Shade light perceived in cotyledons induces de novo auxin biosynthesis and transport to hypocotyl cells leading to their elongation.
Abstract
Plant architecture is optimized for the local light environment. In response to foliar shade or neighbor proximity (low red to far-red light), some plant species exhibit shade-avoiding phenotypes, including increased stem and hypocotyl growth, which increases the likelihood of outgrowing competitor plants. If shade persists, early flowering and the reallocation of growth resources to stem elongation ultimately affect the yield of harvestable tissues in crop species. Previous studies have shown that hypocotyl growth in low red to far-red shade is largely dependent on the photoreceptor phytochrome B and the phytohormone auxin. However, where shade is perceived in the plant and how auxin regulates growth spatially are less well understood. Using the oilseed and vegetable crop species Brassica rapa, we show that the perception of low red to far-red shade by the cotyledons triggers hypocotyl cell elongation and auxin target gene expression. Furthermore, we find that following shade perception, elevated auxin levels occur in a basipetal gradient away from the cotyledons and that this is coincident with a gradient of auxin target gene induction. These results show that cotyledon-generated auxin regulates hypocotyl elongation. In addition, we find in mature B. rapa plants that simulated shade does not affect seed oil composition but may affect seed yield. This suggests that in field settings where mutual shading between plants may occur, a balance between plant density and seed yield per plant needs to be achieved for maximum oil yield, while oil composition might remain constant.
In close proximity to neighboring plants, shade-intolerant plants exhibit a suite of phenotypes collectively referred to as the shade avoidance syndrome (SAS). The SAS includes hypocotyl and stem elongation, reduced root and leaf growth, and reduced defenses against herbivores and pathogens. This adaptive response can be viewed as a competitive strategy to allocate resources toward growth that alters plant architecture to enable better light harvesting. If shade persists, shade-intolerant plants typically transition to early flowering with reduced seed set, ensuring genetic survival, albeit at the cost of fecundity (Casal, 2013).
Shade from neighboring plants is perceived as a reduction in the ratio of red (R) to far-red (FR) light (R:FR). Neighboring plants preferentially absorb R light for photosynthesis, while FR light is reflected (Casal, 2013). The major photosensor of low R:FR shade is phytochrome B (phyB; Reed et al., 1993), although other phytochromes play additional roles (Robson et al., 1993; Franklin et al., 2003). phyB photoconverts between two states: an inactive, cytosolic R light-absorbing form (Pr; λmax = 660 nm) and a nuclear FR light-absorbing form (Pfr; λmax = 730 nm). Under shade conditions (R:FR < 1), Pr is the major form, while in sunlight (R:FR ∼ 1.1), Pfr is the predominant form.
In Arabidopsis (Arabidopsis thaliana), the growth hormone auxin plays a major role in driving hypocotyl elongation in low R:FR shade. Auxin levels are rapidly elevated by shade, and plants carrying mutations in the auxin biosynthetic pathway have reduced hypocotyl growth (Tao et al., 2008). Auxin synthesis in shade is regulated by the interaction of phyB with specific members of the phytochrome-interacting factor (PIF) family of basic helix-loop-helix transcription factors. Under high R:FR light, nuclear Pfr interacts directly with PIFs to induce PIF phosphorylation. This results either in PIF destruction via the ubiquitin-proteasome system (Al-Sady et al., 2006; Shen et al., 2007, 2008; Lorrain et al., 2008) or inhibition of PIF binding to its target gene promoters (Li et al., 2012). In low R:FR shade, the Pr form of phyB predominates and nonphosphorylated PIFs accumulate, resulting in the up-regulation of target gene expression. PIF targets include genes encoding the YUCCA family of flavin monooxygenases that catalyze the rate-limiting step in auxin biosynthesis and other growth-promoting genes (Mashiguchi et al., 2011; Won et al., 2011; Hornitschek et al., 2012; Li et al., 2012).
Previous studies have established the importance of the leaves/cotyledons as sites of phytochrome-mediated perception of R and FR signals that modulate stem growth. For example, shielding the cotyledons of cucumber (Cucumis sativus) seedlings from light abrogates R light-mediated hypocotyl growth inhibition, whereas shielding the hypocotyl has no effect (Black and Shuttleworth, 1974). Other studies, however, have suggested that the opposite may also be the case: that the stem itself is the major site of R and FR light perception. For example, debladed Vigna sinensis epicotyls show growth responses to end-of-day FR light, suggesting that the epicotyl itself is the primary light-sensing organ (García-Martínez et al., 1987). These mechanisms need not be mutually exclusive, and the perception of R and FR light in both leaves/cotyledons and hypocotyls/stems may play additive roles in directing stem growth. In sunflower (Helianthus annuus), FR light control of internode elongation requires irradiance almost equally on the internode as well as other aerial tissues of the plant (Garrison and Briggs, 1975). Similarly, in light-grown Sinapis alba, both internodes and other tissues perceive the addition of supplemental FR light and contribute to internode growth, but with different kinetics (Morgan et al., 1980; Child and Smith, 1987; Casal and Smith, 1988a). By contrast, end-of-day FR treatments induced internode growth in S. alba only when applied to the leaves and cotyledons (Casal and Smith, 1988a, 1988b). This suggests that a complicated interaction between signals from the leaf and stem may be taking place and might be dependent on the specific light environment.
Experiments in Arabidopsis indicate that for this species, the cotyledons are the major sites of R and FR perception and auxin synthesis regulating hypocotyl growth in response to changes in the R:FR ratio. Enhancer trap lines that express phyB-GFP specifically in the cotyledons of Arabidopsis seedlings can complement the elongated hypocotyl phenotype of phyB-deficient mutants (Endo et al., 2005), while spotlight FR irradiation localized to the cotyledons induces the expression of an auxin-dependent gene in the hypocotyl (Tanaka et al., 2002). In addition, the auxin biosynthesis gene SHADE AVOIDANCE3/TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (SAV3/TAA1), which is required for growth responses to shade, is predominantly expressed in the margins and vasculature of cotyledons and emerging leaves (Tao et al., 2008). Treatment of Arabidopsis seedlings with the auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) or mutations in the PIN-FORMED3 auxin efflux transporter both result in reduced hypocotyl growth in shade, suggesting that auxin transport is necessary for responses to shade (Keuskamp et al., 2010). However, it is unknown if all shade-regulated genes in the Arabidopsis hypocotyl require cotyledon-derived signals, and an understanding of how low R:FR shade modifies gene expression spatially in the plant is lacking.
Mutual shading within densely sown, shade-intolerant crop species might affect crop growth and yield (Ballare et al., 1997). Elongated plants are more susceptible to physical damage (Morinaka et al., 2006), and shade responses may diminish the allocation of photosynthate and other growth resources to harvested tissues (Ballare et al., 1997). Brassica rapa and other species of the Brassica genus are important vegetable and oilseed crops, which in low R:FR light display phyB-dependent SAS phenotypes (Devlin et al., 1992, 1997). The recent sequencing of the B. rapa genome (Wang et al., 2011), its close relationship with the well-studied model plant Arabidopsis, its economic value, a large seedling size amenable to spatial studies, and the availability of mutants and rapid-cycling varieties (Williams and Hill, 1986; Stephenson et al., 2010) make this an attractive model system for the study of shade avoidance.
Here, using B. rapa seedlings, we show directly that low R:FR shade perceived by cotyledons regulates hypocotyl cell elongation and that cotyledon-generated auxin drives a gradient of auxin-dependent gene induction down the hypocotyl in shade. However, not all shade-induced genes in the hypocotyl are auxin or cotyledon dependent. Interestingly, studies of soybean (Glycine max) grown under low blue light suggested that spectral quality may alter the oil composition of oilseed crops (Britz and Cavins, 1993). However, we show for B. rapa grown under simulated low R:FR shade that this is not the case and that oil composition might be constant in field settings, even when plant density is high.
RESULTS
Low R:FR Shade Induces Phenotypic and Transcriptional Changes in B. rapa
Previous work has shown that low R:FR light can induce shade avoidance phenotypes in the Wisconsin Fast Plants (WFP) variety of rapid-cycling B. rapa (Devlin et al., 1992, 1997). We find that this is a general attribute of B. rapa varieties: seedlings of an inbred line of B. rapa subsp. trilocularis (Yellow Sarson), R-o-18, also exhibit typical shade avoidance phenotypes when grown in simulated shade (Fig. 1A; here, simulated shade approximates neighbor proximity; see “Materials and Methods” and Tao et al., 2008). R-o-18 was the primary B. rapa strain employed in this study. It is a Yellow Sarson-type oilseed crop grown in Pakistan that is self-fertile with high fecundity and is similar to other genus Brassica oilseed crops (Rana et al., 2004; Stephenson et al., 2010). As such, it is likely that findings derived using R-o-18 will be generally applicable to oilseed crop varieties.
Figure 1.
B. rapa seedlings display auxin-dependent shade avoidance phenotypes. A, Representative image of 7-d-old B. rapa R-o-18 seedlings grown in constant W light (Wc) or 4 d of constant W light followed by 3 d of low R:FR light (↓R:FR). Bar = 1 cm. B to D, Hypocotyl length (B), cotyledon area (C), and lateral root formation (D) of 7-d-old seedlings treated as in A. E, Hypocotyl length of 7-d-old seedlings grown for 4 d in constant W light then 3 d in liquid culture in constant W light or low R:FR light in the presence of dimethyl sulfoxide (DMSO; vehicle), 10 μm auxinole, or 10 μm NPA. Data show means ± se. Student’s t test: **P < 0.005, *P < 0.05. [See online article for color version of this figure.]
We find that, in response to simulated shade, R-o-18 B. rapa seedlings exhibited increased hypocotyl growth, reduced cotyledon expansion, and fewer lateral roots (Fig. 1, B–D). Hypocotyl growth was reduced in the presence of the auxin transport inhibitor NPA and in the presence of the TIR1 auxin receptor antagonist auxinole (Hayashi et al., 2008, 2012; Fig. 1E), suggesting that B. rapa hypocotyl growth in response to shade requires auxin transport and signaling.
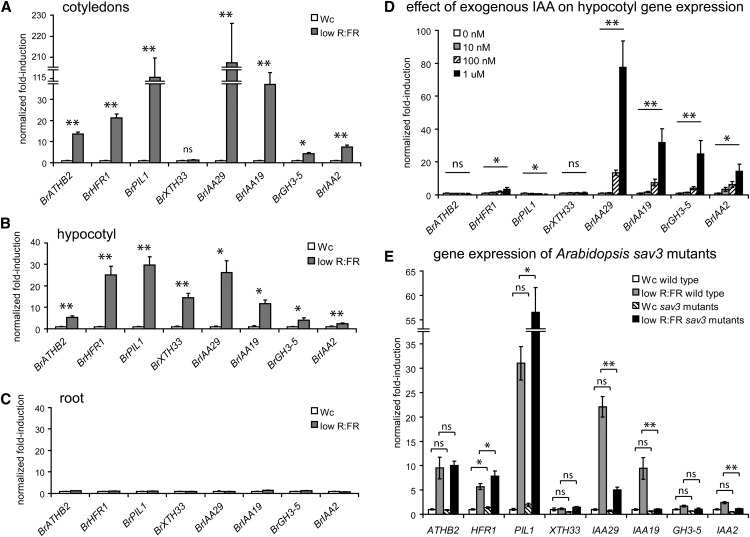
Transcriptional analysis of Arabidopsis seedlings shifted to low R:FR conditions has identified numerous genes whose expression is induced by shade (Devlin et al., 2003). We analyzed the B. rapa whole-genome sequence (accession Chiifu-401-42; Wang et al., 2011) using a combination of methods based on homology and syntenic relationships to identify presumptively orthologous loci that might also show increased expression in B. rapa. Indeed, we found by using quantitative PCR (qPCR) assays (Figs. 2A and 3, A and B; data not shown) that B. rapa orthologs of the Arabidopsis genes ARABIDOPSIS THALIANA HOMEOBOX PROTEIN2 (ATHB2), LONG-HYPOCOTYL IN FAR-RED1 (HFR1), and PHYTOCHROME INTERACTING FACTOR3-LIKE1 (PIL1; hereafter termed BrATHB2 [BraA.ATHB2.a], BrHFR1 [BraA.HFR1.a], and BrPIL1 [BraA.PIL1.a]) are up-regulated following 2 h of exposure to low R:FR shade. In Arabidopsis, the promoters of these genes are directly bound by PIF transcription factors (Hornitschek et al., 2012). In addition, we identified five other shade-induced genes representing possible orthologs to other known shade targets: the cell wall-modifying enzyme BrXTH33 (BraA.XTH33.a [related to Arabidopsis gene XYLOGLUCAN XYLOGLUCOSYL TRANSFERASE33]) and the likely auxin-dependent genes BrIAA29 (BraA.IAA29.a [for INDOLE-3-ACETIC ACID INDUCIBLE29]), BrIAA19 (Br.IAA19.a), the indole-3-acetic acid (IAA)-amido synthase BrGH3-5 (BraA.GH3-5.a), and BrIAA2 (BraA.IAA2.a). Our results suggest that many aspects of the transcriptional response to shade are conserved between Arabidopsis and B. rapa.
Figure 2.
qPCR expression analysis of low R:FR shade-induced marker genes. Four-day-old W light-grown wild-type (A; FPsc strain) and phyB mutant (B; ein194) seedlings were treated for 2 h in constant W light (Wc) or 2 h in low R:FR light. For each gene, expression is shown relative to the 2-h constant W light treatment ± se. Student’s t test: **P < 0.005, *P < 0.05, ns = not significant.
Figure 3.
Shade-induced genes are expressed in distinct organs and can be auxin dependent or independent. A to C, qPCR expression analysis of shade-induced marker genes in the cotyledons (A), hypocotyl (B), and roots (C) of 7-d-old W light-grown B. rapa R-o-18 seedlings treated for 2 h with constant W light (Wc) or low R:FR light. For each gene, expression is shown relative to the 2-h constant W light treatment. D, Gene expression in the hypocotyl of 7-d-old R-o-18 seedlings treated for 150 min in liquid culture with the indicated concentrations of IAA. Normalized fold induction for each gene is shown relative to the vehicle control (0 nm; ethanol). E, Expression of orthologous genes in whole seedlings of the wild type (Columbia-0) and sav3-1 mutants of Arabidopsis. Seven-day-old W light-grown seedlings were treated for 1 h with constant W light or low R:FR light. Expression is shown relative to the constant W light-treated wild-type plants ± se. Asterisks indicate significant differences for Student’s t test (A–C and E) and one-way ANOVA (D): **P < 0.005, *P < 0.05, ns = not significant. In C, all comparisons are not significant. In E, sav3 mutants are compared with wild-type seedlings grown in equivalent conditions.
B. rapa Responses to Shade Are phyB Dependent
The elongated internode (ein) mutants of the rapid-cycling WFP variety of B. rapa lack detectable expression of phyB protein and display constitutive shade-avoiding hypocotyl growth in white (W) light (Devlin et al., 1992; Supplemental Fig. S1, A and B). An additional B. rapa phyB mutant, ein194, was recovered in an ethyl methanesulfonate mutant screen of M2 families derived from the Fast Plants, self-compatible (FPsc) variety. FPsc is a highly inbred and rapid-cycling analog of the self-incompatible WFP variety (wild-type FPsc and mutant ein194 seeds were provided by Scott Woody and Rick Amasino). While the rapid growth of FPsc is unlike B. rapa oilseed crop varieties, we have employed the strain when it was desirable to compare wild-type seedlings with phyB mutants. We also took advantage of the rapid growth of FPsc and its small stature when measuring adult traits of plants grown in simulated shade chambers (see below). Like the R-o-18 variety, FPsc seedlings exhibited a typical hypocotyl SAS response to low R:FR light; however, due to greater variation among the seedlings, the response was less (Supplemental Fig. S2).
We sequenced the PHYB alleles of wild-type and ein194 FPsc plants and verified that a premature stop mutation exists in the ein194 background (Supplemental Fig. S3; see below). The predicted B. rapa FPsc phyB protein (Bra022192) is more than 90% identical to that encoded by Arabidopsis locus AT2G18790, with most divergence in the N-terminal region (approximately 60% identity over the first 55 amino acids). Previous work has shown that deleting amino acids 1 to 57 has little effect on the function of Arabidopsis phyB, suggesting that this less-conserved region is nonessential (Wagner et al., 1996). We find that ein194 seedlings have reduced induction of shade-regulated genes in low R:FR light (Fig. 2B). For many of these genes, this may be because ein194 mutants have a higher basal level of expression (Supplemental Fig S4), an observation reported previously for some shade-induced transcripts in Arabidopsis phyB mutants (Devlin et al., 2003). However, we do not expect all shade-induced genes to have higher basal expression in phyB mutants, as compensatory mechanisms might occur when plants lack phyB signaling for their entire lifespan.
In addition, we find that overexpression of the highly conserved Arabidopsis PHYB sequence (AtPHYB) in transgenic B. rapa FPsc plants resulted in a stereotypical PHYB overexpression phenotype (Wagner et al., 1996), with short hypocotyls in W light (Supplemental Fig. S5; a single line was generated, likely with two insertions of the transgene). These results confirm that phyB is a major photoreceptor mediating low R:FR shade responses in B. rapa.
Transcriptional Responses to Shade Are Both Auxin Dependent and Independent as Well as Spatially Distinct
To examine spatial patterns of changes in gene expression induced by shade, we performed qPCR analysis of mRNAs isolated separately from cotyledon, hypocotyl, and root tissues after 2 h of low R:FR shade treatment of R-o-18 seedlings. Cotyledons and hypocotyls showed a largely similar pattern of gene induction in response to shade (Fig. 3, A and B). One notable exception was BrXTH33, a cell wall-modifying enzyme with a role in cell expansion (Sasidharan et al., 2010) that was uniquely up-regulated in the hypocotyl cell types treated under these simulated shade conditions. This observation may explain why little induction of BrXTH33 was observed in whole-plant FPsc gene expression assays (Fig. 2A) when hypocotyl RNA was diluted among the RNA of other organs. Alternatively, the low induction of BrXTH33 in FPsc seedlings may be due to a lower response of this variety to shade.
Perhaps surprisingly, none of the marker genes we examined were induced in root tissue (Fig. 3C), even though shade affects root growth and the seedlings were grown on agar and thus all organs were equally exposed to light. This lack of response was not due to a delay in induction kinetics, since even after 24 h of low R:FR treatment no change in expression of these marker genes was observed (Supplemental Fig. S6). Neither was the lack of induction in the root due to an absence of gene expression compared with the other organs. While some of the genes were expressed only at low levels in the root compared with the hypocotyl in W light-grown seedlings (Supplemental Fig. S7; e.g. BrATHB2 and BrHFR1), other shade-regulated genes were expressed at similar levels but failed to be induced. These findings suggest that transcriptional responses to shade are distinct within different organs and tissue types of the plant.
To determine which of the shade-regulated loci in B. rapa might be dependent on auxin signaling, we treated whole seedlings with exogenous IAA, the main auxin found in plants, and examined gene expression in hypocotyl tissues. We found that of the eight marker genes, BrIAA29, BrIAA19, BrGH3-5, and BrIAA2 were significantly up-regulated in a dose-dependent manner by IAA, while no or only marginal effects were observed on the other genes tested (Fig. 3D). Unlike IAA treatment, exogenous brassinosteroid or GA did not cause changes in expression, suggesting that auxin is primarily responsible for the responses observed (Supplemental Fig. S8). To verify that up-regulation of these genes in response to shade is auxin dependent, we measured transcript levels of the related sequences in Arabidopsis sav3-1 mutants, which are defective in auxin synthesis (Tao et al., 2008). For three of the four genes whose up-regulation in shade was shown to be auxin dependent in B. rapa (IAA29, IAA19, and IAA2), we observed a loss of induction in the sav3 mutant background in response to shade (Fig. 3E). The GH3-5 marker gene followed a similar trend, although the change was not significant (Student’s t test). By contrast, the induction of expression at loci presumed to be auxin independent (ATHB2, HFR1, PIL1, and XTH33) was essentially unaffected in the sav3-1 mutant background; in fact, shade-mediated induction of PIL1 and HFR1 expression exceeded that observed in wild-type seedlings. As such, we propose that shade-induced changes in transcription in B. rapa in response to perturbation of the R:FR light environment proceed through both auxin-dependent and auxin-independent pathways.
Cotyledon Perception of R and FR Light Regulates Hypocotyl Growth and Auxin Gene Expression
Deetiolated Arabidopsis seedlings carrying a PHYBpro::GUS transcriptional reporter transgene display GUS expression in cotyledons, hypocotyls, and roots (Somers and Quail, 1995). In our hands, GUS expression in the hypocotyl was strongest in the vasculature, while in the root the strongest staining was observed at the root tip (Supplemental Fig. S1C). Similarly, we found that phyB protein was expressed in all three organs of W light-grown B. rapa seedlings (Supplemental Fig. S1D). Thus, all three organs might have the potential to affect responses to changes in R:FR light ratios. However, as demonstrated above, root cells have no change in transcription for the shade-induced genes that we examined here, suggesting that the root may have limited responses to light.
To assess the role of cotyledon and hypocotyl cell types in mediating the B. rapa response to shade, we removed the cotyledons and petioles of B. rapa R-o-18 seedlings immediately prior to simulated shade treatment (Fig. 4A). To compensate for the loss of photosynthate derived from the cotyledons, the plants were grown on medium supplemented with 1% (w/v) Suc. Four-day-old operated seedlings grown for an additional 2 d in shade showed no measurable change in hypocotyl length compared with operated seedlings maintained in W light (Fig. 4B). This is unlikely due to a general lack of growth in the absence of the cotyledons, as the hypocotyls were marginally longer than they were at the time of operation (Fig. 4B). After an additional 1 d of growth, shade-treated operated seedlings did exhibit a small but significant increase in hypocotyl length over W light-treated controls; however, the emergence of developing true leaves at this later time point confounds the interpretation of these results (data not shown).
Figure 4.
The cotyledons regulate hypocotyl growth and gene expression. A, Experimental setup of B to D. The cotyledons (C) of W light-grown R-o-18 B. rapa seedlings were removed immediately prior to light treatment. Light treatments consisted of continued constant W light (Wc) or simulated shade (low R:FR). The cotyledons of operated seedlings were removed at the base of the petioles, while the hypocotyl (H) and root (R) were left intact. B, Hypocotyl lengths of 6-d-old seedlings grown for 4 d in constant W light and then an additional 2 d in constant W light or low R:FR light. In operated seedlings, the cotyledons of 4-d-old seedlings were removed as described in A. Data show means ± se. C and D, Gene expression in the hypocotyls of 7-d-old W light-grown R-o-18 seedlings treated with either 2 h in constant W light or low R:FR light, in intact seedlings (C) or seedlings where the cotyledons were removed immediately prior to the 2-h light treatment (D). For each gene, expression is shown relative to the 2-h constant W light treatment, as measured by qPCR analysis, ± se. Student’s t test in B to D: **P < 0.005, *P < 0.05, ns = not significant.
The removal of cotyledons might eliminate not only signals induced by shade but also other growth signals generated by the organ. Therefore, to assess the role of the cotyledons in shade perception in intact plants, we grew B. rapa R-o-18 seedlings in a split-light chamber. The cotyledons and upper petioles were exposed to either W light (high R:FR) or simulated shade (low R:FR; W light supplemented with an FR light-emitting diode bulb [LumiGrow ECC-FR]). The hypocotyl and root were exposed separately to W light or shade (Fig. 5A; Supplemental Fig. S9, A–F). We found that when the hypocotyl alone experienced simulated shade, there was only a minor, nonsignificant increase in hypocotyl length (Fig. 5B; Student’s t test). This was not due to an inability of the chamber conditions in which the hypocotyl was exposed to induce growth, as hypocotyls elongated when the cotyledons were exposed to the same chamber conditions (Supplemental Fig. S9G). By contrast, exposure of the cotyledons to shade induced hypocotyl growth, even under circumstances in which the hypocotyl simultaneously experienced high R:FR light (Fig. 5B). Furthermore, the response observed with cotyledon-only shade-treated seedlings was not statistically different from that seen when both cotyledons and the hypocotyl were simultaneously exposed to a low R:FR environment (Student’s t test; however, P < 0.05 in a two-way ANOVA when light treatment of the hypocotyl was compared against all cotyledon treatments). Together, our results show that in B. rapa, the cotyledons (and/or upper petioles) are the primary sites for the perception of low R:FR light and, thus, the source of a signal that drives hypocotyl elongation.
Figure 5.
The cotyledons regulate hypocotyl growth and gene expression in intact seedlings. A, Experimental setup of B to D. W light-grown R-o-18 B. rapa seedlings were positioned in a split-light chamber. The cotyledons (C) or the hypocotyl (H) and root (R) were exposed to either high R:FR light (W light; R:FR > 15) or low R:FR light (W light supplemented with FR light; R:FR < 0.8). The cotyledons were fixed in position, while the roots were suspended in liquid growth medium to allow vertical growth of the hypocotyl down. For a complete description of the light conditions used, see Supplemental Figure S9. B, Hypocotyl lengths of R-o-18 seedlings grown as described in A. Four-day-old seedlings were transferred to the split-light chamber and grown for an additional 3 d. Data show means ± se. C and D, Gene expression in the cotyledons (C) and hypocotyls (D) of seedlings grown in the split-light chamber. W light-grown plants were transferred to the split-light chamber at 4 d and allowed to equilibrate to W light conditions (high R:FR). At 6.5 d, the ratio of R:FR light was adjusted as described in B, and gene expression levels were measured by qPCR 12 h later. The light treatments of each column (white, gray, striped, and black) are as indicated in B. For each gene, expression is shown relative to seedlings where both the cotyledons and hypocotyl plus root were exposed to high R:FR light ± se. Asterisks indicate significant differences for Student’s t test (B) and one-way ANOVA (C and D). **P < 0.005, *P < 0.05, ns = not significant.
To test if the signal from the cotyledons driving hypocotyl growth might be auxin related, we removed the cotyledons and petioles of B. rapa seedlings immediately prior to a 2-h low R:FR shade treatment and tested the effect on gene expression in the hypocotyl (the region of the hypocotyl immediately above the root-hypocotyl junction and below the petioles). We found that the auxin-dependent shade response genes were not induced in dissected seedlings under low R:FR conditions as compared with dissected seedlings maintained in W light (Fig. 4, C and D). By contrast, most presumptively auxin-independent loci were still up-regulated to approximately wild-type levels. Intriguingly, B. rapa genes whose expression was unaffected by removal of the cotyledons (BrATHB2, BrHFR1, and BrPIL1) are thought to be direct targets of the PIF gene family in Arabidopsis (Hornitschek et al., 2012). Only the auxin-independent gene BrXTH33, which might be directly involved in elongation growth (Sasidharan et al., 2010), also failed to be induced in the absence of the cotyledons.
To verify that auxin-dependent shade-regulated genes required signals from the cotyledons in intact plants, we measured gene expression changes in the cotyledons and hypocotyls of seedlings grown in our split-light chamber setup. In the cotyledons, we observed that some of the genes representing both auxin-dependent and independent target genes were induced when the cotyledons were exposed to simulated shade, irrespective of the light environment in which the hypocotyl was placed (Fig. 5C). By contrast, in the hypocotyl, the auxin-independent target gene BrPIL1 was dependent on the light quality perceived directly by the hypocotyl and not that experienced by the cotyledons (Fig. 5D). BrATHB2 and BrHFR1 followed a similar pattern; however, their levels were only marginally and, in the case of BrHFR1, nonsignificantly increased by the shade conditions used in this experiment. Consistent with our gene expression data of seedlings in which we had removed the cotyledons, we found that the hypocotyl expression of the auxin target genes BrIAA19, BrIAA2, BrIAA29, and BrGH3-5, as well as the presumptive auxin-independent gene BrXTH33, is instead dependent on the light quality perceived by the cotyledons (Fig. 5D). Low R:FR treatment of the hypocotyl had little to no effect on the expression of these genes.
Together, our results suggest that auxin-mediated transcription in the hypocotyl is dependent on a mobile signal(s) produced in the cotyledons, likely auxin, while most of the auxin-independent shade-induced genes do not require signals derived from the cotyledons.
Shade Results in a Gradient of Auxin-Induced Gene Expression Extending Downward in the Hypocotyl
The median speed of auxin movement along the eudicot hypocotyl is 7 mm h−1 (Kramer et al., 2011). If we assume this speed to be true for the Brassica genus, it would take greater than 2 h for newly synthesized apical auxin to move to the base of a typical 7-d-old B. rapa hypocotyl. As such, if the cotyledons are the sites of increased auxin production in shade, it is likely that a gradient of increased free auxin and auxin-dependent transcriptional responses would occur downward along the hypocotyl and away from the cotyledons during the early shade response. This may be particularly apparent in species such as B. rapa, whose seedlings have a relatively large hypocotyl compared with Arabidopsis.
To test this hypothesis, hypocotyls of B. rapa R-o-18 seedlings were dissected into three segments following low R:FR shade treatment (Fig. 6A). This treatment was associated with an increase in free auxin levels in the cotyledons (Fig. 6B). In the hypocotyl, we did not observe a detectable increase in free IAA levels after 2 h of low R:FR shade (data not shown), although robust gene expression changes are already apparent at this time (Fig. 3B). This may be due to a lack of sensitivity in our measurements of free IAA levels. However, after 6 h of exposure, we did observe increased levels of IAA in the hypocotyl, and those increases were manifest as a basipetal gradient extending downward (high IAA to low IAA) with respect to the cotyledons (Fig. 6C). Consistent with this finding, we observed that rapid changes in auxin-dependent gene expression occurred in a basipetal gradient along the hypocotyl axis. After 2 h of low R:FR shade, BrGH3-5, BrIAA19, and BrIAA2 were all induced in shade-treated hypocotyl segments as compared with equivalent segments from non-shade-treated controls, with greatest induction in the apical segment (Fig. 6D). BrIAA29 followed a similar pattern, although the effect of hypocotyl segment on gene induction was not significant. By 6 h of low R:FR light, the effect of shade on transcript levels is greater still, although a clear basipetal pattern of induction is less apparent and not statistically significant (Fig. 6E). By contrast, the presumptive auxin-independent target genes BrATHB2, BrHFR1, and BrPIL1, as well as BrXTH33, did not exhibit any clear collective pattern of gene expression along the hypocotyl in response to shade (Supplemental Fig. S10). Taken together, our data suggest that auxin is synthesized in the cotyledons and is transported or diffuses into the hypocotyl, thereupon triggering the up-regulation of genes associated with elaboration of the shade avoidance response.
Figure 6.
Low R:FR shade induces a basipetal gradient of auxin-dependent gene expression. A, Experimental setup of C to E. The hypocotyl of 7-d-old W light-grown seedlings (R-o-18) was sectioned after the indicated light treatments into apical, middle, and basal fragments. C, Cotyledons; H, hypocotyl; R, root. B, Free IAA levels in the cotyledons following 6 h of W or low R:FR light treatment. C, Free IAA levels in apical, middle, and basal segments of the hypocotyl following 6 h of W or low R:FR light treatment. In B and C, data show means ± se (n = 5). Student’s t test: **P < 0.005, *P < 0.05, ns = not significant. D and E, qPCR expression analysis of auxin-dependent genes in the hypocotyl following 2 h (D) or 6 h (E) of low R:FR light treatment. Expression is shown for low R:FR-treated hypocotyl segments relative to the W light-treated control segments ± se. In D, asterisks indicate a significant interaction between light treatment and the hypocotyl segment using a two-factor ANOVA with repeated measures on one factor (hypocotyl segment): **P < 0.005, ns = not significant. By 6 h, no significant interaction is observed for any of the genes.
Shade-Induced Hypocotyl Growth Is Correlated with Epidermal Cell Elongation
To determine the regions of the B. rapa hypocotyl that are responsible for shade-induced elongation, we marked hypocotyls of 4-d-old W light-grown R-o-18 seedlings to delineate three segments of approximately equal length. The length of each segment was then measured after 3 d of additional growth in either W light or shade (Fig. 7A). Growth was greatest in apical segments under both light conditions, while shade-treated plants had significantly more growth across all three segments than W light-grown controls.
Figure 7.
B. rapa hypocotyl growth occurs in a basipetal gradient. A, R-o-18 seedlings were grown for 4 d in constant W light (Wc) and the hypocotyl was marked into three divisions of equal size (apex, middle, and base). After a further 3 d of growth in either constant W light or low R:FR shade, the length of each segment was measured. B, Length of hypocotyl epidermal cells of 4- and 7-d-old W light-grown plants, as determined by scanning electron microscopy. The hypocotyls of imaged plants were divided into five equal segments (1–5) for analysis. C, Epidermal cell lengths at the hypocotyl apex (0–2 mm below the hypocotyl-petiole junction) and base (1–3 mm from hypocotyl-root junction). Four-day-old W light-grown seedlings were treated with W or low R:FR light for 3 d. In A to C, data show means ± se. Student’s t test: **P < 0.005, *P < 0.05, ns = not significant. D and I, Overlaid scanning electron microscopy images of full-length hypocotyls of constant W light (D) and low R:FR (I) seedlings grown under the conditions described in C. Boxes mark equivalent positions of the images shown in E to H. E to H, Representative scanning electron microscopy images of epidermal cells from the apex (E and G) and base (F and H) of the hypocotyl of constant W light-grown (E and F) and low R:FR light-grown (G and H) seedlings. White outlines show examples of the large, bulbous epidermal cells measured in B and C. In all images, the apex is up. Bars = 0.5 cm (D and I) and 500 μm (E–H).
Using scanning electron microscopy, we measured the lengths of epidermal cells along the hypocotyls of W light-grown plants and found a similar pattern: epidermal cell length decreased in a basipetal gradient, and hypocotyl cell elongation after 7 d of growth was greatest in apical regions compared with 4-d-old plants (Fig. 7, B and D–F). Following shade, epidermal cells were longer in both apical and basal regions of the hypocotyl, with most growth at the apex (Fig. 7, C and G–I). This growth pattern may reflect a gradient of auxin and/or other growth signals along the apical-basal axis from the shoot apical meristem and/or cotyledons, consistent with our findings above. Alternatively, it might result from the decreased malleability of basal cells or a combination of these two mechanisms.
Low R:FR Shade Does Not Affect the Seed Oil Composition of Mature B. rapa Plants
Stem elongation phenotypes similar to those studied here may be of agronomic importance. For example, increased stem length can make plants more susceptible to physical damage (Morinaka et al., 2006), while elongation growth may reallocate photosynthate and growth resources away from harvested tissues (Ballare et al., 1997; Casal, 2013). In addition, the reallocation of resources toward growth may make plants more susceptible to herbivory and infection (Ballaré, 2009). Many Brassica spp. varieties are important oilseed crops; as such, we sought to further explore how shade signals might affect oilseed-related traits. Specifically, in addition to our studies above, we sought to test how shade might affect B. rapa seed oil quality and yield in mature plants.
To test how low R:FR light might affect Brassica spp. seed oil quality, FPsc plants were grown in long-day conditions (16 h of light and 8 h of dark) under W light in the absence or presence of supplemental FR light during daylight hours (high and low R:FR, respectively; see “Materials and Methods”). The FPsc variety was chosen for these experiments due to the availability of a phyB mutant as well as the plant’s smaller stature compared with mature R-o-18 plants, which facilitated growth in the limited physical space of our shade light chambers. Under these conditions, we observed little difference in the height of 60-d-old wild-type plants grown in W light or W light supplemented with FR light (Fig. 8, A and E) or flowering time (31 ± 1 d until first bud opening under W light versus 30 ± 1 d in low R:FR; Student’s t test, not significant). It should be noted that more rapid flowering times (approximately 18 d) were observed among FPsc plants grown in constant light at higher temperature (Scott Woody, personal communication). By contrast, Arabidopsis plants grown under these same conditions had a marked reduction in flowering time (6.9 ± 0.1 rosette leaves at the time of bolting under W light versus 2.9 ± 0.1 in low R:FR; Student’s t test, P < 0.005; Landsberg erecta [Ler] accession). Notwithstanding this apparent exception of B. rapa FPsc flowering time in the elaboration of certain aspects of SAS, the leaf chlorophyll content of B. rapa plants was strongly reduced in the shade environment (Fig. 8F), and siliques were noticeably paler and longer, produced fewer mature seeds, and those seeds were smaller by weight (Fig. 8, D and G–I). We were unable to assess total spontaneous seed yield per FPsc plant, as we had intervened to facilitate self-crossing in order to maximize seed yield for oil analysis. However, Arabidopsis plants grown under the same conditions had significantly reduced seed yield per plant in shade (14.1 ± 1.5 mg of total seed weight per plant in W light compared with 7.1 ± 0.6 mg in low R:FR light; Student’s t test, P < 0.005; Ler accession). In addition, previous field experiments using B. rapa have shown that silique production (and therefore likely seed yield) is reduced when plants are grown at high density where mutual shading occurs (Dechaine et al., 2007).
Figure 8.
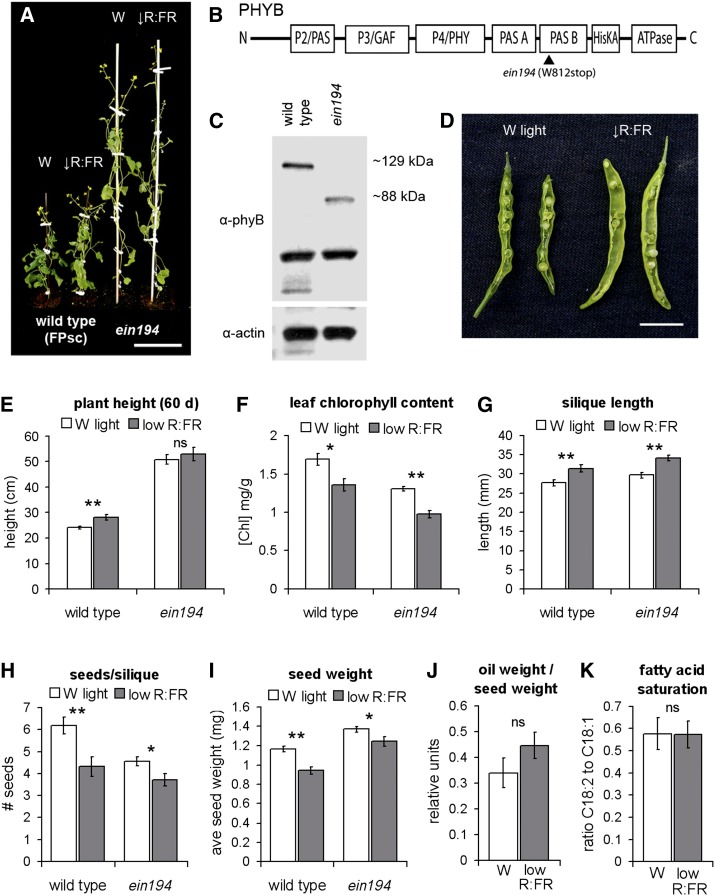
Mature B. rapa plants show defects in seed number and weight in low R:FR light but not in oil content. A, Representative image of 60-d-old wild-type (FPsc) and phyB mutant (ein194) plants grown in long days (16 h of light and 8 h of dark) in W light (W) or W light supplemented with FR (↓R:FR). Bar = 10 cm. B and C, Schematic of PHYB apoprotein, showing the position of the ein194 mutation (B). Domains are represented by boxes. ein194 codes for a truncated form of the protein, seen by western blot (C). D, Representative image of dissected siliques 14 d after pollination from wild-type plants grown in W light or low R:FR shade. Bar = 1 cm. E to K, Plant height at 60 d (E), leaf chlorophyll content (F), silique length (G), number of seeds per silique (H), seed weight (I), oil weight per seed weight (J), and ratio of C18:2 to C18:1 fatty acids (K) of wild-type and ein194 plants grown under W or low R:FR light. Data show means ± se. Student’s t test: **P < 0.005, *P < 0.05, ns = not significant.
To test which of these defects might be phyB dependent, we grew ein194 mutant plants expressing a truncated form of the phyB protein (Figure 8, B and C; Supplemental Fig. S3). In W light conditions, ein194 mutants displayed dramatically elongated stature, reduced chlorophyll content, and reduced seed number per silique compared with wild-type plants, suggesting that some of these phenotypes are in part determined by phyB signaling (Fig. 8, A and E–I). phyB activity did not appear to play a significant role in the determination of other phenotypes examined (silique length and seed weight); however, it is possible that these traits are regulated by other phytochromes encoded in the B. rapa genome or that this observation is a result of the taller ein194 plants receiving more light from the overhead lighting than the wild-type strain.
Seed oil composition was determined by gas chromatography-mass spectrometry (GC-MS) analysis. In initial experiments, five major peaks representing different fatty acids were separated (Table I; Supplemental Table S1). No difference in oil content was observed between seeds harvested from siliques of different plants in W light or between siliques growing at different heights on the same plant (Supplemental Table S1). This shows that interplant and intraplant variation in seed oil composition is minimal. When seeds were compared from both wild-type and ein194 mutant plants growing across the two light environments, we observed no change in the relative amounts of the five fatty acid groups (Table I).
Table I. Seed oil composition of B. rapa (FPsc) is not altered by low R:FR light.
| Genotype | Condition | Percentage Abundancea |
||||
|---|---|---|---|---|---|---|
| 16:0 | 18:2 and 18:1 | 18:0 | 20:1b | 22:1c | ||
| Wild type | W light | 6.3 ± 3.1 | 60 ± 2.3 | 4.9 ± 0.5 | 11.6 ± 1.1 | 16.8 ± 0.4 |
| Wild type | Low R:FR light | 10.1 ± 1.1 | 60.3 ± 1.2 | 5.2 ± 0.5 | 9.6 ± 0.9 | 14.7 ± 1.6 |
| ein194 | W light | 9.1 ± 0.2 | 58.6 ± 0.1 | 3.8 ± 0.2 | 10.5 ± 0.1 | 18.0 ± 0.7 |
| ein194 | Low R:FR light | 10.3 ± 0.6 | 58.1 ± 0.2 | 4.6 ± 0.4 | 8.9 ± 1.4 | 18.1 ± 0.2 |
The percentage abundance was calculated using the total ion count of a given fatty acid peak divided by the sum of the total ion counts of all fatty acid peaks multiplied by 100. bEicosenoic acid monounsaturated at carbon 9, 11, or 13. cDocosenoic acid monounsaturated at carbon 11 or 13.
It had previously been reported that low blue light altered the saturation of 18-carbon-chain fatty acids in soybean (Britz and Cavins, 1993). In the experiments described above, we were unable to adequately separate peaks for linoleic acid (18:2) from monounsaturated 18:1 fatty acids. Therefore, we tested additional diluted oil samples from wild-type plants (Fig. 8, J and K) and found no difference between the ratio of 18:2 to 18:1 fatty acids from seeds of plants grown in either W light or W light supplemented with FR light nor a significant change in relative oil content per seed weight. As such, we conclude that the seed oil composition of B. rapa FPsc plants is stable across changing ratios of R:FR light, even though photosynthesis and total seed yield per plant are affected. This suggests that fatty acid synthesis is a highly regulated process, even in nonoptimal light environments.
DISCUSSION
The Cotyledons Regulate Shade-Induced Hypocotyl Growth in B. rapa
Studies using Arabidopsis have indicated that the cotyledons are the most likely source of increased auxin synthesis in low R:FR shade (Tanaka et al., 2002; Tao et al., 2008) and that phyB functions specifically in cotyledons to modulate hypocotyl growth (Endo et al., 2005). By contrast, experiments using other systems have suggested that the direct perception of R and/or FR light by the stem can also affect stem growth (see introduction). For example, it has been reported that R and FR light regulates the growth of excised hypocotyl segments of bean (Phaseolus vulgaris), and spectral information perceived by one region of the hypocotyl can be transmitted to another, either through the movement of a phytohormone or light funneling (Gotô and Suzuki, 1980). In S. alba, a species of the Brassicaceae family closely related to B. rapa, the addition of supplemental FR light is perceived by both internodes and other tissues to induce internode growth, while end-of-day FR light treatment induced elongation growth only when perceived by the leaves and cotyledons (Morgan et al., 1980; Child and Smith, 1987; Casal and Smith, 1988a, 1988b). This suggests that specific light treatments may also alter the interplay of light-sensing organs.
The cause of the discrepancy in these studies is unclear. Differences in the species examined, light conditions, and tissues (internode, epicotyl, or hypocotyl) all might provide some explanation for the different contributions observed of cotyledons/leaves and hypocotyls/stems in perceiving changes in R:FR light that drive stem growth. In addition, it is possible that the cotyledons of large plant species are unable to synthesize and transport enough auxin or another growth-promoting signal into the hypocotyl to cause robust changes in elongation. However, our results with B. rapa suggest that this need not be the case. Instead, we favor a simple model, even in large plants, where perception by the cotyledons of light quality indicative of neighbor proximity increases cotyledonary auxin synthesis, which in turn is transported or diffuses into the hypocotyl to trigger growth. In support of this model, first, we have shown directly that the perception of shade light by the cotyledons drives hypocotyl growth, while in seedlings where the cotyledons have been removed the hypocotyl fails to elongate in response to shade. Second, we have shown that, following a shift to shade, a gradient of free auxin and auxin-dependent gene induction occurs in a basipetal gradient along the hypocotyl, consistent with an apical auxin maxima. Third, in the absence of the cotyledons or of shade perception by the cotyledons, auxin target genes fail to be induced by shade.
While the R:FR ratio perceived by the cotyledons largely determined hypocotyl length in our split-light chamber experiments, we did observe an additional slight increase in hypocotyl growth when the hypocotyl was also irradiated with supplemental FR light (Fig. 5B). This increase in hypocotyl growth was significant by two-way ANOVA (P < 0.05). This may indicate that a minor contribution of shade perception in the hypocotyl to growth does exist. In addition, our studies do not preclude the possibility that shade perception by the hypocotyl alters the dynamics of the growth response (Morgan et al., 1980). Alternatively, the slight increase in growth might be a result of light piping from the hypocotyl to the cotyledons. However, the observation that hypocotyl length is determined largely by the light quality perceived by the cotyledons, irrespective of hypocotyl treatment, suggests that the effects of light piping from the hypocotyl to cotyledons are minimal. Neither do we observe strong effects of light piping from the cotyledons to the hypocotyl; for example, induction of BrPIL1 and BrATHB2 expression in the hypocotyl is only evident when the hypocotyl is exposed to shade, irrespective of the light quality perceived by the cotyledons.
Although we cannot rule out the possibility that a nonauxin signal moves from the cotyledons to direct auxin synthesis in the hypocotyl, this is unlikely in light of previous studies using Arabidopsis. For example, the SAV3 aminotransferase required for auxin synthesis in shade is expressed in cotyledons (Tao et al., 2008), and the expression of an auxin reporter transgene increases in cotyledons and decreases in hypocotyls when auxin movement is blocked by NPA treatment, consistent with cotyledonary auxin synthesis and accumulation (Tao et al., 2008). Interestingly, however, Tanaka et al. (2002) showed that transcriptional reporters that display increased expression in the Arabidopsis hypocotyl in response to end-of-day FR light treatment can be either auxin dependent or independent. FR irradiation of cotyledons could induce the expression of both reporter types, suggesting that the cotyledons may also be the source of a nonauxin signal. In our studies with B. rapa, we have shown that the removal of the cotyledons prior to simulated shade resulted in a loss of auxin-dependent gene induction. In addition, we saw a loss of induction of the cell wall-modifying enzyme BrXTH33, even though this gene was not responsive to exogenous auxin (Figs. 3D and 4, C and D). As such, the expression of BrXTH33 could require the unknown cotyledon-derived signal proposed by Tanaka et al. (2002), perhaps in addition to auxin. This may be a photoassimilate or other phytohormone.
Consistent with our observation that the cotyledons direct hypocotyl elongation in shade, wag1 wag2 pinoid (pid) triple mutants of Arabidopsis, which fail to develop cotyledons (Cheng et al., 2008), display no hypocotyl growth response to low R:FR shade (Supplemental Fig. S11). However, these mutants have defects in the localization of auxin efflux transporters, and as such we cannot rule out the possibility that the lack of hypocotyl growth is not due to a general defect in auxin movement in these plants (Friml et al., 2004).
In addition, our findings are consistent with the previously reported observation that phyB-GFP expression specifically in the cotyledons can complement the elongated hypocotyl phenotype of phyB-deficient Arabidopsis mutants (Endo et al., 2005). We hypothesize that phyB likely functions within the cells of the cotyledon that are responsible for auxin synthesis, in turn regulating hypocotyl growth.
The fact that the cotyledons perceive R and FR light signals that direct hypocotyl growth might be advantageous in a natural setting. The hypocotyl of young seedlings might be embedded almost entirely underground when the cotyledons first perceive light at the soil surface or might be self-shaded by the cotyledons in older seedlings. As such, we argue that the cotyledons are best poised to transmit signals about the seedling’s light environment.
Not All Shade-Regulated Genes in Hypocotyl Cells Require Cotyledon-Derived Signals
Surprisingly, not all shade-regulated genes in cells of the B. rapa hypocotyl required the cotyledons for their induction (Figs. 4, C and D, and 5D). These genes (BrHFR1, BrPIL1, and BrATHB2) are all likely orthologs of genes in Arabidopsis that are bound directly by PIF transcription factors (Hornitschek et al., 2012). This suggests that the hypocotyl can respond to shade independently of the cotyledons and auxin. However, this response is neither necessary nor sufficient for stimulated growth in our experiments (Figs. 4B and 5B). In addition, the expression of at least one of these genes, BrPIL1, displayed an acropetal pattern of induction along the hypocotyl in response to shade (Supplemental Fig. S10), opposite of hypocotyl growth. This provides additional support that the expression of these genes, at least in the hypocotyl, does not directly correlate with growth. Interestingly, a study of Arabidopsis mutants defective in hypocotyl elongation in shade previously noted that HFR1 and PIL1 expression in whole seedlings does not always correlate with hypocotyl length (Cifuentes-Esquivel et al., 2013). However, that study analyzed gene expression in whole seedlings rather than just the growing organ (the hypocotyl) or the site of shade perception (the cotyledons). Indeed, we hypothesize that the expression of these genes might correlate with growth, but only in the auxin-producing cells of the cotyledons, where the genes might function to alter auxin production, as has been postulated for HFR1 (Hersch et al., 2014).
If the cotyledon-independent transcriptional response to shade is neither necessary nor sufficient for stimulated growth, as our studies would suggest, what is the role of this response? One possibility is that this response regulates other aspects of the shade avoidance phenotype; for example, the down-regulation of defense genes in the hypocotyl (Cerrudo et al., 2012) or the reduction of stem photosynthetic capacity (Cagnola et al., 2012). Alternatively, the cotyledon-independent response might potentiate the response of hypocotyl cells to cotyledon-derived growth signals. For example, in S. alba, it has been shown that R light irradiation of the internode inhibits growth-promoting signals from leaf tissue, perhaps by altering auxin transport (Casal and Smith, 1988a). Similarly, in Arabidopsis, low R:FR shade leads to PIF-dependent up-regulation of the AFB1 auxin receptor gene in hypocotyl tissue, suggesting that there is increased sensitivity of the hypocotyl to auxin in shade (Hersch et al., 2014). Another possibility is that light perception by the hypocotyl is important only under certain environmental conditions and might be a means for integrating multiple push-and-pull growth signals. Such a mechanism may allow the hypocotyl to integrate information about the R:FR light environment with other environmental and/or developmental cues that are perceived directly by the hypocotyl.
We also find that the transcriptional response of the root to low R:FR shade is very different from the cotyledons and hypocotyl. These results are consistent with previous observations that light- and dark-grown Arabidopsis seedlings have distinct changes in gene expression in root, hypocotyl, and cotyledon, even though all three organs likely share the same phyB-dependent photoperception machinery (Ma et al., 2005; Bou-Torrent et al., 2008). In addition, our observation here that auxin target genes are not induced in the root following shade perception may indicate that the shoot is a closed system of auxin signaling and transport in shade. How, then, phytochrome-dependent signals from the shoot regulate lateral root formation in shade remains unclear (Salisbury et al., 2007).
B. rapa Hypocotyl Growth Occurs in a Basipetal Gradient
Generally, plant elongation occurs in a gradient along the stem, with most growth occurring at the apex (Gotô and Suzuki, 1980; Martinez-Garcia and Garcia-Martinez, 1992; Shinkle et al., 1992). These results are consistent with our observation that hypocotyl growth in B. rapa occurs in a basipetal gradient. This may be explained by a gradient of a growth signal from the apex, which may include auxin or photoassimilates, or, rather, a gradient in cellular growth potential and amenability along the hypocotyl. In V. sinensis, elongation in response to end-of-day FR light treatment has been observed in derooted and debladed epicotyl explants (Martinez-Garcia and Garcia-Martinez, 1992). The region of the epicotyl that displayed the greatest change in growth was localized 5 to 20 mm below the apex (the entire epicotyl was 40–80 mm long). Similarly, bean hypocotyl explants have been shown to respond to FR light, and the region that showed the greatest growth response was localized away from the apex, 5 to 15 mm from the top of the shank (the total length of the shank was 40 mm; Gotô and Suzuki, 1980). These findings are not entirely inconsistent with our own; generally, the region near the apex of the growing organ elongates most. However, the localization of the growing region specifically to 5 mm or more below the apex in V. sinensis epicotyl and in bean hypocotyl may be due to species specificity, a lack of resolution in defining the hypocotyl regions most sensitive to growth in our own studies, or a difference in using excised epicotyl/hypocotyl explants that lack additional growth signals from the leaves/cotyledons. In our studies using B. rapa, we only observe shade-induced hypocotyl growth in intact plants, wherein the major source of signals regulating elongation in response to shade is the apex-localized cotyledons.
Stem growth is either a result of cell elongation or cell elongation and cell division, depending on the plant species. In the case of B. rapa, our measurements indicate that most growth resulting in hypocotyl elongation is likely due to cell elongation rather than division (with the caveat that we only measured epidermal cell length and not other cell types of the hypocotyl). This was true whether seedlings were grown under W light or simulated shade conditions. However, we have not examined directly the possibility that increased rates of cell division also contribute to hypocotyl elongation.
Low R:FR Shade May Contribute to Loss of Oil Yield in Agriculture
Brassica spp. are important oilseed crops, and breeding efforts have developed varieties that have fatty acid compositions optimized for human benefit. Stem elongation phenotypes may affect traits of agronomic importance in some plant species by reallocating resources toward stem growth and increasing the likelihood of mechanical damage (Morinaka et al., 2006; Casal, 2013). Here, we sought to explore how shade signals also might affect Brassica spp. seed oil quality and yield. For example, the effect of environmental temperature on oil composition has been well studied, but little is known about how a plant’s spectral environment might also play a role (Izquierdo et al., 2009). The study of changing oil composition in different light environments may be particularly relevant for the low R:FR mutual shading found in densely planted crops (Casal, 2013).
We took advantage of the FPsc strain and specifically the availability of phyB mutants in this background to test the effects of low R:FR shade on B. rapa seed oil composition. FPsc is a self-compatible and highly inbred analog of the self-incompatible WFP variety (Williams and Hill, 1986). Like WFP, the FPsc variety was developed primarily to serve as a model system for hands-on student exploration of plant biology and genetics. In such settings, rapid progression through the plant life cycle is useful, and that consideration was a high priority in the selective breeding program that produced the FPsc B. rapa accession. The fact that we did not observe a detectable advance in flowering time among FPsc plants exposed to low R:FR light may simply be due to the fact that FPsc flowers as rapidly as possible under all circumstances. We report here, to our knowledge, the first transformant and transformation protocol of the new FPsc educational model system (Supplemental Fig. S5).
Here, we find that low R:FR simulated shade causes a reduction in the chlorophyll content of mature FPsc plants, reduced seed number per silique, and reduced seed weight. In Arabidopsis, we also observed that seed yield per plant was reduced. Previous work has shown that many plant species exhibit reduced chlorophyll content in shade and that this response is in part phyB dependent (McLaren and Smith, 1978; Reed et al., 1993), while field studies of B. rapa reported fewer siliques per plant when grown at high density (Dechaine et al., 2007). However, we observed no change in B. rapa seed oil composition either in shade light or in the ein194 phyB mutant. This suggests that fatty acid synthesis in B. rapa is a highly compartmentalized process, as the balance of molecular species is unchanged even when chlorophyll content/photosynthesis is perturbed. It may be that reduced fecundity in response to shade is an evolved and adaptive trait: by reducing the total number of progeny seeds produced, the available energy resources can be delivered to fewer seeds, but each would be equipped with energy stores best able to promote successful germination and growth in the succeeding generation.
Natural environments are complex, and plant density can affect not only light quality (R:FR ratio) but also light quantity and other aspects of the environment aboveground and belowground. Determination of the optimal balance between planting density, seed quality, and seed yield is a challenge faced by all growers. If our results are generalizable to B. rapa oilseed crop varieties growing in such complex field conditions, then the proper balance may be relatively straightforward in this species, as seed yield may be optimized independent of seed oil quality.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Brassica rapa strains used were R-o-18 (Stephenson et al., 2010), WFP (standard; Williams and Hill, 1986) and the ein mutant (Williams and Hill, 1986), and FPsc and ein194 mutants (a gift of Scott Woody and Rick Amasino). Arabidopsis (Arabidopsis thaliana) strains used were Columbia-0, Ler, sav3-1 (Tao et al., 2008), and the wag1 wag2 pid triple mutant (T-DNA insertion alleles SALK_002056, SALK_070240 and SALK_049736; Cheng et al., 2008). Seeds were sterilized, stratified, and germinated on solid agar plates with one-half-strength Linsmaier and Skoog salts and vitamins and without Suc (1% [w/v] Suc was added in the experiment described in Fig. 4B). After stratification, plates were incubated in either constant W light or simulated low R:FR shade chambers, using the light conditions described (Tao et al., 2008) unless indicated otherwise. Temperature was 22°C.
The mature FPsc plants used for fatty acid analysis were grown on soil under long-day conditions (16 h of light and 8 h of dark) at 22°C. Photosynthetically active radiation (400–700 nm) of W light was 75 to 90 μE m−2 s−1 at a height of 10 cm above soil level, with an R:FR ratio of approximately 10. Lighting was provided by Cool-white and Gro-Lux fluorescent bulbs. Low R:FR conditions were W light supplemented with FR light-emitting diode bulbs (LumiGrow ECC-FR); photosynthetically active radiation was 70 to 85 μE m−2 s−1, and R:FR ratio was approximately 0.75. A consequence of the chamber setups used in this study is that the ratios of R to FR light in our W light conditions were typically greater than that encountered in natural environments (we used R:FR ratios greater than 10, while natural sunlight is approximately 1.1; Casal, 2013). However, our shade conditions have ratios of less than 0.8, which are typical of those experienced by plants under natural foliar shade or in the proximity of neighboring plants (Casal, 2013).
Determination of Hypocotyl Length
Quantitative measurements of hypocotyl length and cotyledon area were performed using ImageJ software analysis of scanned images of seedlings. At least 10 plants were scored for each data point. To measure the hypocotyl length of FPsc plants carrying UBIQUITIN10pro::AtPHYB and their controls, only seeds that had germinated within 24 h after stratification and transfer to W light were scored.
Transformation of B. rapa FPsc
The UBQ10pro::AtPHYB plasmid (pCP12.22) was made using the multisite Gateway system (Invitrogen). Donor vectors pDONR-P4P1R-UBQ10pro (Jaillais et al., 2011), pDONR221-AtPHYB (a gift of Kazu Nito), and pDONR-P2RP3-6xHis3xFlag tag were recombined with destination vector pK7m34GW. FPsc plants for transformation were grown on sterile agar plates, and young cotyledon explants were transformed with Agrobacterium tumefaciens GV3101 carrying the UBQ10pro::AtPHYB construct. Hormone conditions used to generate callus and regenerate shoots were as described (Kunvshinov et al., 1999). We were unable to regenerate roots under a variety of conditions tested; however, when we transferred shoots to soil, one of these regenerated a root to provide transgenic seeds.
Western-Blot Analysis and GUS Staining
Western blotting and GUS staining were performed using standard protocols. Primary antibodies used were anti-Flag (Sigma-Aldrich), anti-actin (MP Biomedicals), anti-UDP-Glc pyrophosphorylase (Agrisera), and anti-phyB (a gift of Akira Nagatani).
qPCR and Statistical Analysis
qPCR was performed using standard techniques using three biological replicates. Fold changes in gene expression were calculated using the comparative threshold cycle (Ct) method (2−ΔΔCt method; Schmittgen et al., 2008). At present, no consistent statistical analysis for qPCR results is presented in the literature. Here, we have assumed that the ΔCt values fit a normal distribution and have tested ΔCt values using Student’s t test or the appropriate ANOVA test (Yuan et al., 2006). Primers for Arabidopsis gene expression studies using complementary DNA template were as follows: IPP2 (the reference gene ISOPENTENYL PYROPHOSPHATE:DIMETHYLALLYL PYROPHOSPHATE ISOMERASE2; 5′-GTATGAGTTGCTTCTCCAGCAAAG-3′ and 5′-GAGGATGGCTGCAACAAGTGT-3′), PIL1 (5′-TGGACTAATTCCAAACACTCCTATCTT-3′ and 5′-CACACGAAGGCACCACGA-3′), IAA29 (5′-ACCGTGTGCATATACAAGATGTTTG-3′ and 5′-TCCGATTTGAACGCCTATCCT-3′), XTH33 (5′-TACTAGAACGGGAAGAGAAGAG-3′ and 5′-AATGAGGGTATAGTCGTGAAAGG-3′), IAA2 (5′-ACAACAAGCGTCTATTTGAGG-3′ and 5′-CTTACGGGAAGATCTCACTGG-3′), GH3-5 (5′-CTTACACCAACTACACAAGCC-3′ and 5′-GACATAAGCCACAAAGCATCTG-3′), IAA19 (5′-TGCTCTTGATAAGCTCTTCGG-3′ and 5′-AGTCTCCATCTTTGTCTTCGT-3′), ATHB2 (5′-CAGTCTCAAGCTCTACAGGG-3′ and 5′-GGAGTTATCACCATCTTCATCGT-3′), and HFR1 (5′-TCCCACATTCATCTAGTCAATCTC-3′ and 5′-GGAAATAAGGAACCAAACCGTG-3′). Orthologous gene sequences in B. rapa were found using the Brassica Database (http://brassicadb.org/brad/). qPCR primers for gene expression studies were designed based on the Brassica Database sequences and were as follows: BrGAPDH (the reference gene GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE; BraA.GAPDH.a/Bra016729, 5′-CACCACCGAGTACATGACGTACA-3′ and 5′-TGCCCGTGAACACTGTCGTA-3′), BrPIL1 (BraA.PIL1.a/Bra004489, 5′-TTACGCCTTCATCTATGCTTTC-3′ and 5′-TCTCCACACTTCCTCTTCTTTG-3′), BrIAA29 (BraA.IAA29.a/Bra011332, 5′-GCCAGTCAGACAAGGAAGAT-3′ and 5′-CCTCGTCGTACACAACAGAA-3′), BrXTH33 (BraA.XTH33.a/Bra01843, 5′-TGGTGACAAAGAACACGTATCAT-3′ and 5′-TTGACAAATAGAAAGCAACCACA-3′), BrIAA2/Bra001899, 5′-GTGAATACAGCGAAAGAGAAGG-3′ and 5′-ACCAACCAACATCCAATCTCC-3′), BrGH3-5 (BraA.GH3-5.a/Bra019060, 5′-GACCCTTACACCGACTACAC-3′ and 5′-CATAAGCCACAAAGCATCTGAG-3′), BrIAA19 (Br.IAA19.a/Bra001598, 5′-TCTCGATAAGCTCTTCAGTTTCC-3′ and 5′-CAGTCTCCATCTTTGTCTTCGT-3′), BrATHB2 (BraA.ATHB2.a/Bra040094, 5′-CGCAAGGCTCAAGAGGTATCA-3′ and 5′-TCCTCGAGTTATCACCGTCTTCAT-3′), and BrHFR1 (BraA.HFR1.a/Bra032610, 5′-GCACAACCACCTTTTGAGGGCG-3′ and 5′-GCCGGGTTTAGGCCGTGAGC-3′). qPCR primers used for determining UBQ10pro::AtPHYB copy number from genomic DNA template were as follows: control (Bra011332, 5′-CTTGATCTTTCTCTTTCACCTCAT-3′ and 5′-CCTCGTCGTACACAACAGAA-3′) and UBQ10pro::AtPHYB transgene (5′-TGTGCGATCGAATTTGTCG-3′ and 5′-CGGTTATTAGGAGTGTGACTTGA-3′).
Free IAA Measurements
Samples containing 10 to 20 mg of plant material (fresh weight) were analyzed by gas chromatography-tandem mass spectrometry as described previously (Andersen et al., 2008) with minor modifications. A [13C6]IAA internal standard (500 pg) was added to each sample before purification, and five biological replicates were analyzed per tissue type and treatment.
Scanning Electron Microscopy
Whole plants were carefully removed from the agar by the roots and placed into ice-cold ethanol for 10 min before being loaded and dried in an automated critical point drier (Leica EM CPD300), which was set to perform 25 exchange cycles of CO2 at medium speed and 40% stirring. The fill, heating, and venting steps were performed at medium speed. After drying, the cotyledons were carefully removed with forceps, and the sample was adhered to a glass slide with carbon adhesive. The mounted samples were then sputter coated (Leica SCD500) with approximately 7 nm of platinum while being rotated and imaged on an environmental scanning electron microscope (EVO HD; Zeiss). Three plants from each light condition were imaged, and for each plant, the lengths of 25 epidermal cells in each segment of the hypocotyl being analyzed were measured. Only the large, bulbous epidermal cells were measured.
Alternatively, still hydrated samples were removed from the agar and suspended by the roots and cotyledons between a set of split toothpicks. The samples were then imaged in the variable-pressure mode of a field emission-scanning electron microscope (Sigma VP; Zeiss) at 5 Pa of nitrogen with the variable-pressure secondary electron detector.
Fatty Acid Measurements
Samples for fatty acid extraction and GC-MS analysis were collected from individual siliques. For Table I, three biological replicates were sampled. For Figure 8, J and K, five biological replicates were sampled. Flowers were self-pollinated by hand to increase yield for analysis. Cross-pollination between FPsc plants was very occasionally observed. To ensure that the seeds sampled were not cross progeny between plants of different genotypes, some seeds from each silique used for GC-MS were sequenced.
The fatty acids were extracted and analyzed according to previously published methods (Ngaki et al., 2012). Briefly, frozen seeds were homogenized by agitation with ball bearings and then dissolved in 0.5 mL of 10% (w/v) barium hydroxide, adding nonadecanoic acid as an internal standard (20 μg mL−1 final concentration). The mixture was transferred to a glass tube, and 500 μL of 1,4-dioxane was added and vortexed. Saponification was carried out over 24 h at 105°C. The cooled mixture was acidified using 6 m aqueous HCl, and fatty acids were extracted into 4 mL of n-hexane. This organic phase was evaporated, and any remaining fatty acids were derivatized by the addition of 2 mL of HCl:methanol (1:5.25, v/v) at 80°C for 1 h. The cooled mixture was extracted with 2 mL of n-hexane after the addition of 2 mL of 0.9% (w/v) NaCl. The organic phase was then transferred to a glass vial and evaporated, and 500 μL of acetonitrile and 35 μL of N,O-bis(trimethylsilyl) trifluoroacetamide were added to the residue and heated for 20 min at 60°C. After evaporation of the mixture, the derivatized fatty acids were dissolved in chloroform in a volume that scaled based on the original weights of the seeds (approximately 200 μL).
GC-MS analyses were performed on an Agilent 6890N device coupled with an Agilent 5973 inert mass selective detector (MSD). Samples were injected (1 μL) on an HP-5MS fused silica column (30 m × 250 μm, 0.25 μm film thickness). Initial temperatures of the injector and MSD interface were set at 275°C and 280°C, respectively. Metabolites were separated at a flow rate of 1.2 mL min−1 using helium as the carrier gas and ramping temperature from 80°C (2 min) to 260°C at 4°C min−1 (held for 5 min) and then to 320°C at 5°C min−1. Data were acquired and analyzed in MSD Chemstation (Agilent Technologies). Fatty acids were identified via mass fragmentation pattern alignment with standards from the National Institute of Standards and Technology Mass Spectral Search Program. Peak areas were determined from total ion count.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. B. rapa hypocotyl elongation is phyB dependent.
Supplemental Figure S2. B. rapa FPsc seedlings respond to simulated shade.
Supplemental Figure S3. PHYB gene (Bra022192) and predicted protein sequence from B. rapa FPsc plants.
Supplemental Figure S4. qPCR expression analysis of low R:FR shade-induced marker genes in phyB mutants (ein194).
Supplemental Figure S5. Overexpression of Arabidopsis phyB causes short hypocotyls in B. rapa.
Supplemental Figure S6. qPCR time course of shade-induced genes.
Supplemental Figure S7. Shade-induced genes are expressed in distinct organs of B. rapa.
Supplemental Figure S8. Effect of exogenous GA and brassinosteroid on hypocotyl gene expression.
Supplemental Figure S9. Experimental setup of the split light chamber.
Supplemental Figure S10. Induction of presumptive auxin-independent shade-regulated genes in the hypocotyl.
Supplemental Figure S11. The hypocotyls of Arabidopsis wag1 wag2 pid mutants do not elongate in low R:FR shade.
Supplemental Table S1. Seed oil composition does not change between siliques grown in the same environment.
Supplementary Material
Acknowledgments
We thank Scott Woody and Rick Amasino for providing FPsc wild-type and ein194 mutant seeds and for sharing information pertaining to the FPsc strain; Scott Woody also provided extensive critical comments on the article. We also thank Roger Granbom for technical assistance with free IAA measurements; Matthew Joens and James Fitzpatrick for assistance in the use of electron microscopy resources at the Waitt Advanced Biophotonics Center; Tsegaye Dabi and Ryan Phillipe for technical assistance with B. rapa transformation and GC-MS protocols, respectively; Carol Huang for guidance on statistical analysis; and Ben Cole, Ken-ichiro Hayashi, Akira Nagatani, Kazu Nito, Ullas Pedmale, and Yunde Zhao for providing reagents and strains.
Glossary
- SAS
shade avoidance syndrome
- R
red
- FR
far-red
- PIF
phytochrome-interacting factor
- NPA
naphthylphthalamic acid
- qPCR
quantitative PCR
- FPsc
Fast Plants, self-compatible
- W
white
- IAA
indole-3-acetic acid
- Ler
Landsberg erecta
- GC-MS
gas chromatography-mass spectrometry
- WFP
Wisconsin Fast Plants
Footnotes
This work was supported by the National Institutes of Health (grant no. GM52413 to J.C. and fellowship no. 1F32GM101876–01 to C.P.), the National Science Foundation (grant no. IOS–0649389 to J.C.), the Swedish Governmental Agency for Innovation Systems (to K.L.), the Swedish Research Council and the Marianne and Marcus Wallenberg Foundation (to K.L.), and the Howard Hughes Medical Institute (to C.M.C, J.P.N., J.C.). Scanning electron microscopy was performed at the Waitt Advanced Biophotonics Center, which is supported by the Waitt Foundation, the W.M. Keck Foundation, the National Cancer Institute (P30 Cancer Center grant no. CA014195–40), and the National Institute of Neurological Disorders and Stroke (P30 Neuroscience Center Core grant no. NS072031–03A1).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH. (2006) Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell 23: 439–446 [DOI] [PubMed] [Google Scholar]
- Andersen SU, Buechel S, Zhao Z, Ljung K, Novák O, Busch W, Schuster C, Lohmann JU. (2008) Requirement of B2-type cyclin-dependent kinases for meristem integrity in Arabidopsis thaliana. Plant Cell 20: 88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL. (2009) Illuminated behaviour: phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ 32: 713–725 [DOI] [PubMed] [Google Scholar]
- Ballare CL, Scopel AL, Sanchez RA. (1997) Foraging for light: photosensory ecology and agricultural implications. Plant Cell Environ 20: 820–825 [Google Scholar]
- Black M, Shuttleworth JE. (1974) The role of the cotyledons in the photocontrol of hypocotyl extension in Cucumis sativus L. Planta 117: 57–66 [DOI] [PubMed] [Google Scholar]
- Bou-Torrent J, Roig-Villanova I, Martínez-García JF. (2008) Light signaling: back to space. Trends Plant Sci 13: 108–114 [DOI] [PubMed] [Google Scholar]
- Britz SJ, Cavins JF. (1993) Spectral quality during pod development modulates soybean seed fatty acid desaturation. Plant Cell Environ 16: 719–725 [Google Scholar]
- Cagnola JI, Ploschuk E, Benech-Arnold T, Finlayson SA, Casal JJ. (2012) Stem transcriptome reveals mechanisms to reduce the energetic cost of shade-avoidance responses in tomato. Plant Physiol 160: 1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. (2013) Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol 64: 403–427 [DOI] [PubMed] [Google Scholar]
- Casal JJ, Smith H. (1988a) Persistent effects of changes in phytochrome status on internode growth in light-grown mustard: occurrence, kinetics and locus of perception. Planta 175: 214–220 [DOI] [PubMed] [Google Scholar]
- Casal JJ, Smith H. (1988b) The loci of perception for phytochrome control of internode growth in light-grown mustard: promotion by low phytochrome photoequilibria in the internode is enhanced by blue light perceived by the leaves. Planta 176: 277–282 [DOI] [PubMed] [Google Scholar]
- Cerrudo I, Keller MM, Cargnel MD, Demkura PV, de Wit M, Patitucci MS, Pierik R, Pieterse CM, Ballaré CL. (2012) Low red/far-red ratios reduce Arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol 158: 2042–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Qin G, Dai X, Zhao Y. (2008) NPY genes and AGC kinases define two key steps in auxin-mediated organogenesis in Arabidopsis. Proc Natl Acad Sci USA 105: 21017–21022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child R, Smith H. (1987) Phytochrome action in light-grown mustard: kinetics, fluence-rate compensation and ecological significance. Planta 172: 219–229 [DOI] [PubMed] [Google Scholar]
- Cifuentes-Esquivel N, Bou-Torrent J, Galstyan A, Gallemí M, Sessa G, Salla Martret M, Roig-Villanova I, Ruberti I, Martínez-García JF. (2013) The bHLH proteins BEE and BIM positively modulate the shade avoidance syndrome in Arabidopsis seedlings. Plant J 75: 989–1002 [DOI] [PubMed] [Google Scholar]
- Dechaine JM, Johnston JA, Brock MT, Weinig C. (2007) Constraints on the evolution of adaptive plasticity: costs of plasticity to density are expressed in segregating progenies. New Phytol 176: 874–882 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Rood SB, Somers DE, Quail PH, Whitelam GC. (1992) Photophysiology of the elongated internode (ein) mutant of Brassica rapa: ein mutant lacks a detectable phytochrome B-like polypeptide. Plant Physiol 100: 1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Somers DE, Quail PH, Whitelam GC. (1997) The Brassica rapa elongated internode (EIN) gene encodes phytochrome B. Plant Mol Biol 34: 537–547 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Yanovsky MJ, Kay SA. (2003) A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol 133: 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Nakamura S, Araki T, Mochizuki N, Nagatani A. (2005) Phytochrome B in the mesophyll delays flowering by suppressing FLOWERING LOCUS T expression in Arabidopsis vascular bundles. Plant Cell 17: 1941–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Praekelt U, Stoddart WM, Billingham OE, Halliday KJ, Whitelam GC. (2003) Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol 131: 1340–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, et al. (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- García-Martínez JL, Keith B, Bonner BA, Stafford AE, Rappaport L. (1987) Phytochrome regulation of the response to exogenous gibberellins by epicotyls of Vigna sinensis. Plant Physiol 85: 212–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison R, Briggs WR. (1975) The growth of internodes in Helianthus in response to far-red light. Bot Gaz 136: 353–357 [Google Scholar]
- Gotô N, Suzuki M. (1980) Far red light control of elongation in light-grown bean hypocotyl: effect on different age zones and interaction with indole-3-acetic acid. Plant Cell Physiol 21: 105–113 [Google Scholar]
- Hayashi K, Neve J, Hirose M, Kuboki A, Shimada Y, Kepinski S, Nozaki H. (2012) Rational design of an auxin antagonist of the SCF(TIR1) auxin receptor complex. ACS Chem Biol 7: 590–598 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Tan X, Zheng N, Hatate T, Kimura Y, Kepinski S, Nozaki H. (2008) Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proc Natl Acad Sci USA 105: 5632–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch M, Lorrain S, de Wit M, Trevisan M, Ljung K, Bergmann S, Fankhauser C. (2014) Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc Natl Acad Sci USA 111: 6515–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, López-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, et al. (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J 71: 699–711 [DOI] [PubMed] [Google Scholar]
- Izquierdo NG, Aguirrezabal LAN, Andrade FH, Geroudet C, Valentinuz O, Pereyra Iraola M. (2009) Intercepted solar radiation affects oil fatty acid composition in crop species. Field Crops Res 114: 66–74 [Google Scholar]
- Jaillais Y, Hothorn M, Belkhadir Y, Dabi T, Nimchuk ZL, Meyerowitz EM, Chory J. (2011) Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibitor. Genes Dev 25: 232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LA, Peeters AJ, Pierik R. (2010) Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA 107: 22740–22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM, Rutschow HL, Mabie SS. (2011) AuxV: a database of auxin transport velocities. Trends Plant Sci 16: 461–463 [DOI] [PubMed] [Google Scholar]
- Kunvshinov V, Koivu K, Kanerva A, Pehu E. (1999) Agrobacterium tumefaciens-mediated transformation of greenhouse-grown Brassica rapa ssp. oleifera. Plant Cell Rep 18: 773–777 [Google Scholar]
- Li L, Ljung K, Breton G, Schmitz RJ, Pruneda-Paz J, Cowing-Zitron C, Cole BJ, Ivans LJ, Pedmale UV, Jung HS, et al. (2012) Linking photoreceptor excitation to changes in plant architecture. Genes Dev 26: 785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Ma L, Sun N, Liu X, Jiao Y, Zhao H, Deng XW. (2005) Organ-specific expression of Arabidopsis genome during development. Plant Physiol 138: 80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia JF, Garcia-Martinez JL. (1992) Interaction of gibberellins and phytochrome in the control of cowpea epicotyl elongation. Physiol Plant 86: 236–244 [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al. (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA 108: 18512–18517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren JS, Smith H. (1978) Phytochrome control of the growth and development of Rumex obtusifolius under simulated canopy light environments. Plant Cell Environ 1: 61–67 [Google Scholar]
- Morgan DC, O'Brien TO, Smith H. (1980) Rapid photomodulation of stem extension in light-grown Sinapis alba L.: studies on kinetics, site of perception and photoreceptor. Planta 150: 95–101 [DOI] [PubMed] [Google Scholar]
- Morinaka Y, Sakamoto T, Inukai Y, Agetsuma M, Kitano H, Ashikari M, Matsuoka M. (2006) Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol 141: 924–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngaki MN, Louie GV, Philippe RN, Manning G, Pojer F, Bowman ME, Li L, Larsen E, Wurtele ES, Noel JP. (2012) Evolution of the chalcone-isomerase fold from fatty-acid binding to stereospecific catalysis. Nature 485: 530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana D, van den Boogaart T, O’Neill CM, Hynes L, Bent E, Macpherson L, Park JY, Lim YP, Bancroft I. (2004) Conservation of the microstructure of genome segments in Brassica napus and its diploid relatives. Plant J 40: 725–733 [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P, Whitelam GC, Smith H. (1993) Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol 102: 1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury FJ, Hall A, Grierson CS, Halliday KJ. (2007) Phytochrome coordinates Arabidopsis shoot and root development. Plant J 50: 429–438 [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Chinnappa CC, Staal M, Elzenga JT, Yokoyama R, Nishitani K, Voesenek LA, Pierik R. (2010) Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases. Plant Physiol 154: 978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhu L, Castillon A, Majee M, Downie B, Huq E. (2008) Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20: 1586–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Khanna R, Carle CM, Quail PH. (2007) Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol 145: 1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle JR, Sooudi SK, Jones RL. (1992) Adaptation to dim-red light leads to a nongradient pattern of stem elongation in Cucumis seedlings. Plant Physiol 99: 808–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108 [DOI] [PubMed] [Google Scholar]
- Somers DE, Quail PH. (1995) Temporal and spatial expression patterns of PHYA and PHYB genes in Arabidopsis. Plant J 7: 413–427 [DOI] [PubMed] [Google Scholar]
- Stephenson P, Baker D, Girin T, Perez A, Amoah S, King GJ, Østergaard L. (2010) A rich TILLING resource for studying gene function in Brassica rapa. BMC Plant Biol 10: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Nakamura S, Mochizuki N, Nagatani A. (2002) Phytochrome in cotyledons regulates the expression of genes in the hypocotyl through auxin-dependent and -independent pathways. Plant Cell Physiol 43: 1171–1181 [DOI] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Koloszvari M, Quail PH. (1996) Two small spatially distinct regions of phytochrome B are required for efficient signaling rates. Plant Cell 8: 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun JH, Bancroft I, Cheng F, et al. (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43: 1035–1039 [DOI] [PubMed] [Google Scholar]
- Williams PH, Hill CB. (1986) Rapid-cycling populations of Brassica. Science 232: 1385–1389 [DOI] [PubMed] [Google Scholar]
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y. (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci USA 108: 18518–18523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN., Jr (2006) Statistical analysis of real-time PCR data. BMC Bioinformatics 7: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.