Abstract
Cognitive impairment is a core feature of schizophrenia. However, the longitudinal course and pattern of this impairment, and its relationship to functional outcome is not fully understood. Among the likely factors in the persistence of cognitive deficits in schizophrenia are brain tissue changes over time, which in turn appear to be related to antipsychotic medication adherence.
Cognitive deficits are viewed as a core feature of schizophrenia primarily because cognitive deficits clearly exist before the onset of psychosis and can predict illness onset among those at high risk of developing the illness. Additionally, these deficits often persist during symptomatic remissions in patients, and are relatively stable across time both in patients and in individuals at risk for schizophrenia.
Despite clear evidence that cognitive impairment can predict functional outcome in chronic schizophrenia, results of studies examining this relationship in the early phase of psychosis have been mixed. Recent data, however, strongly suggest that interventions targeting early cognitive deficits may be crucial to the prevention of chronic disability, and thus should be a prominent target for therapy.
Finally, it is vital to keep schizophrenia patients consistently on their antipsychotic medications. A novel method of examining intracortical myelin volume indicated that the choice of antipsychotic treatment had a differential impact on frontal myelination. These data suggest that long acting injectable antipsychotic medication may prevent patients from declining further through a combination of better adherence and pharmacokinetics.
Introduction
As discussed in a previous section of this supplement, cognitive impairment is a core feature in the diagnosis of schizophrenia. In this section, we will focus on the early course of cognitive deficits in schizophrenia patients. Specifically, this paper will focus on the persistence and stability of cognitive deficits over time, and the relationship of these deficits to functional outcome. Likely factors in the persistence of cognitive deficits in schizophrenia are brain tissue changes over time, which in turn appear to be related to antipsychotic medication adherence. The data reviewed here will address the question of whether the early cognitive deficits are a core feature of schizophrenia.
What Evidence Indicates that Early Cognitive Deficits are a Core Feature of Schizophrenia?
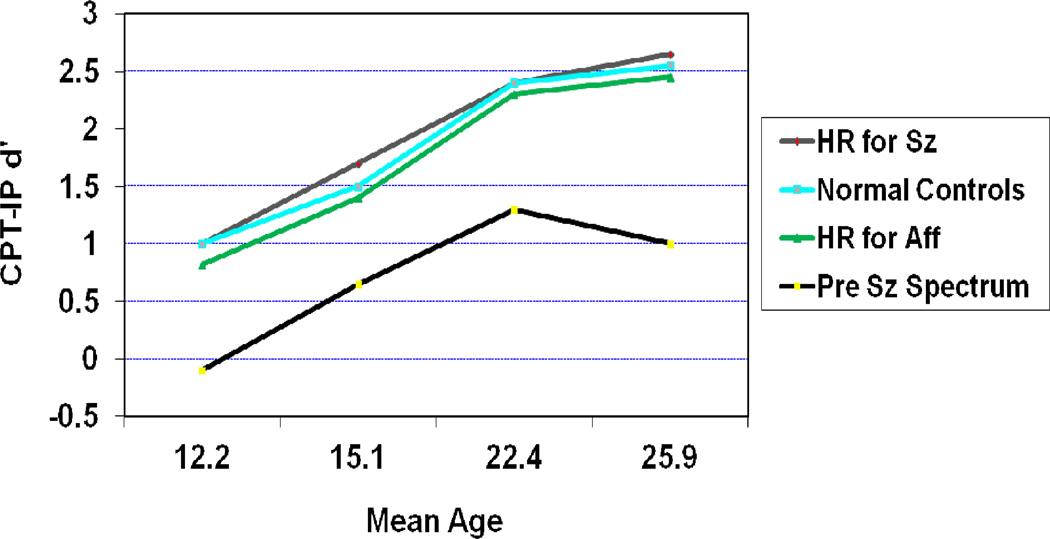
There are two main reasons that cognitive deficits are viewed as a core feature of schizophrenia. The first one centers on the fact that many studies find that cognitive deficits existed before the onset of psychosis and are capable of predicting schizophrenia in groups at high risk. The second reason is that cognitive deficits persist during symptomatic remissions and are relatively stable across time in schizophrenia patients. A good example of the first type is from the New York High Risk Study. In this study individuals who were born to a parent with schizophrenia were followed to determine who later developed a schizophrenia spectrum disorder.1 Results from this study indicated that the children of parents with schizophrenia who went on to develop a schizophrenia spectrum disorder showed major deficits on attention/vigilance tests as early as age 12 (Figure 1). These deficits were seen compared with all other groups, including those children who had a parent with an affective disorder. Though this deficit appeared to increase marginally across the period of time just before illness onset, these deficits were relatively stable over time. These data clearly indicate a decade of attentional deficit preceding illness onset.
Figure 1.
Trait-Like Deficits in Attention/Vigilance Occur Before Illness Onset (CPT-Identical Pairs Version) and Predict Schizophrenia Spectrum Disorder (reprinted from Cornblatt et al, 1999)1
Another study that clearly demonstrated these early onset cognitive deficits was part of the Developmental Processes in Schizophrenic Disorders project at UCLA. This project was a longitudinal study of schizophrenia patients who recently had a first episode of psychosis. The project focused on identifying cognitive characteristics of schizophrenia patients and discriminating: 1) Stable vulnerability indicators; 2) Mediating vulnerability factors; and 3) Episode indicators. These factors were examined by comparing healthy subjects to schizophrenia patients assessed in clinically remitted and psychotic states.2 The hypothesized vulnerability factors and the potential environmental stressors were examined initially during a standardized maintenance period of antipsychotic medication for at least a year. Schizophrenia patients who demonstrated stable remission after the first year of treatment were included in a follow up study examining the need for continuous antipsychotic medication. Results indicated that, compared with healthy subjects, schizophrenia patients had quite severe deficits on tests assessing attention/vigilance, with mean differences that were 1.3 standard deviations below the control group during remission. Further, most of the cognitive deficits were relatively stable across the psychotic and clinically remitted states in schizophrenia patients. These data indicated that the cognitive deficits are not limited to psychotic states.
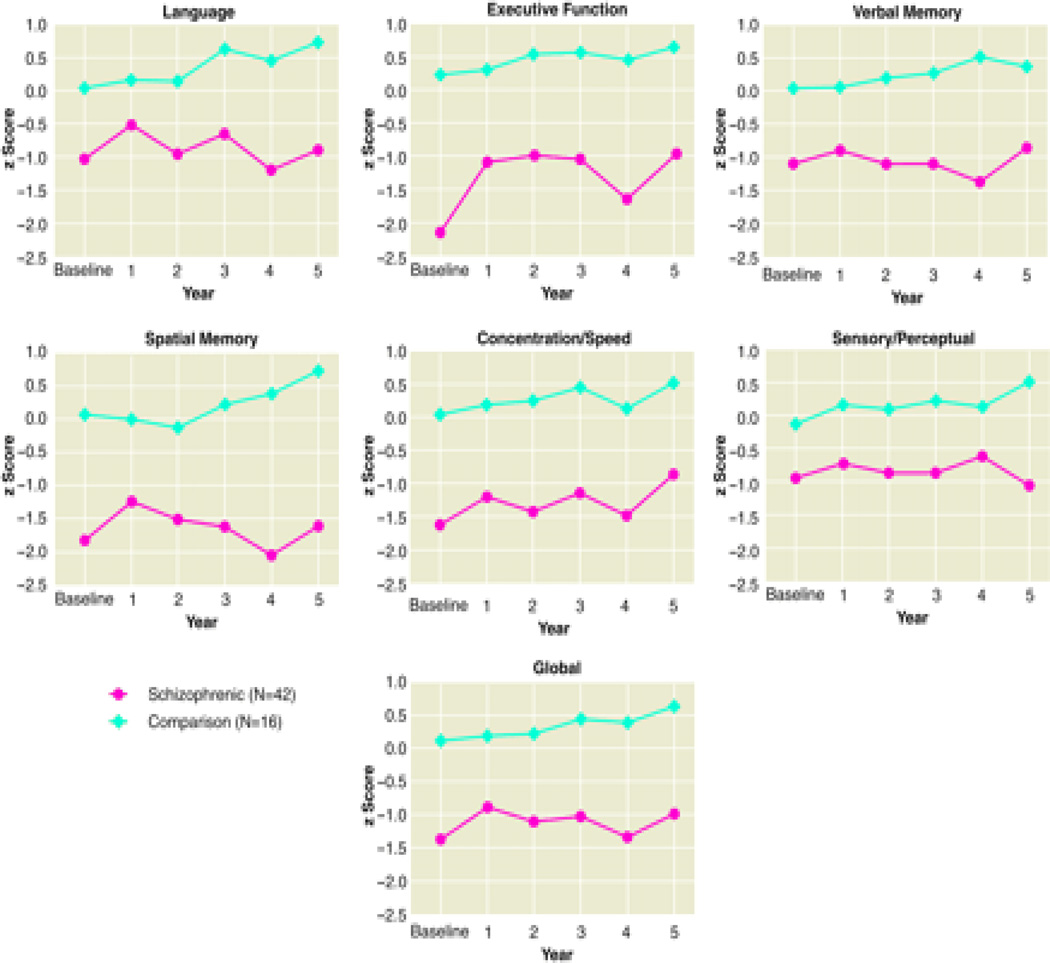
A final study reviewed attempted to determine if cognitive abilities declined, remained unchanged, or improved across the course of illness. To evaluate this, 42 patients with a first hospitalization for schizophrenia were compared with and 16 healthy controls using a battery of neuropsychological tests.3 These groups were evaluated at approximately yearly intervals for up to five years. Resulted indicated that schizophrenia patients exhibited large deficits compared with controls on virtually all neuropsychological tests, with deficits beginning at baseline (Figure 2). Furthermore, these cognitive deficits remained reasonably stable across the first five years. The conclusions from this study were that schizophrenia patients had considerable cognitive dysfunctions that were evident at the beginning of the psychotic illness. These deficits changed very little across the five year study, with stable differences of approximately 1 to 2 standard deviations below controls. Globally across all of these studies, there was little evidence supporting deterioration of cognitive abilities over the first few years of illness.
Figure 2.
Cognitive Deficits in First Episode Schizophrenia Patients across a Five Year Period (reprinted from Hoff et al, 1999)3
Are the initial levels of cognitive deficit in schizophrenia related longitudinally to functional outcome?
The previous section highlighted examples of evidence indicating that cognitive deficits are a core feature of schizophrenia. This section will examine the evidence for a longitudinal relationship of cognitive deficits to functional outcome. Despite clear evidence that cognitive impairment can predict functional outcome in chronic schizophrenia, results of studies examining this relationship in the early phase of psychosis have been mixed.4 A predictive relationship in this early period is critical because it would suggest that early intervention that improved cognitive functioning might prevent the development of chronic disability.
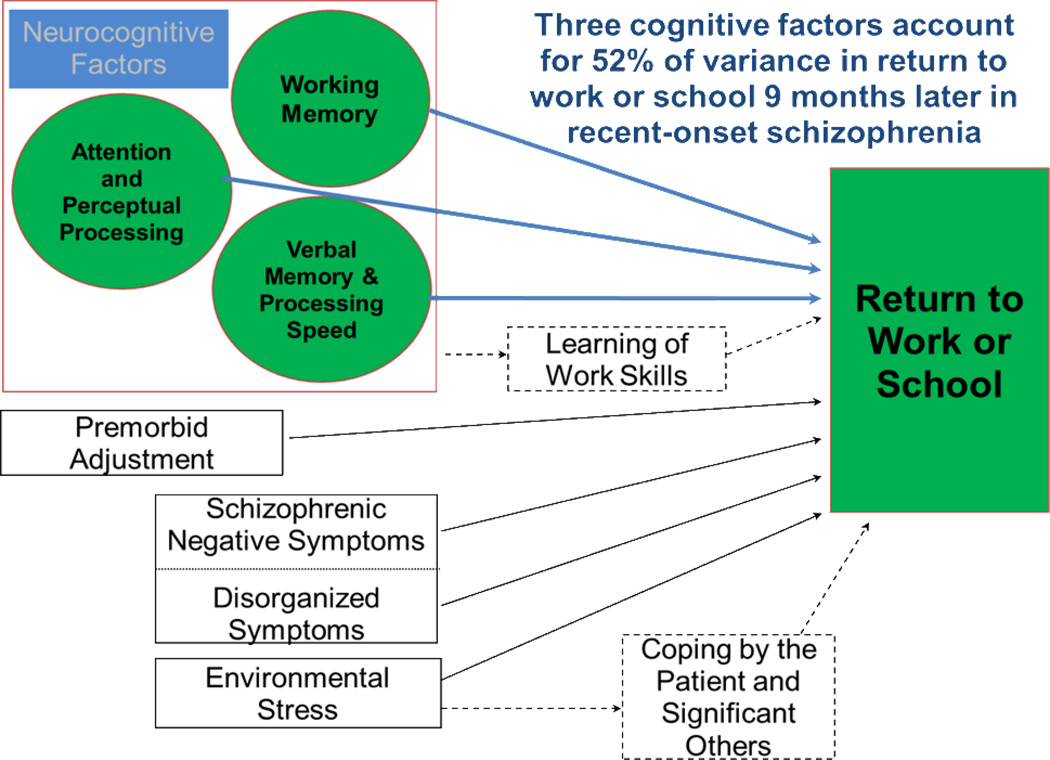
The study that we will examine assessed schizophrenia patients with a recent first episode of psychosis. Patients were assessed at a starting point when the patients were clinically stabilized, and then followed for a nine month continuous treatment period.5 Predictor variables were derived from a neurocognitive battery and included assessments of working memory, attention, early perceptual processing, and verbal memory/processing speed. A modified version of the Strauss-Carpenter Outcome Scale was used to assess return to work or school.
Results of the study indicated that the battery of neurocognitive factors predicted 52% of the variance in work outcome nine months after outpatient clinical stabilization (Figure 3). These results clearly indicated that neurocognitive factors are critical to functional outcome after an onset of schizophrenia. These data strongly suggest that interventions targeting early cognitive deficits may be crucial to the prevention of chronic disability.
Figure 3.
Neurocognitive Predictors of Work Outcome in Recent-Onset Schizophrenia (reprinted from Nuechterlein et al, 2011)5
What are the Separable Cognitive Domains in Schizophrenia and Are They All Relatively Stable?
The evidence just reviewed that supported cognitive deficits being enduring core features of schizophrenia and predictors of everyday functioning indicated that these factors should be a prominent target for treatment development. In order to develop new treatments for the core cognitive deficits in schizophrenia, the key separable cognitive domains needed to be identified in schizophrenia. These domains could then be used to develop a consensus assessment battery. In order to identify these domains, the National Institute of Mental Health (NIMH) created the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative.
An expert panel first evaluated the empirical evidence from prior studies and concluded that the consensus assessment battery should include seven cognitive domains.6 Then it evaluated more than 90 different cognitive tests.7 From these measures a final beta battery of 20 tests were administered to schizophrenia patients at baseline and then four weeks later. Based on these data, 10 were selected to represent 7 cognitive domains that make up the MATRICS Consensus Cognitive Battery (MCCB). The seven cognitive domains are: 1) Processing speed; 2) Attention/vigilance; 3) Working memory; 4) Verbal learning; 5) Visual learning; 6) Reasoning and problem solving; and 7) Social cognition.7 The final outcome from this initiative was a consensus recommendation sent to and accepted by the FDA. These recommendations were that the MCCB should be the standard cognitive assessment in clinical trials of potential cognitive enhancers and that the MCCB should always be administered in its entirety in clinical trials. To aid interpretation of scores, a computerized scoring program provides standardized scores (T-scores) based on a community comparison sample.
An important question to be answered after the development of the MCCB is now that we have a consensus battery, how stable are the data across time? We recently examined this question in a sample of first episode schizophrenia patients at UCLA.8 The goal of this randomized controlled trial was to improve cognition with treatments that included both thorough cognitive training and long acting injectable antipsychotic medications. Results indicated an overall one-year stability correlation of 0.91 for the Overall Composite MCCB score. The individual domains also all had reasonably good one-year stability, with correlations ranging from 0.65 (Verbal Learning) to 0.83 (Working Memory). These data indicated high overall stability across a year of assessment, supporting the properties of the MCCB for clinical trials. In addition, both cognitive training and long acting injectable antipsychotic medication improved cognitive functioning.
Are Changes in Intracortical Myelination in the Early Course of Schizophrenia Related to Antipsychotic Medication Adherence?
A final topic concerns the impact of antipsychotic medication adherence on changes in intracortical myelination. This area of research is very important because of the potential impact of these changes on cognitive functioning in the early course of schizophrenia.
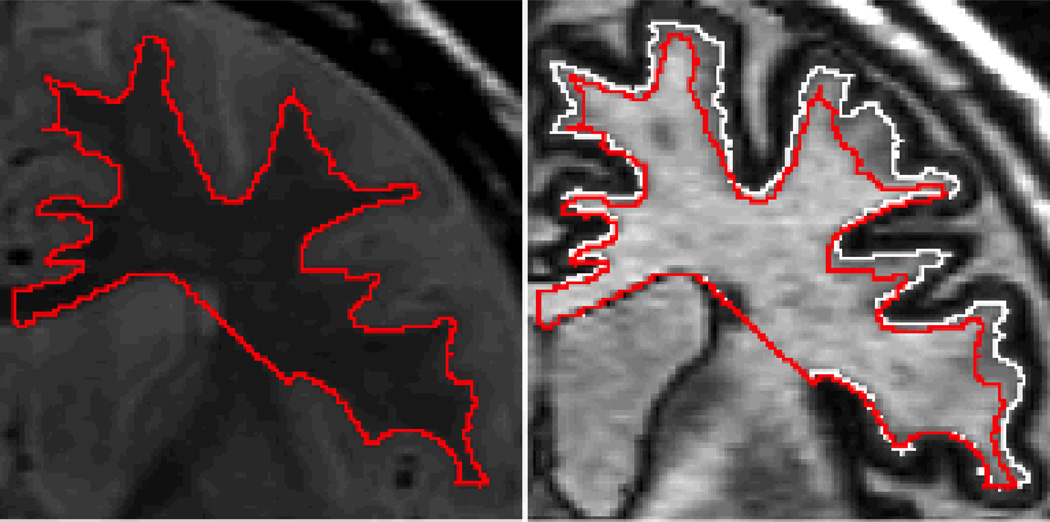
Before examining data on this topic, a brief description of the measurement process is warranted. In order to examine intracortical myelination, two magnetic resonance images are evaluated and then superimposed. The first information to examine is the proton density (PD) image (Figure 4, left panel).9 This image is sensitive to the gray/white boundary but not to the cholesterol in the myelin. In this image, the red line depicts the border between the gray and white matter. Once this border is established, it is then superimposed over the inversion recovery (IR) image (Figure 4, right panel). The IR image is very sensitive to cholesterol in myelin. The white line in this image separates myelinated from unmyelinated gray matter. The difference between the red and white lines is a measure of intracortical myelination. This method of combining the distinct tissue contrasts provided by IR and PD images can thus be used to estimate intracortical myelin (ICM) volume.
Figure 4.
Measurement of Intracortical Myelin Volume in the Frontal Lobe (reprinted from Bartzokis et al, 2009)9
The first study reviewed using this novel technology examined male schizophrenia patients treated with either oral risperidone or fluphenazine decanoate.9 These two groups were compared to healthy controls using the ICM metric and then compared to each other. The risperidone-treated group had higher ICM volume than the group treated with the typical antipsychotic, fluphenazine decanoate. These results suggest that the choice of antipsychotic treatment may have differentially impacted ICM. Further, this novel method appeared to be able to distinguish subtle differences in brain tissue characteristics and thus help clarify the effect of pharmacologic treatments on developmental and pathologic processes.
The Bartzokis et al (2009) study9 indicated that antipsychotic medications initially increased ICM volume. However, additional cross-sectional data (unpublished) also suggested that after the first year of treatment the ICM volume declined prematurely in chronic stages of the disease. The goal of the final study reviewed was to determine whether there was a relationship between changes in brain tissue and medication adherence. Further, given that the trajectory of ICM decline in chronic schizophrenia patients may be due to medication non-adherence or pharmacokinetics, the potential existed that long acting injection formulations may present an opportunity to alter the trajectory of ICM decline that typically occurs in chronic stages of the disease.
In a subsequent longitudinal randomized controlled trial, two groups of schizophrenia patients and a group of healthy controls were prospectively examined at baseline and 6 months later.10 One group of schizophrenia patients was receiving daily oral risperidone while the other was receiving twice-monthly long acting injections of risperidone. Results indicated that the schizophrenia patients who received the long acting injectable antipsychotic medication had significantly increased initial ICM volume compared with controls. In contrast, the oral dosing group had no ICM volume change versus controls. Further, patients receiving long acting injections had better medication adherence and more showed increased ICM. These data provided the first prospective evidence that long acting injectable antipsychotic medication may promote ICM development in first-episode schizophrenia patients. The better adherence possible with these injections may be able to modify the ICM trajectory.
Conclusions
There are several important conclusions to be drawn from the studies reviewed in this paper. First, cognitive deficits clearly precede the onset of psychosis and are relatively stable across 10 years of assessment of individuals at risk for schizophrenia.
Second, these initial cognitive deficits are related longitudinally to functional outcome. A recent study determined that over half of the variance in work outcome was explained by three cognitive factors after a nine month outpatient clinical stabilization period. These data strongly suggest that neurocognitive factors are critical to functional outcome, indicating that interventions targeting early cognitive deficits may be crucial to preventing chronic disability.
Third, the recently developed MATRICS Consensus Cognitive Battery (MCCB) measures seven key domains of cognitive functioning in schizophrenia. All seven of these domains in the MBBC demonstrated moderate to good stability across a one year period.
Finally, it is clear that it is vital to keep schizophrenia patients consistently on their antipsychotic medications. A novel method of examining intracortical myelin volume has provided evidence suggesting that long acting injectable antipsychotic medication may prevent patients from declining further through a combination of providing better adherence and pharmacokinetics. This consistency of antipsychotic medication may also have advantages for cognitive functioning.
Acknowledgments
This article is derived from a meeting entitled “Understanding the lifetime course of schizophrenia: A longitudinal perspective on neurobiology to promote better outcomes and recovery,” which was held in October 2013 and supported by an educational grant from Otsuka Pharmaceuticals and Lundbeck.
Footnotes
Disclosure Statement: In the last year, Dr. Nuechterlein has received consulting fees from Otsuka Pharmaceuticals and Genentech. Dr. Nuechterlein has received research grants from PositScience, Inc., Genentech, and Janssen Scientific Affairs.
References
- 1.Cornblatt B, Obuchowski M, Roberts s, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Dev and Psychopath. 1999;3:487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- 2.Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J. Developmental Processes in Schizophrenic Disorders: Longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- 3.Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am J Psychiatry. 1999;156:1336–1341. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- 4.Allott K, Liu P, Proffitt TM, Killackey E. Cognition at illness onset as a predictor of later functional outcome in early psychosis: systematic review and methodological critique. Schizophr Res. 2011;125:221–235. doi: 10.1016/j.schres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asamow RF, Gitlin MJ, Yee CM, Gretchen-Doorly D, Mintz J. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull. 2011;37:S33–S40. doi: 10.1093/schbul/sbr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 8.Nuechterlein KH, Venture J, Subotnik KL, Gretchen-Doorly G, Turner L, Casaus L, et al. Cognitive Remediation in the Initial Phase of Schizophrenia. Paper presented at the Cognitive Remediation in Psychiatry Conference; June 8, 2012; New York, NY. [Google Scholar]
- 9.Bartzokis G, Lu PH, Stewart SB, Oluwadara B, Lucas AJ, Pantages J, Pratt E, Sherin JE, Altshuler LL, Mintz J, Gitlin MJ, Subotnik KL, Nuechterlein KH. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113:322–331. doi: 10.1016/j.schres.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartzokis G, Lu PH, Raven EP, Amar CP, Detore NR, Couvrette AJ, Mintz J, Ventura J, Casaus LR, Luo JS, Subotnik KL, Nuechterlein KH. Impact on intracortical myelination trajectory of long acting injection versus oral risperidone in first-episode schizophrenia. Schizophr Res. 2012;140:122–128. doi: 10.1016/j.schres.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Research. 2004;72(1):29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]