Abstract
We assessed the long-term efficacy of imatinib dose escalation in 84 patients with chronic myeloid leukemia in chronic phase who met the criteria of failure to standard-dose imatinib. Twenty-one patients with hematologic failure and 63 with cytogenetic failure had their imatinib dose escalated from 400 to 800 mg daily (n = 72) or from 300 to 600 mg daily (n = 12). After a median follow-up of 61 months from dose escalation, 69% remained alive. Complete cytogenetic responses were achieved in 40%; including 52% of patients with cytogenetic failure and 5% of those with hematologic failure. The estimated 2- and 3-year event-free survival and overall survival rates were 57% and 47%, and 84% and 76%, respectively. Responses were long-lasting; 88% of patients with major cytogenetic response sustained their response beyond 2 years. Treatment was well tolerated, with 76% of patients, at 12 months, continuing to receive imatinib at 100% of the intended dose. In conclusion, imatinib dose escalation can induce sustained responses in a subset of patients with cytogenetic failure and a previous cytogenetic response to standard-dose imatinib.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder characterized by the expansion of hematopoietic cells carrying the Philadelphia chromosome (Ph), resulting from a reciprocal translocation of the long arms of chromosomes 9 and 22. A novel fusion gene is formed, BCR-ABL, which encodes a constitutively active tyrosine kinase.1,2
Imatinib (Gleevec, Glivec; Novartis Pharmaceuticals, Florham Park, NJ), a Bcr-Abl tyrosine-kinase inhibitor, has dramatically improved outcome in CML.3–6 In newly diagnosed patients with CML, imatinib is associated with a complete cytogenetic response (CCyR) rate of 82%, an estimated 5-year probability of survival without transformation to accelerated or blast phase of 93%, and overall survival (OS) rate of 89%.3–6
In the International Randomized Study of Interferon versus STI571 (IRIS), events (ie, loss of major cytogenetic response [MCyR], loss of complete hematologic response [CHR], transformation to accelerated or blast phase, or death from any cause) occurred at an annual rate of 4% to 7% during the first 2-3 years and less than 1% after that.7 In addition, other patients may have resistance as defined by the European LeukemiaNet recommendations.8
Resistance can be mediated through BCR-ABL–dependent mechanisms, often through mutations in the ABL kinase domain (40%-50%) or by mechanisms independent of BCR-ABL.9 Several strategies to overcome imatinib failure are being investigated. The first approach used to overcome resistance to imatinib was to increase the dose of imatinib. More recently, more potent tyrosine kinase inhibitors (TKIs) such as nilotinib, dasatinib, and bosutinib have shown to be effective in this setting.10–12
The rationale for imatinib dose escalation is (1) the overexpression of Bcr-Abl and amplification of BCR-ABL as mechanisms of resistance that could potentially be overcome by higher concentrations of imatinib7,9; (2) the fact that some mutations are still sensitive to imatinib at slightly higher concentrations13–16; (3) the clinical experience showing a dose-response effect in the phase I study17; and (4) the experience in CML-accelerated phase in which a dose of 600 mg imatinib was independently associated with significantly better time to transformation and better survival compared with 400 mg.18
Imatinib dose escalation has been reported to be of benefit in some patients after standard-dose imatinib failure.19,20 This benefit was based on small series of patients with short follow-up. It has been suggested that the beneficial effect of dose escalation is short-lived.20,21 Herein, we assess the long-term efficacy of imatinib dose escalation in patients with CML in chronic phase who demonstrated a poor response or relapse on standard-dose imatinib therapy.
Methods
Patients and response criteria
Between April 2000 and March 2007, 626 patients with CML in chronic phase received treatment with standard-dose imatinib either after interferon failure (n = 380) or as initial therapy (n = 246). Among them, 84 consecutive patients (13%) had their dose escalated because of failure to imatinib standard dose (54 previously reported).19 Dose escalation was from 400 mg daily to 800 mg daily (n = 72) or from 300 mg daily (in patients with prior dose reduction because of toxicity) to 600 mg daily (n = 12). Imatinib failure was defined according to the recent recommendations of the European LeukemiaNet.8 Briefly, for CML chronic phase, treatment failure was defined as loss of a cytogenetic or CHR defined by an increasing white blood cell (WBC) count on at least 2 occasions (with a doubling of the count from the nadir to ≥ 20 × 109/L or an absolute increase of ≥ 50 × 109/L), failure to achieve a CHR after 3 months of therapy, persistence of 100% Ph-positive metaphases after 6 months of therapy, 35% or more Ph-positive metaphases after 12 months, or 5% or more Ph-positive metaphases after 18 months of therapy.
Although these definitions were created for patients with previously untreated CML, in the absence of uniform definition for failure for patients receiving imatinib after interferon failure, the same definitions were applied to both groups. Patients were treated on protocols approved by the institutional review board (IRB) of Anderson Cancer Center, and informed consent was obtained in accordance with the Declaration of Helsinki.
All patients had a pretreatment cytogenetic analysis, fluorescent in situ hybridization (FISH), and real-time polymerase chain reaction (PCR). Cytogenetic analysis was done in bone marrow cells by the G-banding technique. At least 20 metaphases were analyzed, and marrow specimens were examined on direct or short-term (24 hour) cultures. Marrow cells were analyzed by FISH using the LSI BCR/ABL dual-color extra-signal probe according to the manufacturer's instructions (Vysis, Downers Grove, IL). Cytogenetics (FISH when routine cytogenetic analysis is not analyzable) and PCR were repeated every 3 months for the first year, and every 6 months thereafter. Patients were followed for survival every 3 months.
Response criteria were as previously described.22 A CHR was defined as a WBC count of less than 10 × 109/L, a platelet count of less than 450 × 109/L, no immature cells (blasts, promyelocytes, myelocytes) in the peripheral blood, and disappearance of all signs and symptoms related to leukemia (including palpable splenomegaly). A partial hematologic response (PHR) was similar to CHR except for persistence of peripheral immature cells or persistence but more than 50% improvement in splenomegaly or in degree of thrombocytosis. CHR was further categorized by the best cytogenetic response as CCyR (0% Ph-positive), partial (PCyR; 1%-35% Ph-positive), minor (36%-65% Ph-positive), and minimal (66%-95% Ph-positive). A MCyR included CCyR plus PCyR (ie, ≤ 35% Ph-positive). Major molecular response (MMR) was defined as a BCR-ABL/ABL ratio of 0.05% or less by real-time TaqMan-based quantitative PCR done in peripheral blood samples. A complete molecular response was defined as undetectable levels of BCR-ABL with level of detection of at least 4.5 logs.23
Mutation analysis
For mutational analysis screening, the entire BCR-ABL KD was sequenced using nested PCR. The BCR-ABL fusion transcript was amplified followed by 2 separate PCRs that cover exons 221 to 390 and codons 350 to 500 of the ABL KD, respectively.24 Standard dideoxy chain termination cycle sequencing was done using a Model 3100 or 3130 genetic analyzer (Applied Biosystems, Foster City, CA) with analysis using Seqscape version 2.0 software (Applied Biosystems). Mutations were confirmed by sequencing of forward and reverse strands, with sensitivity of 10% to 20% mutation-bearing cells in the analyzed population.
Statistical analysis
Event-free survival (EFS) was measured from start of imatinib dose escalation until loss of a CHR or a MCyR, progression to the accelerated or blastic phases of CML, or death from any cause during treatment. Failure-free survival (FFS) was measured from start of imatinib dose escalation until loss of a CHR or a cytogenetic response, failure to achieve a CHR after 3 months of therapy, persistence of 100% Ph-positive metaphases after 6 months of therapy, 35% or more Ph-positive metaphases after 12 months, lack of achievement of a complete cytogenetic response after 18 months of therapy, progression to the accelerated or blastic phases of CML, or death from any cause during treatment. Transformation-free survival (TFS) was measured from start of imatinib dose escalation until progression to the accelerated or blast phases or death from any cause. OS was defined from imatinib dose escalation to date of death or last follow-up. Survival probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test.25 Duration of major cytogenetic response was estimated via the Kaplan-Meier product-limit method and was measured from the first day the criteria of response were met until the date treatment was discontinued as a result of progressive disease or death or the date of last follow-up if response was ongoing. Patients who discontinued for other reasons were censored on the date of their last cytogenetic assessment. Differences among variables were evaluated by the χ2 test and Mann-Whitney U test for categorical and continuous variables, respectively.26
Univariate and multivariate analyses were performed to identify potential prognostic factors associated with EFS. The χ2 test was used to identify prognostic factors, which were then included as variables in a multivariate regression model for response. Factors retaining significance in the multivariate model were interpreted as being independently predictive of EFS. Multivariate analysis of survival used the Cox proportional hazard model.25,27,28
Results
Patients
Patient characteristics are shown in Table 1. Their median age was 54 years (range, 18-79 years). Most patients (95%) had received prior interferon therapy. The median time from diagnosis to imatinib therapy was 28 months (range, 0-184 months). The median duration of standard-dose imatinib therapy was 20 months (range, 3-76 months). The best response to imatinib was CHR only in 36% (n = 30) and cytogenetic response in 60% (26% complete, 19% partial). Two patients had a PHR, and 2 others were primary resistant to standard-dose imatinib. Reasons for imatinib resistance were hematologic failure in 21 patients (25%) (relapse in 17 [20%], resistance in 4 [5%]), and cytogenetic failure in 63 patients (75%) (relapse in 33 [39%], resistance in 30 [36%]). Among the 30 patients with cytogenetic resistance, 18 had never achieved any cytogenetic response. The median follow-up time from dose escalation was 61 months (range, 7-89 months).
Table 1.
Patients' characteristics: N = 84
| Variables | Median (range) | No. (%) |
|---|---|---|
| Age, y | 54 (18-79) | |
| Prior interferon therapy | 80 (95) | |
| Median time from diagnosis to treatment with imatinib, mo | 28 (0-184) | |
| Median duration on imatinib therapy, mo | 20 (3-76) | |
| Best response to imatinib | ||
| Complete hematologic response | 30 (36) | |
| Cytogenetic response | 50 (60) | |
| Major | 38 (45) | |
| Complete | 22 (26) | |
| Disease group at imatinib dose escalation | ||
| Hematologic relapse | 17 (20) | |
| Hematologic resistance | 4 (5) | |
| Cytogenetic relapse | 33 (39) | |
| Loss of CCyR but still in MCyR | 8 (10) | |
| Cytogenetic resistance | 30 (36) | |
| Resistance with no cytogenetic response | 18 (21) | |
| MCyR with no CCyR | 5 (6) | |
| Imatinib dose escalation from | ||
| 400 to 800 mg daily | 72 (86) | |
| 300 to 600 mg daily | 12 (14) | |
| Median follow-up from imatinib escalation, mo | 61 (7-89) | |
MCyR indicates major cytogenetic response; and CCyR, complete cytogenetic response.
Efficacy and outcome: overall population
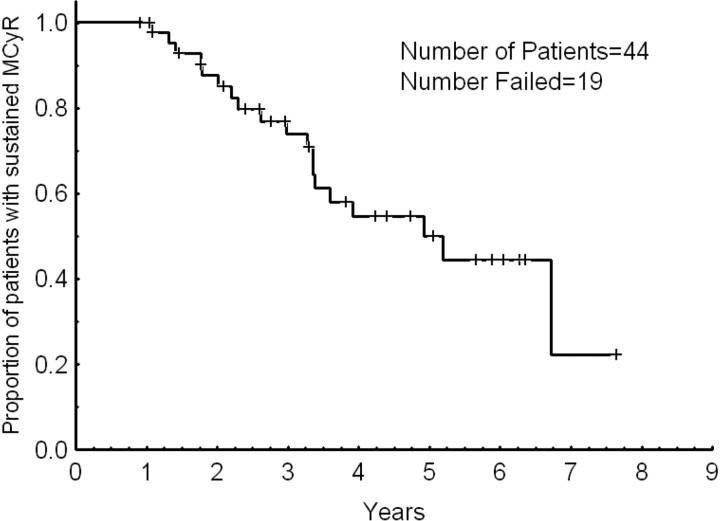
Overall, 57 patients responded: 50 achieved a cytogenetic response, and 7 achieved a hematologic response only. Hematologic response is only evaluable among patients with hematologic failure, and 48% of them had a CHR; one additional patient achieved a PHR. A cytogenetic response was achieved in 60%, including 75% of patients with cytogenetic failure and 14% of those with hematologic failure. A CCyR was achieved in 34 patients (40%) including 33 patients (52%) with cytogenetic failure and one of those patients (5%) with hematologic failure. In addition, a PCyR was achieved in 10 of 71 patients (14%) with greater than 35% Ph-positive metaphases at the time of dose escalation, including 8 of 50 evaluable patients (16%) with cytogenetic failure and 2 of those patients (10%) with hematologic failure. Table 2 details the response rates. The median time to cytogenetic response was 9 months (range, 2-54 months). Thirty-five of the 44 patients (80%) who achieved PCyR or CCyR (including patients with PCyR at the start of imatinib dose escalation who achieved CCyR) did so within 12 months from the start of dose escalation. Responses occurred in 7 of 12 patients (67%) who had their dose escalated from 300 to 600 mg daily and in 50 of 72 of those patients (69%) who had their dose escalated from 400 to 800 mg daily (P = .45). Responses were long-lasting. At 2 and 3 years, 88% and 74% of the patients sustained their MCyR, respectively (Figure 1). The median duration of MCyR was 5 years. All 4 patients in early chronic phase with no previous exposure to interferon therapy achieved a CCyR that remained sustained in all of them for a median of 22+ months (range, 13+-36+ months).
Table 2.
Response after dose increase in patients with imatinib failure
| Outcome | Total, n = 84 | Cytogenetic failure, n = 63 | Hematologic failure, n = 21 | P |
|---|---|---|---|---|
| Cytogenetic response, n (%) | ||||
| Any | 50 (60) | 47 (75) | 3 (14) | < .001 |
| Partial* | 10 (14) | 8 (16) | 2 (10) | .77 |
| Complete | 34 (40) | 33 (52) | 1 (5) | < .001 |
| % 2-year | ||||
| EFS | 57 | 65 | 36 | < .001 |
| FFS | 29 | 38 | 5 | < .001 |
| TFS | 73 | 80 | 51 | .004 |
| OS | 84 | 90 | 67 | < .001 |
EFS indicates event-free survival; FFS, failure-free survival; TFS, transformation-free survival; and OS, overall survival.
Only patients (n = 71) not in MCyR at the time of imatinib dose escalation were evaluable.
Figure 1.
Major cytogenetic response duration.
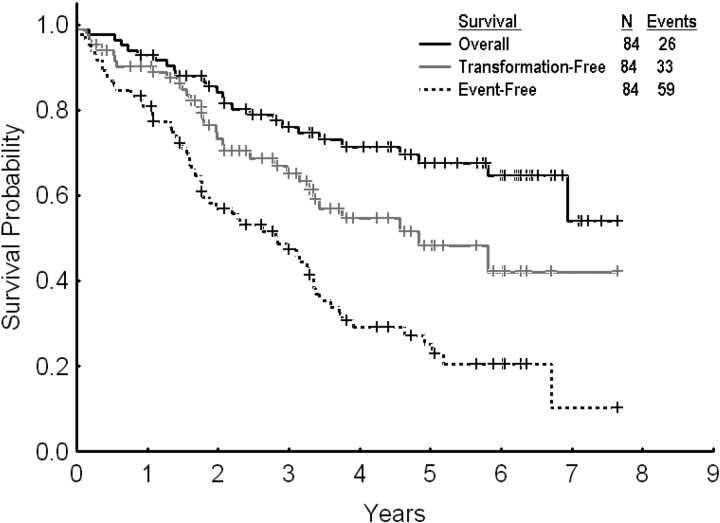
After a median follow-up of 61 months (range, 7-89 months) from dose escalation, 59 events (70%) were reported among the 84 patients treated. Twenty-five patients (30%) remained in complete cytogenetic response and were still receiving therapy with high-dose imatinib. Among the other patients, 26 (31%) were alive in chronic phase, with 18 of them receiving therapy with second-generation TKI after imatinib dose escalation failure, 2 in CMR after allogeneic stem cell transplantation. Seven patients (8%) were alive in advanced phases receiving therapy with second-generation TKI. Twenty-six patients (31%) died (12 in chronic phase and 14 in advanced phases); 2 of them after allogeneic stem cell transplantation. The estimated 2- and 3-year EFS, TFS, and OS rates for all 84 patients were 57% and 47%, 73% and 65%, and 84% and 76%, respectively (Figure 2). By excluding the 13 patients with PCyR at the start of imatinib dose escalation, the 2- and 3-year EFS and OS rates were 53% and 44%, and 81% and 72%, respectively. The 2- and 3-year FFS were 29% and 26%, respectively.
Figure 2.
Overall, event-free, and transformation-free survival for all patients receiving imatinib dose escalation after imatinib failure.
Patients with cytogenetic failure to standard-dose imatinib
Sixty-three patients received imatinib dose escalation for cytogenetic failure. Thirty-three patients had a cytogenetic relapse after having achieved a CCyR (n = 20), PCyR (n = 11), or minor cytogenetic response (n = 2). Among the 20 patients who lost their CCyR, 8 remained in PCyR. The other 30 patients had primary cytogenetic resistance; 18 never achieved a cytogenetic response, 5 achieved a best response of PCyR, and 7 achieved a minor cytogenetic response. Imatinib dose escalation was done at a median of 28 months (range, 3-67 months) from the achievement of the best cytogenetic response. Rate of MCyR after dose escalation was 60% in 50 evaluable patients (i.e. with > 35% Ph+ metaphases before escalation) with cytogenetic failure. CCyR was achieved in 33 of 63 patients (52%). PCyR was achieved in 8 of 50 patients (16%). Among the 18 patients who never achieved any cytogenetic response, 7 (39%) responded, with 2 of them achieving a PCyR and 5 a minor response. In contrast, in patients who had achieved some cytogenetic response before dose escalation, the rates of PCyR and CCyR were 25% (8 of 32 evaluable patients) and 73% (33 of 45 patients), respectively, after dose escalation.
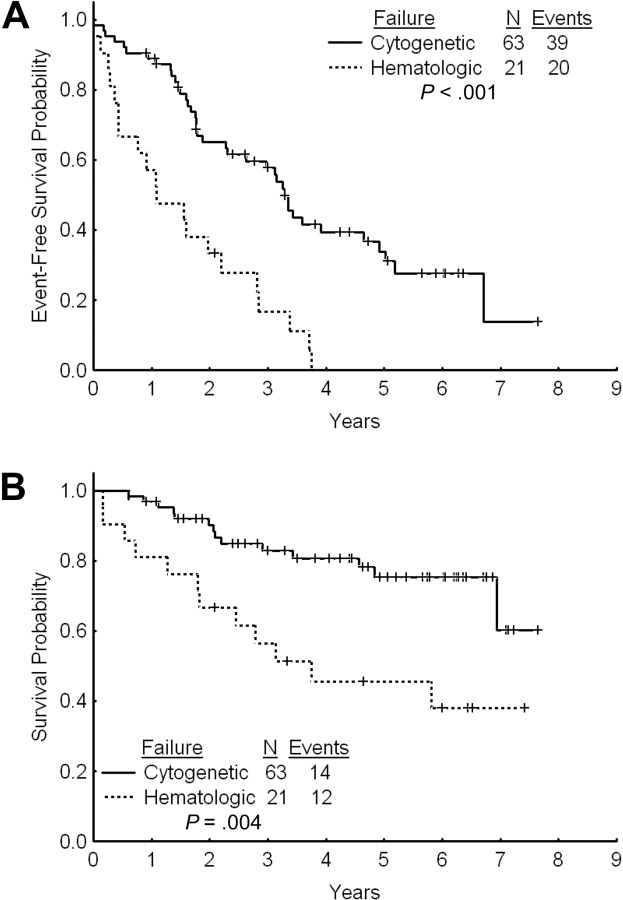
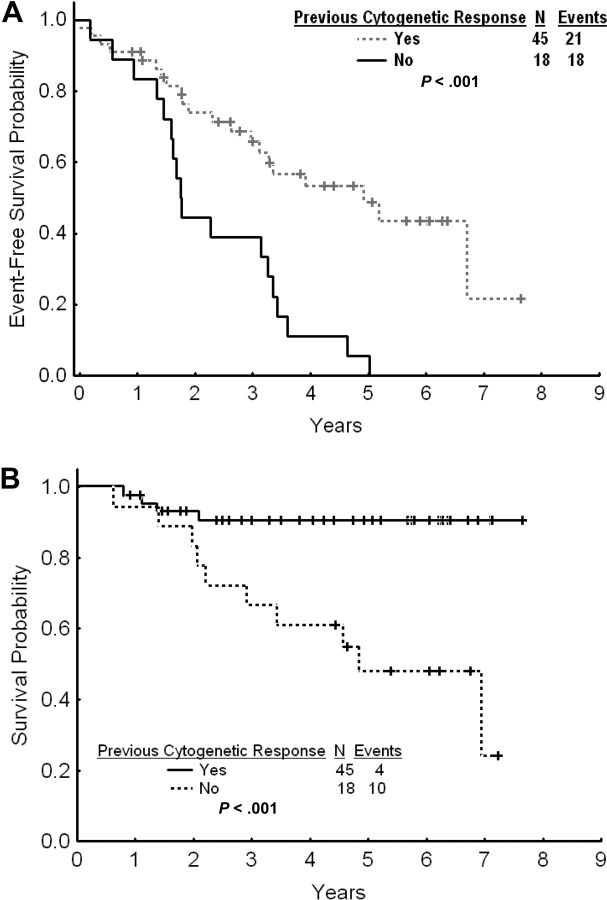
The estimated 2- and 3-year EFS rates for patients with cytogenetic failure were 65% and 58%, respectively (Figure 3A). The estimated 2- and 3-year TFS and OS rates were 80% and 74% and 90% and 83%, respectively. The estimated 2- and 3-year FFS were 38% and 34%, respectively. Among patients with cytogenetic failure, those who had never achieved any cytogenetic response on standard-dose imatinib had a worse outcome with a 2- and 3-year EFS rates of 44% and 39% compared with 74% and 66% for patients with previous cytogenetic response (P < .001; Figure 4A). The estimated 2-year FFS rates were 0% and 53%, respectively. The estimated 2- and 3-year TFS and OS rates were 64% and 58% versus 87% and 81% (P = .004), and 93% and 91% versus 83% and 67% (P < .001), respectively (Figure 4B).
Figure 3.
Outcome by type of failure. (A) Event-free survival after imatinib dose escalation by type of failure to standard-dose imatinib. (B) Overall survival after imatinib dose escalation by type of failure to standard-dose imatinib.
Figure 4.
Outcome by previous cytogenetic response. (A) Event-free survival after imatinib dose escalation by previous cytogenetic response to standard-dose imatinib. (B) Overall survival after imatinib dose escalation by previous cytogenetic response to standard-dose imatinib.
Patients with hematologic failure to standard-dose imatinib
CCyR was achieved in only one patient (5%) with hematologic failure (achieved also an MMR), while PCyR was achieved in 2 patients (10%).
The estimated 2- and 3-year EFS rates were 36% and 19%, respectively (Figure 3A). The estimated 2- and 3-year OS rates were 67% and 56%. These rates are significantly inferior to those for patients with cytogenetic failure to standard-dose imatinib (P = .004; Figure 3B). The 2- and 3-year TFS rates for patients receiving imatinib dose increase after failure to standard-dose imatinib were 51% and 37%, respectively. The 2-year FFS was 5%.
Outcome by baseline BCR-ABL mutational status
Baseline analysis of the ABL kinase domain was retrospectively performed in 25 patients (5 with primary and 20 with secondary resistance) in whom samples were available. Eight patients (32%) harbored 6 different mutations: F317L (n = 2), E355G (n = 2), D276G, N331S, F359V, and H396R. The PCyR and CCyR rates after imatinib dose escalation were 63% and 37% versus 32% and 39%, for patients with and without mutations, respectively (P = .617). There was no difference in the EFS and TFS after dose escalation between the 2 groups (P = .96 and P = .51, respectively). At the time of this report, 6 of the 17 patients (32%) with no mutations sustained their CCyR for 32+ (range, 13+-61+ months). Two of the 8 patients (25%) with mutations also maintained their CCyR (26+ and 31+ months, respectively).
Prognostic factors for EFS
We then analyzed the characteristics that might be associated with long-term EFS. By univariate analysis, adverse factors for EFS were a long CML duration, leucocytosis, higher peripheral basophil and blast percentages, higher percentage of Ph-positive metaphases, clonal evolution, and hematologic failure (vs cytogenetic resistance-recurrence). The achievement of a previous MCyR on standard-dose imatinib and the achievement of a MCyR after imatinib dose escalation were positive prognostic factors. By multivariate analysis, leucocytosis (P < .001), higher blast percentage (P < .001), and higher Ph-positive metaphases percents (P < .001) were independently associated with a shorter EFS. The presence of mutation at the time of imatinib standard-dose failure and dose reduction after imatinib dose escalation had no impact on EFS (Table 3). Imatinib dose reduction that took place in 28 patients after a median of 7 months (range, 1-43 months) from dose escalation had no impact as well on the achievement of a MCyR after dose escalation.
Table 3.
Effect on event-free survival by univariate and multivariate analysis
| Factors/variables* | Effect | Univariate P | Multivariate P, coefficient |
|---|---|---|---|
| Age | NS | ||
| Months on imatinib 400 mg | Better | .02 | NS |
| CML duration | Worse | .01 | NS |
| Previous major cytogenetic response | Better | .003 | NS |
| Higher hemoglobin | NS | ||
| Higher platelets count | NS | ||
| Leukocytosis | Worse | < .001 | < .001, .049 |
| Higher pb basophils % | Worse | .02 | NS |
| Higher pb blasts % | Worse | < .001 | NS |
| Higher marrow basophils % | Worse | .05 | NS |
| Higher marrow blasts % | Worse | < .001 | < .001, .317 |
| Clonal evolution | Worse | .01 | NS |
| Higher Philadelphia-metaphases % | Worse | < .001 | < .001, .021 |
| 400 mg failure (vs hematologic failure) | |||
| Cytogenetic resistance | Better | .001 | NS |
| Cytogenetic relapse | Better | .001 | NS |
| Mutation versus no mutation | NS | ||
| Dose reduction | Better | .01 | NS |
| Achievement of MCyR after dose escalation† | Better | .03 | NS |
CML indicates chronic myeloid leukemia; pb, peripheral blood; MCyR, major cytogenetic response; and NS, not significant.
Values were obtained at the time of 400 mg failure.
Time-dependent variable.
Safety
Imatinib dose escalation was well tolerated in most patients, with 28 patients (33%) having their imatinib dose reduced: from 800 to 600 mg daily (n = 17), from 800 to 400 mg daily (n = 8), and from 600 to 400 mg daily (n = 3) after the initial dose escalation. Imatinib dose reduction took place within 7 months from escalation (range, 1-43 months). The intended dose after dose escalation was sustained in 91%, 88%, 76%, and 54% of evaluable patients at 3, 6, 12, and 24 months, respectively. Imatinib was administered at the dose of 600 mg daily and higher in 79 of 84 evaluable patients (95%) at 3 months, in 70 of 73 evaluable patients (96%) at 6 months, in 69 of 72 evaluable patients (96%) at 9 months, in 67 of 71 evaluable patients (94%) at 12 months, and in 42 of 45 evaluable patients (93%) at 24 months (Figure 5). Dose reduction of imatinib resolved the toxicity in most patients. Interestingly, dose reduction for toxicity after initial dose escalation had no impact on the EFS nor on the probability of achieving a MCyR. The most common causes for dose reduction were myelosuppression in 13 patients (15%), fluid retention in 7 patients (8%), gastrointestinal toxicities in 4 patients (5%), dizziness in 2 patients (2%), skin rash in 2 patients (2%), and bone pain in 1 patient (1%). Table 4 summarizes the most common side effect observed.
Figure 5.
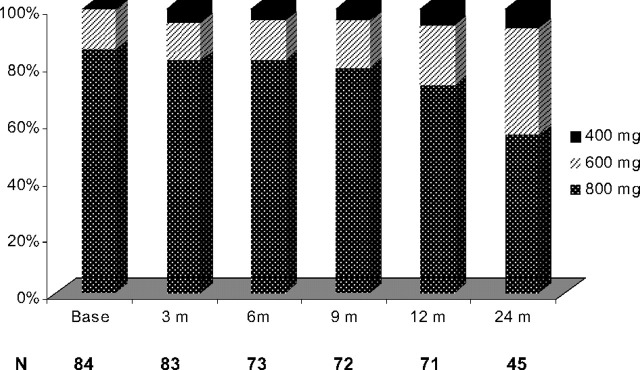
Diagram of imatinib dose administered over time.
Table 4.
Adverse effects associated with imatinib dose escalation in the total population: N = 84
| Adverse events | Patients, no. (%) |
|
|---|---|---|
| All grades | Grades 3/4 | |
| Nonhematologic toxicity | ||
| Fluid retention | 10 (12) | 0 (0) |
| Muscle cramps | 7 (8) | 1 (1) |
| Diarrhea | 5 (6) | 0 (0) |
| Nausea | 4 (5) | 0 (0) |
| Fatigue | 4 (5) | 0 (0) |
| Rash | 4 (5) | 1 (1) |
| Extremity pain | 2 (2) | 0 (0) |
| Dizziness | 2 (2) | 0 (0) |
| Increase creatinine | 1 (1) | 0 (0) |
| Bleeding | 1 (1) | 1 (1) |
| Hematologic toxicity | ||
| Thrombocytopenia | 11 (13) | 4 (5) |
| Leukopenia | 4 (5) | 4 (5) |
| Anemia | 11 (13) | 1 (1) |
Discussion
Overcoming resistance to imatinib mesylate therapy in CML can be achieved through several approaches, including imatinib dose escalation and the use of the second-generation TKIs.10,11 The long-term results of the present study suggest that imatinib dose escalation is an effective therapy in a significant fraction of patients with CML, particularly those with cytogenetic failure to standard-dose imatinib. Overall, PCyR was observed in 14% of patients and CCyR in 40%. Responses were durable, with 88% of patients with MCyR sustaining their response for at least 2 years.
Given the availability of second-generation TKIs that have shown significant efficacy also in this setting, it is important to put our results in perspective with the available data with dasatinib and nilotinib after imatinib failure. At 24 months, dasatinib induced in patients with imatinib failure MCyR and CCyR rates of 55% and 45%, respectively.11 With a follow-up of 24 months, 75% of patients treated with dasatinib for imatinib resistance remained progression-free.11 At 18 months of therapy, nilotinib induced MCyR and CCyR rates of 55% and 41%, respectively,10 with 64% of the patients treated remaining progression-free.10 Contrary to what has been previously suggested,20,21 responses to dose escalation were sustained with 88% of patients who achieved a MCyR, maintaining their response beyond 24 months of therapy. The 2-year survival in our series was 84% (81% if patients with PCyR at the time of dose escalation are excluded from the analysis), whereas the 2-year survival with dasatinib after imatinib failure was reported at 92%, and 91% (at 18 months) with nilotinib.10,29 It is important however to consider that comparisons between independent studies performed at different times may not be accurate. In addition, most patients reported in the studies with second-generation TKIs had already failed imatinib dose escalation. It is also important to underscore that the definitions proposed for failure were created for patients receiving imatinib as initial therapy, and it is unclear whether they apply equally to patients who receive imatinib after failing other therapies that constitute the majority of patients in our series and those of dasatinib and nilotinib after imatinib failure. Still, our results suggest that dose escalation may be an acceptable strategy for some patients with resistance to standard-dose imatinib.
Imatinib dose escalation was particularly effective in patients with cytogenetic failure who had achieved a cytogenetic response with standard-dose imatinib. Among them, the rates of CCyR and MCyR were 73% and 87% for those with 35% or more Ph+ metaphases before escalation, respectively, compared with 52% and 60% for the overall group of similar patients with cytogenetic failure. In a recent randomized phase II study, dasatinib improved the cytogenetic and molecular response rates and the progression-free survival compared with imatinib dose escalation.29 However, in a subset analysis, the difference in favor of dasatinib was considerably smaller among patients who failed standard-dose imatinib and those who had achieved a previous cytogenetic response. In those, the rates of MCyR were 70% and 60%, respectively.30 In addition, this randomized trial of dasatinib versus imatinib dose escalation had a crossover design that allowed crossover for lack of MCyR at the 3 months evaluation. As we report here, the median time to cytogenetic response after imatinib dose escalation is 9 months. Thus, the early crossover may have prevented full evaluation of the potential benefit of this strategy. Thus, with a longer follow-up and a larger number of patients, our study confirmed the sustained efficacy of imatinib dose escalation in patients with cytogenetic failure. In contrast, these results also show that patients who never achieved a cytogenetic response to standard dose imatinib or those with hematologic failure are highly unlikely to benefit from imatinib dose escalation.
In our study, only 25 of 84 patients were tested for BCR-ABL mutation at the time of standard-dose imatinib failure; all 8 patients harboring non-P–loop mutations responded to imatinib dose escalation. There was no difference in response, EFS, and TFS rates between patients with or without mutations. The presence of mutations at the time of standard-dose imatinib failure did not impact the EFS. These needs to be interpreted with caution in view of the lack of some highly resistant mutations (eg, P-loop, T315I) detected in this population. The incidence of BCR-ABL KD mutations at the time of standard-dose imatinib failure has been reported to be approximately 40% to 60%,31–33 with several mutations within the ABL structure in BCR-ABL conferring relative rather than absolute resistance to standard-dose imatinib.16 Non P-loop mutations retain relative sensitivity to imatinib,34 which may explain the response to dose escalation seen in this small cohort of patients. Second-generation TKIs have shown activity against all kinds of mutations except for the notable T315I mutation.10–12 In the randomized study, dasatinib was particularly superior to high-dose imatinib in patients harboring BCR-ABL KD mutations associated with more than 5-fold increase in imatinib resistance (such as the P-loop mutations), where the CHR and major cytogenetic response rates were 84% and 48% versus 29% and 14%, respectively.29 This is supported by our limited experience that shows imatinib dose escalation to be effective in patients harboring no or, relatively, sensitive BCR-ABL KD mutations.
High-dose imatinib was generally well tolerated in this study, with a third of the patients having their dose reduced, mostly for myelosuppression. The rate of myelosuppresion was still lower than the rates observed with the second-generation TKIs.10,11 After 12 months of therapy, 76% of patients continued to receive 100% of the intended daily dose. Thus, higher imatinib dosages could be delivered to most patients after failure of imatinib standard dose.
These data suggest that imatinib dose escalation is well tolerated and may be effective in overcoming resistance to standard-dose imatinib, inducing long-lasting cytogenetic responses in some patients with imatinib resistance, particularly those with cytogenetic relapse and less disease burden. However, these results have to be taken in the context of other available therapies, including second-generation TKIs and stem cell transplantation.
Acknowledgments
The authors have received support from the Betty Foster Leukemia Research Fund (Henderson, NV).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J. analyzed data and wrote and approved the manuscript; H.K. designed the study and wrote and approved the manuscript; D.J. and N.R. performed DNA sequencing and approved the manuscript; J.S. and M.B.R. analyzed data and approved the manuscript; S.O.B., W.W., S.F., G.G.M., and S.V. treated patients and approved the manuscript; and J.C. analyzed data, designed the study, and wrote and approved the manuscript.
Conflict-of-interest disclosure: H.K. and J.C. received research grants from Novartis and Bristol-Myers Squibb (BMS). E.J. is a member of the speakers' bureaus of Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Jorge Cortes, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 428, Houston, TX 77030; e-mail: jcortes@mdanderson.org.
References
- 1.Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 2.Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Talpaz M, O'Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 5.Hochhaus A, Druker B, Larson RA, et al. IRIS 6-year follow-up: sustained survival and declining annual rate of transformation in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract]. Blood. 2007;110:15a. [Google Scholar]
- 6.Druker B, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 7.Hochhaus A, La Rosée P. Imatinib therapy in chronic myelogenous leukemia: strategies to avoid and overcome resistance. Leukemia. 2004;18:1321–1331. doi: 10.1038/sj.leu.2403426. [DOI] [PubMed] [Google Scholar]
- 8.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 9.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Hochhaus A, Cortes J, et al. Nilotinib is highly active and safe in chronic phase chronic myelogenous leukemia (CML-CP) patients with imatinib resistance or intolerance [abstract]. Blood. 2007;110:226a. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 11.Stone RM, Kantarjian H, Baccarani M, et al. Efficacy of dasatinib in patients with chronic-phase chronic myeloid leukemia with resistance or intolerance to imatinib: 2-year follow-up data from START-C (CA180-013) [abstract]. Blood. 2007;110:225a. [Google Scholar]
- 12.Cortes J, Bruemmendorf T, Kantarjian H, et al. Efficacy and safety of bosutinib (SKI-606) among patients with chronic phase Ph+ chronic myeloid leukemia (CML) [abstract]. Blood. 2007;110:225a. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisberg E, Griffin JD. Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR/ABL-transformed hematopoietic cell lines. Blood. 2000;95:3498–3505. [PubMed] [Google Scholar]
- 14.Mahon FX, Deininger MW, Schultheis B, et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood. 2000;96:1070–1079. [PubMed] [Google Scholar]
- 15.le Coutre P, Tassi E, Varella-Garcia M, et al. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood. 2000;95:1758–1766. [PubMed] [Google Scholar]
- 16.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 17.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 18.Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928–1937. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian HM, Talpaz M, O'Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–475. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- 20.Zonder JA, Pemberton P, Brandt H, Mohamad AN, Schiffer CA. The effect of dose increase to imatinib mesylate in patients with chronic or accelerated phase chronic myeloid leukemia with inadequate hematologic or cytogenetic response to initial treatment. Clin Cancer Res. 2002;9:2092–2097. [PubMed] [Google Scholar]
- 21.Marin D, Goldman JM, Olavarria E, Apperley J. Transient benefit only from increasing the imatinib dose in CML patients who do not acheive cytogenetic remissions on conventional doses. Blood. 2003;102:2702–2703. doi: 10.1182/blood-2003-06-2042. [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 23.Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 24.Cortes J, Jabbour E, Kantarjian H, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. 2007;110:4005–4011. doi: 10.1182/blood-2007-03-080838. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Maier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1965;53:457–481. [Google Scholar]
- 26.Snedecor G, Cochran W. Statistical Methods. 7th ed. Ames IA: Iowa State University Press; 1980. [Google Scholar]
- 27.Agresti A. Categorical Data Analysis. 2nd Ed. Hoboken, NJ: John Wiley & Sons; 1990. [Google Scholar]
- 28.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 29.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 30.Kantarjian H, Rousselot P, Pasquini R, et al. Dasatinib or high-dose imatinib for patients with chronic-phase chronic myeloid leukemia resistant to standard-dose imatinib: a 2-year follow-up data from START-R (CA-180-017) [abstract]. Blood. 2007;110:226a. [Google Scholar]
- 31.Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–1773. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- 32.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 33.Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA working party on chronic myeloid leukemia. J Clin Oncol. 2005;23:4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- 34.Corbin AS, La Rosee P, Stoffregen EP, Druker BJ, Deininger MW. Several Bcr-Abl kinase domain mutants associated with imatinib mesylate resistance remain sensitive to imatinib. Blood. 2003;101:4611–4614. doi: 10.1182/blood-2002-12-3659. [DOI] [PubMed] [Google Scholar]