The dose of a ribosomal protein affects the level of plant fertility.
Abstract
Ribosomal protein mutations in Arabidopsis (Arabidopsis thaliana) result in a range of specific developmental phenotypes. Why ribosomal protein mutants have specific phenotypes is not fully known, but such defects potentially result from ribosome insufficiency, ribosome heterogeneity, or extraribosomal functions of ribosomal proteins. Here, we report that ovule development is sensitive to the level of Ribosomal Protein L27a (RPL27a) and is disrupted by mutations in the two paralogs RPL27aC and RPL27aB. Mutations in RPL27aC result in high levels of female sterility, whereas mutations in RPL27aB have a significant but lesser effect on fertility. Progressive reduction in RPL27a function results in increasing sterility, indicating a dose-dependent relationship between RPL27a and female fertility. RPL27a levels in both the sporophyte and gametophyte affect female gametogenesis, with different developmental outcomes determined by the dose of RPL27a. These results demonstrate that RPL27aC and RPL27aB act redundantly and reveal a function for RPL27a in coordinating complex interactions between sporophyte and gametophyte during ovule development.
Eukaryotic cytoplasmic ribosomes are comprised of two subunits, a large 60S and a small 40S subunit. The 60S subunit includes 25S or 28S, 5.8S, and 5S ribosomal RNA (rRNA) and approximately 47 ribosomal proteins, whereas the 40S subunit includes an 18S rRNA and approximately 33 ribosomal proteins. In plants and animals, reduced ribosomal protein function results in specific developmental phenotypes (Byrne, 2009; Warner and McIntosh, 2009; McCann and Baserga, 2013; Terzian and Box, 2013; Tsukaya et al., 2013). Currently, it is not known how ribosomal proteins modulate development. Potentially specific developmental phenotypes in ribosomal protein mutants are an outcome of ribosome haploinsufficiency and reduced global protein synthesis or reduced translation of specific proteins. Alternatively, ribosomal proteins, in addition to their role in translation, may have extraribosomal function required for specific developmental processes.
In Arabidopsis (Arabidopsis thaliana), cytoplasmic ribosomal proteins are encoded by two to five genes (Barakat et al., 2001; Giavalisco et al., 2005; Carroll et al., 2008). Mutations in single ribosomal protein genes are sometimes gametophyte or embryo lethal (Weijers et al., 2001; Tzafrir et al., 2004). However, many ribosomal protein mutants are viable. These mutants typically display a subtle change in leaf shape and may also have distinct developmental defects affecting embryo morphogenesis, inflorescence development, the transition to flowering, and plant stature (Van Lijsebettens et al., 1994; Ito et al., 2000; Pinon et al., 2008; Yao et al., 2008; Byrne, 2009; Fujikura et al., 2009; Falcone Ferreyra et al., 2010; Rosado et al., 2010; Horiguchi et al., 2011; Szakonyi and Byrne, 2011a, 2011b; Stirnberg et al., 2012). Female fertility is also reduced in several ribosomal protein mutants. Mutations in the ribosomal protein genes SHORT VALVE1 (STV1)/RPL24B, SUPPRESSOR OF ACAULIS52 (SAC52)/RPL10A, ARABIDOPSIS MINUTE-LIKE1 (AML1)/RPS5B, and the Ribosomal Protein L27a gene RPL27aC reduce female fertility (Weijers et al., 2001; Nishimura et al., 2005; Imai et al., 2008; Szakonyi and Byrne, 2011b). aml1 and sac52-t1 are partially and fully gametophyte lethal, respectively. Although lower fertility in stv1 and rpl27ac is associated with defective ovules, the nature of the fertility defect in these mutants has not been fully explored.
Female gametophyte development is also disrupted by mutations in a number of genes predicted to be involved in ribosome biogenesis. SLOW WALKER1 (SWA1), SWA3/Arabidopsis thaliana RNA HELICASE36 (AtRH36), and NUCLEOLAR FACTOR1 (NOF1) encode nucleolar-localized proteins required for processing 18S pre-rRNA (Shi et al., 2005; Harscoët et al., 2010; Huang et al., 2010; Liu et al., 2010). Mutations in other genes encoding proteins predicted to be involved in pre-rRNA processing and ribosome maturation or in export of preribosomes from the nucleus to the cytoplasm also reduce female fertility (Li et al., 2009, 2010; Chantha et al., 2010; Wang et al., 2012; Missbach et al., 2013). These mutants share similar phenotypes, where female gametophyte development is delayed and there is a failure in progression through gametophyte mitotic cell divisions. Transmission of these ribosome biogenesis mutants through the female is often reduced. This ostensibly reflects a requirement for active ribosome synthesis and sufficient ribosome levels to support morphogenesis of the gametophyte.
Here, we show that mutations in a number of different ribosomal protein genes lead to reduced seed set and an increase in the number of defective ovules in siliques. This is particularly apparent in mutants affecting ribosomal protein RPL27a. We show the two RPL27a genes, RPL27aC and RPL27aB, act redundantly and that ovule development is sensitive to the dose of RPL27a. rpl27ac and rpl27ab mutations are together female and male gametophyte lethal. Single rpl27ac mutants also result in some female gametophyte lethality. In the homozygous rpl27ac-2 mutant, the mature embryo sac is frequently expelled from the ovule, suggesting RPL27a is necessary for maintaining a viable gametophyte. However, in the heterozygous rpl27ac-2/+, gametogenesis frequently fails early in development. This occurs independent of the genotype of the gametophyte, indicating somatic sporophyte cells in the mutant affect gametophyte development. Together, our data demonstrate that appropriate levels of RPL27a in the sporophyte and gametophyte are required for female gametophyte development and plant fertility.
RESULTS
Insertion Mutations in the Paralogs RPL27aC and RPL27aB Result in Altered Leaf Shape
Arabidopsis has three RPL27a genes. RPL27aC and RPL27aB are transcriptionally active, whereas RPL27aA is not expressed and is likely a pseudogene (Barakat et al., 2001; Szakonyi and Byrne, 2011b). The RPL27aC- and RPL27aB-encoded proteins are highly conserved, differing in only two of 146 amino acids, and in most tissues, RPL27aC expression is approximately twice that of RPL27aB (Rhee et al., 2003; Laubinger et al., 2008). To compare the function of RPL27aC and RPL27aB, we examined the phenotypes of transfer DNA (T-DNA) insertion mutations in both genes. As reported previously, two alleles of RPL27aC, rpl27ac-2 and rpl27ac-3, had T-DNA insertions in the promoter region and the 5′ leader sequence of RPL27aC, respectively, and transcript levels of both alleles were reduced relative to the wild type (Fig. 1A; Szakonyi and Byrne, 2011b). Plants homozygous for either rpl27ac-2 or rpl27ac-3 had rosette leaves that were pointed and serrated compared with the wild type and were similar to the semidominant heterozygous rpl27ac-1d/+ mutant (Fig. 1, B, D, and E; Szakonyi and Byrne, 2011b).
Figure 1.
Leaf phenotypes of rpl27ac and rpl27ab mutants. A, Diagrammatic representation of RPL27aC and RPL27aB genes, showing exons, 5′ and 3′ leader sequences, sites of T-DNA insertions, and the location of the point mutation in rpl27ac-1d. B to I, Rosettes of the wild type (WT; B), rpl27ac-2/+ (C), rpl27ac-2 (D), rpl27ac-3 (E), rpl27ab-1 (F), rpl27ab-2 (G), rpl27ac-2/+ rpl27ab-1/+ (H), and rpl27ac-2/+ rpl27ab-2/+ (I). [See online article for color version of this figure.]
Two alleles of RPL27aB, rpl27ab-1 and rpl27ab-2, had T-DNA insertions in the 5′ leader sequence and in the coding region of RPL27aB, respectively (Fig. 1A). Transcript levels of RPL27aB in rpl27ab-1 were slightly reduced relative to the wild type, indicating this is a weak allele (Supplemental Fig. S1). The insertion in rpl27ab-2 is within the coding sequence and is predicted to be a null allele. Leaf shape in rpl27ab-1 and rpl27ab-2 was changed to a more pointed shape, but this phenotype was less severe than the rpl27ac mutants, indicating that RPL27aB has a less significant role in leaf development than RPL27aC (Fig. 1, F and G). This result is consistent with the lower level of expression of RPL27aB compared with RPL27aC. To test for redundancy between RPL27aC and RPL27aB, we crossed homozygous mutants in each gene and analyzed the F1 transheterozygotes rpl27ac-2/+ rpl27ab-1/+ and rpl27ac-2/+ rpl27ab-2/+ for phenotypes. Both transheterozygotes had pointed and serrated leaves (Fig. 1, H and I). Thus, there is functional overlap between RPL27aC and RPL27aB in leaf development.
Insertion Mutations in RPL27aC and RPL27aB Reduce Fertility
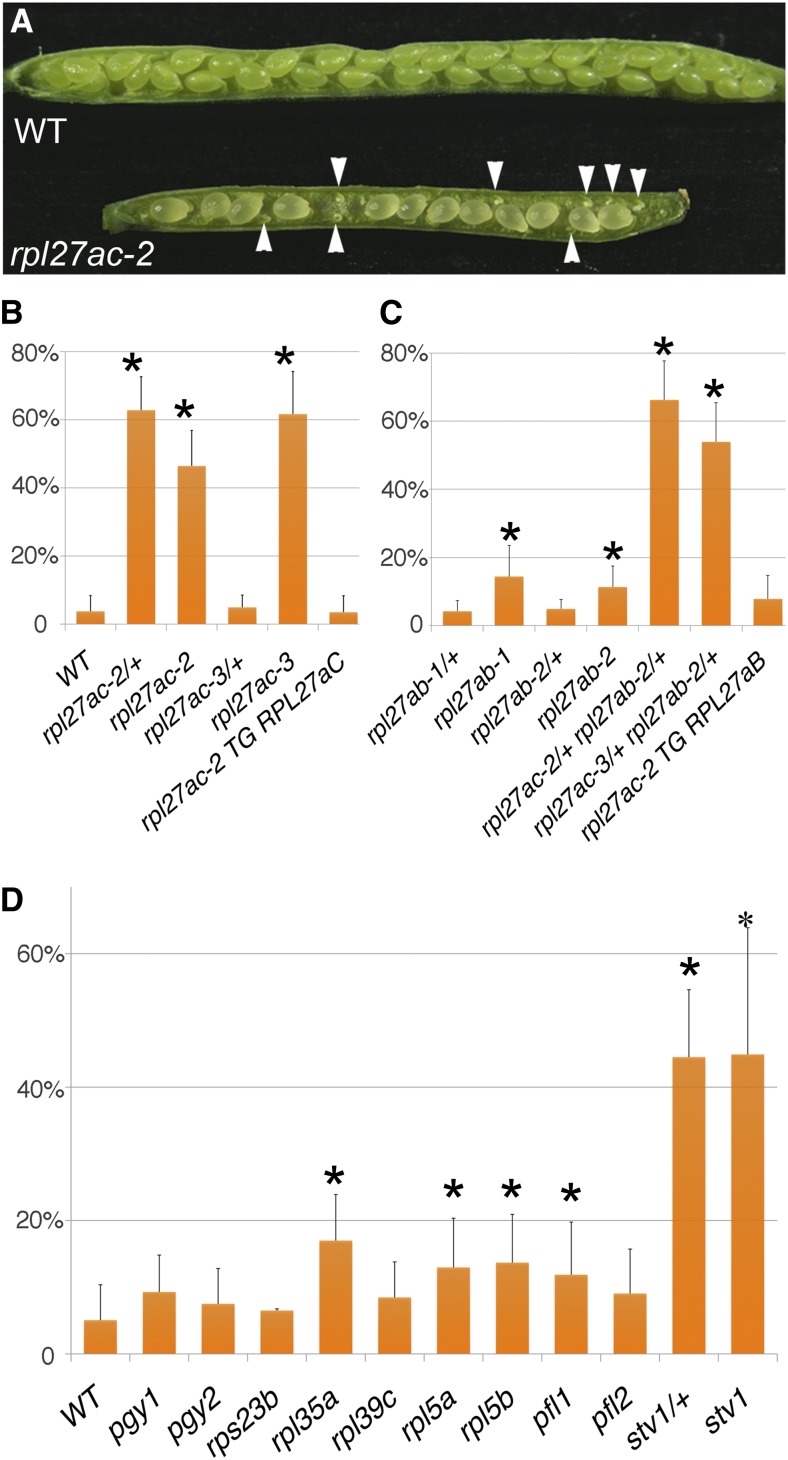
rpl27ac-2 and rpl27ac-3 had a change in leaf shape and were slightly slower growing than the wild type. Both mutants also had shorter siliques than the wild type. Examination of siliques in the mutants revealed a reduced number of seed and the presence of aborted or unfertilized ovules (Fig. 2A). In wild-type siliques, only 3.8% of ovules were defective and 96.2% of ovules were fertile and set seed. As reported previously, rpl27ac-2 and rpl27ac-3 siliques had 46.4% and 61.7% defective ovules, respectively (Fig. 2B; Szakonyi and Byrne, 2011b). The heterozygous rpl27ac-3/+ mutant was indistinguishable from the wild type (Fig. 2B). By contrast, the heterozygous rpl27ac-2/+ had wild type-shaped leaves (Fig. 1C) and was indistinguishable from the wild type, except siliques were found to have a high number of defective ovules (Fig. 2B). Heterozygous plants generated using a homozygous mutant as either the female or male parent did not lead to significant differences in the frequency of defective ovules. Heterozygous plants generated from rpl27ac-2 crossed with wild-type pollen had 62.8% defective ovules (n = 565), and heterozygous plants generated from the wild type crossed with rpl27ac-2 pollen had 60.6% defective ovules (n = 676). Surprisingly, the frequency of defective ovules was higher in the rpl27ac-2/+ heterozygote than in the rpl27ac-2 homozygous. Possibly, expression levels of RPL27aC in the ovule regulate expression of one or both RPL27a paralogs, and this regulation is disrupted to different extents in heterozygotes and homozygotes. A single copy of the transgene RPL27aC:RPL27aC, which carried the genomic promoter and coding region of RPL27aC, restored fertility in the rpl27ac-2 mutant, confirming that the ovule defect is due to a reduction in RPL27a (Fig. 2B). Although there was a dramatic ovule defect in the heterozygous rpl27ac-2/+, reciprocal crosses between rpl27ac-2/+ and the wild type demonstrated that the mutant allele was efficiently transmitted maternally (transmission efficiency = 54.1%, n = 74) and paternally (transmission efficiency = 56%, n = 75). This indicates ovule development depends on appropriate levels of RPL27a in the sporophyte.
Figure 2.
Fertility defects in rpl27a and ribosomal protein mutants. A, Wild-type (WT; top) and rpl27ac-2 (bottom) open siliques, with defective ovules in the mutant marked (arrowheads). B, Percentage of defective ovules in siliques of the wild type (n = 581), rpl27ac-2/+ (n = 565), rpl27ac-2 (n = 521), rpl27ac-3/+ (n = 587), rpl27ac-3 (n = 579), and rpl27ac-2 carrying the transgene RPL27aC:RPL27aC (rpl27ac-2 TG RPL27aC; n = 824). C, Percentage of defective ovules in siliques of rpl27ab-1/+ (n = 572), rpl27ab-1 (n = 549), rpl27ab-2/+ (n = 626), rpl27ab-2 (n = 548), rpl27ac-2/+ rpl27ab-2/+ (n = 612), rpl27ac-3/+ rpl27ab-2/+ (n = 594), and rpl27ac-2 carrying the transgene RPL27aB:RPL27aB (rpl27ac-2 TG RPL27aB; n = 932). D, Percentage of defective ovules in siliques of the wild type (n = 690), pgy1 (n = 603), pgy2 (n = 598), rps23b (n = 630), rpl35a (n = 604), rpl39c (n = 601), rpl5a (n = 532), rpl5b (n = 562), pfl1 (n = 556), pfl2 (n = 585), stv1/+ (n = 668), and stv1 (n = 560). Error bars are the sd (Student’s t test, *P < 0.05). [See online article for color version of this figure.]
Mutations in RPL27aB also affected ovule development. Heterozygous rpl27ab-1/+ and rpl27ab-2/+ plants were indistinguishable from the wild type, and the number of defective ovules in siliques was not significantly different from the wild type (Fig. 2C). Homozygous rpl27ab-1 and rpl27ab-2 mutants were not noticeably different from the wild type, except siliques showed 14.4% and 11.3% defective ovules, respectively (Fig. 2C). The frequency of defective ovules in rpl27ab mutants was higher than the wild type but less than that in rpl27ac mutants.
Given that RPL27aC and RPL27aB acted redundantly in leaf development, we examined whether these genes are redundant in ovule development by testing whether RPL27aB can complement the rpl27ac-2 ovule phenotype. A single copy of the transgene RPL27aB:RPL27aB carrying the promoter and coding region of RPL27aB completely restored fertility in rpl27ac-2 (Fig. 2C). We also determined whether these two ribosomal protein genes act redundantly in ovule development by comparing fertility in the transheterozygotes rpl27ac-2/+ rpl27ab-2/+ and rpl27ac-3/+ rpl27ab-2/+ relative to each single mutant. Ovule defects were significantly increased in both transheterozygotes compared with either single heterozygous or homozygous mutants (Fig. 2, B and C). rpl27ac-2/+ rpl27ab-2/+ and rpl27ac-3/+ rpl27ab-2/+ had 66% and 54% defective ovules, respectively. Like rpl27ac-2/+, these defective ovules may have been due to sporophytic effects but may also have included ovules that were gametophyte lethal. A screen of F2 progeny from the two transheterozygotes failed to recover plants that were double homozygous rpl27ac rpl27ab. Mutants that were homozygous for rpl27ac and heterozygous for rpl27ab or homozygous for rpl27ab and heterozygous for rpl27ac were also not recovered. This indicated that the two mutant alleles were not transmitted together through either the female or the male and were therefore gametophyte lethal. In the transheterozygotes, 25% of the defective ovules were therefore likely to be the result of gametophytic lethality. The remaining defective ovules are predicted to be due to reduced RPL27a in the sporophyte. Transcriptional fusions of the RPL27a gene promoters to a nuclear-localized venus yellow fluorescent protein reporter (nls-vYFP) showed both genes were expressed in all cells of the ovule (Supplemental Fig. S2). The two RPL27a genes therefore have overlapping expression and both contribute to ovule development.
Mutations Disrupting Different Ribosomal Proteins Reduce Fertility
Although mutants in the ribosomal protein genes RPL27aC, RPS5B, STV1, and SAC52 have ovule defects, this phenotype has not been reported for mutations in other ribosomal protein genes (Weijers et al., 2001; Nishimura et al., 2005; Imai et al., 2008; Szakonyi and Byrne, 2011b). To determine whether other ribosomal protein mutants affect fertility, the frequency of defective ovules in siliques of several ribosomal protein mutants was examined. Five of the ribosomal protein mutants were isolated as enhancers of the leaf shape mutant asymmetric leaves1 (as1). All of these mutants had pointed, serrated leaves and, in an as1 mutant background, had a piggyback phenotype with ectopic lamina outgrowths on the adaxial side of the leaf. These included the previously reported mutants piggyback1-1 (pgy1-1; corresponding to rpl10ab) and pgy2-1 (corresponding to rpl9c; Pinon et al., 2008) and three new pgy mutants, designated rps23b-1, rpl35a-1, and rpl39c-1, which were found to have mutations in ribosomal protein genes RPS23B, RPL35A, and RPL39C, respectively (Supplemental Fig. S3). rps23b had a point mutation that changed the second amino acid from Gly to Asp. rpl35a and rpl39c had point mutations in the 5′ leader sequence and at the first exon-intron junction, respectively, and both mutations reduced the level of wild-type transcript (Supplemental Fig. S3). In addition, previously reported mutations rpl5a-2, rpl5b-3, pointed first leaf1-1 (pfl1-1), pfl2, and stv1-2 were included in this analysis (Van Lijsebettens et al., 1994; Ito et al., 2000; Nishimura et al., 2005; Yao et al., 2008; Horiguchi et al., 2011). Compared with the wild type, these nine ribosomal protein mutants had variable effects on fertility. pgy1, pgy2, rps23b, rpl39c, and pfl2 did not reduce fertility, whereas rpl5a, rpl5b, rpl35a, pfl1, and stv1 had significantly more defective ovules than the wild type (Fig. 2D). The number of defective ovules was most extreme in stv1. Homozygous stv1 had a high frequency of defective ovules, as previously reported (Nishimura et al., 2005). As with rpl27ac-2/+, heterozygous stv1/+ plants had siliques with a high proportion of defective ovules (Fig. 2D). Based on these results, it can be concluded that a number of different ribosomal proteins promote plant fertility.
The Dose of RPL27a Determines the Severity of Leaf and Ovule Defects
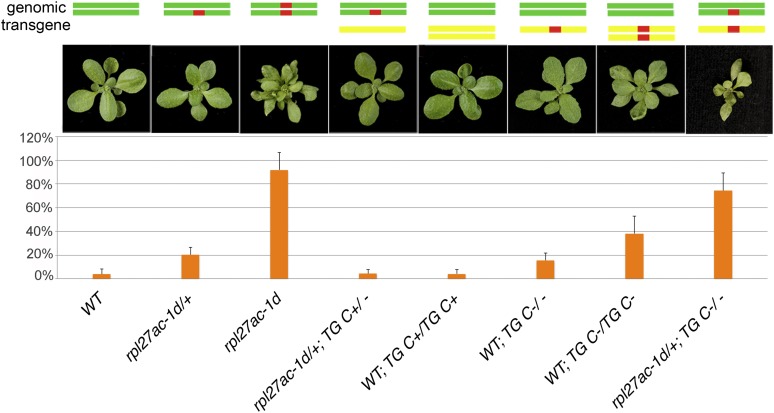
To examine the potential effect of RPL27a dose on development, we took advantage of the dominant-negative rpl27ac-1d allele, which is predicted to produce a protein that interferes with wild-type function (Szakonyi and Byrne, 2011b). Plants in which the copy number of RPL27aC varied relative to mutant rpl27ac-1d were generated by placing transgenes that carried either the wild-type (RPL27aC:RPL27aC) or mutant allele (RPL27aC:rpl27ac-1d) into wild-type or rpl27ac-1d/+ genetic backgrounds (Fig. 3). In the heterozygous rpl27ac-1d/+ and homozygous rpl27ac-1d mutants, there were 20.1% and 91.9% defective ovules, respectively (Fig. 3). RPL27aC:RPL27aC fully suppressed rpl27ac-1d/+ leaf and ovule phenotypes. Furthermore, plants homozygous for RPL27aC:RPL27aC in a wild-type background were indistinguishable from the wild type, indicating there are no phenotypic consequences from an increased copy number of RPL27aC. Wild-type plants hemizygous for RPL27aC:rpl27ac-1d had pointed, serrated leaves and 16.6% defective ovules. Wild-type plants homozygous for RPL27aC:rpl27ac-1d had more severe changes in leaf shape, and siliques had 38.1% defective ovules. rpl27ac-1d/+ plants hemizygous for RPL27aC:rpl27ac-1d had the most severe leaf shape change and had siliques with 74.3% defective ovules. We did not recover rpl27ac-1d/+ plants homozygous for RPL27aC:rpl27ac-1d. Because wild-type plants homozygous for RPL27aC:rpl27ac-1d had more severe phenotypes than rpl27ac-1d/+, we speculated that the mutant transgene was more potent than the wild-type gene, either due to higher levels of transcription or due to differences in levels or stability of the proteins. To test whether this was due to differences in transcript levels, we took advantage of an HpaII polymorphism that distinguished transcripts from the two alleles. Semiquantitative reverse transcription (RT)-PCR analysis demonstrated that wild-type and mutant transcript levels were similar in rpl27ac-1d/+ (Supplemental Fig. S4A). By contrast, in wild-type plants that were also homozygous for RPL27aC:rpl27ac-1d, the level of mutant transcript was higher than that of the wild type alone (Supplemental Fig. S4A). To obtain a more accurate estimate of wild-type and rpl27ac-1d transcript levels in the different genetic backgrounds, RT-PCR products were cloned and individual clones were genotyped. Consistent with the semiquantitative data, the alleles represented in the cloned RT-PCR products showed the level of mutant transcript was 3 times higher than that of the wild type in plants carrying the RPL27aC:rpl27ac-1d transgene (Supplemental Fig. S4B). Although the mutant allele was expressed at higher levels than the transgene, it can be concluded that changes in the ratio of RPL27aC to rpl27ac-1d results in a gradual change in phenotype severity, with changes in leaf shape paralleling changes in the frequency of defective ovules.
Figure 3.
Leaf and fertility defects reciprocally correlate with relative levels of functional RPL27a. Diagrams at the top represent the genomic wild-type (WT) RPL27aC and rpl27ac-1d alleles (top) and RPL27aC:RPL27aC and RPL27aC:rpl27ac-1d transgenes (bottom). Plant rosettes and the percentage of defective ovules for each genotype are shown below the diagrams. Genotypes are, from left to right, the wild type (n = 581), rpl27ac-1d/+ (n = 578), rpl27ac-1d (n = 401), rpl27ac-1d/+ hemizygous for the transgene RPL27aC:RPL27aC (TG C+; n = 2,109), wild-type homozygous for the transgene RPL27aC:RPL27aC (n = 1,836), wild-type hemizygous for the transgene RPL27aC:rpl27ac-1d (TG C–; n = 1,239), wild-type homozygous for the transgene RPL27aC:rpl27ac-1d (n = 1,801), and rpl27ac-1d/+ hemizygous for the transgene RPL27aC:rpl27ac-1d (n = 1,366). Error bars are the sd. [See online article for color version of this figure.]
Ovule and Female Gametophyte Defects in rpl27ac-2
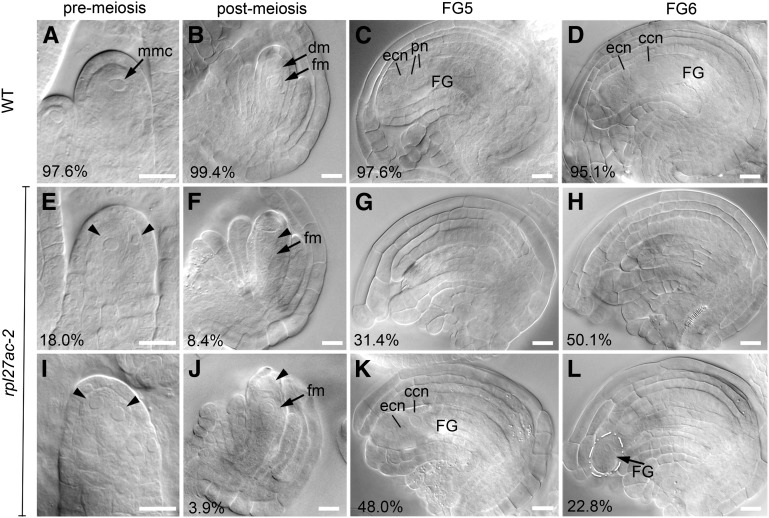
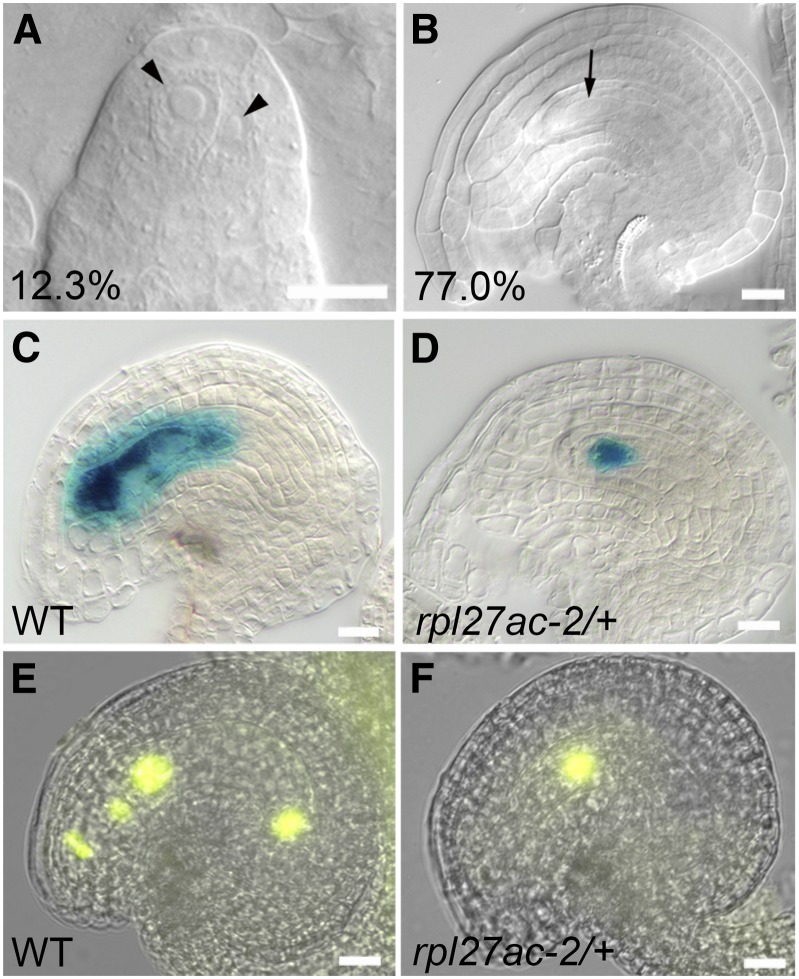
To understand the role of RPL27a in ovule development, we examined the morphology of developing and mature rpl27ac-2 ovules. In the wild type, the megaspore mother cell is a single large subepidermal cell at the distal end of ovule primordium in 97.6% of ovules (Fig. 4A), and only 2.4% of primordia had alterations in this pattern, most often with more than one large subepidermal cell. Postmeiosis, the ovule has a single functional megaspore and three degenerate megaspores (Fig. 4B). The functional megaspore undergoes three rounds of mitosis, leading to female gametophyte stage 5 (FG5) embryo sac with eight nuclei (Fig. 4C). Subsequently, in the FG6 stage of development, the two polar nuclei fuse to form a central cell nucleus (Fig. 4D). In rpl27ac-2, the frequency of an abnormal cell arrangement was 18% of ovule primordia, somewhat higher than that of the wild type (Fig. 4, E and I). Despite this defect, subsequent early stages of female gametophyte development in rpl27ac-2 were not readily distinguished from the wild type. After meiosis, 12.3% of rpl27ac-2 ovules had a large cell in the position of a degenerate megaspore (Fig. 4, F and J). Because these cells were not apparent in subsequent stages of gametogenesis, they may represent delayed degeneration of nonfunctional megaspores. Defects in rpl27ac-2 ovules became more obvious in the final stages of ovule development. At FG5, when the wild type had completed mitotic divisions, 31.4% of rpl27ac-2 ovules had no embryo sac (Fig. 4G). Another 48% of ovules had short integuments and/or an embryo sac in an abnormal position in the ovule (Fig. 4K). At FG6, when the wild type had a large seven-cell embryo sac, 50.1% of rpl27ac-2 ovules did not have an embryo sac (Fig. 4H) and 22.8% of ovules had a small and displaced embryo sac (Fig. 4L). To rule out the potential influence of the Landsberg erecta (Ler) background, we also examined rpl27ac-2 ovule development in the Columbia (Col-0) background and found no significant differences compared with rpl27ac-2 in Ler (Supplemental Fig. S5). The increase in frequency of ovules without an embryo sac in late stages of development indicates that sufficient levels of RPL27a are required to maintain a viable female gametophyte.
Figure 4.
Ovule phenotypes in rpl27ac-2. A to D, Wild-type (WT) ovules. Percentage of normal ovules is indicated. A, Premeiosis ovule (n = 371). B, Postmeiosis ovule (n = 313). FG5 stage ovule with two integuments surrounding an eight-cell embryo sac or functional gametophyte (n = 460; C) and FG6 stage ovule with polar nuclei fused to form a seven-cell gametophyte (n = 425; D). E to L, Abnormal ovules from rpl27ac-2. Percentage of abnormal ovules is indicated. E and I, Premeiosis ovules with enlarged cells (arrowheads; n = 323). F to J, Postmeiosis ovules with functional megaspore and enlarged cell (arrowhead; n = 310). FG5 stage ovules with normal integuments and no embryo sac (G) and with short integuments and protruding embryo sac (n = 523; K). FG6 stage ovules with no embryo sac (H) and with a small embryo sac (outlined; n = 587; L). FG, Functional gametophyte; ccn, central cell nucleus; dm, degenerate megaspore; ecn, egg cell nucleus; fm, functional megaspore; mmc, megaspore mother cell; pn, polar nucleus. Bars = 10 µm.
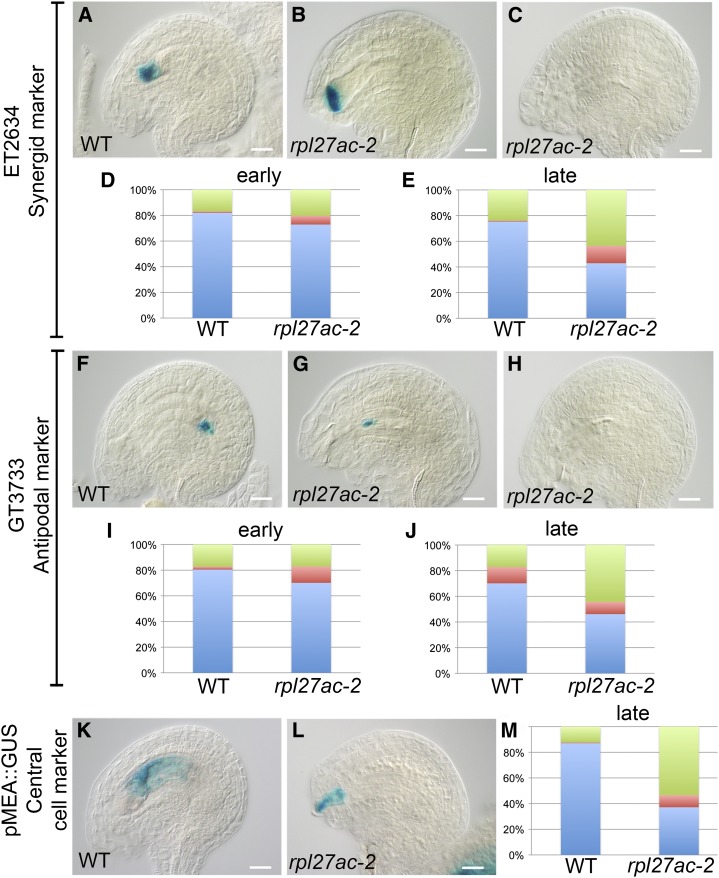
Female gametophyte synergid-, antipodal-, and central cell-specific markers (Huanca-Mamani et al., 2005; Groß-Hardt et al., 2007; Olmedo-Monfil et al., 2010; Tucker et al., 2012) were used to confirm the rpl27ac-2 ovule phenotype. In maturing ovules, GUS expression from the synergid cell marker enhancer trap ET2634 and antipodal cell marker gene trap GT3733 was initially detected in the majority of wild-type and mutant ovules (Fig. 5, A, D, F, and I). In slightly older pistils, the frequency of ovules expressing synergid and antipodal markers decreased in rpl27ac-2 (Fig. 5, C, E, H, and J). The pattern of both markers was abnormal in some rpl27ac-2 ovules, with expression displaced distally toward the micropyle (Fig. 5, B and G). Likewise, GUS expression from the central cell promoter of MEDEA (pMEA:GUS) occasionally showed abnormal expression in rpl27ac-2 and was reduced to a small region toward the micropyle of the ovule (Fig. 5, K–M). The frequency of ovules expressing the central cell marker pMEA:GUS was also reduced in rpl27ac-2 compared with the wild type. The expression pattern of these gametophyte cell markers suggests that the primary defect in rpl27ac-2 is not due to a lack of cellular differentiation, delay in development, or arrest of development and, instead, is due to physical displacement of the mature gametophyte from the ovule.
Figure 5.
Maturing female gametophytes are expelled from the ovule in rpl27ac-2. A to C, Synergid marker ET2634 expression pattern in wild-type (WT) ovule (A) and abnormal rpl27ac-2 ovules (B and C). D and E, Percentage of wild-type synergid marker expression pattern (blue), abnormal expression pattern (red), and no expression (green) in ovules at early-stage (wild type, n = 176; rpl27ac-2, n = 175; D) and late-stage (wild type, n = 823; rpl27ac-2, n = 850; E) development following cell specification. F to H, Antipodal marker GT3733 expression pattern in wild-type ovule (F) and abnormal rpl27ac-2 ovules (G and H). I and J, Percentage of wild-type antipodal marker expression pattern (blue), abnormal expression pattern (red), and no expression (green) in ovules at early-stage (wild type, n = 170; rpl27ac-2, n = 158; I) and late-stage (wild type, n = 854; rpl27ac-2, n = 892; J) development following cell specification. K and L, Central cell marker pMEA:GUS expression pattern in wild-type ovule (K) and abnormal rpl27ac-2 ovule (L). M, Percentage of wild-type pMEA:GUS expression pattern (blue), abnormal expression pattern (red), and no expression (green) in ovules at late-stage development (wild type, n = 192; rpl27ac-2, n = 520). Bars = 10 µm.
Female Gametophyte Defects in rpl27ac/+
rpl27ac-2/+ heterozygote plants had no obvious phenotypes except for short siliques with a high frequency of defective ovules. To determine whether defective ovules in the heterozygote rpl27ac-2/+ and homozygote rpl27ac-2 were the same, we examined the morphology of developing and mature ovules in rpl27ac-2/+ plants. The morphology of young premeiosis ovule primordia was similar in rpl27ac-2/+ and rpl27ac-2 mutants. As with rpl27ac-2 siliques, premeiosis ovules with an abnormal arrangement of cells occurred at a slightly higher frequency in rpl27ac-2/+ compared with the wild type (Fig. 6A). At later stages, a significant proportion of ovules in rpl27ac-2/+ siliques was found to have only a single nucleus. At maturity, all ovules in rpl27ac-2/+ heterozygotes had normal integuments. Thirty-three percent of ovules had a normal FG6 stage embryo sac, whereas 77% of ovules had gametophytes with only a single nucleus (Fig. 6B). The postmeiosis cell-specific markers pFM2:GUS and pAt1g21670:nls-vYFP, which are expressed in the functional megaspore and in the developing gametophyte in the wild type (Fig. 6, C and E; Olmedo-Monfil et al., 2010; Tucker et al., 2012), were used to determine the identity of the single cell in rpl27ac-2/+-defective ovules. These markers were expressed in the single cell of rpl27ac-2/+ ovules, indicating that gametophyte development had undergone meiosis but had not proceeded to mitotic divisions (Fig. 6, D and F). Because transmission of rpl27ac-2 is not affected in the rpl27ac-2/+ mutant, arrested gametogenesis occurs irrespective of the contribution of the genotype of the gametophyte and depends on reduced levels of RPL27a in the sporophyte.
Figure 6.
Ovule phenotypes in rpl27ac-2/+. A, Abnormal ovule from rpl27ac-2/+ plant at the premeiosis stage with several enlarged cells (arrowheads; n = 349). The percentage of abnormal ovules is indicated. B, Ovule from rpl27ac-2/+ plant at the FG6 stage with normal integuments and an embryo sac with a single nucleus (arrow; n = 370). The percentage of abnormal ovules is indicated. C to F, Postmeiosis cell-specific markers pFM2:GUS (C and D) and pAt21670:nls-vYFP (E and F) in wild-type (C and E) and abnormal rpl27ac-2/+ (D and F) ovules. Bars = 10 µm. [See online article for color version of this figure.]
DISCUSSION
In plants and animals, mutations in cytoplasmic ribosomal protein genes result in delayed growth as well as specific developmental defects (Byrne, 2009; Bhavsar et al., 2010; Gilbert, 2011; Horiguchi et al., 2012; Tsukaya et al., 2013). In Arabidopsis, most ribosomal protein mutants have pointed rosette leaves, and this shared phenotype suggests defects are due to reduced ribosome function. However, phenotypic differences between ribosomal protein mutants have been reported, and this may be due to different patterns of gene expression or to extraribosomal function of some ribosomal proteins (Falcone Ferreyra et al., 2013).
Here, we show variable effects of mutations in different ribosomal protein genes on female fertility. Mutations in five ribosomal protein genes, PGY1, PGY2, RPL39C, RPS23B, and PFL2, did not reduce fertility. pgy2-1 and rpl39c-1 are likely weak alleles, whereas pgy1-1 and pfl2 are predicted null alleles. Mutations in five other ribosomal protein genes, RPL5A, RPL5B, RPL27aB, RPL35A, and PFL1, showed a moderate reduction in fertility. rpl5b-3 and rpl35a-1 are predicted to be weak alleles, and rpl27ab-2, rpl5a-2, and pfl1 are predicted null alleles. Potentially, residual expression from weak alleles and redundancy with other ribosomal protein gene family members mask or limit the contribution of these ribosomal proteins to ovule development. By contrast, stv1-2, which is likely a null allele, and rpl27ac mutants had dramatic semidominant effects on fertility. Two other semidominant ribosomal protein mutants induce gametophyte defects. aml1 and sac52-t1 mutants are homozygous embryo lethal, but heterozygotes display reduced transmission of the mutant allele through female and male gametes (Weijers et al., 2001; Imai et al., 2008; Falcone Ferreyra et al., 2013). The effect of multiple ribosomal protein mutants on fertility is consistent with an essential role for the ribosome in cell viability. However, the phenotype of rpl27ac-2/+ mutants also indicates this ribosomal protein has a more specific role in development. RPL27a may be involved in ribosome regulation of translation of genes required for ovule development or may have an extraribosomal function that serves to regulate expression of genes required for ovule development. STV1, RPL4, and RPL5 ribosomal proteins promote the translation of the AUXIN RESPONSE FACTOR (ARF) genes ETTIN/ARF3, MONOPTEROS/ARF5, and ARF7 via upstream open reading frames in the 5′ leader sequences of these target genes (Nishimura et al., 2005; Rosado et al., 2012). Potentially, ribosomal proteins that have extreme effects on fertility and ovule development are disrupting the control of genes involved in auxin signaling.
The ovule defects resulting from mutation in RPL27a highlight general and specific roles for a ribosomal protein in development. The two RPL27a genes in Arabidopsis are functionally redundant. Both genes have a role in fertility, and we propose that both genes contribute to the total cellular pool of RPL27a. Gradual reduction in the level of RPL27a results in a progressive and stochastic reduction in the level of fertility. The consequences for female gametophyte development appear to depend on the level of ribosomal protein in the sporophyte and gametophyte. Mutations in the two RPL27a genes are not transmitted together through female or male gametes, suggesting minimum levels of RPL27a are necessary for gametophyte viability. Likewise, the ribosomal proteins RPL4, RPL5, RPL36a, and RPS6 are each encoded by two genes, and for each of these genes, mutations in both paralogs are not transmitted together through gametes (Yao et al., 2008; Fujikura et al., 2009; Creff et al., 2010; Rosado et al., 2010; Casanova-Sáez et al., 2014). This indicates that a minimal threshold of ribosomes is required for gametophyte viability and reflects an essential role for ribosomes in cell viability.
In the homozygous rpl27ac-2 mutant, female gametophyte development proceeds through megagametogenesis, but there is a subsequent failure to maintain the mature embryo sac within the ovule. The large multinucleate gametophyte may require sufficient ribosome levels to carry out essential cellular and metabolic functions. However, this explanation is difficult to reconcile with ovule defects in the rpl27ac-2/+ mutant, where gametophyte development appears to have arrested following meiosis. Multiple factors appear to be involved in coordinating sporophytic tissues and the gametophyte during ovule development (Bencivenga et al., 2011). Potentially, the distinct heterozygote and homozygote phenotypes reflect the need for balanced levels of RPL27a in the sporophyte and gametophyte.
In rpl27ac-2/+ mutants, it appears that the functional megaspore fails to undergo mitotic division. By comparison, ribosome biogenesis mutants swa1, swa2, swa3/atrh36, and nof1 that are female gametophyte lethal initiate megagametogenesis but do not complete mitotic cell divisions (Shi et al., 2005; Li et al., 2009; Harscoët et al., 2010; Huang et al., 2010; Liu et al., 2010). In swa1, defective embryo sacs are asynchronous with development arrested at the functional megaspore stage or at two, four, or eight nuclei stages of development. Defective embryo sacs in swa2 and swa3 are also asynchronous and arrest with two to eight nuclei, whereas defective embryo sacs in nof1 have four nuclei. In a number of ways, rpl27ac-2/+ is phenotypically similar to plants that are heterozygous for a novel semidominant allele of ARGONAUTE5 (AGO5; Tucker et al., 2012). AGO5 is a putative effector of small RNA silencing pathways that is expressed in somatic cells during megasporogenesis. Heterozygous ago5-4/+ mutants are partially sterile, and like rpl27ac-2/+, female gametophyte development aborts after meiosis and prior to mitotic divisions. This defect is, in part, mediated by AGO5 expression in sporophyte tissues (Tucker et al., 2012). The similarity in phenotypes suggests a potential link between pathways targeted by AGO5 and RPL27a function that remains to be explored.
Comparisons can be made between loss of ribosomal protein function in plants and animals. In Drosophila melanogaster, ribosomal protein mutants are the classic Minute mutants that are slow growing and small in size but also have specific developmental defects, including eye- and wing-patterning defects, and reduced fertility (Lambertsson, 1998; Marygold et al., 2007). In zebrafish and mice, reduced ribosomal protein function results in defects affecting brain, skeleton, eye, and ear development (Amsterdam et al., 2004; Oliver et al., 2004; Uechi et al., 2006; McGowan et al., 2008; Kondrashov et al., 2011; Watkins-Chow et al., 2013). In humans, Diamond-Blackfan anemia and isolated congenital asplenia are associated with mutations in ribosomal proteins (Draptchinskaia et al., 1999; Willig et al., 1999; Gazda et al., 2006, 2008; Farrar et al., 2008; Bolze et al., 2013). Different models have been proposed to explain ribosomal protein mutant phenotypes, including ribosome heterogeneity, extraribosomal function, and targeting of specific transcripts for translational regulation (Byrne, 2009; Warner and McIntosh, 2009; Horiguchi et al., 2012; Xue and Barna, 2012). Although the mechanism of RPL27a function in development is to be established, the data we present demonstrating that female fertility is determined in a dose-sensitive manner by the level of RPL27a further define specific developmental phenotypes of ribosomal proteins.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) mutants rpl27ac-1d, pgy1-1, and pgy2-1 have been described previously (Pinon et al., 2008; Szakonyi and Byrne, 2011b). rpl27ac-2 (SALK_142534), rpl27ac-3 (GABI_066_H03), rpl27ab-1 (SAIL_540_E01), rpl27ab-2 (GABI_104A06), pfl-1, pfl2, and stv1-2 were obtained from The European Arabidopsis Stock Centre and GABI-Kat (Van Lijsebettens et al., 1994; Ito et al., 2000; Scholl et al., 2000; Sessions et al., 2002; Alonso et al., 2003; Nishimura et al., 2005; Szakonyi and Byrne, 2011b; Kleinboelting et al., 2012). rpl5a-2 (Salk_089798), which has been published (Yao et al., 2008; Fujikura et al., 2009), was also obtained from The European Arabidopsis Stock Centre. rpl5b-3 (GT16460) was obtained from Cold Spring Harbor Laboratory (Sundaresan et al., 1995). rps23b-1, rpl35a-1, and rpl39c-1 were isolated in the mutagenesis screen described previously (Byrne et al., 2002; Pinon et al., 2008). The mutations in these genes were identified by map-based cloning and confirmed by complementation of the as1 pgy phenotype with a transgene carrying the full-length genomic region of the respective gene. Lines carrying RPL27aC:RPL27aC in the wild type and rpl27ac-1d/+ and RPL27aC:rpl27ac-1d in the wild type were previously reported (Szakonyi and Byrne, 2011b). rpl27ac-1d carrying RPL27aC:rpl27ac-1d was generated by crossing with RPL27aC:rpl27ac-1d in the wild type to the heterozygous mutant. ET2634, GT3733, pMEA:GUS, pFM2:GUS, and AT1g21670:nls-vYFP were as previously reported (Huanca-Mamani et al., 2005; Groß-Hardt et al., 2007; Olmedo-Monfil et al., 2010; Tucker et al., 2012). rpl27ac-2, rpl27ac-3, rpl27ab-1, rpl27ab-2, and rpl5a-2 in the Col-0 background were backcrossed four to five times to Ler, and unless otherwise stated, these alleles in the Ler background were used in analyses. All mutants and marker lines were in the Ler background, except pfl-1 (C24), pfl2 (No-O), pFM2:GUS, and AT1g21670:nls-vYFP (Col-0). rpl27ac-2 in the Col-0 background was used for crosses to pFM2:GUS and to AT1g21670:nls-vYFP. Plants were grown either in soil or on Murashige and Skoog media at 22°C with a day length of 16 h.
Molecular Biology
RPL27aC and RPL27aB promoters were amplified with the primers 5′-ATGCGAATTCGTCACGTAAGGAAGAATCGTGTC-3′ and 5′-ATGCGGATCCTTTCGCCGATCTGCTACACT-3′ (RPL27aC) and 5′-ATGCGAATTCCCCGGTGAAGCTTGAAAATA-3′ and 5′-ATGCGGATCCTTTTAGTATCAGATCTAGGGTTTTGAA-3′ (RPL27aB). Each PCR product was cloned upstream of the reporter gene 3XNLS-vYFP. The promoter and reporter genes were cloned into the binary vector pBARMAP and transformed into Arabidopsis (Adamski et al., 2009; Tucker et al., 2012). rps23b-1, rpl35a-1, and rpl39c-1 were cloned using Ler × Col-0 mapping populations. To confirm gene cloning by complementation, 2,399-, 1,607-, and 1,137-bp genomic regions encompassing RPS23B, RPL35A, and RPL39C, respectively, were cloned into pMDC123, and the resulting constructs were transformed into Ler (Clough and Bent, 1998; Curtis and Grossniklaus, 2003). For RPL27aC gene expression analysis, mRNA was isolated from 10-d-old plants and used for RT-PCR amplification with the primers 5′-ATGACAACCAGATTCAAGAAGAAC-3′ and 5′-CGCTCTTTCCAAAATCCAAA-3′. PCR products were digested with HpaII and separated by agarose gel electrophoresis HpaII cleaves the wild type but not the rpl27ac-1d mutant allele. Undigested PCR products were also cloned into pCR8 (Invitrogen), and the allele in individual clones was determined by HpaII digestion.
Microscopy
For whole-mount analysis of ovules, gynoecia were dissected from flowers and fixed overnight in ethanol:acetic acid (9:1, v/v) and then dehydrated with 80% and then 70% ethanol prior to clearing with chloral hydrate:glycerol:water solution (8:3:1, w/v/v). Cytochemical staining for GUS activity was performed on developing gynoecia as previously described (Byrne et al., 2002). Following staining, ovules were dissected from gynoecia and mounted in 10% glycerol. Cleared and GUS-stained tissues were analyzed on a Zeiss Axiophot microscope and imaged with an Olympus DP72 digital camera. For fluorescence analysis, ovules were mounted in 10% glycerol and examined on a Zeiss Zeiss AxioImager microscope.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers RPL27aC (At1g70600), RPL27aB (At1g23290), PGY1 (At2g27530), PGY2 (At1g33140), RPS23B (At5g02960), RPL35A (At3g09500), RPL39C (At4g31985), RPL5A (At3g25520), RPL5B (At5g39740), PFL1 (At1g22780), PFL2 (At4g00100), and STV1 (At3g53020).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. rpl27ab-1 has reduced transcript levels.
Supplemental Figure S2. Expression pattern of RPL27aB and RPL27aC in developing ovules.
Supplemental Figure S3. Mutations in rps23b, rpl35a, and rpl39c.
Supplemental Figure S4. Relative transcript levels of the wild type and RPL27aC:rpl27ac-1d.
Supplemental Figure S5. rpl27ac-2 ovules’ phenotypes in Col-0 background.
Supplementary Material
Acknowledgments
We thank Ueli Grossniklaus, Jean-Philippe Vielle-Calzada, and Anna Koltunow for gene reporter lines and reporter plasmids.
Glossary
- T-DNA
transfer DNA
- RT
reverse transcription
- Ler
Landsberg erecta
- Col-0
Columbia
Footnotes
This work was supported by the University of Sydney and the Australian Research Council (Discovery grant no. DP130101186).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Adamski NM, Anastasiou E, Eriksson S, O’Neill CM, Lenhard M. (2009) Local maternal control of seed size by KLUH/CYP78A5-dependent growth signaling. Proc Natl Acad Sci USA 106: 20115–20120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Sadler KC, Lai K, Farrington S, Bronson RT, Lees JA, Hopkins N. (2004) Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol 2: E139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat A, Szick-Miranda K, Chang IF, Guyot R, Blanc G, Cooke R, Delseny M, Bailey-Serres J. (2001) The organization of cytoplasmic ribosomal protein genes in the Arabidopsis genome. Plant Physiol 127: 398–415 [PMC free article] [PubMed] [Google Scholar]
- Bencivenga S, Colombo L, Masiero S. (2011) Cross talk between the sporophyte and the megagametophyte during ovule development. Sex Plant Reprod 24: 113–121 [DOI] [PubMed] [Google Scholar]
- Bhavsar RB, Makley LN, Tsonis PA. (2010) The other lives of ribosomal proteins. Hum Genomics 4: 327–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolze A, Mahlaoui N, Byun M, Turner B, Trede N, Ellis SR, Abhyankar A, Itan Y, Patin E, Brebner S, et al. (2013) Ribosomal protein SA haploinsufficiency in humans with isolated congenital asplenia. Science 340: 976–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ME. (2009) A role for the ribosome in development. Trends Plant Sci 14: 512–519 [DOI] [PubMed] [Google Scholar]
- Byrne ME, Simorowski J, Martienssen RA. (2002) ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development 129: 1957–1965 [DOI] [PubMed] [Google Scholar]
- Carroll AJ, Heazlewood JL, Ito J, Millar AH. (2008) Analysis of the Arabidopsis cytosolic ribosome proteome provides detailed insights into its components and their post-translational modification. Mol Cell Proteomics 7: 347–369 [DOI] [PubMed] [Google Scholar]
- Casanova-Sáez R, Candela H, Micol JL. (2014) Combined haploinsufficiency and purifying selection drive retention of RPL36a paralogs in Arabidopsis. Sci Rep 4: 4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantha SC, Gray-Mitsumune M, Houde J, Matton DP. (2010) The MIDASIN and NOTCHLESS genes are essential for female gametophyte development in Arabidopsis thaliana. Physiol Mol Biol Plants 16: 3–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Creff A, Sormani R, Desnos T. (2010) The two Arabidopsis RPS6 genes, encoding for cytoplasmic ribosomal proteins S6, are functionally equivalent. Plant Mol Biol 73: 533–546 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani I, Ball S, Tchernia G, Klar J, Matsson H, et al. (1999) The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet 21: 169–175 [DOI] [PubMed] [Google Scholar]
- Falcone Ferreyra ML, Casadevall R, Luciani MD, Pezza A, Casati P. (2013) New evidence for differential roles of L10 ribosomal proteins from Arabidopsis. Plant Physiol 163: 378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone Ferreyra ML, Pezza A, Biarc J, Burlingame AL, Casati P. (2010) Plant L10 ribosomal proteins have different roles during development and translation under ultraviolet-B stress. Plant Physiol 153: 1878–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JE, Nater M, Caywood E, McDevitt MA, Kowalski J, Takemoto CM, Talbot CC, Jr, Meltzer P, Esposito D, Beggs AH, et al. (2008) Abnormalities of the large ribosomal subunit protein, Rpl35a, in Diamond-Blackfan anemia. Blood 112: 1582–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura U, Horiguchi G, Ponce MR, Micol JL, Tsukaya H. (2009) Coordination of cell proliferation and cell expansion mediated by ribosome-related processes in the leaves of Arabidopsis thaliana. Plant J 59: 499–508 [DOI] [PubMed] [Google Scholar]
- Gazda HT, Grabowska A, Merida-Long LB, Latawiec E, Schneider HE, Lipton JM, Vlachos A, Atsidaftos E, Ball SE, Orfali KA, et al. (2006) Ribosomal protein S24 gene is mutated in Diamond-Blackfan anemia. Am J Hum Genet 79: 1110–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazda HT, Sheen MR, Vlachos A, Choesmel V, O’Donohue MF, Schneider H, Darras N, Hasman C, Sieff CA, Newburger PE, et al. (2008) Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am J Hum Genet 83: 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavalisco P, Wilson D, Kreitler T, Lehrach H, Klose J, Gobom J, Fucini P. (2005) High heterogeneity within the ribosomal proteins of the Arabidopsis thaliana 80S ribosome. Plant Mol Biol 57: 577–591 [DOI] [PubMed] [Google Scholar]
- Gilbert WV. (2011) Functional specialization of ribosomes? Trends Biochem Sci 36: 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groß-Hardt R, Kägi C, Baumann N, Moore JM, Baskar R, Gagliano WB, Jürgens G, Grossniklaus U. (2007) LACHESIS restricts gametic cell fate in the female gametophyte of Arabidopsis. PLoS Biol 5: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harscoët E, Dubreucq B, Palauqui JC, Lepiniec L. (2010) NOF1 encodes an Arabidopsis protein involved in the control of rRNA expression. PLoS ONE 5: e12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G, Mollá-Morales A, Pérez-Pérez JM, Kojima K, Robles P, Ponce MR, Micol JL, Tsukaya H. (2011) Differential contributions of ribosomal protein genes to Arabidopsis thaliana leaf development. Plant J 65: 724–736 [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Van Lijsebettens M, Candela H, Micol JL, Tsukaya H. (2012) Ribosomes and translation in plant developmental control. Plant Sci 191-192: 24–34 [DOI] [PubMed] [Google Scholar]
- Huanca-Mamani W, Garcia-Aguilar M, León-Martínez G, Grossniklaus U, Vielle-Calzada JP. (2005) CHR11, a chromatin-remodeling factor essential for nuclear proliferation during female gametogenesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 102: 17231–17236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CK, Huang LF, Huang JJ, Wu SJ, Yeh CH, Lu CA. (2010) A DEAD-box protein, AtRH36, is essential for female gametophyte development and is involved in rRNA biogenesis in Arabidopsis. Plant Cell Physiol 51: 694–706 [DOI] [PubMed] [Google Scholar]
- Imai A, Komura M, Kawano E, Kuwashiro Y, Takahashi T. (2008) A semi-dominant mutation in the ribosomal protein L10 gene suppresses the dwarf phenotype of the acl5 mutant in Arabidopsis thaliana. Plant J 56: 881–890 [DOI] [PubMed] [Google Scholar]
- Ito T, Kim GT, Shinozaki K. (2000) Disruption of an Arabidopsis cytoplasmic ribosomal protein S13-homologous gene by transposon-mediated mutagenesis causes aberrant growth and development. Plant J 22: 257–264 [DOI] [PubMed] [Google Scholar]
- Kleinboelting N, Huep G, Kloetgen A, Viehoever P, Weisshaar B. (2012) GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res 40: D1211–D1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov N, Pusic A, Stumpf CR, Shimizu K, Hsieh AC, Xue S, Ishijima J, Shiroishi T, Barna M. (2011) Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell 145: 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsson A. (1998) The minute genes in Drosophila and their molecular functions. Adv Genet 38: 69–134 [DOI] [PubMed] [Google Scholar]
- Laubinger S, Zeller G, Henz SR, Sachsenberg T, Widmer CK, Naouar N, Vuylsteke M, Schölkopf B, Rätsch G, Weigel D. (2008) At-TAX: a whole genome tiling array resource for developmental expression analysis and transcript identification in Arabidopsis thaliana. Genome Biol 9: R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Liu NY, Shi DQ, Liu J, Yang WC. (2010) YAO is a nucleolar WD40-repeat protein critical for embryogenesis and gametogenesis in Arabidopsis. BMC Plant Biol 10: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Yuan L, Liu N, Shi D, Li X, Tang Z, Liu J, Sundaresan V, Yang WC. (2009) SLOW WALKER2, a NOC1/MAK21 homologue, is essential for coordinated cell cycle progression during female gametophyte development in Arabidopsis. Plant Physiol 151: 1486–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Shi DQ, Yuan L, Liu J, Yang WC. (2010) SLOW WALKER3, encoding a putative DEAD-box RNA helicase, is essential for female gametogenesis in Arabidopsis. J Integr Plant Biol 52: 817–828 [DOI] [PubMed] [Google Scholar]
- Marygold SJ, Roote J, Reuter G, Lambertsson A, Ashburner M, Millburn GH, Harrison PM, Yu Z, Kenmochi N, Kaufman TC, et al. (2007) The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol 8: R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann KL, Baserga SJ. (2013) Genetics. Mysterious ribosomopathies. Science 341: 849–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan KA, Li JZ, Park CY, Beaudry V, Tabor HK, Sabnis AJ, Zhang W, Fuchs H, de Angelis MH, Myers RM, et al. (2008) Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet 40: 963–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missbach S, Weis BL, Martin R, Simm S, Bohnsack MT, Schleiff E. (2013) 40S ribosome biogenesis co-factors are essential for gametophyte and embryo development. PLoS ONE 8: e54084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Wada T, Yamamoto KT, Okada K. (2005) The Arabidopsis STV1 protein, responsible for translation reinitiation, is required for auxin-mediated gynoecium patterning. Plant Cell 17: 2940–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver ER, Saunders TL, Tarlé SA, Glaser T. (2004) Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development 131: 3907–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo-Monfil V, Durán-Figueroa N, Arteaga-Vázquez M, Demesa-Arévalo E, Autran D, Grimanelli D, Slotkin RK, Martienssen RA, Vielle-Calzada JP. (2010) Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 464: 628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon V, Etchells JP, Rossignol P, Collier SA, Arroyo JM, Martienssen RA, Byrne ME. (2008) Three PIGGYBACK genes that specifically influence leaf patterning encode ribosomal proteins. Development 135: 1315–1324 [DOI] [PubMed] [Google Scholar]
- Rhee SY, Beavis W, Berardini TZ, Chen G, Dixon D, Doyle A, Garcia-Hernandez M, Huala E, Lander G, Montoya M, et al. (2003) The Arabidopsis Information Resource (TAIR): a model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucleic Acids Res 31: 224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado A, Li R, van de Ven W, Hsu E, Raikhel NV. (2012) Arabidopsis ribosomal proteins control developmental programs through translational regulation of auxin response factors. Proc Natl Acad Sci USA 109: 19537–19544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado A, Sohn EJ, Drakakaki G, Pan S, Swidergal A, Xiong Y, Kang BH, Bressan RA, Raikhel NV. (2010) Auxin-mediated ribosomal biogenesis regulates vacuolar trafficking in Arabidopsis. Plant Cell 22: 143–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl RL, May ST, Ware DH. (2000) Seed and molecular resources for Arabidopsis. Plant Physiol 124: 1477–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al. (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14: 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi DQ, Liu J, Xiang YH, Ye D, Sundaresan V, Yang WC. (2005) SLOW WALKER1, essential for gametogenesis in Arabidopsis, encodes a WD40 protein involved in 18S ribosomal RNA biogenesis. Plant Cell 17: 2340–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Liu JP, Ward S, Kendall SL, Leyser O. (2012) Mutation of the cytosolic ribosomal protein-encoding RPS10B gene affects shoot meristematic function in Arabidopsis. BMC Plant Biol 12: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V, Springer P, Volpe T, Haward S, Jones JDG, Dean C, Ma H, Martienssen R. (1995) Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev 9: 1797–1810 [DOI] [PubMed] [Google Scholar]
- Szakonyi D, Byrne ME. (2011a) Involvement of ribosomal protein RPL27a in meristem activity and organ development. Plant Signal Behav 6: 712–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakonyi D, Byrne ME. (2011b) Ribosomal protein L27a is required for growth and patterning in Arabidopsis thaliana. Plant J 65: 269–281 [DOI] [PubMed] [Google Scholar]
- Terzian T, Box N. (2013) Genetics of ribosomal proteins: “curiouser and curiouser”. PLoS Genet 9: e1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaya H, Byrne ME, Horiguchi G, Sugiyama M, Van Lijsebettens M, Lenhard M. (2013) How do ‘housekeeping’ genes control organogenesis? Unexpected new findings on the role of housekeeping genes in cell and organ differentiation. J Plant Res 126: 3–15 [DOI] [PubMed] [Google Scholar]
- Tucker MR, Okada T, Hu Y, Scholefield A, Taylor JM, Koltunow AM. (2012) Somatic small RNA pathways promote the mitotic events of megagametogenesis during female reproductive development in Arabidopsis. Development 139: 1399–1404 [DOI] [PubMed] [Google Scholar]
- Tzafrir I, Pena-Muralla R, Dickerman A, Berg M, Rogers R, Hutchens S, Sweeney TC, McElver J, Aux G, Patton D, et al. (2004) Identification of genes required for embryo development in Arabidopsis. Plant Physiol 135: 1206–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uechi T, Nakajima Y, Nakao A, Torihara H, Chakraborty A, Inoue K, Kenmochi N. (2006) Ribosomal protein gene knockdown causes developmental defects in zebrafish. PLoS ONE 1: e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lijsebettens M, Vanderhaeghen R, De Block M, Bauw G, Villarroel R, Van Montagu M. (1994) An S18 ribosomal protein gene copy at the Arabidopsis PFL locus affects plant development by its specific expression in meristems. EMBO J 13: 3378–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SQ, Shi DQ, Long YP, Liu J, Yang WC. (2012) GAMETOPHYTE DEFECTIVE 1, a putative subunit of RNases P/MRP, is essential for female gametogenesis and male competence in Arabidopsis. PLoS ONE 7: e33595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JR, McIntosh KB. (2009) How common are extraribosomal functions of ribosomal proteins? Mol Cell 34: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins-Chow DE, Cooke J, Pidsley R, Edwards A, Slotkin R, Leeds KE, Mullen R, Baxter LL, Campbell TG, Salzer MC, et al. (2013) Mutation of the Diamond-Blackfan anemia gene Rps7 in mouse results in morphological and neuroanatomical phenotypes. PLoS Genet 9: e1003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Franke-van Dijk M, Vencken RJ, Quint A, Hooykaas P, Offringa R. (2001) An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 128: 4289–4299 [DOI] [PubMed] [Google Scholar]
- Willig TN, Draptchinskaia N, Dianzani I, Ball S, Niemeyer C, Ramenghi U, Orfali K, Gustavsson P, Garelli E, Brusco A, et al. (1999) Mutations in ribosomal protein S19 gene and Diamond Blackfan anemia: wide variations in phenotypic expression. Blood 94: 4294–4306 [PubMed] [Google Scholar]
- Xue S, Barna M. (2012) Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol 13: 355–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Ling Q, Wang H, Huang H. (2008) Ribosomal proteins promote leaf adaxial identity. Development 135: 1325–1334 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.