ROS signaling is crucial for lateral root emergence and root growth, and it regulates distinct sets of genes in these processes.
Abstract
Overall root architecture is the combined result of primary and lateral root growth and is influenced by both intrinsic genetic programs and external signals. One of the main questions for root biologists is how plants control the number of lateral root primordia and their emergence through the main root. We recently identified S-phase kinase-associated protein2 (SKP2B) as a new early marker for lateral root development. Here, we took advantage of its specific expression pattern in Arabidopsis (Arabidopsis thaliana) in a cell-sorting and transcriptomic approach to generate a lateral root-specific cell sorting SKP2B data set that represents the endogenous genetic developmental program. We first validated this data set by showing that many of the identified genes have a function during root growth or lateral root development. Importantly, genes encoding peroxidases were highly represented in our data set. Thus, we next focused on this class of enzymes and showed, using genetic and chemical inhibitor studies, that peroxidase activity and reactive oxygen species signaling are specifically required during lateral root emergence but, intriguingly, not for primordium specification itself.
Plants are sessile organisms that continuously need to adapt their growth to the surrounding environment. Root growth relies on the proliferative activity of cells located in the root apical meristem (RAM) and on the expansion and differentiation of these cells in the elongation and maturation zone (Ubeda-Tomás et al., 2012). In Arabidopsis (Arabidopsis thaliana) and many crop species, most of the overall root architecture is generated by the de novo formation of lateral roots (LRs). These lateral organs are specified at regular intervals along the main root from a limited number of pericycle cells, called founder cells (Dolan et al., 1993; Casimiro et al., 2003). These cells are specified in a zone immediately above the RAM called the basal meristem, and this process is correlated with an oscillating auxin response (De Smet et al., 2007; Moreno-Risueño et al., 2010) in an INDOLE-3-ACETIC ACID28 (IAA28)-dependent manner (De Rybel et al., 2010). These specified cells retain the ability to restart the cell division program to initiate a lateral root primordium (LRP). In a mature region of the root, distal from the basal meristem, these founder cells, in response to an auxin-dependent mechanism that involves IAA14/SOLITARY ROOT, undergo anticlinal cell divisions to initiate the formation of an LRP (Malamy and Benfey, 1997; Dubrovsky et al., 2008). IAA14/SOLITARY ROOT controls the entry of these cells into the cell cycle, as dominant negative mutations in this gene inhibit the first anticlinal divisions (Fukaki et al., 2002; Vanneste et al., 2005; De Rybel et al., 2010).
As very few pericycle cells located deep inside the primary root contribute to LR formation (Kurup et al., 2005), an LR-inducible system was developed previously that allows synchronized LRP formation along the entire pericycle (Himanen et al., 2002, 2004). More recently, cell sorting of GFP-labeled root cells (e.g. using the enhancer trap lines J0121 and RM1000; Brady et al., 2007) coupled with transcriptomics have contributed to our understanding of LRP development (De Smet et al., 2008). Interestingly, genes oscillating in phase or antiphase with the synthetic auxin response promoter DR5 fused to the LUCIFERASE reporter gene (DR5:LUC) in the upper region of the root meristem were identified (Moreno-Risueño et al., 2010), suggesting that they might be involved in LR specification. Although these studies have provided the community with an important resource with which to study LR development, they lack the specificity needed to unravel the transcriptional changes that specifically take place within the developing LRP.
To further refine these existing transcriptomic data sets by focusing only on those cells that actually participate in LRP development, we have used the S-phase kinase-associated protein2 (SKP2B):GFP marker line (Manzano et al., 2012) to isolate only those cells that are intimately associated with LRP development. SKP2B encodes an F-box ubiquitin ligase that regulates cell division (Ren et al., 2008) and founder cell division (Manzano et al., 2012). In this study, we isolated SKP2Bp:GFP-expressing cells, without any exogenous drug or hormonal treatment to promote LRP formation, through cell sorting. In the subsequent transcriptomics analysis (cs-SKP2B data set), we identified a large number of genes that are likely involved in LR development. Loss-of-function mutants of many of these genes caused changes in root length, LR number, or development. Interestingly, we found a large proportion of genes involved in redox activity (reactive oxygen species [ROS] signaling). Genetic analyses and peroxidase activity inhibitor studies showed that peroxidase activity is needed for LR emergence but not for LRP specification itself. Taken together, we have identified a novel set of LRP regulators and highlighted an important role for ROS signaling in this developmental process.
RESULTS
Identification and Validation of New Genes Associated with the LRP
We previously showed that SKP2Bp:GUS is expressed in all stages of the LRP, including founder cells and RAM cells (Manzano et al., 2012). We generated a SKP2Bp:GFP reporter that showed a similar expression pattern (Fig. 1, A–D) and used this transgenic line to identify SKP2B-coexpressed genes by means of cell sorting and subsequent whole-genome transcript expression analysis. To carry out the sorting experiment, we used 5-d-old SKP2Bp:GFP roots, which contained mostly LRPs in stages ranging from 0 to III and few in stages IV and V (Fig. 1E). To identify genes expressed in LRP-associated cells, and to prevent contamination due to asynchronous root growth, we decided to sort GFP-positive cells from root tips only and subtracted the data from the whole-root data set (see “Materials and Methods”) to end up with a set of genes that are expressed in LRP cells (hereafter called the cs-SKP2B data set, for cell sorting-SKP2B). Using this approach, we found that SKP2B was 1.35-fold enriched in the whole root with respect to the root apical region. Thus, to define the cs-SKP2B data set, we selected genes that were more than 1.5-fold enriched (P < 0.05). Using these parameters, we identified over 600 genes that are coexpressed with SKP2B in LRPs (Supplemental Table S1).
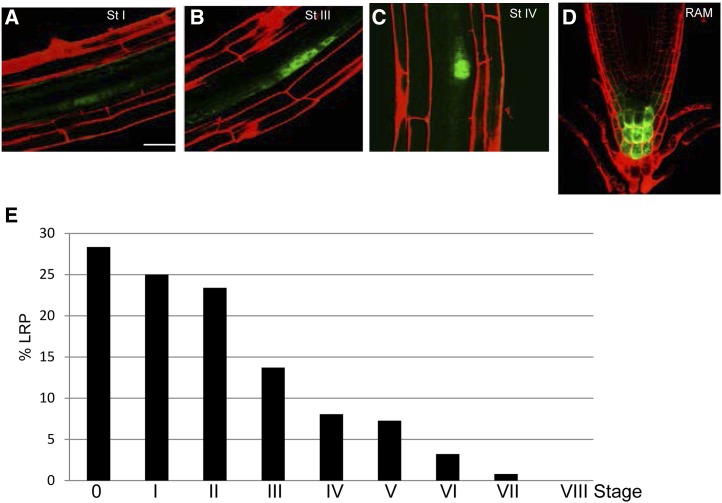
Figure 1.
SKP2Bp:GFP expression. A to D, Confocal images of the SKP2Bp:GFP reporter in the pericycle founder cells and LRPs in different stages of development (cell walls are counterstained using propidium iodide; A–C) and in the columella cell in the RAM (D). Bar = 20 μm. E, Percentage of LRP stages, according to Malamy and Benfey (1997), in 5-d-old seedlings grown in similar conditions to those used for the cell-sorting experiment (n > 30). Stage 0 corresponds to pericycle cells that showed GUS staining but did not divide yet. [See online article for color version of this figure.]
Next, to validate the cs-SKP2B data set, we overlapped our list of genes with previously published data sets (using Affymetrix ATH1 arrays) related to LR specification, initiation, or formation (Edgar et al., 2002). Two of these data sets used sorted cells from xylem pole pericycle cells (J1021; Brady et al., 2007; De Smet et al., 2008) or from LR initials (RM1000; Brady et al., 2007), while others used entire roots in the LR-inducible system (Vanneste et al., 2005). In addition, we also overlapped our list of genes with the naxillin data set, since this compound, unlike the auxin effect, promotes LR formation without affecting root growth (De Rybel et al., 2012). We also included two data sets of genes that coexpressed in phase or in antiphase with the oscillating DR5:LUC marker (Moreno-Risueño et al., 2010). Depending on the experiment, we found that 76% or 69% of our cs-SKP2B genes were represented in the xylem pole pericycle cells, which are the precursor cells of LRs, and 77% were present in the RM1000 set, which labels LR initials (Table I; Supplemental Table S1). Using the VisuaLRTC program (Parizot et al., 2010), we found that the cs-SKP2B data set contains auxin-induced (12.9%) and auxin-repressed (18.9%) genes associated with LR formation (Supplemental Table S2). In addition, a large number of the cs-SKP2B data set genes (118 genes) were not expressed in J0121- or RM1000-marked pericycle cells (Table I). We next explored the possibility that these genes are specifically enriched in other tissues types (endodermis, cortex, or epidermis; Brady et al., 2007). Over 60 genes were not enriched in either of these radial tissues, indicating that they likely correspond to genes that are specifically associated with SKP2B-expressing cells (Supplemental Table S1).
Table I. Comparison of the cs-SKP2B data set with other microarray data sets related to LR formation, founder cell specification, and pericycle expression.
The percentage in each case was calculated by dividing the number of common genes in the cs-SKP2B data set and the experiment. The P values for differences between observed and predicted random coincidences were calculated using the Binomial.N function. Boldface values highlight the most significant differences. NPA/IAA, Treatment with 1-N-naphthylphthalamic acid for 5 d and for 2 or 6 h with IAA; NPA/naxillin, treatment with 1-N-naphthylphthalamic acid for 3 d and for 6 h with naxillin.
| Data Source | Genotype | Cell Type | Treatment | Time | Expression | Total No. of Genes | Common Genes | Percentage | Random Percentage | P |
|---|---|---|---|---|---|---|---|---|---|---|
| cs-SKP2B data set | SKP2Bp:GFP | SKP2B-expressing cells | MS | 5 d | Present | 605 | ||||
| Vanneste et al. (2005) | Wild type | Whole root | NPA/IAA | 5 d/2 h | Induced | 750 | 70 | 10.00 | 3.29 | 2.4399E-17 |
| Repressed | 261 | 25 | 3.80 | 1.24 | 1.7605E-07 | |||||
| 5 d/6 h | Induced | 1,849 | 106 | 16.00 | 8.80 | 4.1405E-11 | ||||
| Repressed | 1,463 | 124 | 18.80 | 6.90 | 1.4768E-25 | |||||
| Solitary root | Whole root | 5 d/2 h | Induced | 508 | 61 | 9.20 | 2.40 | 1.5634E-20 | ||
| Repressed | 227 | 22 | 3.30 | 1.08 | 7.4296E-07 | |||||
| 5 d/6 h | Induced | 456 | 47 | 7.10 | 2.17 | 9.6032E-14 | ||||
| Repressed | 250 | 23 | 3.50 | 1.20 | 1.0319E-06 | |||||
| De Smet et al. (2008) | J0121 | Cell-sorting pericycle | NPA | 5 d | Present | 10,099 | 501 | 76.00 | 48.29 | 5.993E-30 |
| Cell-sorting pericycle | NPA/IAA | 5 d/2 h | Induced | 1,053 | 62 | 9.40 | 5.01 | 1.2187E-07 | ||
| Repressed | 603 | 67 | 10.00 | 2.87 | 1.4887E-20 | |||||
| 5 d/6 h | Induced | 1,704 | 85 | 12.90 | 8.11 | 7.8625E-07 | ||||
| Repressed | 1,666 | 125 | 18.90 | 7.93 | 2.7046E-21 | |||||
| De Rybel et al. (2012) | Wild type | Whole root | NPA/naxillin | 3 d/6 h | Induced | 401 | 79 | 13.00 | 1.75 | 1.02E-43 |
| NPA/naxillin | 3 d/6 h | Repressed | 149 | 5 | 0.80 | 0.65 | 0.15457 | |||
| Brady et al. (2007) | RM1000 | Cell-sorting LRP | MS | 7 d | Present | 12,283 | 513 | 77.80 | 58.47 | 3.6506E-16 |
| J0121 | Cell-sorting pericycle | MS | 6 d | Present | 12,221 | 449 | 68.00 | 58.19 | 1.0603E-07 | |
| Moreno-Risueño et al. (2010) | DR5:LUC | Upper RAM | MS | 5 d | DR5 phase | 2,084 | 41 | 6.20 | 9.92 | 0.00171517 |
| Upper RAM | DR5 antiphase | 1,409 | 38 | 6.40 | 6.18 | 0.05907853 | ||||
| Tsukagoshi et al. (2010) | 35:UPBEAT1(UPB1)-GFP | Root meristem region | MS | 6 d | Induced | 2,347 | 75 | 11.30 | 11.18 | 0.03263331 |
| Repressed | 1,805 | 176 | 27.00 | 8.59 | 3.2995E-43 | |||||
| Root elongation zone | MS | 6 d | Induced | 790 | 13 | 2.00 | 3.76 | 1.1725E-20 | ||
| Repressed | 906 | 79 | 12.00 | 4.31 | 1.8602E-32 |
cs-SKP2B Genes Function in LRP Development
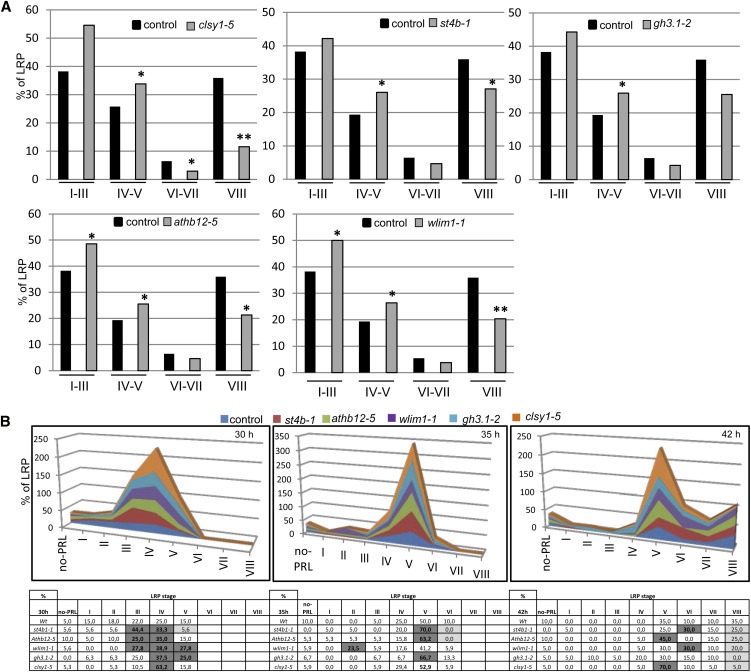
As the primary objective of this work was to identify novel genes that function in LR development, we next evaluated the role of cs-SKP2B genes (including transcription factors, kinases, and genes involved in chromatin remodeling, hormone signaling, or the stress response) in LR formation by studying transfer DNA (T-DNA) insertion lines. Phenotypic analyses revealed that the majority of these genes have different roles in root growth and/or LR development (Table II). We next performed a detailed analysis of the LRP distribution for several of these genes, including SULFOTRANSFERASE 4B (st4b-1; encoding a sulfotransferase), Arabidopsis thaliana HOMEOBOX12 (athb12-5; encoding a transcription factor), Gretchen Hagen3.1 (gh3.1-2; encoding an IAA-amido synthase-like protein), CHROMATIN REMODELING38/CLASSY1 (CHR38/clsy1-5; encoding a chromatin-remodeling protein), and Arabidopsis thaliana WLIM1 (wlim1-1; encoding a member of the actin bundlers family; Fig. 2A). All of these mutants showed an accumulation of LRPs in stages IV to V and a reduction in LR emergence, except for gh3-1-2, in which the reduction in emerged LR was not statistically significant. In summary, these data suggest that these genes might indeed have a role in LR development. To analyze the differences in LRP transitions in detail, we next synchronized LRP initiation by gravistimulation (Péret et al., 2012) and counted LRP stages at 30, 35, and 42 h after gravistimulation (hag; Fig. 2B). In our conditions, at 30 hag, control roots accumulated mainly LRPs in stages III and IV. The analyzed mutants showed a higher percentage of LRPs in stage III and IV, and two of them (wlim1 and chr38) also showed a higher percentage in stage V. This suggests that the transition between early stages of LRPs might be affected. Later, at 35 hag, most mutants showed a clear accumulation in stage V LRPs; and at 42 hag, all mutants developed significantly fewer emerged LRPs (stage VIII) compared with control plants (Fig. 2B). Taken together, these data indicate that mutations in several cs-SKP2B genes affect LRP development.
Table II. cs-SKP2B genes function in root development.
T-DNA mutants were identified for selected genes in the cs-SKP2B data set and grown in MS medium. Main root growth and emerged LRs were quantified at 10 d after germination. They were analyzed in three different groups with respective controls. Data are presented as means ± se. Bold numbers highlight significant differences by two-sided Student’s t test (P < 0.05; n ≥ 15).
| Arabidopsis Genome Initiative Identifier | Gene | Root Growth | Student’s t Test 1 | Emerged LRs per mm | Student’s t Test 2 | LRP Stages Study | Present in Pericycle | Regulated by Auxin | Regulated by ox-UPB1 |
|---|---|---|---|---|---|---|---|---|---|
| mm | |||||||||
| Control 1 | 34.41 ± 3.37 | 0.087 ± 0.029 | Yes | ||||||
| At1g26870 | FEZ | 29.76 ± 3.45 | 0.003487 | 0.102 ± 0.032 | 0.049281 | Yes | No | Up | |
| At1g74500 | TMO7 | 26.34 ± 2.13 | 3.56E-07 | 0.073 ± 0.024 | 0.2398724 | Yes | Yes | Up | Down |
| Control 2 | 35.3 ± 2.68 | 0.088 ± 0.036 | Yes | ||||||
| At3g61890 | ATHB12 | 28.4 ± 1.85 | 1.2738E-06 | 0.011 ± 0.006 | 1.13E-05 | Yes | Yes | Down | |
| At3g42670 | CHR38 | 29.17 ± 2.02 | 5.1386E-05 | 0.032 ± 0.011 | 0.0007398 | Yes | Yes | Up | |
| At2g14960 | GH3.1 | 29.52 ± 3.07 | 9.4372E-05 | 0.030 ± 0.009 | 0.0012735 | Yes | Yes | Up | Up |
| At1g13420 | ST4B | 31.16 ± 2.15 | 0.00228672 | 0.041 ± 0.017 | 0.0055029 | Yes | No | Down | |
| At3g03170 | Unknown | 23.9 ± 4.68 | 8.9202E-08 | 0.0376 ± 0.01 | 0.0079325 | Yes | Up | ||
| At4g36380 | ROTUNDIFOLIA3 | 27.94 ± 2.7 | 2.4387E-06 | 0.048 ± 0.019 | 0.0205045 | Yes | |||
| At1g62800 | ASPARTATE AMINOTRANSFERASE4 | 29.78 ± 1.83 | 3.5185E-05 | 0.056 ± 0.021 | 0.0452885 | Yes | Down | Down | |
| At2g16850 | Plasma membrane intrinsic protein 3B | 29.87 ± 3.19 | 0.00042324 | 0.073 ± 0.03 | 0.4019897 | Yes | Down | ||
| At3g10780 | Endomembrane protein24 | 32.47 ± 2.23 | 0.02411448 | 0.077 ± 0.019 | 0.473432 | Yes | Down | ||
| At5g43890 | YUCCA5 | 33.76 ± 2.2 | 0.23258445 | 0.078 ± 0.02 | 0.5444242 | Yes | Down | ||
| Control 3 | 35.41 ± 3.07 | 0.093 ± 0.032 | Yes | ||||||
| At1g10200 | GATA/WLIN1 | 27.86-3.35 | 0.0034321 | 0.022 ± 0.008 | 0.0012558 | Yes | Yes | Down | Down |
| At1g57560 | MYB DOMAIN PROTEIN50 (MYB50) | 37.89 ± 3.27 | 0.08045866 | 0.046 ± 0.023 | 0.0049299 | No | Up | Down | |
| At2g34070 | TRICHOME BIREFRINGENCE-LIKE37 | 40.93 ± 3.22 | 0.00125521 | 0.129 ± 0.034 | 0.0489991 | Yes | Down | ||
| At1g60010 | Unknown | 42.59 ± 2.89 | 2.0611E-06 | 0.13610.039 | 0.0080971 | Yes | Up | ||
| At3g06035 | Glycoprotein membrane precursor-anchored | 45.06 ± 2.66 | 2.9981E-07 | 0.151 ± 0.029 | 0.0025145 | Yes | Down | ||
| At5g44020 | HAD superfamily-IIIB acid phosphatase | 39.65 ± 5.61 | 0.01316582 | 0.124 ± 0.031 | 0.047023 | Yes | Down | Down | |
| At5g56040 | Leucine-rich repeat protein kinase family protein (LRR-kinase) STERILITY-REGULATING KINASE MEMBER2 | 30.57 ± 2.78 | 0.00088064 | 0.128 ± 0.022 | 0.0242053 | Yes | Up | ||
| At1g48630 | RACK1B_AT-RECEPTOR FOR ACTIVATED C KINASE 1B | 29.24 ± 4.09 | 0.00088943 | 0.062 ± 0.024 | 0.017875 | Yes | Up | ||
| At3g19200 | Unknown | 43.82 ± 2.53 | 7.5501E-07 | 0.102 ± 0.045 | 0.6083854 | Yes | Up | ||
| At5g14750 | MYB DOMAIN PROTEIN66 | 34.84 ± 3.56 | 0.34056745 | 0.087 ± 0.009 | 0.198798 | No | Up | ||
| At4g26890 | MITOGEN-ACTIVATED PROTEIN KINASE KINASE KINASE16 (MAPKKK16) | 38.59 ± 4.91 | 0.05141523 | 0.121 ± 0.06 | 0.1402085 | Yes | |||
| At5g16900 | LRR-kinase | 36.90 ± 4.15 | 0.15403393 | 0.107 ± 0.044 | 0.2633841 | Yes | |||
| At3g13530 | MAPKKK7 | 34.78 ± 4.31 | 0.87683704 | 0.096 ± 0.032 | 0.6467743 | Yes | |||
| At4g12420 | Monocopper oxidase-like protein5 (SKU5) | 37.71 ± 2.75 | 0.9878534 | 0.117 ± 0.038 | 0.2007416 | Yes | |||
| At4g09990 | GLUCURONOXYLAN METHYLTRANSFERASE 2 (GXM2) | 36.38 ± 2.84 | 0.45775026 | 0.074 ± 0.032 | 0.2268636 | No |
Figure 2.
LRP development is affected in cs-SKP2B mutants. A, LRPs at different developmental stages were quantified in T-DNA mutants from selected cs-SKP2B data. Graphs represent the percentage of LRP stages in roots of 8-d-old seedlings grown in MS medium (n = 10). Statistical differences between groups were analyzed by a mixed-model ANOVA. For these analyses, we grouped stages I, II, and III, stages IV and V, stages VI and VII, and stage VIII (see “Materials and Methods”). Asterisks indicate ANOVA values: *P < 0.05, **P < 0.01. B, Three-day-old roots from the wild type and different mutants were induced to a gravitropic response for 30, 35, or 42 h. LRPs were counted and grouped according to their stages (n = 30). Gray boxes highlighted those cases where statistical differences were found by ANOVA: P < 0.01. [See online article for color version of this figure.]
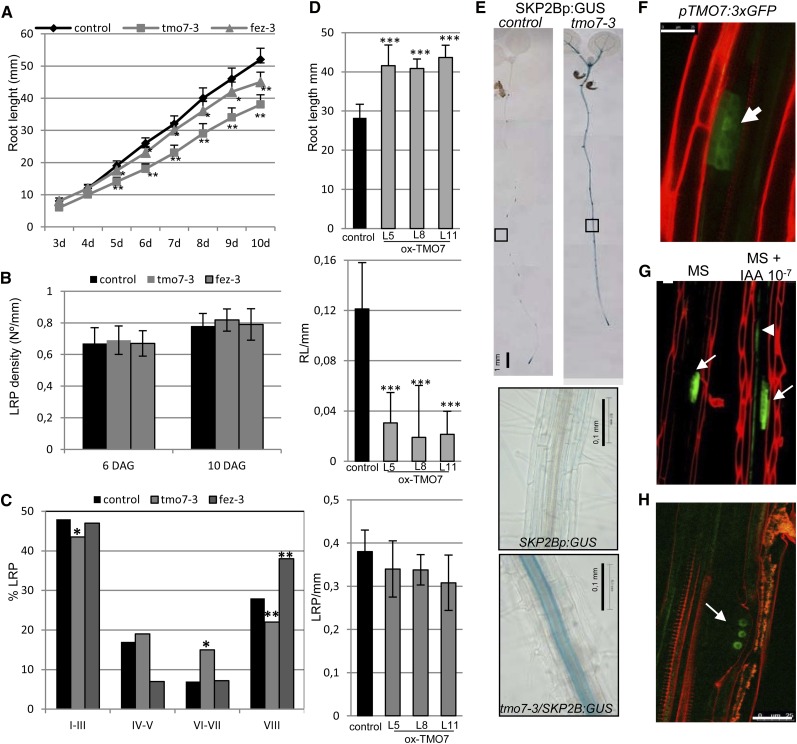
In addition, we further characterized the TARGET OF MONOPTEROS7 (TMO7) transcription factor (Schlereth et al., 2010). Previously, two available T-DNA mutants (tmo7-1 [SALK_058700] and tmo7-2 [SALK_080003]) did not show any significant reduction of TMO7 levels (Schlereth et al., 2010). However, we have analyzed a newly available allele, tmo7-3 (SALK_129876), which showed a significant reduction in TMO7 expression in roots (Supplemental Fig. S1). We found that the tmo7-3 mutant developed shorter roots than the wild type (Fig. 3A). Although the total density of LRPs was similar in both tmo7-3 and control plants (Fig. 3B), the mutant accumulated a significantly lower percentage of LRPs in stage VIII, while the percentage of LRPs in stages VI and VII was higher than in roots of control plants (Fig. 3C). Moreover, ectopically overexpressing TMO7 resulted in significantly fewer emerged LRs than control plants (Fig. 3D), although they showed similar LRP densities to control roots (Fig. 3D), suggesting that TMO7 regulates the emergence of LRs rather than LRP specification. Using the TMO7p:3nGFP line (Schlereth et al., 2010), we found that TMO7 was expressed during the early stages of LRP development and that its expression was induced in pericycle cells upon auxin treatment (Fig. 3, F and G). Interestingly, SKP2B expression was strongly up-regulated in pericycle and vascular cells in the tmo7-3 mutant (Fig. 3E). These data clearly show that TMO7, in addition to its function as a mobile signal to promote root initiation in the embryo (Schlereth et al., 2010), also has a role in LRP development.
Figure 3.
TMO7 and FEZ function in LR emergence. A, Root length of control and tmo7-3 and fez-3 seedlings at the indicated days. *P < 0.05, **P > 0.01 by two-sided Student’s t test (n ≥ 20). Error bars represent se. B, LRP density at 6 or 10 d after germination of control, tmo7-3, and fez-3 seedlings (n = 10). C, Percentage of LRPs at different developmental stages of 8-d-old control, tmo7-3, and fez-3 seedlings. Statistical differences between groups were analyzed by a mixed-model ANOVA: *P < 0.05, ** P > 0.01 (n = 10). D, Root length and number of emerged LRs (eLR) and LRPs per 1-mm of 7-d-old control seedlings and three independent lines of 35S:TMO7. ***P < 0.0001 by two-sided Student’s t test (n ≥ 25). Error bars represent se. E, GUS staining of 7-d-old SKP2Bp:GUS and tmo7-3/SKP2Bp:GUS seedlings. The lowest images show higher magnifications of root sections with GUS staining in the pericycle layer in the tmo7-3 mutant. Bars = 1 mm. F, Confocal image of an LRP expressing pTMO7:3xGFP. Bar = 25 µm. G, Representative confocal images of pTMO7:3xGFP roots treated with or without auxin (10−7 m IAA) for 6 h. Bar = 20 µm. Arrows indicate GFP signal in LRPs, and the arrowhead points to TMO7 induction upon auxin treatment. H, FEZ is expressed in early stages of LRP development. The confocal image shows LRPs of an 8-d-old pFEZ:FEZ-GFP seedling. The arrow points to the FEZ-GFP protein in the nucleus of a developing LRP cell. Bar = 25 µm.
We also analyzed the function of FEZ, a member of the NAC (No Apical Meristem/ATAF/CUP-Shaped Cotyledons) transcription factor family (Willemsen et al., 2008), for a putative role during LR development. FEZ was induced in pericycle cells after 6 h of auxin treatment (De Smet et al., 2008). FEZ:FEZ-GFP was found to be expressed in early stages of LRP development (Fig. 3H). fez mutants developed shorter roots (Fig. 3A) and accumulated higher numbers of emerged LRs than control plants but had similar LRP density (Fig. 3, B and C).
In conclusion, the analyzed cs-SKP2B genes have different roles in LRP development, confirming the predictive nature and validity of the cs-SKP2B data set.
Redox Signaling Controls LR Growth
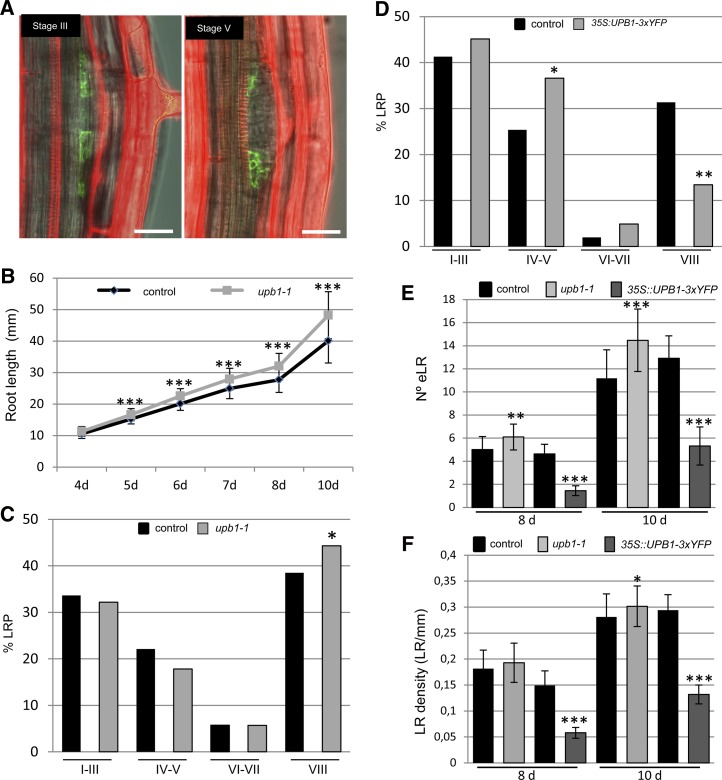
Interestingly, a large number of genes in the cs-SKP2B data set relate to redox activity (Supplemental Table S1). Among them, we found UPB1, a transcription factor that regulates the expression of a subset of PEROXIDASE (PER) genes involved in modulating the balance of ROS between the zones of cell division and cell elongation in the root meristem (Tsukagoshi et al., 2010). Moreover, we found a large number of cs-SKP2B genes that were down-regulated in the meristematic zone of UPB1-overexpressing plants (Supplemental Table S3; Tsukagoshi et al., 2010). Analyses of pUPB1:GFP plants showed that the UPB1 gene is also expressed in early stages of LRPs, although its expression seems to be restricted to the peripheral cells of the primordium (Fig. 4A) and no expression was found in stage I or II upon gravistimulation (data not shown). Next, LRP stage distribution was analyzed in the wild type, upb1-1, and a UPB1-3xYFP overexpressor plant (Tsukagoshi et al., 2010). As published previously (Tsukagoshi et al., 2010), we found that loss of function of UPB1 led to longer roots than in control plants (Fig. 4B). The distribution or percentage of LRPs at different stages was rather similar in both upb1-1 and control plants (Fig. 4C), but the upb1-1 mutant developed a higher number of emerged and mature LRs than control plants (Fig. 4, C and E). Conversely, UPB1-overexpressing roots significantly accumulated a higher number of LRPs in stages IV and V, while the number of LRPs in stage VIII was significantly reduced (Figs. 4D and 5, E and F). These data suggest that UPB1-mediated signaling is important to control LRP emergence.
Figure 4.
UPB1 function regulates LR emergence. A, UPB1 is expressed in early stages of LRP development. Confocal images show two different stages of LRPs of an 8-d-old pUPB1:GFP seedling. Bars = 25 µm. B, Root lengths of control and upb1-1 plants at different days after germination. ***P < 0.001 by two-sided Student’s t test (n = 60). C, Percentage of LRPs at different stages in wild-type and upb1-1 plants. Statistical differences between groups were analyzed by a mixed-model ANOVA: *P < 0.05 (n = 12). D, Percentage of LRPs at different stages in wild-type and UPB1-overexpressing (35S:UPB1-3xYFP) plants (n = 12). E, Number of emerged LRs in 8- and 10-d-old control, upb1-1, and UPB1-overexpressing plants. F, LR density (LR per mm of main root) in 8- and 10-d-old control, upb1-1, and 35S:UPB1-3xYFP plants. *P < 0.05, **P < 0.01, ***P < 0.001 by two-sided Student’s t test (n = 60). [See online article for color version of this figure.]
Figure 5.
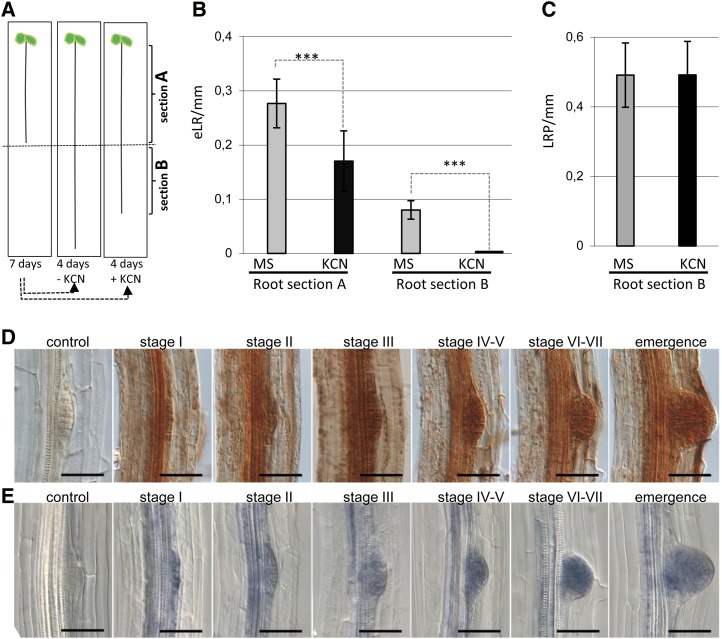
ROS peroxidase signaling is needed for LR development. A, Inhibition of peroxidase activity reduces LR emergence but not LRP specification. SKP2Bp:GUS seedlings were grown in MS medium for 7 d (root section A), transferred to MS medium with or without 100 µm KCN, a peroxidase inhibitor, for an extra 4 d (root section B), and then stained for GUS activity. B, Total number of emerged LRs (eLR) was counted in the two different root sections. C, The total number of LRPs was counted in root section B to analyze the effect of KCN on LRP specification. ***P < 0.001 by two-sided Student’s t test (n ≥ 35). Error bars represent se. D, DAB staining indicates H2O2 localization in the LRP of Arabidopsis seedlings. Photographs show LRPs from an initial stage of development to an organized primordium growing across the cortical tissues of the primary root (stage I to emergence) and a young LRP (control) pretreated for 1 h with 10 mm potassium iodide before DAB staining. Bars = 1 mm. E, NBT staining indicates the presence of superoxide in LRP of Arabidopsis seedlings. Photographs show developmental stages of LRP formation stained with NBT (stage I to emergence) and a control root pretreated for 1 h with 10 mm propyl gallate before NBT staining. Bars = 1 mm.
PER Genes Control LR Emergence
We also found a remarkably large number of PER genes in the cs-SKP2B data set (Supplemental Table S1). Strikingly, several of these PER genes were significantly down-regulated in UPB1-overexpressing plants (Supplemental Table S3). For this reason, we focused our work on the role of PER genes during LR development. We first treated SKP2Bp:GUS seedlings with KCN, a broad peroxidase inhibitor (Fig. 5A). The KCN treatment significantly reduced LR emergence (Fig. 5B), while the number of LRPs, counted as GUS-stained LRPs, was similar (Fig. 5C). These results indicated that PER activities are important for LR development, especially during LR emergence.
PERs promote the oxidation of various compounds using naturally occurring peroxides, especially hydrogen peroxide (H2O2). To inspect the spatial localization of peroxidase activity during LR development, we stained roots with diaminobenzidine (DAB) to visualize H2O2. DAB staining was found from early stages of LRP (initial stage I) up to emergence (Fig. 5D), suggesting that H2O2 is involved throughout LR development. Remarkably, H2O2 accumulation was higher during the later stages of LR emergence. As ROS signaling also involves superoxide, and we identified the ARABIDOPSIS THALIANA RESPIRATORY BURST OXIDASE HOMOLOG C (ATRBOHC) NADPH oxidase in the cs-SKP2B data set, we investigated the presence of superoxide in LRP by staining with nitroblue tetrazolium (NBT). Similar to H2O2, superoxide was also localized in all stages of LRP development, with higher accumulation from stages IV and V to stage VII (Fig. 5E).
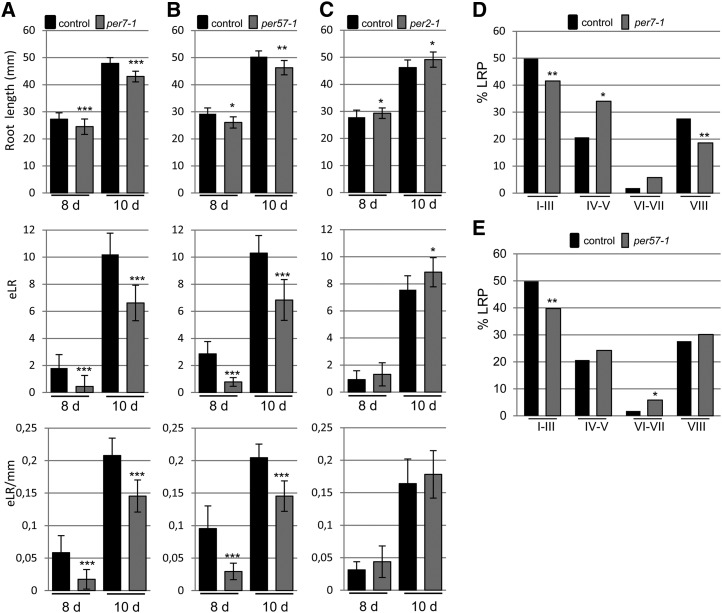
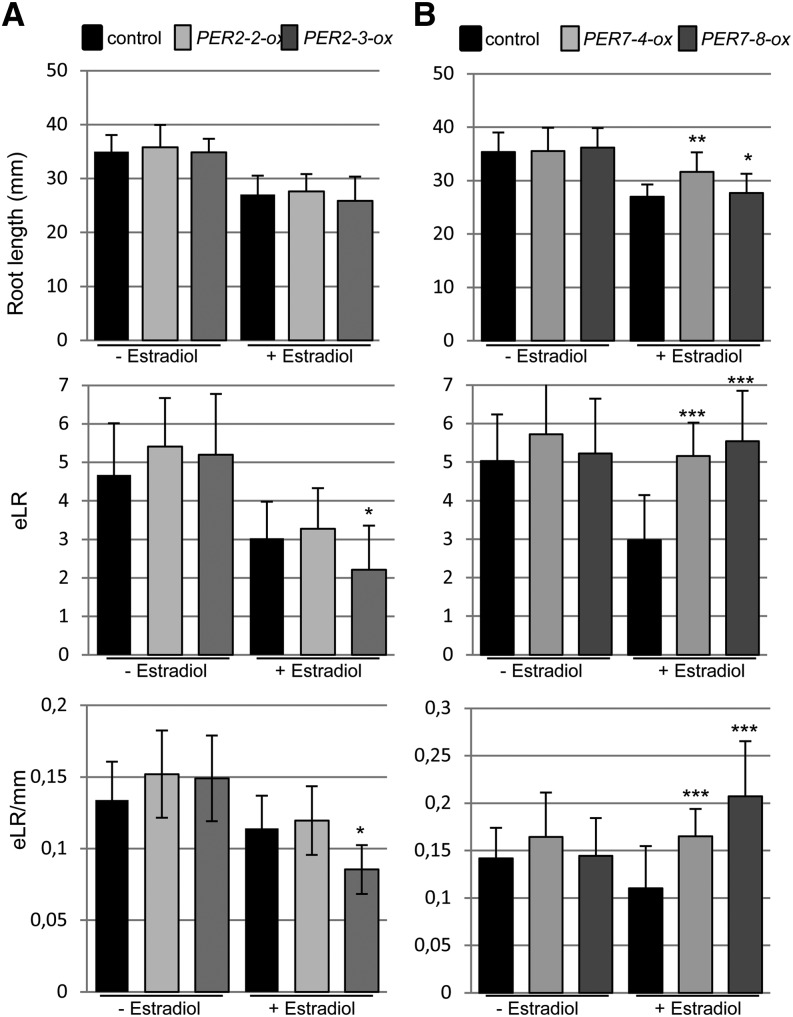
Having established a role for ROS during LR development, we next analyzed T-DNA insertion lines for PER2, PER7, and PER57 genes, which were identified in the cs-SKP2B data set. We found that the per7 and per57 mutants led to shorter roots (Fig. 6, A–C), while per2 developed slightly longer roots. Moreover, the total number of emerged LRs and the LR density were significantly reduced in both per7 and per57 mutants. Since these mutants showed LR defects, we decided to further characterize their LRP development. The per7 mutant showed significant differences in LRP transitions, developing fewer LRPs in early stages (I and II) and also in stage VIII (Fig. 6D), but it accumulated more stage IV and V primordia. The per57 mutant also developed fewer LRPs in early stages, but the number of fully formed LRPs (stage VIII) was similar to that in control plants (Fig. 6E). Overexpression of PER2 did not affect the root length but statistically reduced the number and density of emerged LRs (Fig. 7A). On the other hand, overexpression of PER7 significantly increased the number of emerged LRs and LR density (Fig. 7B). Taken together, these data suggest that specific peroxidase activities contribute to promote LRP emergence.
Figure 6.
Loss of function of a specific peroxidase reduces LR emergence. A to C, Root length (mm), number of total emerged LRs (eLR), and emerged LR density (eLR per mm) were quantified in mutants of three peroxidase genes, PER2 (A), PER7 (B), and PER57 (C). Each mutant was analyzed independently along with its corresponding control. Measurements were made at 8 and 10 d after germination. *P < 0.05, **P < 0.01, ***P < 0.001 by two-sided Student’s t test (n ≥ 60). D, Percentage of LRPs at different stages in wild-type and per7-1 plants. E, Percentage of LRPs at different stages in wild-type and per57-1 plants. Statistical differences between groups were analyzed by a mixed-model ANOVA: *P < 0.05, **P > 0.01 (n = 12).
Figure 7.
Gain of function of peroxidase increases LR emergence. Transgenic plants that express PER2 (iox-PER2; A) or PER7 (iox-PER7; B) under the control of an estradiol-inducible promoter were grown in medium with or without estradiol for 10 d. Root length (mm), number of total emerged LRs (eLR), and emerged LR density (eLR per mm) were quantified. Two different PER2- or PER7-overexpressing lines were analyzed independently along with their corresponding controls. *P < 0.05, **P < 0.01, ***P < 0.001 by two-sided Student’s t test (n ≥ 60).
PER Activity Is Independent from Auxin Signaling
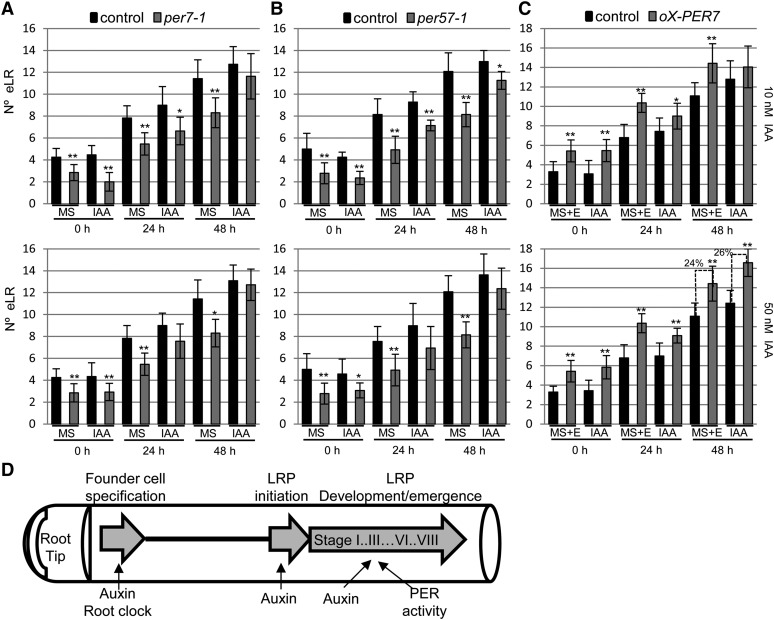
Several genetic and physiological studies have highlighted the role of phytohormones on LR development. Among them, auxin has the predominant role during specification, initiation, and development (Fukaki and Tasaka, 2009). To determine whether PER7 or PER57 functions in the auxin-dependent LR formation process, we treated roots of per7-1 and per57-1 seedlings with the naturally occurring auxin (Fig. 8, A and B). Auxin treatment at different concentrations increased the number of emerged LRs in both per7-1 and per57-1 to the same extent as in control roots after 48 h of treatment, indicating that PER7 and PER57 do not function downstream of auxin signaling. IAA treatment also increased LR production in both control and PER7-overexpressing plants with a similar ratio (Fig. 8C), suggesting that PER7 acts independently of auxin signaling.
Figure 8.
Effects of auxin on LR formation in plants with altered peroxidase activity. Arabidopsis seedlings were grown on control medium for 8 d (root section A) and then transferred to fresh medium with or without IAA (10 or 50 nm as indicated in each graph). The numbers of emerged LRs (eLR) were quantified in root section A (similar to Fig. 4A) at 0, 24, or 48 after seedling transfer. A, Average number of emerged LRs of control (Columbia-0) and per7-1 plants. Error bars represent se. *P < 0.05, **P < 0.01 by two-sided Student’s t test (n ≥ 12). B, Average number of LRs of control (Columbia-0) and per57-1 plants. Error bars represent se. *P < 0.05, **P < 0.01 by two-sided Student’s t test (n ≥ 12). C, Arabidopsis seedlings were grown in MS1/2 medium containing 10 µm estradiol (E) for 8 d and then transferred to fresh MS1/2 medium containing 10 µm estradiol with or without IAA (10 or 50 nm, as indicated in each graph). Average numbers of emerged LRs of control (pER8:GUS) and pER8:PER7 (ox-PER7) plants are shown. Error bars represent se. *P < 0.05, **P < 0.01 by two-sided Student’s t test (n ≥ 12). Percentages above the dashed lines indicate increments in LR due to the effect of auxin treatment in each genotype. D, Simplified model of LR development. Founder cells are specified in the basal meristem by the action of a root clock and auxin signaling. In the differentiation zone, these founder cells enter the LRP formation program in an auxin-dependent manner. LRP development can be arrested or can progress through the different developmental stages, which are controlled by auxin signaling and PER activities, likely through independent pathways.
DISCUSSION
In this work, we took advantage of the newly described LR marker, SKP2B, which is expressed in all stages of LR development, including founder cells and quiescent LRPs (Manzano et al., 2012). Auxin signaling is crucial during all stages of LR development (Benková et al., 2003; Dubrovsky et al., 2008; De Rybel et al., 2010). We have found several auxin response and auxin transport genes, such as SMALL AUXIN UP-REGULATED, AUXIN-INDUCED IN ROOT CULTURES1, GH3, IAA33, IAA19, IAA3, PIN-FORMED3 (PIN3), PIN4, PIN7, LIKE AUX1 3 (LAX3), and LAX2, in the cs-SKP2B data set (Supplemental Table S1). Auxin also plays a key role in reprogramming LRP-overlaying cells of cortex and epidermis to facilitate primordium emergence during the later stages of LRP development. The IAA3/LAX3 module regulates the expression of cell wall-remodeling enzymes that seems to be involved in cell separation (Swarup et al., 2008). We have identified IAA3, LAX3, and LAX2 in the cs-SKP2B data set as well as several enzymes that participate in cell wall-remodeling events. The identification of these genes could be explained, since SKP2B is also expressed in the surrounding cortex and epidermis during LR formation (Manzano et al., 2012). However, the majority of the cs-SKP2B genes are expressed in pericycle cells and early stages of LRP rather than in other surrounding root tissues (Supplemental Table S1).
Among the cs-SKP2B genes, several transcription factors were identified. Two of them, TMO7 and FEZ, were previously shown to be involved in root meristem development but not in LR formation. TMO7 is a basic helix-loop-helix that promotes root initiation in the embryo (Schlereth et al., 2010). Recently, it was shown that overexpression of TMO7/PACLOBUTRAZOL RESISTANCE3 reduced the number of emerged LRs (Castelain et al., 2012). Our results show that TMO7 regulates the emergence of the LR but does not control the specification of LRPs. Interestingly, the tmo7-3 mutant showed an increased ectopic expression of SKP2B in the whole pericycle as well as in the hypocotyl vasculature. Since overexpression of TMO7 does not alter the SKP2B expression pattern (data not shown), we speculate that TMO7 might repress SKP2B indirectly. Considering that SKP2B is a negative cell cycle regulator in LRP development (Manzano et al., 2012), the increased SKP2B expression in tmo7-3 might explain the lower LRP number and the delay between stages found in this mutant. Taken together, these results suggest that TMO7 acts a negative regulator of LR development. Another transcription factor analyzed is FEZ, a member of the NAC transcription factor family. FEZ regulates switches in the cell division plane in the root cap (Willemsen et al., 2008). Recently, FEZ was also proposed to function in LR prebranching, since RAM-decapitated fez-3 mutants develop lower numbers of emerged LRs than the wild type (Moreno-Risueño et al., 2010). Conversely, our data show that lack of FEZ function accelerates LR emergence. These differences might be the consequence of different experimental approaches. However, despite these differences, both experiments clearly showed that FEZ has a role in LR development.
Another highly represented gene family in the cs-SKP2B data set are the PERs belonging to class III, plant-specific oxidoreductases that have been involved in diverse physiological processes. In Arabidopsis, more than 73 genes that encode for class III PERs have been described (Hiraga et al., 2001; Tognolli et al., 2002). This high number is in accordance with the number of physiological functions described for these enzymes (Hiraga et al., 2001; Passardi et al., 2005). PERs are associated with the formation of lignin in the primary cell wall (Ros Barceló, 1997) and cross-linking between extension molecules (Iiyama et al., 1994). Peroxidase activity is also related to cell stem maintenance and cell division and differentiation in the root meristem (Jiang and Feldman, 2005; Tsukagoshi et al., 2010). It is remarkable that plant peroxidase activities have the ability to catalyze IAA (Gazaryan et al., 1996; Cosio et al., 2009), reducing the active auxin pool. Overexpression of an anionic PER in tobacco (Nicotiana tabacum) plants repressed LR formation, likely by oxidizing the IAA and reducing the active auxin needed for the formation of the lateral primordia (Lagrimini et al., 1997). Recently, Passaia and coworkers (2014) showed that the redox-mediated glutathione peroxidase (GPX) family plays an important role in shaping the root architecture rather than in aerial development. Genetic analyses showed that gpx mutants differentially affect LR formation. All gpx mutants, except gpx3-2, developed higher LRP density than control plants, suggesting that GPX activities act as repressors of LR formation. Those authors proposed that GPX1 and GPX7 have a more relevant role in the control of root architecture and LR development than other members of the family and that these two enzymes are involved in the auxin-dependent control of LR formation. In this work, we have shown that H2O2 and superoxide are formed during LR development. We also found in the cs-SKP2B data set several enzymes that could be involved in the formation of ROS, such as cytochromes P450 (12 members of the family), ATRBOHC NADPH oxidase, lipoxygenases, and electron carrier proteins (Supplemental Table S1). Taken together, our data demonstrate that ROS signaling is important for LR development.
In Arabidopsis, UPB1, a basic helix-loop-helix transcription factor, modulates the cell proliferation/differentiation balance in the root meristem, labeling the border of the transition zone between division and differentiation (Tsukagoshi et al., 2010). It has been proposed that UPB1 directly represses PER genes in the transition/elongation zone, increasing the H2O2 signaling needed for differentiation. Here, we show that UPB1 is expressed in the peripheral LRP cells, outside of the proliferative cells in the dome, suggesting a role in cell differentiation by repressing PER genes, as occurs in the root apical meristem (Tsukagoshi et al., 2010). Ectopic overexpression of UPB1 reduces the emergence of LRPs, while the overexpression of PER7 or PER57 promotes the emergence of LRs. Tsukagoshi and collaborators (2010) also showed that the chemical inhibition of peroxidases leads to a decrease in meristematic activity, likely by uncoupling cell division and differentiation, and promotes cell differentiation. In this study, we have identified 15 different PER genes in the cs-SKP2B, suggesting that ROS peroxidase-mediated activity is important for LR development. Inhibition of these activities with KCN, a broad inhibitor (Bestwick et al., 1997), reduces LR emergence but does not seem to affect founder cell priming. Phenotypic analyses of different per mutants revealed that they might have specific functions during LR development. Thus, PER2 does not seem to have a clear role in this process, while PER7 and PER57 act during LR development and emergence. The weak phenotype of per2 on LR development can be explained by the existence of another gene (At2g05250; PER2-like) that encodes for a PER that is identical at the amino acid level to PER2. Morphological analyses of a T-DNA mutant (SAIL_355_A01) for PER2-like did not show any differences with respect to its control (data not shown). In summary, these data provide strong evidence that these PER activities, via UPB1 regulation, regulate LR emergence through ROS signaling, probably by promoting the transition from proliferation to differentiation. This is in agreement with a role for UPB1 as a regulator of LR emergence.
Auxin signaling plays an important role in controlling root growth and LR formation. This hormone regulates cell proliferation and differentiation balance in the root meristem as well as in LRPs. Auxin signaling is involved in both founder cell specification and LR formation (Fukaki et al., 2002; Dubrovsky et al., 2008; De Rybel et al., 2010; Lavenus et al., 2013). Analyses of transcriptomic studies of auxin-treated roots showed that the majority of the PER genes included in the cs-SKP2B data set are not regulated by this hormone (data not shown). Tsukagoshi and collaborators (2010) proposed that PER activity, regulated by UPB1, acts independently of the auxin pathway to regulate growth at the main root meristem. However, there is evidence that the auxin response involves ROS signaling (Ma et al., 2013). Here, we present evidence that, at least, PER7 and PER57 function is not required for the auxin-dependent LR formation. It is remarkable that several PERs included in the cs-SKP2B are induced by naxallin. This chemical compound, which has been shown to promote LR formation, induces the expression of a set of genes that are different from those induced by auxin, including several PERs. Taken together, these data indicate that PERs act in a different pathway from auxin to root branching (Fig. 8D).
In this work, using the promoter of the SKP2Bp:GFP root expression marker, we have identified a large set of genes that participate in root system formation. Among them, a remarkably large number are involved in LR emergence (Supplemental Table S1). Although we still need more information to determine their exact mode of action, our work has highlighted the importance of ROS signaling during LR development.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Cloning
Arabidopsis (Arabidopsis thaliana) T-DNA lines were ordered from the Nottingham Arabidopsis Stock Centre and were genotyped by PCR. For some lines, homozygous lines were generated. The T-DNA insertion lines correspond to the following genes: At1g74500 (N629876), At1g26870 (N655506), At3g19200 (N658274), At4g36380 (N842691), At1g62800 (N586748), At2g16850 (N599098), At3g10780 (N909180), At5g43890 (N879099), At1g10200 (N360084), At1g57560 (N674429), At2g34070 (N678284), At1g60010 (N662224), At3g06035 (N663801), At5g44020 (N842097), At5g44020 (N669825), At5g56040 (N800047), At1g48630 (N873938), At3g61890 (N318701), At3g42670 (N845000), At2g14960 (N803887), At1g13420 (N309576), At5g14750 (N614008), At4g26890 (N664680), At5g16900 (N667068), At3g13530 (N669767), At4g12420 (N672658), At4g09990 (N676028), At1g05250/PER2 (N816472), At1g30870/PER7 (N369159), and At5g17820/PER57 (N305613).
TMO7 overexpression lines were generated by cloning the corresponding coding sequence into the improved pGWB502Ω vector (Nakagawa et al., 2007). Estradiol-inducible PER2 and PER7 lines were made by cloning the complementary DNA sequences into a Gateway-compatible pER8 vector (Zuo et al., 2000), as adapted by Papdi et al. (2008). T3 homozygous lines were selected by hygromycin selection and were next phenotypically analyzed.
All seedlings were sown under sterile conditions on vertically oriented 12-cm square plates containing one-half-strength Murashige and Skoog (MS1/2) medium with 0.05% (w/v) MES, 1% (w/v) Suc, and 1% (w/v) plant agar (Duchefa) under a 16-h-light/8-h-dark photoperiod at 21°C/18°C. For auxin treatment, plants were grown on vertical Murashige and Skoog (MS) plates for 5 d and then transferred to MS medium with 1 µm IAA for the indicated times. To induce PER2 and PER7 expression, transgenic seedlings were cultivated in MS medium containing 10 µm estradiol for the indicated days.
Microarray Analyses on SKP2Bp:GFP-Expressing Root Cells
We previously showed that, in roots, SKP2Bp:GUS was specifically expressed in founder cells and the early stages of LR development and root apical meristem (Manzano et al., 2012). We generated a transgenic line that expressed GFP under the control of the same SKP2B promoter using the pGWB4 vector (Nakagawa et al., 2007). The transcriptional regulation in early LRP development was analyzed by means of specific cell separation and transcriptional analyses by microarray hybridization. SKP2B-expressing cells from the whole root (sample 1) or the apical part of the root (approximately 1 mm from the tip; sample 2) were isolated from the rest of the root throughout fluorescence-activated cell sorting. Total RNA was prepared for individual microarray hybridizations for the different samples using the Affymetrix Arabidopsis ATH-1 Genome Array, representing approximately 24,000 genes in the Arabidopsis genome (Redman et al., 2004). Three independent replicates of these samples were prepared to test the variability between our chip hybridization experiments. To identify genes that were only expressed in the early stages of LRP, we subtracted the expression in the apical root meristem from the expression in the whole root. Statistical analysis using a mixed model of variance was performed to identify differentially expressed genes.
Metaanalyses of Other Published Arrays Used in This Work
Data sets used for comparison were downloaded from the Gene Expression Omnibus (Edgar et al., 2002) as .CEL files. Gene Expression Omnibus accession numbers are GSE3350, GSE6349, GSM226525, GSM226529, GDS3216, GSE21876, GSE21611, and GPL198. The analysis is based on the robust multiarray average expression values as obtained with the affy package of Bioconductor (www.bioconductor.org). They were normalized using the robust multiarray average method (Bolstad et al., 2003) and P value calculation. In those experiments where an experimental condition was compared with a control, each expression value of the experimental replicate of the condition was compared with the average of the control to get the signal-to-log ratio for each gene and calculate the expression fold change. We selected genes that changed their expression more than 2-fold, except for overexpressing UPB1 plants, for which we selected 1.75-fold, with respect to the control experiment, with a false discovery rate less than 0.02. The overlap between different data sets was carried out using a venny program (http://bioinfogp.cnb.csic.es/tools/venny/index.html). In those data sets where there was no experimental condition to compare, we used the MAS 5.0 detection calls to decide whether a signal was significantly above background.
Peroxidase Inhibitor Treatments and per Mutant Analyses
To evaluate the effect of peroxidase inhibition on LR formation, SKP2Bp:GUS seedlings were grown in solid MS medium on vertical plates for 7 d (section A) and then transferred to MS medium with or without KCN (100 µm) for another 4 d (section B). Afterward, roots were exscinded from the shoot and cut into two sections, A and B, and stained separately for GUS activity. The numbers of emerged LRs and LRPs were counted in both sections.
To analyze LR formation in per mutants and overexpressing lines, per7-1 and per57-1 mutant plants were grown on vertical plates containing MS1/2 medium for 8 d (root section A) and then transferred to fresh plates containing MS1/2 supplemented with 10 or 50 nm IAA for an additional 24 or 48 h. Estradiol-inducible PER7 plants were grown on vertical MS1/2 medium with 10 µm estradiol for 8 d and then transferred to fresh plates containing MS1/2 medium with 10 µm estradiol plus 10 or 50 nm IAA for an extra 24 or 48 h. Emerged LRs were counted using a Leica stereomicroscope (MZ9.5) in section A only at the time of the transfer (0 h) and at 24 and 48 h after transfer.
ROS Localization
Arabidopsis seedlings were cultured for 10 d on standard MS medium under a 16-h-light/8-h-dark photoperiod at 21°C/18°C. DAB was used for the detection of H2O2 in root tissues. Whole seedlings were stained from 4 to 6 h in 1 mg mL−1 DAB and 0.05% (w/v) Tween 20 in 20 mm phosphate buffer, pH 7.4, and covered with aluminum foil with gentle agitation. H2O2 was visualized as a reddish-brown coloration. A pretreatment with 10 mm potassium iodide was applied for 1 h before DAB staining and used as a control. For superoxide visualization, roots were stained for 1 h in a solution of 2 mm NBT in 20 mm phosphate buffer, pH 6.1. A pretreatment with 10 mm propyl gallate was applied for 1 h before NBT staining and used as a control. The blue/violet color denotes the presence of superoxide. Seedlings were cleaned according to Malamy and Benfey (1997).
GUS Assays
Histochemical GUS staining was performed as described by del Pozo et al. (2006). Photographs were taken using a Leica stereomicroscope (MZ9.5) with a DCF280 camera or a Leica MD2000 microscope with a DCF300 camera.
Root Growth Assays and Microscopy Analysis
Primary root length was determined as described previously (Lucas et al., 2011). All data are mean values of at least 50 plants, and these experiments were repeated twice, obtaining similar values in each experiment. Data values were statistically analyzed using Student’s t test. Total numbers and stages of LRPs were counted according to methods used previously (Malamy and Benfey, 1997), and root meristem size was calculated based on the number of meristematic cortex cells (Casamitjana-Martínez et al., 2003). To analyze statistical differences in LRP development, we grouped stages I, II, and III (stages where the initial and oriented divisions to form the primordium take place), stages IV and V (stages were the LRP acquired meristem identity), stages VI and VII (stages where cell elongation into the primordium starts), and stage VIII (the final stage of LRP emergence out of the root). These grouped stages were analyzed together by a mixed-model ANOVA.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. TMO7 expression is reduced in tmo7-3.
Supplemental Table S1. Genes identified in the SKP2Bp:GFP Expressing Root Cells (cs-SKP2B data set).
Supplemental Table S2. VisuaLRTC analysis of the cs-SKP2B data set.
Supplemental Table S3. Common genes between cs-SKP2B data set and UPB1-overexpressing plants.
Supplementary Material
Acknowledgments
We thank Sara Navarro for technical assistance, Dr. Dolf Weijers for providing the TMO7:3xGFP lines, Dr. Ben Scheres for pFEZ:FEZ-GFP, and Dr. Philip N. Benfey for upb1-1, 35S:UPB1-3xYFP, and pUPB1:GFP. We also thank the Nottingham Arabidopsis Stock Centre for providing the T-DNA mutants.
Glossary
- RAM
root apical meristem
- LR
lateral root
- LRP
lateral root primordium
- ROS
reactive oxygen species
- hag
hours after gravistimulation
- T-DNA
transfer DNA
- H2O2
hydrogen peroxide
- DAB
diaminobenzidine
- NBT
nitroblue tetrazolium
- IAA
indole-3-acetic acid
- MS1/2
one-half-strength Murashige and Skoog
- MS
Murashige and Skoog
Footnotes
This work was supported by the Spanish Government (grant nos. BIO2008–00639, BIO2011–28184–C02–01, and CDS2007–0057 to J.C.d.P.), by the Interuniversity Attraction Poles Programme and Fonds de la Recherche Scientifique (to X.D.), by the Interuniversity Attraction Poles Programme from the Belgian Federal Science Policy Office and the Research Foundation Flanders (grant no. IUAP P7/29 MARS to T.B.), by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (predoctoral fellowship to C.M.), by the Spanish Education Ministry (Formación Profesorado Universitario fellowship to M.P.-B.), by the Special Research Fund of Ghent University (to B.D.R. and B.O.-L.), and by the Fonds de la Recherche pour l’Industrie et l’Agriculture (fellowship to B.O.-L.).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MH, Mansfield JW. (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193 [DOI] [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. (2007) A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318: 801–806 [DOI] [PubMed] [Google Scholar]
- Casamitjana-Martínez E, Hofhuis HF, Xu J, Liu CM, Heidstra R, Scheres B. (2003) Root-specific CLE19 overexpression and the sol1/2 suppressors implicate a CLV-like pathway in the control of Arabidopsis root meristem maintenance. Curr Biol 13: 1435–1441 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ. (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Castelain M, Le Hir R, Bellini C. (2012) The non-DNA-binding bHLH transcription factor PRE3/bHLH135/ATBS1/TMO7 is involved in the regulation of light signaling pathway in Arabidopsis. Physiologia Plantarum 145: 450–460 [DOI] [PubMed] [Google Scholar]
- Cosio C, Vuillemin L, De Meyer M, Kevers C, Penel C, Dunand C. (2009) An anionic class III peroxidase from zucchini may regulate hypocotyl elongation through its auxin oxidase activity. Planta 229: 823–836 [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Diaz-Trivino S, Cisneros N, Gutierrez C. (2006) The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell 18: 2224–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Audenaert D, Xuan W, Overvoorde P, Strader LC, Kepinski S, Hoye R, Brisbois R, Parizot B, Vanneste S, et al. (2012) A role for the root cap in root branching revealed by the non-auxin probe naxillin. Nat Chem Biol 8: 798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B, Demeulenaere M, Grunewald W, Audenaert D, Van Campenhout J, Overvoorde P, Jansen L, Vanneste S, et al. (2010) A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol 20: 1697–1706 [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, Frei dit Frey N, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al. (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- De Smet I, Vassileva V, De Rybel B, Levesque MP, Grunewald W, Van Damme D, Van Noorden G, Naudts M, Van Isterdael G, De Clercq R, et al. (2008) Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322: 594–597 [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E. (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 105: 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M. (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29: 153–168 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69: 437–449 [DOI] [PubMed] [Google Scholar]
- Gazaryan IG, Lagrimini LM, Ashby GA, Thorneley RN. (1996) Mechanism of indole-3-acetic acid oxidation by plant peroxidases: anaerobic stopped-flow spectrophotometric studies on horseradish and tobacco peroxidases. Biochem J 313: 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T. (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Vuylsteke M, Vanneste S, Vercruysse S, Boucheron E, Alard P, Chriqui D, Van Montagu M, Inzé D, Beeckman T. (2004) Transcript profiling of early lateral root initiation. Proc Natl Acad Sci USA 101: 5146–5151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42: 462–468 [DOI] [PubMed] [Google Scholar]
- Iiyama K, Lam T, Stone BA. (1994) Covalent cross-links in the cell wall. Plant Physiol 104: 315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Feldman LJ. (2005) Regulation of root apical meristem development. Annu Rev Cell Dev Biol 21: 485–509 [DOI] [PubMed] [Google Scholar]
- Kurup S, Runions J, Köhler U, Laplaze L, Hodge S, Haseloff J. (2005) Marking cell lineages in living tissues. Plant J 42: 444–453 [DOI] [PubMed] [Google Scholar]
- Lagrimini LM, Joly RJ, Dunlap JR, Liu TT. (1997) The consequence of peroxidase overexpression in transgenic plants on root growth and development. Plant Mol Biol 33: 887–895 [DOI] [PubMed] [Google Scholar]
- Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18: 450–458 [DOI] [PubMed] [Google Scholar]
- Lucas M, Swarup R, Paponov IA, Swarup K, Casimiro I, Lake D, Péret B, Zappala S, Mairhofer S, Whitworth M, et al. (2011) Short-Root regulates primary, lateral, and adventitious root development in Arabidopsis. Plant Physiol 155: 384–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Wang L, Li J, Samma MK, Xie Y, Wang R, Wang J, Zhang J, Shen W. (2014) Interaction between HY1 and H2O2 in auxin-induced lateral root formation in Arabidopsis. Plant Mol Biol 85: 49–61 [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Manzano C, Ramirez-Parra E, Casimiro I, Otero S, Desvoyes B, De Rybel B, Beeckman T, Casero P, Gutierrez C, Del Pozo JC. (2012) Auxin and epigenetic regulation of SKP2B, an F-box that represses lateral root formation. Plant Physiol 160: 749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Risueño MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN. (2010) Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329: 1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. (2007) Development of series of Gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Papdi C, Abrahám E, Joseph MP, Popescu C, Koncz C, Szabados L. (2008) Functional identification of Arabidopsis stress regulatory genes using the controlled cDNA overexpression system. Plant Physiol 147: 528–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizot B, De Rybel B, Beeckman T. (2010) VisuaLRTC: a new view on lateral root initiation by combining specific transcriptome data sets. Plant Physiol 153: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaia G, Queval G, Bai J, Margis-Pinheiro M, Foyer CH. (2014) The effects of redox controls mediated by glutathione peroxidases on root architecture in Arabidopsis thaliana. J Exp Bot 65: 1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi F, Cosio C, Penel C, Dunand C. (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24: 255–265 [DOI] [PubMed] [Google Scholar]
- Péret B, Li G, Zhao J, Band LR, Voß U, Postaire O, Luu DT, Da Ines O, Casimiro I, Lucas M, et al. (2012) Auxin regulates aquaporin function to facilitate lateral root emergence. Nat Cell Biol 14: 991–998 [DOI] [PubMed] [Google Scholar]
- Redman JC, Haas BJ, Tanimoto G, Town CD. (2004) Development and evaluation of an Arabidopsis whole genome Affymetrix probe array. Plant J 38: 545–561 [DOI] [PubMed] [Google Scholar]
- Ren H, Santner A, del Pozo JC, Murray JA, Estelle M. (2008) Degradation of the cyclin-dependent kinase inhibitor KRP1 is regulated by two different ubiquitin E3 ligases. Plant J 53: 705–716 [DOI] [PubMed] [Google Scholar]
- Ros Barceló A. (1997) Lignification in plant cell walls. Int Rev Cytol 176: 87–132 [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D. (2010) MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916 [DOI] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Tognolli M, Penel C, Greppin H, Simon P. (2002) Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 288: 129–138 [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN. (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616 [DOI] [PubMed] [Google Scholar]
- Ubeda-Tomás S, Beemster GT, Bennett MJ. (2012) Hormonal regulation of root growth: integrating local activities into global behaviour. Trends Plant Sci 17: 326–331 [DOI] [PubMed] [Google Scholar]
- Vanneste S, De Rybel B, Beemster GT, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M, et al. (2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17: 3035–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen V, Bauch M, Bennett T, Campilho A, Wolkenfelt H, Xu J, Haseloff J, Scheres B. (2008) The NAC domain transcription factors FEZ and SOMBRERO control the orientation of cell division plane in Arabidopsis root stem cells. Dev Cell 15: 913–922 [DOI] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Chua NH. (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24: 265–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.