Abstract
The itch-scratch reflex serves as a protective mechanism in everyday life. However, chronic persistent itching can be devastating. Despite the clinical importance of the itch sensation, its mechanism remains elusive. In the past decade, substantial progress has been made to uncover the mystery of itching. Here, we review the molecules, cells, and circuits known to mediate the itch sensation, which, coupled with advances in understanding the pathophysiology of chronic itching conditions, will hopefully contribute to the development of new anti-itch therapies.
Keywords: itch, pruritus, neurons, neural circuits, sensitization, pain
INTRODUCTION
Itch, also known as pruritus, was defined 350 years ago by the German physician Samuel Hafenreffer as an “unpleasant sensation that elicits the desire or reflex to scratch” (54, p. 535). Itch manifests in both acute and chronic forms. Acute itch evoked by insect bites can be easily relieved by scratching. Chronic itch, however, remains a challenge in the clinic. On the basis of clinical classification, chronic itch conditions can be divided into four subtypes: dermatologic, systemic, neuropathic, and psychogenic (15, 153). Dermatologic itch arises from diseases of the skin, such as atopic dermatitis, eczema, psoriasis, urticaria, and xerosis. Systemic itch, by contrast, always accompanies diseases of organs other than the skin. The examples of systemic itch include cholestatic pruritus and uremic pruritus. Neuropathic itch results from nerve injury and can arise from diseases or disorders of the central nervous system (CNS) or peripheral nervous system, such as neuropathy, multiple sclerosis, brain tumors, and nerve compression or irritation. Psychogenic itch is attributed to psychological or psychiatric disorders, such as obsessive-compulsive disorders and delusions of parasitosis. It should be noted that the classification of chronic itch is based on clinical relevance, not on underlying mechanisms. Different subtypes of itch conditions might involve common pruriceptive factors. For example, patients with uremic pruritus often experience changes in skin physiology, such as dehydration (xerosis), which leads to dermatologic itching (70).
Similar to pain, the itch sensation is initiated by pruriceptive primary afferent neurons that transmit information from the periphery to the CNS (13, 54). The cell bodies of primary afferent neurons are located in the dorsal root ganglia (DRG) and the trigeminal ganglion. Primary afferent neurons are categorized into three classes: small-diameter, unmyelinated C fibers with slow conduction velocity; medium-diameter, thinly myelinated Aδ fibers; and larger-diameter, heavily myelinated Aβ fibers with the fastest conduction velocity. C fibers are the primary mediator of the itch sensation (54). A recent report demonstrated that A fibers are also involved in the perception of itch (109). The investigators showed that selectively blocking the conduction of myelinated fibers attenuated the itch sensation. The central projections of sensory neurons form synapses with secondary neurons in the dorsal horn of the spinal cord to convey itch signaling to the brain.
Because of the similarities between the pain and itch sensations, itch has been historically considered a submodality of pain. However, as the study of the neuroscience of itching has blossomed in recent years, our understanding of the mechanism and neuronal circuit mediating the itch sensation has greatly advanced. Emerging evidence suggests that the itch sensation is a distinct sensory modality that employs specific neural pathways. In this review, we first introduce animal models for studying itching. We then discuss several theories about how an itch is encoded and related to the pain sensation. We focus on itch mediators, their corresponding receptors (Table 1), and sensing neurons on both the peripheral and central levels, with the goal of clarifying the specific neural circuit mediating the itch sensation. We also highlight progress made in investigating different subtypes of clinical itchiness, including the systemic itch, neuropathic itch, and psychogenic itch. We discuss the sensitization of the itch sensation in pathological conditions to understand the plasticity of the itch neural pathway. The similarities and cross talk between the pain and itch sensations are also briefly summarized.
Table 1. Pruritogens and receptors in the periphery.
| Pruritogen | Source(s) | Receptor(s) in DRG |
|---|---|---|
| Histamine | Mast cells | H1R, H4R |
| BAM8-22 | Proteolytic cleavage of proenkephalin | MrgprC11 |
| CQ | Antimalaria medicine | MrgprA3 |
| SLIGRL-NH2 | Synthetic peptide or proteolytic cleavage of PAR2 | MrgprC11, PAR2 |
| β-alanine | Muscle-building supplement | MrgprD |
| Mucunain | Cowhage | PAR2, PAR4 |
| Cathepsin S | Immune cells and epithelial cells | PAR2, PAR4 |
| Tryptase | Mast cells | PAR2, PAR4 |
| 5-HT | Mast cells | 5-HT1, 5-HT2 |
| ET-1 | Endothelial cells | ETA |
| Substance P | Primary sensory neurons | NK1 |
| IL-31 | Mainly Th2 lymphocytes | IL-31RA, OSMR |
| Imiquimod | Synthetic immunostimulant | TLR7 (controversial) |
| Polyinosinic:polycytidylic acid | Synthetic immunostimulant | TLR3 |
| Bile acid | Cholestasis | TGR5 |
| Lysophosphatidic acid | Cholestasis | Unknown |
Abbreviations: BAM8-22, bovine adrenal medulla 8–22 peptide; CQ, chloroquine; DRG, dorsal root ganglion; ET-1, endothelin-1; IL-31, interleukin-31; 5-HT, serotonin.
ANIMAL MODELS FOR INVESTIGATING ITCH MECHANISMS
Recent progress in uncovering the mechanisms of the itch sensation has led to enthusiasm for the generation of better animal models for this investigation. By definition, itch is the unpleasant sensation that elicits the desire to scratch. Therefore, scratching behavior is the indication of an itch sensation in animal models. In the traditional rodent scratching assay, pruritogens are injected into the nape of the neck of the mouse, and the number of scratching bouts is measured (69). One limitation of this model is that injection of the algogenic capsaicin into the nape of the back also causes scratching behavior, indicating that this model is not able to differentiate between itch- and pain-related behaviors (119). This is an important issue, especially for itch researchers who are looking for components specific to the itch neural pathway. Recently, LaMotte’s group (119) modified the traditional model to establish the “cheek injection model.” They reported that mice exhibit distinct behaviors in response to cheek injection of pruritogens versus algogens: Pruritogens elicit scratching with the hind paw, whereas algogens evoke facial wiping with the forelimb. This model was amended in rats by Carstens’ group (127) and provides a reliable means of distinguishing itch and pain behaviors.
Pruritogen injection is used to evoke only acute itch-scratching behavior. To investigate the mechanism of itchiness in pathological conditions, some chronic itch models have been established, most of which focus on cutaneous diseases, including atopic dermatitis and xerosis (dry skin). Atopic dermatitis is a common pruritic inflammatory skin disorder that affects 10–20% of children and 1–3% of adults (57). Currently, there are three different types of atopic dermatitis animal models. The first type, and also the first established atopic dermatitis model, is the NC/Nga mouse inbred strain, which spontaneously develop atopic dermatitis–like skin lesions (90). When the NC/Nga mice are maintained in conventional non-air-controlled rooms, spontaneous dermatitis and pruritus occur because of the skin-barrier abnormalities that predispose the mice to skin damage from the environment. The second type of atopic dermatitis model is produced by treating the skin of the mice with an allergen, such as ovalbumin, dinitrobenzene, or squaric acid dibutylester, to induce skin inflammation and pruritus (57). The third type is genetically modified mice that either overexpress or lack specific molecules, for instance, IL-31 transgenic mice (29) and cathepsin E knockout mice (142). These models have been valuable tools for investigating the pathogenesis of atopic dermatitis and the associated mechanism of the itch sensation.
Chronic itch models have also been developed for another cutaneous condition: xerosis. Xerosis is a medical condition in which skin dehydration is accompanied by persistent itching. It might be caused by excessive washing or defects in genes that are important for maintaining the skin-barrier function (23). Dry skin can directly cause pruritus, and in many cases, it is also one of the symptoms of chronic itch conditions, such as atopic dermatitis, cholestatic pruritus, and uremic pruritus. A dry-skin mouse model was established by Kuraishi’s group (94) in 2002. In this model, the skin on the backs of mice was shaved and then dehydrated using a mixture of acetone and ether. After this treatment, the mice displayed robust spontaneous scratching, and the affected skin area exhibited decreased stratum corneum hydration and increased transepidermal water loss, which mimic the symptoms of dry skin in patients. This model was later modified by Carstens’ (4) and Bautisca’s groups (150) to generate dry skin on the hind paw and cheek, respectively. The same treatment can induce spontaneous scratching in mast cell–deficient mice, suggesting that mast cells are not required for the generation of dry-skin pruritus. Histological analyses have shown that the dermis of a treated skin sample is not infiltrated with inflammatory cells, suggesting that different mechanisms are responsible for dry-skin pruritus versus contact dermatitis, which always involves skin inflammation (94).
The mechanisms of pruritus associated with systemic disorders, such as cholestatic pruritus, are poorly understood, partly because of the scarcity of animal models for the investigation. Cholestasis, a condition in which bile cannot flow properly from the liver, can be caused by bile duct obstruction and is modeled in mice or rats via common bile duct ligation (BDL) (14). This model is very effective in causing jaundice and impairment of liver function and has been a useful tool for investigating the pathophysiology of cholestasis. Recently, researchers in Planells-Cases’s group (14) showed that this model can be used as an animal model of cholestatic pruritus. They showed for the first time that BDL can induce scratching behavior in rats. They found that rats that underwent BDL exhibited robust spontaneous scratching behavior 48 hours after surgery and that this behavior persisted for up to 3 months. This study provided a promising animal model to elucidate the mechanisms of cholestatic pruritus in the future.
PERIPHERAL MECHANISMS OF ITCH CODING
Itch has been considered a minor form, or submodality, of pain based on studies in the early twentieth century (75, 147). These studies have shown that there are itch and pain spots coinciding in human skin, leading to hypotheses that both sensations are transmitted by the same neuronal populations and that they would be differentiated by way of intensity or patterns of neuronal activity. However, more recent findings argue against the intensity/pattern theory. Tuckett (143) showed that increased frequency of electrical stimulation on human skin produces increased intensity of itch, with no change in the quality of the sensation. Painful stimulation cannot be converted to itch at lower frequencies either (46, 100). In 1997, Schmelz et al. (114), using a microneurographic study in humans, identified a population of histamine-sensitive C fibers, which have distinct properties from other nociceptive populations, such as mechanoinsensitivity, high electrical activation threshold, low-conduction velocity, and large innervation territories. Subsequently, Andrew & Craig (9) identified a class of lamina I spinothalamic tract (STT) neurons in the spinal cord dorsal horn that responds to histamine but is insensitive to mechanical or thermal stimuli, which typically induce pain. Although subsequent studies demonstrated that all of the histamine-responsive neurons examined also respond to algogens (79, 86, 115, 123), these results lead to the speculation, termed labeled-line theory, that different sets of neuronal populations mediate itch versus pain sensations.
The strongest evidence for labeled-line theory has come from recent studies involving genetic modification. Sun and colleagues (132, 133) showed that gastrin-releasing peptide receptor (GRPR) and GRPR+ neurons in the spinal cord are required for an itch sensation but are dispensable for a pain sensation. Similarly, our group reported that mice lacking mas-related G protein–coupled receptors (Mrgprs) exhibit attenuated itch sensations but normal acute pain responses (79, 80). We also found that ablation of MrgprA3+ neurons in the DRG reduced itch behavior without disturbing pain (44). Moreover, Ji’s group (82) found that toll-like receptor 7 (TLR7) expression in primary sensory neurons was required for inducing itch but not pain. These studies suggest that certain molecules and sensory neurons specifically mediate itch sensations but not pain, providing the molecular and cellular basis of labeled-line theory. However, these studies have some limitations. They do not rule out the possibility that the molecules or neurons investigated may also mediate pain and that their ablation did not affect pain behavior owing to compensation from other pain-sensing neurons. To address this issue, our recent study (44) created a genetically modified mouse line to express the transient receptor potential cation channel V1 (TRPV1), the molecular sensor of noxious heat and capsaicin only in MrgprA3+ neurons (44). Injection of capsaicin, an algogen, into the mice specifically activated MrgprA3+ itch-sensing neurons and induced itch but not pain behavior. This result demonstrated that MrgprA3+ DRG neurons only mediate an itch sensation, not pain (Figure 1). Even when MrgprA3+ neurons were activated by an algogen, the activated neuronal circuit still transmitted itch signaling, rather than pain signaling, to the brain. This result is consistent with Müller’s doctrine of “specific nerve energy,” which proposes that the quality of sensation depends on the specific neuronal pathway that is activated and not on the nature of the stimulus (34). In summary, recent studies suggest that the itch sensation is not a submodality of pain. It is a distinct sensory modality that employs specific molecules and sensory neurons and a specific neural pathway (Figure 1a) (see the “Interactions Between Pain and Itch” section below for an additional discussion of itch coding).
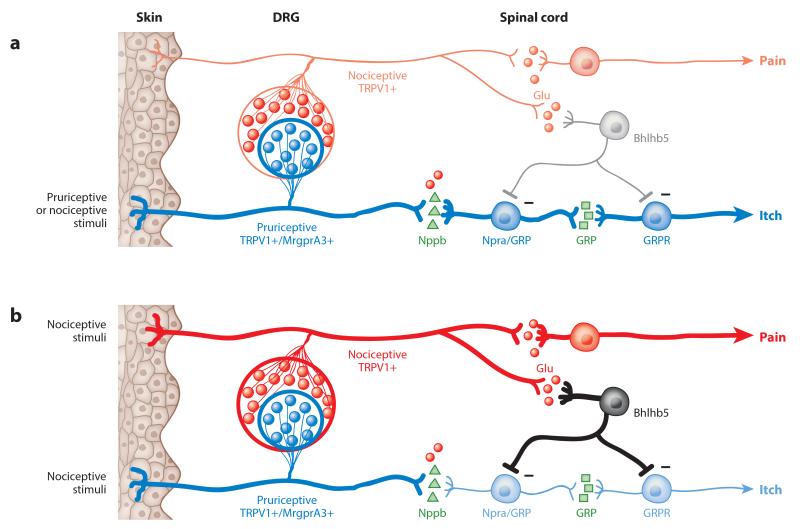
Figure 1.
Schematic drawing of the neuronal interactions between dorsal root ganglia (DRG) neurons and spinal neurons in the itch and pain pathways. The neural pathways for pain (red) and for itch (blue) are shown. The bold lines indicate the activated pathways. (a) When pruriceptive DRG neurons are selectively activated by either pruriceptive or nociceptive (e.g., capsaicin) stimuli, the itch sensation is produced (labeled-line theory) (44). (b) When nociceptive stimuli activate both pruriceptive and nociceptive DRG neurons, the itch sensation is suppressed by the inhibitory spinal interneurons, and only the pain sensation is produced (population theory) (71, 84, 101). For the purposes of this illustration, the sensory fibers of all DRG neurons are bundled into one single line to represent the peripheral and central terminals. Each different population of spinal neurons is represented by a single neuron. Minus signs indicate the inhibitory synapse between the interneuron and the pruriceptive spinal neuron. Figure modified with permission from Reference 105.
PERIPHERAL ITCH MEDIATORS AND RELATED RECEPTORS
Histamine and Histamine Receptors
Histamine is synthesized endogenously from the amino acid histidine and is mainly released from mast cells (124). Application of histamine to human skin induces the itch sensation, accompanied by axon reflex flare (54). Histamine is by far the most closely studied pruritogen, and it has been employed as the itch stimulus to identify pruritoceptive sensory neurons/fibers. Four histamine receptors, all of which are G protein–coupled receptors (GPCRs), have been identified in mammals (124). Among them, H1R has been detected on DRG neurons and has been presumed to be important in histamine-induced itch reactions (45). Indeed, inhibitors of H1R can completely suppress histamine-induced itch in human skin (124). A recent study suggests that H4R also plays an important role in evoking an itch sensation: The H4R antagonist can block the itch sensation in a contact dermatitis mouse model (112). The combination of H1R and H4R antagonists is even more effective than either alone (112). Unfortunately, histamine receptor antagonists are ineffective at relieving itch in many of the chronic itch conditions, such as cholestatic pruritus and uremic pruritus (54). Therefore, itch is often characterized as either histaminergic or nonhistaminergic itch.
H1R is coupled with the G protein Gαq/11 to activate phospholipase C-β3 (PLCβ3), which in turn leads to an increase in intracellular calcium and activation of protein kinase C (124). Mice lacking PLCβ3 showed significant defects in scratching behavior induced by histamine (45). H1R-mediated sensory neuron activation and scratching behavior also require TRPV1 (45), possibly through the activation of phospholipase A2 and 12-lipoxygenase after histamine binding (118).
Mas-Related G Protein–Coupled Receptors
Mrgprs are a family of orphan GPCRs consisting of more than 50 members in the mouse genome; several of these are specifically expressed in small-diameter sensory neurons in the DRG and trigeminal ganglia, indicating their important role in somatosensation (30, 74). In recent studies, several Mrgprs have been identified as receptors that mediate nonhistaminergic itch (78–80, 120). For example, MrgprA3 is a receptor for the antimalarial drug chloroquine (CQ), which can produce a side effect of severe itchiness when taken by black Africans (79). MrgprC11 serves as the receptor for the bovine adrenal medulla 8–22 peptide (BAM8-22), which when injected into human skin induces nonhistaminergic itching (79, 120). In addition, MrgprD is activated by β-alanine, leading to the itch sensation in humans and scratching behavior in mice (78). Furthermore, Liu et al. (80) found that the synthetic peptide SLIGRL-NH2, which had been believed to activate protease-activated receptor (PAR) 2 to induce an itch sensation, actually acts through MrgprC11. Together, these findings suggest that Mrgprs are an itch-sensing receptor family and that some family members serve as the receptors to detect different pruritogens in primary sensory neurons, analogous to other chemosensory systems (e.g., taste and olfaction).
The intracellular signaling pathways underlying Mrgpr-mediated itch sensations are not completely known. A recent study has shown that MrgprA3 and MrgprC11 utilize distinct mechanisms to induce neuronal activation. MrgprA3 is coupled to Gβγ to induce neuronal activation, whereas MrgprC11 is coupled to Gαq/11 to activate PLC and TRPA1. In spite of involving different mechanisms, both MrgprA3 and MrgprC11 require TRPA1 (149). TRPA1-deficient mice exhibited markedly decreased scratching responses to application of CQ and BAM8-22.
More exciting results came from the cellular analysis of MrgprA3+ DRG neurons. The expression of MrgprA3 and MrgprC11 largely overlap in DRG neurons (79), which suggests that MrgprA3+ neurons respond to CQ, BAM8-22, and SLIGRL. Given that histamine can activate all CQ-sensitive neurons (79), MrgprA3+ neurons therefore respond to at least four different pruritogens, suggesting that MrgprA3+ neurons are selective pruriceptive primary sensory neurons. Indeed, ablation of MrgprA3+ neurons significantly attenuated behavioral responses to multiple pruritogens, as well as spontaneous scratching under chronic itch conditions, without affecting pain sensitivity (44). MrgprA3+ neurons also exhibit some characteristics typical of pruriceptive neurons. For example, they exclusively innervate the skin, which provides the anatomical basis of skin-derived itch (44).
It has been suggested that itch-mediating nerve fibers terminate in the superficial skin layer. The cowhage spicule (an itchy plant) induces a stronger itch sensation when it is inserted into the basal membrane between the epidermis and the dermis (117). Capsaicin generates pain when delivered intradermally but can evoke an initial sensation of itch when applied topically or by means of a cowhage spicule to the superficial skin layer (38, 121). Consistent with this finding, both MrgprA3+ and MrgprD+ fibers innervate the stratum granulosum, the superficial epidermis layer (44, 157).
Proteases and Protease-Activated Receptors 2 and 4
Protease-activated receptors (PARs) belong to the GPCR family and are widely expressed in different tissues, including the DRG (125, 156). PARs are very different from most GPCRs in their mechanism of activation: They are activated by proteolytic cleavage of their own extracellular N terminus by a protease. The unmasked N terminus functions as a tethered ligand and activates the receptor. PAR2 and PAR4 can be activated by many endogenous and exogenous proteases that have been shown to induce itch sensations, such as mucunain (107), cathepsin S (108), and tryptase (125). Mucunain is the active pruritogenic component isolated from the spicule of the seed pod of cowhage (Mucuna pruriens), a plant widely found in tropical areas (107, 117). The insertion of a single cowhage spicule into the human skin produces a strong pruritus without wheal-and-flare response, indicating nonhistaminergic itch. Consistent with this finding, cowhage-induced itch cannot be blocked by antihistamines. Therefore, cowhage has been used to investigate the mechanism of nonhistaminergic itch (26, 28, 58, 59, 86, 95, 103). Cathepsin S is a lysosomal enzyme that is important for mediating immune responses. It was recently identified as a new pruritogen because the application of cathepsin S on human skin induced the itch sensation (108). Cathepsin S–overexpressing transgenic mice spontaneously develop atopic dermatitis and severe itching (64). Both mucunain and cathepsin S activate PAR2 and PAR4 in heterologous systems (107, 108). Tryptase is another endogenous agonist of PAR2. The expressions of tryptase and PAR2 were dramatically increased in the skin of atopic dermatitis patients (129, 141), which suggests that PAR2 is one of the key players in evoking the itch sensation. Because PAR2 and PAR4 are expressed on primary sensory neurons, it is likely that the proteases mentioned above directly activate DRG neurons to elicit itch sensations (156). Given that PAR2 and PAR4 are widely expressed in many different cell types and have important roles in allergic inflammation and immune responses (73), overexpression of the proteases can also promote the pathogenesis of atopic dermatitis and induce itch indirectly.
A recent study (80) revealed an unexpected role of MrgprC11 in the PAR2-mediated itch signaling pathway. Injections of the peptide SLIGRL-NH2, the synthesized unmasked N terminus (tethered ligand) of PAR2, induce robust scratching behaviors in mice and the itch sensation in humans. This effect had been believed to be mediated directly by PAR2. However, Liu et al. (80) showed that PAR2 mutant mice exhibit scratching behavior comparable to wild-type (WT) mice in response to SLIGRL-NH2 injections, suggesting that PAR2 is not required for a SLIGRL-NH2–induced itch sensation. They also discovered that SLIGRL-NH2 actually acts directly on MrgprC11 to induce the itch sensation (80). This result leads to the hypothesis that the unmasked N terminus of PAR2, after cleavage by a protease, can activate MrgprC11 in vivo if PAR2 is expressed in the same DRG neurons as MrgprC11 or in the neighboring skin cells. This idea has a precedent in the intermolecular activation model of PARs wherein the cleaved PAR N terminus was employed as a ligand to activate another PAR molecule (125).
Toll-Like Receptors
TLRs are a class of single transmembrane proteins that function as microbial-sensing receptors in the immune system. Once TLRs recognize the invading microbes in the body, they activate immune cell responses, such as the release of proinflammatory cytokines and chemokines (1). Recent studies have shown that some TLR family members are expressed in sensory neurons and are involved in somatosensations (65, 81, 82). TLR7 is one such family member. Topical application of a TLR7 agonist, imiquimod (47), is currently used for treating genital warts via stimulation of the immune system, and itching has been reported as one of the side effects (130). Two groups have published studies investigating the underlying mechanism of the imiquimod-induced itch sensation (65, 82). Both found that imiquimod can excite primary sensory neurons directly and evoke scratching behavior in mice. However, they presented contrasting data about whether the action of imiquimod is mediated by TLR7. Data from the first group demonstrate that neuronal activation and scratching responses evoked by imiquimod are reduced in TLR7 mutant mice, suggesting that TLR7 is directly involved in imiquimod-induced itch (82). The other group, by contrast, reported that imiquimod induced comparable neuronal and behavioral responses in WT and TLR7 mutant mice, suggesting that a TLR7-independent pathway exists (65). Indeed, imiquimod can also activate adenosine receptors, inositol 1,4,5-trisphosphate receptors, and potassium channels, although the roles of these molecules in itch sensations have not been identified (72, 116). An interesting finding from the first study is that mice lacking TLR7 showed a significant reduction in scratching behaviors compared to WT mice in response to multiple pruritogens, including CQ, endothelin-1, and SLIGRL-NH2, suggesting that TLR7 plays a general role in the transduction of itch signaling (82).
In a later study, Liu et al. (81) reported that TLR3 plays a role similar to that of TLR7 in itch sensations. The synthetic TLR3 agonist polyinosinic:polycytidylic acid activates primary sensory neurons directly and evokes scratching behavior in a TLR3-dependent way. The researchers (81) also found that mice lacking TLR3 showed reduced scratching responses evoked by many pruritogens, including histaminergic and nonhistaminergic chemicals. This result can be explained by the fact that TLR3 is critically involved in spinal cord excitatory synaptic transmission and central sensitization, suggesting that TLR3 might not be directly involved in evoking itch sensations. Instead, TLR3 might regulate the transmission of the itch signal in the neural circuit.
Serotonin
The effect of serotonin (5-HT) on the itch sensation appears to be species dependent. In rats, intradermal injection of 5-HT induces scratching with almost no pain behavior (115), whereas in mice and humans, it produces both itch and pain sensations (3, 115). 5-HT is, like histamine, mainly secreted from skin mast cells in the periphery (126) and is able to directly activate sensory neurons. The 5-HT receptors are GPCRs, with the exception of the 5-HT3 receptor, which is a ligand-gated ion channel (17). Pharmacological studies suggest that the 5-HT1 and 5-HT2 receptors are important for itch perception (55, 63, 151). The 5-HT2 receptor is coupled to Gαq/11 to activate PLCs, especially PLCβ3, leading to activation of mitogen-activated protein kinase and protein kinase C (17).
Endothelin-1
Endothelin-1 (ET-1) is a 21–amino acid peptide whose name is based on the fact that it is secreted from endothelial cells. It is a well-known vasoconstrictor that plays a key role in vascular homeostasis (25). In humans, intradermal injection of ET-1 causes a burning pruritus sensation, accompanied by wheal-and-flare responses (60), and in rodents, it induces mixed pain and itch behaviors (3, 77, 91, 140). Two GPCR subtypes serve as the receptors for ET-1: ETA and ETB (25). ETA is responsible for the ET-1–evoked itch sensation (77, 91). It acts via Gs to raise cAMP levels and activate PLC. Although both types of receptors for ET-1 have been found on DRG neurons, direct application of ET-1 to the DRG neurons elicits only weak or no neural activation, suggesting that ET-1 evokes the itch sensation via an indirect mechanism. Because ET-1 causes a wheal-and-flare response in human skin, it is possible that it evokes the itch sensation by inducing the release of endogenous itch mediators (e.g., histamine) from mast cells. In a recent study (8), researchers found that ET-1 is involved in the itch sensation induced by cathepsin E, an aspartic protease. The ETA antagonist blocked the scratching behavior that was induced by intradermal injection of cathepsin E. They also observed increased ET-1 levels in the skin at the injection site, leading to the hypothesis that cathepsin E induces the release of ET-1 in vivo to evoke the itch sensation.
Interleukin-31
Interleukin-31 (IL-31) has been shown to play an important role in the induction of itch sensations. IL-31 is a newly discovered member of the IL-6 family of cytokines, and it is mainly secreted from Th2 lymphocytes (29). IL-31 signals via a heterodimeric receptor consisting of the IL-31 receptor α (IL-31RA) and the oncostatin M receptor (OSMR) (29). This heterodimeric receptor directly activates members of the JAK family of tyrosine kinases, which leads to activation of the transcription factor STAT, as well as to induction of the phosphatidylinositol 3-kinase and mitogen-activated protein kinase signaling cascades (24). IL-31RA and OSMR are expressed in a wide range of cell types and tissues, including DRG neurons (11).
Transgenic mice overexpressing IL-31 either ubiquitously or in lymphocytes experience severe pruritus, as evidenced by excessive scratching behavior. This pruritus leads to skin lesions resembling those in patients with atopic dermatitis (29). These results suggest that IL-31 is involved in the pathogenesis of atopic dermatitis and associated pruritus. Consistent with this hypothesis, an increased level of IL-31 has been reported in both human patients and NC/Nga mice with atopic dermatitis (97, 134, 136), and treatment with the IL-31 antibody can attenuate the scratching behavior in NC/Nga mice (39). In addition, levels of IL-31 correlate with the severity of atopic dermatitis (106). However, the exact role IL-31 plays in the itch sensation is unclear. Currently, there is no evidence showing that IL-31 can directly excite DRG neurons, although IL-31RA and OSMR are expressed in DRG neurons (11). IL-31 is also involved in inflammatory lung disease and inflammatory bowel disease (24), suggesting that it might play a general role in inflammatory immune responses and hence might indirectly evoke the itch sensation.
Substance P
Intradermal injection of substance P into human skin stimulates a histamine release from mast cells to induce the itch sensation (50). Therefore, substance P–evoked itch can be blocked by histamine H1 receptor antagonists (50). The serum level of substance P is increased in contact dermatitis patients (139), and histological analysis revealed that the density of substance P+ nerve fibers is increased in the lesional skin of patients with prurigo nodularis (42). A recent study showed that aprepitant, a substance P receptor (NK1) antagonist, significantly inhibits scratching behavior in the NC/Nga mouse model of atopic dermatitis (101). More importantly, clinical studies demonstrated that aprepitant is effective for treating chronic pruritus, especially in patients with therapy-refractory pruritus (18, 128, 146). The inhibitory effect of this NK1 receptor antagonist could be caused by inhibition of substance P in the periphery or by blockage of NK1+ neurons in the dorsal spinal cord, which are involved in transmitting the itch sensation. Nonetheless, these results suggest that substance P plays an important role in the chronic itch sensation.
Mediators in Systemic Itch
Chronic pruritus is often associated with certain systemic disorders. For example, about 60–70% of patients with cholestatic liver disease report pruritus as a common symptom within 10 years after diagnosis (19). Up to 90% of patients receiving chronic dialysis therapy for renal disease suffer from severe itching (62). This persistent itching significantly affects the quality of the patients’ lives, leading to sleep deprivation and depression. On rare occasions, justification can be made for patients to undergo liver transplantation because of debilitating pruritus. The pathogenesis of chronic pruritus in these conditions is largely unknown, and the current treatment options are all empiric and are not consistently effective.
It has been hypothesized that cholestatic pruritus arises from pruritogenic substances that accumulate as a consequence of impaired secretion of bile (19, 92). Among these substances, bile acid has become an attractive candidate for several reasons. First, application of bile acid on skin causes the itch sensation in humans (145). Second, the levels of bile acid in both serum and skin are elevated in patients with cholestasis (87). Furthermore, the bile acid sequestrant cholestyramine, which is the current first-line therapy for the management of cholestatic pruritus, enhances the clearance of bile acid from the body and relieves pruritus (19, 92).
TGR5 is a GPCR that serves as the cell surface receptor for bile acids. It is a key player in the bile acid signaling pathway, which regulates a number of pathophysiological events, such as energy homeostasis and glucose metabolism (61). TGR5 is expressed in many different tissues, including lung, liver, spleen, and gall bladder, as well as in primary sensory neurons and dorsal horn spinal neurons. Bunnett’s and Corvera’s groups (7) found that TGR5 expression in DRG is restricted to a subset of peptidergic neurons that coexpress GRP, TRPA1, and TRPV1. They also showed that bile acid can activate TGR5 to cause direct excitation and increased intrinsic excitability of DRG neurons. Bile acid–induced scratching behavior is attenuated in TGR5 mutant mice but exacerbated in TGR5 transgenic mice, which exhibit spontaneous scratching, indicating that TGR5 mediates bile acid–induced itch sensation. Furthermore, the authors have shown that TGR5 signals act upstream of GRP-GRPR because TGR5 activation stimulates release of GRP and a GRPR antagonist that can block bile acid–induced scratching behavior. This report revealed the molecular and cellular mechanisms of bile acid–induced itch sensation and advanced our understanding of cholestatic pruritus.
Involvement of lysophosphatidic acid (LPA) has also been proposed in cholestatic pruritus (68). Evidence for its involvement came from an analysis of the serum from pruritic and nonpruritic cholestatic patients. The researchers showed that serum levels of LPA and autotoxin, the enzyme that converts lysophosphatidylcholine into LPA, are elevated only in those patients who suffer from pruritus. It is noteworthy that autotoxin activity significantly correlates with the intensity of pruritus in these patients. However, further investigations are required to clarify whether LPA plays a critical role in cholestatic pruritus.
CENTRAL ITCH PATHWAY
Primary sensory neurons transmit sensory information from the periphery to the spinal cord. The molecular identification of itch-sensing neurons in the spinal cord came from studies about gastrin-releasing peptide (GRP) and its receptor, the GRPR. GRP and GRPR are distributed throughout the mammalian CNS and have been involved in numerous physiological processes, including feeding behavior, learning, and memory, as well as in neurological and psychiatric disorders (88). In the spinal cord, the expression of GRPR is restricted in the lamina I of the superficial dorsal horn (132). Mice lacking GRPR showed reduced responses to pruritogenic stimuli, especially nonhistaminergic pruritogens, but comparable responses to painful stimuli compared to WT mice, suggesting that GRPR is an important component in the itch-sensing neural pathway but is not involved in the pain-sensing neural pathway (132). This conclusion is supported by pharmacological studies in which GRPR antagonists effectively blocked pruritogen-evoked scratching behavior. Furthermore, ablation of GRPR+ neurons by intrathecal injection of bombesin, the high-affinity ligand, coupled with a cytotoxin, almost eliminated the behavioral responses evoked by both histaminergic and nonhistaminergic pruritogens. In contrast, ablation of GRPR+ neurons did not affect behavioral responses to painful stimuli, demonstrating that GRPR+ neurons are the specific itch-sensing neurons in the spinal cord (133). These studies provided the cellular basis of the itch sensation in the spinal cord, supporting the labeled-line theory. It is unclear whether GRPR+ neurons are spinal interneurons or projection neurons; this question can be answered by retrograde tracing from the thalamus.
Compared to WT mice, GRPR knockout mice also exhibit attenuated scratching responses evoked by intrathecal injection of morphine (83). Morphine-induced itch has been presented for years as an example of the cross talk between the pain and itch sensations (54). The underlying mechanism of a morphine-induced itch is presumably attributed to morphine-induced disinhibition of the pain-sensing pathway in the spinal cord. Recent findings from Chen’s group (83) argue against this hypothesis. They found that morphine-induced analgesia and itch are two independent processes. Morphine acts through the μ-opioid receptor (MOR) isoform, MOR1, to induce analgesia. However, it directly activates the heterodimer of MOR1D and GRPR to relay the itch sensation This study further strengthened the notion that GRPR is an itch-specific receptor.
Like GRPR, the substance P receptor neurokinin 1 (NK1) is expressed in the lamina I of the spinal cord dorsal horn; more specifically, it is expressed in the majority of STT neurons in this region (54). However, NK1 does not label the same neuronal population as GRPR because ablation of GRPR+ neurons does not affect the total number of NK1+ neurons (133). NK1+ neurons are involved in both pain and itch sensations. In rats, elimination of NK1+ neurons by the intrathecal injection of substance P-saporin significantly attenuated both pain and itch behaviors, demonstrating that NK1 is expressed in both nociceptive and pruriceptive STT neurons (21, 89).
STT neurons have also been studied by recordings from antidromically identified projection neurons in the dorsal horn. Using this method, a small fraction of STT neurons in lamina I was identified in cats as selective pruriceptive neurons (9). This class of neurons is mechanically and thermally insensitive, and responds to histamine with a discharge pattern that parallels the temporal profile of the histamine-elicited itch sensation in humans. This result supports the labeled-line theory. However, in this study, the response of STT neurons to algogens was not examined. In later studies, researchers found that STT neurons in monkeys are polymodal neurons that respond to pruritogens, algogens, and mechanical and thermal stimuli (28, 123).
Recent studies have examined the responses of STT neurons to histamine and cowhage, a nonhistaminergic itch stimulus. They found that histamine and cowhage activate separate populations of STT neurons in nonhuman primates (26, 28). Additional studies using antidromic mapping revealed that histamine-responsive STT neurons project to narrower brain regions than do cowhage-responsive STT neurons (26). Consistent with this result, human brain imaging studies revealed notable differences in the brain regions activated by histamine versus cowhage, although they can coactivate a core group of brain structures (103). A similar phenomenon was discovered in the periphery. Namer et al. (95) found that histamine and cowhage activate distinct nonoverlapping populations of C fibers in human skin. Together, these results suggest that different neural circuits exist for mediating histaminergic versus nonhistaminergic itch sensations. However, possibly because of species differences and sensitivity of detection, other groups have reported overlapping populations of primary afferent neurons responding to histamine and cowhage in mice and monkeys (58, 86).
When sensory information is transmitted to the spinal cord, the signals are integrated and regulated and then conveyed to projection neurons. It is comprehensible that the perception of sensation by the brain requires the engagement of projection neurons, as well as the excitatory and inhibitory interneurons that regulate the activity of projection neurons. Direct evidence about the role of excitatory interneurons in the behavioral responses evoked by pruritogens came from the analysis of TR4 conditional knockout mice (148). Neuronal deletion of TR4, a testicular orphan nuclear receptor, results in preservation of primary afferent neurons and spinal projection neurons, as well as the loss of many excitatory interneurons in the superficial dorsal horn. Spinal projection neurons in TR4 conditional knockout mice exhibit decreased responsiveness to both noxious stimuli and pruritogens; these changes are associated with the attenuated pain and itch behavioral responses, demonstrating the importance of the concurrent activation of excitatory neurons for transmitting sensory information (148).
NEUROTRANSMITTERS FOR THE ITCH SENSATION
Recent striking evidence implicates neuropeptide natriuretic polypeptide b (Nppb) as a neurotransmitter secreted from pruriceptive primary sensory neurons (93). Nppb is selectively expressed in TRPV1+ and MrgprA3/C11+ DRG neurons. Nppb knockout mice lost almost all behavioral responses to multiple pruritogens. Intrathecal injection of Nppb induced robust scratching behavior. Furthermore, its primary receptor, natriuretic peptide receptor A (Npra), is expressed in a group of dorsal horn neurons in the spinal cord, which is a different neuronal population from GRPR+ neurons. Ablation of Npra+ neurons by Nppb-saporin attenuated the behavioral responses to histamine. These data suggest that Nppb acts as a neurotransmitter that conveys itch signaling from the periphery. Despite these compelling results, there is no direct evidence that Nppb is released in response to pruritic stimuli. In addition, Npra is also expressed in a large population of DRG neurons (155), which leads to the possibility that Nppb-saporin ablates pruriceptive primary sensory neurons, which in turn inhibit the itch sensation. Further studies with a trans-synaptic marker are needed to examine the neuronal connection between primary pruriceptive neurons and Npra+ spinal neurons.
Because of the intriguing role of GRPR in the itch sensation, it is reasonable to consider GRP as a neurotransmitter that possibly mediates the itch sensation. Indeed, intrathecal injection of GRP induces robust scratching behavior, which can be blocked by a GRPR antagonist, demonstrating that GRP is a key player in transmitting the itch sensation (132). There are some controversies about the types of cells that secrete GRPs. Some groups have shown that GRP is expressed in a small subset of peptidergic DRG neurons (81, 96, 132), leading to the hypothesis that GRP is a neurotransmitter secreted from the periphery. Other groups have found that GRP is expressed in the dorsal horn of the spinal cord, instead of the DRG (35, 93). Mishra et al. (93) showed that GRP and Npra are expressed in the same population of dorsal horn neurons in the spinal cord. Ablation of Npra+ neurons with Nppb-saporin attenuated Nppb-induced scratching behavior but not GRP-induced scratching, suggesting that GRP acts downstream of Nppb and Npra+ neurons in the itch neural circuit. The critical role of GRP in the itch sensation is also supported by the recent study from Bunnett’s and Corvera’s groups (7), which shows that bile acid can activate its receptor, G protein–coupled bile acid receptor 1 (GPBAR1 or TGR5), to stimulate release of GRP and induce the itch sensation.
There are limited studies about the role of glutamate in the itch sensation. A recent study performed an electrophysiological recording of the spinal cord dorsal horn neurons that receive monosynaptic inputs from C fibers (67). The results showed that activation of these neurons by histamine application on the skin can be blocked by glutamate receptor antagonists. Although the cell number in this study was too small to make clear conclusions, it provides the possibility that glutamate is a neurotransmitter involved in transmitting the itch sensation. Because deletion of the vesicular glutamate transporter (VGLUT) 2 in pruriceptive DRG neurons leads to enhanced responses to pruritogens (71, 84), it is likely that glutamate is not the only neurotransmitter that transmits the itch sensation.
MECHANICALLY EVOKED ITCH
We know from everyday experience that mechanical stimuli, such as the contact of wool fibers to the skin, can induce itching. However, not every type of mechanical stimulus evokes this response. For example, poking the skin with von Frey filaments or touching it lightly with a cotton swab induces only a tactile sensation, not itching. In a recent study from Ikoma’s group (36), researchers developed an assay to generate mechanically evoked itch sensations. They attached a thin stainless-steel wire loop to a piezoelectric actuator. The actuator was electrically controlled to vibrate at a fixed range and frequency, which caused the wire loop to vibrate and apply a light mechanical stimulus to the vellus hair on human skin. They demonstrated that this light mechanical stimulus can reproducibly induce an intense histamine-independent itch sensation. This is the first report to experimentally show a mechanically evoked itch sensation in healthy human skin. The beauty of this study is that the mechanically evoked itch is the “pure itch” that itch researchers have been seeking. In previous human psychophysical studies, when the subjects were challenged with pruritogens, such as histamine, BAM8-22, and cowhage, they always reported an itch sensation accompanied by pricking, stinging, and burning, which represents a nociceptive (although not necessarily painful) sensation (107, 120). Despite emerging evidence suggesting the existence of a specific neural pathway for itching, the mixed human sensations generated by pruritogens create doubt that the itch sensation has a pain-related sensory component. In this study, Ikoma’s group (36) demonstrated that vibrating vellus hairs on human skin induce only an itch sensation and never involve pain-related sensory components. This method will be very useful for further investigation into the differentiation of itch and pain sensations. The authors did not examine which type of afferent is involved in their mechanically evoked itch, but they propose that C–tactile neurons are possible candidates because these cells have characteristics that are consistent with the observed phenomenon. MrgprA3+ and MrgprD+ fibers have been identified as pruriceptive C-afferents; however, they do not innervate hair follicles and should be excluded from consideration as possible candidates. A recently identified TH+ C–low threshold mechanoreceptor that innervates hair follicles might be a good candidate (76). Further microneurographic studies are required for characterization of the responsible neural pathway.
NEUROPATHIC ITCH
Neuropathic itch arises from nerve damage at any point along the neural pathway (98). Despite fundamental advances in understanding the mechanisms of itch in the normal nervous system, virtually nothing is known about the cellular and molecular mechanisms of neuropathic itch. Our limited knowledge about neuropathic itch is based only on clinical experiences. Neuropathic itch has been associated with most of the major categories of neurological disease, ranging from multiple sclerosis to brain tumors to stroke to vascular malformations and radicular compression. However, only a small proportion of patients with these neurological conditions develop chronic itch. Another puzzle is that particular diseases, zoster, for instance, lead to neuropathic pain in some patients, neuropathic itch in others, and both in yet others. It appears that neuropathic itch has a complicated pathological origin, which involves a specific trigger, neuronal damage, and individual susceptibility (98, 99, 131).
PSYCHOGENIC ITCH
When researchers talk about the basic neural mechanism of itch, they talk about the molecules and cells that are involved in transmitting an itch signal from the periphery to the brain, but an itch is not a simple electrical signal flow: It is a multidimensional sensation that involves discriminative sensory, cognitive, and emotional components. Therefore, the perception of an itch sensation in the brain can be affected by many psychosocial factors (154). Psychological factors such as anxiety, depression, and stress can aggravate pruritus conditions (137). In fact, anxiety level and depression are always correlated with the severity of atopic dermatitis and psoriasis as well as with the itch intensity associated with the diseases (37, 41). Not only can a person’s psychological status aggravate pruritus, but pruritus and scratching can also exacerbate the person’s psychological status, which affects illness cognition and coping mechanisms and, in turn, disease and treatment outcomes. Some studies have suggested that the development or exacerbation of skin disorders is associated with certain personality traits (10, 20). For example, patients with urticaria show higher tendency toward compulsion, a higher level of general psychosomatic symptoms (headache, insomnia, paranoid thoughts, and fears), and higher dissatisfaction with life (10).
Some psychogenic disorders such as obsessive-compulsive disorders and delusions of parasitosis can cause pruritus and scratching behavior directly (137, 154). A psychogenic origin of itching has been found in 2% of patients with chronic pruritus; these cases need to be treated with psychotherapy and antidepressants. However, the perception of itch without real stimuli can also happen to healthy people, for example, “contagious itch.” Daily life experiences suggest that contagious itch occurs when we see other people itch and empathetically scratch. Researchers have recently investigated this phenomenon systematically in both monkey and human subjects (33, 49, 85, 104). They found that exposure to visual cues of an itch can induce itching in healthy subjects and that this is a normal social response experienced by most people (49, 85, 104). Patients with atopic dermatitis reported a higher intensity of itch and more scratching when watching videos of people scratching, consistent with sensitization of the itch sensation in atopic dermatitis patients (104). These studies established a behavioral model in which an itch is a subjective somatosensory perception even in the absence of sensory stimulation (33, 49, 85, 104). This model might provide insights into the central neural mechanisms of psychogenic itch disorders in the future.
INTERACTIONS BETWEEN PAIN AND ITCH
Although pain and itch are two distinct sensory modalities, they have many similarities. In fact, much of our understanding about the itch sensation has arisen from the investigation of pain. Both pain and itch are unpleasant sensations, evoking different behavioral responses. Pain induces a withdrawal reflex, whereas itch evokes a scratching response, and both can serve as protective mechanisms. Both pain and itch are elicited by more than one type of stimulus, including mechanical and chemical stimuli (54). Furthermore, they share certain molecular mechanisms and are transmitted by similar neuronal populations. Most of the pruritogens tested, such as histamine, BAM8-22, and cowhage, generate both itch- and pain-related sensations (stinging and pricking) in human skin, although itching is prevalent (107, 120). In some medical conditions, both symptoms can be evoked (131). Pain and itch sensations employ similar mechanisms for periphery sensitization, such as nerve sprouting and neurotrophin regulation, and share similar patterns of central sensitization: Light touch is perceived as painful in chronic pain conditions and itchy in chronic itch conditions (54).
Pain and itch sensations also interact with each other. An itch is defined as an unpleasant sensation that elicits the desire or reflex to scratch. Scratching, in turn, generates a mild pain that inhibits the itch sensation. Davidson et al. (27) observed that the scratching of the cutaneous receptive field inhibits the neuronal activity of histamine-sensitive STT neurons in nonhuman primates. This inhibition is likely the result of spinal inhibition of the itch circuit instead of direct inhibition of the periphery fibers because noxious stimuli can reduce itching even when they are applied centimeters away from the itchy site (152). A study from Greenberg’s group (111) provided insights into the neural mechanism of spinal inhibition of itch. They found that mice lacking Bhlhb5 exhibit increased scratching in response to pruritic agents and develop self-inflicted skin lesions, but these mice exhibit normal acute nociception in response to mechanical and thermal stimuli. Bhlhb5 is a neural-specific basic helix-loop-helix (bHLH) transcription factor that is transiently expressed in excitatory and inhibitory neurons in the dorsal spinal cord. Deletion of Bhlhb5 in mice results in the loss of a group of Bhlhb5+ inhibitory interneurons and the disinhibition of the itch neural pathway, which in turn leads to excessive scratching behavior.
Not only does disinhibition of itch circuitry induce itching, but recent studies show that inhibition of the pain circuitry can also induce itching. Ma’s group (84) and Kullander’s group (71) published parallel papers describing the phenotype of mice lacking Vglut2, the transporter of glutamate, the critical neural transmitter for mediating pain sensation. Deletion of Vglut2 in primary nociceptors significantly inhibits pain behavior but enhances itch behavior. In addition, with the deletion of Vglut2 in nociceptors, a capsaicin injection no longer induces nocifensive behavior (wiping on the cheek) but instead induces itch behavior (scratching) (84). This result suggests that loss of synaptic release of glutamate from primary nociceptors inhibits the perception of pain but leads to disinhibition of the itch neural pathway. This idea was further supported by a recent study from Woolf’s group (110). They first injected mice with capsaicin together with QX-314, which can enter primary nociceptors through the opened TRP channel pore to selectively block sodium currents and therefore block neuronal activity of TRPV1+ nociceptors. Application of allyl isothiocyanate (AITC) afterward on the same skin area induced scratching behavior. When mice were instead pretreated with allyl isothiocyanate together with QX-314 to block TRPA1+ nociceptors, subsequent capsaicin treatment also induced scratching behavior. The explanation is that a subset of TRPV1+/TRPA1+ sensory neurons normally mask itch via spinal inhibitory interneurons. However, when they are inhibited by QX-314, the spinal inhibition of itch is relieved, and subsequent application of capsaicin/AITC can induce itch.
To incorporate new findings about the spinal inhibition of itching with labeled-line theory (Figure 1a), population theory (or selectivity theory) was proposed (Figure 1b) (105). The theory suggests that itch-sensing neurons may constitute a subset of the nociceptor population. When this subgroup is selectively activated, the itch sensation is generated regardless of the stimuli. However, when a larger population of neurons, including both pain- and itch-sensing neurons, is activated, the itch sensation is occluded by the spinal inhibition from pain-sensing neurons, and only the pain sensation is perceived. In cases in which the pain stimulus is not strong enough to mask the itch, mixed pain and itch sensations may be produced. This model not only highlights the existence of a specific itch neural pathway but can also explain the similarity as well as the antagonistic relationship between pain and itching.
MODULATION OF THE ITCH NEURAL PATHWAY
Peripheral Sensitization
Modulation of the itch neural pathway can occur during pathological conditions, leading to spontaneous itching or hypersensitivity to the itch sensation. For example, human psychophysical studies have shown that pruritogens elicit stronger itching in the lesional skin of patients with atopic dermatitis or psoriasis than in the skin of healthy people (50, 53, 102, 144). Itch sensitization can occur peripherally and centrally. Peripheral sensitization of the itch neural pathway can be attributed to the increased excitability of the primary itch-sensing neurons. Hyperinnervation is one way to increase the excitability or decrease the threshold of primary sensory neurons. The sprouting of epidermal nerve fibers (i.e., hyperinnervation) has been reported in patients with atopic dermatitis and in experimental animal models (66, 138). The increased expression levels of various molecules in itch-sensing receptors also contribute to peripheral sensitization. MrgprA3 and TLR3 expression levels are significantly increased in sensory neurons from mice suffering from dry skin (81, 150), and the PAR2 expression level has been found to be upregulated in patients with atopic dermatitis (129). Researchers in Carstens’ group (2) have shown direct evidence of hypersensitization of primary sensory neurons in animal models. They reported that DRG neurons from dry-skin mice exhibited significantly stronger responses to a PAR2 agonist and 5-HT than did DRG neurons from WT mice (2).
Many endogenous mediators have been shown to sensitize primary sensory neurons (13). Among them, nerve growth factor (NGF) has been proposed to play an important role in itch conditions. In the skin, NGF can be secreted from keratinocytes and mast cells (40). Expression of NGF and its receptor TrkA is upregulated in atopic dermatitis and psoriasis (31, 40), and an increased serum level of NGF is correlated with the severity of the pruritus in atopic dermatitis (139). As a neurotrophic factor, NGF can increase the sensitivity of itch-sensing neurons by promoting nerve sprouting (22), elongation, and survival (51). Rukwied et al. (113) reported that cowhage-induced itch sensation was significantly enhanced when the skin of human subjects was pretreated with NGF. Consistent with this result, in an animal model of contact dermatitis, treatment with anti-NGF antibodies significantly inhibited epidermal hyperinnervation as well as the development of disease symptoms, including skin lesions and scratching behavior (135). These results suggest that NGF is required for the pathogenesis of atopic dermatitis and that NGF inhibition is a promising therapeutic approach for treating pruritic diseases.
Central Sensitization
Similar to peripheral sensitization, central sensitization can be achieved by regulating the expression level of itch-sensing molecules in the CNS. For example, both GRP and GRPR expression levels are significantly increased in nonhuman primates suffering from idiopathic chronic itch (96). Also, itch-sensing neurons in the CNS have hyperexcitability, which leads to enhanced processing of pruriceptive stimuli. This phenomenon has been demonstrated by recent study from Carstens’ group (5). They showed that lumbar superficial dorsal horn neurons exhibited enhanced responses to intradermal injection of the SLIGRL peptide in a mouse hind paw dry-skin itch model (5).
Central sensitization of the pain neural pathway has been extensively studied, and numerous mechanisms have been implicated, such as the alteration in glutamatergic neurotransmission, the loss of tonic inhibitory control, and glial-neuronal interactions (13). Given the striking similarities between pain and itch sensations, similar mechanisms of sensitization might be employed. For example, TLR3 is required for central sensitization of the pain sensation, possibly because of its essential role in synaptic transmission and long-term potentiation in the spinal cord (81). Because mice lacking TLR3 also exhibit dramatically decreased spontaneous scratching behavior after dry-skin treatment compared to WT mice, it is possible that TLR3 is also required for the central sensitization of the itch neural pathway (81). Central sensitization of the itch neural pathway can also occur with the disinhibition of Bhlhb5+ inhibitory interneurons in the dorsal horn, as observed in Bhlhb5 and Vglut2 knockout mice (71, 84, 111).
Innocuous mechanical stimuli, such as light touch, also frequently elicit the itch sensation when applied around pruritogen injection site on human skin (48, 122). This phenomenon was termed alloknesis. Alloknesis is a typical example of sensitization of the itch neural pathway, and it might involve both peripheral and central sensitization mechanisms (122). Recently, Carstens’ group (6) developed an animal model of alloknesis. They showed that the innocuous mechanical stimuli (light touch by von Frey filaments) applied to the skin near pruritogen injection sites or the lesional dry-skin region can induce scratching behavior in mice. Consistent with the previous human psychophysical finding that a μ-opioid antagonist attenuated alloknesis in humans (48), they also showed that a μ-opioid antagonist inhibited touch-evoked scratching in the mice, suggesting that this is a reliable animal model of alloknesis.
It should be noted that, in pathological conditions, the distinction between the pain and itch neural pathways becomes blurred. In the lesional skin of atopic dermatitis patients, normally painful electrical, chemical, mechanical, and thermal stimulation is perceived as itching (50, 52). Consistent with these data, repetitive scratching, the most common behavioral response that inhibits itch, enhances itch in these patients (56). Another example is histamine iontophoresis, which induces burning pain rather than itch in patients with neuropathic pain (12, 16). These results suggest that, although pain and itch are transmitted by distinct neural pathways under normal circumstances, certain pathological conditions might change the neural processing that discriminates between pain and itch sensations. Both peripheral and central mechanisms might be involved.
CLOSING REMARKS
During the past several years, we have experienced rapid progress in and expansion of itch research. The identification of novel pruritogens, receptors, and itch-sensing neurons greatly expanded our understanding of the itch neural pathway. However, the study of the mechanisms and circuits of itch sensation is still in its infancy, and many key questions remain to be answered. The most important task in the field is to treat chronic itch conditions, especially those associated with systemic disorders. The next frontier in our investigation of itch mechanism should discover the essential endogenous itch mediators and receptors in chronic itch conditions to look for the trigger of chronic itch signaling. In addition, itch-sensing nerve fibers in the skin can be activated by molecules secreted by neighboring cell types. For example, mast cells secrete histamine and serotonin to initiate itch signaling. Whether and how itch-sensing nerve fibers communicate with other cell types in the skin (e.g., keratinocytes and epithelial cells) to induce itch sensation requires further investigation. Moreover, new research must further elucidate how the itch neural pathway is regulated and modulated under pathological conditions. Although itch is a distinct somatosensation from pain, the similarities between these sensations (e.g., the fact that they are mediated by similar neuronal cell types, neural circuits, and molecules) suggest that they might employ similar mechanisms. Therefore, the valuable knowledge on pain mechanisms such as peripheral and central sensitization will help to facilitate our understanding of itch mechanisms. Finally, all the expectations mentioned above require better animal models that mimic chronic itch conditions in patients. We wish to witness exciting research breakthroughs in the itch field, both in deciphering the mechanisms and in translating the discoveries into treatments with clinical impact.
SUMMARY POINTS.
The itch sensation is not a submodality of pain. It is a distinct sensory modality that employs specific molecules, sensory neurons, and neural pathways.
Itch signaling is initiated by endogenous or exogenous pruriceptive stimuli, which activate itch-sensing receptors expressed on primary sensory neurons.
Several groups of spinal neurons have been identified as mediating itch sensations, including STT neurons, GRPR+ neurons, and Npra+/GRP+ neurons. Nppb and GRP are promising candidates as the neurotransmitters that activate spinal itch-sensing neurons.
Pure itch can be induced by light mechanical stimuli on human skin.
The molecular and cellular mechanisms of systemic itch, such as cholestatic pruritus, are beginning to be explored.
Perception of an itch sensation in the brain can be affected by many psychosocial factors, and psychogenic itch can be generated by solely visual stimuli.
Pain and itch sensations have many similarities as well as an antagonistic relationship, which can be explained by the population coding theory.
In pathological conditions, the itch neural pathway is modulated by regulation of the excitability of the itch-sensing neurons in both the peripheral and central nervous systems.
ACKNOWLEDGMENTS
This work was supported by grants (DE022750, NS054791, and GM087369) from the National Institutes of Health to X.D. X.D. is an early career scientist of the Howard Hughes Medical Institute.
Glossary
- Pruritogens
substances that induce an itching sensation
- Algogens
substances that induce a pain sensation
- STT
spinothalamic tract
- GRPR
gastrin-releasing peptide receptor
- Mrgprs
mas-related G protein–coupled receptors
- TLRs
toll-like receptors
- TRP
transient receptor potential cation channel
- PAR
protease-activated receptor
- NK1
neurokinin 1
- Nppb
neuropeptide natriuretic polypeptide b
- Npra
natriuretic peptide receptor A
- TGR5 (also called GPBAR1)
G protein–coupled bile acid receptor 1
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–83. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J. Neurophysiol. 2010;104:2442–50. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama T, Carstens MI, Carstens E. Spontaneous itch in the absence of hyperalgesia in a mouse hindpaw dry skin model. Neurosci. Lett. 2010;484:62–65. doi: 10.1016/j.neulet.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama T, Carstens MI, Carstens E. Enhanced responses of lumbar superficial dorsal horn neurons to intradermal PAR-2 agonist but not histamine in a mouse hindpaw dry skin itch model. J. Neurophysiol. 2011;105:2811–17. doi: 10.1152/jn.01124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) J. Investig. Dermatol. 2012;132:1886–91. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, et al. The TGR5 receptor mediates bile acid–induced itch and analgesia. J. Clin. Investig. 2013;123:1513–30. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andoh T, Yoshida T, Lee JB, Kuraishi Y. Cathepsin E induces itch-related response through the production of endothelin-1 in mice. Eur. J. Pharmacol. 2012;686:16–21. doi: 10.1016/j.ejphar.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat. Neurosci. 2001;4:72–77. doi: 10.1038/82924. A pioneering work on searching the itch-specific neuronal pathway. Also see Reference 114.
- 10.Bahmer JA, Kuhl J, Bahmer FA. How do personality systems interact in patients with psoriasis, atopic dermatitis and urticaria? Acta Derm. Venereol. 2007;87:317–24. doi: 10.2340/00015555-0246. [DOI] [PubMed] [Google Scholar]
- 11.Bando T, Morikawa Y, Komori T, Senba E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor βin the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. 2006;142:1263–71. doi: 10.1016/j.neuroscience.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Baron R, Schwarz K, Kleinert A, Schattschneider J, Wasner G. Histamine-induced itch converts into pain in neuropathic hyperalgesia. Neuroreport. 2001;12:3475–78. doi: 10.1097/00001756-200111160-00020. [DOI] [PubMed] [Google Scholar]
- 13.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belghiti M, Estevez-Herrera J, Gimenez-Garzo C, Gonzalez-Usano A, Montoliu C, et al. Potentiation of the transient receptor potential vanilloid 1 channel contributes to pruritogenesis in a rat model of liver disease. J. Biol. Chem. 2013;288:9675–85. doi: 10.1074/jbc.M113.455162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernhard JD. Itch and pruritus: What are they, and how should itches be classified? Dermatol. Ther. 2005;18:288–91. doi: 10.1111/j.1529-8019.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- 16.Birklein F, Claus D, Riedl B, Neundorfer B, Handwerker HO. Effects of cutaneous histamine application in patients with sympathetic reflex dystrophy. Muscle Nerve. 1997;20:1389–95. doi: 10.1002/(sici)1097-4598(199711)20:11<1389::aid-mus6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–72. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 18.Booken N, Heck M, Nicolay JP, Klemke CD, Goerdt S, Utikal J. Oral aprepitant in the therapy of refractory pruritus in erythrodermic cutaneous T-cell lymphoma. Br. J. Dermatol. 2011;164:665–67. doi: 10.1111/j.1365-2133.2010.10108.x. [DOI] [PubMed] [Google Scholar]
- 19.Bunchorntavakul C, Reddy KR. Pruritus in chronic cholestatic liver disease. Clin. Liver Dis. 2012;16:331–46. doi: 10.1016/j.cld.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Buske-Kirschbaum A, Ebrecht M, Kern S, Gierens A, Hellhammer DH. Personality characteristics in chronic and non-chronic allergic conditions. Brain Behav. Immun. 2008;22:762–68. doi: 10.1016/j.bbi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–8. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc. Res. 2001;50:409–16. doi: 10.1016/s0008-6363(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 23.Cheong WK. Gentle cleansing and moisturizing for patients with atopic dermatitis and sensitive skin. Am. J. Clin. Dermatol. 2009;10(Suppl. 1):13–17. doi: 10.2165/0128071-200910001-00003. [DOI] [PubMed] [Google Scholar]
- 24.Cornelissen C, Luscher-Firzlaff J, Baron JM, Luscher B. Signaling by IL-31 and functional consequences. Eur. J. Cell Biol. 2012;91:552–66. doi: 10.1016/j.ejcb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Davenport AP, Maguire JJ. Endothelin. Handb. Exp. Pharmacol. 2006;176:295–329. doi: 10.1007/3-540-32967-6_9. [DOI] [PubMed] [Google Scholar]
- 26.Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, et al. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J. Neurophysiol. 2012;108:1711–23. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat. Neurosci. 2009;12:544–46. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J. Neurosci. 2007;27:10007–14. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 30.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–32. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 31.Dou YC, Hagstromer L, Emtestam L, Johansson O. Increased nerve growth factor and its receptors in atopic dermatitis: an immunohistochemical study. Arch. Dermatol. Res. 2006;298:31–37. doi: 10.1007/s00403-006-0657-1. [DOI] [PubMed] [Google Scholar]
- 32.Ernsberger U. Role of neurotrophin signalling in the differentiation of neurons from dorsal root ganglia and sympathetic ganglia. Cell Tissue Res. 2009;336:349–84. doi: 10.1007/s00441-009-0784-z. [DOI] [PubMed] [Google Scholar]
- 33.Feneran AN, O’Donnell R, Press A, Yosipovitch G, Cline M, et al. Monkey see, monkey do: contagious itch in nonhuman primates. Acta Derm. Venereol. 2013;93:27–29. doi: 10.2340/00015555-1406. [DOI] [PubMed] [Google Scholar]
- 34.Finger S, Wade NJ. The neuroscience of Helmholtz and the theories of Johannes Müller. Part 2: Sensation and perception. J. Hist. Neurosci. 2002;11:234–54. doi: 10.1076/jhin.11.3.234.10392. [DOI] [PubMed] [Google Scholar]
- 35.Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, Luo W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol. Pain. 2012;8:52. doi: 10.1186/1744-8069-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuoka M, Miyachi Y, Ikoma A. Mechanically evoked itch in humans. Pain. 2013;154:897–904. doi: 10.1016/j.pain.2013.02.021. Demonstrated mechanically evoked itch on human skin.
- 37.Ginsburg IH. Psychological and psychophysiological aspects of psoriasis. Dermatol. Clin. 1995;13:793–804. [PubMed] [Google Scholar]
- 38.Green BG. Spatial summation of chemical irritation and itch produced by topical application of capsaicin. Percept. Psychophys. 1990;48:12–18. doi: 10.3758/bf03205007. [DOI] [PubMed] [Google Scholar]
- 39.Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp. Dermatol. 2009;18:35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 40.Groneberg DA, Serowka F, Peckenschneider N, Artuc M, Grutzkau A, et al. Gene expression and regulation of nerve growth factor in atopic dermatitis mast cells and the human mast cell line-1. J. Neuroimmunol. 2005;161:87–92. doi: 10.1016/j.jneuroim.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Gupta MA, Gupta AK. Depression modulates pruritus perception: a study of pruritus in psoriasis, atopic dermatitis and chronic idiopathic urticaria. Ann. N. Y. Acad. Sci. 1999;885:394–95. doi: 10.1111/j.1749-6632.1999.tb08697.x. [DOI] [PubMed] [Google Scholar]
- 42.Haas S, Capellino S, Phan NQ, Bohm M, Luger TA, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J. Dermatol. Sci. 2010;58:193–97. doi: 10.1016/j.jdermsci.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Hagermark O, Hokfelt T, Pernow B. Flare and itch induced by substance P in human skin. J. Investig. Dermatol. 1978;71:233–35. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- 44.Han L, Ma C, Liu Q, Weng HJ, Cui Y, et al. A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 2013;16:174–82. doi: 10.1038/nn.3289. Demonstrated that MrgprA3+ DRG neurons are itch-sensing neurons.
- 45.Han SK, Mancino V, Simon MI. Phospholipase Cβ 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 46.Handwerker HO, Forster C, Kirchhoff C. Discharge patterns of human C-fibers induced by itching and burning stimuli. J. Neurophysiol. 1991;66:307–15. doi: 10.1152/jn.1991.66.1.307. [DOI] [PubMed] [Google Scholar]
- 47.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 48.Heyer G, Dotzer M, Diepgen TL, Handwerker HO. Opiate and H1 antagonist effects on histamine induced pruritus and alloknesis. Pain. 1997;73:239–43. doi: 10.1016/S0304-3959(97)00098-5. [DOI] [PubMed] [Google Scholar]
- 49.Holle H, Warne K, Seth AK, Critchley HD, Ward J. Neural basis of contagious itch and why some people are more prone to it. Proc. Natl. Acad. Sci. USA. 2012;109:19816–21. doi: 10.1073/pnas.1216160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–42. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 52.Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62:212–17. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- 53.Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain. 2005;113:148–54. doi: 10.1016/j.pain.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat. Rev. Neurosci. 2006;7:535–47. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 55.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA. 2009;106:11330–35. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishiuji Y, Coghill RC, Patel TS, Dawn A, Fountain J, et al. Repetitive scratching and noxious heat do not inhibit histamine-induced itch in atopic dermatitis. Br. J. Dermatol. 2008;158:78–83. doi: 10.1111/j.1365-2133.2007.08281.x. [DOI] [PubMed] [Google Scholar]
- 57.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J. Neurosci. 2008;28:7659–69. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J. Neurosci. 2007;27:7490–97. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katugampola R, Church MK, Clough GF. The neurogenic vasodilator response to endothelin-1: a study in human skin in vivo. Exp. Physiol. 2000;85:839–46. [PubMed] [Google Scholar]
- 61.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, et al. A G protein–coupled receptor responsive to bile acids. J. Biol. Chem. 2003;278:9435–40. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 62.Kfoury LW, Jurdi MA. Uremic pruritus. J. Nephrol. 2012;25:644–52. doi: 10.5301/jn.5000039. [DOI] [PubMed] [Google Scholar]
- 63.Kim DK, Kim HJ, Kim H, Koh JY, Kim KM, et al. Involvement of serotonin receptors 5-HT1 and 5-HT2 in 12(S)-HPETE-induced scratching in mice. Eur. J. Pharmacol. 2008;579:390–94. doi: 10.1016/j.ejphar.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Kim N, Bae KB, Kim MO, Yu DH, Kim HJ, et al. Overexpression of cathepsin S induces chronic atopic dermatitis in mice. J. Investig. Dermatol. 2012;132:1169–76. doi: 10.1038/jid.2011.404. [DOI] [PubMed] [Google Scholar]
- 65.Kim SJ, Park GH, Kim D, Lee J, Min H, et al. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc. Natl. Acad. Sci. USA. 2011;108:3371–76. doi: 10.1073/pnas.1019755108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinkelin I, Motzing S, Koltenzenburg M, Brocker EB. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000;302:31–37. doi: 10.1007/s004410000202. [DOI] [PubMed] [Google Scholar]
- 67.Koga K, Chen T, Li XY, Descalzi G, Ling J, et al. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol. Pain. 2011;7:47. doi: 10.1186/1744-8069-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kremer AE, Martens JJ, Kulik W, Rueff F, Kuiper EM, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–18.e1. doi: 10.1053/j.gastro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur. J. Pharmacol. 1995;275:229–33. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- 70.Kuypers DR. Skin problems in chronic kidney disease. Nat. Clin. Pract. Nephrol. 2009;5:157–70. doi: 10.1038/ncpneph1040. [DOI] [PubMed] [Google Scholar]
- 71.Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–42. doi: 10.1016/j.neuron.2010.09.016. Demonstrated the spinal inhibition of itch by pain. Also see References 83 and 110.
- 72.Lee J, Kim T, Hong J, Woo J, Min H, et al. Imiquimod enhances excitability of dorsal root ganglion neurons by inhibiting background (K2P) and voltage-gated (Kv1.1 and Kv1.2) potassium channels. Mol. Pain. 2012;8:2. doi: 10.1186/1744-8069-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med. J. 2010;51:808–22. doi: 10.3349/ymj.2010.51.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lembo PM, Grazzini E, Groblewski T, O’Donnell D, Roy MO, et al. Proenkephalin A gene products activate a new family of sensory neuron–specific GPCRs. Nat. Neurosci. 2002;5:201–9. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- 75.Lewis T, Grant RT, Marvin HM. Vascular reactions of the skin to injury. X. The intervention of a chemical stimulus illustrated especially by the flare: the response to faradism. Heart. 1927;14:139–60. [Google Scholar]
- 76.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–27. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang J, Kawamata T, Ji W. Molecular signaling of pruritus induced by endothelin-1 in mice. Exp. Biol. Med. (Maywood) 2010;235:1300–5. doi: 10.1258/ebm.2010.010121. [DOI] [PubMed] [Google Scholar]
- 78.Liu Q, Sikand P, Ma C, Tang Z, Han L, et al. Mechanisms of itch evoked by β-alanine. J. Neurosci. 2012;32:14532–37. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–65. doi: 10.1016/j.cell.2009.11.034. Identified MrgprA3 and MrgprC11 as itch-sensing receptors.
- 80.Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci. Signal. 2011;4:ra45. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu T, Berta T, Xu ZZ, Park CK, Zhang L, et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Investig. 2012;122:2195–207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010;13:1460–62. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu XY, Liu ZC, Sun YG, Ross M, Kim S, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–58. doi: 10.1016/j.cell.2011.08.043. Revealed the mechanism of morphine-induced itch.
- 84.Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–56. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lloyd DM, Hall E, Hall S, McGlone FP. Can itch-related visual stimuli alone provoke a scratch response in healthy individuals? Br. J. Dermatol. 2013;168:106–11. doi: 10.1111/bjd.12132. [DOI] [PubMed] [Google Scholar]
- 86.Ma C, Nie H, Gu Q, Sikand P, Lamotte RH. In vivo responses of cutaneous C-mechanosensitive neurons in mouse to punctate chemical stimuli that elicit itch and nociceptive sensations in humans. J. Neurophysiol. 2012;107:357–63. doi: 10.1152/jn.00801.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Magyar I, Loi HG, Feher T. Plasma bile acid levels and liver disease. Acta Med. Acad. Sci. Hung. 1981;38:109–15. [PubMed] [Google Scholar]
- 88.Majumdar ID, Weber HC. Biology of mammalian bombesin-like peptides and their receptors. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18:68–74. doi: 10.1097/MED.0b013e328340ff93. [DOI] [PubMed] [Google Scholar]
- 89.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–79. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 90.Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997;9:461–66. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- 91.McQueen DS, Noble MA, Bond SM. Endothelin-1 activates ETA receptors to cause reflex scratching in BALB/c mice. Br. J. Pharmacol. 2007;151:278–84. doi: 10.1038/sj.bjp.0707216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mela M, Mancuso A, Burroughs AK. Pruritus in cholestatic and other liver diseases. Aliment. Pharmacol. Ther. 2003;17:857–70. doi: 10.1046/j.1365-2036.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- 93.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–71. doi: 10.1126/science.1233765. Presented the specific pruriceptive neural circuits from DRG to the spinal cord.
- 94.Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn. J. Pharmacol. 2002;88:285–92. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- 95.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J. Neurophysiol. 2008;100:2062–69. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nattkemper LA, Zhao ZQ, Nichols AJ, Papoiu AD, Shively CA, et al. Overexpression of the gastrin-releasing peptide in cutaneous nerve fibers and its receptor in the spinal cord in primates with chronic itch. J. Investig. Dermatol. 2013;133:2489–92. doi: 10.1038/jid.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J. Allergy Clin. Immunol. 2006;118:930–37. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 98.Oaklander AL. Neuropathic itch. Semin. Cutan. Med. Surg. 2011;30:87–92. doi: 10.1016/j.sder.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oaklander AL. Common neuropathic itch syndromes. Acta Derm. Venereol. 2012;92:118–25. doi: 10.2340/00015555-1318. [DOI] [PubMed] [Google Scholar]
- 100.Ochoa J, Torebjork E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J. Physiol. 1989;415:583–99. doi: 10.1113/jphysiol.1989.sp017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohmura T, Hayashi T, Satoh Y, Konomi A, Jung B, Satoh H. Involvement of substance P in scratching behaviour in an atopic dermatitis model. Eur. J. Pharmacol. 2004;491:191–94. doi: 10.1016/j.ejphar.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 102.Ozawa M, Tsuchiyama K, Gomi R, Kurosaki F, Kawamoto Y, Aiba S. Neuroselective transcutaneous electrical stimulation reveals neuronal sensitization in atopic dermatitis. J. Am. Acad. Dermatol. 2009;60:609–14. doi: 10.1016/j.jaad.2008.11.900. [DOI] [PubMed] [Google Scholar]
- 103.Papoiu AD, Coghill RC, Kraft RA, Wang H, Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59:3611–23. doi: 10.1016/j.neuroimage.2011.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Papoiu AD, Wang H, Coghill RC, Chan YH, Yosipovitch G. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br. J. Dermatol. 2011;164:1299–303. doi: 10.1111/j.1365-2133.2011.10318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patel KN, Dong X. An itch to be scratched. Neuron. 2010;68:334–39. doi: 10.1016/j.neuron.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raap U, Weissmantel S, Gehring M, Eisenberg AM, Kapp A, Folster-Holst R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr. Allergy Immunol. 2012;23:285–88. doi: 10.1111/j.1399-3038.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- 107.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J. Neurosci. 2008;28:4331–35. doi: 10.1523/JNEUROSCI.0716-08.2008. Demonstrated that a cysteine protease is the key ingredient of cowhage-evoked itch by activating PARs.
- 108.Reddy VB, Shimada SG, Sikand P, Lamotte RH, Lerner EA. Cathepsin S elicits itch and signals via protease-activated receptors. J. Investig. Dermatol. 2010;130:1468–70. doi: 10.1038/jid.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ringkamp M, Schepers RJ, Shimada SG, Johanek LM, Hartke TV, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J. Neurosci. 2011;31:14841–49. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat. Neurosci. 2013;16:910–18. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–98112. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rossbach K, Wendorff S, Sander K, Stark H, Gutzmer R, et al. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp. Dermatol. 2009;18:57–63. doi: 10.1111/j.1600-0625.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 113.Rukwied RR, Main M, Weinkauf B, Schmelz M. NGF sensitizes nociceptors for cowhage- but not histamine-induced itch in human skin. J. Investig. Dermatol. 2013;133:268–70. doi: 10.1038/jid.2012.242. [DOI] [PubMed] [Google Scholar]
- 114.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J. Neurosci. 1997;17:8003–8. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J. Neurophysiol. 2003;89:2441–48. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 116.Schön MP, Schön M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J. Investig. Dermatol. 2006;126:1338–47. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 117.Shelley WB, Arthur RP. Mucunain, the active pruritogenic proteinase of cowhage. Science. 1955;122:469–70. doi: 10.1126/science.122.3167.469. [DOI] [PubMed] [Google Scholar]
- 118.Shim WS, Tak MH, Lee MH, Kim M, Koo JY, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 2007;27:2331–37. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–87. doi: 10.1016/j.pain.2008.08.002. Established an animal model that differentiates between pain and itch behaviors.
- 120.Sikand P, Dong X, LaMotte RH. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J. Neurosci. 2011;31:7563–67. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens. Mot. Res. 1991;8:271–79. doi: 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- 123.Simone DA, Zhang X, Li J, Zhang JM, Honda CN, et al. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J. Neurophysiol. 2004;91:213–22. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- 124.Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J. Allergy Clin. Immunol. 2011;128:1139–50.e4. doi: 10.1016/j.jaci.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br. J. Pharmacol. 2010;160:191–203. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sommer C. Serotonin in pain and analgesia: actions in the periphery. Mol. Neurobiol. 2004;30:117–25. doi: 10.1385/MN:30:2:117. [DOI] [PubMed] [Google Scholar]
- 127.Spradley JM, Davoodi A, Carstens MI, Carstens E. Opioid modulation of facial itch- and pain-related responses and grooming behavior in rats. Acta Derm. Venereol. 2012;92:515–20. doi: 10.2340/00015555-1364. [DOI] [PubMed] [Google Scholar]
- 128.Stander S, Siepmann D, Herrgott I, Sunderkotter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS ONE. 2010;5:e10968. doi: 10.1371/journal.pone.0010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J. Neurosci. 2003;23:6176–80. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stern PL, van der Burg SH, Hampson IN, Broker TR, Fiander A, et al. Therapy of human papillomavirus-related disease. Vaccine. 2012;30(Suppl. 5):F71–82. doi: 10.1016/j.vaccine.2012.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stumpf A, Stander S. Neuropathic itch: diagnosis and management. Dermatol. Ther. 2013;26:104–9. doi: 10.1111/dth.12028. [DOI] [PubMed] [Google Scholar]
- 132.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–3. doi: 10.1038/nature06029. Identified the first molecule and neuronal population in the spinal cord specific for mediating itch.
- 133.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–34. doi: 10.1126/science.1174868. Identified the first molecule and neuronal population in the spinal cord specific for mediating itch.
- 134.Szegedi K, Kremer AE, Kezic S, Teunissen MB, Bos JD, et al. Increased frequencies of IL-31-producing T cells are found in chronic atopic dermatitis skin. Exp. Dermatol. 2012;21:431–36. doi: 10.1111/j.1600-0625.2012.01487.x. [DOI] [PubMed] [Google Scholar]
- 135.Takano N, Sakurai T, Kurachi M. Effects of anti-nerve growth factor antibody on symptoms in the NC/Nga mouse, an atopic dermatitis model. J. Pharmacol. Sci. 2005;99:277–86. doi: 10.1254/jphs.fp0050564. [DOI] [PubMed] [Google Scholar]
- 136.Takaoka A, Arai I, Sugimoto M, Yamaguchi A, Tanaka M, Nakaike S. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur. J. Pharmacol. 2005;516:180–81. doi: 10.1016/j.ejphar.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 137.Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin. Dermatol. 2013;31:31–40. doi: 10.1016/j.clindermatol.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tominaga M, Ozawa S, Ogawa H, Takamori K. A hypothetical mechanism of intraepidermal neurite formation in NC/Nga mice with atopic dermatitis. J. Dermatol. Sci. 2007;46:199–210. doi: 10.1016/j.jdermsci.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 139.Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Dermatol. 2002;147:71–79. doi: 10.1046/j.1365-2133.2002.04803.x. [DOI] [PubMed] [Google Scholar]
- 140.Trentin PG, Fernandes MB, D’Orleans-Juste P, Rae GA. Endothelin-1 causes pruritus in mice. Exp. Biol. Med. 2006;231:1146–51. [PubMed] [Google Scholar]
- 141.Tsujii K, Andoh T, Ui H, Lee JB, Kuraishi Y. Involvement of tryptase and proteinase-activated receptor-2 in spontaneous itch-associated response in mice with atopy-like dermatitis. J. Pharmacol. Sci. 2009;109:388–95. doi: 10.1254/jphs.08332fp. [DOI] [PubMed] [Google Scholar]
- 142.Tsukuba T, Okamoto K, Okamoto Y, Yanagawa M, Kohmura K, et al. Association of cathepsin E deficiency with development of atopic dermatitis. J. Biochem. 2003;134:893–902. doi: 10.1093/jb/mvg216. [DOI] [PubMed] [Google Scholar]
- 143.Tuckett RP. Itch evoked by electrical stimulation of the skin. J. Investig. Dermatol. 1982;79:368–73. doi: 10.1111/1523-1747.ep12529734. [DOI] [PubMed] [Google Scholar]
- 144.van Laarhoven AI, Kraaimaat FW, Wilder-Smith OH, van Riel PL, van de Kerkhof PC, Evers AW. Sensitivity to itch and pain in patients with psoriasis and rheumatoid arthritis. Exp. Dermatol. 2013;22:530–34. doi: 10.1111/exd.12189. [DOI] [PubMed] [Google Scholar]
- 145.Varadi DP. Pruritus induced by crude bile and purified bile acids. Experimental production of pruritus in human skin. Arch. Dermatol. 1974;109:678–81. [PubMed] [Google Scholar]
- 146.Vincenzi B, Fratto ME, Santini D, Tonini G. Aprepitant against pruritus in patients with solid tumours. Support Care Cancer. 2010;18:1229–30. doi: 10.1007/s00520-010-0895-9. [DOI] [PubMed] [Google Scholar]
- 147.von Frey M. Zur Physiologie der Juckempfindung. Arch. Néerl. Physiol. 1922;7:142–45. [Google Scholar]
- 148.Wang X, Zhang J, Eberhart D, Urban R, Meda K, et al. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78:312–24. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, et al. TRPA1 is required for histamine-independent, Mas-related G protein–coupled receptor-mediated itch. Nat. Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, et al. The ion channel TRPA1 is required for chronic itch. J. Neurosci. 2013;33:9283–94. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci. Res. 1999;35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 152.Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br. J. Dermatol. 2007;156:629–34. doi: 10.1111/j.1365-2133.2006.07711.x. [DOI] [PubMed] [Google Scholar]
- 153.Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690–94. doi: 10.1016/S0140-6736(03)12570-6. [DOI] [PubMed] [Google Scholar]
- 154.Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol. Ther. 2008;21:32–41. doi: 10.1111/j.1529-8019.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 155.Zhang FX, Liu XJ, Gong LQ, Yao JR, Li KC, et al. Inhibition of inflammatory pain by activating B-type natriuretic peptide signal pathway in nociceptive sensory neurons. J. Neurosci. 2010;30:10927–38. doi: 10.1523/JNEUROSCI.0657-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhu WJ, Yamanaka H, Obata K, Dai Y, Kobayashi K, et al. Expression of mRNA for four subtypes of the proteinase-activated receptor in rat dorsal root ganglia. Brain Res. 2005;1041:205–11. doi: 10.1016/j.brainres.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 157.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]