclusion was activated after three fragmentation events for 0.3 min.
Data were analyzed using PinPoint 1.3 (Thermo Fisher Scientific), and the signal-to-noise ratio was set at less than 10. In general, three transitions per peptide for each light and heavy form were used for peak area summation [except Flaveria pringlei peptide LASIDAQLR (Phos), which had two transitions per peptide]. The phosphorylation of PEPC on Ser-11 was monitored using LASIDAQLR and LA(pS)IDAQLR. The absolute concentration was calculated as light(area)/heavy(area) × concentration of the specific standard peptide, and the ratios of these two concentrations were taken as the ratio of phosphorylated to nonphosphorylated PEPC.
DNA sequences of PPCK from C4 Flaveria spp. are available at the GenBank/EMBL data libraries with the accession numbers AB065100 (F. trinervia PPCK) and AB272061 (F. bidentis PPCK). The Arabidopsis genes used as references for the transcriptome mapping are annotated as At1g08650 (PPCK1) and At3g04530 (PPCK2). Accession numbers for the phylogram were taken from deposited GenBank/EMBL data libraries as described (Marsh et al., 2003; Nimmo, 2003; Sullivan et al., 2004; Fukayama et al., 2006; Shenton et al., 2006).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental File S1. Sequences.
Supplemental File S2. Phylogenetic analysis of plant PPCKs.
Supplemental File S3. Alignment of PPCKs from various species.
Supplemental File S4. RNAseq and SRM data.
Supplemental File S5. Recombinant PPCKA expression and anti-PPCK antibodies.
Supplemental File S6. SRM peptide information.
Supplemental File S7. PEPC N-terminal alignment.
Supplemental File S8. Primers.
Supplementary Material
Acknowledgments
We thank Judith K. Paulus of the group of Dr. Georg Groth for providing the PEPC recombinant proteins and Bianca Jacobs for providing the PPCKA cDNA from F. trinervia. The single-read sequencing was performed with the Illumina HiSeq 2000 by the Biologisch-Medizinisches Forschungszentrum at Heinrich-Heine-Universität.
Glossary
- CAM
Crassulacean acid metabolism
- RNAseq
RNA sequencing
- SRM
selected reaction monitoring
- cDNA
complementary DNA
- UTR
untranslated region
- A-loop
activation loop
- RPMK
reads per million mapped reads and kilobase transcript length
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft through the International Research Training Group (grant no. IRTG 1525 to S.H.A., B.A., and P.W.), by the Excellence Cluster (grant no. EXC 1028 to P.W.), and by Michigan AgBioResearch.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Agetsuma M, Furumoto T, Yanagisawa S, Izui K. (2005) The ubiquitin-proteasome pathway is involved in rapid degradation of phosphoenolpyruvate carboxylase kinase for C4 photosynthesis. Plant Cell Physiol 46: 389–398 [DOI] [PubMed] [Google Scholar]
- Akyildiz M, Gowik U, Engelmann S, Koczor M, Streubel M, Westhoff P. (2007) Evolution and function of a cis-regulatory module for mesophyll-specific gene expression in the C4 dicot Flaveria trinervia. Plant Cell 19: 3391–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22: 195–201 [DOI] [PubMed] [Google Scholar]
- Arsova B, Zauber H, Schulze WX. (2012) Precision, proteome coverage, and dynamic range of Arabidopsis proteome profiling using (15)N metabolic labeling and label-free approaches. Mol Cell Proteomics 11: 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry S, Brown NJ, Hibberd JM. (2011) The role of proteins in C(3) plants prior to their recruitment into the C(4) pathway. J Exp Bot 62: 3049–3059 [DOI] [PubMed] [Google Scholar]
- Bailey TL, Elkan C. (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2: 28–36 [PubMed] [Google Scholar]
- Benkert P, Biasini M, Schwede T. (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27: 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsing OE, Ernst K, Streubel M, Westhoff P, Svensson P. (2002) The non-photosynthetic phosphoenolpyruvate carboxylases of the C4 dicot Flaveria trinervia: implications for the evolution of C4 photosynthesis. Planta 215: 448–456 [DOI] [PubMed] [Google Scholar]
- Bläsing OE, Westhoff P, Svensson P. (2000) Evolution of C4 phosphoenolpyruvate carboxylase in Flaveria, a conserved serine residue in the carboxyl-terminal part of the enzyme is a major determinant for C4-specific characteristics. J Biol Chem 275: 27917–27923 [DOI] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Jenkins GI, Wilkins MB, Nimmo HG. (1999) Metabolite control overrides circadian regulation of phosphoenolpyruvate carboxylase kinase and CO2 fixation in Crassulacean acid metabolism. Plant Physiol 121: 889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bräutigam A, Mullick T, Schliesky S, Weber AP. (2011) Critical assessment of assembly strategies for non-model species mRNA-Seq data and application of next-generation sequencing to the comparison of C(3) and C(4) species. J Exp Bot 62: 3093–3102 [DOI] [PubMed] [Google Scholar]
- Brown NJ, Newell CA, Stanley S, Chen JE, Perrin AJ, Kajala K, Hibberd JM. (2011) Independent and parallel recruitment of preexisting mechanisms underlying C4 photosynthesis. Science 331: 1436–1439 [DOI] [PubMed] [Google Scholar]
- Budde RJA, Chollet R. (1986) In vitro phosphorylation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol 82: 1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O’Leary MH. (1996) Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 273–298 [DOI] [PubMed] [Google Scholar]
- Christin PA, Boxall SF, Gregory R, Edwards EJ, Hartwell J, Osborne CP. (2013) Parallel recruitment of multiple genes into C4 photosynthesis. Genome Biol Evol 5: 2174–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Osborne CP, Sage RF, Arakaki M, Edwards EJ. (2011) C(4) eudicots are not younger than C(4) monocots. J Exp Bot 62: 3171–3181 [DOI] [PubMed] [Google Scholar]
- de Brevern AG, Etchebest C, Hazout S. (2000) Bayesian probabilistic approach for predicting backbone structures in terms of protein blocks. Proteins 41: 271–287 [DOI] [PubMed] [Google Scholar]
- Duff S, Chollet R. (1995) In vivo regulation of wheat-leaf phosphoenolpyruvate carboxylase by reversible phosphorylation. Plant Physiol 107: 775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SMG, Andreo CS, Pacquit V, Lepiniec L, Sarath G, Condon SA, Vidal J, Gadal P, Chollet R. (1995) Kinetic analysis of the non-phosphorylated, in vitro phosphorylated, and phosphorylation-site-mutant (Asp8) forms of intact recombinant C4 phosphoenolpyruvate carboxylase from sorghum. Eur J Biochem 228: 92–95 [PubMed] [Google Scholar]
- Echevarria C, Vidal J. (2003) The unique phosphoenolpyruvate carboxylase kinase. Plant Physiol Biochem 41: 541–547 [DOI] [PubMed] [Google Scholar]
- Echevarría C, Vidal J, Jiao JA, Chollet R. (1990) Reversible light activation of the phosphoenolpyruvate carboxylase protein-serine kinase in maize leaves. FEBS Lett 275: 25–28 [DOI] [PubMed] [Google Scholar]
- Edgar RC. (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GE, Walker DA (1983) C3, C4 Mechanisms and Cellular and Environmental Regulation of Photosynthesis. Blackwell Scientific, Oxford [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR. (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112: 285–299 [DOI] [PubMed] [Google Scholar]
- Engelmann S, Bläsing OE, Westhoff P, Svensson P. (2002) Serine 774 and amino acids 296 to 437 comprise the major C4 determinants of the C4 phosphoenolpyruvate carboxylase of Flaveria trinervia. FEBS Lett 524: 11–14 [DOI] [PubMed] [Google Scholar]
- Engelmann S, Zogel C, Koczor M, Schlue U, Streubel M, Westhoff P. (2008) Evolution of the C4 phosphoenolpyruvate carboxylase promoter of the C4 species Flaveria trinervia: the role of the proximal promoter region. BMC Plant Biol 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolova NV, Cushman MA, Taybi T, Condon SA, Cushman JC, Chollet R. (2003) Expression, purification, and initial characterization of a recombinant form of plant PEP-carboxylase kinase from CAM-induced Mesembryanthemum crystallinum with enhanced solubility in Escherichia coli. Protein Expr Purif 29: 123–131 [DOI] [PubMed] [Google Scholar]
- Ernst K, Westhoff P. (1997) The phosphoenolpyruvate carboxylase (ppc) gene family of Flaveria trinervia (C4) and F. pringlei (C3): molecular characterization and expression analysis of the ppcB and ppcC genes. Plant Mol Biol 34: 427–443 [DOI] [PubMed] [Google Scholar]
- Facella P, Lopez L, Carbone F, Galbraith DW, Giuliano G, Perrotta G. (2008) Diurnal and circadian rhythms in the tomato transcriptome and their modulation by cryptochrome photoreceptors. PLoS ONE 3: e2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine V, Hartwell J, Jenkins GI, Nimmo HG. (2002) Arabidopsis thaliana contains two phosphoenolpyruvate carboxylase kinase genes with different expression patterns. Plant Cell Environ 25: 115–122 [Google Scholar]
- Fukayama H, Tamai T, Taniguchi Y, Sullivan S, Miyao M, Nimmo HG. (2006) Characterization and functional analysis of phosphoenolpyruvate carboxylase kinase genes in rice. Plant J 47: 258–268 [DOI] [PubMed] [Google Scholar]
- Furumoto T, Izui K, Quinn V, Furbank RT, von Caemmerer S. (2007) Phosphorylation of phosphoenolpyruvate carboxylase is not essential for high photosynthetic rates in the C4 species Flaveria bidentis. Plant Physiol 144: 1936–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelly JC, Joseph AP, Srinivasan N, de Brevern AG. (2011) iPBA: a tool for protein structure comparison using sequence alignment strategies. Nucleic Acids Res 39: W18–W23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglioli-Guivarc’h N, Pierre JN, Brown S, Chollet R, Vidal J, Gadal P. (1996) The light-dependent transduction pathway controlling the regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase in protoplasts from Digitaria sanguinalis. Plant Cell 8: 573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowik U, Bräutigam A, Weber KL, Weber AP, Westhoff P. (2011) Evolution of C4 photosynthesis in the genus Flaveria: how many and which genes does it take to make C4? Plant Cell 23: 2087–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M, Tikkanen M, Paakkarinen V, Kangasjärvi S, Aro EM. (2012) Steady-state phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol 160: 1896–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford NG, Hardie DG. (1998) SNF1-related protein kinases: global regulators of carbon metabolism in plants? Plant Mol Biol 37: 735–748 [DOI] [PubMed] [Google Scholar]
- Hall BG. (2013) Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol 30: 1229–1235 [DOI] [PubMed] [Google Scholar]
- Hartwell J, Gill A, Nimmo GA, Wilkins MB, Jenkins GI, Nimmo HG. (1999) Phosphoenolpyruvate carboxylase kinase is a novel protein kinase regulated at the level of expression. Plant J 20: 333–342 [DOI] [PubMed] [Google Scholar]
- Hartwell J, Smith LH, Wilkins MB, Jenkins GI, Nimmo HG. (1996) Higher plant phosphoenolpyruvate carboxylase kinase is regulated at the level of translatable mRNA in response to light or a circadian rhythm. Plant J 10: 1071–1078 [Google Scholar]
- Hatch MD. (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895: 81–106 [Google Scholar]
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber AP, Lercher MJ. (2013) Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153: 1579–1588 [DOI] [PubMed] [Google Scholar]
- Hermans J, Westhoff P. (1990) Analysis of expression and evolutionary relationships of phosphoenolpyruvate carboxylase genes in Flaveria trinervia (C4) and F. pringlei (C3). Mol Gen Genet 224: 459–468 [DOI] [PubMed] [Google Scholar]
- Hermans J, Westhoff P. (1992) Homologous genes for the C4 isoform of phosphoenolpyruvate carboxylase in a C3 and a C4 Flaveria species. Mol Gen Genet 234: 275–284 [DOI] [PubMed] [Google Scholar]
- Hrabak EM, Chan CW, Gribskov M, Harper JF, Choi JH, Halford N, Kudla J, Luan S, Nimmo HG, Sussman MR, et al. (2003) The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol 132: 666–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B, Engelmann S, Westhoff P, Gowik U. (2008) Evolution of C(4) phosphoenolpyruvate carboxylase in Flaveria: determinants for high tolerance towards the inhibitor L-malate. Plant Cell Environ 31: 793–803 [DOI] [PubMed] [Google Scholar]
- Jiao J, Echevarría C, Vidal J, Chollet R. (1991) Protein turnover as a component in the light/dark regulation of phosphoenolpyruvate carboxylase protein-serine kinase activity in C4 plants. Proc Natl Acad Sci USA 88: 2712–2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao JA, Chollet R. (1988) Light/dark regulation of maize leaf phosphoenolpyruvate carboxylase by in vivo phosphorylation. Arch Biochem Biophys 261: 409–417 [DOI] [PubMed] [Google Scholar]
- Jiao JA, Chollet R. (1989) Regulatory seryl-phosphorylation of C4 phosphoenolpyruvate carboxylase by a soluble protein kinase from maize leaves. Arch Biochem Biophys 269: 526–535 [DOI] [PubMed] [Google Scholar]
- Jiao JA, Chollet R. (1990) Regulatory phosphorylation of serine-15 in maize phosphoenolpyruvate carboxylase by a C4-leaf protein-serine kinase. Arch Biochem Biophys 283: 300–305 [DOI] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. (1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8: 275–282 [DOI] [PubMed] [Google Scholar]
- Joseph AP, Agarwal G, Mahajan S, Gelly JC, Swapna LS, Offmann B, Cadet F, Bornot A, Tyagi M, Valadié H, et al. (2010) A short survey on protein blocks. Biophys Rev 2: 137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. (2009) The SWISS-MODEL repository and associated resources. Nucleic Acids Res 37: D387–D392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lange V, Picotti P, Domon B, Aebersold R. (2008) Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol 4: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RD, Plaxton WC. (1997) Regulatory phosphorylation of banana fruit phosphoenolpyruvate carboxylase by a copurifying phosphoenolpyruvate carboxylase-kinase. Eur J Biochem 247: 642–651 [DOI] [PubMed] [Google Scholar]
- Lepiniec L, Vidal J, Chollet R, Gadal P, Cretin C. (1994) Phosphoenolpyruvate carboxylase: structure, regulation and evolution. Plant Sci 99: 111–124 [Google Scholar]
- Leport L, Kandlbinder A, Baur B, Kaiser WM. (1996) Diurnal modulation of phosphoenolpyruvate carboxylation in pea leaves and roots as related to tissue malate concentrations and to the nitrogen source. Planta 198: 495–501 [DOI] [PubMed] [Google Scholar]
- Li B, Chollet R. (1994) Salt induction and the partial purification/characterization of phosphoenolpyruvate carboxylase protein-serine kinase from an inducible Crassulacean-acid-metabolism (CAM) plant, Mesembryanthemum crystallinum L. Arch Biochem Biophys 314: 247–254 [DOI] [PubMed] [Google Scholar]
- Li B, Zhang XQ, Chollet R. (1996) Phosphoenolpyruvate carboxylase kinase in tobacco leaves is activated by light in a similar but not identical way as in maize. Plant Physiol 111: 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M. (2011) The molecular evolution of β-carbonic anhydrase in Flaveria. J Exp Bot 62: 3071–3081 [DOI] [PubMed] [Google Scholar]
- Mallmann J, Heckmann D, Brautigam A, Lercher MJ, Weber AP, Westhoff P, Gowik U. (June 16, 2014) The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. Elife http://dx.doi.org/10.7554/eLife.02478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lanczycki CJ, et al. (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41: D348–D352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JT, Sullivan S, Hartwell J, Nimmo HG. (2003) Structure and expression of phosphoenolpyruvate carboxylase kinase genes in solanaceae. A novel gene exhibits alternative splicing. Plant Physiol 133: 2021–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Moncalvo JM, Dengler NG. (2005) Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. Am J Bot 92: 1911–1928 [DOI] [PubMed] [Google Scholar]
- McNaughton GA, MacKintosh C, Fewson CA, Wilkins MB, Nimmo HG. (1991) Illumination increases the phosphorylation state of maize leaf phosphoenolpyruvate carboxylase by causing an increase in the activity of a protein kinase. Biochim Biophys Acta 1093: 189–195 [DOI] [PubMed] [Google Scholar]
- Meimoun P, Gousset-Dupont A, Lebouteiller B, Ambard-Bretteville F, Besin E, Lelarge C, Mauve C, Hodges M, Vidal J. (2009) The impact of PEPC phosphorylation on growth and development of Arabidopsis thaliana: molecular and physiological characterization of PEPC kinase mutants. FEBS Lett 583: 1649–1652 [DOI] [PubMed] [Google Scholar]
- Mockler TC, Michael TP, Priest HD, Shen R, Sullivan CM, Givan SA, McEntee C, Kay SA, Chory J. (2007) The DIURNAL project: DIURNAL and circadian expression profiling, model-based pattern matching, and promoter analysis. Cold Spring Harb Symp Quant Biol 72: 353–363 [DOI] [PubMed] [Google Scholar]
- Narsai R, Howell KA, Millar AH, O’Toole N, Small I, Whelan J. (2007) Genome-wide analysis of mRNA decay rates and their determinants in Arabidopsis thaliana. Plant Cell 19: 3418–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo GA, Nimmo HG, Hamilton ID, Fewson CA, Wilkins MB. (1986) Purification of the phosphorylated night form and dephosphorylated day form of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi. Biochem J 239: 213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo HG. (2000) The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci 5: 75–80 [DOI] [PubMed] [Google Scholar]
- Nimmo HG. (2003) Control of the phosphorylation of phosphoenolpyruvate carboxylase in higher plants. Arch Biochem Biophys 414: 189–196 [DOI] [PubMed] [Google Scholar]
- O’Leary B, Park J, Plaxton WC. (2011) The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem J 436: 15–34 [DOI] [PubMed] [Google Scholar]
- Osuna L, Pierre JN, Gonzalez MC, Alvarez R, Cejudo FJ, Echevarria C, Vidal J. (1999) Evidence for a slow-turnover form of the Ca2+-independent phosphoenolpyruvate carboxylase kinase in the aleurone-endosperm tissue of germinating barley seeds. Plant Physiol 119: 511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Zachos JC, Freeman KH, Tipple B, Bohaty S. (2005) Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science 309: 600–603 [DOI] [PubMed] [Google Scholar]
- Paulus JK, Niehus C, Groth G. (2013a) Evolution of C4 phosphoenolpyruvate carboxylase: enhanced feedback inhibitor tolerance is determined by a single residue. Mol Plant 6: 1996–1999 [DOI] [PubMed] [Google Scholar]
- Paulus JK, Schlieper D, Groth G. (2013b) Greater efficiency of photosynthetic carbon fixation due to single amino-acid substitution. Nat Commun 4: 1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan AV, Devi MT, Raghavendra AS. (1994) Molecular biology of C4 phosphoenolpyruvate carboxylase: structure, regulation and genetic engineering. Photosynth Res 39: 115–135 [DOI] [PubMed] [Google Scholar]
- Sage RF. (2004) The evolution of C-4 photosynthesis. New Phytol 161: 341–370 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63: 19–47 [DOI] [PubMed] [Google Scholar]
- Saze H, Ueno Y, Hisabori T, Hayashi H, Izui K. (2001) Thioredoxin-mediated reductive activation of a protein kinase for the regulatory phosphorylation of C4-form phosphoenolpyruvate carboxylase from maize. Plant Cell Physiol 42: 1295–1302 [DOI] [PubMed] [Google Scholar]
- Schulz MH, Zerbino DR, Vingron M, Birney E. (2012) Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics 28: 1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede T, Kopp J, Guex N, Peitsch MC. (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31: 3381–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, Fedosejevs ET, Plaxton WC. (2013) Reciprocal control of anaplerotic phosphoenolpyruvate carboxylase by in vivo monoubiquitination and phosphorylation in developing proteoid roots of phosphate-deficient harsh hakea. Plant Physiol 161: 1634–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. (1999) C4 gene expression. Annu Rev Plant Physiol Plant Mol Biol 50: 187–217 [DOI] [PubMed] [Google Scholar]
- Shenton M, Fontaine V, Hartwell J, Marsh JT, Jenkins GI, Nimmo HG. (2006) Distinct patterns of control and expression amongst members of the PEP carboxylase kinase gene family in C4 plants. Plant J 48: 45–53 [DOI] [PubMed] [Google Scholar]
- Sullivan S, Jenkins GI, Nimmo HG. (2004) Roots, cycles and leaves: expression of the phosphoenolpyruvate carboxylase kinase gene family in soybean. Plant Physiol 135: 2078–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Shenton M, Nimmo HG. (2005) Organ specificity in the circadian control of plant gene expression. Biochem Soc Trans 33: 943–944 [DOI] [PubMed] [Google Scholar]
- Svensson P, Bläsing OE, Westhoff P. (1997) Evolution of the enzymatic characteristics of C4 phosphoenolpyruvate carboxylase: a comparison of the orthologous PPCA phosphoenolpyruvate carboxylases of Flaveria trinervia (C4) and Flaveria pringlei (C3). Eur J Biochem 246: 452–460 [DOI] [PubMed] [Google Scholar]
- Svensson P, Bläsing OE, Westhoff P. (2003) Evolution of C4 phosphoenolpyruvate carboxylase. Arch Biochem Biophys 414: 180–188 [DOI] [PubMed] [Google Scholar]
- Takahashi-Terada A, Kotera M, Ohshima K, Furumoto T, Matsumura H, Kai Y, Izui K. (2005) Maize phosphoenolpyruvate carboxylase: mutations at the putative binding site for glucose 6-phosphate caused desensitization and abolished responsiveness to regulatory phosphorylation. J Biol Chem 280: 11798–11806 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N (2010) Amino acids and proteins. In J Baynes, M Dominiczak, eds, Medical Biochemistry, Ed 3. Elsevier, China, pp 5–21 [Google Scholar]
- Taybi T, Patil S, Chollet R, Cushman JC. (2000) A minimal serine/threonine protein kinase circadianly regulates phosphoenolpyruvate carboxylase activity in Crassulacean acid metabolism-induced leaves of the common ice plant. Plant Physiol 123: 1471–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipple BJ, Pagani M. (2007) The early origins of terrestrial C4 photosynthesis. Annu Rev Earth Planet Sci 35: 435–461 [Google Scholar]
- Tovar-Méndez A, Mújica-Jiménez C, Muñoz-Clares RA. (2000) Physiological implications of the kinetics of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol 123: 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi KE, Turner WL, Gennidakis S, Plaxton WC. (2005) In vivo regulatory phosphorylation of novel phosphoenolpyruvate carboxylase isoforms in endosperm of developing castor oil seeds. Plant Physiol 139: 969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida Y, Furumoto T, Izumida A, Hata S, Izui K. (2001) Phosphoenolpyruvate carboxylase kinase involved in C(4) photosynthesis in Flaveria trinervia: cDNA cloning and characterization. FEBS Lett 507: 318–322 [DOI] [PubMed] [Google Scholar]
- Ubersax JA, Ferrell JE., Jr (2007) Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol 8: 530–541 [DOI] [PubMed] [Google Scholar]
- Ueno Y, Imanari E, Emura J, Yoshizawa-Kumagaye K, Nakajima K, Inami K, Shiba T, Sakakibara H, Sugiyama T, Izui K. (2000) Immunological analysis of the phosphorylation state of maize C4-form phosphoenolpyruvate carboxylase with specific antibodies raised against a synthetic phosphorylated peptide. Plant J 21: 17–26 [DOI] [PubMed] [Google Scholar]
- Uhrig RG, She YM, Leach CA, Plaxton WC. (2008) Regulatory monoubiquitination of phosphoenolpyruvate carboxylase in germinating castor oil seeds. J Biol Chem 283: 29650–29657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Quy L, Foyer C, Champigny ML. (1991) Effect of light and NO3 on wheat leaf phosphoenolpyruvate carboxylase activity: evidence for covalent modulation of the C3 enzyme. Plant Physiol 97: 1476–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J, Chollet R. (1997) Regulatory phosphorylation of C-4 PEP carboxylase. Trends Plant Sci 2: 230–237 [Google Scholar]
- Wang X, Gowik U, Tang H, Bowers JE, Westhoff P, Paterson AH. (2009) Comparative genomic analysis of C4 photosynthetic pathway evolution in grasses. Genome Biol 10: R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Duff SMG, Lepiniec L, Crétin C, Sarath G, Condon SA, Vidal J, Gadal P, Chollet R. (1992) Site-directed mutagenesis of the phosphorylatable serine (Ser8) in C4 phosphoenolpyruvate carboxylase from sorghum: the effect of negative charge at position 8. J Biol Chem 267: 16759–16762 [PubMed] [Google Scholar]
- Willige BC, Isono E, Richter R, Zourelidou M, Schwechheimer C. (2011) Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell 23: 2184–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiludda C, Schulze S, Gowik U, Engelmann S, Koczor M, Streubel M, Bauwe H, Westhoff P. (2012) Regulation of the photorespiratory GLDPA gene in C4 Flaveria: an intricate interplay of transcriptional and posttranscriptional processes. Plant Cell 24: 137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.