An Arabidopsis mitogen-activated protein triple kinase subfamily is required for elicitor-triggered immunity and reactive oxygen species (ROS) generation, as well as for response to ROS, but is not required for elicitor/auxin antagonism.
Abstract
Plant immunity is activated through complex and cross-talking transduction pathways that include a mitogen-activated protein kinase phosphorylation cascade. Here, we have investigated the role in immunity of the Arabidopsis (Arabidopsis thaliana) gene subfamily that encodes the mitogen-activated protein triple kinases indicated as ARABIDOPSIS NUCLEUS- AND PHRAGMOPLAST-LOCALIZED KINASE1-RELATED PROTEIN KINASE1 (ANP1), ANP2, and ANP3. For this study, we used representative danger signals (elicitors) belonging to the classes of the damage- and pathogen-associated molecular patterns, i.e. oligogalacturonides, linear fragments derived from the plant cell wall homogalacturonan, and the peptide elf18 derived from the bacterial elongation factor thermo-unstable. Analyses of single and double as well as conditional triple mutants show that ANPs are required for elicitor-triggered defense responses and protection against the necrotrophic fungus Botrytis cinerea. Notably, ANPs are also required for both the elicitor-induced oxidative burst and the transduction of the hydrogen peroxide signal but not for the inhibition of auxin-induced gene expression, indicating that this response can be uncoupled from the activation of defense responses. Our findings point to ANPs as key transduction elements that coordinate damage- and pathogen-associated molecular pattern-triggered immunity and orchestrate reactive oxygen species accumulation and signaling.
Plants are continually exposed to microbial pathogens and, like animals, activate the innate immune system to respond properly and in a timely manner (Boller and He, 2009). Plants also rely on the structure of the cell wall that acts as a physical barrier against the microbial invasion (De Lorenzo et al., 2001). In their attempts to penetrate plant tissues, pathogens need to efficiently degrade the cell wall (Lionetti et al., 2010). Once the plant cell wall is breached, pathogens encounter the host plasma membrane, where pattern recognition receptors (PRRs) sense the presence of nonself molecules (pathogen-associated molecular patterns [PAMPs]) and activate the so-called PAMP-triggered immunity (PTI; Dodds and Rathjen, 2010). Endogenous molecules, released during infection or mechanical wounding and usually referred to as damage-associated molecular patterns (DAMPs), are also recognized by PRRs as danger signals and contribute to the activation of the plant immune response (Schwessinger and Ronald, 2012). Representative PAMPs are the peptides elf18 and flg22, derived from the bacterial elongation factor thermo-unstable (EF-Tu) and flagellin, respectively (Gómez-Gómez and Boller, 2000; Zipfel et al., 2006). Among the best characterized DAMPs are oligogalacturonides (OGs), linear molecules of 10 to about 16 α-1,4-d-galactopyranosyluronic acid residues released upon fragmentation of homogalacturonan, which is an important component of the plant cell wall (Ferrari et al., 2013). In Arabidopsis (Arabidopsis thaliana), elf18 and flg22 are recognized by the transmembrane leucine-rich repeat receptor kinases EF-TU RECEPTOR (EFR) and FLAGELLIN-SENSING2, respectively (Gómez-Gómez and Boller, 2000; Zipfel et al., 2006). OGs, instead, are recognized by the WALL-ASSOCIATED KINASE1 (WAK1), a receptor kinase containing epidermal growth factor-like repeats (Brutus et al., 2010).
Activation of PRRs leads to a prompt induction of ion fluxes, an oxidative burst, and defense gene expression and to late responses such as callose deposition, seedling growth inhibition, and protection against further pathogen attack. An overlap but also some distinctive features exist between responses induced by PAMPs and DAMPs. For example, flg22 and OGs generate an extracellular oxidative burst mediated by RESPIRATORY BURST OXIDASE HOMOLOG D (RbohD) and induce protection against the necrotrophic fungus Botrytis cinerea independently of the ethylene, jasmonic acid, and salicylic acid pathways and of the RbohD-mediated production of reactive oxygen species (ROS; Zhang et al., 2007; Galletti et al., 2008). The inhibition of auxin responses is another feature shared by PAMPs and DAMPs (Savatin et al., 2011); in the case of OGs, the inhibition of auxin responses has been described as a true antagonism (Branca et al., 1988; Bellincampi et al., 1993; Savatin et al., 2011). On the other hand, microarray analyses indicate that late responses to the two classes of elicitors are considerably different (Denoux et al., 2008).
In plants, as in animals, immunity is activated through complex and cross-talking transduction pathways that include a mitogen-activated protein (MAP) kinase (MAPK) phosphorylation cascade (Rodriguez et al., 2010). A MAPK cascade consists of a core module of three kinases that perform sequential phosphorylation reactions: a MAP kinase kinase kinase (MAP3K) activates, by phosphorylation, a MAP kinase kinase (MAP2K), which activates a MAPK. Sixty MAP3Ks, 10 MAP2Ks, and 20 MAPKs are encoded by the Arabidopsis genome (Ichimura et al., 2002), leading to a complexity that hampers the characterization of this transduction system. Three immune-related MAPK modules have been identified. A module comprising the MAP3K MEKK1, the MAP2Ks MKK1 and MKK2, and the MAPK MPK4 (MEKK1/MKK1-MKK2/MPK4) negatively controls defense responses (Kong et al., 2012; Rasmussen et al., 2012) by negatively regulating the expression of the MAP3K MEKK2, which triggers a salicylate (SA)-dependent autoimmunity response when the cascade is compromised (Berriri et al., 2012; Su et al., 2013). Two other modules, MEKK1-MAPKKKα/MKK4-MKK5/MPK3-MPK6 (Ren et al., 2008) and MKK9/MPK3-MPK6 (Xu et al., 2008), mediate the activation of defense responses, including the synthesis of ethylene and camalexin, i.e. a phytoalexin with antimicrobial activity. The only MAPK elements shown so far to participate in the response to DAMPs are the Arabidopsis MPK3 and MPK6. Both are phosphorylated within minutes upon elicitation with OGs and flg22, but only MPK6 is required for full elicitor-induced up-regulation of defense genes and protection against B. cinerea (Galletti et al., 2011).
The subfamily of MAP3Ks indicated as ARABIDOPSIS NUCLEUS- AND PHRAGMOPLAST-LOCALIZED KINASE1 (NPK1)-RELATED PROTEIN KINASEs (ANPs) includes three members, ANP1, ANP2, and ANP3, that were initially identified for their homology with the tobacco (Nicotiana tabacum) NPK1 (Jouannic et al., 1999; Sasabe and Machida, 2012). NPK1 regulates cytokinesis (Nakashima et al., 1998) as well as the effector-triggered immunity and development in Nicotiana benthamiana (Jin et al., 2002). ANPs have also been reported to be involved in the inhibition of auxin-induced gene expression. Constitutively active forms of ANPs, obtained by deletion of the C-terminal regulatory domain (ΔANPs) and expressed in protoplasts, negatively regulate the activity of the auxin-inducible soybean (Glycine max) GRETCHEN HAGEN3 promoter and activate the hydrogen peroxide signaling pathway (Kovtun et al., 2000). Therefore, ANPs have been proposed as a molecular link between oxidative stress and auxin signal transduction. However, we have previously shown that double knockout (KO) anp mutants exhibit a normal auxin/OG antagonism (Savatin et al., 2011), thus providing no support to this conclusion. Our work left unanswered whether this was due to the presence of a functional third ANP member or to a lack of involvement of ANPs in elicitor/auxin antagonism and, in general, in the response to OGs, because a main role of ANP1 in response to flg22 had been previously ruled out (Asai et al., 2002).
The anp2 anp3 double mutant displays developmental defects related to cytokinesis as well as up-regulation of stress-related genes, while an anp triple KO mutant was never obtained, probably because ANPs are essential for plant development (Krysan et al., 2002). The phenotype of the anp2 anp3 mutant is similar to that of mpk4 mutant, suggesting that ANPs and MPK4 may be part of the same transduction pathway and act as negative regulators of defense responses (Beck et al., 2011).
We show here that ANP genes are not involved in elicitor-auxin antagonism but are required for DAMP and PAMP signal transduction. Single and double mutants as well as conditional triple mutants, which were generated in this work, are defective in defense responses to both OGs and elf18. Notably, ANPs are required for both elicitor-induced generation of ROS and response to ROS. Our study points to ANPs as key regulators of plant immunity.
RESULTS
ANPs Do Not Play a Major Role in OG-Auxin Antagonism
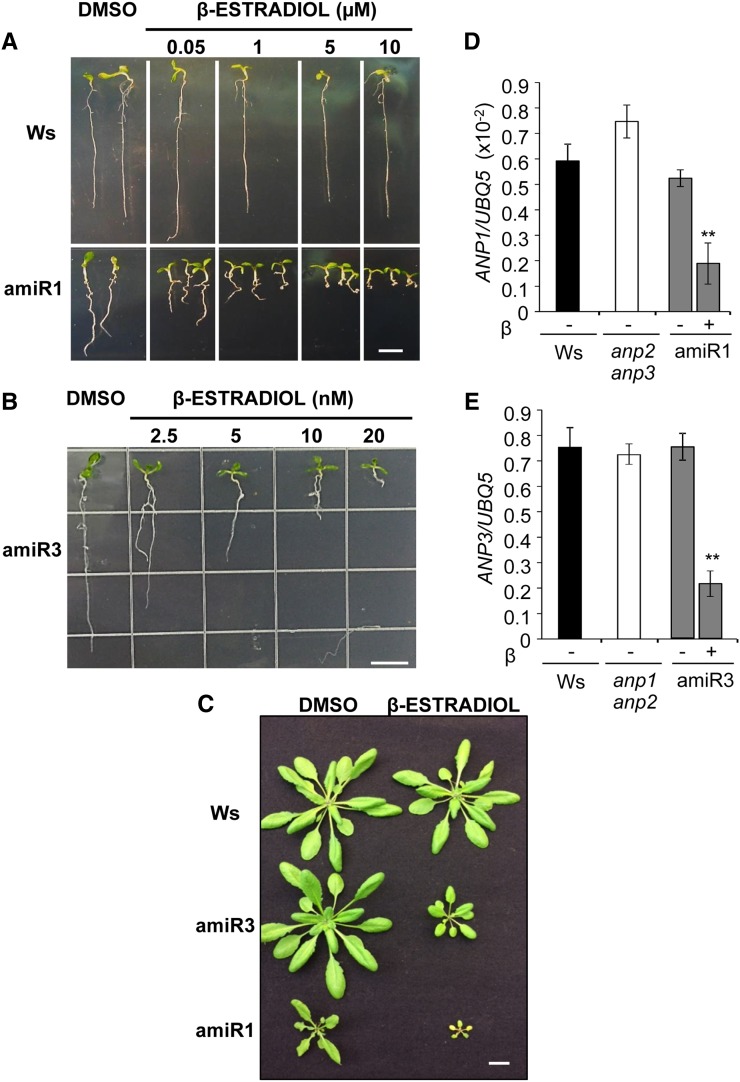
To clarify whether ANPs play a role in the inhibition of auxin-induced gene expression by OGs, we decided to generate conditional triple mutants, hereon simply indicated as anp triple mutants. In fact, previous data show that (1) the normal auxin-OG antagonism behavior of the anp double KO mutants (Wassilewskija [Ws] background) may be due to a redundant role of ANPs and the presence of a functional third member (Savatin et al., 2011) and (2) an anp triple mutant could not be obtained by crossing (Krysan et al., 2002). Triple mutants were constructed by down-regulating, in an inducible manner, the expression of the third gene family member in both the double mutants anp1 anp2 and anp2 anp3. Only the latter double mutant exhibits developmental defects (Krysan et al., 2002; Beck et al., 2010, 2011). Conditional silencing was obtained by individually expressing ANP3- and ANP1-specific artificial microRNAs (amiRNAs), respectively, under the control of a β-estradiol-inducible promoter. Two independent transgenic lines for each background (hereon indicated as triple mutant amiR1[lines 2.5 and 8.7], and triple mutant amiR3 [lines 5.3 and 4.1]), each homozygous for a single insertion, were isolated.
Morphological phenotype and expression of the ANP1- and ANP3-specific primary amiRNAs as well as their silencing effectiveness were examined in silenced triple mutant seedlings. Increasing concentrations of β-estradiol resulted in an increasingly severe alteration of root development (Fig. 1, A and B); similarly, in adult plants, spraying with β-estradiol caused growth inhibition (Fig. 1C). In the two sets of lines, very different concentrations of the β-estradiol inducer (in the micromolar and nanomolar range for the amiR1 and amiR3 mutants, respectively) were required to obtain a similar morphological phenotype and a reduction of the target transcript levels higher than 50% (Fig. 1, D and E). In noninduced conditions (treatment with dimethyl sulfoxide [DMSO] alone), target transcripts were similar to those in the corresponding mutant background and in the wild type. Control seedlings and plants transformed with the empty pMDC7 vector for β-estradiol-inducible expression were indistinguishable from wild-type ones, when treated with β-estradiol up to a concentration of 5 µm (Supplemental Fig. S1, A–C).
Figure 1.
Developmental phenotype and silencing degree of anp triple mutants. A and B, Ten-day-old triple mutant amiR1 (line 2.5) and amiR3 (line 5.3) seedlings, germinated and grown in the absence (DMSO control) or in the presence of β-estradiol at the indicated concentrations. C, Two-week-old adult plants sprayed with 5 µm (amiR1) and 50 nm β-estradiol (amiR3) every 3 d for three times and grown further for 2 weeks. D and E, Levels of ANP1 (D) and ANP3 (E) transcripts, relative to UBQ5 transcripts, in 10-d-old amiR1 (D) and amiR3 (E) seedlings grown in the absence (–; DMSO control) or in the presence (+) of β-estradiol. Asterisks indicate statistically significant differences between DMSO-treated and β-estradiol-treated seedlings according to Student’s t test (**P < 0.01). Bars = 1 cm.
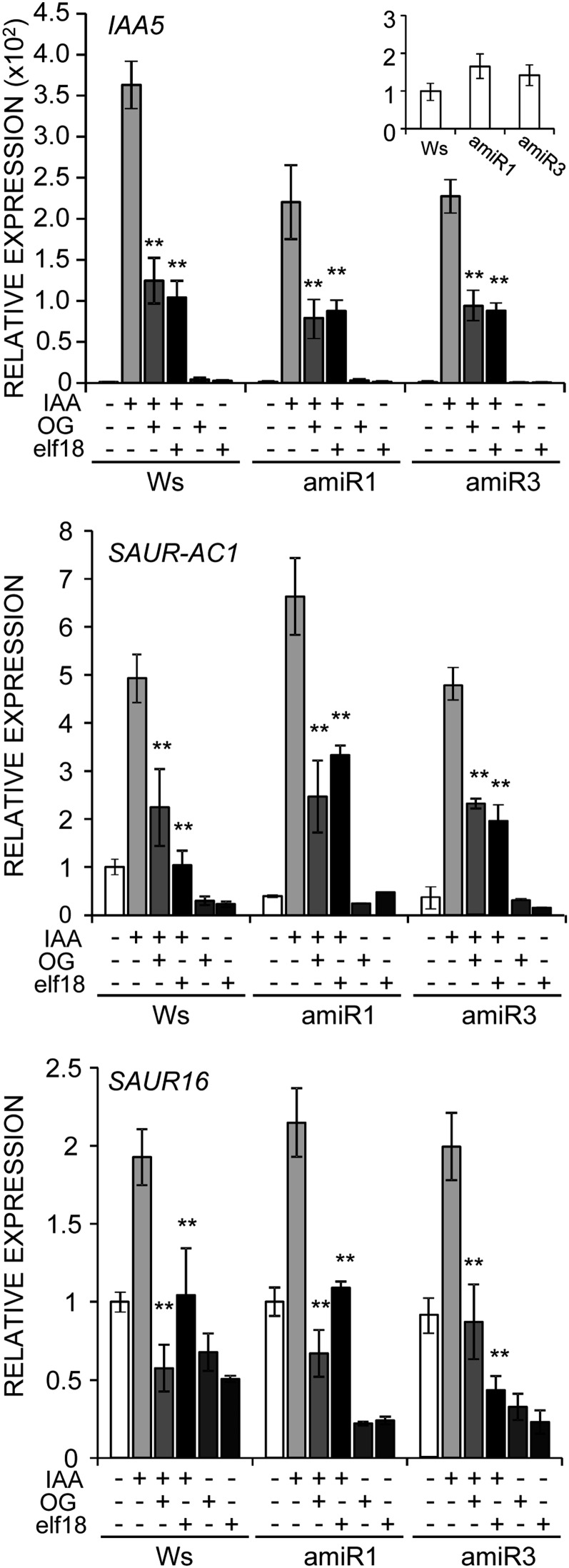
Next, expression of the auxin-regulated genes INDOLE-3-ACETIC ACID-INDUCED PROTEIN5 (IAA5), SMALL AUXIN UP RNA (SAUR) AC1 (for Arabidopsis Columbia SAUR gene1; SAUR-AC1) and SAUR16 was analyzed in wild-type and β-estradiol-induced triple mutant seedlings in the presence and in the absence of IAA and OGs; in parallel, the effect of elf18, a peptide PAMP derived from the bacterial EF-Tu to which the Ws ecotype is sensitive (Kunze et al., 2004), was analyzed. In the triple mutant lines, transcripts of the three genes increased, in the presence of IAA alone, at levels that were comparable to those observed in Ws (SAUR-AC1 and SAUR16) or slightly lower (IAA5). IAA-induced expression of all three genes was inhibited by both OGs and elf18 in a similar manner in the triple mutant and in the wild type (Fig. 2). These results indicate that the triple mutants are capable of perceiving OGs and elf18 and that an impairment of the ANP function that severely affects development does not compromise the inhibitory effect of the elicitors on auxin-regulated gene expression, suggesting that ANPs do not play a major role in this effect.
Figure 2.
Inhibition of auxin-regulated gene expression by OGs and elf18 is not affected in anp triple mutants. Expression of the indicated auxin-regulated genes in the wild type (Ws) and amiR1 (line 2.5) and amiR3 (line 5.3) mutant seedlings grown in the presence of β-estradiol. Seedlings were treated for 1 h with water (mock), OGs, elf18, IAA (1.5 µm), IAA plus OGs, or IAA plus elf18, as indicated. Analyses were performed by qRT-PCR, and transcript levels are shown as the mean of at least three independent experiments (± se; n = 20 in each experiment) normalized to UBQ5 expression and plotted relative to expression in mock-treated Ws. Asterisks indicate statistically significant differences between cotreatment with IAA plus elicitor and treatment with IAA alone according to Student’s t test (**P < 0.01). In inset, levels of IAA5 transcripts in mock-treated seedlings are shown.
Elicitor-Induced Expression of Defense Response Genes Is Reduced in anp Mutant Plants
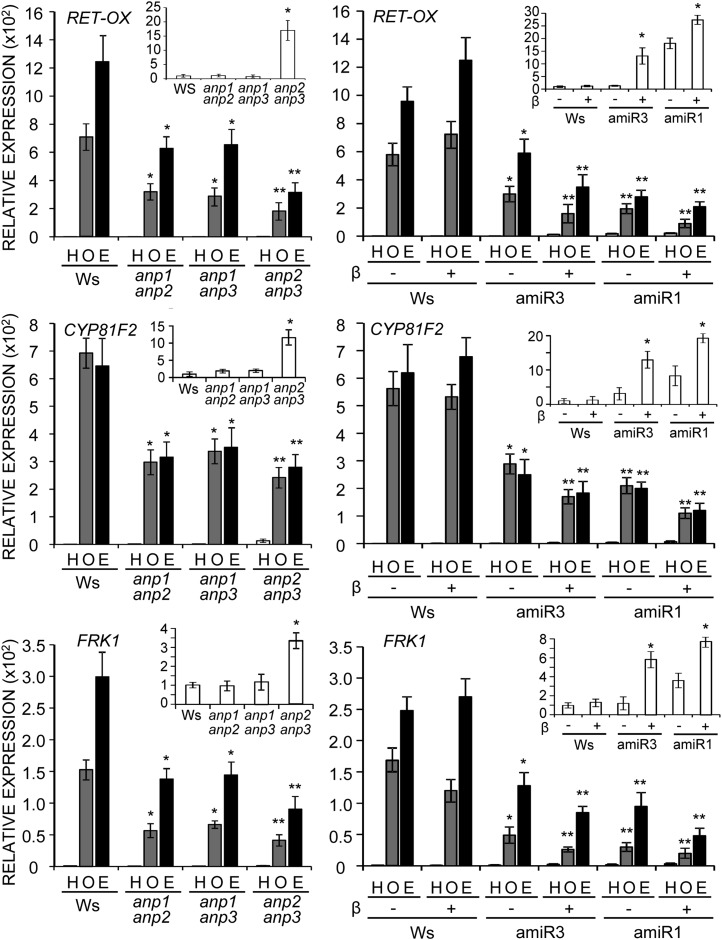
Because information about the role of ANPs in immunity induced by elicitors is scarce, we investigated whether defense responses typically induced by OGs and elf18 are affected in anp mutants. Seedlings were examined for the expression of genes that are induced early in response to both OGs and elf18 (Kunze et al., 2004; Denoux et al., 2008). We chose FRK1, a flagellin-responsive receptor-like kinase gene, the expression of which is considered to be strictly regulated by the MAPK cascade (Boudsocq et al., 2010), RET-OX, encoding a protein with homology to reticuline oxidases (Dittrich and Kutchan, 1991), and CYP81F2, encoding a cytochrome P450 involved in indol-3-yl-methyl glucosinolate catabolism (Clay et al., 2009). Both RET-OX and CYP81F2 are proposed to be induced mainly but not exclusively through the MAPK cascade (Boudsocq et al., 2010). Expression of these genes was examined in homozygous single null anp mutants (insertional mutants in the Columbia [Col-0] background; Supplemental Fig. S2A) and anp double mutants, as well as in wild-type plants (Col-0 and Ws). In response to both elicitors, transcript accumulation of the three genes examined was slightly but not significantly reduced in the single mutants (Supplemental Fig. S2B), whereas accumulation was significantly reduced in all the double mutants, with a more marked defect in anp2 anp3 (Fig. 3). Basal transcript levels were normal in single mutants and in anp1 anp2 and anp1 anp3, whereas levels were increased in anp2 anp3 compared with wild-type seedlings (about 17-fold for RET-OX, 12-fold for CYP81F2, and 3.5-fold for FRK1; Fig. 3, insets). This observation is in agreement with a previous report of a constitutively high expression of defense genes in this mutant (Krysan et al., 2002). The defective response to the elicitors of the double mutants likely reflects an impairment of the signal transduction cascade, because in these mutants, transcript levels of WAK1 and EFR, i.e. the receptors of OGs and elf18, respectively, were not reduced (results for the double mutant anp2 anp3 are shown in Supplemental Fig. S2C). The triple mutant lines showed, in the presence of β-estradiol, a further impairment of the elicitor-induced expression of all three genes compared with the double mutants (Fig. 3), as well as an impairment of the expression of Phosphate Induced1, which is considered to be regulated specifically through the Ca+2-dependent protein kinase cascade (Boudsocq et al., 2010; Supplemental Fig. S2D). Both amiR1 and amiR3 mutants showed an increased basal expression of all marker genes (insets in Fig. 3 and Supplemental Fig. S2D) in the presence of β-estradiol compared with the DMSO control treatment. The defective response of the double and triple mutants points to ANPs as positive and redundant regulators of the elicitor-induced defense gene expression.
Figure 3.
Induction of defense response genes by elicitors is defective in anp double and triple mutant seedlings. Expression of RET-OX, CYP81F2, and FRK1 in double (left) and triple mutants (amiR3 line 5.3 and amiR1 line 2.5; right). Seedlings were treated for 1 h with OGs (O), elf18 (E), or water (H; mock). Analyses were performed by qRT-PCR, and transcript levels are shown as the mean of at least three independent experiments (± se; n = 20 in each experiment) normalized to UBQ5 expression and plotted relative to expression in water-treated Ws. Asterisks indicate statistically significant differences between elicitor treatment of mutant seedlings and Ws according to Student’s t test (*P < 0.05 and **P < 0.01). In insets, transcript levels in mock-elicited seedlings are shown; asterisks indicate statistically significant differences between mutants and Ws (left) and DMSO- and β-estradiol-treated seedlings (right; *P < 0.05).
High Basal Expression of RET-OX, CYP81F2, and FRK1 Genes Is Independent of MEKK2
The increased basal expression of the defense-related marker genes in anp2 anp3 and in the silenced triple mutants recalls the phenotype of another MAP3K mutant, mekk1, which also shows a reduced growth (Suarez-Rodriguez et al., 2007). Both phenotypes of mekk1 have been demonstrated to be caused by an increased expression of the MAP3K MEKK2 (At4g08480) that takes place as a guard mechanism for the integrity of the MAPK cascade, leading to the activation of an SA-dependent autoimmune response (Kong et al., 2012; Su et al., 2013). We therefore investigated whether the higher basal expression of defense genes observed in the anp mutants is mediated by the MEKK2 pathway. If this is the case, higher levels of MEKK2 transcripts are expected in the anp2 anp3 mutant and in the silenced triple mutants but not in the other double mutants. On the other hand, a higher expression of RET-OX, CYP81F2, and FRK1 is expected to occur in the mutants defective in the MEKK1/MKK1-MKK2/MPK4 pathway compared with wild-type plants.
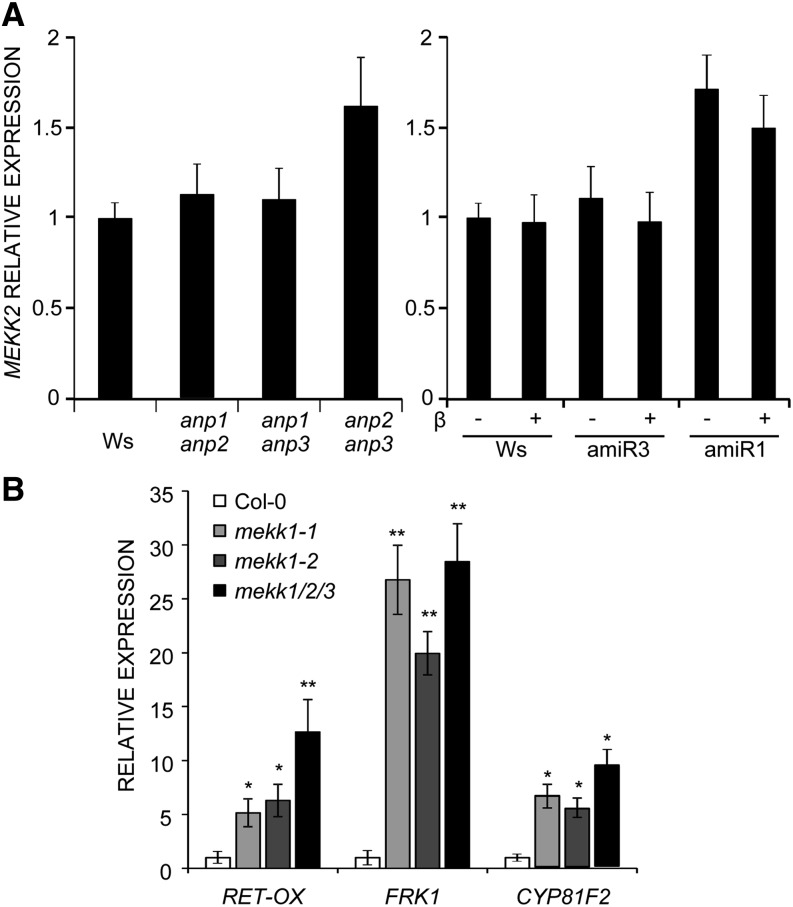
We found that, in the anp1 anp2 and anp1 anp3 double mutants, MEKK2 transcripts do not differ from the wild type. This result suggests that their defective elicitor-triggered defense gene expression does not depend on the activation of the MEKK2-mediated autoimmunity cascade. Instead, an increase of about 1.6-fold of MEKK2 transcripts was observed in the anp2 anp3 mutant (Fig. 4A). In noninduced amiR1 and amiR3 mutant seedlings (treatment with DMSO alone), MEKK2 transcripts were similar to those in the corresponding double mutant background, i.e. like the wild type in amiR3 (anp1 anp2 background) and about 1.5-fold higher in amiR1 (anp2 anp3 background). MEKK2 transcripts did not increase in both mutants in the presence of β-estradiol concentrations that caused similar severe developmental defects (10 nm amiR3 and 1 µm for amiR1; Fig. 4A). Thus, in silenced amiR3 mutants, the elevated basal defense gene expression is not accompanied by an increase of MEKK2 transcripts, strongly suggesting that MEKK2 does not mediate this effect. On the other hand, the moderate increase of MEKK2 transcripts in anp2 anp3 and amiR1 mutants, albeit lower than that observed in the mekk1 mutant (4-fold higher than that of the wild type; Su et al., 2013), may still reflect a role of MEKK2 in the increased basal expression of the defense marker genes of these mutants.
Figure 4.
MEKK2-mediated autoimmunity cascade is not responsible for the altered basal expression levels of the defense genes. A, Expression of MEKK2 was analyzed in anp double mutant seedlings and in anp triple mutant (amiR3 line 5.3 and amiR1 line 2.5) seedlings grown in the absence (DMSO) or in the presence of β-estradiol. B, Expression of the indicated defense response genes was measured in two allelic mekk1 loss-of-function mutants and in the mekk1/mekk2/mekk3 deletion mutant. All analyses were performed by qRT-PCR, and results are shown as the mean of at least three independent experiments (± se; n = 20 in each experiment) normalized to UBQ5 expression and plotted relative to expression in the corresponding wild type (Ws for anp mutants and Col-0 for mekk1-1, mekk1-2, and mekk1/mekk2/mekk3 mutants). Asterisks indicate statistically significant differences between gene expression in mutant seedlings and Col-0 according to Student’s t test (*P < 0.05 and **P < 0.01).
To elucidate this, we investigated whether MEKK2-activated autoimmunity includes up-regulation of RET-OX, CYP81F2, and FRK1 by analyzing their expression in the null allelic mutants mekk1-1 and mekk1-2 as well as in a mutant deleted in the whole MEKK gene subfamily (mekk1/mekk2/mekk3). This mutant, which lacks the activation of the MEKK2-dependent autoimmunity response, exhibits both a normal morphological phenotype and strongly decreased levels of SA-dependent responses compared with mekk1 (Su et al., 2013). As expected, in the mekk1/mekk2/mekk3 mutant, mekk2 expression was absent (data not shown). However, in both mekk1 and mekk1/mekk2/mekk3, basal expression of defense genes was comparable and higher than in the wild type: RET-OX and CYP81F2 transcript levels were approximately 5-fold higher than in the wild type, while those of FRK1 were more than 20-fold higher (Fig. 4B). These results indicate that loss of MEKK1 function leads to a higher constitutive expression of RET-OX, CYP81F2, and FRK1, independently of MEKK2. Moreover, because the morphological phenotype of mekk1/mekk2/mekk3 is normal, they also indicate that increased basal expression of these genes is not correlated with developmental defects. These results lead to the conclusion that the activation of the MEKK2-mediated autoimmunity cascade is unlikely to be responsible for the altered basal expression levels of the defense genes examined as well as for the developmental defects of the silenced anp triple mutants.
Taken together, our observations confirm the importance of ANPs in development and strongly point to a role of ANPs also in the regulation of immunity.
ANPs Are Required for Elicitor-Induced Protection against B. cinerea
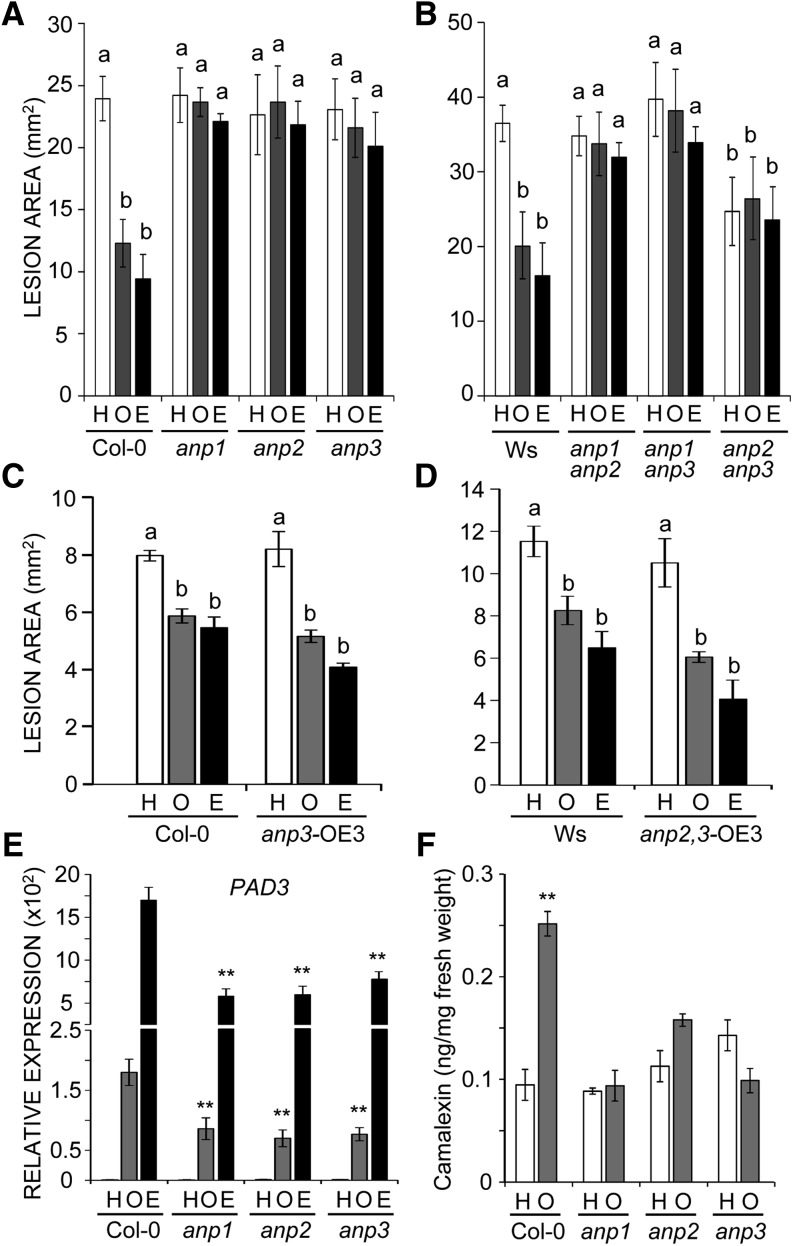
To assess whether the altered regulation of defense gene expression affects the response of anp mutants to pathogens, we examined susceptibility to B. cinerea as well as the protection response induced by OG (Ferrari et al., 2007) and elf18 against this fungus. Adult single and double mutant plants as well as wild-type (Col-0 and Ws) plants were sprayed with OGs, elf18, or water, and excised leaves were infected with B. cinerea after 24 h. All mutants exhibited a basal susceptibility to the fungus similar to that of the wild type, with the exception of the anp2 anp3 double mutant, which was more resistant, likely due to the basal up-regulation of stress-related genes (Fig. 5, A and B). However, neither the single nor the double mutants were protected by treatment with either OGs or elf18, unlike the corresponding wild-type genotypes. The defective protection was rescued in complemented anp3 mutants expressing a wild-type copy of the ANP3 gene driven by either its native promoter (anp3compl plants) or the Cauliflower mosaic virus (CaMV) 35S promoter (anp3 plants overexpressing ANP3 [anp3-OE3 plants]; Fig. 5C, data are shown for the anp3-OE3 plants). It was also rescued in a complemented anp2 anp3 double mutant expressing the wild-type ANP3 driven by the CaMV 35S promoter (anp2,3-OE3 plants; Fig. 5D). Levels of ANP3 transcripts were 1.2-fold, 10-fold, and 40-fold higher in anp3compl, anp3-OE3, and anp2,3-OE3 seedlings, respectively, compared with wild-type seedlings. In anp2,3-OE3 plants, developmental defects were also rescued (Supplemental Fig. S1D). These data indicate that all three ANPs are required for elicitor-induced protection against B. cinerea and that a higher level of ANP3 expression can restore the OG-induced protection even in the absence of ANP2.
Figure 5.
anp single and double mutant plants are defective in elicitor-induced protection against B. cinerea. Protection conferred by pretreatment with water (H; control), OGs (O), or elf18 (E) was determined in anp single (A) and double (B) mutant plants, in transgenic anp3 (C) and anp2 anp3 double (D) mutant plants overexpressing ANP3, and in corresponding wild-type plants. Different letters above bars indicate statistically significant differences (P < 0.05) between samples, as determined by ANOVA with Tukey’s honestly significant difference mean-separation test. The experiments were repeated three times with similar results (n = 20 in each experiment). E, Expression of the PAD3 gene was determined by qRT-PCR in adult Col-0 and anp single mutant leaves 6 h after spraying with water, OGs, or elf18. Analyses were performed by qRT-PCR, and transcript levels are shown as the mean of at least three independent experiments (± se; n = 5 in each experiment) normalized to UBQ5 expression and plotted relative to expression in mock-treated Col-0. Asterisks indicate statistically significant differences between elicitor-treated mutants and corresponding treatment of the wild type according to Student’s t test (P < 0.01). F, Amount of camalexin measured in infected leaves of Col-0 and anp single mutant plants pretreated with water or OGs at 18 h after B. cinerea inoculation. Mean values (± se; n = 4) are shown. Asterisks indicate statistically significant differences between OG and water pretreatment according to Student’s t test (P < 0.01). The experiment was repeated three times with similar results.
It is known that the expression of PHYTOALEXIN DEFICIENT3 (PAD3), encoding the cytochrome CYP71B15 that catalyzes the last step of camalexin biosynthesis, is required for OG-induced protection against B. cinerea (Ferrari et al., 2007). We therefore analyzed whether elicitor-induced expression of PAD3 is affected in the anp single mutants. Levels of PAD3 transcripts, examined by quantitative reverse transcription (qRT)-PCR in OG- and elf18-sprayed leaves, were lower in the mutant plants than in the wild type (Fig. 5E), suggesting that an impaired response to the elicitors likely causes the lack of protection against B. cinerea. Analysis of camalexin levels at 18 h postinoculation, i.e. during the early phase of camalexin production (Ferrari et al., 2003), showed (1) a higher amount of camalexin in the wild-type OG-pretreated leaves compared with the water (mock) pretreated leaves and (2) camalexin levels similar to those of the wild-type mock-pretreated leaves in the anp single mutants (Fig. 5F). These results reveal not only that treatment with OGs prompts (primes) tissue to produce camalexin either more rapidly and/or at higher levels in response to B. cinerea, but also that ANPs are required for this priming effect, thus providing a possible explanation to the observed defect in protection.
ANPs Are Required for Both Elicitor-Induced Generation of ROS and Response to ROS
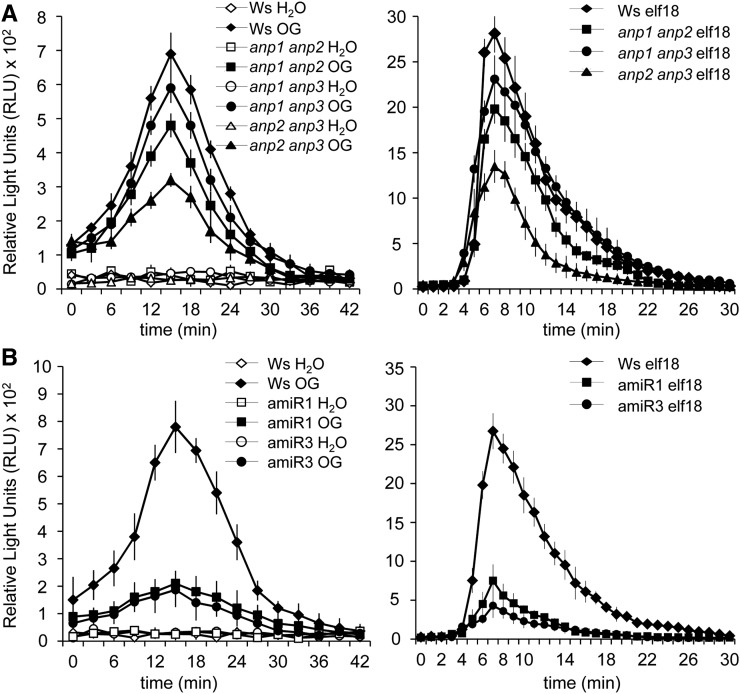
The rapid generation of ROS is one of the early responses induced by pathogen attack and elicitors (Mittler et al., 2011; O’Brien et al., 2012). We therefore investigated whether elicitor-induced generation of extracellular ROS requires the function of ANPs. Leaf discs and seedlings of anp single mutants did not significantly differ from the wild type in their response to both OGs and elf18 (data not shown), whereas a compromised elicitor-triggered oxidative burst was observed in the anp double mutants. OG- and elf18-induced hydrogen peroxide production by leaf discs was reduced by about 20% and 35%, respectively, in the anp1 anp2 double mutant and by more than 50% in the anp2 anp3 mutant compared with the wild type (Fig. 6A). Water-treated mutant and wild-type leaf discs produced similar levels of hydrogen peroxide. Analysis of seedlings confirmed the defective oxidative burst of the double mutants (Supplemental Fig. S3A). In both amiR1 and amiR3 mutants, elicitor-induced ROS production was also strongly reduced in leaf discs (Fig. 6B). Because elicitor-induced extracellular ROS production is mainly mediated by the NADPH oxidase RbohD (Marino et al., 2012), we ascertained that the defective ROS production was not due to a lower expression of the RbohD gene caused by the lack of ANPs. In both rosette leaves and seedlings, levels of RhohD transcripts in the mutants were similar or even higher than in the wild type (Supplemental Fig. S3B).
Figure 6.
The elicitor-induced oxidative burst is defective in anp mutants. ROS production was measured using a luminol-based assay in leaf discs from anp double mutant (A) and amiR1 and amiR3 triple mutant (B) plants and in wild-type plants after elicitation with water, OGs, or elf18. Results are mean ± se (n = 12). Experiments were repeated at least three times with similar results.
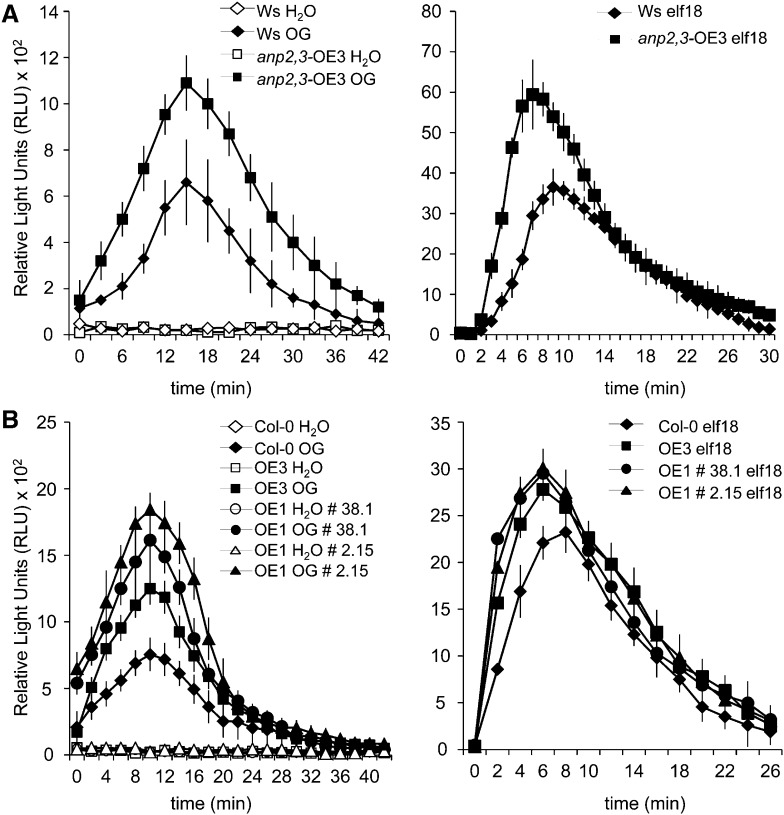
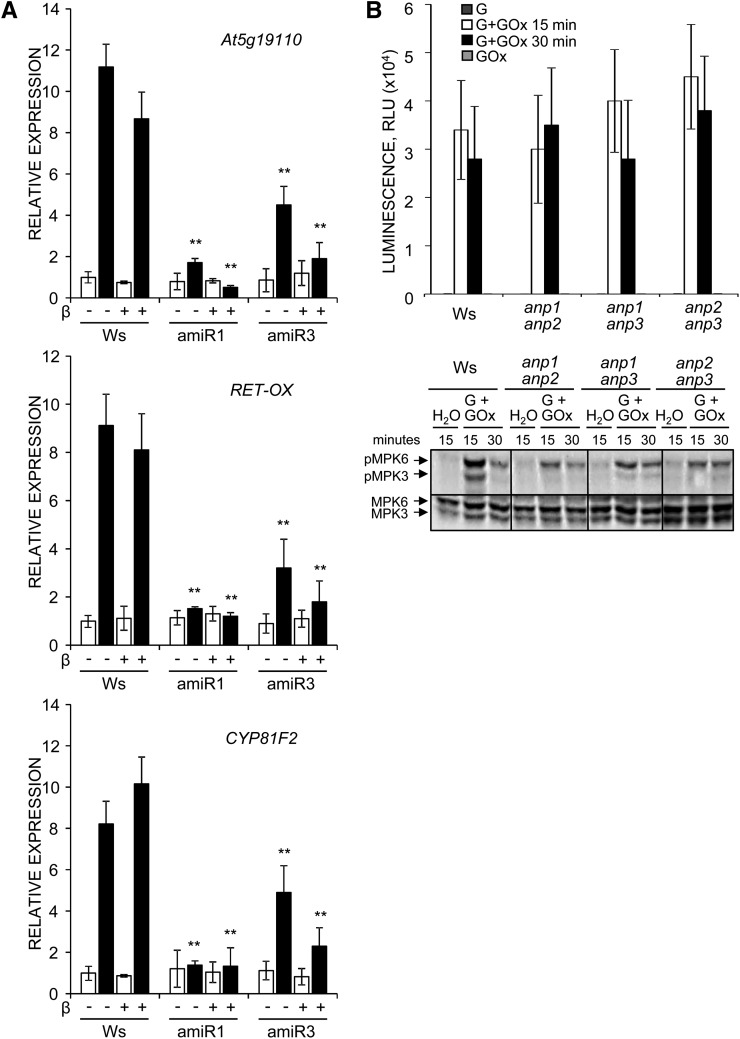
To corroborate the conclusion that ANPs play a role in the regulation of the oxidative burst, we generated homozygous transgenic Col-0 seedlings that overexpress either ANP1 or ANP3 under the control of the CaMV 35S promoter (OE1 and OE3 plants). OE1 and OE3 seedlings and plants did not exhibit macroscopic developmental and growth defects (data not shown). Both OE1 and OE3 plants as well as homozygous anp2,3-OE3 plants produced higher amounts of hydrogen peroxide in response to both OGs and elf18 (Fig. 7, A and B), confirming the hypothesis that ANPs are required for the oxidative burst induced by OG and elf18, in addition to being involved in response to hydrogen peroxide, as previously proposed on the basis of experiments performed using the protoplast transient expression system (Kovtun et al., 2000). To obtain evidence in planta for the role of ANPs in the transduction of the ROS signal, we analyzed the expression of hydrogen peroxide-responsive genes, selected on the basis of available microarray data, in anp mutant seedlings. Hydrogen peroxide was artificially generated using a combination of Glc and Glc oxidase that generates levels of extracellular hydrogen peroxide (20–40 μm g–1 fresh weight, measured by a xylenol orange-based assay) slightly higher than those induced by elicitors (Supplemental Fig. S3A). The genes examined were GST6 (At2g47730), which encodes a glutathione S-transferase (Kovtun et al., 2000), ZAT12, which encodes a zinc finger protein (Iida et al., 2000), and At5g19110, which encodes a putative aspartyl protease; in addition, we analyzed the expression of RET-OX and CYP81F2, although their up-regulation is considered to occur independently of hydrogen peroxide (Galletti et al., 2008). Unexpectedly, no up-regulation of GST6 and ZAT12 expression was observed in response to the amount of hydrogen peroxide generated in our conditions, both in the wild type and in the mutants (data not shown). Instead, expression of At5g19110, RET-OX, and CYP81F2 in wild-type plants was induced (by about 11-, 9-, and 8-fold, respectively, with respect to water-treated seedlings). Up-regulation of RET-OX and CYP81F2 was about 155× and 75×, respectively, lower than that observed in response to elicitors (Figs. 3 and 8A), confirming that elicitor-induced expression of these genes takes place mainly through pathways distinct from that mediated by extracellular hydrogen peroxide. No up-regulation of At5g19110, RET-OX, and CYP81F2 by hydrogen peroxide occurred in the anp double mutants (data not shown) and in the triple mutants (Fig. 8A), in agreement with the notion that ANPs mediate the gene expression response to hydrogen peroxide (Kovtun et al., 2000). We conclude that ANPs are required for both the oxidative burst induced by the elicitors and the transduction of the hydrogen peroxide signal.
Figure 7.
Transgenic plants overexpressing ANPs show an increased production of hydrogen peroxide in response to elicitors. A, Hydrogen peroxide produced by leaf discs from wild-type (Ws) and transgenic anp2,3-OE3 plants after elicitation with OGs or elf18. B, Hydrogen peroxide produced by leaf discs from wild-type (Col-0) and OE1 (lines 38.1 and 2.15) and OE3 transgenic plants in response to OGs or elf18. Results are mean ± se (n = 12). Experiments were repeated at least three times with similar results.
Figure 8.
anp mutant seedlings show a defective response to hydrogen peroxide. A, Wild-type (Ws) and anp triple mutant (amiR1 line 2.5 and amiR3 line 5.3) seedlings grown in DMSO alone (beta, –) or β-estradiol (β, +) were treated for 1 h with Glc (0.25 mm, white bars) in the absence or in the presence of Glc oxidase (0.01 units; black bars). Expression of the indicated genes was analyzed by qRT-PCR analysis, normalized to UBQ5 expression, and plotted relative to expression in Ws treated only with Glc and grown in DMSO. Results are the mean of at least three independent experiments (± se; n = 20 in each experiment). Asterisks indicate statistically significant differences between mutant and Ws plants treated in the same way according to Student’s t test (**P < 0.01). B, anp double mutant seedlings were treated for the indicated minutes with Glc (G) in the absence or in the presence of Glc oxidase (GOx). The amount of hydrogen peroxide produced was assessed by a luminol-based assay (top), and MPK3 and MPK6 phosphorylation was determined by immunoblot analysis using an antip44/42-ERK antibody (bottom).
ANPs Act Upstream of MPK3 and MPK6 in the Response to Hydrogen Peroxide and OGs But Not to elf18
ANPs have been shown to function upstream of the MAP single kinases MPK3 and MPK6. In the protoplast expression system, the constitutively active ΔANP1 form, obtained by deletion of the C-terminal regulatory domain, is capable of activating these kinases, and expression of ANP1 preferentially enhances their activation in response to hydrogen peroxide (Kovtun et al., 2000). To obtain evidence in planta for the ANP-MPK3/MPK6 functional relationship, we investigated whether a defective activation of these kinases might explain the defective gene expression response to hydrogen peroxide of anp mutants. anp double mutant seedlings treated with artificially generated hydrogen peroxide showed an evident reduction of MPK3/MPK6 phosphorylation (Fig. 8B).
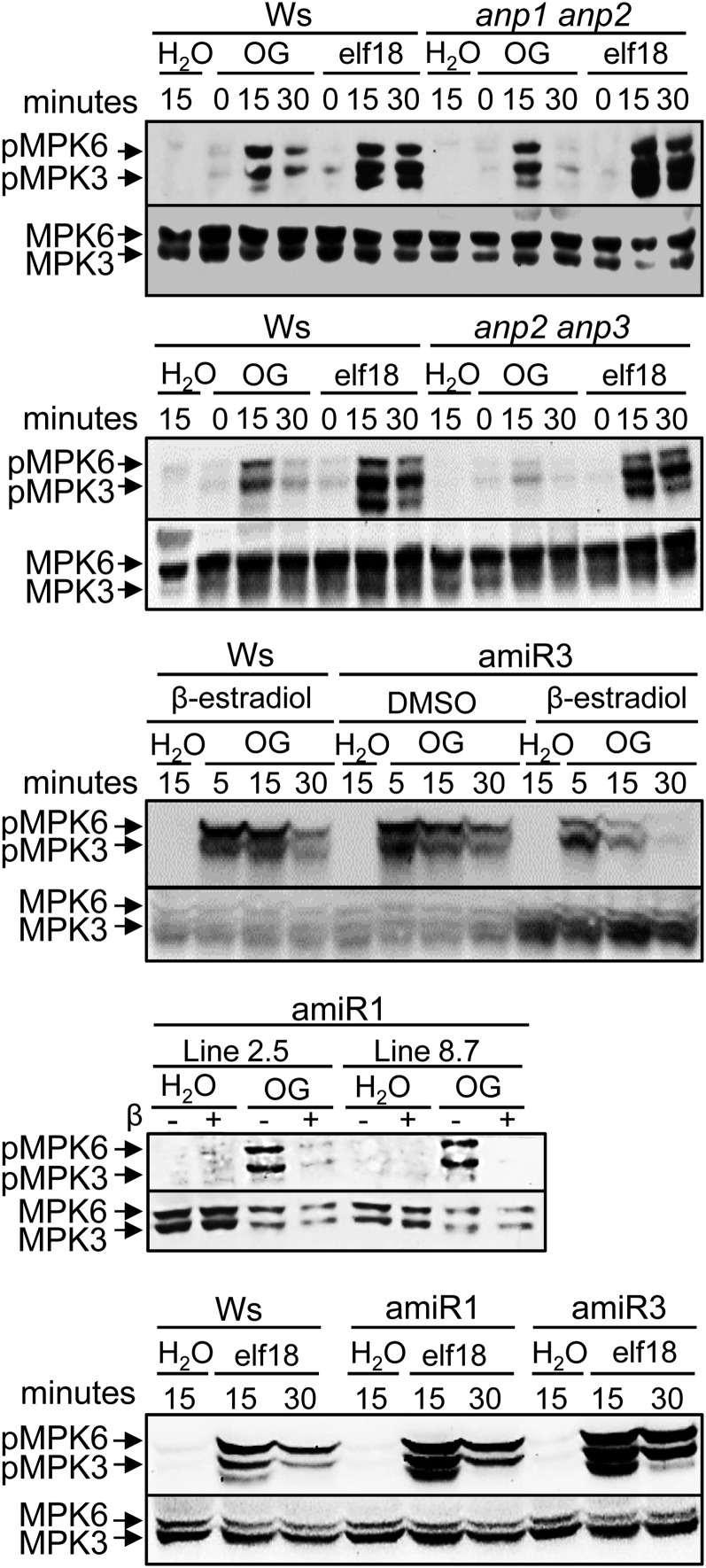
MPK3 and MPK6 are activated also in response to both OGs (Galletti et al., 2011) and elf18 (Zipfel et al., 2006; Rasmussen et al., 2012), and MPK6 has been shown to be required for OG- and flg22-induced resistance to B. cinerea (Galletti et al., 2011). We investigated whether a compromised phosphorylation of MPK3/MPK6 in the anp mutants determines the defect in elicitor-induced defense responses. In the single mutants (Supplemental Fig. S4A) as well as in the anp1 anp2 (Fig. 9) and anp1 anp3 double mutants (Supplemental Fig. S4B), MAPK phosphorylation was similar to that observed in the wild type in response to both OGs and elf18. In the anp2 anp3 double mutant, a reduction of MPK6/MPK3 phosphorylation was observed, unexpectedly, only in response to OGs and not to elf18 (Fig. 9). Analysis of the conditional triple mutants, grown in the presence of β-estradiol, showed a reduction of the OG-induced MAPK phosphorylation compared with the respective background and confirmed the normal phosphorylation in response to elf18 (Fig. 9). β-Estradiol treatment alone did not affect MAPK phosphorylation in the wild type and anp2 anp3 double mutants treated either with water, OGs, or elf18 (Supplemental Fig. S4C).
Figure 9.
MPK3 and MPK6 phosphorylation is defective in anp mutant seedlings in response to OGs but not to elf18. Levels of phosphorylated MAPKs (pMPK3 and pMPK6) after elicitation with OGs or elf18 in the wild type (Ws), anp1 anp2 and anp2 anp3 double mutants, and amiR1 and amiR3 anp triple mutants were determined by immunoblot analysis using an antip44/42-ERK antibody (top). Levels of MPK3 and MPK6 total proteins were determined using specific antibodies (bottom). The identity of individual MAPKs as determined by size is indicated by arrows. Experiments were repeated at least three times with similar results.
DISCUSSION
The ANP subfamily of MAP3Ks has been implicated in the regulation of cytokinesis and in the inhibition of auxin-induced gene activation in Arabidopsis protoplasts, the latter effect likely occurring through the transduction of the hydrogen peroxide signal (Kovtun et al., 2000). The tobacco ANP homolog, i.e. NPK1, was reported to play a similar role (Kovtun et al., 1998). However, we have previously reported that elicitor-induced inhibition of early auxin-induced gene expression is not affected in the anp double KO mutant plants. Whether this is due to the function of a third ANP member, which compensates the loss of the other two members, remained unclear. To clarify this point, we generated conditional triple mutants in the anp1 anp2 double mutant background, which is phenotypically normal, as well as in the anp2 anp3 double mutant background, which is associated with both developmental defects, such as dwarfism and altered root development, and constitutive activation of stress-related genes. In these mutants, we silenced the third ANP member through the β-estradiol-inducible expression of amiRNAs. The analyses reported in this study show that even when the expression of the third ANP member is silenced by more than 50%, a condition that leads to severe developmental defects and to an impairment of elicitor-induced defense responses (see below), seedlings respond to OGs and elf18 to inhibit auxin-regulated expression similarly to the wild type. Thus, an involvement of the three ANPs in the elicitor-auxin antagonism still appears unlikely.
On the other hand, our analyses reveal that ANPs are required for OG- and elf18-induced defense-related gene expression and protection against B. cinerea. Elicitor-induced expression of both MAPK- and Ca+2-dependent protein kinase-dependent gene expression is affected, apparently placing ANPs upstream of both types of cascades. Elicitor-induced gene expression is impaired not only in the silenced triple mutants, but also in all the double mutants, including anp1 anp2 and anp1 anp3, which display a normal morphological phenotype, ruling out the possibility that such an impairment is a consequence of the stress caused by the developmental defects rather than a primary effect of the lack of ANP function.
A role of ANPs as positive regulators of immunity is supported by the observation that the anp mutants lack the protection against B. cinerea induced by elicitor spraying. This defect is observed in all three single mutants and appears to be a consequence of the impaired response to the elicitors. OG-induced expression of PAD3, required for camalexin synthesis and the protection response, and accumulation of camalexin are reduced in the anp single mutant leaves. Notably, we also show that protection is likely due to the ability of OGs to prime plants, allowing higher levels of camalexin accumulation early during infection, and that, in single anp mutants, the priming effect is lacking. The defective protection phenotype is rescued by the expression of the wild-type gene either under its native promoter or the CaMV 35S promoter. We also found that overexpression of the wild-type ANP3 gene rescues not only the developmental phenotype of the double mutant anp2 anp3, but also its defective protection. This result confirms the redundant action of ANPs in elicitor-induced immunity and suggests a gene dosage effect. Thus, a decreased ANP level in the anp single mutants is likely the cause of the impaired protection response. Furthermore, we observed an increase of basal expression of RET-OX, CYP81F2, and FRK1 when the extent of the loss of ANP function was such that it caused severe phenotypical defects. This effect, however, may not be a specific consequence of the lack of the ANP function but rather a generic stress response to the altered developmental phenotype. Alternatively, it may be caused by the MEKK2-triggered response that is consequent to the impairment of the MAPK cascade, although this hypothesis appears unlikely because the three genes examined are regulated mainly through SA-independent pathways. In the attempt to clarify this, we analyzed the MAP3K mutant mekk1, which also shows dwarfism as well as a constitutive expression of MEKK2- and SA-dependent defense response genes. We found that the basal expression of RET-OX, CYP81F2, and FRK1 is enhanced in this mutant. The analysis of the mekk1/mekk2/mekk3 mutant, which carries a deletion encompassing MEKK1, MEKK2, and MEKK3, allowed us to conclude that this up-regulation is not part of the MEKK2-mediated response and is uncoupled from the developmental defects, because the mutant maintains the elevated basal expression of the three genes and has a wild-type phenotype.
Thus, we can argue that the altered basal expression of defense response genes in the ANP mutants is a specific effect of the lack of function of these kinases. This possibility is supported by the observation that MEKK2 transcripts do not increase in the silenced amiR3 triple mutant, slightly increase in the anp2 anp3 double mutant, and do not further increase in the silenced amiR1 mutants (anp2 anp3 background). On the other hand, increase of MEKK2 expression up to 2.4-fold, as previously shown, is not sufficient to cause activation of the downstream autoimmunity cascade and dwarfism (Kong et al., 2012; Su et al., 2013). These data also suggest that cytokinesis defects of ANP mutants are independent of MEKK2, in agreement with what has been found in mpk4 (Kong et al., 2012; Su et al., 2013).
The role of ANPs in immunity includes the regulation of ROS signaling. We confirmed, with in planta experiments, that ANPs are required for response to hydrogen peroxide, as previously proposed on the basis of data obtained in transfected protoplasts (Kovtun et al., 2000). However, we observed that the genes GST6 and ZAT12, often used as markers for the response to hydrogen peroxide, were not induced in wild-type seedlings in our experimental conditions, likely because the expression of these genes requires levels of extracellular hydrogen peroxide much higher than those normally induced by elicitors. Instead, the genes RET-OX and CYP81F2 responded to the artificially generated hydrogen peroxide, although at a level much lower than that observed upon elicitor treatment. This result suggests that induction of these genes by elicitors is mediated mainly, but not exclusively, by pathways independent of extracellular hydrogen peroxide, which is not in contrast with the previous observation that elicitor-induced expression of these genes is hardly affected in the presence of catalase or in the rbohD KO mutant (Galletti et al., 2008). Those data were obtained by semiquantitative PCR analyses that may not have been sensitive enough to reveal the moderate contribution of hydrogen peroxide to the elicitor-triggered up-regulation of these genes. In the anp double and triple mutants, hydrogen peroxide-induced accumulation of RET-OX, CYP81F2, and At5g19110 was abolished, confirming the role of ANPs in the transduction of hydrogen peroxide, as previously proposed.
In this work, we also showed, both in leaf discs and seedlings, that ANPs are required for elicitor-induced accumulation of extracellular ROS. Defects in the elicitor-induced oxidative burst were observed in the double mutants, including those that are phenotypically normal, and in the triple mutants. The increased production of ROS upon treatment with the elicitors of plants overexpressing single ANPs, which do not show developmental defects, supports our conclusion that ANPs are important regulators of the elicitor-induced extracellular oxidative burst. Taken together, our findings suggest that ANPs act both upstream and downstream of ROS, as key elements of a regulatory circuitry that likely leads to the amplification of the oxidative burst during PTI. They also suggest that ANPs regulate defense gene expression independently of apoplastic hydrogen peroxide.
On the other hand, our results do not support the hypothesis that ANPs and ROS act as molecular links between elicitors and auxin signal transduction (Kovtun et al., 2000), because the strong impairment of ROS production and signaling due to the lack of ANPs does not affect auxin/elicitor antagonism. This is in agreement with previous observations showing that the antagonism mediated by OGs is independent of the accumulation of extracellular hydrogen peroxide (Bellincampi et al., 2000; Savatin et al., 2011). Notably, the loss of ANP function reveals that the inhibition of auxin-induced gene expression can be uncoupled from the activation of defense response and suggests that the former effect is mediated by an ANP-independent signal transduction pathway. The mechanistic basis of the elicitor/auxin antagonism remains obscure. So far, this antagonism has been shown to be independent of salicylic acid, jasmonic acid, and ethylene and does not involve the silencing of TRANSPORT INHIBITOR RESPONSE1/AUXIN F-BOX genes nor miR393 activity or posttranscriptional gene silencing (Savatin et al., 2011).
Response to hydrogen peroxide and to both OGs and elf18, as well as to flg22, involves the activation of MPK3 and MPK6 (Zipfel et al., 2006; Galletti et al., 2011; Rasmussen et al., 2012). MPK3 has been shown to be required for basal resistance to B. cinerea and does not significantly affect elicitor-induced resistance (Ren et al., 2008), whereas MPK6 is required for elicitor-induced resistance to B. cinerea and full expression of a subset of defense response genes but not for basal resistance to B. cinerea (Galletti et al., 2011). Our in planta analyses provide further evidence for the role of ANPs upstream of MPK3 and MPK6 in the gene expression response to hydrogen peroxide (Kovtun et al., 2000). The phosphorylation of these MAPKs is reduced in the double mutants that are defective in the up-regulation of hydrogen peroxide-regulated genes. Moreover, in agreement with the notion that these kinases are involved in the response to OGs (Galletti et al., 2011), a reduced phosphorylation also accompanies the defective elicitor-induced protection and gene expression response induced by OGs in the anp2 anp3 double mutant and in the triple mutant. Unexpectedly, we observed no defect in the elicitor-induced MPK3/MPK6 phosphorylation (1) in the anp single mutants, in spite of their impaired elicitor-induced protection against B. cinerea and OG-primed camalexin production during infection, (2) in the anp1 anp2 and anp1 anp3 double mutants, in spite of their defective protection against B. cinerea and the lower expression of defense response genes in response to both OGs and elf18, and (3) in the anp2 anp3 double mutant and the triple mutant, in spite of their severely impaired expression of defense response genes induced by elf18. A defective elicitor-induced defense response gene expression in the presence of an apparently unaffected phosphorylation of MPK3 and MPK6 is not unprecedented and has been previously reported for the Arabidopsis brassinosteroid insensitive1-associated kinase1-5 mutant (Schwessinger et al., 2011). However, these apparent inconsistencies are still unexplained and suggest that MPK3 and MPK6 phosphorylation is necessary but not sufficient for full activation of the immune gene expression response by elicitors. It is possible that ANP-dependent posttranslational modification of additional regulatory elements is required for this response. The behavior of the anp2 anp3 double mutant and the triple mutant, which showed a defect in MPK3/MPK6 phosphorylation in response to OGs but not to elf18, also points to an important difference between the signaling cascades activated by these elicitors. Possibly, elf18 induces MPK3/MPK6 phosphorylation through multiple pathways, some of which may be ANP independent.
From our study, ANPs clearly emerge as key positive regulatory elements for response to both PAMPs and DAMPs and for PTI, rather than as mere repressors of defense responses, as initially hypothesized on the basis of the phenotypic similarities with the mpk4 mutant (Berriri et al., 2012). The action of MPK4 is placed downstream of MEKK1 in one branch of the MAPK cascade that is activated in response to flagellin, and accordingly, loss-of-function mutants in these kinases display similar PTI-related defects (Ichimura et al., 2006; Suarez-Rodriguez et al., 2007). As in the case of MEKK1, lack of ANPs results in dwarfism, insensitivity to hydrogen peroxide (Nakagami et al., 2006), and basal up-regulation of RET-OX, CYP81F2, and FRK1 (this work). However, lack of ANPs and MPK4 result in cytokinesis and primary root developmental defects, which are both independent of MEKK2 (Beck et al., 2010). Thus, ANPs and MPK4, but not MEKK1, may functionally interact during plant growth and development (Su et al., 2007). On the other hand, MEKK1 may functionally interact with ANPs and MPK4, either linearly, as part of the same pathway, or in parallel and cross-talking pathways in the regulation of basal and elicitor-induced expression of defense responses. Interestingly, ANPs play a positive role in the regulation of expression of defense response genes by elicitors but a negative role on the basal expression of the same genes, suggesting different routes of regulations. Clearly, the complexity of this scenario remains to be unraveled.
In ANP mutants, cytokinetic defects and root defects are similar to those of loss-of-function mutants for MPK4, which, like NPK1, localizes to the phragmoplast and physically interacts with MAP65-1, a major microtubule-cross-linking protein that regulates the stability and turnover of phragmoplast microtubules (Krysan et al., 2002; Beck et al., 2010, 2011). ANPs have been shown to functionally interact with proteins that are required for cytokinesis and thought to control stability and turnover of microtubules (Takahashi et al., 2010; Komis et al., 2011). Whether ANPs localize to the phragmoplast is not yet known.
In conclusion, our findings have uncovered a key role of ANPs as redundant and positive regulators of PAMP- and DAMP-triggered oxidative burst and immunity, in addition to their previously proposed role as transduction elements in the response to hydrogen peroxide. ANPs therefore emerge as central hubs in the orchestration of the immune response. Furthermore, these MAP3Ks are likely to be key elements in the growth defense trade off, because they have been shown to play a role in the regulation of cell division and development.
MATERIALS AND METHODS
Plant Growth and Treatment
Arabidopsis (Arabidopsis thaliana) Col-0 and Ws wild-type seeds were purchased from Lehle Seeds. anp1 (SALK_083439), anp2 (SALK_144973), and anp3 (SALK_026009) transfer DNA insertional mutants (in the Col-0 background) were obtained from the Nottingham Arabidopsis Stock Centre (School of Biosciences, University of Nottingham). The anp1 anp2, anp1 anp3, and anp2 anp3 (in the Ws background) and mekk1-1, mekk1-2, and mekk1/mekk2/mekk3 (in the Col-0 background) mutants were kindly provided by Patrick J. Krysan (Department of Horticulture, University of Wisconsin).
For analyses performed on seedlings, seeds were surface sterilized and germinated in multiwell plates (approximately 10 seeds per well) containing one-half-strength Murashige and Skoog (Murashige and Skoog, 1962) medium (1 mL per well; Sigma-Aldrich) supplemented with 0.5% (w/v) Suc. After 9 d, the incubation medium was replaced with fresh medium, and treatments with elicitors were performed after 24 h. For anp triple mutants, seeds were germinated and seedlings grown in one-half-strength Murashige and Skoog containing β-estradiol (1 µm for the wild type and amiR1 and 10 nm for amiR3) or DMSO (mock; 0.01% [v/v]) for 9 d. Culture medium was then replaced with fresh medium containing β-estradiol at the same concentrations, and seedlings were grown for additional 24 h before treatments or analyses. Elicitor treatment of seedlings was performed using OGs and elf18 at a concentration of 100 μg mL–1 and 10 nm, respectively. For protection assay against Botrytis cinerea, 4-week-old plants were sprayed with water, OGs (200 μg mL–1), or elf18 (1 µm). For analysis of ROS production, leaf discs were obtained from 4-week-old plants. For amiR1 and amiR3 triple mutants and the wild type (Ws), plants were sprayed with 5 µm (amiR1 and Ws) and 50 nm β-estradiol (amiR3) three times every 24 h before leaf disc preparation. OGs and elf18 were used at a concentration of 200 μg mL–1 and 100 nm, respectively.
Seedlings and plants were grown at 22°C and 70% relative humidity under a 16-h-light/8-h-dark cycle (approximately 120 μmol m–2 s–1).
OGs with an average degree of polymerization of 10 to 16, as assessed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, were prepared as previously described (Bellincampi et al., 2000). The elf18 peptide was synthesized by Maria Eugenia Schininà (Sapienza, Università di Roma).
Generation of Transgenic Plants
Conditional triple anp mutants were generated by expressing an amiRNA having as specific targets ANP1 and ANP3 transcripts in the anp2 anp3 and anp1 anp2 double mutants, respectively. The amiRNAs were designed using a Web-based tool (http://wmd.weigelworld.org). The primary amiRNAs were obtained as previously described (Schwab et al., 2006) and inserted in the pMDC7 binary vector downstream of a β-estradiol-inducible promoter (Curtis and Grossniklaus, 2003) by using the Gateway Recombination Cloning Technology (Life Technologies). T3 progenies of two independent homozygous transgenic lines (2.5 and 8.7 for amiR1 and 4.1 and 5.3 for amiR3) carrying a single insertion were obtained; in these lines, ANP1 and ANP3 transcripts were reduced by more than 50% in the presence of β-estradiol.
Complemented anp3 mutants were obtained by expressing a wild-type copy of the ANP3 gene driven by either its native promoter (1-kb upstream start codon) cloned in pCAMBIA1391z or the CaMV 35S promoter by using the Gateway Recombination Cloning Technology (Life Technologies) in pB7m34GW,0 destination vector. This clone was also used to complement the anp2 anp3 double mutant. The Gateway system was also used to obtain clones for transgenic Col-0 plants that overexpress either ANP1 or ANP3 under the control of the CaMV 35S promoter. In all cases, homozygous complemented T3 lines carrying a single insertion of the wild-type gene were obtained.
All Gateway-compatible vectors were previously described (Karimi et al., 2002) and obtained from Plant System Biology (Ghent University; http://gateway.psb.ugent.be/).
Gene Expression Analysis
Gene expression analysis was performed as previously described (Galletti et al., 2011) with slight modifications. Seedlings or leaf tissues were frozen in liquid nitrogen and homogenized with a MM301 Ball Mill (Retsch). Total RNA was extracted from at least three independent replicates, each composed by 20 seedlings or at least five adult leaves from different plants, with Isol-RNA Lysis Reagent (5 Prime) according to the manufacturer’s protocol. Total RNA (2 µg) was treated with RQ1 DNase (Promega), and first-strand complementary DNA was synthesized using ImProm-II reverse transcriptase (Promega) according to the manufacturer’s instructions. qRT-PCR analysis was performed by using a CFX96 Real-Time System (Bio-Rad). Complementary DNA (corresponding to 50 ng of total RNA) was amplified in a 30-μL reaction mix containing 1× GoTaq Real-Time PCR System (Promega) and 0.4 μm of each primer. Three technical replicates were performed for each sample, and data analysis was done using LinRegPCR software. Expression levels of each gene, relative to UBIQUITIN5, were determined using a modification of the Pfaffl method (Pfaffl, 2001) as previously described (Ferrari et al., 2006) and expressed in arbitrary units or referred to as mock treatment in the wild type. Primer sequences are shown in Supplemental Table S1.
B. cinerea Protection Assay
Protection assays against B. cinerea were performed as previously described (Ferrari et al., 2007) with slight modifications. Four-week-old intact rosettes were sprayed with water, OGs (200 μg mL–1), or elf18 (1 µm), and after 24 h, fully expanded leaves were detached and placed in petri dishes containing 0.8% (w/v) plant agar, with the petiole embedded in the medium. Inoculation was performed by placing 5-µL drops of a conidiospore suspension (5 × 105 mL–1) in potato dextrose broth (Difco; 24 g L–1) regularly spaced on each side of the middle vein. Plates were incubated at 22°C with a 12-h photoperiod. High humidity was maintained by covering the plates with a clear plastic lid. Lesion area was determined 48 h after inoculation.
Camalexin Determination
Camalexin was extracted as previously described (Pan et al., 2010) from leaves of 4-week-old Col-0 and anp single mutant plants (approximately 100 mg) and collected 18 h after infection with B. cinerea conidia (5 × 105 mL–1). Samples were dissolved in methanol and analyzed by liquid chromatography coupled to mass spectrometry using an Ultimate 3000 HPLC system connected to an Orbitrap XL Discovery (Thermo Fisher Scientific) equipped with an electrospray ionization source operating in positive mode. Samples were separated by reversed-phase HPLC using an Acclaim 120 C18 column (3 μm, 200 Å, 2.1 × 150 mm; Thermo Fisher Scientific) equipped with a guard column and eluted using, as mobile phases, water containing 0.1% (v/v) formic acid (eluent A) and methanol containing 0.1% (v/v) formic acid (eluent B). A 45-min gradient, from 30% to 100% B, was performed. The effluent from the HPLC was directly electrosprayed into the mass spectrometer. The spray voltage was set at 4.5 kV, with the heated capillary temperature set at 350°C. The Orbitrap mass spectrometer instrument operated in full-scan mass spectrometry with resolution R = 30,000 at a mass-to-charge ratio of 400. The quantification was obtained using the calibration curve method.
Measurement of ROS
Hydrogen peroxide produced by leaf discs was measured by a luminol-based assay as previously described (Roux et al., 2011). Discs (0.125 cm2) obtained from 4-week-old plants were washed for 2 h with water and left to recover in a 96-well titer plate (one disc per well). After about 12 h, water was replaced with a solution of luminol (Sigma-Aldrich; 30 μg mL–1) and horseradish peroxidase (Sigma-Aldrich; 20 μg mL–1) containing elf18. For elicitation by OGs, discs were vacuum infiltrated with the OG solution or water as a control for 2 min before addition of the luminol/peroxidase solution. Plates were analyzed for 40 min using a GloMax 96 microplate luminometer with dual injectors (Promega) and a signal integration time of 1 s. Luminescence was expressed in relative light units.
Hydrogen peroxide generated in the incubation medium by seedlings in response to OGs and elf18 was measured by a colorimetric assay based on the xylenol orange dye (o-cresolsulfonephthalein 3′,3″-bis[methylimino] diacetic acid, sodium salt; Sigma) as previously described (Galletti et al., 2008).
Protein Extraction and Immunoblot Analyses
Protein extraction and immunoblotting were performed as described previously (Galletti et al., 2011) using primary antibodies against MPK3 and MPK6 (Sigma-Aldrich) and against phospho-p44/42-EXTRACELLULAR SIGNAL-REGULATED KINASES (Cell Signaling Technologies). Signal detection was performed using the ChemiDoc MP imaging system (Bio-Rad).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phenotype of control plants transformed with the empty vector for β-estradiol-inducible expression and of anp2,3-OE3 transgenic plants.
Supplemental Figure S2. Expression analyses in anp mutant seedlings.
Supplemental Figure S3. ANPs are involved in elicitor-triggered hydrogen peroxide production in seedlings.
Supplemental Figure S4. MPK3 and MPK6 phosphorylation in response to OGs or elf18 in anp single and double mutant seedlings.
Supplemental Table S1. Primers used in this work.
Supplementary Material
Acknowledgments
We thank Dr. Simone Ferrari for helpful discussions.
Glossary
- PRR
pattern recognition receptor
- PTI
pathogen-associated molecular pattern-triggered immunity
- PAMP
pathogen-associated molecular pattern
- DAMP
damage-associated molecular pattern
- OG
oligogalacturonide
- ROS
reactive oxygen species
- MAP
mitogen-activated protein
- MAPK
mitogen-activated protein kinase
- MAP2K
mitogen-activated protein kinase kinase
- MAP3K
mitogen-activated protein kinase kinase kinase
- KO
knockout
- Ws
Wassilewskija
- amiRNA
artificial microRNA
- DMSO
dimethyl sulfoxide
- IAA
indole-3-acetic acid
- SA
salicylate
- CaMV
Cauliflower mosaic virus
- qRT
quantitative reverse transcription
- Col-0
Columbia
Footnotes
This work was supported by the European Research Council (advanced grant no. 233083).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Beck M, Komis G, Müller J, Menzel D, Samaj J. (2010) Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization. Plant Cell 22: 755–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Komis G, Ziemann A, Menzel D, Samaj J. (2011) Mitogen-activated protein kinase 4 is involved in the regulation of mitotic and cytokinetic microtubule transitions in Arabidopsis thaliana. New Phytol 189: 1069–1083 [DOI] [PubMed] [Google Scholar]
- Bellincampi D, Dipierro N, Salvi G, Cervone F, De Lorenzo G. (2000) Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol 122: 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D, Salvi G, De Lorenzo G, Cervone F, Marfà V, Eberhard S, Darvill A, Albersheim P. (1993) Oligogalacturonides inhibit the formation of roots on tobacco explants. Plant J 4: 207–213 [Google Scholar]
- Berriri S, Garcia AV, Frei dit Frey N, Rozhon W, Pateyron S, Leonhardt N, Montillet JL, Leung J, Hirt H, Colcombet J. (2012) Constitutively active mitogen-activated protein kinase versions reveal functions of Arabidopsis MPK4 in pathogen defense signaling. Plant Cell 24: 4281–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, He SY. (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324: 742–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. (2010) Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca C, De Lorenzo G, Cervone F. (1988) Competitive inhibition of the auxin-induced elongation by a-d-oligogalacturonides in pea stem segments. Physiol Plant 72: 499–504 [Google Scholar]
- Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. (2010) A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci USA 107: 9452–9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo G, D’Ovidio R, Cervone F. (2001) The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu Rev Phytopathol 39: 313–335 [DOI] [PubMed] [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J. (2008) Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol Plant 1: 423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich H, Kutchan TM. (1991) Molecular cloning, expression, and induction of berberine bridge enzyme, an enzyme essential to the formation of benzophenanthridine alkaloids in the response of plants to pathogenic attack. Proc Natl Acad Sci USA 88: 9969–9973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP. (2010) Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet 11: 539–548 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J. (2007) Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol 144: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Vairo D, Cervone F, De Lorenzo G. (2006) Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase-inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea. Mol Plant Microbe Interact 19: 931–936 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. (2003) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35: 193–205 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Savatin DV, Sicilia F, Gramegna G, Cervone F, Lorenzo GD. (2013) Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front Plant Sci 4: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti R, Denoux C, Gambetta S, Dewdney J, Ausubel FM, De Lorenzo G, Ferrari S. (2008) The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol 148: 1695–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti R, Ferrari S, De Lorenzo G. (2011) Arabidopsis MPK3 and MPK6 play different roles in basal and oligogalacturonide- or flagellin-induced resistance against Botrytis cinerea. Plant Physiol 157: 804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Ichimura K, Casais C, Peck SC, Shinozaki K, Shirasu K. (2006) MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. J Biol Chem 281: 36969–36976 [DOI] [PubMed] [Google Scholar]
- Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang S, Hirt H, Wilson C, et al. (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7: 301–308 [DOI] [PubMed] [Google Scholar]
- Iida A, Kazuoka T, Torikai S, Kikuchi H, Oeda K. (2000) A zinc finger protein RHL41 mediates the light acclimatization response in Arabidopsis. Plant J 24: 191–203 [DOI] [PubMed] [Google Scholar]
- Jin H, Axtell MJ, Dahlbeck D, Ekwenna O, Zhang S, Staskawicz B, Baker B. (2002) NPK1, an MEKK1-like mitogen-activated protein kinase kinase kinase, regulates innate immunity and development in plants. Dev Cell 3: 291–297 [DOI] [PubMed] [Google Scholar]
- Jouannic S, Hamal A, Leprince AS, Tregear JW, Kreis M, Henry Y. (1999) Plant MAP kinase kinase kinases structure, classification and evolution. Gene 233: 1–11 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Komis G, Illés P, Beck M, Šamaj J. (2011) Microtubules and mitogen-activated protein kinase signalling. Curr Opin Plant Biol 14: 650–657 [DOI] [PubMed] [Google Scholar]
- Kong Q, Qu N, Gao M, Zhang Z, Ding X, Yang F, Li Y, Dong OX, Chen S, Li X, et al. (2012) The MEKK1-MKK1/MKK2-MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell 24: 2225–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J. (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Zeng W, Sheen J. (1998) Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature 395: 716–720 [DOI] [PubMed] [Google Scholar]
- Krysan PJ, Jester PJ, Gottwald JR, Sussman MR. (2002) An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell 14: 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Francocci F, Ferrari S, Volpi C, Bellincampi D, Galletti R, D’Ovidio R, De Lorenzo G, Cervone F. (2010) Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc Natl Acad Sci USA 107: 616–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N. (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17: 9–15 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. (2011) ROS signaling: the new wave? Trends Plant Sci 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) Revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15: 437–479 [Google Scholar]
- Nakagami H, Soukupová H, Schikora A, Zárský V, Hirt H. (2006) A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem 281: 38697–38704 [DOI] [PubMed] [Google Scholar]
- Nakashima M, Hirano K, Nakashima S, Banno H, Nishihama R, Machida Y. (1998) The expression pattern of the gene for NPK1 protein kinase related to mitogen-activated protein kinase kinase kinase (MAPKKK) in a tobacco plant: correlation with cell proliferation. Plant Cell Physiol 39: 690–700 [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Daudi A, Butt VS, Bolwell GP. (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236: 765–779 [DOI] [PubMed] [Google Scholar]
- Pan X, Welti R, Wang X. (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc 5: 986–992 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MW, Roux M, Petersen M, Mundy J. (2012) MAP kinase cascades in Arabidopsis innate immunity. Front Plant Sci 3: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang S. (2008) A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 105: 5638–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MC, Petersen M, Mundy J. (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61: 621–649 [DOI] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C. (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe M, Machida Y. (2012) Regulation of organization and function of microtubules by the mitogen-activated protein kinase cascade during plant cytokinesis. Cytoskeleton (Hoboken) 69: 913–918 [DOI] [PubMed] [Google Scholar]
- Savatin DV, Ferrari S, Sicilia F, De Lorenzo G. (2011) Oligogalacturonide-auxin antagonism does not require posttranscriptional gene silencing or stabilization of auxin response repressors in Arabidopsis. Plant Physiol 157: 1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. (2006) Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Ronald PC. (2012) Plant innate immunity: perception of conserved microbial signatures. Annu Rev Plant Biol 63: 451–482 [DOI] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C. (2011) Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet 7: e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SH, Bush SM, Zaman N, Stecker K, Sussman MR, Krysan P. (2013) Deletion of a tandem gene family in Arabidopsis: increased MEKK2 abundance triggers autoimmunity when the MEKK1-MKK1/2-MPK4 signaling cascade is disrupted. Plant Cell 25: 1895–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SH, Suarez-Rodriguez MC, Krysan P. (2007) Genetic interaction and phenotypic analysis of the Arabidopsis MAP kinase pathway mutations mekk1 and mpk4 suggests signaling pathway complexity. FEBS Lett 581: 3171–3177 [DOI] [PubMed] [Google Scholar]
- Suarez-Rodriguez MC, Adams-Phillips L, Liu Y, Wang H, Su SH, Jester PJ, Zhang S, Bent AF, Krysan PJ. (2007) MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol 143: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Soyano T, Kosetsu K, Sasabe M, Machida Y. (2010) HINKEL kinesin, ANP MAPKKKs and MKK6/ANQ MAPKK, which phosphorylates and activates MPK4 MAPK, constitute a pathway that is required for cytokinesis in Arabidopsis thaliana. Plant Cell Physiol 51: 1766–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li Y, Wang Y, Liu H, Lei L, Yang H, Liu G, Ren D. (2008) Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J Biol Chem 283: 26996–27006 [DOI] [PubMed] [Google Scholar]
- Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, et al. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1: 175–185 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G. (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.