A fasciclin-like arabinogalactan protein mediates Arabidopsis seed coat mucilage adherence primarily through its influence on pectin.
Abstract
Interactions between cell wall polymers are critical for establishing cell wall integrity and cell-cell adhesion. Here, we exploit the Arabidopsis (Arabidopsis thaliana) seed coat mucilage system to examine cell wall polymer interactions. On hydration, seeds release an adherent mucilage layer strongly attached to the seed in addition to a nonadherent layer that can be removed by gentle agitation. Rhamnogalacturonan I (RG I) is the primary component of adherent mucilage, with homogalacturonan, cellulose, and xyloglucan constituting minor components. Adherent mucilage contains rays composed of cellulose and pectin that extend above the center of each epidermal cell. CELLULOSE SYNTHASE5 (CESA5) and the arabinogalactan protein SALT-OVERLY SENSITIVE5 (SOS5) are required for mucilage adherence through unknown mechanisms. SOS5 has been suggested to mediate adherence by influencing cellulose biosynthesis. We, therefore, investigated the relationship between SOS5 and CESA5. cesa5-1 seeds show reduced cellulose, RG I, and ray size in adherent mucilage. In contrast, sos5-2 seeds have wild-type levels of cellulose but completely lack adherent RG I and rays. Thus, relative to each other, cesa5-1 has a greater effect on cellulose, whereas sos5-2 mainly affects pectin. The double mutant cesa5-1 sos5-2 has a much more severe loss of mucilage adherence, suggesting that SOS5 and CESA5 function independently. Double-mutant analyses with mutations in MUCILAGE MODIFIED2 and FLYING SAUCER1 that reduce mucilage release through pectin modification suggest that only SOS5 influences pectin-mediated adherence. Together, these findings suggest that SOS5 mediates adherence through pectins and does so independently of but in concert with cellulose synthesized by CESA5.
Cellulosic cell walls are a defining feature of land plants. Primary cell walls are composed of three major classes of polysaccharides: cellulose, hemicelluloses, and pectins. In addition, approximately 10% of the primary cell wall is composed of protein (Burton et al., 2010). Cell walls provide mechanical support for the cell, and cell wall carbohydrates in the middle lamellae mediate cell-cell adhesion (Caffall and Mohnen, 2009). Current models of cell wall structure depict a cellulose-hemicellulose network embedded in an independent pectin gel (for review, see Albersheim et al., 2011). These components are believed to interact through both covalent and noncovalent bonds to provide structure and strength to the cell wall, although the relative importance of pectin and its interactions with the hemicellulose-cellulose network remain unclear (for review, see Cosgrove, 2005).
Another gap in our understanding of cell wall structure and assembly is the role of arabinogalactan proteins (AGPs). AGPs are a family of evolutionarily conserved secreted proteins highly glycosylated with type II arabinogalactans, and they can be localized to the plasma membrane by a C-terminal glycophosphatidylinositol (GPI) lipid anchor (for review, see Schultz et al., 2000; Showalter, 2001; Johnson et al., 2003; Seifert and Roberts, 2007; Ellis et al., 2010). AGPs can be extensively modified in the cell wall; many glycosyl hydrolases can affect AGP function by cleaving their glycosyl side chains (Sekimata et al., 1989; Cheung et al., 1995; Wu et al., 1995; Kotake et al., 2005). The GPI anchor can also be cleaved, releasing the AGPs from the membrane into the cell wall (Schultz et al., 2000). Although their exact roles are still unclear, AGPs have been proposed to interact with cell wall polysaccharides, initiate intracellular signaling cascades, and influence a wide variety of biological processes (for review, see Seifert and Roberts, 2007; Ellis et al., 2010; Tan et al., 2013).
Many fasciclin-like AGPs (FLAs), which contain at least one fasciclin domain (FAS) associated with protein-protein interactions, have been suggested to influence cellulose biosynthesis or organization (Seifert and Roberts, 2007; Li et al., 2010; MacMillan et al., 2010). FLA3 RNA interference lines have reduced intine cell wall biosynthesis and loss of Calcofluor white (a fluorescent dye specific for glycan molecules) staining in aborted pollen grains (Li et al., 2010). A fla11 fla12 double mutant was shown to have reduced cellulose deposition, altered cellulose microfibril angle, and reduced cell wall integrity (MacMillan et al., 2010). The fla11 fla12 double mutant also had reductions in arabinans, galactans, and rhamnose (MacMillan et al., 2010). FLA4/SALT-OVERLY SENSITIVE5 (SOS5) was identified in a screen for salt sensitivity in roots. The SOS5 gene encodes an FLA protein with a GPI anchor, two AGP-like domains, and two FAS domains (Shi et al., 2003). Plants homozygous for the loss-of-function conditional allele sos5-1 have thinner root cell walls that appear less organized (Shi et al., 2003). The presence of the two FAS domains has led to the suggestion that SOS5 may interact with other proteins, forming a network that strengthens the cell wall (Shi et al., 2003). SOS5 is involved in regulation of cell wall rheology through a pathway involving two Leu-rich repeat receptor-like kinases, FEI1 and FEI2 (Xu et al., 2008). SOS5 and FEI2 are also required for normal seed coat mucilage adherence and hypothesized to do so by influencing cellulose biosynthesis (Harpaz-Saad et al., 2011, 2012).
Arabidopsis (Arabidopsis thaliana) seed coat mucilage is a powerful model for studying cell wall biosynthesis and polysaccharide interactions (Arsovski et al., 2010; Haughn and Western, 2012). Seed coat epidermal cells sequentially produce two distinct types of secondary cell walls with unique morphologies and properties (Western et al., 2000; Windsor et al., 2000). Between approximately 5 and 9 d approximate time of fertilization (DPA), seed coat epidermal cells synthesize mucilage and deposit it in the apoplast, creating a donut-shaped mucilage pocket that surrounds a central cytoplasmic column (Western et al., 2000, 2004; Haughn and Chaudhury, 2005). From 9 to 13 DPA, the cytoplasmic column is gradually replaced by a cellulose-rich, volcano-shaped secondary cell wall called the columella (Beeckman et al., 2000; Western et al., 2000; Windsor et al., 2000; Stork et al., 2010; Mendu et al., 2011).
Seed mucilage is composed primarily of relatively unbranched rhamnogalacturonan I (RG I) with minor amounts of homogalacturonan (HG), cellulose, and hemicelluloses (for review, see Haughn and Western, 2012). When mucilage is hydrated, it expands rapidly from the apoplastic pocket, forming a halo that surrounds the seed. Mucilage separates into two fractions: a loose nonadherent fraction and an inner adherent fraction that can only be released by vigorous shaking, strong bases, or glycosidases (for review, see North et al., 2014). Galactans and arabinans are also present in mucilage, and their regulation by glycosidases is required for correct mucilage hydration (Dean et al., 2007; Macquet et al., 2007b; Arsovski et al., 2009). For example, β-XYLOSIDASE1 encodes a bifunctional β-d-xylosidase/α-l-arabinofuranosidase required for arabinan modification in mucilage, and β-xylosidase1 mutant seeds have a delayed mucilage release phenotype (Arsovski et al., 2009). MUCILAGE MODIFIED2 (MUM2) encodes a β-d-galactosidase, and mum2 seeds fail to release mucilage when hydrated in water (Dean et al., 2007; Macquet et al., 2007b). MUM2 is believed to modify RG I galactan side chains but may also affect the galactan component of other mucilage components (Dean et al., 2007; Macquet et al., 2007b). Galactans are capable of binding to cellulose in vitro and could affect mucilage hydration through pectin-cellulose interactions (Zykwinska et al., 2005, 2007a, 2007b; Dick-Pérez et al., 2011; Wang et al., 2012), although carbohydrate linkage analysis suggests that the galactan side chains are very short.
Several studies indicate that seed mucilage extrusion and expansion are also influenced by methylesterification of HG. For example, both SUBTILISIN-LIKE SER PROTEASE1.7 and PECTIN METHYLESTERASE INHIBITOR6 are required for proper methyl esterification of mucilage (Rautengarten et al., 2008; Saez-Aguayo et al., 2013). Mutations in another gene, FLYING SAUCER1 (FLY1; a transmembrane E3 ubiquitin ligase), reduce the degree of pectin methylesterification in mucilage and cause increased mucilage adherence and defective mucilage extrusion (Voiniciuc et al., 2013). fly1 seeds have disc-like structures at the edge of the mucilage halo, which are outer primary cell wall fragments that detach from the columella during extrusion and are difficult to separate from the adherent mucilage (Voiniciuc et al., 2013).
Recently, CELLULOSE SYNTHASE5 (CESA5) and SOS5 were proposed to facilitate cellulose-mediated mucilage adherence (Harpaz-Saad et al., 2011; Mendu et al., 2011; Sullivan et al., 2011). A simple hypothesis for the role of CESA5 in mucilage adherence is that it synthesizes cellulose, which interacts with the mucilage pectin to mediate adherence. Loss of CESA5 function results in a reduction of mucilage cellulose biosynthesis and a less adherent mucilage cell wall matrix (Mendu et al., 2011; Sullivan et al., 2011). The role of SOS5 in mucilage adherence is more difficult to explain. SOS5 null mutations cause a loss-of-adherence phenotype similar to cesa5-1 seeds, suggesting that SOS5 may regulate mucilage adherence by influencing CESA5 function (Harpaz-Saad et al., 2011). However, the mechanism through which SOS5 could influence CESA5 and/or cellulose biosynthesis is not clear.
To better understand the role of SOS5 in mucilage adherence and its relationship to CESA5, we thoroughly investigated the seed coat epidermal cell phenotypes of the cesa5-1 and sos5-2 single mutants as well as those of the cesa5-1 sos5-2 double mutant. We also investigated how cellulose, SOS5, and pectin interact to mediate mucilage adherence by constructing double mutants with either cesa5-1 or sos5-2 together with either mum2-1 or fly1. Our results suggest that SOS5 mediates mucilage adherence independently of CESA5. Furthermore, compared with CESA5, SOS5 has a greater influence on mucilage pectin structure, suggesting that SOS5 mediates mucilage adherence through pectins, not cellulose.
RESULTS
Both SOS5 and CESA5 Are Expressed in the Seed Coat Epidermal Cells
CESA5 is expressed in seed coat epidermal cells throughout seed development, with a peak in expression at approximately 7 DPA (Sullivan et al., 2011). Using RNA extracted from seed coats, we examined the expression of SOS5 during seed development. SOS5 is highly expressed at 4 and 7 DPA, whereas expression levels are reduced at 10 DPA (Supplemental Fig. S1A). This expression pattern is similar to that observed for SOS5 using microarray analysis of expression during seed coat development (Supplemental Fig. S1B; Dean et al., 2011; http://bar.utoronto.ca/efp_seedcoat/cgi-bin/efpWeb.cgi).
The sos5 and cesa5 Mutant Seed Mucilage Phenotypes Are Distinct, and the Double-Mutant Phenotype Is More Severe
sos5-2 and cesa5-1 are strong loss-of-function alleles (Xu et al., 2008; Sullivan et al., 2011), both showing a loss of mucilage adherence after seed hydration. To better understand the functions of CESA5 and SOS5, both single mutants were characterized in detail, and cesa5-1 sos5-2 double mutants were isolated and examined.
When hydrated directly in ruthenium red (RR), a dye that binds pectins (Sterling, 1970), mucilage extrudes rapidly from wild-type, cesa5-1, and sos5-2 seeds (Fig. 1, A–C), whereas cesa5-1 sos5-2 seeds did not extrude mucilage as uniformly (Fig. 1D, arrow). After 1 h of shaking in water, wild-type seeds have a strong RR-stained adherent layer (Fig. 1E), whereas in cesa5-1, sos5-2, and cesa5-1 sos5-2 seeds, the RR-stained halo is almost completely absent (Fig. 1, F–H). During mucilage extrusion, the outer primary cell wall of the wild-type seed coat epidermal cells ruptures at the radial wall and remains attached to the columella, where it extends perpendicularly to the seed surface (Fig. 1I, arrow). The outer primary wall of seed coat epidermal cells of both cesa5-1 and sos5-2 ruptures similarly to the wild type (arrows in Fig. 1, J and K), whereas those of cesa5-1 sos5-2 seeds did not (Fig. 1L). In cesa5-1 sos5-2 seeds, the outer walls of most cells either remain intact or partially detach from the parent seed as a sheet (Fig. 1L, arrow). Additionally, the double mutant had smaller and fewer columellae compared with wild-type, cesa5-1, and sos5-2 seeds (arrowheads in Fig. 1, I–L).
Figure 1.
cesa5-1 sos5-2 has a more severe mucilage phenotype. A to D, Seeds stained with RR (unshaken). The arrow indicates the region with reduced mucilage release. E to H, Seeds shaken in water and stained with RR. I to L, Higher magnification of RR-stained seeds. Arrows indicate the primary wall fragments, and arrowheads indicate the columellae. M to P, Seeds shaken in 0.05 m EDTA and then stained with RR. Q to T, S4B-stained seeds illustrating the cellulosic ray (arrows) above the columellae and the diffuse staining mucilage. Arrowhead indicates brightly staining primary wall fragments. Bars in A to H and M to P = 100 μm. Bars in I to L and Q to T = 10 μm.
Wild-type seeds hydrated in EDTA, a divalent cation chelator, display a larger mucilage halo that stains less intensely with RR compared with water-treated seeds (Fig. 1M). EDTA-treated cesa5-1 and sos5-2 mutant seeds also show a larger mucilage halo and increased RR staining than the same seeds hydrated in water (Fig. 1, N and O) but had a smaller halo and reduced RR staining intensity compared with the wild type. EDTA-treated cesa5-1 sos5-2 seeds show very little RR staining, and the halo size is much smaller than either single mutant (Fig. 1P).
The differences between cesa5-1 and sos5-2 seeds are most obvious when seeds are stained with Pontamine fast scarlet S4B (S4B), a fluorescent dye that binds preferentially to cellulose (Fig. 1, Q–T; Anderson et al., 2010; Harpaz-Saad et al., 2011; Mendu et al., 2011). Wild-type seeds are surrounded by a large S4B-stained halo composed of several distinct regions (Fig. 1Q). The primary wall remnants, which remain attached to the columella, stain very brightly (Fig. 1Q, arrowhead). Above each columella, cellulosic rays are clearly observable, extending to the periphery of the adherent mucilage halo (Fig. 1Q, arrow). Wild-type mucilage also has a diffuse S4B-stained region between the rays (Mendu et al., 2011). S4B-stained cesa5-1 seeds lack this diffuse staining pattern but retain the rays that are located above the columella, albeit at lower intensity (Fig. 1R, arrow). In contrast to cesa5-1 seeds, sos5-2 seeds lack distinct rays entirely but retain the diffuse staining pattern (Fig. 1S), even immediately after hydration (Supplemental Fig. S2), suggesting that SOS5 is needed to establish and/or maintain the ray structure. The cesa5-1 sos5-2 double-mutant seed mucilage is distinct from that of both single mutants in that it lacks both the rays and the diffuse regions of S4B labeling (Fig. 1T). These results reveal that the single-mutant phenotypes are distinct from each other and that the double mutant has a more severe phenotype than either of the single mutants.
SOS5 and CESA5 Act Independently to Promote Seed Mucilage Adherence
To investigate the differences between sos5 and cesa5 seed mucilage phenotypes more thoroughly, we used an indirect immunolabeling approach with cellulose binding modules and antipectin antibodies. We used two carbohydrate-binding modules (CBMs; CBM3a and CBM28) in parallel with S4B staining to further examine the distribution of cellulose in the adherent mucilage. CBM3a binds preferentially to crystalline cellulose structures, whereas CBM28 binds preferentially to amorphous cellulose structures (Blake et al., 2006; Dagel et al., 2011). In wild-type adherent mucilage, there are obvious differences in the staining pattern of S4B and CBM3a (Fig. 2A). CBM3a strongly labeled the entire adherent mucilage halo with slightly stronger labeling at the outer periphery and along the rays, whereas S4B stains the inner adherent layer and the rays above the columella (Fig. 2A; Supplemental Fig. S3). In cesa5-1 seeds, CBM3a labeling seemed disorganized and reduced in width compared with the wild type, consistent with a general reduction in cellulose biosynthesis and loss of mucilage adherence (Fig. 2B). CBM3a labeling of cesa5-1 mucilage appeared concentrated around the ray structures (Fig. 2B, arrow) and is less intense than the wild type between the rays. In contrast to cesa5-1, CBM3a-labeled sos5-2 seeds lacked the ray-like structures (Fig. 2C). The cesa5-1 sos5-2 seeds had more severe defects than either single mutant, with an almost complete loss of CBM3a and S4B labeling (Fig. 2D; Supplemental Fig. S3). Some areas of cesa5-1 sos5-2 mucilage show small, weakly labeling, disorganized tufts (Fig. 2D, arrow).
Figure 2.
cesa5-1 and sos5-2 have differences in adherent mucilage cell wall epitopes. Images of CBM or pectin antibody seed mucilage immunolabeling (green) merged with S4B staining (magenta). Seed mucilage labeled for crystalline cellulose with CBM3a (A–D), amorphous cellulose with CBM28 (E–H), unsubstituted RG I with CCRC-M36 (I–L), and low-methylesterified HG with JIM5 (M-P). Bar = 10 μm.
CBM28 labeling of mucilage was reduced compared with CBM3a and other pectin-specific antibodies (signal intensities for CBM28 were increased relative to other antibody treatments). Wild-type mucilage labeled with CBM28 was diffuse; the signal appeared punctate, with a slight increase in labeling around the rays (Fig. 2E; Supplemental Fig. S4). CBM28 labeling of cesa5-1 seeds had a similar pattern as the wild type, with reduced intensity (Fig. 2F). CBM28 labeling of sos5-2 seeds was reduced compared with wild-type and cesa5-1 seeds, whereas in cesa5-1 sos5-2 double mutant, mucilage labeling was almost entirely absent (Fig. 2, G and H).
Two pectin antibodies, John Innes Monoclonal-antibody5 (JIM5) and Complex Carbohydrate Research Center-Monoclonal-antibody36 (CCRC-M36), were used in conjunction with S4B staining to further examine the distribution of pectin relative to cellulose in the adherent mucilage. JIM5 is specific for partially methylesterified HG (up to 40%; Vandenbosch et al., 1989; Knox et al., 1990; Willats et al., 2000), whereas CCRC-M36 is specific for the unsubstituted RG I backbone that is found in Arabidopsis seed mucilage (Young et al., 2008; Arsovski et al., 2009; Pattathil et al., 2010). CCRC-M36 strongly labeled the periphery of the mucilage halo in wild-type seeds (Fig. 2I; Supplemental Fig. S5). CCRC-M36 labeling in wild-type seeds appeared to surround the ray structures at the periphery of the mucilage halo (Fig. 2I, arrow; Supplemental Fig. S5). The CCRC-M36 labeling signal of cesa5-1 seeds appeared concentrated around the S4B-labeled rays (Fig. 2J, arrow) and is reduced at the edge of the mucilage halo and between the rays. CCRC-M36 labeling is almost absent in sos5-2 seeds and was not concentrated in a ray-like manner (compare Fig. 2I with Fig. 2K). Finally, CCRC-M36 labeling was completely absent in cesa5-1 sos5-2 double-mutant seeds (Fig. 2L).
The distribution of partially methylesterified HG was also examined in these mutant lines with JIM5 (Fig. 2; Supplemental Fig. S6). In wild-type seeds, JIM5 specifically labeled the rays that extend above the columella and throughout the adherent layer (Fig. 2M, arrow). JIM5 labeling is reduced in cesa5-1 mutant seed mucilage, located only at the base of the rays just above the columella (Fig. 2N, arrow). sos5-2 and cesa5-1 sos5-2 mucilage did not label with JIM5 at all; only the primary wall that remains attached to the columella showed any signal (Fig. 2, O and P). Overall, the distribution of cellulose and pectins in adherent mucilage was clearly different between cesa5-1 and sos5-2, and cellulose staining was entirely absent in cesa5-1 sos5-2 seeds. sos5-2 seemed to have a greater effect on the adherence of pectin and the organization of cellulose than cesa5-1. These results suggest that CESA5 and SOS5 function through independent mechanisms to mediate mucilage adherence.
Because SOS5 is an AGP, we wanted to investigate the distribution of AGP epitopes in mucilage using arabinogalactan and AGP-specific antibodies. Wild-type, cesa5-1, and sos5-2 seeds were labeled with JIM13 [epitope: β-d-GlcA-(1,3)-α-d-GalA-(1,2)-α-l-Rha; Knox et al., 1991; Yates and Knox, 1994; Yates et al., 1996; Majewska-Sawka and Nothnagel, 2000], MAC207 [epitope: α-GlcA-(1,3)-α-GalA-(1,2)-α-Rha; Bradley et al., 1988; Pennell et al., 1989; Yates and Knox, 1994; Yates et al., 1996; Pattathil et al., 2010], JIM8 (epitope: arabinogalactan; Pennell et al., 1991; Majewska-Sawka and Nothnagel, 2000), and CCRC-M7 (epitope: recognizes 6-linked β-d-Gal oligomers that contain arabinose; Puhlmann et al., 1994; Steffan et al., 1995; Pattathil et al., 2010) antibodies (Supplemental Figs. S7 and S8). Of these antibodies, only JIM13 showed any significant signal in the adherent mucilage halo, which appeared to be localized to the ray structures above the columella (Supplemental Fig. S8). Interestingly, JIM13 label was absent in sos5-2 seeds, whereas cesa5-1 seeds showed minor labeling located at the base of the rays (Supplemental Fig. S8, arrow). The fact that JIM13 recognizes a β-d-GlcpUA-(1-3)-α-d-GalpUA-(1-2)-l-Rha trisaccharide indicates that this trisaccharide is part of the ray structure and suggests the presence of AGP proteins in mucilage.
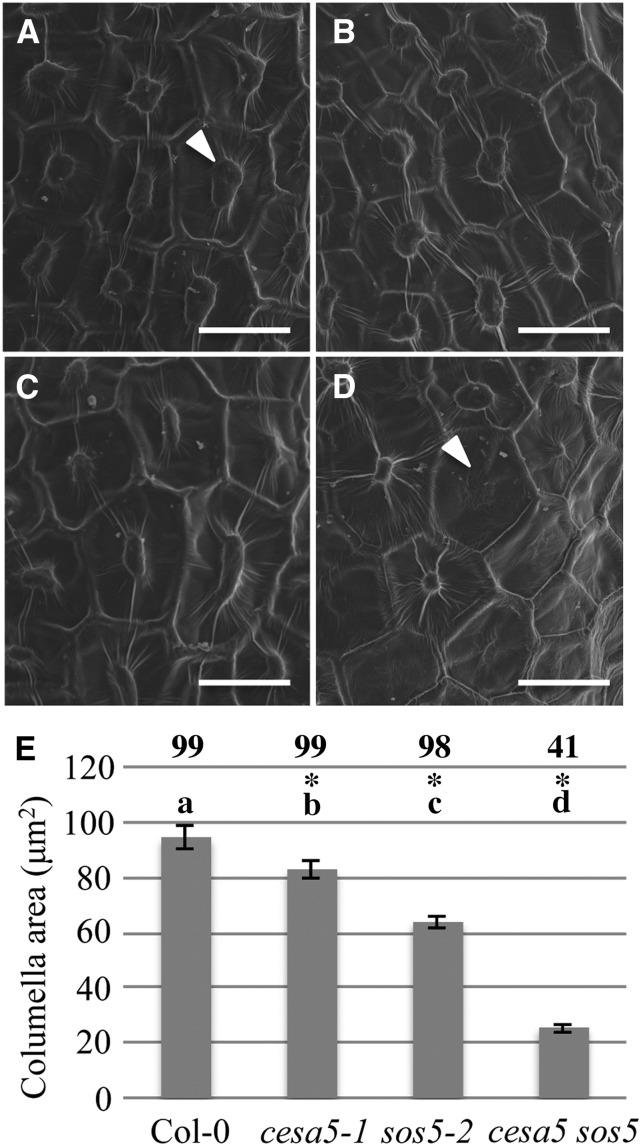
CESA5 and SOS5 Are Both Required for Normal Columella Formation
During the analysis of seed mucilage, cesa5-1 sos5-2 columellae often appeared smaller than in the wild type and sometimes completely absent. We, therefore, examined the surface of seed coat epidermal cells using scanning electron microscopy. Wild-type seed coat epidermal cells were hexagonal in shape and delineated by the radial wall, which appeared as an elevated ridge between cells (Fig. 3). In the wild type, columellae were present in the center of each seed coat epidermal cell (Fig. 3A, arrowhead) surrounded by a recessed mucilage pocket. Columellae in cesa5-1 seed coat epidermal cells appeared morphologically similar to the wild type, whereas in sos5-2 seeds, they appeared slightly smaller. Consistent with the observations made during mucilage staining experiments, 59% of the cesa5-1 sos5-2 epidermal cells lacked observable columellae (Fig. 3, D, arrowhead and E), and when columellae were present, they appeared much smaller than in the wild type.
Figure 3.
Columella formation is severely impaired in cesa5-1 sos5-2 mutant seeds. Scanning electron microscopy of the seed coat surface of Col-0 (A), cesa5-1 (B), sos5-2 (C), and cesa5-1 sos5-2 (D). Bar = 25 μm. E, Quantification of columella area. Numbers above each bar represent the percentage of cells that contain a visible columella for each genotype. Means for each genotype not sharing the same letter are significantly different (Tukey's HSD, P < 0.05). *, Significant difference from the wild type (two-way ANOVA, P < 0.05).
The size of the columella top was quantified by measuring the surface area of 80 columellae from at least six different seeds for each genotype (Fig. 3E). Compared with the wild type, cesa5-1 seeds had approximately 10% smaller columellae, consistent with previous results (Mendu et al., 2011), whereas sos5-2 showed an approximately 30% decrease in columella area (Fig. 3E). A two-way ANOVA, with CESA5 and SOS5 as independent variables, shows that the columella area of cesa5-1 and sos5-2 seeds is significantly smaller than that of the wild type (two-way ANOVA, P < 0.0001; Fig. 3E). The columellae present in cesa5-1 sos5-2 seeds were approximately 70% smaller than the wild type. A two-way ANOVA analysis was used to detect interactions between the effects of cesa5 and sos5 in the double mutant. Because the columella shape for cesa5-1 sos5-2 seeds was significantly reduced relative to that of all other genotypes (two-way ANOVA, P < 0.0001), cesa5-1 and sos5-2 mutations affect columella area in a synergistic manner in double-mutant seeds. A Tukey’s honestly significant difference (HSD) post hoc test showed that the effects of each genotype (the wild type, cesa5-1, sos5-2, and cesa5-1 sos5-2) on columella area were significantly different from each other (P < 0.05). No significant reduction in epidermal cell surface area was detected between wild-type and mutant seeds, indicating that the observed changes are specific to the columella (two-way ANOVA, P > 0.05; Supplemental Fig. S9).
The smaller columellae observed in cesa5-1 sos5-2 seeds could result from changes in the shape of the cytoplasmic column or deposition of the secondary cell wall. To determine the time of onset of columella defects, seed coat epidermal cells were examined before (8 DPA), during (10 DPA), and after (mature) columella deposition. Cryo-fixed developing seeds and glutaraldehyde-fixed mature seeds were resin embedded, sectioned, and stained with toluidine blue (Fig. 4). At 8 DPA, Columbia-0 (Col-0), cesa5-1, and sos5-2 seed coat epidermal cells displayed a large purple-stained mucilage pocket surrounding a central cytoplasmic column filled with amyloplasts (Fig. 4). Mucilage pockets in cesa5-1 sos5-2 double mutants were apparent, but they were broader in shape, and the cytoplasmic column was typically narrower than in wild-type and single-mutant seeds (Fig. 4D). By 10 DPA, wild-type seeds had nearly completed columella deposition. At this stage, columellae stained a darker purple than the mucilage pockets. Both cesa5-1 and sos5-2 seeds at 10 DPA had columellae similar in size to the wild type, whereas cesa5-1 sos5-2 columellae were either narrower or entirely absent at this developmental stage (Fig. 4H). Glutaraldehyde fixation of mature seeds causes the mucilage pocket to burst, leaving the columella and radial walls. Some cesa5-1 sos5-2 seed epidermal cells lacked a columella (Fig. 4L), despite substantial secondary cell wall deposition in the basal region of the cell. Similar observations of epidermal cell development were obtained using live imaging of seeds treated with propidium iodide at 8, 10, and 12 DPA (Supplemental Fig. S10). In summary, analysis of developing and mature seeds indicates that cesa5-1 sos5-2 seed coat epidermal cells had reduced or absent columellae. However, the columella defects seem to originate from an altered morphology of the cytoplasmic column before the onset of columella deposition.
Figure 4.
Cytoplasmic columns are smaller in cesa5-1 sos5-2 seed coat epidermal cells. Columella development in cryo-fixed (A to H) and glutaraldehyde-fixed (I–L) resin-embedded seed coat epidermal cells stained with toluidine blue. A to D, 8 DPA. E to H, 10 DPA. I to L, Mature seeds. A, E, and I, Col-0. B, F, and J, cesa5-1. C, G, and K, sos5-2. D, H, and L, cesa5-1 sos5-2. C, Columella; cc, cytoplasmic column; m, mucilage; mp, mucilage pocket. Bar = 10 μm.
SOS5 Affects Mucilage Adherence But Not Cellulose Deposition in Seeds
If the role of SOS5 in mucilage adherence is because of its effect on cellulose deposition, then changes in the amount or pattern of cellulose analogous to that of cesa5 should be evident in sos5 mutant seeds. To test this hypothesis, we determined the monosaccharide composition (alcohol insoluble residue) of whole seeds using high-performance anion-exchange chromatography (HPAEC; Fig. 5; Supplemental Table S1). Mucilage contains a significant percentage of the Rha and GalA found in whole seed, and therefore, large changes in the synthesis of mucilage components can be detected in whole-seed monosaccharide levels. We again used a two-way ANOVA to analyze the effects of CESA5 and SOS5 on monosaccharide levels. The amount of Rha and GalA in cesa5-1, sos5-2, and cesa5-1 sos5-2 whole seeds was similar to that of the wild type, suggesting that neither SOS5 nor CESA5 affected the overall amount of mucilage produced (Fig. 5A). However, there was a statistically significant reduction in Glc levels in cesa5-1 seeds relative to the wild type (two-way ANOVA, P < 0.05; Fig. 5A; Supplemental Table S1), consistent with CESA5 being involved in cellulose biosynthesis. In contrast, the amount of Glc in sos5-2 seeds was not significantly different from the wild type (two-way ANOVA, P > 0.05; Supplemental Table S1), and no significant interaction was detected between CESA5 and SOS5 (two-way ANOVA, cesa5-1 sos5-2, P > 0.05; Supplemental Table S1), suggesting that, unlike CESA5, SOS5 does not directly affect the amount of cellulose biosynthesis.
Figure 5.
SOS5 is not involved in cellulose biosynthesis in seeds. A, Whole-seed alcohol-insoluble residue (AIR) monosaccharide amounts. B, Crystalline cellulose amount in whole seeds. C, Nonadherent mucilage Rha and GalA amounts. D, Nonadherent mucilage Glc amounts. Means not sharing the same letter are significantly different (Tukey's HSD, P < 0.05). *, Significant difference from the wild type for single mutants and a significant interaction between genes for double mutants (two-way ANOVA, P < 0.05).
To more specifically investigate cellulose biosynthesis in cesa5-1 and sos5-2 seeds, the crystalline cellulose content of whole seeds was quantified using a modified method by Updegraff (1969; Fig. 5B). Consistent with the pattern observed for Glc in whole seeds, cellulose quantities were significantly reduced in cesa5-1 seeds compared with the wild type (two-way ANOVA, P < 0.005; Supplemental Table S2) but not in sos5-2 seeds (two-way ANOVA, P > 0.05). No significant interaction was detected (cesa5-1 sos5-2, two-way ANOVA, P > 0.05), indicating that only CESA5 significantly affects crystalline cellulose quantities, a conclusion supported by Tukey’s HSD post hoc analysis (P < 0.05; Supplemental Table S2). These data suggest that SOS5 has a limited, if any, role in the amount of cellulose synthesized in seeds and that it is mediating its effects on mucilage adherence and columella morphology through other mechanism(s).
We also examined the monosaccharide levels of nonadherent mucilage. Rha and GalA are the major monosaccharides found in mucilage carbohydrates (approximately 80%) and representative of total mucilage adherence in general. In cesa5-1 and sos5-2 seeds, the nonadherent mucilage fraction contained approximately 20% more Rha and GalA than wild-type seeds (two-way ANOVA, P < 0.05; Fig. 5C; Supplemental Table S3), indicating that mucilage is less adherent in both mutants. Similar increases in nonadherent Rha and GalA were also observed in cesa5-1 sos5-2 double-mutant seeds. Rha and GalA values for cesa5-1 sos5-2 double mutants were significant (two-way ANOVA, P < 0.05), indicating a synergistic interaction between CESA5 and SOS5 in mucilage adherence. Tukey’s HSD post hoc analysis confirmed the significant increases in nonadherent Rha and GalA amounts in cesa5-1, sos5-2, and cesa5-1 sos5-2 seeds compared with the wild type (P < 0.05) but did not detect any differences between the mutant genotypes (P > 0.05), likely owing to the fact that the percent increase in nonadherent mucilage of the double mutant is relatively small compared with the single mutants. Because similar increases in Rha and GalA were not observed in whole seeds (Fig. 5A; Supplemental Table S1), these data suggest that, consistent with previous reports (Harpaz-Saad et al., 2011; Mendu et al., 2011; Sullivan et al., 2011), both cesa5-1 and sos5-2 mutations result in more nonadherent mucilage.
Unlike Rha and GalA, Glc levels were significantly decreased in cesa5-1 nonadherent mucilage (two-way ANOVA, P < 0.05), consistent with a decrease in mucilage cellulose synthesis. In contrast, sos5-2 nonadherent mucilage had levels of Glc that were not statistically different from the wild type (two-way ANOVA, P > 0.05; Fig. 5D; Supplemental Table S3). Glc levels in nonadherent mucilage for the cesa5-1 sos5-2 double mutant seemed intermediate between cesa5-1 and sos5-2 levels, and these values were not significantly different from either single mutant (two-way ANOVA and Tukey’s HSD, P > 0.05). These data suggest that, unlike cesa5-1, sos5-2 is not impaired in cellulose synthesis.
Finally, we examined how cellulose was deposited during mucilage biosynthesis to determine if SOS5 had any effects on CESA5 movement and velocity. We crossed pCESA5:GFP-CESA5 with sos5-2 plants and isolated individuals expressing GFP-CESA5 with homozygous sos5-2 alleles in the F2 generation. In time-lapse images of approximately 7 DPA seed coat epidermal cells, GFP-CESA5 is localized around the cytoplasmic column, appearing as small punctae occasionally arranged in longer striations perpendicular with the outer surface of the seed coat (Supplemental Fig. S11A). In the sos5-2 background, the localization and distribution of GFP-CESA5 appeared similar to the wild type (Supplemental Fig. S11B). Kymographs of GFP-CESA5 in wild-type and sos5-2 backgrounds appeared similar (Supplemental Fig. S11, C and D). We calculated the velocity of GFP-CESA5 particles for the wild type and sos5-2 and found no major differences between the two genotypes (Supplemental Fig. S11E). Thus, SOS5 does not seem to be required for the arrangement or movement of CESA5 in the seed coat epidermal cells.
sos5 But Not cesa5 Alters the Mucilage Pectin Defects of fly1 and mum2
Plants with loss-of-function mutations in both MUM2 and FLY1 have seeds with mucilage that extrudes poorly and adheres more strongly, respectively, because of changes in mucilage pectin (Dean et al., 2007; Macquet et al., 2007b; Voiniciuc et al., 2013). Because mutation of both CESA5 and SOS5 result in mucilage that is less adherent, we questioned whether one or both would be able to suppress the phenotypes of either mum2-1 or fly1-1 mutant mucilage. We, therefore, constructed four double-mutant lines (cesa5-1 mum2-1, sos5-2 mum2-1, cesa5-1 fly1-1, and sos5-2 fly1-2) and examined their seed mucilage phenotype.
cesa5 mum2 and sos5 mum2
mum2-1 seeds fail to release mucilage in water but release a small mucilage halo when treated with Na2CO3 (Dean et al., 2007; Macquet et al., 2007b), a mild base that weakens the cell wall structure (Thimm et al., 2009). When hydrated in water, seeds of both double mutants, cesa5-1 mum2-1 and sos5-2 mum2-1, failed to release mucilage (Fig. 6, C and E), similar to the mum2-1 single mutant (Fig. 6D) but unlike the wild type, cesa5-1, and sos5-2 (Fig. 6, A, B, and F). When treated with Na2CO3, seeds of all genotypes had a larger RR-stained mucilage halo compared with those treated with water (Fig. 6, G–L). The amount of mucilage released from Na2CO3-treated cesa5-1 mum2-1 seeds most closely resembled that of mum2-1 seeds (compare Fig. 6I with Fig. 6J). However, the sos5-2 mum2-1 Na2CO3-treated seeds showed large adherent mucilage halos that appeared most similar to sos5-2 (compare Fig. 6K with Fig. 6L). Thus, in Na2CO3, sos5-2 was able to suppress the reduced extrusion phenotype of mum2-1, whereas cesa5-1 was not.
Figure 6.
SOS5 is required for the mum2 phenotype. A to F, RR-stained seeds hydrated in water. G to R, RR-stained seeds hydrated in Na2CO3. Bars in A to L = 100 μm. Bars in M to R = 10 μm. S, Monosaccharide quantification of nonadherent Na2CO3-soluble mucilage. Letters not connected by the same letters are significantly different (Tukey’s HSD post hoc analysis, P < 0.05).
Mucilage release and adherence were quantified by measuring Rha and GalA levels in whole seeds and nonadherent mucilage from Na2CO3-treated seeds of the wild type, cesa5-1, sos5-2, and mum2-1 single mutants, and cesa5-1 mum2-1 and sos5-2 mum2-1 double mutants using HPAEC (Supplemental Tables S1 and S3). Except for the statistically significant reduction of Glc levels in cesa5-1 seeds noted above, whole-seed monosaccharide compositions of all single and double mutants were largely similar (ANOVA, P > 0.05; Supplemental Table S1). As expected, in nonadherent mucilage of Na2CO3-treated seeds, cesa5-1 and sos5-2 had increased levels of Rha and GalA relative to the wild type, whereas that of mum2-1 seeds was reduced (ANOVA on a linear model with three factors [CESA5, SOS5, and MUM2] with all possible interaction terms; Rha, P < 0.05; GalA, P < 0.05; Fig. 6S; Supplemental Table S3). Two-way ANOVA and Tukey’s HSD post hoc analysis indicated that, as measured by Rha and GalA levels, cesa5-1 mum2-1 had low levels of nonadherent mucilage most similar to mum2-1 (cesa5-1 mum2-1, P < 0.05). In contrast, sos5-2 mum2-1 had higher levels of nonadherent mucilage more similar to sos5-2 (sos5-2 mum2-1, P < 0.05; Fig. 6S; Supplemental Table S3). These data support the hypothesis that sos5-2 is more effective at suppressing the phenotype of mum2-1 seeds than cesa5-1.
cesa5 fly1 and sos5 fly1
Loss-of-function mutations in FLY1 result in a lower degree of HG methylesterification in mucilage and increased mucilage cohesiveness (Voiniciuc et al., 2013). The most obvious aspect of the fly1 mutant phenotype is detachment of the seed coat epidermal cell primary walls, forming cell wall discs that remain attached at the periphery of the adherent mucilage halo (Fig. 7D, arrow; Voiniciuc et al., 2013). S4B staining of fly1-2 seeds indicates that the discs are located precisely at the distal end of rays (Fig. 7L, arrow). We generated fly1-1 cesa5-1 and fly1-2 sos5-2 double mutants to examine the effects of CESA5 and SOS5 on the adherence of fly1 discs. RR-stained fly1-1 cesa5-1 seeds released a large number of discs that remained attached to the edge of the mucilage halo similar to fly1 seeds, although the size of the RR-stained adherent mucilage halo was reduced in the double mutant relative to fly1 (compare Fig. 7C with Fig.7D). In contrast, the fly1-2 sos5-2 seeds also released discs on hydration (Fig. 7G, arrow), but unlike in the fly1 single mutant or the fly1-1 cesa5-1 double mutant, the majority of discs in the fly1-2 sos5-2 double mutant were easily detached from the seed after gentle shaking (Fig. 7E) and observed in the nonadherent mucilage fraction (Fig. 7K, arrow). These results show that sos5, but not cesa5, suppresses the fly1 phenotype and that SOS5, but not CESA5, is required for attachment of fly1 discs to the adherent mucilage halo.
Figure 7.
SOS5 is required for fly disc adherence. A to G, RR-stained seeds. H to K, Nonadherent mucilage fraction of RR-stained seeds. Col-0 (A), cesa5-1 (B), cesa5-1 fly1-1 (C), fly1-1 (D), sos5-2 fly1-2 (E), sos5-2 (F), and initial hydration of sos5-2 fly1-2 (G) seeds. Col-0 mucilage (H), cesa5-1 fly1-1 mucilage (I), fly1-1 mucilage (J), and sos5-2 fly1-2 mucilage (K). L, S4B-stained fly1-2 seeds. Bars in A to K = 100 μm. Bar in L = 10 μm.
DISCUSSION
SOS5 and CESA5 Influence Mucilage Structure through Distinct Mechanisms
The requirement of both SOS5 and CESA5 for seed coat mucilage adherence has led to the suggestion that SOS5 interacts with CESA complexes to promote cellulose production (Harpaz-Saad et al., 2011). If this hypothesis is correct, then it is expected that the phenotypes of sos5, cesa5 , and cesa5 sos5 mutants should be similar and that interactions of sos5 and cesa5 with other genetic loci affecting mucilage adherence should be the same. However, we have shown that, although both single-mutant phenotypes include a decrease in mucilage adherence and impact both cellulose and pectin in the mucilage, the phenotypes are distinctly different in the quantity, organization, and adherence of these mucilage components. Furthermore, the cesa5 sos5 double-mutant phenotype has a more severe mucilage adherence phenotype than either single mutant and includes deficiencies in columella formation not seen in either single mutant (discussed below). Finally, sos5 is able to modify the mucilage phenotypes of mum2 and fly1, whereas cesa5 cannot. It is unlikely that the observed differences result from the severity of the mutant alleles, because both sos5-2 and cesa5-1 are strong loss-of-function mutations. Therefore, taken together, our results suggest that SOS5 plays a role in mucilage structure/adherence that is relatively independent of and distinct from that of CESA5.
SOS5 Has a Greater Impact on Mucilage Pectin, Whereas CESA5 Has a Greater Impact on Mucilage Cellulose
Several studies have indicated that CESA5, a CESA, is involved in synthesizing cellulose in the mucilage and that this cellulose is critical for mucilage adherence (Harpaz-Saad et al., 2011; Mendu et al., 2011; Sullivan et al., 2011; this study). In contrast, SOS5 is not required for normal amounts of mucilage cellulose, although it is required for the distribution of cellulose into ray-like structures (Harpaz-Saad et al., 2011; this study). If SOS5 does not mediate its effects on cell wall structure through cellulose, it must function through other mechanisms. The composition and modification of mucilage pectin (RG I and HG) have also been shown to influence the structure and adherence of mucilage (Dean et al., 2007; Macquet et al., 2007b; Rautengarten et al., 2008; Arsovski et al., 2009; Saez-Aguayo et al., 2013; Voiniciuc et al., 2013). Although both SOS5 and CESA5 are required for the adherence of mucilage pectin, several lines of evidence suggest that SOS5 has a much stronger effect than CESA5. Qualitative assessment of RG I and HG in seed mucilage using the appropriate antibodies indicated that cesa5 seed mucilage had detectable pectin epitopes, whereas sos5 was substantially lower (Fig. 2; Supplemental Figs. S3 and S4). A similar result was found using JIM13 (Supplemental Fig. S8), an antibody that detects an AGP-related glycan. Both MUM2 and FLY1 are proteins that modify mucilage pectin so that it can expand when exposed to water. The sos5 mutation is able to partially suppress the phenotypes of both mum2 and fly1, whereas the cesa5 mutation was unable to suppress either. These data suggest that SOS5 has a more direct role in establishing the structure of mucilage pectin than CESA5. Taken together, our results imply a structure for adherent mucilage involving two distinct but interdependent networks (one of cellulose and one of pectin), each of which contributes significantly to the strength of mucilage adherence.
The Role of SOS5 in Pectin Adherence
The molecular mode of action of AGPs in cell walls is poorly understood. Like other AGPs, SOS5 has been shown to play a role in cell wall structure (Shi et al., 2003; Xu et al., 2008; Harpaz-Saad et al., 2011), and like other AGPs, the mechanism through which SOS5 acts is under debate. It has been suggested that SOS5 serves as a structural component of the wall (Shi et al., 2003), a cell-cell signaling molecule (Xu et al., 2008; Seifert et al., 2014), or a cofactor for CESA5 complexes (Harpaz-Saad et al., 2011). Here, we have shown that SOS5 is required for mucilage adherence and the formation of ray structure but seems to do so through an influence on pectin rather than for cellulose biosynthesis. How could SOS5 have a role in pectin structure? Multiple lines of evidence show associations between AGPs and pectins. Pectins frequently colocalize with AGPs in pollen tubes (Li et al., 1995; Jauh and Lord, 1996; Mollet et al., 2002). Treatment of cell wall fractions with pectin-degrading enzymes allows for the increased release of AGPs (Immerzeel et al., 2006; Lamport et al., 2006). AGPs have also been shown to bind specifically to pectins in a calcium-dependent manner (Baldwin et al., 1993). Most recently, an AGP has been shown to be covalently linked to rhamnosyl residues of RG I and an arabinoxylan polysaccharide (Tan et al., 2013). This linkage suggests that noncellulosic carbohydrates in the cell wall form extensive networks that contribute to cell wall strength (Tan et al., 2013). Therefore, SOS5 could serve as a structural component in the formation of a pectin or pectin-hemicellulose network in the mucilage. The facts that SOS5 localizes to the apoplast (Shi et al., 2003; G.J. Seifert and H. Xue, unpublished data) and that JIM13, an antibody that recognizes carbohydrate moieties associated with AGPs, binds mucilage rays are consistent with this hypothesis. However, if SOS5 plays such a structural role, it should be present in mature mucilage. Although we have been able to detect AGP glycans in extruded mucilage using antibodies and FLAs through proteomic analysis of mature mucilage, SOS5 was not among them (A.Y.-L. Tsai, G.W. Haughn, and B.E. Ellis, unpublished data). An alternative hypothesis consistent with the available data is that membrane-anchored SOS5 plays a role in mediating pectin assembly but is not included in the final structure. In addition, we cannot completely exclude the possibility that SOS5 subtly influences the pattern of cellulose deposition by interacting with the CESA complex (see below), thereby influencing pectin indirectly. Experiments to identify SOS5 interacting partners during mucilage biosynthesis may help resolve these questions.
Mucilage Rays Are a Macromolecular Assembly Composed of Pectin and Cellulose
Cytological analysis has shown that extruded adherent mucilage has a distinct structure with ray-like projections extending outward from each columella perpendicular to the seed surface that forms during mucilage expansion. These rays are composed of both cellulose and pectins (Macquet et al., 2007a; Harpaz-Saad et al., 2011; Mendu et al., 2011; Sullivan et al., 2011; Figs. 1 and 2). Mucilage cellulose appears to be deposited in a specific orientation circumferential to the cytoplasmic column that predetermines a ray-like structure after extrusion (J.S. Griffiths, R. Kushwaha, K. Šola, P. Lam, C. Voiniciuc, G. Dean, S.D. Mansfield, S. DeBolt, and G.W. Haughn, unpublished data). Moreover, SOS5 is required for mucilage ray structure after extrusion (Harpaz-Saad et al., 2011; Figs. 1 and 2; Supplemental Fig. S1) but does not have any obvious effects on the localization or movement of GFP-CESA5 or the amount of cellulose synthesized during mucilage biosynthesis (Supplemental Fig. S9). Because SOS5 seems to influence the structure of mucilage pectin and because pectin is an integral component of the ray, it is possible that SOS5 influences the ray structure through its role in establishing a pectin network that maintains cellulose in ray-like bundles after extrusion. The loss of SOS5 by mutation would, therefore, result in a more dispersed pattern of both pectin and cellulose, eliminating the rays, although the amounts of pectin and cellulose remain the same. In this context, it is interesting to note that a role of pectin in establishing cellulose microfibril orientation has been previously identified (Yoneda et al., 2010).
The Adherent Properties of Mucilage Are Important for Normal Formation of the Cytoplasmic Column
One of the most dramatic aspects of the cesa5-1 sos5-2 double-mutant phenotype is the drastic change observed in columella structure. Scanning electron microscopy analysis of mature seeds shows a reduction in columella area in both sos5-2 and cesa5-1 seeds, but the double-mutant phenotype is much more severe. The columella shape defect does not originate during deposition of the columella but rather, during the formation of the mucilage pocket and corresponding cytoplasmic column. The abnormal shape of the cesa5 sos5 double-mutant seed coat epidermal cells is reflected in the shape of the mucilage pocket/cytoplasmic column that clearly precedes columella formation. Changes in the shape of the mucilage pocket are known to influence the shape of the columella. For example, a decrease in the amount of mucilage synthesized results in a smaller mucilage pocket and a much flatter columella (Western et al., 2004). Given the effect of sos5 and cesa5 on mucilage adherence, the expanded cytoplasmic pocket and concomitant deformation of the cytoplasmic column could be a consequence of an increase in the expansion of the cesa5-1 sos5-2 double-mutant mucilage. This proposed overexpansion would increase the size of the mucilage pocket at the expense of the shape of the cytoplasmic column. These data support the hypothesis that the amount and/or properties of the mucilage determine the morphology of the cytoplasmic column and subsequently, the shape of the columella.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All Arabidopsis (Arabidopsis thaliana) mutants used in this study were from the Col-0 ecotype, except for mum2-1 and fly1-1, which are in the Col-2 ecotype. Transfer DNA insertions or ethyl methanesulfonate mutants were obtained from previous studies (Dean et al., 2007; Harpaz-Saad et al., 2011; Mendu et al., 2011; Voiniciuc et al., 2013; Supplemental Table S3). Seeds were germinated on plates with Arabidopsis medium (Haughn and Somerville, 1986) and 7% (w/v) agar, and seedlings were transferred to soil (Sunshine Mix 4; SunGro, Kelowna) after 7 d. Plants were grown with continuous fluorescent illumination of 80 to 140 μEm−2 s−1 at 20°C to 22°C. Developing seeds were staged as previously described (Western et al., 2001). All double mutants (cesa5-1 sos5-2, cesa5-1 mum2-1, sos5-2 mum2-1, cesa5-1 fly1-1, and sos5-2 fly1-2) were isolated from an F2 population (n = 72) from a cross between the two respective single-mutants parents. Mutant loci were identified using a PCR-based assay using allele-specific primers (Supplemental Table S5).
Expression Analysis
The expression of SOS5 was determined using gene-specific primers (Supplemental Table S5) to amplify complementary DNA (cDNA) isolated from excised seed coat tissue at 4, 7, and 10 DPA. RNA extraction, cDNA synthesis, and reverse transcription PCR conditions were performed as in Voiniciuc et al. (2013). SOS5 expression during seed coat development was also examined using the Arabidopsis seed coat-specific expression browser (http://bar.utoronto.ca/efp_seedcoat/cgi-bin/efpWeb.cgi; Dean et al., 2011).
Microscopy and Image Analysis
Confocal images were acquired on a Perkin-Elmer Ultraview VoX Spinning Disk Confocal System (PerkinElmer). pCESA5:GFP-CESA5 (GFP-CESA5) was a gift from Helen North and Stuart Sullivan (Bischoff et al., 2011; Sullivan et al., 2011). Time-lapse images are projections of multiple time points of six time points per minute for 5 min.
For RR staining, mature dry seeds were hydrated in distilled water for 1 to 2 h with head-over-tail rotation, rinsed one time with water, and stained with 0.01% (w/v) RR (Sigma-Aldrich) for 30 min or as described. mum2-1 mutant seeds were hydrated in either water for 2 h with shaking or 100 μL of 1 m Na2CO3 for 15 min followed by dilution to 100 mm Na2CO3 with shaking for 2 h, two water rinses, and then RR staining. Bright-field micrographs of stained samples were taken with QCapture software and digital camera (QImaging; Surrey) equipped on a Zeiss AxioSkop 2 upright light microscope (Carl Zeiss AG). For cellulose staining, seeds were shaken in water for 1 to 2 h, stained with 0.01% (w/v) Pontamine Fast Scarlet 4B (S479896; Sigma-Aldrich Rare Chemical Library) with 100 mm NaCl for 1 h, and rinsed two times before imaging (Anderson et al., 2010; Mendu et al., 2011).
Whole-seed immunolabeling was conducted according to a published method, except that seeds were shaken in water before immunolabeling and that seeds were stained with S4B after immunolabeling (Harpaz-Saad et al., 2011). The specificities of the primary antibodies JIM5 and JIM7 (CarboSource) have been extensively described (Liners et al., 1989; Knox et al., 1990; Knox, 1997; Willats et al., 2001a, 2001b, 2001c; Macquet et al., 2007a; Pattathil et al., 2010; Xu et al., 2011). JIM13, MAC207, JIM8, and CCRC-M7 (CarboSource) have also been previously described (Bradley et al., 1988; Pennell et al., 1989, 1991; Knox et al., 1991; Puhlmann et al., 1994; Yates and Knox, 1994; Steffan et al., 1995; Yates et al., 1996; Majewska-Sawka and Nothnagel, 2000; Pattathil et al., 2010). CBM3a, specific to more crystalline cellulose, and CBM28, specific to more amorphous cellulose regions (Blake et al., 2006), were treated as primary antibodies in identical solutions before treatment with mouse anti-histidine (Qiagen). Goat anti-rat secondary antibody conjugated to AlexaFluor488 was used against JIM5, JIM7, JIM8, JIM13, and MAC207, whereas goat anti-mouse conjugated to AlexaFluor488 (Molecular Probes; Invitrogen) was used as a tertiary antibody against the CBMs, CCRC-M36, and CCRC-M7. The protocol was carried out without primary antibody as a negative control. Seeds were imaged using a 488-nm laser (antibody fluorescence) and 561-nm laser (seed intrinsic fluorescence and S4B fluorescence) on a PerkinElmer Ultraview VoX Spinning Disk Confocal System (PerkinElmer). Signal intensities for each antibody treatment were preserved across genotypes; however, the signal intensity was varied between treatments and between two- and three-dimensional images.
Seed coat morphology was investigated using a Hitachi S4700 scanning electron microscope (Hitachi High-Technologies). Seeds were mounted on standard electron microscope stubs and sputter coated with a gold-palladium alloy using a Cressington 208C high-resolution sputter coater (Ted Pella Inc.).
All confocal micrographs were processed with ImageJ (Abramoff et al., 2004). Columella width and cell size were also measured using ImageJ by outlining the columella or cell using the freehand selection tool. Confocal images containing signals from multiple optical stacks or time-lapse images were rendered using the Z-project maximum intensity method. Kymographs were created from time-lapse images at 10-s image intervals using the kymograph plugin for ImageJ (J. Rietdorf and A. Seitz, EMBL Heidelberg). Time-lapse images were averaged for three frames using the Walking Average program, and spiral tracks of GFP-CESAs were used to create kymopgraphs using the Multiple Kymograph program. The slope of GFP-CESA particle velocity from the kymograph was analyzed using the read velocities from tsp macro (http://www.embl.de/eamnet/downloads/macros/tsp050706.txt).
Seed Coat Development Analysis
Seed staging, high-pressure freezing, freeze substitution, resin embedding, sectioning, and imaging were performed as described (Rensing et al., 2002; Mendu et al., 2011). Live-cell imaging was performed by staining dissected seeds in a 10µg/mL propidium iodide solution for 15 min. Seeds were then rinsed two times with distilled water and imaged on a PerkinElmer Ultraview VoX Spinning Disk Confocal system (PerkinElmer) with a 488-nm laser.
Determination of Monosaccharide Composition by HPAEC
For nonadherent mucilage extractions, four technical replicates of approximately 20 mg of seeds (exact weight recorded) were mixed with 1.4 mL of distilled water and 10 µL of 5 mg mL−1 d-erythritol internal standard. Samples were shaken head to tail using a tube rotator for 2 h. One milliliter of mucilage was transferred to a glass tube and dried at 60°C under nitrogen gas. For whole seeds, sugar standards were dissolved in water, and the dried mucilage samples were processed according to published methods (Dean et al., 2007; Mendu et al., 2011; Voiniciuc et al., 2013). Neutral and acidic cell wall sugars were identified and quantified by pulsed electrochemical detection using a Dionex ED50 apparatus. Sugar separation was achieved using a BioLC GS50 HPLC device and a CarboPAC-PAl pellicular anion exchange column with column guard (Dionex).
Crystalline Cellulose Determination
Crystalline cellulose levels were determined based on a microscale modification by Updegraff (1969). Ten to twenty milligrams of seeds (exact weight recorded) was frozen in liquid nitrogen, ground using mortar and pestle, and then dried at 50°C overnight. Ground seeds were then treated with 2 mL of the acetic-nitric acid reagent (stock solution, 150 mL of 80% [v/v] glacial acetic acid diluted with water and 15 mL of [70%] concentrated nitric acid) and vortexed. Samples were heated at 100°C for 1 h, centrifuged for 5 min at 3,000 rpm, and washed. Samples were then treated with 1 mL 72% (w/v) H2SO4 vortex, incubated at room temperature for 90 min, centrifuged for 5 min at 10,000 rpm, and then diluted 10 times in distilled water (duplicated samples). One hundred microliters of diluted sample was treated with 200 μL of cold (4°C) anthrone reagent (0.2% [w/v] anthrone [Sigma-Aldrich] in concentrated H2SO4) and vortexed. Anthrone mixtures were incubated for 15 min at 100°C, and duplicate samples were measured two times for A620 in a spectrophotometer. A standard curve was prepared from a standard dilution of 1.2 mg mL−1 Avicel microcrystalline cellulose that was treated identically to samples. Total amounts of cellulose were calculated per weight of dry seed mass.
Statistical Analysis
ANOVAs and Tukey’s HSD post hoc analysis were performed using JMP 11 software (SAS). Tukey’s HSD analysis was performed using CESA5, SOS5, and/or MUM2 as independent variables to determine significant differences between values and interaction effects between the different genotypes in double mutants. For the two-way ANOVA analysis, significant values for single mutants indicate a difference from the wild type, whereas significant values for double mutants indicate interactions between both genes.
AGI numbers for genes used in this study can be found in Supplemental Table S4.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. SOS5 is expressed early during seed coat development.
Supplemental Figure S2. sos5-2 seeds fail to form rays immediately after hydration.
Supplemental Figure S3. CBM3a immunolabeling (green) and S4B-stained (magenta) wild-type, cesa5-1, sos5-2, and cesa5-1 sos5-2 double-mutant seeds showing individual light channels and three-dimensional reconstructions of multiple optical stacks.
Supplemental Figure S4. CBM28 immunolabeling (green) and S4B-stained (magenta) wild-type, cesa5-1, sos5-2, and cesa5-1 sos5-2 double-mutant seeds showing individual light channels and three-dimensional reconstructions of multiple optical stacks.
Supplemental Figure S5. CCRC-M36 immunolabeling (green) and S4B-stained (magenta) wild-type, cesa5-1, sos5-2, and cesa5-1 sos5-2 double-mutant seeds showing individual light channels and three-dimensional reconstructions of multiple optical stacks.
Supplemental Figure S6. JIM5 immunolabeling (green) and S4B-stained (magenta) wild-type, cesa5-1, sos5-2, and cesa5-1 sos5-2 double-mutant seeds showing individual light channels and three-dimensional reconstructions of multiple optical stacks.
Supplemental Figure S7. Arabinogalactan-specific antibodies JIM8 (AGP), CCRC-M7 (RG I and AGP), and MAC207 (AGP) fail to significantly label wild-type adherent mucilage.
Supplemental Figure S8. Wild-type, cesa5-1, and sos5-2 seeds immunolabeled with JIM13.
Supplemental Figure S9. Surface cell area is not significantly different in cesa5-1 sos5-2 epidermal cells.
Supplemental Figure S10. Live cell imaging of columella development.
Supplemental Figure S11. SOS5 does not affect GFP-CESA5 direction of movement or velocity.
Supplemental Table S1. Whole-seed monosaccharide composition.
Supplemental Table S2. Acid-insoluble cellulose amounts.
Supplemental Table S3. Nonadherent mucilage monosaccharide composition.
Supplemental Table S4. Genes, accession numbers, and mutant lines used in this study.
Supplemental Table S5. Sequences of primers used in this study.
Supplementary Material
Acknowledgments
We thank Helen North and Volker Bischoff for the gift of GFP-CESA5 seeds, Patrick Martone and Sam Yeaman for assistance with statistical analysis, members of the laboratory of S.D.M. for aid in running the HPLC, the University of British Columbia Bioimaging Facility for technical assistance, Erin Gilchrist for design of the mum2-1 genotyping primers, Gabriel Levesque-Tremblay for the gift of seed coat-specific cDNA, and Gillian Dean for critical reading of this work.
Glossary
- AGP
arabinogalactan protein
- CBM
carbohydrate-binding module
- cDNA
complementary DNA
- CESA
CELLULOSE SYNTHASE
- DPA
day approximate time of fertilization
- FLA
fasciclin-like
- FLY
FLYING SAUCER
- GPI
glycophosphatidylinositol
- HG
homogalacturonan
- HPAEC
high-performance anion-exchange chromatography
- HSD
honestly significant difference
- MUM
MUCILAGE MODIFIED
- RG I
rhamnogalacturonan I
- RR
ruthenium red
- SOS
SALT-OVERLY SENSITIVE
- S4B
Pontamine fast scarlet S4B
- Col
Columbia
Footnotes
This work was supported by the National Sciences and Engineering Research Council (Postgraduate Scholarship-Doctoral Scholarship to J.S.G. and Discovery Grant to S.D.M. and G.W.H.) and the Austrian Science Fund (grant nos. P21782–B12 and I1182–B22 to G.J.S.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Abramoff MD, Magalhaes PJ, Ram SJ. (2004) Image processing with ImageJ. Biophotonics Int 11: 36–42 [Google Scholar]
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. (2011) Plant Cell Walls. Garland Science, New York [Google Scholar]
- Anderson CT, Carroll A, Akhmetova L, Somerville C. (2010) Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol 152: 787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsovski AA, Haughn GW, Western TL. (2010) Seed coat mucilage cells of Arabidopsis thaliana as a model for plant cell wall research. Plant Signal Behav 5: 796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsovski AA, Popma TM, Haughn GW, Carpita NC, McCann MC, Western TL. (2009) AtBXL1 encodes a bifunctional β-D-xylosidase/α-L-arabinofuranosidase required for pectic arabinan modification in Arabidopsis mucilage secretory cells. Plant Physiol 150: 1219–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin TC, McCann MC, Roberts K. (1993) A novel hydroxyproline-deficient arabinogalactan protein secreted by suspension-cultured cells of Daucus carota: purification and partial characterization. Plant Physiol 103: 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman T, De Rycke R, Viane R, Inze D. (2000) Histological study of seed coat development in Arabidopsis thaliana. J Plant Res 113: 139–148 [Google Scholar]
- Bischoff V, Desprez T, Mouille G, Vernhettes S, Gonneau M, Höfte H. (2011) Phytochrome regulation of cellulose synthesis in Arabidopsis. Curr Biol 21: 1822–1827 [DOI] [PubMed] [Google Scholar]
- Blake AW, McCartney L, Flint JE, Bolam DN, Boraston AB, Gilbert HJ, Knox JP. (2006) Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. J Biol Chem 281: 29321–29329 [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Wood EA, Larkins AP, Galfre G, Butcher GW, Brewin NJ. (1988) Isolation of monoclonal antibodies reacting with peribacteriod membranes and other components of pea root nodules containing Rhizobium leguminosarum. Planta 173: 149–160 [DOI] [PubMed] [Google Scholar]
- Burton RA, Gidley MJ, Fincher GB. (2010) Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6: 724–732 [DOI] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D. (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344: 1879–1900 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wang H, Wu HM. (1995) A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82: 383–393 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Dagel DJ, Liu YS, Zhong L, Luo Y, Himmel ME, Xu Q, Zeng Y, Ding SY, Smith S. (2011) In situ imaging of single carbohydrate-binding modules on cellulose microfibrils. J Phys Chem B 115: 635–641 [DOI] [PubMed] [Google Scholar]
- Dean G, Cao Y, Xiang D, Provart NJ, Ramsay L, Ahad A, White R, Selvaraj G, Datla R, Haughn G. (2011) Analysis of gene expression patterns during seed coat development in Arabidopsis. Mol Plant 4: 1074–1091 [DOI] [PubMed] [Google Scholar]
- Dean GH, Zheng H, Tewari J, Huang J, Young DS, Hwang YT, Western TL, Carpita NC, McCann MC, Mansfield SD, et al. (2007) The Arabidopsis MUM2 gene encodes a β-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell 19: 4007–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick-Pérez M, Zhang Y, Hayes J, Salazar A, Zabotina OA, Hong M. (2011) Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 50: 989–1000 [DOI] [PubMed] [Google Scholar]
- Ellis M, Egelund J, Schultz CJ, Bacic A. (2010) Arabinogalactan-proteins: key regulators at the cell surface? Plant Physiol 153: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz-Saad S, McFarlane HE, Xu S, Divi UK, Forward B, Western TL, Kieber JJ. (2011) Cellulose synthesis via the FEI2 RLK/SOS5 pathway and cellulose synthase 5 is required for the structure of seed coat mucilage in Arabidopsis. Plant J 68: 941–953 [DOI] [PubMed] [Google Scholar]
- Harpaz-Saad S, Western TL, Kieber JJ. (2012) The FEI2-SOS5 pathway and CELLULOSE SYNTHASE 5 are required for cellulose biosynthesis in the Arabidopsis seed coat and affect pectin mucilage structure. Plant Signal Behav 7: 285–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn G, Chaudhury A. (2005) Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci 10: 472–477 [DOI] [PubMed] [Google Scholar]
- Haughn GW, Somerville C. (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204: 430–434 [Google Scholar]
- Haughn GW, Western TL. (2012) Arabidopsis seed coat mucilage is a specialized cell wall that can be used as a model for genetic analysis of plant cell wall structure and function. Front Plant Sci 3: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immerzeel P, Eppink MM, De Vries SC, Schols HA, Voragen AGJ. (2006) Carrot arabinogalactan proteins are interlinked with pectins. Physiol Plant 128: 18–28 [Google Scholar]
- Jauh GY, Lord EM. (1996) Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 199: 251–261 [Google Scholar]
- Johnson KL, Jones BJ, Bacic A, Schultz CJ. (2003) The fasciclin-like arabinogalactan proteins of Arabidopsis: a multigene family of putative cell adhesion molecules. Plant Physiol 133: 1911–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP. (1997) The use of antibodies to study the architecture and developmental regulation of plant cell walls. Int Rev Cytol 171: 79–120 [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K. (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512–521 [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, Peart J, Cooper C, Roberts K. (1991) Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J 1: 317–326 [DOI] [PubMed] [Google Scholar]
- Kotake T, Dina S, Konishi T, Kaneko S, Igarashi K, Samejima M, Watanabe Y, Kimura K, Tsumuraya Y. (2005) Molecular cloning of a β-galactosidase from radish that specifically hydrolyzes β-(1→3)- and β-(1→6)-galactosyl residues of Arabinogalactan protein. Plant Physiol 138: 1563–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport DT, Kieliszewski MJ, Showalter AM. (2006) Salt stress upregulates periplasmic arabinogalactan proteins: using salt stress to analyse AGP function. New Phytol 169: 479–492 [DOI] [PubMed] [Google Scholar]
- Li J, Yu M, Geng LL, Zhao J. (2010) The fasciclin-like arabinogalactan protein gene, FLA3, is involved in microspore development of Arabidopsis. Plant J 64: 482–497 [DOI] [PubMed] [Google Scholar]
- Li YQ, Faleri C, Geitmann A, Zhang HQ, Cresti M. (1995) Immunogold localization of arabinogalactan proteins, unesterified and esterified pectins in pollen grains and pollen tubes of Nicotiana tabacum L. Protoplasma 189: 26–36 [Google Scholar]
- Liners F, Letesson JJ, Didembourg C, Van Cutsem P. (1989) Monoclonal antibodies against pectin: recognition of a conformation induced by calcium. Plant Physiol 91: 1419–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan CP, Mansfield SD, Stachurski ZH, Evans R, Southerton SG. (2010) Fasciclin-like arabinogalactan proteins: specialization for stem biomechanics and cell wall architecture in Arabidopsis and Eucalyptus. Plant J 62: 689–703 [DOI] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Kronenberger J, Marion-Poll A, North HM. (2007a) In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol 48: 984–999 [DOI] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Loudet O, Kronenberger J, Mouille G, Marion-Poll A, North HM. (2007b) A naturally occurring mutation in an Arabidopsis accession affects a β-D-galactosidase that increases the hydrophilic potential of rhamnogalacturonan I in seed mucilage. Plant Cell 19: 3990–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska-Sawka A, Nothnagel EA. (2000) The multiple roles of arabinogalactan proteins in plant development. Plant Physiol 122: 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendu V, Griffiths JS, Persson S, Stork J, Downie AB, Voiniciuc C, Haughn GW, DeBolt S. (2011) Subfunctionalization of cellulose synthases in seed coat epidermal cells mediates secondary radial wall synthesis and mucilage attachment. Plant Physiol 157: 441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet JC, Kim S, Jauh GY, Lord EM. (2002) Arabinogalactan proteins, pollen tube growth, and the reversible effects of Yariv phenylglycoside. Protoplasma 219: 89–98 [DOI] [PubMed] [Google Scholar]
- North HM, Berger A, Saez-Aguayo S, Ralet MC. (March 7, 2014) Understanding polysaccharide production and properties using seed coat mutants: future perspectives for the exploitation of natural variants. Ann Bot http://dx.doi.org/10.1093/aob/mcu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Baldwin D, Swennes AG, McGill JA, Popper Z, Bootten T, Albert A, Davis RH, Chennareddy C, et al. (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart JM, Roberts K. (1991) Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 3: 1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Knox JP, Scofield GN, Selvendran RR, Roberts K. (1989) A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J Cell Biol 108: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhlmann J, Bucheli E, Swain MJ, Dunning N, Albersheim P, Darvill AG, Hahn MG. (1994) Generation of monoclonal antibodies against plant cell-wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal alpha-(1—>2)-linked fucosyl-containing epitope. Plant Physiol 104: 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C, Usadel B, Neumetzler L, Hartmann J, Büssis D, Altmann T. (2008) A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant J 54: 466–480 [DOI] [PubMed] [Google Scholar]
- Rensing KH, Samuels AL, Savidge RA. (2002) Ultrastructure of vascular cambial cell cytokinesis in pine seedlings preserved by cryofixation and substitution. Protoplasma 220: 39–49 [DOI] [PubMed] [Google Scholar]
- Saez-Aguayo S, Ralet MC, Berger A, Botran L, Ropartz D, Marion-Poll A, North HM. (2013) PECTIN METHYLESTERASE INHIBITOR6 promotes Arabidopsis mucilage release by limiting methylesterification of homogalacturonan in seed coat epidermal cells. Plant Cell 25: 308–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CJ, Johnson KL, Currie G, Bacic A. (2000) The classical arabinogalactan protein gene family of Arabidopsis. Plant Cell 12: 1751–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. (2007) The biology of arabinogalactan proteins. Annu Rev Plant Biol 58: 137–161 [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Xue H, Acet T. (March 5, 2014) The Arabidopsis thaliana FASCICLIN LIKE ARABINOGALACTAN PROTEIN 4 gene acts synergistically with abscisic acid signaling to control root growth. Ann Bot http://dx.doi.org/10.1093/aob/mcu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimata M, Ogura K, Tsumuraya Y, Hashimoto Y, Yamamoto S. (1989) A β-galactosidase from radish (Raphanus sativus L.) seeds. Plant Physiol 90: 567–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kim Y, Guo Y, Stevenson B, Zhu JK. (2003) The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell 15: 19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM. (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58: 1399–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan W, Kovác P, Albersheim P, Darvill AG, Hahn MG. (1995) Characterization of a monoclonal antibody that recognizes an arabinosylated (1→6)-beta-D-galactan epitope in plant complex carbohydrates. Carbohydr Res 275: 295–307 [DOI] [PubMed] [Google Scholar]
- Sterling C. (1970) Crystal structure of ruthenium red and stereochemistry of its pectic stain. Am J Bot 57: 172–175 [Google Scholar]
- Stork J, Harris D, Griffiths J, Williams B, Beisson F, Li-Beisson Y, Mendu V, Haughn G, Debolt S. (2010) CELLULOSE SYNTHASE9 serves a nonredundant role in secondary cell wall synthesis in Arabidopsis epidermal testa cells. Plant Physiol 153: 580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Ralet MC, Berger A, Diatloff E, Bischoff V, Gonneau M, Marion-Poll A, North HM. (2011) CESA5 is required for the synthesis of cellulose with a role in structuring the adherent mucilage of Arabidopsis seeds. Plant Physiol 156: 1725–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Eberhard S, Pattathil S, Warder C, Glushka J, Yuan C, Hao Z, Zhu X, Avci U, Miller JS, et al. (2013) An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25: 270–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm JC, Burritt DJ, Ducker WA, Melton LD. (2009) Pectins influence microfibril aggregation in celery cell walls: an atomic force microscopy study. J Struct Biol 168: 337–344 [DOI] [PubMed] [Google Scholar]
- Updegraff DM. (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32: 420–424 [DOI] [PubMed] [Google Scholar]
- Vandenbosch KA, Bradley DJ, Knox JP, Perotto S, Butcher GW, Brewin NJ. (1989) Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J 8: 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiniciuc C, Dean GH, Griffiths JS, Kirchsteiger K, Hwang YT, Gillett A, Dow G, Western TL, Estelle M, Haughn GW. (2013) Flying saucer1 is a transmembrane RING E3 ubiquitin ligase that regulates the degree of pectin methylesterification in Arabidopsis seed mucilage. Plant Cell 25: 944–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zabotina O, Hong M. (2012) Pectin-cellulose interactions in the Arabidopsis primary cell wall from two-dimensional magic-angle-spinning solid-state nuclear magnetic resonance. Biochemistry 51: 9846–9856 [DOI] [PubMed] [Google Scholar]
- Western TL, Burn J, Tan WL, Skinner DJ, Martin-McCaffrey L, Moffatt BA, Haughn GW. (2001) Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol 127: 998–1011 [PMC free article] [PubMed] [Google Scholar]
- Western TL, Skinner DJ, Haughn GW. (2000) Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol 122: 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western TL, Young DS, Dean GH, Tan WL, Samuels AL, Haughn GW. (2004) MUCILAGE-MODIFIED4 encodes a putative pectin biosynthetic enzyme developmentally regulated by APETALA2, TRANSPARENT TESTA GLABRA1, and GLABRA2 in the Arabidopsis seed coat. Plant Physiol 134: 296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WG, Limberg G, Buchholt HC, van Alebeek GJ, Benen J, Christensen TM, Visser J, Voragen A, Mikkelsen JD, Knox JP. (2000) Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res 327: 309–320 [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Mackie W, Knox JP. (2001a) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47: 9–27 [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Knox JP. (2001b) In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 213: 37–44 [DOI] [PubMed] [Google Scholar]
- Willats WGT, Orfila C, Limberg G, Buchholt HC, van Alebeek GJWM, Voragen AGJ, Marcus SE, Christensen TMIE, Mikkelsen JD, Murray BS, et al. (2001c) Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J Biol Chem 276: 19404–19413 [DOI] [PubMed] [Google Scholar]
- Windsor JB, Symonds VV, Mendenhall J, Lloyd AM. (2000) Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J 22: 483–493 [DOI] [PubMed] [Google Scholar]
- Wu HM, Wang H, Cheung AY. (1995) A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell 82: 395–403 [DOI] [PubMed] [Google Scholar]
- Xu SL, Rahman A, Baskin TI, Kieber JJ. (2008) Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20: 3065–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Zhao L, Pan X, Samaj J. (2011) Developmental localization and methylesterification of pectin epitopes during somatic embryogenesis of Banana (Musa spp. AAA). PLoS One 6: e22992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates EA, Knox JP. (1994) Investigations into the occurrence of plant cell surface epitopes in exudate gums. Carbohydr Polym 24: 281–286 [Google Scholar]
- Yates EA, Valdor JF, Haslam SM, Morris HR, Dell A, Mackie W, Knox JP. (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6: 131–139 [DOI] [PubMed] [Google Scholar]
- Yoneda A, Ito T, Higaki T, Kutsuna N, Saito T, Ishimizu T, Osada H, Hasezawa S, Matsui M, Demura T. (2010) Cobtorin target analysis reveals that pectin functions in the deposition of cellulose microfibrils in parallel with cortical microtubules. Plant J 64: 657–667 [DOI] [PubMed] [Google Scholar]
- Young RE, McFarlane HE, Hahn MG, Western TL, Haughn GW, Samuels AL. (2008) Analysis of the Golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. Plant Cell 20: 1623–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zykwinska A, Gaillard C, Buléon A, Pontoire B, Garnier C, Thibault JF, Ralet MC. (2007a) Assessment of in vitro binding of isolated pectic domains to cellulose by adsorption isotherms, electron microscopy, and X-ray diffraction methods. Biomacromolecules 8: 223–232 [DOI] [PubMed] [Google Scholar]
- Zykwinska A, Thibault JF, Ralet MC. (2007b) Organization of pectic arabinan and galactan side chains in association with cellulose microfibrils in primary cell walls and related models envisaged. J Exp Bot 58: 1795–1802 [DOI] [PubMed] [Google Scholar]
- Zykwinska AW, Ralet MC, Garnier CD, Thibault JF. (2005) Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiol 139: 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.