Abstract
A 42-year-old man presented with right-sided epiphora, a fleshy lesion emanating from the right inferior punctum and a painless mass below the medial canthal tendon. Biopsy of the lacrimal sac mass disclosed papillary squamous cell carcinoma in situ. The patient underwent wide local excision with clear surgical margins and remained disease free until 28 months later when he returned with hemorrhagic epiphora of the OS and fullness overlying the left lacrimal sac. Biopsy confirmed papillary squamous cell carcinoma in situ. He underwent similar excision and has remained disease free for 6 months. To the best of the authors’ knowledge, this is the first report of bilateral squamous cell carcinoma of the lacrimal sac.
Lacrimal sac tumors are rare with approximately 300 cases described in the literature.1 The malignancy rate of all lacrimal sac masses has been reported to be 55% to 75%.1,2 The most common malignant tumors are of epithelial origin, with squamous cell carcinoma predominating.3
This is a report of a case of bilateral squamous cell carcinoma of the lacrimal sac. Using PubMed on the National Library of Medicine, the authors were unable to identify bilateral cases of squamous cell carcinoma of the lacrimal sac with search terms “lacrimal sac masses,” “squamous cell carcinoma of lacrimal sac,” and “bilateral lacrimal sac masses.”
CASE REPORT
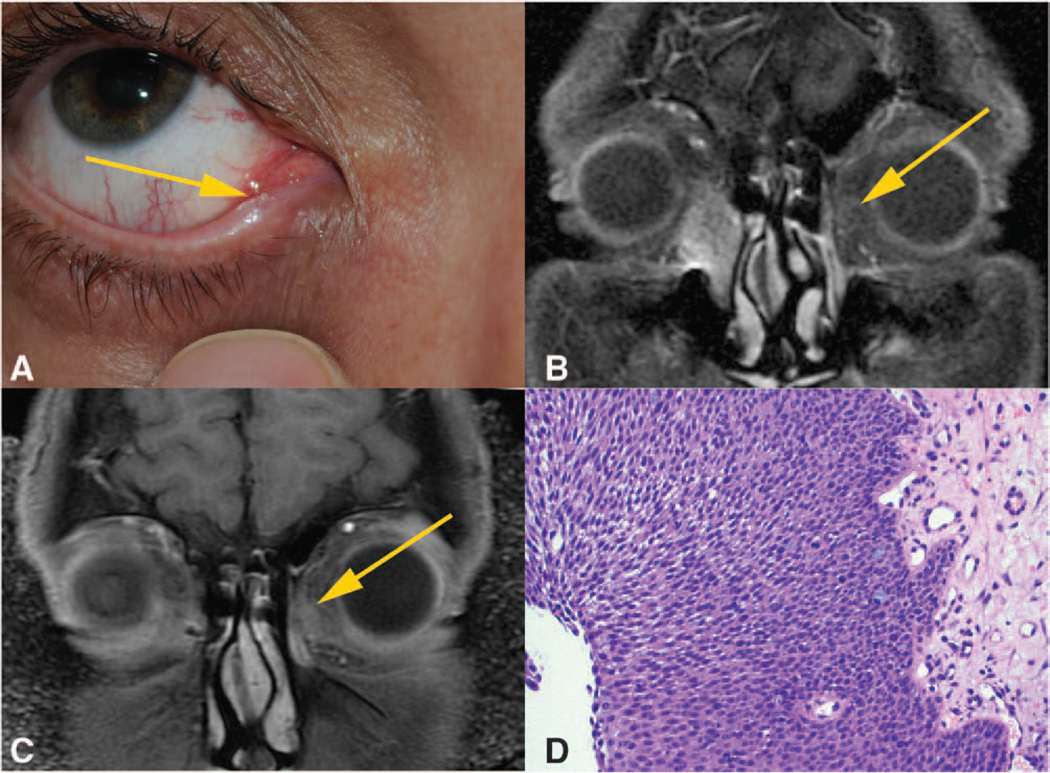
A 42-year-old man presented with a 15-year history of right-sided epiphora and a 3-month history of a fleshy painless lesion at the right inferior puncta (Fig. A). There was no purulent or hemorrhagic discharge from the right tear duct. His medical history was notable for hepatitis B. He denied a history of human papilloma virus (HPV) and human immunodeficiency virus (HIV) and a family history of cancer. On clinical examination, he was noted to have a firm mass inferior to the right medial canthal tendon, in the region of the lacrimal sac. Slit lamp examination and dilated fundoscopic examination were unremarkable. MRI of the orbit was obtained, given concern for mass and revealed a 2.5-cm homogeneously enhancing lesion arising from the right lacrimal sac, extending inferiorly into the nasolacrimal duct (Fig. B). CT imaging of the orbit confirmed the lacrimal sac mass and did not show any evidence of bony erosion (images not shown). A systemic workup was performed by oncology. Local and regional lymph nodes were unremarkable, and full body imaging did not disclose any abnormalities. He then underwent incisional biopsy, which revealed papillary squamous cell carcinoma in situ. Tumor node metastasis staging was determined to be stage 0 as he had no cutaneous lesions or enlarged regional lymph nodes. HIV serology was obtained given the age of presentation and was negative.
FIG.
A, External photograph showing the lesion emanating from inferior punctum. B, T1 coronal MRI scan showing enhancement of right lacrimal sac with extension into the nasolacrimal duct. Yellow arrow indicating left-sided signal flare suggestive of developing neoplasm. C, T1 coronal MRI scan showing a 7-mm enhancing lesion of the left lacrimal sac. D, High-power hematoxylin and eosin stain of the left lacrimal sac biopsy which reveals proliferation of atypical squamous cells (hematoxylin-eosin).
Prior to definitive surgical resection, he was treated with topical mitomycin C (MMC) 0.04% four times daily for 4 cycles to prophylactically treat any lesions which may have seeded the ocular surface from the carcinoma emerging from the inferior punctum. Following topical MMC therapy, the patient underwent medial maxillectomy and dacryocystectomy through a lateral rhinotomy approach. Surgical margins were all negative. Primary closure was performed with the placement of a silicone stent in the lacrimal system.
He remained disease free until 28 months after initial presentation when he presented with left-sided hemorrhagic epiphora. Clinical examination revealed blood-tinged reflux on palpation of the lacrimal sac. Nasolacrimal irrigation revealed no obstruction. MRI of the orbit revealed a 7-mm mildly enhancing soft tissue mass in the left nasolacrimal sac (Fig. C). Secondary review of the original MRI for the right-sided mass in 2009 demonstrated small left-sided signal flare possibly suggestive of developing neoplasm (Fig. B). Incisional biopsy was performed and was consistent with papillary squamous cell carcinoma in situ (Fig. D). No lesions were noted in the puncta. A similar treatment regimen was instituted on the left side with 4 cycles of topical MMC followed by medial maxillectomy and dacryocystectomy. Surgical margins were free of tumor, and the patient remained disease free on the left side for 6 months and for 36 months on the right side.
Immunohistochemistry was performed on the pathologic specimens for HPV p16 marker. Staining with p16 was negative in both sections for this marker. In situ hybridization was positive for low-risk HPV strains 6 and 11 but negative for high-risk HPV strains 16 and 18.
DISCUSSION
There are no known reported cases of bilateral squamous cell carcinoma of the lacrimal sac. Bilateral SCC of the lacrimal sac in a young patient raises suspicion for infectious risk factors, including HPV and HIV. The association of infectious agents with the pathogenesis of squamous cell carcinoma has been well established in anogenital cancers. In greater than 99% of invasive cervical cancers, specific subtypes of HPV strains can be readily identified.4 HPV has also been implicated in the etiology of epithelial tumors of the lacrimal sac with recent studies demonstrating the presence of HPV DNA and HPV RNA in lacrimal sac carcinomas.5,6 Surprisingly, when the authors assayed for HPV in this sections, p16 staining was negative in their patient. Furthermore, in situ hybridization was positive for low-risk HPV strains 6 and 11 associated with papillomas and was negative for high-risk strains 16 and 18 classically associated with SCC.7 There is, however, a prior report of a lacrimal sac carcinoma that tested positive only for the low-risk HPV strain 11 on in situ hybridization.6 HIV has been associated with increased incidence of HPV infection and anogenital squamous cell carcinoma.8 HIV was not implicated in this case, given negative serology.
Several other possibilities may explain the bilateral nature of the SCC. First, it is possible that the SCC arose de novo, independently in both lacrimal sacs. A second possibility is that with a 15-year history of the right-sided epiphora and growth of the SCC from the inferior punctum, eye rubbing could have led to seeding of the tumor on the contralateral side. Despite no visible ocular surface lesions suggestive of SCC, the authors pretreated the patient’s ocular surface with topical MMC prior to definitive surgical resection to sterilize the ocular surface. Finally, it is possible that the contralateral side represents a metastasis as evidenced by the suggestive enhancement on MRI. The removal of the primary SCC on the right side could have led to the loss of an antiangiogenic factor leading to unregulated growth of the SCC on the left side as was first postulated by Holmgren et al.9 Once molecular genetic testing is established for SCC, clonality can be established and used to determine if the bilateral SCC originated from a single cell.
Lacrimal sac carcinoma has been reported to have an ipsilateral recurrence rate of 50% necessitating close follow up.9 Tumor recurrence has been described locally despite wide excision techniques, and it is unclear if this is a case of local-regional metastasis or separate de novo carcinomas. This case highlights the need to maintain a high index of suspicion for SCC of the lacrimal system in a patient who presents with contralateral tearing and a prior history of SCC in the fellow eye.
Acknowledgments
This study was supported by a grant from the Research to Prevent Blindness and the William and Marilyn Fields Family Fund.
Footnotes
This study was approved by the University of California, San Diego Human Research Protections Program.
The authors have conflicts of interest to disclose.
References
- 1.Parmar DN, Rose GE. Management of lacrimal sac tumours. Eye (Lond) 2003;17:599–606. doi: 10.1038/sj.eye.6700516. [DOI] [PubMed] [Google Scholar]
- 2.Stefanyszyn MA, Hidayat AA, Pe’er JJ, et al. Lacrimal sac tumors. Ophthal Plast Reconstr Surg. 1994;10:169–184. doi: 10.1097/00002341-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Pang CS, Brown JD, Ganote CE, et al. A mass of the right lacrimal sac in a 53-year-old man. Arch Pathol Lab Med. 2005;129:1493–1494. doi: 10.5858/2005-129-1493-AMOTRL. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Lorincz A, Munoz N, et al. The causal relationship between human papillomaviris and cervical cancer. J. Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjö NC, von Buchwald C, Cassonnet P, et al. Human papillomavirus: cause of epithelial lacrimal sac neoplasia? Acta Ophthalmol Scand. 2007;85:551–556. doi: 10.1111/j.1600-0420.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- 6.Madreperla SA, Green WR, Daniel R, et al. Human papillomavirus in primary epithelial tumors of the lacrimal sac. Ophthalmology. 1993;100:569–573. doi: 10.1016/s0161-6420(93)31629-5. [DOI] [PubMed] [Google Scholar]
- 7.Doxtader EE, Katzenstein AL. The relationship between p16 expression and high-risk human papillomavirus infection in squamous cell carcinomas from sites other than uterine cervix: a study of 137 cases. Hum Pathol. 2012;43:327–332. doi: 10.1016/j.humpath.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Piketty C, Selinger-Leneman H, Bouvier AM, et al. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the French hospital database on HIV. J Clin Oncol. 2012;30:4360–4366. doi: 10.1200/JCO.2012.44.5486. [DOI] [PubMed] [Google Scholar]
- 9.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]