Strigolactone hormones signal through a specific receptor to induce particular responses in Arabidopsis, whereas their stereoisomers induce different responses by signaling through a closely related receptor that also perceives karrikins from wildfires.
Abstract
Two α/β-fold hydrolases, KARRIKIN INSENSITIVE2 (KAI2) and Arabidopsis thaliana DWARF14 (AtD14), are necessary for responses to karrikins (KARs) and strigolactones (SLs) in Arabidopsis (Arabidopsis thaliana). Although KAI2 mediates responses to KARs and some SL analogs, AtD14 mediates SL but not KAR responses. To further determine the specificity of these proteins, we assessed the ability of naturally occurring deoxystrigolactones to inhibit Arabidopsis hypocotyl elongation, regulate seedling gene expression, suppress outgrowth of secondary inflorescences, and promote seed germination. Neither 5-deoxystrigol nor 4-deoxyorobanchol was active in KAI2-dependent seed germination or hypocotyl elongation, but both were active in AtD14-dependent hypocotyl elongation and secondary shoot growth. However, the nonnatural enantiomer of 5-deoxystrigol was active through KAI2 in growth and gene expression assays. We found that the four stereoisomers of the SL analog GR24 had similar activities to their deoxystrigolactone counterparts. The results suggest that AtD14 and KAI2 exhibit selectivity to the butenolide D ring in the 2′R and 2′S configurations, respectively. However, we found, for nitrile-debranone (CN-debranone, a simple SL analog), that the 2′R configuration is inactive but that the 2′S configuration is active through both AtD14 and KAI2. Our results support the conclusion that KAI2-dependent signaling does not respond to canonical SLs. Furthermore, racemic mixtures of chemically synthesized SLs and their analogs, such as GR24, should be used with caution because they can activate responses that are not specific to naturally occurring SLs. In contrast, the use of specific stereoisomers might provide valuable information about the specific perception systems operating in different plant tissues, parasitic weed seeds, and arbuscular mycorrhizae.
Strigolactones (SLs) are carotenoid-derived phytohormones that mediate various aspects of plant development in addition to symbiotic and parasitic interactions in the rhizosphere. Originally identified as seed germination stimulants of root-parasitic weeds (Cook et al., 1966, 1972), SLs have now been implicated in several processes, including inhibition of bud outgrowth to decrease shoot branching, regulation of leaf morphology, regulation of root architecture, control of secondary growth in the cambium, and association of plant roots with symbiotic fungi and nodulating bacteria (for review, see Brewer et al., 2013; Waldie et al., 2014).
The isolation of several mutants in pea (Pisum sativum; rms), rice (Oryza sativa; d), petunia (Petunia hybrida; dad), and Arabidopsis (Arabidopsis thaliana; max) that exhibit dwarfism and an increased number of secondary shoots or tillers has enabled the identification of genes involved in four steps in SL biosynthesis and another three involved in signal transduction. The initial step of SL biosynthesis involves the conversion of all-trans-β-carotene into 9-cis-β-carotene by the isomerase D27. The sequential cleavage of the D27 product by two carotenoid cleavage dioxygenases (CCDs), CCD7 (also known as RAMOSOUS5 [RMS5], DWARF17 [D17], DECREASED APICAL DOMINANCE3 [DAD3], and MORE AXILLARY BRANCHING3 [MAX3]) and CCD8 (also known as RMS1, D10, DAD1, and MAX4), results in the formation of an intermediate, carlactone (Alder et al., 2012). A cytochrome P450, originally identified in Arabidopsis as MAX1 (Booker et al., 2005), is necessary for the conversion of carlactone into functional SLs (Scaffidi et al., 2013; Seto et al., 2014).
The components required for SL signal transduction include an α/β-fold hydrolase (known as D14, DAD2, or Arabidopsis thaliana DWARF14 [AtD14]), an F-box protein (RMS4, D3, or MAX2), and a protein (D53) with similarity to Clp ATPases and heat shock proteins. In Arabidopsis and rice, both d14 and max2 (d3) mutants exhibit increased numbers of secondary shoots, a phenotype similar to that of SL biosynthetic mutants (Brewer et al., 2013; Waldie et al., 2014). The rice d53 mutant also has multiple secondary shoots (Jiang et al., 2013; Zhou et al., 2013). However, the shoot phenotypes of d14, max2 (d3), and d53 are unresponsive to the application of exogenous SLs, confirming a role for these proteins in SL signal transduction.
D14/DAD2 is a globular protein with enzymatic activity that depends on a Ser-Asp-His catalytic triad located at the bottom of a hydrophobic active site cavity. The catalytic triad is essential for SL signaling and yeast two-hybrid interaction of D14/DAD2 with MAX2 (Hamiaux et al., 2012) and with D53 (Jiang et al., 2013; Zhou et al., 2013). Based on recent x-ray structural data that reveal the hydrolyzed D ring of the synthetic SL GR24 bound to the catalytic Ser (Zhao et al., 2013), D14 is proposed to cleave the ABC ring and D ring by direct nucleophilic attack on the butenolide carbonyl moiety (Scaffidi et al., 2012) to result in a change in D14 protein tertiary structure. This change, perhaps coupled with an SL degradation product located at the cavity opening, is assumed to modulate protein-protein interactions (Hamiaux et al., 2012; Nakamura et al., 2013; Zhao et al., 2013). The subsequent step in SL signaling in rice shoots is the ubiquitination and degradation of D53. This protein is thought to promote expression of genes, leading to the growth of secondary shoots (Jiang et al., 2013; Zhou et al., 2013). Another report provides evidence that D14 and SL together stimulate the MAX2-dependent ubiquitination and degradation of the transcriptional regulator brassinolide-insenitive1-EMS-suppressor1 (BES1), which is a key component of brassinosteroid signaling in Arabidopsis (Wang et al., 2013). Yet another report provides evidence that D14 exhibits SL-dependent interaction with the rice transcriptional regulator protein SLENDER1 (SLR1), which also mediates responses to gibberellins (Nakamura et al., 2013). Therefore, SL signal transduction is profoundly important in the control of plant growth, development, and productivity.
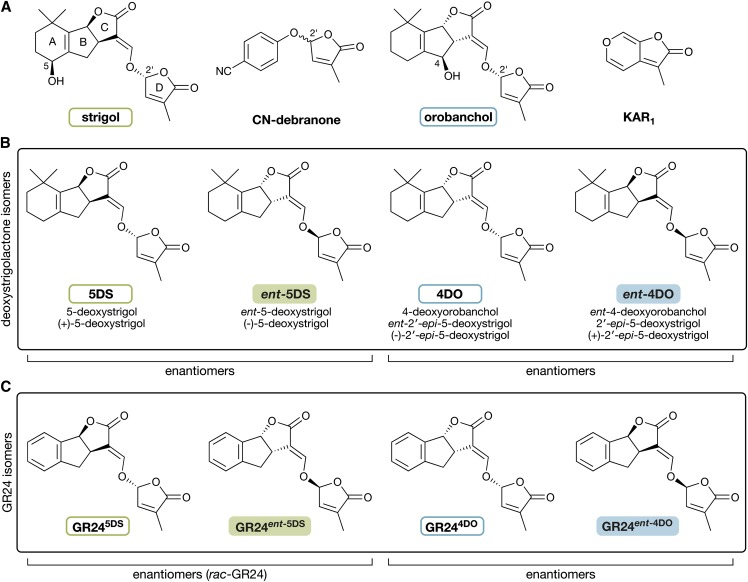
All naturally occurring SLs share a unique core structure comprising an ABC-ring system linked to a 2′R-configured butenolide D ring. It has been proposed that only a few steps are needed to convert carlactone into the simplest SL, 5-deoxystrigol (5DS; Alder et al., 2012). As a result, 5DS was thought to be the precursor from which all SLs are derived; however, the recent structural amendment of (−)-orobanchol has required a revision of this theory (Ueno et al., 2011; Vurro and Yoneyama, 2012). It is now clear that SLs are divided into two distinct classes: one defined by (+)-strigol, and the other defined by (−)-orobanchol (Xie et al., 2013). These two classes differ by having opposite stereochemistry at the BC-ring junction to give diastereomers with the same 2′R D-ring configuration (Fig. 1A). SL nomenclature has adopted the use of ent as an abbreviation for enantiomer (meaning mirror image) and epi for epimer (meaning the opposite stereochemistry at one carbon). This notation is only applicable when referenced back to a parent structure, which has caused some confusion in recent years given the reclassification of SLs into two families. To resolve this issue, we propose that SLs should be referenced back to their parent scaffold. In the case of (+)-strigol, 5DS is a suitable reference compound, and for (−)-orobanchol, 4-deoxyorobanchol (4DO; ent-2′-epi-5DS) is an appropriate reference. Both of these deoxystrigolactones (deoxySLs) have been identified from root exudates of rice and tobacco (Nicotiana tabacum; Xie et al., 2013). We suggest the name 4DO as a reference structure in its own right, replacing the confusing strigol-derived name of ent-2′-epi-5DS. Furthermore, we have chosen to refrain from using optical notation, because these descriptors offer no information on the spatial arrangement of substituents attached to the chiral centers within the compound.
Figure 1.
Chemical structures of SL stereoisomers and related butenolide compounds. A, Two naturally occurring SLs, strigol and orobanchol, with the characteristic ABC-ring to D-ring structure. Note the hydroxyl groups at the C5 and C4 positions and the differing stereochemistry at the B-ring to C-ring junctions. The stereochemistry at C2′ is the same in both strigol and orobanchol. KAR1 is an achiral seed germination stimulant found in burnt plant material. CN-debranone is a compound with SL-like activity. Note the single chiral center at the C2′ position; both stereoisomers are depicted in the single structure. B, The deoxySLs 5DS and 4DO and their corresponding enantiomers (mirror images). Below each compound is a list of possible synonyms. The enantiomers ent-5DS and ent-4DO have not been reported to be produced in nature. C, The GR24 group of artificial SL analogs. Each is an analog of a corresponding deoxySL, which is denoted by the superscript suffix.
This classification system for natural SLs is also helpful when considering the stereochemistry of SL analogs, such as GR24. This synthetic SL analog is commonly used as a racemic mixture of two 5DS-configured enantiomers and can be referenced back to their deoxySL counterparts: GR245DS and GR24ent-5DS. For the purpose of this work, we refer to an equimolar mixture of these compounds as rac-GR24. The chemical synthesis of GR24 also generates two 4DO-like enantiomers (GR244DO and GR24ent-4DO); however, these compounds are typically not used in biological assays (Mangnus et al., 1992). Nevertheless, these enantiomers can serve as synthetic equivalents of the orobanchol class of natural SLs. A reference guide for all structures and synonyms is presented in Figure 1.
The stereochemistry of SLs can play an important role in regulating various biological functions, including parasitic weed seed germination, hyphal branching in the arbuscular mycorrhizal fungus Gigaspora margarita, and tillering in rice. Stereospecificity can be observed not only between genera but even between species within the same genus. For example, analogs with strigol-like stereochemistry, including strigol, ent-2′-epi-orobanchol, sorgolactone, demethylsorgolactone, sorgomol, 5DS, and GR245DS, show the greatest activity toward stimulating germination of Striga hermonthica seed, with their enantiomers inducing a lower response (Thuring et al., 1997; Sugimoto et al., 1998; Nomura et al., 2013; Zwanenburg and Pospísil, 2013). However, germination in Striga gesnerioides is inhibited by isomers that promote germination in S. hermonthica and stimulated by orobanchol-type analogs (Nomura et al., 2013). Interestingly, S. gesnerioides can also respond to SL analogs possessing the nonnatural S-configured D ring (Nomura et al., 2013). The general preference for natural configured isomers also holds true for hyphal branching, and in this case, orobanchol derivatives display the greatest level of activity (Akiyama et al., 2010). Recently, ent-2′-epi-GR7, which has the same stereochemistry as orobanchol, was reported to inhibit the outgrowth of the second tiller in rice more effectively than ent-GR7, which has a nonnatural D-ring configuration (Nakamura et al., 2013). These results suggest that analogs with stereochemistry similar to those of natural SLs often return the highest activity. However, the differing biological systems used in these studies make direct comparisons between similar compounds difficult.
Related to SLs are the abiotic signals known as karrikins (KARs), which are found in plant-derived smoke (Flematti et al., 2004). Although achiral, they share a 3-methyl-substituted butenolide ring in common with SLs and can promote the germination of various plant species from around the world (Nelson et al., 2012; Waters et al., 2013). Unlike SLs, KARs do not promote the germination of parasitic weed seeds or inhibit shoot branching (Nelson et al., 2011). The KAR signaling pathway has been partially resolved by the identification of a paralog of AtD14 in Arabidopsis known as KARRIKIN INSENSITIVE2 (KAI2; Waters et al., 2012a) and SUPPRESSOR OF MAX2 1 (SMAX1), an Arabidopsis homolog of rice D53 (Stanga et al., 2013). Like AtD14, KAI2 possesses an essential conserved catalytic triad within a hydrophobic cavity (Waters et al., 2014), and the overall structures of both proteins are generally very similar (Bythell-Douglas et al., 2013; Kagiyama et al., 2013; Zhao et al., 2013). Nonetheless, there is a clear distinction between KAI2 and AtD14 functions: whereas the former mediates seed and seedling development, the latter is involved in postjuvenile development, most notably axillary or secondary bud outgrowth. Similarly, SMAX1 is required for KAR responses and seedling development but not for secondary shoot growth in Arabidopsis (Stanga et al., 2013).
Unlike Atd14 and SL biosynthetic mutants, the kai2 mutant exhibits increased seed dormancy and abnormal seedling photomorphogenesis, suggesting that these processes are under the control of a unique signal independent from the canonical SL pathway (Waters et al., 2012a). Additionally, exogenously supplied carlactone is a very weak inhibitor of hypocotyl elongation in Arabidopsis, further suggesting that natural SLs are not physiologically relevant during seedling establishment (Scaffidi et al., 2013). Interestingly, rac-GR24 is able to effect the light-dependent inhibition of hypocotyl elongation in both a KAI2-dependent and an AtD14-dependent manner, showing that rac-GR24 can signal through both proteins (Waters et al., 2012a). One interpretation of this result is that the GR24ent-5DS component of rac-GR24, representing the stereochemistry of the nonnatural SL ent-5DS, may have activity distinct from that of GR245DS. To test this possibility, we evaluated endogenous SLs, their nonnatural enantiomers, and their GR24 counterparts for their ability to regulate hypocotyl elongation, gene expression levels, shoot branching, and seed germination in Arabidopsis.
RESULTS
KAI2 and AtD14 Exhibit Differential Stereoselectivity toward SL and SL-Like Compounds
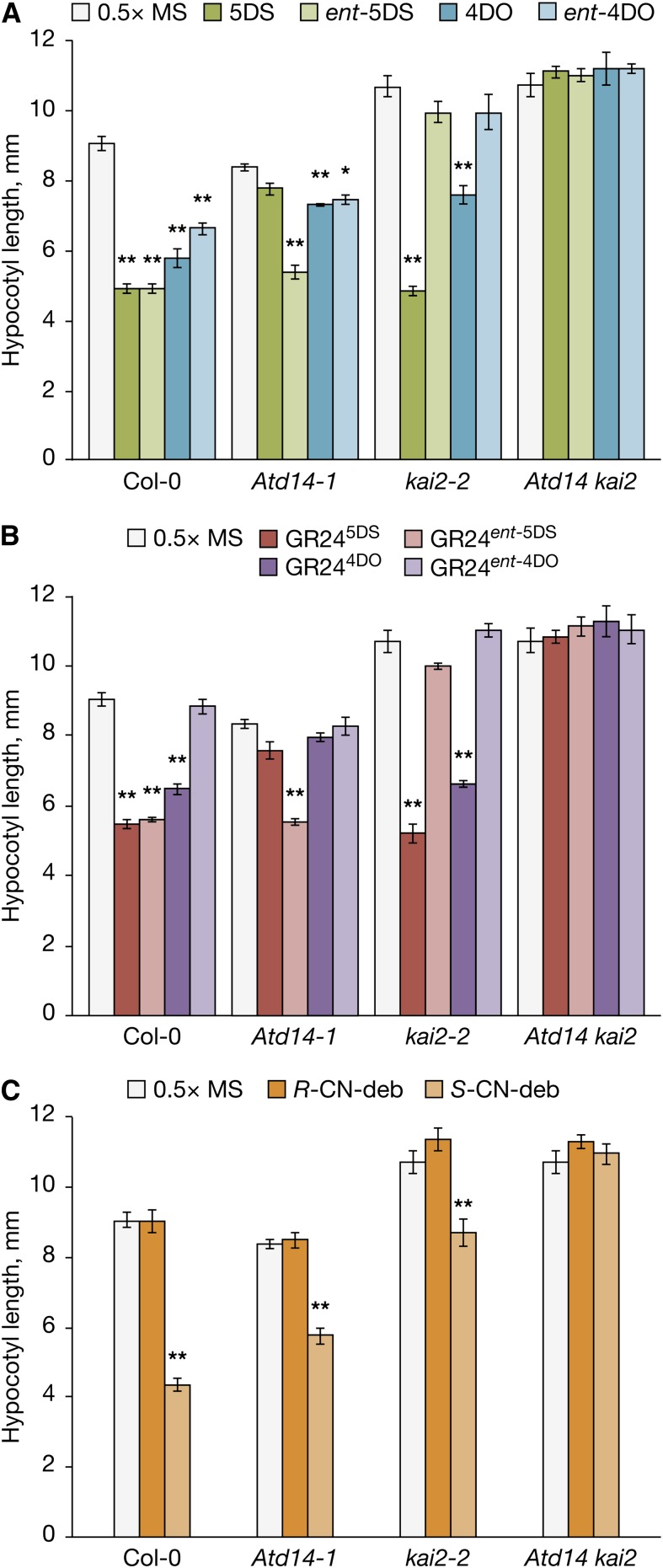
Previously, we have exploited Arabidopsis genetics and the growth of seedling hypocotyls to dissect the relative contributions of KAI2 and AtD14 in response to butenolide-containing compounds (Waters et al., 2012a, 2012b; Scaffidi et al., 2013). Unlike AtD14, KAI2 is essential for normal hypocotyl growth and responses to KARs. Although not required for early development, AtD14 is still present in seedlings, and similar to KAI2, it can mediate responses to rac-GR24. Because rac-GR24 can signal through both KAI2 and AtD14, we hypothesized that it might reflect specificity for distinct enantiomers both present in rac-GR24. Alternatively, the activity of rac-GR24 on seedlings might result from the synthetic nature of the compound, which may be different from the activity observed with naturally occurring SLs. To investigate these possibilities, we first chemically synthesized two naturally occurring deoxySLs, 5DS and 4DO, along with their nonnatural enantiomers, ent-5DS and ent-4DO, and separated all four stereoisomers (Supplemental Figs. S1 and S2). We assessed the bioactivity of each stereoisomer in hypocotyl elongation assays with Columbia-0 (Col-0) wild-type, Atd14-1, kai2-2, and Atd14 kai2 double mutants. Wild-type seedlings exhibited substantial responses to all four stereoisomers, although the response to ent-4DO was less than the response to the other three (Fig. 2A). Whereas Atd14 showed the strongest response to ent-5DS and weak but significant responses to 4DO and ent-4DO, kai2 exhibited significant inhibition of hypocotyl growth on 5DS and 4DO. Atd14 kai2 double mutants were insensitive to all four stereoisomers, showing that KAI2 and AtD14 α/β-fold hydrolases together account for all activity (Fig. 2A). These findings confirm that exogenously applied deoxySLs are regulators of hypocotyl development. In addition, we infer that AtD14 and KAI2 exhibit specificity toward different stereoisomers of deoxySLs: AtD14 preferentially mediates responses to natural 5DS and 4DO isomers, and KAI2 preferentially mediates responses to the nonnatural isomer ent-5DS.
Figure 2.
Seedling hypocotyl elongation responses to stereoisomers of SLs and their analogs. Seeds were sown on one-half-strength Murashige and Skoog (MS) agar media supplemented with 1 µm each compound as indicated, dark stratified at 4°C, and incubated for 4 d under continuous red light at 21°C. A, Hypocotyl elongation responses to 5DS, 4DO, and their respective enantiomers. B, Responses to four stereoisomers of GR24. C, Responses to each enantiomer of CN-debranone (CN-deb). The data for mock-treated controls are the same in each graph. Data are means ± ses (n = 3 independent experimental replicates of 20 seedlings per sample). Asterisks denote significant differences within a genotype relative to untreated samples (ANOVA). *, P < 0.05; **, P < 0.01.
Next, we investigated whether these stereoisomer-specific responses to deoxySLs were also reflected among the stereoisomers of GR24. Once again, wild-type seedlings responded strongly to all GR24 stereoisomers except GR24ent-4DO (Fig. 2B). Atd14-1 mutants showed a significant response only to GR24ent-5DS, whereas kai2-2 responded to GR245DS and GR244DO (Fig. 2B). Notably, the relative strength of the response of kai2 to GR245DS and GR244DO is the same as the response of Col-0 and the same as for the equivalently configured deoxySLs (5DS and 4DO). Atd14 kai2 double mutants were again insensitive to all four GR24 stereoisomers. Thus, the broad pattern of response is similar between the various stereoisomers of GR24 and their deoxySL equivalents, supporting the interpretation that AtD14 and KAI2 exhibit substrate stereoselectivity.
A difference between the 5DS and ent-5DS enantiomers is the C2′ configuration of the D ring (Fig. 1). The 2′R-configured isomer (5DS) signals preferentially through AtD14, and the 2′S-isomer (ent-5DS) signals preferentially through KAI2. To test whether it is the configuration of the D ring that determines the specificity of AtD14 and KAI2 for specific substrates, we separated the two stereoisomers of nitrile debranone (CN-debranone), a simple bioactive SL analog with only one chiral center located at the D ring (Fukui et al., 2011). The absolute stereochemistry of the two R- and S-CN-debranone enantiomers was established by single-crystal x-ray diffraction experiments (Supplemental Fig. S3; Supplemental Table S2). We evaluated the relative impacts of R- and S-CN-debranone on hypocotyl growth. Surprisingly, only the S-configured compound showed any activity, which was the case in both Atd14 and kai2 mutants, whereas the Atd14 kai2 double mutant was again completely unresponsive (Fig. 2C). Thus, S-CN-debranone acts through both AtD14 and KAI2 and implies that chirality at the C2′ position alone is insufficient to account for substrate specificity of AtD14 versus KAI2. Nevertheless, these data also show that stereochemistry is an important determinant of bioactivity.
SL Stereoisomers Induce Differential Gene Expression Changes
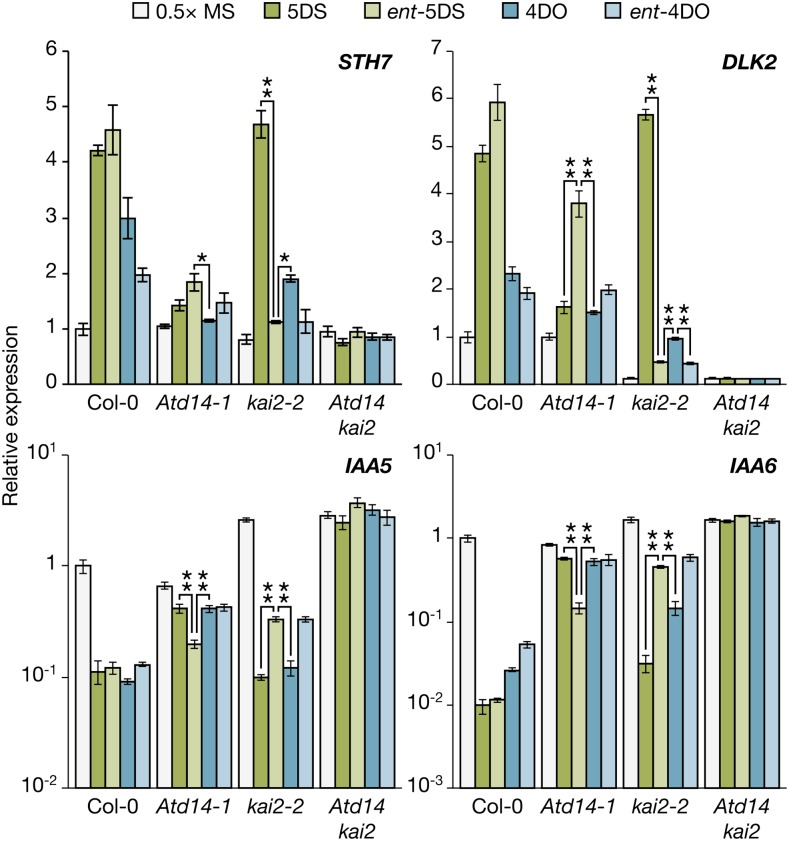
To further investigate the role of AtD14 and KAI2 in determining stereoisomer-specific responses, we measured the changes in abundance of four transcripts in seedlings treated with each of four deoxySL stereoisomers. SALT TOLERANCE HOMOLOG7 (STH7; At4g39070) and DWARF14-LIKE2 (DLK2; At3g24420) increase in abundance in response to KARs and rac-GR24, whereas INDOLEACETIC ACID-INDUCED5 (IAA5; At1g15580) and IAA6 (At1g52830) decrease (Nelson et al., 2010; Waters et al., 2012a). We found that 5DS and ent-5DS were generally more active than 4DO and ent-4DO in wild-type seedlings based on the magnitude of change of STH7, DLK2, and IAA6 transcripts (Fig. 3). In particular, 5DS and ent-5DS induced a 100-fold reduction in IAA6 transcript levels compared with a 30-fold and 20-fold reduction for 4DO and ent-4DO, respectively. This difference between the deoxySL stereoisomers in the regulation of STH7, DLK2, and IAA6 transcripts in wild-type seedlings correlates with their effectiveness in inhibiting hypocotyl elongation. As expected, Atd14 kai2 double mutants did not respond to any of four stereoisomers tested (Fig. 3).
Figure 3.
KAI2 and AtD14 differentially mediate responses of multiple transcripts to SL stereoisomers. Seed were sown, stratified, and incubated as described in Figure 2, and RNA was isolated from whole seedlings after 4 d. Two transcripts known to respond positively to rac-GR24 (STH7 and DLK2) and two that respond negatively (IAA5 and IAA6) were measured. Target transcripts were normalized to CLATHRIN ADAPTOR COMPLEX SUBUNIT reference transcripts, and expression was scaled relative to Col-0 grown on one-half-strength Murashige and Skoog (MS). Data are means ± ses of three biological replicates of >100 seedlings each. Asterisks denote significant differences between selected treatments as indicated (ANOVA); for clarity, not all significant differences are shown. *, P < 0.05; **, P < 0.01.
Substantial differences in transcript responses to the four stereoisomers between Atd14 and kai2 mutants were discernible. Responses of all four transcripts to 5DS and 4DO, especially DLK2, IAA5, and IAA6, were substantially weakened in Atd14 but mostly normal in kai2 (Fig. 3). Conversely, responses to ent-5DS and ent-4DO were dramatically suppressed in kai2 mutants but much less so in Atd14 mutants. Although there was some overlap between AtD14- and KAI2-mediated responses (e.g. IAA5 and IAA6 transcripts were still substantially repressed in kai2 mutants treated with ent-5DS and ent-4DO), overall, these data support a broad division in substrate preferences between AtD14 and KAI2 and corroborate the hypocotyl elongation data above. Specifically, AtD14 is most active toward naturally configured SLs (5DS and 4DO), whereas KAI2 preferentially mediates responses to their nonnatural enantiomers, especially ent-5DS.
Stereoisomer-Specific Control of Shoot Branching
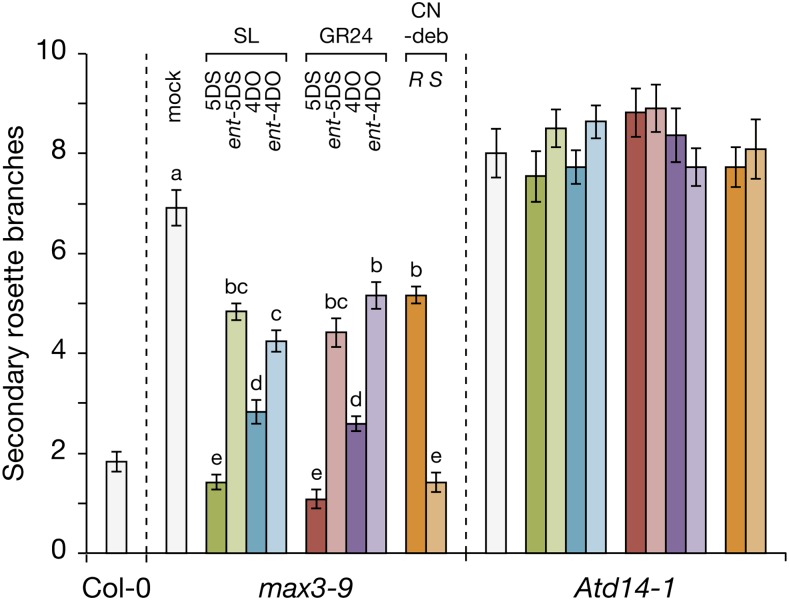
Inhibition of outgrowth of secondary branches arising from the rosette requires AtD14 but not KAI2, and therefore, we investigated whether SL stereochemistry also affects branching in the SL-deficient mutant max3-9 and the SL-insensitive mutant Atd14-1, both of which display enhanced numbers of inflorescences relative to wild type. All four GR24 stereoisomers and their equivalent deoxySL stereoisomers repressed shoot branching in max3 but not Atd14, confirming that their activity was AtD14 dependent (Fig. 4). However, among the four deoxySL stereoisomers, 5DS exhibited significantly more activity than 4DO, and both were more active than their respective enantiomers. The pattern of activity observed for deoxySLs was repeated among the four GR24 stereoisomers: in particular, GR245DS was more active than GR244DO, and both were more active than their enantiomers (Fig. 4). Notably, both 5DS and GR245DS were able to fully restore the max3 branching phenotype to wild-type levels.
Figure 4.
Stereoisomers of SLs and their analogs are differential inhibitors of shoot branching. Secondary rosette branches of max3-9 and Atd14-1 mutants grown hydroponically and treated with 0.01% (v/v) acetone (mock) or 1 µm each compound as indicated. As a baseline reference for branch number, Col-0 plants were untreated. Data are means ± ses (n = 10–12 plants). Treatments sharing the same lowercase letter are not significantly different (P < 0.01, ANOVA). There were no significant differences between treatments among Atd14-1 plants. CN-deb, CN-debranone.
There was a dramatic difference in activity between the two CN-debranone stereoisomers. The S-configured debranone was as active as 5DS and GR245DS (which have R-configured D rings); however, in this case, the R-isomer was also active, albeit at lower levels, but equivalent to ent-5DS and GR24ent-5DS (which have 2′S-configured D rings; Fig. 4). Again, therefore, the D ring configuration alone is insufficient to explain the relative bioactivity of each compound. Nevertheless, assuming that all control of secondary shoot growth is AtD14 dependent (Waters et al., 2012a), the differences in activity between the various stereoisomers may reflect the affinity of AtD14 to each isomer. Consistent with this hypothesis, the activity of each stereoisomer in secondary shoot growth corresponds largely to the relative strengths of each isomer in inhibiting hypocotyl elongation in kai2 mutants by AtD14.
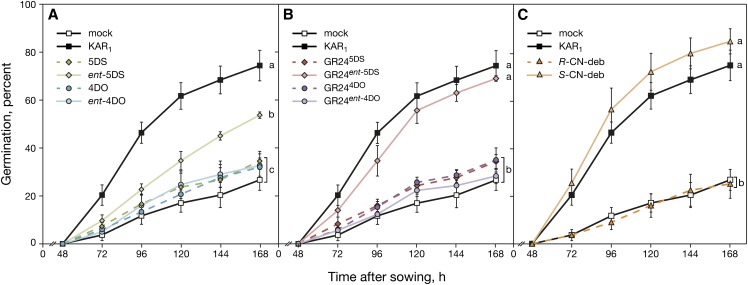
Arabidopsis Primary Seed Dormancy Is Not Overcome by Naturally Configured SLs
Freshly harvested Arabidopsis seed exhibits primary dormancy that can be overcome by many treatments, such as a period of cold imbibition or exposure to nitrate (Ali-Rachedi et al., 2004). Exogenously supplied KARs and rac-GR24 can also promote the germination of primary dormant seed, and this activity is dependent on functional KAI2 (Nelson et al., 2009; Waters et al., 2012a). The observation that, in seedlings, KAI2 mostly mediates responses to ent-5DS and GR24ent-5DS over their naturally configured enantiomers 5DS and GR245DS led us to predict that only those stereoisomers that act through KAI2 would promote the germination of primary dormant Arabidopsis seed. We tested the ability of each stereoisomer of deoxySLs and GR24 to promote seed germination in the ecotype Landsberg erecta of Arabidopsis (Ler) wild type. Of four deoxySL stereoisomers, only ent-5DS had activity, albeit significantly less than KAR1, whereas activities of the other three isomers were not significantly different from mock-treated controls (Fig. 5A). Consistent with this observation, GR24ent-5DS was the only GR24 isomer to promote germination, and it was comparable with KAR1 (Fig. 5B). The difference in effectiveness of ent-5DS and GR24ent-5DS may reflect differences in chemical stability under the elevated temperature and pH conditions of the germination assay (neutral pH, 28°C; Akiyama et al., 2010), because in hypocotyl assays (pH 5.9, 21°C ± 1°C) and branching (pH 5.9, 22°C day and 16°C night, with media replaced every 3 d after treatment commenced), these two isomers are broadly equivalent (Figs. 2–4). We also tested the two CN-debranone isomers, and only the S-isomer was active in promoting seed germination (Fig. 5C), consistent with its activity in hypocotyl elongation assays. Overall, these results indicate that only those stereoisomers that act through KAI2 are capable of overcoming primary seed dormancy. These findings are consistent with our previous observation that carlactone is unable to overcome seed dormancy (Scaffidi et al., 2013), because carlactone-derived SLs, which have conventional 2′R-configured D rings, do not act through KAI2.
Figure 5.
Alleviation of Arabidopsis primary seed dormancy by SLs and their analogs shows stereoisomeric specificity. Primary dormant Ler seed was sown on water-agar (1% [w/v] phytagel supplemented with 1.5 mm MgCl2 as a gelling agent) containing either 0.1% (v/v) acetone (mock) or 1 µm each compound as indicated. A, Germination responses to natural deoxySLs and their enantiomers. B, Germination responses to the four stereoisomers of GR24. C, Germination responses to the two stereoisomers of CN-debranone. Values are means ± ses of three independent samples of 100 seeds. Plates were incubated at 28°C under constant light, and germination was scored daily. Treatments with significantly different germination levels after 168 h are indicated by different lowercase letters (P < 0.01, ANOVA). The data for mock-treated and KAR1-treated controls are the same in each graph. CN-deb, CN-debranone.
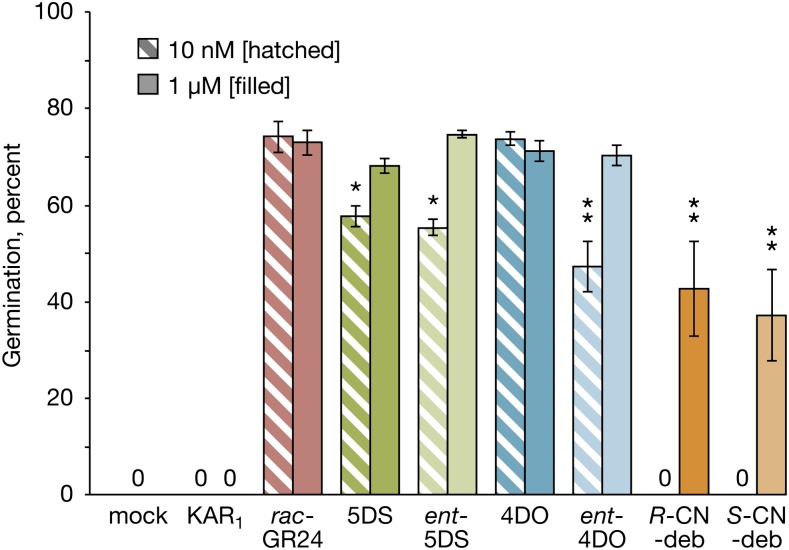
Seeds of Orobanche minor Respond to Both Natural and Nonnatural Configured SL Isomers
The finding that Arabidopsis seed germination responds only to deoxySL isomers with 2′S-configured D rings led us to consider whether seeds of root-parasitic plants of the Orobanchaceae also show a consistent selectivity for particular stereoisomers and whether it could help to indicate whether they use a D14-type or KAI2-type receptor. Seeds of S. hermonthica respond to all stereoisomers of GR24 (Zwanenburg and Pospísil, 2013) and deoxystrigol (Nomura et al., 2013), with apparent preference for isomers with the same configuration as strigol; notably, 4DO is comparatively inactive in S. hermonthica (Nomura et al., 2013). However, such studies have not been reported for O. minor. To allow direct comparison with the work by Nomura et al. (2013), we tested deoxySL stereoisomers at 10 nm and 1 µm on O. minor seed germination. As previously reported (Nelson et al., 2009), rac-GR24 stimulated O. minor seed germination, whereas KAR1 did not (Fig. 6). All four deoxySL stereoisomers were active at 10 nm, with 4DO being the most active and its enantiomer being the least active, indicating that the D ring configuration is noncritical for promoting O. minor seed germination. This result was confirmed with both CN-debranone stereoisomers, both of which are equally active but only at the highest concentration (Fig. 6). We conclude that the stereo selectivity of S. hermonthica and O. minor toward deoxySLs is quite different from each other and also different from that of Arabidopsis. Thus, we are not able to draw any conclusions from this evidence alone about whether KAI2-type or D14-type proteins are preferentially active in the seeds of these parasitic plants.
Figure 6.
O. minor seed is differentially responsive to stereoisomers of deoxySLs but not debranones. Germination of O. minor seed after 1 week of dark incubation in the presence of butenolide compounds at either 10 nm (hatched bars) or 1 μm (filled bars). Samples with zero germination are marked with 0 (data are means ± ses of n = 3–6 samples). Each sample comprised 100 to 120 seeds. Asterisks indicate a significant difference from rac-GR24 at the same concentration (ANOVA). CN-deb, CN-debranone. *, P < 0.05; **, P < 0.01.
DISCUSSION
In this study, we examined multiple physiological traits within a single plant species to elucidate the activity of two natural deoxySLs and their enantiomers plus four GR24 and two debranone stereoisomers. Stereochemistry emerges as a significant determinant of SL activity, and differences in activity can now be linked to signaling through specific receptor proteins. In general, AtD14 mediates those traits that are known to be regulated by canonical 2′R-configured SLs, such as shoot branching. The surprising observation that nonnatural SL enantiomers with 2′S-configured butenolide rings are also active but primarily through KAI2 raises the question of whether similar compounds are made by plants as signaling molecules. If plants do produce these precise SL enantiomers, they must do so at extremely low levels, because one would expect them to copurify with the canonical SLs, and no such stereoisomeric mixtures have been described to date. The relatively weak response of Arabidopsis hypocotyl growth and seedling gene expression to the ent-4DO configuration compared with ent-5DS (Figs. 2 and 3) shows that subtle changes in substrate chirality (in this case, at the junction of B and C rings) can dramatically affect KAI2 activity.
Two observations indicate that the substrate stereo selectivity of AtD14 is not absolute. First, ent-5DS and ent-4DO exhibit relatively weak but significant AtD14-dependent activity as inhibitors of shoot branching (Fig. 4). Second, R-CN-debranone is entirely inactive in seedlings and thus, cannot operate through AtD14 at this stage of development, but it does exhibit some AtD14-dependent activity in shoot branching assays.
Because the structures of D14 and KAI2 proteins have been determined by x-ray crystallography in several separate studies, it raises the possibility that their stereo selectivity could be explained in terms of their ability to accommodate substrates in the active site pocket. The pocket of D14 is reported to be larger than that of KAI2, but both could theoretically accommodate GR24 (Bythell-Douglas et al., 2013; Guo et al., 2013; Zhao et al., 2013), consistent with the observed biological activity. However, the position of an intact SL in the active site pocket of D14 and the exact binding of KAR in KAI2 remain unclear. In the case of deoxySLs, it is clear that D14 prefers R-configured butenolide rings and that KAI2 prefers the S-configuration. The ability of S-CN-debranone to regulate both KAI2 and AtD14 functions at various stages of development suggests that the ABC ring of SLs imposes a constraint to determine the preference of AtD14 for the R-configured butenolide ring. We know from studies of rice D14 that the SL butenolide must occupy a position close to the catalytic Ser during catalysis (Zhao et al., 2013) but can potentially relocate after hydrolysis (Nakamura et al., 2013). In the case of KAI2, the positioning of KAR1 at the opening of the active site pocket has been reported (Guo et al., 2013); however, its position is inconsistent with the required involvement of the catalytic triad in KAR response (Waters et al., 2014). Another consideration for activity that must be taken into account is that D14 and KAI2 proteins undergo conformational change during substrate binding, which is assumed to be necessary for protein partner interactions during signaling (Hamiaux et al., 2012; Guo et al., 2013; Kagiyama et al., 2013; Nakamura et al., 2013). It is interesting that the same stereoisomer has differing activities through the same receptor protein depending on developmental period, consistent with the possibility of different interacting partners operating in distinct tissue at various stages of development. Given such uncertainty, it is not surprising that molecular modeling of SLs in D14 and KAI2 proteins has produced multiple theoretical solutions and therefore, failed to provide us with an explanation for the discrimination of stereoisomers by D14 and KAI2.
SL signaling is thought to depend on the formation of a complex between the D14 α/β-fold hydrolase and MAX2 to trigger the ubiquitin-mediated degradation of target proteins (Hamiaux et al., 2012; Waters et al., 2013). In rice, D53 is a member of a family of nine closely related proteins and has been identified as a target protein for SL-dependent degradation (Jiang et al., 2013; Zhou et al., 2013). In rice, the transcriptional regulator FINE CULM1 (FC1) is necessary for SL-dependent control of secondary shoot outgrowth (Minakuchi et al., 2010). FC1 transcripts are down-regulated in d53 mutants, providing a possible mechanism for D53-mediated repression of shoot outgrowth by gene transcription. Arabidopsis also contains a D53-related protein family with eight members. The first characterized protein of this family, SMAX1, is required for KAI2-dependent signaling in seeds and seedlings but does not apparently play a role in the control of shoot growth (Stanga et al., 2013). Loss-of-function mutations in SMAX1 restore numerous seedling phenotypes of max2, such as elongated hypocotyl, aberrant cotyledon morphology, and misexpression of KAR-related marker transcripts (Stanga et al., 2013). Notably, they are KAI2-dependent functions of MAX2 (Waters et al., 2012a). Critically, the other max2 phenotypes, such as increased secondary shoot branching and decreased primary inflorescence height, are not suppressed by smax1. These other functions of MAX2, which are likely dependent on SL and AtD14, may be mediated by other SMAX1-LIKE (SMXL) family members, possibly orthologs of rice D53. Transcripts of several SMXL genes are down-regulated in max2 and positively regulated by KARs and rac-GR24 (Stanga et al., 2013). Considering also the close functional relationship between SMAX1 and MAX2 in the same physiological processes, SMXL proteins are, thus, good candidates to be negative regulators of KAI2-dependent, AtD14-dependent, and MAX2-dependent signaling (Stanga et al., 2013).
More recently, it has been reported that, on hydrolysis of rac-GR24, rice D14 interacts directly with gibberellin signaling protein SLR1 and undergoes conformational change (Nakamura et al., 2013). Another study shows that AtD14 and rac-GR24 together stimulate the direct interaction of MAX2 with brassinosteroid signaling protein BES1, triggering its ubiquitination and destruction (Wang et al., 2013). It remains to be investigated whether the strength of protein interactions within the KAI2/D14-SCFMAX2 complex or the D14/SLR1 complex is dependent on the stereochemistry of the signaling substrate.
Although exogenously applied deoxySLs are potent inhibitors of seedling hypocotyl elongation and repressors of IAA5 and IAA6 expression, it is unlikely that endogenous canonical SLs derived from carlactone are physiologically relevant in the context of Arabidopsis seedling development. Both KAI2 and AtD14 have the capacity to act in the regulation of hypocotyl growth but only when supplied with a suitable exogenous substrate, such as rac-GR24. In such a response, KAI2 and AtD14 effects are additive, implying that they are independent. In the absence of such an exogenous substrate, only KAI2-dependent signaling affects hypocotyl growth, because Atd14 kai2 double-mutant seedlings resemble kai2 seedlings in this respect. Although AtD14 is expressed and has the potential to signal at this stage, carlactone is largely ineffective on seedlings, and all Arabidopsis SL biosynthetic mutants have a normal seedling phenotype (Shen et al., 2012; Flematti et al., 2013; Scaffidi et al., 2013). However, the rice SL-deficient mutants d10, d17, and d27 have elongated mesocotyl when grown in darkness, a phenotype that can be rescued with exogenous rac-GR24 (Hu et al., 2010). This phenotype is also shared with rice d14 mutants. Thus, in at least one monocot system, endogenous SLs seem to have a role in the development of juvenile plants. It is possible that the absence of a role for SLs in Arabidopsis seedlings reflects the anatomical distinction between the hypocotyl in epigeal dicots and the mesocotyl of monocots. In Arabidopsis, the cells that comprise the hypocotyl are established in the embryo, and cell expansion is responsible for postgermination elongation (Gendreau et al., 1997). In contrast, rice mesocotyls elongate, at least in part, by substantive cell division after germination (Hu et al., 2010). SLs seem to affect rice mesocotyl growth through cell division rather than cell expansion (Hu et al., 2010). In pea, SLs affect internode growth by controlling cell number rather than length (de Saint Germain et al., 2013), and SLs promote cell division in the cambium to promote secondary growth (Agusti et al., 2011). Thus, in rice, SLs may have been recruited to regulate cell division as a means to modulate seedling growth, but no such role is apparent in Arabidopsis. Nevertheless, it is still possible that a KAI2-based system for regulating seed germination and seedling development exists in rice, evidence for which is currently lacking. In the absence of genetic evidence, the effects of nonnatural SL enantiomers may be useful for inferring KAI2-based activity in species, such as rice.
Genetic evidence indicates that KAI2, but not AtD14, is required for normal seed germination in Arabidopsis and that KAI2 transcripts are much more abundant in Arabidopsis seeds than AtD14 transcripts (Waters et al., 2012a). Thus, it is not surprising that only ent-5DS and its GR24 counterpart are very active in promoting Arabidopsis seed germination. Furthermore, this observation helps to explain why carlactone and/or carlactone-derived SLs cannot promote Arabidopsis seed germination: the relevant receptor is presumably not present in the seed. However, Striga spp. parasitic weed seeds are extremely sensitive to canonical SLs and also respond positively to carlactone (Cook et al., 1972; Alder et al., 2012). We now know that O. minor responds to both natural and nonnatural SLs, indicating that this species has a more flexible signaling system that is not highly constrained by ligand stereochemistry. Nevertheless, considerable specialization toward SLs might have occurred in O. minor, because debranones are only active at high concentrations and KAR1 (Nelson et al., 2009) is totally inactive.
KAI2 and D14 proteins apparently arose in seed plants by duplication of an ancestral KAI2-like gene, resulting in two conserved clades (Delaux et al., 2012; Waters et al., 2013). Although Arabidopsis uses KAI2 to control seed germination, we do not know if other taxa use KAI2 or D14 for this function. In the event that root-parasitic weed seeds have adopted a KAI2-dependent or D14-dependent mechanism for regulating germination, there are two likely explanations for achieving specificity toward SL substrates. One possibility is that a bona fide D14 ortholog is used, which in the case of O. minor, also confers sensitivity to ent-SLs. Alternatively, a KAI2 ortholog is used, but it has undergone selection to respond to canonical SLs with 2′R-configured D rings and in doing so, has lost (or failed to acquire) the capacity to respond to KARs. Whichever scenario is the case, it seems that the perception system in O. minor is relaxed somewhat relative to S. hermonthica to permit responses to ent-SLs.
A number of open questions remain regarding the diversity and function of SLs. How do strigol and orobanchol both derive from the same carlactone precursor but fall into two different stereochemical forms? Clearly, there are differences in SL profiles between species; rice, for example, produces almost exclusively orobanchol-type SLs, whereas tobacco also produces the strigol class (Xie et al., 2013). What determines the relative abundance of each, and what is the physiological relevance, if any? Our data show that 5DS and GR245DS were generally more active in Arabidopsis than 4DO and GR244DO (Figs. 2 and 3). The basis and biological significance for these observations are unclear, especially considering that Arabidopsis reportedly produces both classes of SL (Kohlen et al., 2011), although other unidentified SLs are also produced by Arabidopsis (Seto et al., 2014). Additional investigations of the identities and effects of each type of SL, in various species, will be necessary to address these issues.
In conclusion, our findings show that the stereochemistry of SLs is crucial to interpreting their activity. The subtle differences in apparent substrate specificities of KAI2 and AtD14 in just a single plant species underline the importance of comparing like with like when drawing specific conclusions about individual proteins and substrates. When examining the functions of SLs in diverse systems, especially when using synthetic SL analogs in the context of agriculturally relevant parasitic weeds and mychorrhizal symbioses, stereochemistry must be considered and verified. Finally, the use of rac-GR24 as a generic SL analog requires caution in the absence of additional genetic resources because of potential specific effects attributable to noncanonical SLs. In the same vein, we urge the use of Atd14 rather than max2 as a specific SL-insensitive mutant.
MATERIALS AND METHODS
Preparation of SL Isomers
Racemic mixtures of GR24 (Mangnus et al., 1992), 2′-epi-GR24 (Mangnus et al., 1992), 5DS (Akiyama et al., 2005), 4DO (Akiyama et al., 2005), and CN-debranone (Fukui et al., 2011) were prepared as previously described. The racemic mixtures were separated into their individual enantiomers using semipreparative HPLC performed on a Hewlett-Packard 1050 system fitted with an Astec Cellulose DMP Chiral HPLC Column (250 mm long, 10-mm internal diameter, 5-μm particle size). Separation was achieved for rac-GR24 using a flow rate of 3 mL min−1 of a 3:1:1 mixture of hexane:methanol:methyl-tert-butyl ether; rac-2′-epi-GR24 using a flow rate of 2 mL min−1 of a 6:1:1 mixture of hexane:methanol:methyl-tert-butyl ether; rac-5DS and rac-4DO using a flow rate of 4 mL min−1 of a 6:1:1 mixture of hexane:methanol:methyl-tert-butyl ether; and rac-CN-debranone using a flow rate of 2.5 mL min−1 of a 3:2 mixture of hexane:isopropanol. All circular dichroism spectra were recorded on a Jasco J-810 spectropolarimeter in acetonitrile with stereochemistry assigned based on previously reported data (Akiyama et al., 2010). Circular dichroism spectra of all purified stereoisomers synthesized for this study are shown in Supplemental Figure S2. Test compounds were added to growth media from 1,000× or 10,000× stock solutions in acetone (1–10 mm); an equivalent volume of acetone was added to untreated controls.
Chiral Liquid Chromatography-Mass Spectrometry
Chiral liquid chromatography-mass spectrometry was conducted with a Waters Alliance e2695 HPLC connected to a Waters LCT Premier XE time-of-flight mass spectrometer with an atmospheric chemical ionization source. Separation was achieved with an Astec Cellulose DMP Chiral HPLC Column (250 mm long, 4.6-mm internal diameter, 5-μm particle size) using a flow rate of 0.5 mL min−1. For 5DS stereoisomers, separation was achieved with a 6:1:1 ratio of hexane:methanol:methyl-tert-butyl ether. For GR24 stereoisomers, separation was achieved with a 14:3:3 ratio of hexane:methanol:methyl-tert-butyl ether. The column was held at 25°C, and sample injection volume was 5 to 20 µL. The mass spectrometer was operated in positive atmospheric chemical ionization source mode. Cone and desolvation gas flows were set to 250 and 650 L h−1, respectively. The corona voltage was set at 3.0 kV, the source temperature was at 100°C, and the desolvation temperature was at 350°C.
Determination of CN-Debranone Structures
The crystal data for R-CN-debranone and S-CN-debranone are summarized in Supplemental Table S1. Structures in which ellipsoids have been drawn at the 50% probability level are depicted in Supplemental Figure S3. Crystallographic data for the structure were collected at 100(2) K on an Oxford Diffraction Gemini diffractometer fitted with Cu Kα radiation. After multiscan absorption corrections and solution by direct methods, the structure was refined against F2 with full-matrix least squares using the program SHELXL-97 (Sheldrick, 2008). All hydrogen atoms were added at calculated positions and refined by use of a riding model with isotropic displacement parameters based on those of the parent atoms. Anisotropic displacement parameters were used throughout for the nonhydrogen atoms. CCDC974002 and CCDC974003 contain supplementary information for R-CN-debranone and S-CN-debranone, respectively, and can be obtained free of charge from Cambridge Crystallographic Data Centre at http://www.ccdc.cam.ac.uk/.
Plant Material
Seeds of max3-9 (Booker et al., 2004) were obtained from the European Arabidopsis Stock Centre (http://arabidopsis.info/). Atd14–1 (in Col-0 background), kai2–2 (in Ler background), and an Ler/Col-0 hybrid Atd14 kai2 double mutant were described previously (Waters et al., 2012a). To generate near-isogenic kai2-2 and Atd14-1 kai2-2 in the Col-0 background, the hybrid Atd14 kai2 double mutant was backcrossed to Col-0, and kai2-2/KAI2 heterozygotes among the F1 were selected by PCR as described (Waters et al., 2012a). The heterozygote was crossed again to Col-0, an F1 heterozygote was selected, and the process was repeated two more times. Finally, a kai2-2/KAI2 heterozygote from the fourth backcross was crossed one more time with Atd14-1, yielding a total of six crosses of the kai2-2 allele into the Col-0 background. The resulting F1 was allowed to self-pollinate. Homozygous wild-type, Atd14-1, kai2-2, and Atd14 kai2 siblings were isolated from the segregating F2 progeny, and F3 seeds were used for experiments. Seeds of Orobanche minor were obtained from Kings Park and Botanic Garden.
Hypocotyl Elongation Assays
Hypocotyl elongation assays were performed as described previously (Waters et al., 2012a).
Arabidopsis Shoot Branching Assays
Growth of Arabidopsis (Arabidopsis thaliana) plants was established under hydroponics as described previously (Waters et al., 2012a). A step-by-step protocol is available online (http://www.bio-protocol.org/wenzhang.aspx?id=264). Plants were grown in Hoagland nutrient solution under light provided by white fluorescent tubes emitting 150 μmol photons m−2 s−1 with a 16-h-light/8-h-dark photoperiod and a 22°C light/16°C dark temperature cycle with constant 60% humidity. Germination was initiated on 0.25× Hoagland nutrient solution, and treatment commenced 18 d later by supplementing 0.5× Hoagland nutrient solution with a 1:10,000 dilution of GR245DS, GR24ent-5DS, GR244DO, GR24ent-4DO, 5DS, ent-5DS, 4DO, ent-4DO, R-CN-debranone, or S-CN-debranone in acetone, yielding a final concentration of 1 μm. Control plants were treated with 100% acetone, yielding a final concentration of 0.01% (v/v). Treatment was continued for 3.5 weeks, fully replacing the supplemented nutrient solution every 3 d. Secondary rosette branches more than 5 mm in length were counted at the completion of the treatment period.
Arabidopsis Germination Assays
Germination profile of Ler seed was taken from a separate pool of seed, with each pool derived from three parents grown simultaneously under identical conditions (16-h day at 22°C/8-h night at 16°C cycle and constant 60% relative humidity, with light provided by fluorescent tubes emitting 100–120 μmol photons m−2 s−1). Primary dormant seeds were harvested promptly after silique ripening and stored at −80°C until use. Seeds were sown on 1% (w/v) phytagel containing 1.5 mm MgCl2 supplemented with 1 μm KAR1, GR245DS, GR24ent-5DS, GR244DO, GR24ent-4DO, 5DS, ent-5DS, 4DO, ent-4DO, R-CN-debranone, or S-CN-debranone or mock treated with 0.1% (v/v) acetone. Petri dishes containing seed were incubated at 28°C under constant light for 1 week, and germination was monitored every 24 h.
O. minor Seed Germination Assays
O. minor seeds were surface sterilized and preconditioned in the dark at 21°C for 2 weeks on filter paper dampened with 2 mL of water. Petri dishes were sealed with micropore tape. A second filter paper dampened with 2 mL of 2 μm solution of rac-GR24, KAR1, 5DS, ent-5DS, 4DO, ent-4DO, R-CN-debranone, or S-CN-debranone or mock treated with 0.1% (v/v) acetone was then placed on top of the preconditioned seeds. Germination was scored for three replicates (each of approximately 100–120 seeds) after an additional 7 d of imbibition in the dark at 21°C.
RNA Isolation and Transcript Analysis
Seedlings were grown and harvested as described previously (Waters and Smith, 2013). Total RNA was isolated using the Spectrum Plant Total RNA Kit (Sigma) following Protocol A as outlined by the manufacturer combined with on-column DNase treatment. Complementary DNA synthesis and quantitative reverse transcription PCR were performed as described previously (Waters and Smith, 2013). Oligonucleotide sequences for quantitative reverse transcription PCR are listed in Supplemental Table S2.
Statistical Analysis
For comparisons of shoot branching numbers and hypocotyl responses within a single genotype, one-way, two-sided ANOVA (Fisher’s lsd t test) was performed in conjunction with Dunnett’s adjustment for multiple pairwise comparisons with a single untreated control. For comparison of transcript abundance, expression ratios were log transformed before ANOVA to compensate for large differences in expression level between samples, and Tukey’s adjustment was used for pairwise comparisons. For germination data, percentages were arcsine transformed before ANOVA. Statistical computation was performed using SAS Enterprise Guide 4.3 (www.sas.com).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Chiral liquid chromatography-mass spectrometry separation of four deoxySL and GR24 stereoisomers.
Supplemental Figure S2. Circular dichroism spectra of purified stereoisomers.
Supplemental Figure S3. Crystal structures of CN-debranone stereoisomers.
Supplemental Table S1. Crystal data and structure refinement for CN-debranone stereoisomers.
Supplemental Table S2. Oligonucleotides used for quantitative reverse transcription PCR.
Supplementary Material
Acknowledgments
We thank David Nelson (University of Georgia) for critical comments on the manuscript.
Glossary
- CCD
carotenoid cleavage dioxygenase
- Col-0
Columbia-0
- deoxySL
deoxystrigolactone
- 4DO
4-deoxyorobanchol
- 5DS
5-deoxystrigol
- KAR
karrikin
- Ler
ecotype Landsberg erecta of Arabidopsis
- SL
strigolactone
- SMAX
SUPPRESSOR OF MAX2
Footnotes
This work was supported by the Australian Research Council (grant nos. LP0882775, DP1096717, and FT110100304).
The online version of this article contains Web-only data.
References
- Agusti J, Herold S, Schwarz M, Sanchez P, Ljung K, Dun EA, Brewer PB, Beveridge CA, Sieberer T, Sehr EM, et al. (2011) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci USA 108: 20242–20247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Akiyama K, Ogasawara S, Ito S, Hayashi H. (2010) Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol 51: 1104–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S. (2012) The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335: 1348–1351 [DOI] [PubMed] [Google Scholar]
- Ali-Rachedi S, Bouinot D, Wagner MH, Bonnet M, Sotta B, Grappin P, Jullien M. (2004) Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 219: 479–488 [DOI] [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 14: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O. (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8: 443–449 [DOI] [PubMed] [Google Scholar]
- Brewer PB, Koltai H, Beveridge CA. (2013) Diverse roles of strigolactones in plant development. Mol Plant 6: 18–28 [DOI] [PubMed] [Google Scholar]
- Bythell-Douglas R, Waters MT, Scaffidi A, Flematti GR, Smith SM, Bond CS. (2013) The structure of the karrikin-insensitive protein (KAI2) in Arabidopsis thaliana. PLoS ONE 8: e54758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Coggon P, Mcphail AT, Wall ME, Whichard LP, Egley GH, Luhan PA. (1972) Germination stimulants: II. The structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea Lour.). J Am Chem Soc 94: 6198–6199 [Google Scholar]
- Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. (1966) Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154: 1189–1190 [DOI] [PubMed] [Google Scholar]
- de Saint Germain A, Ligerot Y, Dun EA, Pillot JP, Ross JJ, Beveridge CA, Rameau C. (2013) Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiol 163: 1012–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaux PM, Xie X, Timme RE, Puech-Pages V, Dunand C, Lecompte E, Delwiche CF, Yoneyama K, Bécard G, Séjalon-Delmas N. (2012) Origin of strigolactones in the green lineage. New Phytol 195: 857–871 [DOI] [PubMed] [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. (2004) A compound from smoke that promotes seed germination. Science 305: 977. [DOI] [PubMed] [Google Scholar]
- Flematti GR, Waters MT, Scaffidi A, Merritt DJ, Ghisalberti EL, Dixon KW, Smith SM. (2013) Karrikin and cyanohydrin smoke signals provide clues to new endogenous plant signaling compounds. Mol Plant 6: 29–37 [DOI] [PubMed] [Google Scholar]
- Fukui K, Ito S, Ueno K, Yamaguchi S, Kyozuka J, Asami T. (2011) New branching inhibitors and their potential as strigolactone mimics in rice. Bioorg Med Chem Lett 21: 4905–4908 [DOI] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H. (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Zheng Z, La Clair JJ, Chory J, Noel JP. (2013) Smoke-derived karrikin perception by the α/β-hydrolase KAI2 from Arabidopsis. Proc Natl Acad Sci USA 110: 8284–8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamiaux C, Drummond RSM, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC. (2012) DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol 22: 2032–2036 [DOI] [PubMed] [Google Scholar]
- Hu Z, Yan H, Yang J, Yamaguchi S, Maekawa M, Takamure I, Tsutsumi N, Kyozuka J, Nakazono M. (2010) Strigolactones negatively regulate mesocotyl elongation in rice during germination and growth in darkness. Plant Cell Physiol 51: 1136–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y, et al. (2013) DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504: 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagiyama M, Hirano Y, Mori T, Kim SY, Kyozuka J, Seto Y, Yamaguchi S, Hakoshima T. (2013) Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells 18: 147–160 [DOI] [PubMed] [Google Scholar]
- Kohlen W, Charnikhova T, Liu Q, Bours R, Domagalska MA, Beguerie S, Verstappen F, Leyser O, Bouwmeester H, Ruyter-Spira C. (2011) Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol 155: 974–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangnus EM, Dommerholt FJ, Dejong RLP, Zwanenburg B. (1992) Improved synthesis of strigol analog GR24 and evaluation of the biological-activity of its diastereomers. J Agric Food Chem 40: 1230–1235 [Google Scholar]
- Minakuchi K, Kameoka H, Yasuno N, Umehara M, Luo L, Kobayashi K, Hanada A, Ueno K, Asami T, Yamaguchi S, et al. (2010) FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol 51: 1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Xue YL, Miyakawa T, Hou F, Qin HM, Fukui K, Shi X, Ito E, Ito S, Park SH, et al. (2013) Molecular mechanism of strigolactone perception by DWARF14. Nat Commun 4: 2613. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Flematti GR, Ghisalberti EL, Dixon KW, Smith SM. (2012) Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Annu Rev Plant Biol 63: 107–130 [DOI] [PubMed] [Google Scholar]
- Nelson DC, Flematti GR, Riseborough JA, Ghisalberti EL, Dixon KW, Smith SM. (2010) Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc Natl Acad Sci USA 107: 7095–7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DC, Riseborough JA, Flematti GR, Stevens J, Ghisalberti EL, Dixon KW, Smith SM. (2009) Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol 149: 863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DC, Scaffidi A, Dun EA, Waters MT, Flematti GR, Dixon KW, Beveridge CA, Ghisalberti EL, Smith SM. (2011) F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc Natl Acad Sci USA 108: 8897–8902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S, Nakashima H, Mizutani M, Takikawa H, Sugimoto Y. (2013) Structural requirements of strigolactones for germination induction and inhibition of Striga gesnerioides seeds. Plant Cell Rep 32: 829–838 [DOI] [PubMed] [Google Scholar]
- Scaffidi A, Waters MT, Bond CS, Dixon KW, Smith SM, Ghisalberti EL, Flematti GR. (2012) Exploring the molecular mechanism of karrikins and strigolactones. Bioorg Med Chem Lett 22: 3743–3746 [DOI] [PubMed] [Google Scholar]
- Scaffidi A, Waters MT, Ghisalberti EL, Dixon KW, Flematti GR, Smith SM. (2013) Carlactone-independent seedling morphogenesis in Arabidopsis. Plant J 76: 1–9 [DOI] [PubMed] [Google Scholar]
- Seto Y, Sado A, Asami K, Hanada A, Umehara M, Akiyama K, Yamaguchi S. (2014) Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc Natl Acad Sci USA 111: 1640–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick GM. (2008) A short history of SHELX. Acta Crystallogr A 64: 112–122 [DOI] [PubMed] [Google Scholar]
- Shen H, Zhu L, Bu QY, Huq E. (2012) MAX2 affects multiple hormones to promote photomorphogenesis. Mol Plant 5: 750–762 [DOI] [PubMed] [Google Scholar]
- Stanga JP, Smith SM, Briggs WR, Nelson DC. (2013) SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol 163: 318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Wigchert SCM, Thuring JWJF, Zwanenburg B. (1998) Synthesis of all eight stereoisomers of the germination stimulant sorgolactone. J Org Chem 63: 1259–1267 [Google Scholar]
- Thuring JWJF, Heinsman NWJT, Jacobs RWAWM, Nefkens GHL, Zwanenburg B. (1997) Asymmetric synthesis of all stereoisomers of demethylsorgolactone. Dependence of the stimulatory activity of Striga hermonthica and Orobanche crenata seed germination on the absolute configuration. J Agric Food Chem 45: 507–513 [Google Scholar]
- Ueno K, Nomura S, Muranaka S, Mizutani M, Takikawa H, Sugimoto Y. (2011) Ent-2′-epi-Orobanchol and its acetate, as germination stimulants for Striga gesnerioides seeds isolated from cowpea and red clover. J Agric Food Chem 59: 10485–10490 [DOI] [PubMed] [Google Scholar]
- Vurro M, Yoneyama K. (2012) Strigolactones—intriguing biologically active compounds: perspectives for deciphering their biological role and for proposing practical application. Pest Manag Sci 68: 664–668 [DOI] [PubMed] [Google Scholar]
- Waldie T, McCulloch H, Leyser O. (February 25, 2014) Strigolactones and the control of plant development: lessons from shoot branching. Plant J [DOI] [PubMed] [Google Scholar]
- Wang Y, Sun S, Zhu W, Jia K, Yang H, Wang X. (2013) Strigolactone/MAX2-induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Dev Cell 27: 681–688 [DOI] [PubMed] [Google Scholar]
- Waters MT, Nelson DC, Scaffidi A, Flematti GR, Sun YKM, Dixon KW, Smith SM. (2012a) Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139: 1285–1295 [DOI] [PubMed] [Google Scholar]
- Waters MT, Scaffidi A, Flematti GR, Smith SM. (2012b) Karrikins force a rethink of strigolactone mode of action. Plant Signal Behav 7: 969–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Scaffidi A, Flematti GR, Smith SM. (2013) The origins and mechanisms of karrikin signalling. Curr Opin Plant Biol 16: 667–673 [DOI] [PubMed] [Google Scholar]
- Waters MT, Scaffidi A, Sun YK, Flematti GR, Smith SM. (January 16, 2014) The karrikin response system of Arabidopsis. Plant J http://dx.doi.org/10.1111/tpj.12430 [DOI] [PubMed] [Google Scholar]
- Waters MT, Smith SM. (2013) KAI2- and MAX2-mediated responses to karrikins and strigolactones are largely independent of HY5 in Arabidopsis seedlings. Mol Plant 6: 63–75 [DOI] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Kisugi T, Uchida K, Ito S, Akiyama K, Hayashi H, Yokota T, Nomura T, Yoneyama K. (2013) Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol Plant 6: 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LH, Zhou XE, Wu ZS, Yi W, Xu Y, Li S, Xu TH, Liu Y, Chen RZ, Kovach A, et al. (2013) Crystal structures of two phytohormone signal-transducing α/β hydrolases: karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res 23: 436–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, et al. (2013) D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 504: 406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanenburg B, Pospísil T. (2013) Structure and activity of strigolactones: new plant hormones with a rich future. Mol Plant 6: 38–62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.